Abstract
The placenta forms the maternal-fetal interface to protect the developing fetus from xenobiotics or pathogens. However, the understanding of complex placental features and responses to pathogens are hindered due to the lack of near-physiological models. Here, we present an engineered human placental organoid microphysiological system (MPS) incorporated with vascular endothelium, which allows to recapitulate early placental features in a vascular niche. The MPS comprises a customized insert-based organ chip and a rocker, enabling in situ differentiation and formation of placental organoids from human trophoblast stem cells under dynamic culture conditions. By incorporating vascular endothelium, trophoblast organoids (TOs) maintain improved cell viability, long-term trophoblast proliferation and differentiation. Moreover, trophoblast organoids cocultured with endothelium (EndTOs) show the activation of innate immune-related signaling pathways and high-level secretion of distinct immunomodulatory factors, including antiviral type I and III interferons and trophoblast-specific factors. We further demonstrate that EndTOs exhibit attenuated susceptibility to Zika virus (ZIKV) than single cultured TOs, indicating the crucial role of vascular niche in enhancing intrinsic antiviral defenses functions of trophoblasts. This bioinspired placental organoid MPS provides a useful platform for studying placental physiology and relevant diseases.
Similar content being viewed by others
Introduction
The placenta serves as a physical barrier to mediate maternal-fetal communication and provide immunologic defenses to protect the developing fetus from xenobiotics or pathogens. The early placenta consists of a multi-layer structure, including the trophoblast lineages, basement membrane, and fetal capillary1. During early placental development, villous cytotrophoblasts (CTBs) proliferate and fuse to form the multinucleated syncytiotrophoblasts (STBs) covering the surfaces of the human placental villous trees soaked in blood flow 2,3. The proliferative cell columns of anchoring villi give rise to extravillous trophoblasts (EVTs), which invade the maternal decidua to remodel and connect to maternal spiral arteries4. The formation of intravillous fetal capillaries are hallmarks of tertiary villus development, which plays an essential role in the development of adequate fetal circulation5. The hemochorial placentation depends on a competent fetoplacental vascular network formed by vasculogenesis and branching angiogenesis in the first trimester, allowing the exchange of gas, nutrients, and waste between the mother and the fetus6,7.
In addition to mediating maternal-fetal communication, the placenta acts as an immunological barrier to protect the developing fetus from the vertical transmission of viruses. It possesses intrinsic mechanisms of innate immune defense and is mainly resistant to infections8,9,10. The innate immune system is a primary host defense strategy to suppress viral infections and converges on the induction of interferons (IFNs). Previous studies demonstrated that primary human trophoblast (PHT) cells isolated from full-term placentas can release antiviral IFNs that restrict infection by diverse viruses in autocrine and paracrine manners11,12,13. However, the lack of biomimetic 3D placental models to study the human maternal-fetal interface during the first trimester has limited our understanding of the immune responses against congenital pathogens and the pathogenesis of pregnancy diseases.
At present, studies of placental biology and pathogen infections primarily rely on animal models, cell lines, isolated explants and organoids. Given the species difference, animal models’ placental cellular composition, anatomy, and pathological responses significantly differ from human placenta14,15. No equivalent animal systems can accurately represent human placental development. Trophoblast cell lines are easy to culture and manipulate, including immortalized or tumor cells (e.g., HTR8/SVneo, JEG-3, and BeWo). Still, they have abnormal phenotypes and genomes that differ from those of in vivo trophoblast cells. In addition, human placental cotyledon perfusion system can be used to study placental transfer and complex function, but they are limited to term placentas that may not reflect placental development at earlier stages. Recently, trophoblast organoids (TOs) have been established via 3D cultures of induced pluripotent stem cells (iPSCs)16,17, human trophoblast stem cells (hTSCs) or primary trophoblasts, recapitulating the essential morphology and functions of early placental villi18,19. These models have brought new insights into studying human placental development, viral infections, and pregnancy complications. Although organoid models represent a significant step forward in studying human trophoblasts in vitro, several limitations exist. They recapitulate only the trophoblast component of the placenta but lack other cellular components and intercellular communications, such as vascular cells, which may play an important role in regulating placental development and function. Future work is necessary to build more complex and high-fidelity models.
Recently, the convergence of organs-on-chips technology (often also referred to as microphysiological system, MPS) with organoids has led to the development of organoids-on-chips with more physiological relevance20,21,22,23. We previously developed a bioengineering macrofluidic system to generate trophoblast-like 3D tissues from iPSCs17, demonstrating the role of fluid flow in promoting the trophoblast differentiation. The synergistic technology has added advantage of providing biochemical and mechanical cues that further control stem cell differentiation and organization of organoids with high fidelity and improved reproducibility. Here, we propose a strategy to engineer hTSC-derived placental organoids by incorporating a vascular niche in a microfluidic organ chip system with periodic flow. A customized KabellyInsert™ chip consisting of multiple culture units was placed on a rocker, allowing in situ differentiation and generation of placental organoids from hTSCs and co-culture of placental organoids with endothelium under gravity-driven flow conditions. The TOs cocultured with endothelium (EndTOs) displayed long-term trophoblast proliferation, differentiation, and improved viability. EndTOs were characterized by secretome profiles and RNA-sequencing (RNA-seq) analysis, which revealed the activation of innate immune responses-related signaling pathways and enhanced secretion of antiviral IFNs. In addition, the differentiation of EVT-enriched organoids (EOs) cocultured with endothelium were identified. Upon ZIKV infection, we analyzed the susceptibility and immune response of EndTOs to the virus. The established placental organoid MPS may provide a robust platform to study early placental physiology and placenta-related gestational diseases.
Results
Design and operation of the placental organoid MPS
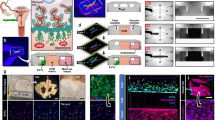
Human placenta development in vivo is a dynamic and complex process, relying on the coordinated control of inherent cues and the extracellular environment, such as the matrix, multiple cell types, and blood flow 24 (Fig. 1a). In vitro, the process of placental organoids formation from hTSCs involves stem cell self-renewal, cell aggregation formation, and differentiation of TOs or EOs (Fig. 1b). In this study, we designed and fabricated a facile and scalable MPS to engineer hTSC-derived placenta organoids in a biomimetic vascular niche. The MPS is composed of a customized KabellyInsert™ chip and a rocker. The chip is manufactured from polycarbonate, a biocompatible inert polymer. It consists of one coverlid, cell culture inserts and one baseplate that made up of 12 functional units. Each unit contains four cylindrical hole chambers with two side medium reservoirs and two central loading chambers with open access ports, in which four chambers are connected by a bottom flow channel (Fig. 1c, d and Fig. S1a–c). Two chambers in the middle can be loaded with cell culture inserts that enables the differentiation and culture of placental organoids from hTSCs, mimicking the placental villi structure. Prior to organoid formation, hTSC aggregates were embedded in Matrigel and the gel droplet was seeded on chip and subsequently in situ differentiated into 3D placental organoids at stage one (Fig. 1d). Fresh medium was introduced into the reservoirs of the cell culture unit, followed by the KabellyInsert™ chip was placed onto a rocker to achieve a gravity-driven flow that mimics blood recirculation. At stage two, endothelial cells were seeded on the lower side of the porous membrane in the insert to form an endothelial barrier, mimicking the fetal capillaries (Fig. 1d). The porous membrane can separate the placental organoids and vascular endothelium, which enables the interconnection of the medium flow in the upper and lower channels of the chip. The vasculature-like fluid flow conditions in the lower channel permitted the sufficient exchange of nutrients and the diffusion of secreted factors between endothelium and placental organoids.
a Schematic representation of the in vivo placental villi, which are formed and located at the interface between maternal and fetal blood during the first trimester. The placental villi are composed of fetal endothelial layer, basal membrane and trophoblastic epithelium layer. The trophoblastic epithelium consists of the STB layer and the underlying CTB layer. b The development process of trophoblast organoids (TOs) and extravillous trophoblast organoids (EOs) derived from hTSCs in vitro. The hTSCs were derived from trophectoderm of blastocysts, which can differentiate into three major trophoblast lineages (CTB, STB and EVT). c Configuration of the organ chip system containing a customized KabellyInsert™ chip and rocker. The chip plate contains multiply culture units, in which one unit consists of medium reservoir, cell culture chamber and bottom flow channel. A transwell insert was loaded into the middle culture chamber. d The placental organoids-on-a-chip was constructed by coculture of placental organoids and vascular endothelium layer (HUVECs) under dynamic cultures by gravity-driven flow. The coculture medium was perfused through the microchannel to deliver nutrients to organoids and to expose the endothelium layer to shear stress. The phenotype and function of placental organoids model were analyzed using the different indicated methods. This image and every element of this image was created by the authors.
The organ chip system was capable of achieving pumpless gravity-driven flow for recirculating culture media between the chambers at a set flow rate on a bi-directional laboratory rocker. To analyze and characterize the fluid flow in the organ chip, we performed computational fluid dynamics simulations (Fig. S1d and S1e). Due to the fluidic resistance of the porous membrane, the convective fluid flow is confined to the media channels. We conducted a time-resolved model of velocity and flow shear stress in the upper and lower channels of the chip within one cycle (6 s). The design of central chambers is modular and scalable, and two chambers can be connected to model different physiological scenarios under the same outside geometry and customized interiors. The modular design allows organoids to be placed into the organ chip with customized configurations, such as tissue types, replicates, and tissue-tissue interactions. We next evaluated the development and generation of placental organoids under dynamic cultures to validate the organ chip utility.
Generation and characterization of trophoblast stem cell-derived 3D placental organoids on chip
To directly differentiate hTSCs into 3D placental organoids in a controlled way, uniform hTSC aggregates were initially formed via 3D culture in the concave well. HTSCs aggregates were then embedded in Matrigel, and the mixture droplet was transferred into the upper chamber of the insert on chip (Fig. 2a (i)), which promotes trophoblasts self-renewal, differentiation, and formation of TOs with Wnt inducers (Wnt+). As shown in Fig. 2b, small cell aggregates rapidly grew and developed into TOs with cavity structures after six days of culture with defined chemical factors. The average size of organoids was increased due to trophoblast epithelium differentiation and expansion over time. One of the placental hormones, human chorionic gonadotropin (hCG), is secreted by STBs and serves as a biochemical marker of trophoblast differentiation. The positive detection of hCG secreted by TOs (approximately 60 TOs per milliliter) at day 6 using ELISA and test strip suggested the secretory activity in STBs (Fig. 2c, d). Immunofluorescence data showed that the CTB marker (GATA3 and TEAD4) and proliferative marker Ki67 expressed in the out layers of TOs at day 6 (Fig. 2e). The TOs also expressed the STB marker CGB, confirming their trophoblast identity. The TOs formed polarized trophoblast epithelium surrounding a cavity with STB marker ENDOU at the apical surface and basal E-cadherin expression, reminiscent of the placental villi structure (Fig. 2a (ii) and 2e). In vivo, STB contacts maternal blood in the intervillous space. However, STB forms in the center of organoids and the inside-out architecture of TOs is different from native placental villous epithelium (Fig. 2a (ii)). Human trophoblasts can also differentiate to EVTs, which is crucial for proper placentation. In TOM with Wnt+, TOs showed only sporadic expression of EVT marker HLA-G. Moreover, flow cytometry was performed in TOs to confirm the trophoblast subtypes. The percentage of cell populations revealed ~87% proliferative ITGA2+ (CD49b) cells (Fig. 2f) and ~52% CGB+ cells (Fig. S2f). A high proportion of proliferating trophoblast precursors implied the high potential of these organoids to generate villous lineages.
a The illustration of the development process of TOs on chip. The hTSCs aggregates were embedded in Matrigel and the droplet was seeded on a porous membrane of the insert in the chip device, followed by differentiated into TO in differentiation medium (TOM) with Wnt activators (Wnt+) under dynamic cultures (i). In vivo, STB contacts maternal blood in the intervillous space but forms in the center of organoids in vitro (ii). b Representative bright-field images of TOs in differentiation medium at different development stages. Scale bars: 100μm. c Over the counter pregnancy tests for hCG in TOs. d Levels of hCG in conditioned medium isolated from TOs as determined by ELISA kit (bottom). e Confocal microscopy in TOs immunostained for GATA3, TEAD4, ENDOU, CGB (in red) and Ki67, CGB, E-cadherin, HLA-G (in green) on day 6. DAPI stained nuclei are shown in blue. Scale bars: 50μm. f FACS analysis of TOs stained with anti-ITGA2 (Red), representing the cells at the base of the cytotrophoblast cell columns. The experiments have been performed twice. g Relative mRNA expression of GATA3, TEAD4, ITGA6, CGB3, CGA, SDC1, HLA-G and ITGA5 in hTSCs and TO mRNA expression normalized to GAPDH RNA level was analyzed by real-time PCR (n = 3). h The illustration of the development process of EO on chip. The hTSCs aggregates were maintained in TOM for 3 days and switched to differentiation medium withdrawal of Wnt activators (Wnt-) under dynamic cultures, then promoting EVT trophoblast outgrowth in organoids (EOs). Representative bright-field images of TOs and EOs on day 6. Scale bars: 100 μm. i Confocal microscopy in EOs immunostained for GATA3 (in red) and HLA-G, Ki67 (in green) on day 6. DAPI stained nuclei are shown in blue. Scale bars: 50μm. j Levels of MMP-2 in the media of TOs and EOs as assessed by Luminex assays (n = 3). k Relative mRNA expression of CGB3, TEAD4, HLA-G, ELF5, TCF7L1, EBRR2 and NOTCH1 in TOs and EOs. mRNA expression normalized to GAPDH RNA level was analyzed by real-time PCR (n = 3). In (g), (j), and (k), The data are presented as the mean ± SD from three independent experiments. Data significance was assessed by unpaired two-tailed Student’s t test; *p < 0.05,**p < 0.01,***p < 0.001.
To further identify the formation of TOs, the respective mRNAs were detectable in TOs using RT-qPCR. As expected, the expression of the pluripotency (GATA3) and CTB markers (TEAD4 and ITGA6) were decreased in TOs at day 6 of differentiation relative to the control of hTSCs (Fig. 2g). In contrast, the STB-specific markers (CGB3, CGA and SDC1) were significantly upregulated (Fig. 2g). Similarly, immunostaining images of TOs at days 4 and 6 showed increased expression of CGB as organoids grew in size during the developmental period (Fig. S2d, e). These data indicate the efficient differentiation of trophoblast lineages in organoids from hTSCs during development. However, when TOs were differentiated up to day 11, the expression of TEAD4, STB-related genes (CGB3 and CGA) and EVT markers (ITGA5) were downregulated compared to that in TOs at day 6 (Fig. S2a–c). It indicates that TOs cannot be cultured for extended periods without being passaged.
Generally, invasive trophoblast-derived EVT is crucial for pregnancy success as it invades the maternal decidua to transform the spiral arteries. According to previous studies, withdrawing Wnt inducers (R-spondin and CHIR99021) promoted EVT outgrowth and differentiation18. In our organoid on chip system, after being cultured in TOM for three days, trophoblast spheroids were transferred into the differentiation medium without Wnt inducers (Wnt-) to generate EOs with additional three days of differentiation (Fig. 2h). The EOs showed obvious trophoblast outgrowth from the outer CTB layers and HLA-G expression through microscopy and immunofluorescence analyses (Fig. 2i). Luminex assay showed a significantly higher level of MMP-2 in the conditioned media from EOs than that from TOs (Fig. 2j). Further, EOs under Wnt- condition showed decreased expression of the CTB self-renewal markers (ELF5, TEAD4) and upregulated EVT markers (HLA-G, TCF7L1, and ERBB2) and EVT progenitor marker NOTCH1 (Fig. 2k). These data suggest the efficient EVT differentiation in EOs in the perfused MPS.
Long-term survival and differentiation of trophoblast organoids in a vascular niche
The developmental placenta is highly vascularized, crucial for viable fetal growth and well-being. During the first trimester, extraembryonic mesoderm cells migrate from the embryo and differentiate into endothelial cells, forming fetal capillaries. The vascular network in the placental villi increases the blood supply and facilitates placental villi development and mother-fetus nutrient exchange. To mimic the vascular niche, we initially generate an endothelial barrier by seeding HUVEC underneath of the porous membrane in the insert (Fig. 3a). We found that various sizes of membrane pores in the insert can support the formation of confluent endothelium with the expression of vascular gap junctions (Fig. S3a). Membranes with different pore size (0.4 μm and 3 μm) showed minor but significant TEER differences (Fig. S3b). We also evaluated the barrier’s permeability with FITC-dextran and determined the formation of tight endothelial barrier on the membrane with different pore sizes (Fig. S3c and S3d). In vivo, human placental villi are exposed to low rate of maternal blood flow in the first trimester14,24. In order to recapitulate the physiologically relevant microenvironment of earlier placental development, we chose a low flow rate under dynamic cultures. The data showed that low flow rate (2 rpm/min) could maintain more stable vascular barrier integrity compared to static cultures (Fig. S3e and S3f).
a Schematic of the coculture of TOs with vascular endothelium on chip, mimicking the maternal side and fetal side, representatively. The vascular endothelial cells (HUVECs) were seeded on the other side of insert membrane to form an endothelium monolayer, then TOs on day 6 were transferred into the upper side of insert membrane cocultured up to 6 days. b Representative bright-field images of TOs and EndTOs on day 12 on chip. Scale bars: 100 μm. c Size distribution was assessed by the average diameter of TOs (n = 25) and EndTOs (n = 25) on day 12. d Confocal microscopy in TOs and EndTOs immunostained for E-cadherin (in red) and Caspase 3 (in green). Scale bars: 50μm. e Quantifications for the percentage of Caspase 3+ cells in TOs and EndTOs by Image J software. The measurements were taken from distinct organoid samples (n = 9). f Relative mRNA expression of Caspase 3 in TOs and EndTOs (n = 3). g–i Confocal microscopy in TOs and EndTOs immunostained for TEAD4, CGB, KRT7 (in red) and GATA3, E-cadherin, Ki67 (in green) on day 12. DAPI stained nuclei are shown in blue. Scale bars: 50 μm. j Quantifications for the percentage of TEAD4+, Ki67+ and CGB+ cells in TOs and EndTOs based on fluorescence images by Image J software. Six distinct organoid samples were randomly analyzed. k Relative mRNA expression of ITGA6, ITGA2, TEAD4, SDC1, CGB3 and ITGA5 in TOs and EndTOs. mRNA expression normalized to GAPDH RNA level was analyzed by real-time PCR (n = 3). In (d–f), (j) and (k), the data are presented as the mean ± SD from three independent experiments. Data significance was assessed by unpaired two-tailed Student’s t test; *p < 0.05,**p < 0.01,***p < 0.001.
To include placental organoids in a vascular niche, we generated TOs incorporated with vascular endothelium (EndTOs) in the organoid MPS. TOs were added into the upper chamber of the insert to enable co-culture of TOs and endothelium (Fig. 3a). We initially optimized the common medium of cocultured TOs and endothelium on chip. We found that HUVEC showed markedly proliferation and formation of confluent endothelium layer after coculture of TOs in the TOM/EGM2 mixture (1:1) media rather than TOM (Fig. S4a). In addition, the expression of trophoblast genes including GATA3, TEAD4, CGB3, and SDC1 showed no significant changes in EndTOs under TOM/EGM2 medium compared to TOM conditions, while HLA-G expression was upregulated in EndTOs in TOM/EGM2 medium (Fig. S4b). Notably, over six days of coculture in the optimized TOM/EGM2 media, organoid maintained growth and proliferation and formed large and characteristic cavity-like structures (Fig. 3b). The analysis of organoid size distribution showed that EndTOs can grow up to about 600 μm average diameter without passage culture during the long-term period, larger than single-cultured TOs on day 12 (Fig. 3c). Moreover, EndTOs exhibited higher cell viability with reduced cell death (~8% caspase3-positive of cells) under perfused cocultures on chip compared to single-cultured TOs (~28% caspase3-positive of cells) as determined by immunostaining for active caspase 3 on day 12 (Fig. 3d, e). Similarly, the expression of caspase 3 was markedly upregulated in TOs (Fig. 3f). Electron microscopy showed the formation of surface microvilli and transport vesicles in the multinucleated cells of EndTOs resembling in vivo villi structure (Fig. S4c). In addition, the secretion of hCG in the supernatants of EndTOs was detected by ELISA (Fig. S4d). These data indicated that endothelium cocultures could facilitate favorable cell viability, trophoblast epithelium proliferation, and long-term culture of organoids with good architecture and functions by providing a biomimetic vascular niche.
To further examine the features of the trophoblast differentiation in EndTOs, immunohistochemical analysis of specific markers was performed. As expected, high proportions of positive cells for TEAD4, Ki67, and KRT7 were presented in EndTOs at day 12 (Fig. 3g, i, j), forming proliferative trophoblast populations. Compared with TOs, EndTOs revealed larger and more continuous trophoblast epithelium cavity-like organization. Different from the architecture of native placental villi, E-cadherin+ CTBs lined along the CGB+ STBs with an outside-in cavity in both TOs and EndTOs (Fig. 3h). Moreover, 3D volume render of the organoid structure showed the tight junctions distribution on the surface (Fig. 3h). The percentage of CGB+ cells decreased to ~38% in EndTOs compared to TOs (~47% of CGB+ cells) during long-term cultures, accompanied by the increase of proliferative cell markers Ki67 and TEAD4 (Fig. 3j). In addition, the expression profiles of genes representative of trophoblast lineage were analyzed in TOs and EndTOs at day 12 of differentiation by RT-qPCR. Consistently, the expressions of CTB markers (ITGA6, TEAD4 and ITGA2) were increased in EndTOs as the differentiation of organoids progression, while the expressions of STB markers (CGB3 and SDC1) were decreased (Fig. 3k). Similarly, the expression of syncytia gene ADAM12 was decreased while KRT7 showed no significant change (Fig. S8f). These data validated the maintenance of proliferative trophoblasts and proper differentiation of STB and cell fusion in EndTOs, which may facilitate the integrity of trophoblast epithelium, long-term survival and growth of organoids.
Enhanced differentiation of EVT-enriched organoids (EOs) in a vascular niche
EVTs invade the maternal decidua to remodel and connect to maternal spiral arteries, which is crucial for pregnancy success. Early placental development involves coordinated trophoblast outgrowth and angiogenesis, mediated by niche growth factors. To investigate the interactions between EVT and vascular endothelium, we integrated endothelium cocultures with EOs (EndEOs) for up to 6 days in the MPS (Fig. S5a). The differentiation of EVTs in EOs and EndEOs was identified by immunohistochemical analysis and RT-qPCR. Notably, the expressions of EVT marker HLA-G were significantly increased in EndEOs, while STB markers (CGB3 and SDC1) were decreased by RT-qPCR (Fig. S5b). There was no significant change in the CTB marker ITGA6 expression. Similarly, immunohistochemical analysis identified a high expression of HLA-G along the peripheral organoids (Fig. S5c–e), suggesting the differentiation of invasive EVTs in EndEOs. Quantitative analysis showed an increased proportion of EVT+ cells (~27% to 7% of HLA-G+ cells) and a decreased proportion of CGB+ cells (~28% to 40% of CGB + cells) in EndEOs (Fig. S5f). These data implied that the presence of endothelial cells enhanced the induction of EVTs in EndEOs.
In addition, we evaluated the morphology and angiogenic response of vascular endothelium under different culture conditions. Immunostaining analysis showed the outgrowth and high dense distribution of HUVECs under TOs or EOs cocultures compared to HUVECs single cultures (Fig. S6a). Quantitative image analysis showed the number of endothelial cells per unit area was significantly increased in the presence of trophoblasts, suggesting that trophoblasts promoted the proliferation of endothelial cells (Fig. S6b). Notably, the endothelial cell number was relatively deceased in EndEOs cultures than that in EndTOs cultures, possibly due to EVT migration reducing vascular network formation. Furthermore, the levels of PDGF-BB, one of potent angiogenic factors25, secreted by HUVEC, EC cocultured with TOs (TO-EC) and EC cocultured with EOs (EO-EC) were examined by ELISA (Fig. S6c). Moreover, other angiogenic factors, such as PDGF-AA, angiogenin, FGF, PIGF, endostatin and TIMP-1, generated from TO-EC and EO-EC were analyzed using Luminex-based multianalyte. The data showed significantly upregulated levels of these angiogenic factors in the TO-EC and EO-EC compared to HUVEC (Fig. S6d), indicating the augmented angiogenic responses. Similarly, the mRNA level of angiogenic-related markers such as VEGFA, PECAM1, and VEGFR were significantly upregulated in endothelial cells with trophoblast cocultures (Fig. S6e). These results revealed autocrine or paracrine role in synergy cell communications between vascular endothelium and trophoblasts during early gestation.
Transcriptional profiling and secretome analysis of EndTOs
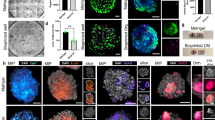
To further characterize the established engineered placental organoids, we performed transcriptional profiling of EndTOs using bulk RNA-Seq. Hierarchical clustering of differentially expressed genes (DEGs) in the heatmap showed TOs and EndTOs were transcriptionally distinct (Fig. 4a). Volcano plots showed that 184 down-regulated genes and 278 upregulated genes were notably modulated in EndTOs (Fig. 4b). Notably, the enriched interferon-inducible genes, such as IFIT1, IFIT2, IFIT3, and DDX60, were up-regulated in EndTOs (Fig. 4b), which are closely associated with immune regulation during early placentation. In addition, the up-regulated gene LGALS3BP (a galectin-3 binding protein) is associated with cell adhesion and abundantly expressed at the maternal-fetal interface (Fig. 4b), playing a crucial role in successful implantation and maintenance of pregnancy. Gene ontology (GO) analysis identified enhanced biological processes, including type I interferon signaling pathway and innate immune response in EndTOs (Fig. 4c, Fig. S7a). Moreover, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis showed that Hippo, PI3K-Akt, focal adhesion, cell adhesion molecules and RIG-I-like receptor signaling pathways were enriched in EndTOs (Fig. 4d, Fig. S7b–d). These pathways were related to trophoblast proliferation, differentiation, and innate immune functions. Genes related to type I interferon signaling pathway and innate immune response were also verified in EndTOs by RT-qPCR (Fig. 4e). We further compared the IFNs genes (IFN-α1, IFN-β, IFN-λ1 and IFN-λ2) expression in TOs and EndTOs during development. The data showed significantly increased expression of all IFNs in EndTOs at days 10 and 12 while no obvious changes of IFN expression in TOs at distinct stages (Fig. S8a–d), suggesting the crucial rule of vascular niche in improving innate immune functions of trophoblasts. In addition, angiogenesis-related genes (e.g., PDGFA, ANGPTL6, FLT4) were up-regulated in EndTOs (Fig. S7e), which may play important roles in regulating trophoblast development.
a Hierarchical clustering heat map for differentially expressed genes (DEGs) between trophoblast cells of TOs and EndTOs. The gradient color scale at the right top indicates the log2 (fold change) in the expression of the treatment case compared with the control case. b Volcano plots showing the differential gene expression of EndTOs versus TOs. Genes differentially expressed with fold change over 2.0 and p < 0.05 were marked in color. P values were calculated using a two-sided, unpaired Student’s t test with equal variance assumed (n = 3). c GO analysis between TOs and EndTOs. d KEGG functional classification of the DEGs between TOs and EndTOs. The color of the dots represents the rich factor, while the size represents the input number of genes for each KEGG term. e Validation of selected DEGs associated with innate immune response identified by RNA-seq using RT-qPCR (n = 3). The expression values were normalized to GAPDH. Data were normalized against mono-culture expression values and are shown as mean ± SD; Unpaired two-tailed Student’s t test, *p < 0.05, ***p < 0.001. f, h Heatmap of 3 conditioned medium preparations generated from the TOs and EndTOs on day 12 on chip analyzed by Luminex-based multianalyte profiling for pentraxin-3, IL-8 (f) and the other indicated cytokines, chemokines, and growth factors (h). Scale is shown at right. g Comparison of the levels of pentraxin-3 and IL-8 in conditioned medium from TOs and EndTOs (n = 3). h Heatmap (based on log2 RPKM values) of transcripts expressed in TOs and EndTOs that are associated with DEGs of type I interferon signaling pathway. Red indicates high levels of expression, blue indicates low levels of expression. Hierarchical clustering is shown on left and top. i Comparison of the levels of type I IFNs IFN-α2 and IFN-β and type III IFNs IFN-λ1 and IFN-λ2 in conditioned medium from TOs and EndTOs (n = 3). In (g) and (i), each symbol represents an individual medium preparation, and data significance was assessed by unpaired two-tailed Student’s t test; *p < 0.05, ***p < 0.001.
Previous studies have shown that human chorionic villous explants or PHTs release numerous cytokines, chemokines, and growth factors that impact maternal and fetal antimicrobial/antiviral defenses12,26,27. Therefore, we profiled the immunological secretome TOs and EOs to define basal differences in trophoblasts under different culture conditions. We performed multianalyte Luminex-based profiling of 37 cytokines, chemokines, growth, and other factors from conditioned media isolated from TOs and EOs at day 12. Factors detected over 50 pg/mL were defined as present in conditioned media. We found that TOs secreted cytokines (IL-6, IL-26, IL-32, and IL-28A/IFN-λ2), immune-regulated secreted soluble receptors (sTNF-R1, sTNF-R2, gp130/sIL-6Rβ, BAFF/TNFSF13B, sCD163) and Osteopontin (OPN) at baseline (Fig. S9a). EOs secreted a high level of MMP2 apart from other similar cytokines and soluble factors TOs (Fig. S9b). Previous studies showed that PHTs isolated from full-term placentas and mid-gestation chorionic villi constitutively release type III IFNs, which protect trophoblasts from viral infections11,12. We found TOs recapitulated this phenotype and released high levels of IFN-λ2 and less detectable IFN-λ1, but not type I IFNs (IFN-α2, IFN-β) and IFN-γ, while EOs only basally secrete IFN-λ2 (Fig. S9c, d). We further performed multianalyte Luminex profiling in EndTOs and EndEOs under coculture conditions. Cytokines and chemokines induced by endothelium coculture with levels >10-fold above TOs or EOs were highlighted. The topmost induced factors in EndTOs and EndEOs were the cytokines Pentraxin-3 and IL-8 (Fig. 4f, g, and Fig. S10a). EndTOs released a larger number of immune factors including immune-regulated secreted soluble receptors (gp130/sIL-6Rβ, BAFF/TNFSF13B, sCD30/TNFSF13B), inflammatory cytokines (IL-22, IL-11, IL-20, IL-12, LIGHT/TNFSF14 and TWEAK/TNFSF12) and trophoblastic factors (MMP2, Chitinase 3-like 1, TSLP) (Fig. 4h, i). Notably, the secretion of type I (IFN-α2, IFN-β) and III IFNs (IFN-λ2 and IFN-λ1) were significantly enhanced in EndTOs (Fig. 4j), indicating the improved innate immune function of trohpoblasts. In addition, EndEOs exhibited higher level of secreted MMP2 compared to that in EndTOs or EOs (Fig. S10b–d), revealing the efficient EVT differentiation in EndEOs and enhanced EVTs development induced by vascular niche. Above data suggested that the vascular niche could promote placental organoids’ proliferation and differentiation and enhance inherent immune functions of trophoblasts.
Susceptibility of EndTOs to ZIKV infection
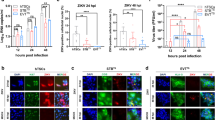
ZIKV is one of the common vertically transmitted pathogens, which is associated with high rates of placental dysfunction and adverse pregnancy outcomes, including preterm birth, fetal/neonatal defects and congenital disease. To further characterize the susceptibility of trophoblasts to ZIKV infection in the vascular niche, we examined the biomarker expression and viral infectivity in TOs and EndTOs (Fig. 5a). Initially, TOs at day 6 were infected with ZIKV at a MOI of 0.1 or 1, followed by re-embedding in Matrigel and cocultured with endothelium layer for additional 6 days. We examined the level of ZIKV RNA by RT-qPCR and found increased ZIKV infection in TOs and EndTOs in a virus titer-dependent manner, while a lower level of viral RNA in EndTOs than that in TOs (Fig. 5b). It indicates the less susceptibility of EndTOs than TOs to ZIKV infection. Immunofluorescent staining showed the co-expression of ZIKV NS2B and E-cadherin proteins in TOs and EndTOs (Fig. 5c), determining the viral infection and replication mainly in trophoblast epithelium. Moreover, more ZIKV NS2B-positive cells were observed TOs (~10%) than that in EndTOs (~3%) (Fig. 5d), which is consistently with gene expression analysis.
a Schematic of ZIKV infections of TOs and EndTOs on chip that assessed by IF, RT-qPCR and RNA-seq analysis. b Level of ZIKV replication was assessed by viral RNA as determined by qRT-PCR in TOs and EndTOs infected with ZIKV (KU501215) at 0.1MOI (red) and 1MOI (blue). Data in all panels are mean ± SD (n = 3). Significance was determined by one-way ANOVA with Tukey’s post-test, ***p < 0.001. c TOs and EndTOs were infected with ZIKV for 6 days and assessed by immunostaining for E-cadherin (green) and ZIKV NS2B (red). Scale bars: 50μm. d Quantifications for the percentage of ZIKV NS2B+ cells in TOs and EndTOs with ZIKV infection (n = 7). e Read coverage of viral reads along the ZIKV genome for the infected TO, EndTO and TO-EC. The graph indicated the viral reads number per position of the viral genome in the infected cells. This graph is representative of three independent experiments. f The ratio of virus-aligned reads over total reads is indicated for the viral replication level for each sample (n = 3). In (d) and (f), The data are presented as the mean ± SD from three independent experiments. Data significance was assessed by unpaired two-tailed Student’s t test; ***p < 0.001. g Relative mRNA expression of IFN-λ1, IFN-λ2 and IFN-β in TOs and EndTOs with or without ZIKV infection. The data are presented as the mean ± SD (n = 3). Significance was determined by one-way ANOVA with Tukey’s post-test, *p < 0.05, **p < 0.01, ***p < 0.001.
In order to check the viral infection efficiency in trophoblasts under different culture conditions and endothelium, we performed RNA-seq analysis of TO, EndTO and TO-EC following viral infection on chip, respectively. The ratio of virus-aligned reads over total reads in each sample was calculated to estimate the viral replication levels in these three cell types. The results showed that the ratio of viral reads in TOs is much higher than EndTOs (Fig. 5e, f), which are consistent with the immunostaining analysis. Besides, the ratio of viral reads in EndTOs is much higher than TO-EC (Fig. 5e, f), suggesting that ZIKV can primarily infect in the trophoblasts under coculture conditions, but not in endothelial cells. In general, ZIKV infection can trigger antiviral or immune defense responses in PHTs, especially induce IFNs production11. In order to identify the specific host defense responses to ZIKV infection, we examined the gene expression of IFNs in EndTOs induced by viral infection. The data showed that TOs expressed high levels of IFN-λ1, IFN-λ2 and IFN-β mRNA in a virus titer-dependent manner (Fig. 5g). Although the fold change of IFN expression was lower in viral-infected EndTOs compared to TOs, the expressions of Type I and III IFNs were significantly upregulated in EndTOs induced by 1MOI ZIKV infection (Fig. 5g), suggesting that EndTOs were highly resistant to viral infection. TAM family including AXL, TYRO3 and MERTK were suggested as potential viral entry receptors in ZIKV infection of human host cells28. To explore the role of these potential host factors in ZIKV infection of EndTOs, we examined the expression of AXL, TYRO3 and MERTK in TOs and EndTOs by RT-qPCR. The data showed reduced expression of TYRO3 and MERTK in EndTOs compared to TOs, while AXL expression showed no significant changes (Fig. S8e). It indicates that AXL may not confer permissiveness to ZIKV infection in EndTOs.
In addition, we examined the responses of endothelial cells to ZIKV infection under different conditions, including single HUVEC and TO-EC. The data showed that HUVECs were susceptible to ZIKV identified by ZIKV NS2B staining, while TO-ECs showed weak expression of ZIKV NS2B (Fig. S11a, b). Moreover, endothelial cells in the coculture system did not induce IFNs production after viral infection identified by IFNs mRNA expression (Fig. S11c), indicating the antiviral responses of cocultured endothelium. To evaluate the barrier function of endothelium cocultured with the mock- and ZIKV-infected TOs, the TEER analysis showed no significant effects of ZIKV infection on the integrity of endothelium in the coculture system (Fig. S11d). These results suggest that endothelial cells cocultured with TOs were less susceptible to ZIKV infection, which may be explained by the high resistant to viral infection in EndTOs.
Evaluation of transcriptional and immune response of EndTOs to ZIKV infection
To fully understand the transcriptional responses of EndTOs to ZIKV infection, we next profiled the transcriptional changes induced by ZIKV treatment TOs and EndTOs by RNA-seq analysis. We found that ZIKV infection induced significant transcriptional changes in TOs and EndTOs (Fig. 6a). Comparison of the transcripts TOs and EndTOs induced by ZIKV infection revealed that 656 DEGs (423 down-regulated genes and 233 upregulated genes) were significantly modulated TOs, while 258 DEGs (100 down-regulated genes and 158 upregulated genes) were significantly modulated in EndTOs. Of these, only 53 overlapping upregulated DEGs and 6 downregulated DEGs zzwere shared between TOs and EndTOs (Fig. 6b). GO enrichment analysis was further performed to identify the host responses to ZIKV infection. It is noted, ZIKV infection induced a broad innate immune response and defense responses to virus, including type I interferon, RIG-I-like receptor and cytokine-mediated signaling pathways in EndTOs, which was similar to that in TOs (Fig. 6c, d, Fig. S12b–e). Moreover, among the upregulated genes associated with type I interferon signaling pathway, we identified the enhanced induction of IFN-stimulated genes (ISGs) in infected TOs and EndTOs, including IFIT1, OAS1, ISG15 (Fig. 6e, f). Comparison of the DEGs related to type I interferon signaling pathway suggested the enhanced properties of immune defense to infection with reduced induction of ISGs in EndTOs. In addition, ZIKV infection has a slight effect on trophoblast-related genes expression (Fig. S12a). To confirm the innate immune responses to ZIKV infection in EndTOs, we performed parallel multianalyte Luminex profiling of 37 cytokines and chemokines in EndTOs infected with ZIKV. Cytokines and chemokines with levels >1.5-fold above the mock group were defined as induced by ZIKV infection. We found that EndTOs secreted only four cytokines in response to ZIKV infection, including pentraxin-3 (PTX3), IFN-λ1, IFN-λ2 and IFN-β (Fig. 6g, h). Other cytokines or chemokines were basally expressed at high levels in TOs, but were not further induced by ZIKV. These findings confirmed that high-level secretion of IFNs and enhanced innate immune defense functions in EndTOs under biomimetic vascular niche, which could be resistant to viral infections in trophoblasts.
a Scatter plots of differentially expressed genes (DEGs) in TO ZIKV and EndTO ZIKV at day 6 post-infection. Genes differentially expressed with fold change over 2.0 and p < 0.05 were marked in color. b Venn diagrams of up- and down-regulated DEGs in TO ZIKV (blue circle) or EndTO ZIKV (orange circle). c, d GO analysis of the up-regulated DEGs between TO ZIKV (c) and EndTO ZIKV (d). e, f Heatmap (based on log2 RPKM values) of transcripts expressed in TO ZIKV (e) and EndTO ZIKV (f) that are associated with DEGs of type I interferon signaling pathway. g Heatmap of the induction of factors at left (shown as fold change from mock-treated controls) from EndTO ZIKV by Luminex-based multianalyte profiling. Asterisks indicate factors induced >1.5-fold change. Scale is shown at right. h Levels of IFN-λ1, IFN-λ2, IFN-β and pentraxin-3 in EndTO ZIKV (blue) compared to mock-infected controls (red) (n = 3). Significance was determined using unpaired two-tailed Student’s t test. *p < 0.05, **p < 0.01.
Discussion
In this work, we established engineered placental organoids in the vascular niche using an organ chip system, which allows to recapitulate the key features of human early hemochorial placenta at the maternal-fetal interface and model viral infections. By integrating hTSCs self-organization, multicellular interactions, dynamic flow with engineered organ on chip technology, the interdisciplinary strategy enabled us to create physiologically relevant placental organoids with complex functions and structure including trophoblast epithelium layer and intravillous fetal capillaries. In the first trimester, the formation of competent fetoplacental vascular network is essential for the normal function and growth of placental villi by orchestrated interactions. We demonstrated that the vascular niche facilitated the trophoblast cell viability, proliferation and intrinsic innate immune functions in organoids. Moreover, vascular niche could enable the long-term culture and survival of placental organoids by physiological transport of nutrients and metabolic products in a paracrine manner under dynamic culture conditions. In addition, endothelium cocultures enhanced EVT differentiation from hTSCs. The applicability of the engineered placental organoids for modeling viral infection was demonstrated by the ZIKV-induced high-level secretion of IFNs and enhanced innate immune defenses in EndTOs, which reveals the protective effect of trophoblasts and possible intrinsic mechanisms of resistance to infections in a biomimetic vascular microenvironment during early gestation.
Establishing fetal-maternal blood circulation is crucial for hemochorial placentation and successful pregnancy. hTSCs have the capacity to differentiate into major trophoblast subpopulations, which provides a valuable cell source for placental research. Although current hTSC-derived organoid systems recapitulate placental development and function, they often lack vascular endothelium or dynamic microenvironment. We designed a gravity-driven perfused MPS composed of a multilayered array chip that enabled in situ generation of hTSC-derived placental organoids and coculture of organoids with endothelium in a vascular niche. Compared to the conventionally placental organoid model, the established organoid MPS offers several advantages and potentials. First, the pumpless design of the chip using gravity-driven flow allows high-throughput and bubble-free construction of the placental organoid model in a dynamic culture microenvironment. The constant-rate flow on chip is optimized by the adjustment of channel size and tilting frequency, which offers physiologically relevant range of flow shear stress. Our data demonstrated an intact endothelial barrier identified by the TEER values and permeability under lower flow rate. Our previous work demonstrated that fluid flow facilitated the differentiation and formation of hiPSC-derived liver or brain organoids with improved tissue-specific functions29,30. Similarly, we have shown that the flow conditions were helpful to the differentiation of trophoblast lineages from hTSCs in organoids through sufficient substance exchange. Second, the MPS allows coculture of organoids and endothelial barrier that can simultaneously maintain the complex architecture and mature functions of organoids and evaluate vasculature structure or permeability in normal or disease states. Third, this system is scalable to generate placental organoids derived from iPSCs or other types of organoids, which enables in-situ lineage differentiation of stem cells and organization of organoids in dynamic 3D cultures. Fourth, the chip enables real-time monitoring such as the TEER value in transwell insert, which offers a scalable and convenient approach for downstream analysis. Finally, the customized chip plate is universally adapted to common laboratories and can be designed to be compatible with high-throughput apparatus.
The fetal placental vascular network is essential for normal hemochorial placentation and the function of trophoblast invasion in the first trimester. In this model, we observed high proportions of Ki67-positive cells in EndTOs at day 12, suggesting that vascular niche may lead to increased cell-cycle entry of trophoblasts. It also explained that endothelium benefits trophoblast organoids’ proliferation, long-term survival, and specific functions, especially the enhanced innate immune functions. Interestingly, we observed that endothelium showed marked proliferation and more endothelial network formation when cocultured with TOs. This suggests the endothelium-trophoblasts crosstalk may affect angiogenesis and trophoblast development. It is known that matrix metalloproteinases (MMPs) play a significant role in regulating angiogenesis31. The higher level of secretion of MMP1 and MMP2 in EndTOs may dissolve ECM and initiate and promote angiogenesis (Fig. 4h). Previous studies have demonstrated that conditioned medium from primary cytotrophoblasts or sub-cultured placental tissue promoted HUVEC angiogenesis in vitro via paracrine angiogenic factors27. Similarly, our results suggest that trophoblasts could play an important role in placental vascular development possibly by paracrine way. Moreover, HUVECs promoted the differentiation of EVTs in EndEOs, suggesting the process of trophoblast endovascular invasion. Investigating the EVT-endothelium interactions is crucial for pregnancy success as it invades the maternal decidua to transform the spiral arteries. There is also potential to use endometrial-specific endothelial cells to create a tissue-specific model of endometrial angiogenesis to determine the effects of EVT migration on vascular endothelial network complexity.
The placenta trophoblasts, a pregnancy-specific component of the innate immune system, act as important host defenses against fetal pathogen infections32. PHTs from full-term placentas or mid-gestation chorionic villi in normal states can secrete cytokines and immunological factors that mediate innate immune defenses11,26. Similarly, we observed the basal secretomes of these cytokines in TOs. Interestingly, our studies show the elevated levels of cytokines, especially the induction of IFNs by the activation of antiviral innate immune signaling and high expression of interferon (IFN)-induced antiviral genes (IFIT1, IFIH1, OAS1, etc.) in EndTOs in the presence of endothelium. These data revealed that the vascular niche mainly facilitated the constitutively release of antiviral type III and I IFNs in trophoblasts, which may protect the trophoblasts from viral infections. Other mostly induced cytokines, such as IL-8 and pentraxin-3, were detected in EndTOs and essential for successful early gestation33. In addition, we found that EndEOs exhibited significantly reduced levels of several cytokines such as pentraxin-3, IL-8, and IFN-λ2 but higher level of secreted MMP2 compared to that in EndTOs, suggesting that endothelium cocultures primarily induced the differentiation of EVTs in EndEOs rather than innate immune response.
During pregnancy, vertical ZIKV transmission can cause placental dysfunction and elicit severe fetal defects. Some work using first-trimester placental explants or PHTs isolated from full-term placentas have revealed important insights into placental ZIKV transmission and antiviral immunity11,34. A recent study demonstrated that ZIKV targets hTSCs and reduced syncytialization in trophoblast organoids35. In this work, we have determined the distinct susceptibility and responses to viral infections between trophoblasts and endothelium using engineered placental models. STBs were reported to be less-permissive to ZIKV compared to other cell types at the maternal-fetal interface34. Consistently, we found CTBs are ZIKV-permissive rather STBs in TOs or EndTOs defined by immunostaining analysis. Moreover, EVTs are also ZIKV-permissive in EOs or EndEOs (data not shown), which is consistent with previous study using early gestation chorionic villus explants36. These results suggested that ZIKV replication in trophoblasts may mediate its transmission to the fetus during early pregnancy. Notably, we found EndTOs displayed weakly permissive to ZIKV compared to TOs. The relative resistance to ZIKV infection in EndTOs may be related to the high production of type III IFNs, particularly IFNλ2 induced by ZIKV. Moreover, trophoblast and endothelium showed different susceptibilities to ZIKV, suggesting that the trophoblasts preventing ZIKV transmission to the underlying fetal endothelium in the villi to some extent during early placentation. Collectively, these results imply a robust innate immune function in EndTOs including upregulation of IFN-induced antiviral genes in response to ZIKV infection at a biomimetic maternal-fetal interface. The placental organoid MPS supports a crosstalk between endothelium and trophoblasts and work in autocrine/paracrine to suppress viral infection in placental cells in the first trimester.
Despite the potential applications of the placental organoid MPS, there is still space for improvement. Although such a dynamic placental organoid MPS showed great potential to understand the infection events at the fetal-maternal interface, they did not recapitulate a continuous placental barrier containing trophoblast epithelium and endothelium, which may limit their applications. Moreover, the maternal peripheral immune milieu, including natural killer cell, regulatory T lymphocytes or fetal Hofbaur cells were important in host response, which is lacking in this model. Thus, the incorporation of more physiologically relevant placental immune cells may contribute to study the host-pathogen interaction from an immunological standpoint and reflect more accurate responses to external stimuli at maternal-fetal interface. In addition, the system is amenable for the interconnection with other reproductive organoids/tissues, such as endometrial organoids enabling the study of systemic responses and disease mechanism. The endothelial barrier and controllable fluid perfusion could enable the crosstalk between distinct organs/tissues in their local media environments without affected the tissue-specific functions. These integrated bioengineered strategies may advance the development of placental models with high fidelity.
Collectively, this work proposed a placental organoid MPS incorporated with vascular niche by combining stem cell biology and organs-on-chips technology. We show that this system has ability to recapitulate the key features of trophoblast development and fetal trophoblast-endothelium crosstalk in a biomimetic vascular niche, which is not possible by conventional approaches. Our work also highlights the utilization of engineered placental model in understanding the effects of ZIKV infection on early placental development and the immune response at the maternal-fetal interface. Collectively, these studies define the engineered organoid model can be used to study placental development and viral infections, thereby contributing to their applications in studies of human reproductive health and disease.
Methods
Cell culture
The hTSCs were kindly gifted by Prof. Tianqing Li (Kunming University of Science and Technology)37,38. The hTSCs were established from human blastocysts according to the published protocol39. Human blastocyst was obtained with signed informed consent of the donors, and the approval of the Medicine Ethics Committee of The First People’s Hospital of Yunnan Province (2017LS[K]NO.035)37. Based on the guiding principles in the International Society for Stem Cell Research (ISSCR), written informed consent was obtained from all donor couples for voluntary donations of embryos. All ethical regulations relevant to human research participants were followed. hTSCs were cultured on Collagen IV (cat. no. C5533, Sigma-Aldrich)-coated plates and maintained in TS medium containing DMEM/F12 basic medium (cat. no. C11330500BT, Gibco), 0.1 mM 2-mercaptoethanol (cat. no. M3148, Sigma-Aldrich), 0.2% FBS, 0.5% Penicillin-Streptomycin (cat. no. 15140122, Gibco), 0.3% HSA (cat. no. A1653, Sigma-Aldrich), 1% ITS-X supplement (cat. no. 51500056, Sigma-Aldrich), 1.5 mg/ml L-ascorbic acid (cat. no. A4544, Sigma-Aldrich), 50 ng/ml EGF (cat. no. AF-100-15, PeproTech), 2 mM CHIR99021 (cat. no. S1263, Selleckchem), 0.5 mM A83-01 (cat. no. S7692, Selleckchem), 1 mM SB431542 (cat. no. S1067, Selleckchem), 0.8 mM VPA (cat. no. P4543, Sigma-Aldrich) and 5 mM Y-27632 (cat. no. S6390, Selleckchem). HUVECs were cultured on Collagen I (cat. no. 354249, Corning)-coated plates and maintained in EGM-2 medium (cat. no. CC-3162, Lonza). hTSCs were utilized in experiments before passage 40 and HUVECs were used before passage10.
Fabrication and assembly of the organoid MPS
The organoid MPS is composed of a customized KabellyInsert™ chip and a rocker. The 3D model of multi-well organ chip plate was design by SolidWorks. The KabellyInsert™ chip was made of polycarbonate (PC) using standard computer numerical control machining techniques with ±10 μm tolerance on features. The chip consists of multiple layers including one coverlid, cell culture inserts and one baseplate containing 12 arrays of functional units. Each unit consisted of four cylindrical holes connected with a bottom flow channel. The cylindrical holes on both sides were medium reservoirs and the middle two holes was cell culture chambers. To culture the cells and organoids, a transwell insert with polyethylene terephthalate (PET) porous membrane (pore size: 0.4 or 3 μm) was placed into the middle hole of the device. Detailed information on the chip system fabrication and assembly is reported in the Supporting Information (Fig. S1).
Fluid dynamics measurement and computational simulation
The layout of the organ chip was modeled and optimized by using COMSOL Multiphysics® software (COMSOL, Inc., Burlington, MA, USA). The velocity fields in the whole volume and multiple cross-sections were simulated. Specifically, the simulation was carried out by solving the incompressible Navier-Stokes equation. A no-slip condition was applied for all the surfaces except the inlet and outlet. The dynamic viscosity and density of the culture medium were set to be 0.69 mPa·s and 1 g/cm3, respectively. Furthermore, the shear stress exerted on tissues was simulated and presented using heatmaps and a line graph as shown in the Supporting Information (Fig. S1).
Culture of hTSC-derived placental organoids on chip
To generate TOs in the organ chip system, hTSCs were digested into single cells using TrypLE Express (cat. no. 12605028, Gibco) and resuspended in the TS medium. Then, 2.5 × 105 cells were seeded onto an AggreWell™400 Microwell culture plate (cat. no. 34411, Stemcell) for cell aggregation. The generated cell aggregates (~1200 per well) were then resuspended in 400 µL ice-cold 60% Matrigel (cat. no. 354230, Corning). Next, 20 µL of the aggregates-Matrigel mixture was pipetted into the upper chamber of each transwell insert in the chip plate for continuous culture. MPS was incubated at 37 °C for 25 min to make Matrigel gelation. hTSC aggregates were cultured and differentiated into organoids TOs differentiation medium (TOM) consisting of Advanced DMEM/F12 medium (cat. no. 12634010, Gibco) supplemented with 0.5% N2 supplement (cat. no. 17502048, Gibco), 1% B27 supplement minus vitamin A (cat. no. 12587010, Gibco), 100 μg/mL Primocin (cat. no. ant-pm-1, Invivogen), 1.25 mM N-Acetyl-L-cysteine (cat. no. A9165, Sigma-Aldrich), 2 mM L-glutamine (cat. no. 25030081, Gibco), 50 ng/mL EGF, 1.5 µM CHIR99021, 80 ng/mL R-spondin 1 (cat. no. AF-120-38, PeproTech), 100 ng/mL FGF-2 (cat. no. 100-18C, PeproTech), 50 ng HGF (cat. no. CJ72, Novoprotein, Shanghai, China), 500 nM A83-01, 2.5 µM prostaglandin E2 (cat. no. HY-101952, Selleck) and 2 µM Y-27632. We added 1.6 mL TOM to the side reservoirs and added 200 μL TOM to each central upper chambers. The chip was placed on a rocker system for dynamic culture at 2 rpm/min. Cultures were maintained in 5% CO2 in a humidified incubator at 37 °C. Medium was replaced every 2 days. TOs were either directly analyzed or extracted from the gels for subsequent analysis after cultured for 6 days.
The derivation protocols of EOs from hTSCs were similar to those as previously described18. Briefly, hTSC aggregates were embedded in ice-cold 60% Matrigel and cultured in TOM as described above for initial 3 days. For the differentiation of EOs, the media was replaced with TOM lacking Wnt inducers (R-spondin1 and CHIR99021) for an additional 3 days, when EOs showed obvious EVT outgrowth from the outer layers. EOs were either directly analyzed or extracted from the gels for subsequent analysis.
Coculture of placental organoids with endothelium on chip
To established a vascular niche in the MPS, the porous membrane of transwell insert was coated with collagen I (0.5 mg/mL) at 37 °C for 1 h. HUVECs at a concentration of 2 × 106 cells/mL were then seeded on the lower side of the membrane. The transwell insert was inverted and incubated at 37 °C for 2 h to promote the attachment of HUVECs. For coculture of placental organoids with endothelium, TOs or EOs on day 6 were transferred to the upper chamber with endothelium in the lower channel on chip. For EndTOs cultures, the coculture medium was the mixture of TOM and EGM-2 (1:1). For EndEOs cultures, the coculture medium was the TOM lacking R-spondin1 and CHIR99021, diluted with EGM-2 (1:1). The same media was used for both upper and lower chambers. The chip plate was then placed on a rocker for dynamic coculture for another 6 days at 2 rpm/min. Cultures were maintained in 5% CO2 in a humidified incubator at 37 °C. Medium was replaced every 2 days.
Immunostaining of placental organoids and endothelium
In order to precisely reveal the detailed characterizations of hTSC-derived placental organoids by immunohistochemistry, organoids were harvested from the organ chip system on desired days during the development period. Organoids were fixed in 4% PFA, then dehydrated in 30% sucrose solution. After embedded in OCT compound (SAKURA), 10 mm thick sections of organoids were obtained with a cryostat (Leica). Samples on adhesive slides were permeabilized with 0.25% Triton X-100 and blocked with 10% goat serum (Solarbio). For immunofluorescence staining, cryosections were incubated with primary antibodies and secondary antibodies listed in the Supporting Information (Table S2). Cell nuclei were counterstained with DAPI (Life Technologies, 1:4000). The images were visualized and acquired using a confocal microscope (FV3000, Olympus). ImageJ (NIH) performed the analysis of images. Quantifications for the percentage of marker positive cells in TOs or EndTOs were analyzed based on fluorescence images by Image J software.
Endothelial cells on the chip were fixed in 4% paraformaldehyde for 20 min at room temperature. Next, microchannels were washed with DPBS and filled with 0.2% Triton X-100 for 10 min. Then, samples were blocked with goat serum for 1 h and incubated with primary antibody at 4 °C overnight. Primary antibodies used in the experiment were listed in the Supporting Information (Table S2). The samples were then incubated with Alexa Fluor 488- or 594- (1:500, Cell Signaling Technology) conjugated secondary antibodies at room temperature for 1 h. Cell nuclei were counterstained with DAPI (Life Technologies, 1:4000). The stained samples were imaged using a confocal microscope (FV3000, Olympus). ImageJ (NIH) performed the analysis of images.
Transmission electron microscopy
Organoid samples were collected and then fixed with PFA and 2.5% glutaraldehyde at room temperature for 1 h. After washed with PBS for three times and fixed in 1% OsO4 buffer for 2 h, the samples were dehydrated with graded ethanol solutions, and embedded in SPI-Pon 812 resin (Sigma-Aldrich). Ultrathin sections (70 nm) were stained with 2% uranyl acetate for 30 min and then lead citrate for 10 min. Images were acquired with a JEM-1400 PLUS electron microscope.
Exposure of organoids to ZIKV
TOs, EndTOs, EOs or EndEOs were extracted from the Matrigel with cell recovery solution (cat. no. 354253, Corning) after culture for 6 days in the MPS. Then organoids were incubated with ZIKV strain PRVABC59 (GenBank number KU501215) at MOIs of 0.1 and 1 diluted in organoid growth media, respectively. After 2 h of incubation, organoids were washed three times with PBS and re-embedded in Matrigel. The organoid-matrigel mixture were re-inoculate onto the insert upper chamber in MPS and kept in fresh medium for 6 days. Cultures were maintained in 5% CO2 in a humidified incubator at 37 °C. Medium was replaced every 2 days. At day 6 post-infection, the supernatant was collected for Luminex-based multianalyte profiling and RNA isolated as described for qRT-PCR or RNA-Seq. Endothelium cultured on chip were fixed for immunofluorescence analysis or lysed for RT-qPCR, respectively. The experiments with infectious ZIKV were conducted under biosafety level 2 (BSL2).
Flow cytometry
Placental organoids were extracted from the Matrigel with cell recovery solution. Following incubation at 4 °C for 20 min to depolymerize Matrigel, organoids were dissociated with 0.25% trypsin at 37 °C for 10 min. Single cells were obtained by filtering through 40 μm cell strainers (cat. no. 352340, Falcon) after washing with medium contain FBS. Finally, cells were labeled with PE-ITGA2, CGB or isotype-matched controls for analysis (Table S2). The experiments were conducted using SH800S Cell Sorter (Sony Biotechnology). Data were analyzed in Cell Sorter Software (Sony Biotechnology). Cell debris was excluded using an FSC vs SSC gate, followed by aggregate exclusion through FSC-H vs FSC-A gating. Cells with ITGA2 or CGB signals were defined as positive population and gated based on the isotype control.
Real-time quantitative PCR
Total RNA was isolated from organoids or cells on the chip using Trizol reagent (TAKARA). RNA quality and concentration were determined by NanoPhotometer (IMPLEN). cDNA was produced after RNA was diluted to 50 ng/μL. Then, cDNA was amplified via qPCR using Ex Raq DNA polymerase (TAKARA) under the following reaction conditions (40 cycles): denaturation at 94 °C for 1 min, annealing at 58 °C for 30 s, and extension at 72 °C for 30 s. The primer pairs used are listed in Table S1. GAPDH was used as a reference gene in each sample.
ELISA
To examine the hCG secreted from trophoblast organoids, hCG ELISA kit (cat. no. E-EL-H0175, Elabscience) was utilized. The secretion levels of PDGF-BB of HUVECs under different conditions, including single HUVEC, TO-EC and EO-EC were examined using a commercial human PDGF-BB ELISA Kit (cat. no. E-EL-H1577, Elabscience). The conditioned medium was collected from MPS after culture for 48 h and stored at -80 °C until use. For detection of hCG and PDGF-BB concentration, samples were prepared following the instructions provided by manufacturer and the fluorescence intensity was assessed by a microplate reader.
RNA-sequencing
TOs and EndTOs with or without ZIKV infection in MPS were collected to conduct gene expression analysis. Total RNA was isolated from samples using Trizol reagent. Then, 2 μg RNA was used for stand RNA sequencing library preparation with Ribo-off rRNA Depletion Kit (Illumina) and KC-DigitalTM Standard mRNA Library Prep Kit for Illumina (Seqhealth) following the instructions provided by manufacturer. The library products corresponding to 200-500 bps were enriched, quantified and sequenced on NovaSeq 6000 sequencer (Illumina) with PE150 model. Sequencing data was analyzed through standard RNA-seq protocol. Reads were mapped to the reference genome of Homo sapiens from Homoserines (GRCh38) using STAR software (version 2.5.3a) with default parameters.
Reads mapped to the exon regions of each gene were counted by featureCounts (Subread-1.5.1; Bioconductor) and then RPKMs were calculated. Differentially expressed genes between groups were identified using the edgeR package (version 3.12.1). An FDR-corrected p-value cutoff of 0.05 using the Benjamin-Hochberg method and fold-change cutoff of 2 were used to judge the statistical significance of gene expression differences. GO and KEGG enrichment analysis for differentially expressed genes was implemented by KOBAS software (version: 2.1.1) with a corrected P-value cutoff of 0.05 to judge statistically significant enrichment.
Biomarker profiling by Luminex
Cytokine profiles in cell culture medium samples were analyzed by Luminex array for determination of 37 analytes in total, consisting of cytokines, chemokines, and growth factors using the MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel-Premixed 37 Plex (Merck Millipo USA). Angiogenic factors in cell culture medium samples were examined by Luminex array using customized human angiogenesis array kit (R&D Systems).
Statistical analysis
Data were expressed as the mean ± standard deviation (SD) for at least three independent experiments. Differences between two groups were analyzed using unpaired Student’s t test. Multiple comparisons were performed using one-way analysis of variance (ANOVA) followed by the Tukey’s post hoc test. P values < 0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All relevant data are available in the manuscript or Supporting Information. All the RNA-seq raw data have been deposited SRA under the accession number PRJNA1024681. The source data for the main figures are available as Supplementary Data and all other data are available from the corresponding author on reasonable request.
References
Knofler, M. et al. Human placenta and trophoblast development: key molecular mechanisms and model systems. Cell Mol. Life Sci. 76, 3479–3496 (2019).
Gude, N. M., Roberts, C. T., Kalionis, B. & King, R. G. Growth and function of the normal human placenta. Thromb. Res. 114, 397–407 (2004).
Kingdom, J., Huppertz, B., Seaward, G. & Kaufmann, P. Development of the placental villous tree and its consequences for fetal growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 92, 35–43 (2000).
Hamilton, W. J. & Boyd, J. D. Development of the human placenta in the first three months of gestation. J. Anat. 94, 297–328 (1960).
Sato, Y. Endovascular trophoblast and spiral artery remodeling. Mol. Cell Endocrinol. 503, 110699 (2020).
Moll, W. Structure adaptation and blood flow control in the uterine arterial system after hemochorial placentation. Eur. J. Obstet. Gynecol. Reprod. Biol. 110, S19–S27 (2003). Suppl 1.
Soares, M. J., Varberg, K. M. & Iqbal, K. Hemochorial placentation: development, function, and adaptations. Biol. Reprod. 99, 196–211 (2018).
Ander, S. E., Diamond, M. S. & Coyne, C. B. Immune responses at the maternal-fetal interface. Sci. Immunol. 4, eaat6117 (2019).
Pereira, L. Congenital viral infection: traversing the uterine-placental interface. Annu. Rev. Virol. 5, 273–299 (2018).
Rosenfeld, C. S. Transcriptomics and other omics approaches to investigate effects of xenobiotics on the placenta. Front Cell Dev. Biol. 9, 723656 (2021).
Bayer, A. et al. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 19, 705–712 (2016).
Corry, J., Arora, N., Good, C. A., Sadovsky, Y. & Coyne, C. B. Organotypic models of type III interferon-mediated protection from Zika virus infections at the maternal-fetal interface. Proc. Natl Acad. Sci. USA 114, 9433–9438 (2017).
Lazear, H. M., Schoggins, J. W. & Diamond, M. S. Shared and distinct functions of type I and type III interferons. Immunity 50, 907–923 (2019).
Roberts, R. M., Green, J. A. & Schulz, L. C. The evolution of the placenta. Reproduction 152, R179–R189 (2016).
Maltepe, E., Bakardjiev, A. I. & Fisher, S. J. The placenta: transcriptional, epigenetic, and physiological integration during development. J. Clin. Invest. 120, 1016–1025 (2010).
Cui, K. et al. Establishment of trophoblast-like tissue model from human pluripotent stem cells in three-dimensional culture system. Adv. Sci. 9, 2100031 (2021).
Deng, P. et al. Fluidic flow enhances the differentiation of placental trophoblast-like 3D tissue from hiPSCs in a perfused macrofluidic device. Front. Bioeng. Biotechnol. 10, 907104 (2022).
Haider, S. et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep. 11, 537–551 (2018).
Turco, M. Y. et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 564, 263–267 (2018).
Takebe, T., Zhang, B. & Radisic, M. Synergistic engineering: organoids meet organs-on-a-chip. Cell Stem Cell 21, 297–300 (2017).
Zhu, Y. et al. In situ generation of human brain organoids on a micropillar array. Lab Chip 17, 2941–2950 (2017).
Wang, Y., Wang, L., Zhu, Y. & Qin, J. Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab chip 18, 851–860 (2018).
Park, S. E., Georgescu, A. & Huh, D. Organoids-on-a-chip. Science 364, 960–965 (2019).
Turco, M. Y. & Moffett, A. Development of the human placenta. Development 146 (2019).
Battegay, E. J., Rupp, J., Iruela-Arispe, L., Sage, E. H. & Pech, M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J. Cell Biol. 125, 917–928 (1994).
Megli, C., Morosky, S., Rajasundaram, D. & Coyne, C. B. Inflammasome signaling in human placental trophoblasts regulates immune defense against Listeria monocytogenes infection. J. Exp. Med. 218, e20200649 (2021).
Ma, H. et al. Conditioned medium from primary cytotrophoblasts, primary placenta-derived mesenchymal stem cells, or sub-cultured placental tissue promoted HUVEC angiogenesis in vitro. Stem Cell Res. Ther. 12, 141 (2021).
Ghosh Roy, S. TAM receptors: a phosphatidylserine receptor family and its implications in viral infections. Int Rev. Cell Mol. Biol. 357, 81–122 (2020).
Wang, Y. Q. et al. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab a chip 18, 3606–3616 (2018).
Wang, Y. Q., Wang, L., Guo, Y. Q., Zhu, Y. J. & Qin, J. H. Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC Adv. 8, 1677–1685 (2018).
Sang, Q. X. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 8, 171–177 (1998).
Guleria, I. & Pollard, J. W. The trophoblast is a component of the innate immune system during pregnancy. Nat. Med. 6, 589–593 (2000).
Popovici, R. M. et al. Gene expression profiling of human endometrial-trophoblast interaction in a coculture model. Endocrinology 147, 5662–5675 (2006).
Sheridan, M. A. et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc. Natl Acad. Sci. USA 114, E1587–E1596 (2017).
Wu, H. et al. Zika virus targets human trophoblast stem cells and prevents syncytialization in placental trophoblast organoids. Nat. Commun. 14, 5541 (2023).
Tabata, T. et al. Virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 20, 155–166 (2016).
Xiang, L. et al. A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature 577, 537–542 (2020).
Cao, R., Wang, Y., Liu, J., Rong, L. & Qin, J. Self-assembled human placental model from trophoblast stem cells in a dynamic organ-on-a-chip system. Cell Prolif. 56, e13469 (2023).
Okae, H. et al. Derivation of human trophoblast stem cells. Cell Stem Cell 22, 50–63 e56 (2018).
Acknowledgements
The authors thank Prof. Tianqing Li (Kunming University of Science and Technology) for kindly providing the hTSCs. This research was supported by the National Natural Science Foundation of China (No. 32301206), National Key R&D Program of China (Nos. 2022YFA1104700, 2022YFA1205000). Noncommunicable Chronic Diseases-National Science and Technology Major Project (No. 2024ZD0531000).
Author information
Authors and Affiliations
Contributions
Y.Q.W. and J.H.Q. conceived the study. Y.Q.W. performed the experiments and analyzed results. Y.Q.G. performed chip fabrication. Y.Q.W. and P.W. performed the viral infection in the BSL-2 lab. Y.Q.W., J.Y.L., Q.L., X.Z., L.W., and C.X. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Min Jae Song and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editors: Emily Lee and Dario Ummarino. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Guo, Y., Wang, P. et al. An engineered human placental organoid microphysiological system in a vascular niche to model viral infection. Commun Biol 8, 669 (2025). https://doi.org/10.1038/s42003-025-08057-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08057-0