Abstract
Cardiovascular disease exhibits distinct sex-based differences, yet the mechanisms underlying these differences remain under-explored. The pro-resolving mediator annexin-A1 (ANXA1) is a pivotal regulator in inflammation resolution and tissue homeostasis, including within the cardiovascular system. However, the sex-specific differences in ANXA1 in blood pressure regulation have not been investigated. Here, we demonstrate that deficiency of ANXA1 exacerbates angiotensin II-induced adverse aortic and cardiac structural remodeling, mitochondrial proteome dysregulation, and impaired mitochondrial function in preclinical hypertensive models, exacerbated in females. Mechanistically, we demonstrate that estrogen upregulates ANXA1 levels, associated with dysregulation of inflammatory and mitochondrial networks, suggesting that the estrogen-ANXA1 axis plays a critical role in modulating inflammation and preventing pathological remodeling. In conclusion, this study advances the understanding of female-specific cardiac and aortic tissue and cellular alterations in hypertension, providing a platform for developing therapeutic ANXA1 mimetics that address the unique pathophysiological features of hypertension in females.

Similar content being viewed by others
Introduction
While both men and women develop hypertension, leading to end-organ damage in the heart and blood vessels, well-established sex differences exist in the incidence and severity of this condition. Men typically experience a higher incidence of hypertension than women, with this disparity persisting until the sixth decade of life1. However, there is a significant gap in our understanding of the sex-specific effects of hypertension and antihypertensives, as clinical trials often overlook these differences. Recent evidence suggests that the risk of cardiovascular complications arises at a lower blood pressure (BP) in females than in males, challenging the current approach of using the same BP threshold for both sexes2. This highlights the urgent need to better understand the unique mechanisms driving hypertension and its cardiovascular complications in females as an underexplored population. Addressing this knowledge gap is critical for developing more effective, sex-specific therapeutic strategies.
Inflammation may contribute to the development of hypertension-associated end-organ damage3, and there is increasing evidence that differing immunological profiles may contribute to sex differences in BP control4. Sex differences in BP responses to hypertensive stimuli, such as angiotensin II (Ang II), may be influenced by immune cells5. Moreover, the lower incidence of hypertension in females may be due to a more anti-inflammatory T-cell profile than in males4. Current anti-inflammatory drugs that target the onset of inflammation have been ineffective in reducing BP, largely attributable to their adverse effects6, suggesting that the suppression of inflammation is not a suitable approach to treating hypertension. Importantly, a failure to resolve inflammation may lead to chronic inflammation and exacerbation of the risk of cardiovascular diseases7. Targeting the resolution of inflammation may, therefore, be an alternative approach to treat hypertension-induced end-organ damage.
Annexin-A1 (ANXA18) is a glucocorticoid-regulated protein9 involved in the resolution of inflammation, downstream of the activation of formyl peptide receptors (FPR) 1 and 210,11, which are G-protein coupled receptors critical to the resolution of inflammation12. ANXA1 is expressed in a wide variety of tissues, including the heart, blood vessels, liver, lungs, and spleen, and many cell types such as cardiac fibroblasts, cardiomyocytes10, vascular smooth muscle cells, endothelial cells13,14, and immune cells, with the highest levels detected in neutrophils and macrophages10. ANXA1 deficiency (ANXA1-/-) increases neutrophil migration and expression of pro-inflammatory cytokines, exacerbating inflammatory responses in several experimental disease models, including arthritis15, ulcerative colitis16, systemic lupus erythematosus17, and atherosclerosis18. Qin et al.9, have reported that a deficiency of ANXA1 increased cardiac necrosis, inflammation, hypertrophy, and fibrosis following myocardial infarction (MI). FPR1 appears to be involved in cardiomyocyte survival and the preservation of left ventricular (LV) function, while FPR2 is considered responsible for attenuation of inflammation9,19. Therefore, dual FPR1/FPR2 agonists may be useful for reducing MI injury9,19. We recently reported that the small molecule FPR1/2 agonist Compound17b attenuates hypertension, adverse cardiovascular remodeling, and dysfunction in Ang II-infused male wild-type (WT) C57BL/6 mice20. In addition, ANXA1 deficiency exacerbates adverse vascular remodeling in insulin-resistant mice21 and exacerbates abdominal aortic dissection in mice22.
Taken together, these findings indicate that the endogenous FPR agonist ANXA1 is implicated in systemic aortic and cardiac organization and functionally in resolving inflammation to ultimately provide cardiovascular protection. Despite these observations, the role of ANXA1 in the regulation of BP has not been explored, nor is it known whether any of these actions are sex-dependent. We, therefore, hypothesized that deficiency of ANXA1 exacerbates BP elevation, adverse cardiovascular remodeling, dysfunction, and organ-based proteomic alterations in both male and female hypertensive mice, with a greater impact on females.
Results
Impact of Ang II-induced hypertension on the cardiovascular proteome of male and female WT and ANXA1-/- mice
A detailed workflow of aortic (thoracic aorta, TA) and cardiac (LV) proteomics based on sequential extraction of tissue (n = 4–5 per group), followed by quantitative label-free tandem mass spectrometry and stringent informatics analyses, quantified ~ 3500 proteins (Fig. 1A). In TA, Ang II changed 151 and 366 proteins in male and female WT mice, respectively (P < 0.05, Fig. 1B–D) and 219 and 270 proteins in male and female ANXA1-/- mice respectively (P < 0.05, Fig. 1E–G) compared to saline-infusion (Supplementary Tables 6–7, 10–11). In LV, there was a significant change in 148 and 184 proteins in male and female WT mice respectively (P < 0.05, Fig. 1H–J), and 232 and 108 proteins in male and female ANXA1-/- mice respectively with Ang II-infusion (P < 0.05, Fig. 1K–M) compared to saline-infusion (Supplementary Tables 8–9, 12–13).
Proteomics preparation and analysis workflow of TA (n = 4–5) and LV (n = 4–5) (A). Created in Biorender. Singh, J (2025) https://creativecommons.org/licences/by/3.0/. Significantly upregulated (red/purple) and downregulated (blue/green) cellular components in TA (B and E) and LV (H and K). Distribution of identified proteins (Venn) displaying proteins identified in TA (co-identified proteins: center, non-bold and significantly changed proteins: center, bold) and volcano plot indicating differential abundance of proteins associated with structural (red), calcium regulatory (orange), inflammatory (green), oxidative stress (purple), and mitochondrial (blue) networks in TA of normotensive and hypertensive WT (C, D) and ANXA1-/- (F, G) mice of both sexes. Venn diagram comparing the LV proteome (co-identified proteins: center, non-bold and significantly changed proteins: center, bold) and volcano plot indicating differential abundance of proteins associated with structural (red), calcium regulatory (orange), inflammatory (green), oxidative stress (purple), and mitochondrial (blue) proteins in LV of normotensive and hypertensive WT (I, J) and ANXA1-/- (L, M) mice of both sexes. Fold change of proteins with P < 0.05 (calculated with Student’s t test) was considered statistically significant. Ang II: angiotensin II, ANXA1-/-: annexin-A1-deficient, GO: gene ontology annotation, LV: left ventricle, Sal: saline, TA: thoracic aorta, WT: wild-type.
We first compared the hypertension-impacted TA proteomes of WT and ANXA1-/- mice with their normotensive mouse counterparts of both sexes. A total of 60 proteins were up- and 91 proteins were down-regulated in male WT mice, while 59 proteins were up- and 160 proteins were down-regulated in male ANXA1-/- mice (P < 0.05, Fig. 1C, F). In contrast, 219 proteins were up- and 147 proteins were down-regulated in female WT and 171 proteins were up- and 99 proteins were down-regulated in female ANXA1-/- mice infused with Ang II for 28 days (P < 0.05, Fig. 1D, G). The identified proteins were associated with networks linked with cell structure and organization, calcium regulation, inflammatory response, or mitochondrial-associated proteins (P < 0.05, Fig. 1B–G, Supplementary Tables 14 and 15). Functional enrichment analysis (Gene Ontology (GO) cellular components processes) based on these differential proteome subsets revealed that aortic structural modulation (GO:0062023, collagen-containing extracellular matrix, P < 0.0001, Fig. 1B, E), calcium regulation (GO:0015629, actin cytoskeleton, P < 0.006, Fig. 1B, E) and mitochondrial related GO terms (GO:0031966, mitochondrial membrane, P < 0.0001, Fig. 1B, E) were dysregulated in the Ang II-TA proteome of male and female mice (Supplementary Tables 14 and 15). Structural proteins (Col15a1 and Col4a1 in male WT and ANXA1-/- mice, respectively, and Col18a1 in female WT and ANXA1-/- mice, P < 0.05, Fig. 1C, D, F, G), calcium regulatory proteins (Fbln2 in male and female WT and ANXA1-/- mice, P < 0.05, Fig. 1C, D, F, G) were upregulated in Ang II-TA of both genotypes compared to saline-TA. Chronic Ang II-infusion resulted in dysregulation of inflammatory response proteins (Cr1l and C8g in male WT and ANXA1-/- mice respectively, and Ptgis and Cd47 in female WT and ANXA1-/- mice respectively, P < 0.05, Fig. 1C, D, F, G) and mitochondrial proteins (Suclg1 and Ucp3 in male WT and ANXA1-/- mice respectively, and Suclg2 and Ucp1 in female WT and ANXA1-/- mice respectively, P < 0.05, Fig. 1C, D, F, G) in TA of WT and ANXA1-/- mice.
In addition to Ang II-infusion, TA proteins were compared in both sexes and genotypes that were subjected to saline-infusion for 28 days. Functional enrichment analysis (GO cellular component processes) of TA demonstrated that aortic structure (GO:0005622, intracellular anatomical structure, P < 0.0001, Supplementary Tables 14 and 15), and mitochondrial energetics (GO:0031966, mitochondrial membrane, P < 0.0001, Supplementary Tables 14 and 15) were different in saline-infused male and female WT and ANXA1-/- mice. Structural proteins (Capzb in male and Col18a1 in female ANXA1-/- mice, P < 0.05, Supplementary Fig. 1A, B), calcium regulatory proteins (Capn1 in male and Myl12b in female ANXA1-/- mice, P < 0.05, Supplementary Fig. 1A, B), inflammatory response proteins (Ly6c1 in male and S100a6 in female ANXA1-/- mice, P < 0.05, Supplementary Fig. 1A, B) were upregulated in saline-infused ANXA1-/- than WT mice. In contrast, mitochondrial proteins (Cox7a1 in male and Cox6a1 in female ANXA1-/- mice, P < 0.05, Supplementary Fig. 1A, B) were dysregulated in TA of ANXA1-/- compared to WT mice infused with saline for 28 days.
Chronic Ang II-infusion induced a similar alteration in the structural and mitochondrial processes of LV and TA proteome, and these dysregulated processes were greater in the vascular (TA) proteomes of male and female WT and ANXA1-/- mice. Functional enrichment analysis (GO cellular processes) of LV demonstrated that cardiac structure (GO:0099512, supramolecular fiber, P < 0.0001, Fig. 1H, K), calcium regulation (GO:0015629, actin cytoskeleton, P < 0.0001, Fig. 1H, K) and mitochondrial energetics (GO:0031966, mitochondrial membrane, P < 0.0001, Fig. 1H, K) were dysregulated in Ang II-infused male and female mice of both genotypes (Supplementary Tables 16 and 17). Differential LV proteins belonging to these altered processes revealed that the expression of 110 proteins was up- and 38 proteins were down-regulated in male WT mice, and 139 proteins were up- and 93 proteins were down-regulated in male ANXA1-/- mice (P < 0.05, Fig. 1I, L). In female WT mice, 104 proteins were up- and 80 proteins were down-regulated, and 58 proteins were up- and 50 proteins were downregulated in female ANXA1-/- mice subjected to chronic Ang II-infusion (P < 0.05, Fig. 1J, M).
Cardiac structural proteins (Xirp2 in male and female WT and ANXA1-/- mice, P < 0.05, Fig. 1I, J, L, M), calcium regulatory proteins (Sorbs2 in both male WT and ANXA1-/- mice, and Mylk in female WT and Capn1 in ANXA1-/- mice, P < 0.05, Fig. 1I, J, L, M), inflammatory proteins (Cast and C3 in male WT and ANXA1-/- mice respectively and C4b and Mif in female WT and ANXA1-/- mice respectively, P < 0.05, Fig. 1I, J, L, M) were dysregulated in hypertensive WT and ANXA1-/- mice of both sexes in comparison to their respective normotensive control. Altered mitochondrial proteins (Suclg2 and Ucp3 in male WT and ANXA1-/- mice, respectively, and Suclg2 and Sucla2 in female WT and ANXA1-/- mice, respectively, P < 0.05, Fig. 1I, J, L, M) were identified in LV of both sexes and genotypes subjected to Ang II-infusion. Even in the absence of Ang II-infusion, LV proteomes were different in mice of both sexes (mice subjected to 28 days of saline-infusion, P < 0.05, Supplementary Fig. 1C, D).
Ang II-induced dysregulation of the TA proteome was greater in ANXA1-/- than WT mice and this effect was greater in females than males
We have analyzed the TA proteome in Ang II-infused ANXA1-/- compared to WT mice of both sexes. A total of 2290 proteins of male and 2244 proteins of female mice were identified when comparing both genotypes in male and female hypertensive mice respectively, out of which 84 proteins of male and 349 proteins of female were dysregulated in expression across WT and ANXA1-/- mice-infused with Ang II for 28 days (P < 0.05, Fig. 2A–C and Supplementary Tables 10 and 11). Based on differential expression in hypertensive WT mice, networks associated with structural organization, calcium regulation, and inflammatory response proteins of TA were mildly dysregulated in males (-log10 p-value of GO process: intracellular anatomical structure = 5.6 and mitochondrial envelope = 1.6, Fig. 2A and Supplementary Table 14) and were dysregulated to a greater extent in female hypertensive ANXA1-/- mice (-log10 p-value of GO process: intracellular anatomical structure = 9.3 and mitochondrial envelope = 10.6, Fig. 2A and Supplementary Table 15). The expression of aortic structural (Lgals3, Fig. 2B, D), inflammatory (Lta4h, Fig. 2B, D), and calcium regulatory proteins (Itpr1, Fig. 2B, D) was higher in TA of male ANXA1-/- compared to WT mice-infused with Ang II. Expression of these altered proteins was exaggerated in TA of female ANXA1-/- compared to WT mice in hypertension, i.e., structural (Ablim1, Fig. 2C, E), inflammatory (Cfh, Fig. 2C, E), and calcium regulatory proteins (Smoc1, Fig. 2C, E). In addition, our TA proteomics analysis also demonstrated that expression of mitochondrial proteins (Ndufa3, Glud1, Fig. 2B, D) was downregulated in male ANXA1-/- than in WT mice-infused with Ang II. The altered expression of mitochondrial proteins (Coq10b, Aldh3a2, Fig. 2C, E) was further downregulated in Ang II-infused female ANXA1-/- than in WT mice (Supplementary Tables 10 and 11). The heatmap demonstrated that expression of mitochondrial (Ndufa7) and inflammatory proteins (Femt3) in male TA and structural (Ablim1), calcium regulatory (Itpr1), mitochondrial (Ndufaf1), oxidative stress (Sod3), and inflammatory proteins (Ptges3) in female TA were commonly identified across all groups (Fig. 2D, E). In LV tissue, expression of structural, inflammatory, oxidative stress, calcium regulatory, and mitochondrial proteins was more dysregulated in hypertensive ANXA1-/- than WT mice in both sexes, but this alteration was less than in the TA region (Supplementary Fig. 2A–E, Tables 12, 13, 16, 17).
Upregulated (orange) and downregulated (green) cellular components in TA (A). Venn diagram displaying Ang II-regulated change in proteins identified and their abundance in TA of WT and ANXA1-/- mice of both sexes (B, C). Volcano plot comparing structural (red), calcium regulatory (orange), inflammatory (green), oxidative stress (purple), and mitochondrial (blue) proteins in TA of hypertensive WT and ANXA1-/- mice of both sexes (B, C). Heat map comparing structural (red), calcium regulatory (orange), inflammatory (green), oxidative stress (purple), and mitochondrial (blue) proteins in TA of hypertensive WT (n = 4–5) and ANXA1-/- mice (n = 5) and normotensive WT (n = 4–5) of both sexes (D, E). Heatmap compared the expression of differential proteins in WT mice-infused with Sal or Ang II (light purple), WT and ANXA1-/-mice-infused with Ang II (dark purple), and WT and ANXA1-/- mice-infused with Sal or Ang II (darkest purple) in TA of both sexes (D, E). Fold change of proteins with P < 0.05 (calculated with Student’s t test) was considered statistically significant. Ang II: angiotensin II, ANXA1-/-: annexin-A1-deficient, GO: gene ontology annotation, Sal: saline, TA: thoracic aorta, WT: wild-type.
ANXA1-/- mice exhibit higher BP and exaggerated hypertension
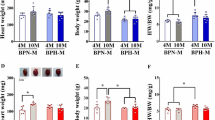
Systolic arterial pressure (SAP) at week 7 was higher in ANXA1-/- than WT mice of both sexes, and this was maintained to the endpoint (14 weeks-of-age). Average SAP from 7–14 weeks was 6 mmHg higher in male ANXA1-/- compared to male WT mice (Fig. 3A, B and Supplementary Table 1A) and 10 mmHg higher in female ANXA1-/- compared to female WT counterparts (Fig. 3A, B and Supplementary Table 1A). SAP was higher in female than male ANXA1-/- mice (Fig. 3A, B and Supplementary Table 1A). Chronic Ang II-infusion resulted in 31 and 19 mmHg higher SAP from baseline in male and female WT mice, respectively, and this was increased to 45 mmHg in male ANXA1-/- mice and 44 mmHg in female ANXA1-/- mice (Fig. 3C, D, Supplementary Table 1B) at end of the week 4. The SAP increase was 12 mmHg lower in Ang II-infused female than in male WT mice (Fig. 3C, D and Supplementary Table 1B). Heart and LV weight normalized to tibia length (TL) was ~ 10% and ~ 20% greater in Ang II-infused male and female WT mice, respectively compared to saline-infused counterparts (Fig. 3E, F, Supplementary Table 2) and this was exaggerated in Ang II-infused ANXA1-/- male but not in female mice (Fig. 3E, F and Supplementary Table 2). Heart and LV weight normalized to TL were not different in either sex (Fig. 3E, F and Supplementary Table 2). Right ventricle (RV) weight normalized to TL was not different between genotypes (Fig. 3G and Supplementary Table 2) but was different in male and female mice of both genotypes (Fig. 3G and Supplementary Table 2). Lung weight normalized to TL was greater in Ang II-infused male mice but not in female mice of either genotype, and this was greater in male than female mice (Fig. 3H and Supplementary Table 2).
The line graph represents tail-cuff blood pressure recording from week 7 to week 14 (A). The bar graph (B) illustrates SAP for male WT mice (blue filled, n = 18), male ANXA1-/- mice (blue filled with pattern, n = 18), female WT mice (green filled, n = 15), and female ANXA1-/- mice (green filled with pattern, n = 16). SAP was recorded over 28 days in male and female WT and ANXA1-/- mice-infused with Sal and Ang II (C). The mean change in SAP at week 4 was measured (D). Heart, LV, RV, and lung weights normalized to TL were recorded (E–H) in saline-infused male WT mice (blue filled, n = 12–17) and male ANXA1-/- mice (blue pattern, n = 12–21) and Ang II-infused male WT mice (red filled, n = 11–19) and male ANXA1-/- mice (red pattern, n = 11–22) and saline-infused female WT mice (green filled, n = 12) and female ANXA1-/- mice (green pattern, n = 12) and Ang II-infused female WT mice (pink filled, n = 12) and female ANXA1-/- mice (pink pattern, n = 12). Results are presented as mean ± SEM. Statistical analysis was conducted with a mixed model split-plot analysis of variance corrected with Bonferroni and Greenhouse Geisser adjustments for SAP and MANOVA, followed by a Bonferroni significant difference post-hoc test for organ weights. *P < 0.05, **P < 0.01, ***P < 0.001 between treatment groups. #P < 0.05, ##P < 0.01, ###P < 0.001 between sexes. Ang II: angiotensin II, ANXA1-/-: annexin-A1-deficient, BP: blood pressure, LV: left ventricle, RV: right ventricle, Sal: saline, SAP: Systolic arterial pressure, TL: tibia length, WT: wild-type.
Ang II-infused ANXA1-/- mice demonstrated exaggerated adverse vascular remodeling
Distensibility of the carotid artery was lower in saline-infused male and female ANXA1-/- than in WT mice, respectively (Fig. 4A, B and Supplementary Table 3). Chronic Ang II-infusion resulted in lower distensibility in WT and ANXA1-/- mice of both sexes compared to their respective saline counterparts (Fig. 4A, B and Supplementary Table 3). This response was enhanced in female but not in male ANXA1-/- mice compared to WT mice (Fig. 4A, B and Supplementary Table 3). Distensibility was not different between sexes (Fig. 4A, B and Supplementary Table 3). Collagen deposition in the abdominal aorta was not different in saline-infused mice of either sex and genotype (Fig. 4C, D). Collagen deposition in the abdominal aorta was greater in Ang II-infused WT and ANXA1-/- mice of both sexes compared to their respective saline counterparts (Fig. 4C, D). This greater collagen deposition in the abdominal aorta of Ang II-infused mice was similar regardless of the genotype and sex (Fig. 4C, D). The elastin content in the abdominal aorta was similar between saline-infused male and female ANXA1-/- and WT mice (Fig. 4E, F). In males, elastin content was lower in Ang II-infused WT and ANXA1-/- mice compared to saline controls, respectively (Fig. 4E, F). In females, chronic Ang II treatment resulted in similar elastin content in WT, while elastin content was lower in ANXA1-/- mice compared to saline counterparts (Fig. 4E, F). Elastin content was lower in male compared to female mice in both genotypes (Fig. 4E, F). Calcium deposition in the abdominal aorta was greater in saline-infused female mice but not in male ANXA1-/- compared with their respective WT mice (Fig. 4G, H). Aortic calcium deposition was not different in Ang II-infused male WT mice but was greater in male ANXA1-/- mice compared with their saline counterparts (Fig. 4G, H). Chronic Ang II-infusion resulted in greater aortic calcification area in female WT mice, which was further exaggerated in ANXA1-/- mice (Fig. 4G, H). Aortic calcification was greater in saline and Ang II-infused female ANXA1-/- mice compared with male ANXA1-/- mice (Fig. 4G, H).
Ultrasound imaging of the carotid artery was performed to measure distensibility. The representative images showed a two-dimensional B-mode segment of the carotid artery (A, B). Quantification of the collagen (fibrotic) area of the abdominal aorta was performed by PSR stain. Red-stained collagen is shown in the representative images of the vessel (C, D). Quantification of elastin was performed by Verhoeff-van Gieson stain. Representative images of the vessel show black stained elastin lining (E, F). Calcification was quantified as the percentage area of calcium deposition in the abdominal aorta using the Von Kossa stain. Black-stained calcification is shown in the representative images of the vessel (G, H). Histograms (B, D, F, H) represent saline-infused male WT mice (blue filled, n = 6–12) and male ANXA1-/- mice (blue pattern, n = 8–12) and Ang II-infused male WT mice (red filled, n = 6–12) and male ANXA1-/- mice (red pattern, n = 5–11) and saline-infused female WT mice (green filled, n = 11–12) and female ANXA1-/- mice (green pattern, n = 12) and Ang II-infused female WT mice (pink filled, n = 12) and female ANXA1-/- mice (pink pattern, n = 12). The scale bar in black represents 1 mm for PSR and Verhoeff-van Gieson stain images and 300 µm for Von Kossa stain images. Results are presented as mean ± SEM. Statistical analysis was conducted with a MANOVA followed by a Bonferroni significant difference post-hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 between treatment groups. #P < 0.05, ###P < 0.001 between sexes. Ang II: angiotensin II, ANXA1-/-: annexin-A1-deficient, PSR: picrosirius red, Sal: saline, WT: wild-type.
Ang II-infused ANXA1-/- mice demonstrated exaggerated adverse collagen deposition and exhibited greater dysfunction and hypertrophy in the LV
Global longitudinal strain (GLS) and ejection fraction (EF) were similar between genotypes in chronic saline infusion (Fig. 5A–D and Supplementary Table 4). Chronic Ang II-infusion resulted in a lower GLS and EF in male but not female mice of either genotype compared to saline counterparts (Fig. 5A–D and Supplementary Table 4). GLS and EF were lower in Ang II-infused male WT and ANXA1-/- mice compared to Ang II-infused female mice of both genotypes (Fig. 5A–D and Supplementary Table 4). Cardiomyocyte area was not different in saline-infused WT and ANXA1-/- mice of either sex (Fig. 5E, F). Cardiomyocyte area was greater in Ang II-infused male and female WT and ANXA1-/- mice compared with their respective saline counterparts (Fig. 5E, F). The extent of cardiomyocyte hypertrophy induced by Ang II was lower in male and female ANXA1-/- mice compared to WT counterparts (Fig. 5E, F). Cardiomyocyte area was lower in saline-infused female than male ANXA1-/- mice (Fig. 5E, F), but was not different in other groups (Fig. 5E, F). Interstitial collagen deposition in LV was not different in saline-infused WT and ANXA1-/- mice of either sex (Fig. 5G, H). Chronic Ang II-infusion resulted in greater interstitial collagen deposition in the LV of male and female WT and ANXA1-/- mice relative to their saline counterparts (Fig. 5G, H). This response was exaggerated in ANXA1-/- mice compared to WT counterparts in both sexes (Fig. 5G, H). Interstitial collagen deposition in LV was comparable between male and female mice (Fig. 5G, H).
B-mode speckle tracking, longitudinal two-dimensional strain of the LV was performed to measure GLS. The representative echocardiography images show myocardial strain (A, B). EF was measured by performing parasternal long-axis LV chamber echocardiography. LV long-axis showed by representative echocardiographic images (C, D). Hematoxylin and eosin staining was used to quantify the cardiomyocyte’s area. Representative images of LV showing cardiomyocytes (E, F). PSR stain quantified the interstitial fibrotic area of LV and showed it as red-stained collagen in representative images (G, H). Histograms (B, D, F, H) represent saline-infused male WT mice (blue filled, n = 8–15) and male ANXA1-/- mice (blue pattern, n = 8–20) and Ang II-infused male WT mice (red filled, n = 8–19) and male ANXA1-/- mice (red pattern, n = 8–22) and saline-infused female WT mice (green filled, n = 8–12) and female ANXA1-/- mice (green pattern, n = 8–12) and Ang II-infused female WT mice (pink filled, n = 8–12) and female ANXA1-/- mice (pink pattern, n = 8–12). The scale bar in black represents 1 mm. Results are presented as mean ± SEM. Statistical analysis was conducted with a MANOVA followed by a Bonferroni significant difference post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 between treatment groups. #P < 0.05 between sexes. Ang II: angiotensin II, ANXA1-/-: annexin-A1-deficient, EF: ejection fraction, GLS: global longitudinal strain, H & E: hematoxylin and eosin, LV: left ventricle, PSR: picrosirius red, Sal: saline, WT: wild-type.
Chronic Ang II-infusion resulted in mitochondrial dysfunction and altered estrogen signaling in male and female ANXA1-/- mice
There were no differences between males and females for cardiac mitochondrial oxygen consumption rates (OCR) by complex 1 or complex 4 in WT mice (Fig. 6A, B, E, F), but the activity of both was greater in Ang II-infused ANXA1-/- mice of both sexes (Fig. 6A, B, E, F). Cardiac complex 2 OCR was similar between saline and Ang II-infused WT mice of both sexes (Fig. 6C, D). Ang II-infusion failed to impact cardiac complex 2 OCR in male ANXA1-/- mice, but the impact of Ang II-infusion on complex 2 activity was exaggerated in female ANXA1-/- mice relative to sex-matched WT, as well as in male ANXA1-/- mice (Fig. 6C, D). As complex 2 activity was markedly elevated in females compared to males, we further explored the mechanistic interaction between estrogen and the FPR-ANXA1 axis in human aortic smooth muscle cells (HASMCs). Administration of 17β-estradiol (10 μM) increased ANXA1 protein levels in HASMCs compared to the vehicle (0.001% dimethyl sulfoxide [DMSO], Fig. 6G). Similarly, the positive control, dexamethasone (DEX, 10 μM), caused a 2-fold increase in ANXA1 protein levels in the supernatant of HASMCs compared to the vehicle (Fig. 6G). DEX, but not 17β-estradiol, elevated ANXA1 gene expression in HASMCs (Fig. 6H). FPR2 gene expression tended to be greater in HASMCs treated with 17β-estradiol, but not DEX (Fig. 6I). The expression of the FPR1 gene was similar in HASMCs of either treatment (Fig. 6J). These results suggest a distinct regulatory effect of estrogen on the FPR-ANXA1 axis.
OCR (max-min) of complexes 1, 2, and 4 was measured for 60 min in mitochondria isolated from LV of WT and ANXA1-/- mice-infused with saline or Ang II in both sexes (A–F). ANXA1 levels and expression of genes (ANXA1, FPR1, FPR2) in HASMCs treated with 17β-estradiol and DEX (G–J). Protein-protein interaction of mouse TA was performed using STRINGApp and visualized biological processes, including blue-colored cellular hormone metabolic processes, green-colored electron transport chain, red-colored estrogen biosynthetic processes, and pink-colored inflammatory responses (K). This network was created in STRING and is freely available under a Creative Commons BY 4.0 license: https://string-db.org/cgi/network?taskId=baXS1LngLYbl&sessionId=bMuZiq96QNPH. Proteins associated with estrogen-inflammatory signaling were co-identified in male and female mice and humans (L). Proteins co-identified in our mouse aorta and published human aortic datasets of both sexes (M). Histograms (B, D, F) represent OCR of complex 1, 2, and 4 of saline-infused male WT mice (blue filled, n = 6) and male ANXA1-/- mice (blue pattern, n = 6) and Ang II-infused male WT mice (red filled, n = 6) and male ANXA1-/- mice (red pattern, n = 6) and saline-infused female WT mice (green filled, n = 7) and female ANXA1-/- mice (green pattern, n = 7) and Ang II-infused female WT mice (pink filled, n = 7) and female ANXA1-/- mice (pink pattern, n = 6). Histograms (G–J) represent HASMCs treated with DMSO (black filled bar, n = 3–4), 17β-estradiol (mild blue filled bar, 0.1 µm, moderate blue filled bar, 1 µm, dark blue filled bar, 10 µm, n = 3–4), and DEX (green filled bar, n = 3–4). Dot plots (M) represent an expression of proteins dysregulated in Ang II vs Sal-infused WT (black circle) and ANXA1-/- (brown circle), and ANXA1-/- vs WT mice infused with Sal (blue circle) and Ang II (red circle). Results are presented as mean ± SEM. Statistical analysis was conducted with one-way ANOVA and MANOVA, followed by a Bonferroni significant difference post-hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 between treatment groups. ###P < 0.001 between sexes. Ang II: angiotensin II, ANXA1-/-: annexin-A1-deficient, DEX: dexamethasone, DMSO: dimethyl sulfoxide, FPR: formyl peptide receptor, HASMCs: human aortic smooth muscle cells, LV: left ventricle, OCR: oxygen consumption rate, Sal: saline, WT: wild-type.
Further, we performed a selective protein interaction mapping using the STRING database to investigate dysregulated protein-protein interactions associated with estrogen signaling and the FPR-ANXA1 axis in the TA proteome. We identify functional enrichment of biological processes (Fig. 6K), including GO:0034754 (cellular hormone metabolic process, false discovery rate [FDR]:1.30e-07), GO:0022900 (electron transport chain, FDR:0.0105), GO:0006703 (estrogen biosynthetic process, FDR:0.00036), and GO:0006954 (inflammatory response, FDR:2.47e-05, Supplementary Table 18). Proteins associated with these processes, such as members of the S100A and HSD17b family, were significantly altered in our mouse TA proteome datasets across both sexes (Supplementary Tables 10 and 11). We performed a STRING analysis to investigate whether our male and female WT mouse TA proteins associated with estrogen signaling and mitochondrial processes were mirrored in patients with aortic complications23. We identified estrogen-inflammatory signaling proteins (S100A1, HSD17B11, Fig. 6K–M) and mitochondrial physiology proteins (HSD17B4, NDUFS4, COQ9, Fig. 6K–M and Supplementary Table 18) co-identified in the aorta of male and female mice and human data23, suggesting important clinical relevance, particularly in understanding sex differences in vascular health and cardiovascular diseases.
Discussion
Cardiovascular disease remains a leading cause of morbidity and mortality globally, with significant differences in prevalence, presentation, and outcomes between men and women. Despite these well-documented disparities, cardiovascular research has historically focused on males, often neglecting sex-specific mechanisms that may influence disease progression and treatment efficacy. Among these mechanisms, BP regulation and cardiovascular remodeling play a critical role in maintaining cardiovascular health, yet unique pathways in females remain underexplored. Given this gap, there is an urgent need to identify potential therapeutic targets to address hypertension-induced cardiovascular complications in both sexes, particularly those involving endogenous pro-resolving mediators such as ANXA1, a target that is yet to be fully investigated. In this study, our quantitative tissue proteomics provides a detailed molecular analysis of the hypertensive cardiac and aortic landscape in male and female WT and ANXA1-/- mice. This advanced proteomic approach offers a systemic level understanding of global changes in the cardiac and aortic proteome due to hypertension, highlighting the translational potential of proteomics in identifying molecular insights into hypertension across both sexes24,25. Our functional analyses, combined with tissue- and cellular-level proteome remodeling, reveal that deficiency of the pro-resolving mediator ANXA1 exacerbates cardiovascular damage in both sexes, with a more pronounced effect in females without this protective pathway. These findings suggest that ANXA1 may play a more protective role in females than in males, particularly in mitigating cardiovascular dysfunction associated with hypertension.
In this study, we demonstrated that conscious ANXA1-/- mice of both sexes exhibited elevated BP from 7 weeks-of-age, which was maintained until 14 weeks-of-age. This reveals that ANXA1 plays a critical physiological role in BP regulation. Ang II induces hypertension via angiotensin receptor type 1 (AT1R) to cause vasoconstriction, sympathetic nerve stimulation, increased aldosterone biosynthesis, and increased renal sodium retention26. Hypertensive responses to Ang II were greater in male than in female WT mice, consistent with previous reports27. The hypertensive response to Ang II was less marked in females than males, and this may be attributable to higher expression of renal AT2R (which functionally opposes AT1R responses) than in males28. The exaggerated hypertensive response observed in ANXA1-/- mice suggests endogenous ANXA1 has a protective effect against hypertension, and its effect is more pronounced in females.
Cardiovascular hypertrophy and fibrosis are characteristic features of structural remodeling during hypertension29. Our previous studies demonstrated that ANXA1-/- mice exhibit exaggerated cardiac fibrosis after MI9 and eutrophic remodeling in mesenteric arteries in mice with insulin deficiency21. In this study, LV fibrosis (demonstrated by collagen deposition) was greater in Ang II-infused WT mice of both sexes, consistent with previous reports26. This was similarly increased in male and female ANXA1-/- mice subjected to chronic Ang II-infusion. Consistent with this, our LV proteomics analysis demonstrated that the expression of the fibrotic protein Fn1 was higher in male and female ANXA1-/- mice-infused with Ang II for 28 days. Collagen deposition in the abdominal aorta was elevated in all Ang II-infused mice but was comparable in both sexes and genotypes, consistent with previous reports in male30 and female29 mice. Our TA proteomics analysis demonstrated that expression of fibrotic proteins Lgals3 in males and Fn1 in females was elevated in Ang II-infused ANXA1-/- mice, which may contribute to aortic fibrosis.
Previous studies demonstrated that Ang II-infusion increases LV mass in male and female WT mice26. Consistent with this, LV mass and cardiomyocyte area were greater in hypertensive WT mice. Interestingly, LV mass was increased, but cardiomyocyte area was not, in hypertensive ANXA1-/- compared to WT mice of either sex, indicating LV hypertrophy but not cardiomyocyte hypertrophy in ANXA1-/- mice of both sexes. The exact mechanism for this difference is unknown, however, we have previously demonstrated that the ANXA1 mimetic Ac2-26 reduced cardiomyocyte death in mice subjected to MI31, suggesting a critical role of ANXA1 in cardiomyocyte survival. In contrast to our results, exogenous human recombinant ANXA1 supplementation reduces cardiomyocyte hypertrophy in male arthritic mice32. In addition, chronic Ang II-infusion resulted in upregulated expression of hypertrophic protein myosin heavy chain 9 (Myh9) in male and myofibrillar biogenesis protein nebulin-related anchoring protein (Nrap1) in female ANXA1-/- mice, as demonstrated in our LV proteomics analysis. In line with our findings, overexpression of Nrap1 was associated with ventricular hypertrophy and dysfunction in female mice33. Taken together, this supports the protective role of ANXA1 in preventing LV hypertrophy in either sex. Chronic Ang II-infusion induces cardiac pathological remodeling, ultimately leading to cardiac dysfunction34. Consistent with this, our two-dimensional LV strain images demonstrated that myocardial GLS strain was lower in male hypertensive mice of either genotype. Surprisingly, GLS was not different in female mice, indicating that LV function was selectively impaired in male mice, regardless of genotype. The mechanism responsible for this difference in cardiac dysfunction between sexes is unknown and warrants further investigation.
It is well-documented that Ang II alters the structure and function of the blood vessel wall, mainly by causing deposition of extracellular matrix, elastin degradation, and increasing vascular inflammation and stiffness35,36. Consistent with this, our TA proteomics analysis demonstrated that structural proteins (Lgals3 and Tbca in males and Tnc and Ablim1 in females) were upregulated to a greater extent in Ang II-infused ANXA1-/- than WT mice, contributing to adverse vascular dysfunction. Further, our vascular ultrasonography analysis demonstrated that distensibility of the carotid artery was lower in ANXA1-/- than in WT mice of either sex. Interestingly, distensibility was lower in female than in male ANXA1-/- mice, suggesting that ANXA1 exhibits a more pronounced vascular protective effect in females. Increased stiffness and decreased compliance of the vascular wall are the main consequences of excessive vascular calcification and inflammation37,38. Calcium deposition in the aorta was greater in female than in male atherosclerotic mice39. Similarly, in this study, aortic calcification was more elevated in female than in male ANXA1-/- mice. This is consistent with higher calcium regulatory proteins (Itpr1 in males and Smoc1 in females) and inflammatory proteins (Lta4h in males and Cfh in females) identified in our TA proteome. Furthermore, saline-infused ANXA1-deficient mice exhibit greater aortic calcification consistent with greater expression of calcium regulatory proteins (Capn1 in males and Myl12b in females). These observations were accompanied by a greater immune cell count, particularly neutrophils, in female than in male ANXA1-/- mice (Supplementary Table 5). Taken together, ANXA1 plays a critical role in the regulation of calcium and inflammation. Continuous Ang II-infusion is associated with the loss of elastin fibers within the vessel wall20. Ang II-infusion in male WT mice exhibited lower elastin content than female WT mice40. In line with this, elastin content was lower in male than in female mice subjected to chronic Ang II-infusion, and this lower elastin content was not affected by either genotype.
Mitochondrial dysfunction has been associated with adverse structural remodeling41, and there is evidence to suggest that those abnormalities in hypertension may be triggered by the renin-angiotensin-aldosterone system42. Increased myocardial oxygen consumption has been reported to be associated with cardiac inefficiency in hypertensive patients and mice with diabetic cardiomyopathy43,44. Our study demonstrated that the cardiac mitochondrial OCR was elevated in ANXA1-deficient hypertensive mice of both sexes, indicating reduced cardiac efficiency. Interestingly, cardiac complex 2 OCR (succinate dehydrogenase activity) was higher in Ang II-infused female ANXA1-/- mice but not in their male counterparts. This suggests that hypertension-induced mitochondrial dysregulation may be more pronounced in females than in males. This finding is consistent with recent clinical evidence showing that female patients with mitochondrial diseases exhibit higher SAP compared to female controls, with this increase being comparable to the differences observed between male patients and control45. Previous studies have shown that a high concentration of succinate induces a reversed electron transfer from complex 2 to complex 1, which is associated with a higher rate of superoxide generation46,47. These observations suggest that the deficiency of ANXA1 induces mitochondrial dysfunction, especially by upregulated complex 2-induced production of reactive oxygen species. Chronic Ang II-infusion in mice resulted in dysregulated expression of mitochondrial genes and proteins in LV and abdominal aorta48,49. Consistent with this, our proteomics data demonstrated that cardiovascular mitochondrial proteins were aberrant in female (Nqo1, Coq8a, Cat in LV and Ndufs4, Coq10b, Sod3 in TA) and male (Nqo1, Cox5a, Cat in LV and Coq7, Slc27a2, Kpna4 in TA) ANXA1-deficient mice-infused with Ang II, consistent with adverse mitochondrial remodeling.
Previous reports demonstrated that ANXA1 expression on polymorphonuclear cells was positively correlated with plasma estrogen levels in females50. Given the more pronounced effects observed in female ANXA1-/- mice, HASMCs were treated with the female sex hormone, estrogen, to investigate the estrogen-ANXA1 axis. Treatment with the estrogen receptor agonist, 17β-estradiol, stimulated the release of ANXA1 without altering mRNA expression. This suggests that there is post-transcriptional regulation of ANXA1 by 17β-estradiol, potentially through post-translational modifications or protein stabilization mechanisms, rather than via direct transcriptional regulation51. In addition, the minimal changes observed in FPR2 expression across all treatments suggest that estrogen may not directly modulate FPR2 in HASMCs. Alternatively, FPR2 regulation may occur through different signaling pathways or be influenced by other cell types (e.g., immune cells)52. 17β-estradiol reverses ovariectomized-induced leukocyte adherence in the cerebral vasculature of ANXA1-/- but not WT mice suggesting estrogen exerts protective ANXA1-dependent actions53. Further, we define primary hormone metabolic signaling and estrogen biosynthetic processes associated with females following TA, based on aortic hypertensive proteome remodeling. Of note, estrogen-mediated upregulation of ANXA1 has been shown to mediate a critical role in maintaining cardiovascular homeostasis, particularly in females, where its protective effects against inflammation and pathological remodeling are more pronounced23. Investigating the mechanistic details of this axis will provide valuable insights for developing both gender-neutral and targeted treatments for hypertension and related systemic complications, focusing on addressing the unique pathophysiology observed in females. We have elucidated the critical role of ANXA1 in Ang II-induced hypertension and end-organ damage through a loss-of-function approach. Further studies involving gain-of-function approaches are now warranted to assess the therapeutic potential of ANXA1 supplementation in preventing hypertension-induced cardiovascular damage. Future studies will assess receptor-mediated constriction (phenylephrine or Ang II) using myography to thoroughly characterize both large conductance and resistance vascular beds in the Ang II-induced hypertension model, examining both male and female WT and ANXA1-deficient mice. This should be considered in future investigations.
In summary, our comprehensive analysis of the mouse cardiac and aortic proteome unveiled insights into the molecular pathways driving pathological remodeling and mitochondrial function, both of which are critical in the development of hypertension. Strikingly, these pathways were more prominently dysregulated in female ANXA1-deficient mice than in males. This study provides unique, compelling evidence that deficiency of the pro-resolving mediator, ANXA1, exaggerates Ang II-induced hypertension, exacerbating adverse cardiovascular remodeling and function in both sexes, with a notably greater impact in females. This sex-specific vulnerability aligns with the observation that the protective effects of endogenous ANXA1 are more pronounced in females. These findings underscore the critical need for advancing pro-resolution ANXA1-based therapies to mitigate systemic hypertension and its severe complications, particularly in women, who experience disproportionately pronounced effects and limited selective therapeutic interventions.
Methods
Animals
Male (n = 79) and female (n = 48) WT mice and ANXA1-/- mice were randomly divided into eight groups: Normotensive (saline-infused) WT male mice (WT+Sal, n = 17), normotensive (saline-infused) ANXA1-/- male mice (ANXA1-/-+ Sal, n = 19), hypertensive (Ang II-infused) WT male mice (WT + Ang II, n = 21), hypertensive (Ang II-infused) male ANXA1-/- mice (ANXA1-/-+Ang II, n = 22), normotensive (saline-infused) female WT mice (WT + Sal, n = 12), normotensive (saline-infused) ANXA1-/- female mice (ANXA1-/-+ Sal, n = 12), hypertensive (Ang II-infused) WT female mice (WT + Ang II, n = 12) and hypertensive (Ang II-infused) ANXA1-/- female mice (ANXA1-/-+ Ang II, n = 12). Mice were group housed in a room with a 12:12 h light-dark cycle and allowed ad libitum access to water and standard mouse chow (Specialty Feeds, Glen Forrest, Western Australia, 19% protein, 5% fat, 5% fiber, 0.2% sodium). We have complied with all relevant ethical regulations for animal use. This study was carried out following the recommendations of the Australian Code for the Care and Use of Animals for Scientific Purposes from the National Health and Medical Research Council. The protocol was approved by the Alfred Medical Research Education Precinct Animal Ethics Committee (#E/1987/2020/B) and was conducted following Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines54. At 15 weeks-of-age, hypertension was induced by subcutaneous infusion of Ang II at a dose of 0.7 mg/kg/day for 28 days as described previously20. Animals were monitored once daily following mini-pump surgery. We have included flow charts for the reporting of animal use and analysis in all the experiments (Supplementary Fig. 3).
Measurement of BP by the tail-cuff method
Tail-cuff BP recording was performed once weekly between 9 a.m. to 12 p.m. using a CODA 8 non-invasive BP acquisition system for mice (Kent Scientific, Torrington, CT) as described previously52. This system uses volume pressure recording (VPR) to detect BP based on volume changes in the tail. Prior to commencing the experiments, the patency of the occlusion and VPR cuffs was checked routinely. The BP recording was performed in a designated quiet area (22 ± 2 °C), with mice acclimatized for 1 h before experiments began. Mice were placed in the restrainer carefully with an adjustable nose piece to prevent excessive movement. The occlusion cuff (O-cuff) was placed through the tail and to the base of the tail and the VPR sensor cuff was placed adjacent to the O-cuff. Heating pads were preheated to 33 to 35 °C for 1 h. The mice were warmed for 10 min before and during BP recordings. To measure BP, the occlusion cuff was inflated to 250 mmHg and deflated over 20 s. As blood flow returned to the tail, a change in the tail volume was detected by the VPR sensor cuff during deflation. The minimum volume change was set to 15 μL. Each BP recording session consisted of 15 deflation and inflation cycles per set, of which the first 5 cycles were acclimation cycles and were not used in the analysis, with the following 10 cycles included in the analysis. In addition, the following criteria were applied to include cycles for BP recording: cycles were excluded for mice with a heart rate of less than 100 bpm, fewer than 5 true cycles, a mean arterial pressure under 60 mmHg, and a standard deviation ±3.
Ultrasound imaging
Cardiac52 and vascular function20 were assessed by ultrasound imaging using a Vevo 2100 High-Resolution Imaging System (FUJIFILM, Visual Sonics Inc., Canada). Measurement of LV and carotid artery function was performed in anesthetized mice (Isoflurane, 1.7-1.8%) at 19 weeks of age. LV function was assessed using B-mode speckle tracking, and vascular function was assessed using two-dimensional, parasternal long-axis B-mode imaging of the carotid artery using Vevo Vasc software (FUJIFILM, VisualSonics Inc., Canada) as previously described20. All ultrasound imaging data were analyzed in a blinded manner and validated using strict quality control measures as previously described52. Vascular distensibility was semi-automatically derived using Vevo Vasc software. Average distensibility was measured from six regions of the vascular lumen using the following formula: [Area (systole)-Area (diastole)/ Area (diastole)]/[Pressure (systole)-Pressure (diastole)].
Differential cell count
Differential cell counts were measured in whole blood as described previously20. A small aliquot of uncoagulated blood (20 µl) was diluted in a 120 µl cell pack for differential cell counts, and blood cell count data were acquired using an XS-1000i automated hematology analyzer (Sysmex, Japan).
Tissue collection
Animals were euthanized with pentobarbital sodium (100 mg/kg, i.p.) between 8 a.m. and 12 p.m. A cardiac puncture was performed, and blood was collected into an ethylenediaminetetraacetic acid (EDTA)-containing tube (Microvette, SARSTEDT, Germany). The heart, kidneys, lungs, liver, spleen, and blood vessels were rinsed prior to collection. All tissues were weighed with the exception of the blood vessels. The heart was further dissected into the LV, RV, and left and right atria, and the weights were recorded. One segment of LV was fixed in 10% formaldehyde for histology, with other segments of the LV as well as the TA, snap-frozen in liquid nitrogen for proteomics analysis. The abdominal aortas were placed into cassettes and submerged in calcium-free Krebs solution, followed by fixing in 10% formaldehyde.
Histology analysis
The Monash Histology Platform performed staining and imaging of all slides. The whole slide was scanned, and the Monash Histology Platform provided raw ScanScope virtual slide files. To extract the whole image, Aperio ImageScope (Leica Microsystems Pty. Ltd.) software was used. All image analysis was carried out using custom-written macros in the Fiji distribution of Image J software (version 1.53c, National Institute of Health, USA)55. LV20, and vessel20 sections were stained with picrosirius red to detect collagen deposition. With the use of Image J software’s color deconvolution and thresholding, the percentage area of collagen-stained red was determined (version 1.53c, National Institute of Health, USA). LV sections were stained with hematoxylin and eosin to measure cardiomyocyte area using a semi-automated macro in Image J software (version 1.53c, National Institute of Health, USA)52. Verhoeff–van Gieson stain identified elastin52, and the Von Kossa stain indicated calcium deposition20 in vessels. Using trained Ilastik models56 in Image J software, stained colors associated with the vessel’s calcium area and elastin content were extracted (version 1.53c, National Institute of Health, USA). Area coverage and percentages were then computed using these extracted regions.
Mitochondrial respiration20
Tissue homogenization
4 mg of frozen LV tissues were thawed, minced, and homogenized in 200 μl of mitochondrial assay solution (MAS) buffer (70 mM sucrose, 220 mM mannitol, 1 mM EGTA, 5 mM MgCl2, 5 mM KH2PO4, 1 mM EGTA, 2 mM HEPES, pH 7.4). LV tissues were incubated at 37 °C for 30 min. All homogenates were centrifuged at 1000 × g for 10 min at 4 °C, and the supernatant was collected. BCA (bicinchoninic acid) was used to determine protein concentration (Thermo Fisher).
Plate loading and substrate injection
LV homogenates were diluted with 20 μl of MAS and loaded into a Seahorse XF96 microplate reader. The plate was then centrifuged at 2000 × g for 5 min at 4 °C (with the brake turned off), and an additional 140 μl of MAS containing cytochrome c (10 μg/ml, final concentration) was carefully added to each well (to avoid disrupting the bottom of the plate). Substrate injection was as follows: NADH (1 mM), or 5 mM succinate + rotenone (5 mM + 2 μM) was injected at port A; rotenone + antimycin A (2 μM + 4 μM) at port B; TMPD [N, N, N, N-tetramethyl-p-phenylenediamine] + ascorbic acid (0.5 mM + 1 mM) at port C; and azide (50 mM) at port D. These conditions determine the respiratory capacity of mitochondria through complex 1, 2, and 4.
Mitochondrial respiration analysis
Wave software (Agilent) was used to export OCR normalized by protein. Complex 1, 2, and 4-dependent respiration was calculated by subtracting OCR values (maximum-minimum).
Quantitative tissue-based proteomics analysis
Sample homogenization, protein reduction, alkylation, and digestion
LV (5 mg) and TA (2 mg) samples were harvested from normotensive (saline-infused) WT male mice (WT + Sal, n = 4–5), normotensive (saline-infused) ANXA1-/- male mice (ANXA1-/-+ Sal, n = 5), hypertensive (Ang II-infused) WT male mice (WT + Ang II, n = 4–5), hypertensive (Ang II-infused) male ANXA1-/- mice (ANXA1-/-+ Ang II, n = 5), normotensive (saline-infused) female WT mice (WT + Sal, n = 5), normotensive (saline-infused) ANXA1-/- female mice (ANXA1-/-+ Sal, n = 5), hypertensive (Ang II-infused) WT female mice (WT + Ang II, n = 5) and hypertensive (Ang II-infused) ANXA1-/- female mice (ANXA1-/-+ Ang II, n = 5) and lysed on ice with lysis buffer (8 M urea in 50 mM HEPES, pH 8.0) containing protease and phosphatase inhibitors (Halt, Life Technologies, #78442). Proteins were extracted by tip-probe sonication and quantified by microBCA (Life Technologies, #23235). Lysates (10 µg protein) were normalized in 50 µL lysis buffer, prior to sample reduction in 10 mM dithiothreitol (DTT) for 1 hr at 25 °C, then alkylated (20 mM iodoacetamide) for 30 min at 25 °C in the dark and quenched with additional DTT (10 mM) before the Sera-Mag-based workflow24,57. A magnetic bead slurry was prepared by mixing SpeedBeads™ magnetic carboxylate modified particles (Cytiva, 65152105050250, 45152105050250) at 1:1 (v:v) ratio, washing with mass spectrometry-grade water, and samples reconstituted to a final concentration of 100 µg/µL. The final concentration of ethanol (EA043, ChemSupply) was added to the beads at a ratio of 10:1 bead to protein (v/v). Protein-bound magnetic beads were washed three times with 200 µL of 80% ethanol and reconstituted in 50 µL of 50 mM triethylammonium bicarbonate (TEAB) pH 8.0. Protein digestion was performed with Lysyl Endopeptidase (enzyme: substrate 1:100, 125-05061, Wako Pure Chemical Industries) and trypsin (enzyme: substrate 1:50, Promega V5113) overnight at 37 °C with agitation (1000 rpm). The peptide mixture was centrifuged at 20,000 × g and dried by vacuum centrifugation, reconstituted in 0.07% trifluoroacetic acid (TFA), and quantified by fluorometric peptide assay (Thermo Scientific, 23290) as per manufacturer’s instructions.
NanoLC and mass spectrometry
Spectra were acquired using data-dependent acquisition on a Q Exactive HF-X benchtop Orbitrap mass spectrometer coupled to an UltiMate™ NCS-3500RS nano-HPLC (Thermo Fisher Scientific) as previously described58. Peptides (360 ng) were loaded (Acclaim PepMap100 C18 3 μm beads with 100 Å pore-size, Thermo Fisher Scientific) and separated (1.9 µm particle size C18, 120 Å, 0.075 × 200 mm, Nikkyo Technos Co. Ltd) with a gradient of 2–28% acetonitrile containing 0.1% formic acid over 95 min followed by 28–80% from 95–98 min at 300 nL per min at 55 °C (butterfly portfolio heater, Phoenix S&T). After acquiring an MS1 scan from 350–1650 m/z (60,000 resolution, 3 × 106 automatic gain control (AGC), 128 msec injection time), followed by MS/MS data-dependent acquisition (top 25) with collision-induced dissociation and detection in the ion trap (30,000 resolution, 1 × 105 AGC target, 28% normalized collision energy, 1.3 m/z quadrupole isolation width, 60 msec injection time). Peptide match was disabled for unassigned precursor ions, charge states, and slightly charged species. For thirty seconds, the chosen sequenced ions were dynamically excluded. Data was acquired using Xcalibur (v4.5, Thermo Fisher Scientific). For spectral library generation and high-pH fractionation, anhydrous peptide digests from LV (pooled) and TA (pooled) were individually reconstituted in 25 mM ammonium formate, pH 10, for high-pH reversed-phase microscale fractionation. In-house X-RPS Stagetips (#66886-U, Sigma) were used for peptide binding, and peptides were eluted using acetonitrile (2–50%, v/v) in 25 mM ammonium formate, pH 10. A total of 8 fractions from each tissue region were lyophilized by SpeedVac. Peptide samples were reconstituted in 0.07% TFA and analyzed in single-shot proteomics. A list of samples and RAW data (including spectral libraries) is available in ProteomeXchange Consortium via the PRIDE partner repository; #PXD035034.
Data processing and bioinformatics
Identification and quantification of peptides were performed using MaxQuant (v1.6.14.0)59 and Andromeda60 as described61. For each tissue region (LV or TA), tandem mass spectra were searched against the Mus musculus (mouse) reference proteome (55,398) supplemented with common contaminants and a generated spectral library. Search parameters were as follows: carbamidomethylated cysteine as fixed modification, oxidation of methionine and N-terminal protein acetylation as variable modifications, trypsin/P as a proteolytic enzyme with ≤2 missed cleavage sites, search tolerance 7 ppm, fragment ion mass tolerance 0.15 Da; minimum peptide length was defined at 6, <1% FDR on peptide spectrum match with target-decoy approach at peptide and protein levels, match between runs selected, and label-free quantification (LFQ) algorithm employed. Contaminants and reverse identification were excluded from further data analysis. High confident protein identification required more than one unique or razor peptide per protein group. Data analysis was performed using Perseus via the MaxQuant computational platform62 and the R programming language. By using Perseus software, protein intensities were log2 transformed and subjected to PCA with missing values imputed from a normal distribution (width 0.3, downshift 1.8) and Student’s t test. Hierarchical clustering was performed using Euclidean distance and average linkage clustering. By using the R package ggplot2, the violin plot of the protein intensity distribution and the boxplot of the coefficient of variations per sample group were visualized. The volcano plot of Student’s t test p-value versus log2 fold change was generated. g: Profiler and Reactome databases were utilized for functional enrichment and network/pathway analysis, with significance P < 0.05 as described previously58. In addition, the protein interaction networks were selected and visualized using STRING functional enrichment via STRINGApp63. Our TA proteome datasets of male and female mice were compared with published datasets of patients with adverse aortic remodeling in both sexes23. Proteins associated with estrogen-inflammatory signaling and mitochondrial physiology were co-identified in mice and humans. To maintain relevance specifically to sex-dependent changes in vascular remodeling, we have selected the human studies of Gunnarsson et al.23. This study performed transcriptomics analysis of the aorta and reported that estrogen regulates inflammatory signaling in male and female patients with aortic complications23.
Cell culture
Female HASMCs were obtained from the American Type Culture Collection (ATCC Manassas, VA, USA)20. HASMCs were cultured in a Vascular Cell Basal Medium containing 5% fetal bovine serum (FBS) at 37 °C in a humidified 5% CO2 atmosphere. Cells from passages 5 to 8 were used for the experiments. Once confluency reached over 70%, cells were washed with Phosphate-Buffered Saline, detached using 0.25% Trypsin-EDTA, and incubated for 5 min at 37 °C. After detachment, an equal volume of trypsin neutralizing solution was added and incubated for another 5 min at 37 °C, and the suspension was centrifuged at 200 x g for 5 min. HASMCs were then resuspended in fresh medium, counted using a hemocytometer, and their viability was assessed using Trypan Blue. The plating concentration was set at 2 × 105 cells per well in 6-well plates. HASMCs were treated with 17β-estradiol (0.1 to 10 μM) and DEX (10 μM)64 was included as a positive control standard to evaluate its known effects on ANXA1 levels. DMSO (0.001%) was used as a vehicle control. Each condition was performed in four replicates and incubated for 48 h. Supernatants were collected to measure protein levels, and cells were collected using TRIzol reagent to quantify mRNA expression.
Protein quantification by ELISA
ANXA1 levels were quantified in supernatants by a human ANXA1 ELISA Kit (Thermo Fisher Scientific, USA). The procedure followed the manufacturer’s instructions, adding 100 μL of supernatant sample to pre-coated ELISA plates. The plates were incubated for 2.5 h at room temperature. After incubation, the plates were washed four times with a wash buffer, and 100 μL of biotin-conjugated anti-ANXA1 antibody was added, followed by incubation for 1 h. Streptavidin-HRP solution (100 μL) was then added to each well, followed by the addition of the TMB substrate for color development. Absorbance was measured by a CLARIO star ELISA Plate Reader at 450 nm, and a standard curve was generated to quantify the concentration of ANXA1 in the samples. The results were averaged across four replicates for each treatment condition and expressed as mean ± SEM.
Quantification of gene expression52
RNA was extracted from cells (lysates using TRIzol reagent) according to the TRIzol reagent manufacturer’s protocol. Frozen cell lysates were thawed on ice, and chloroform (C6074, Thermo Scientific) was added to each tube (100 µL of chloroform per 500 µL of TRIzol reagent). After vortexing for 15 sec at room temperature (RT), samples were incubated on ice for 10 min and centrifuged at 12,000 x g for 15 min at 4 °C. The colorless upper aqueous phase was transferred to a fresh 2 mL safe-lock microcentrifuge tube, and 500 µL of isopropanol (BP26181, Thermo Scientific) was added. Tubes were capped, inverted several times to mix the sample, and stored at -20 °C for 48 h to precipitate RNA. Precipitated RNA was centrifuged at 12,000 x g for 15 min at 4 °C to pellet the RNA. The supernatant was discarded, and 500 µL of cold 75% ethanol (75 mL of 100% ethanol [E7023, Sigma-Aldrich] diluted in 25 mL of Milli-Q water) was added to the tubes, allowed to stand for 2 min (to dissolve any salts), and briefly vortexed at RT. Samples were centrifuged at 7500 x g for 5 min at 4 °C, and the supernatant was discarded. RNA pellets were allowed to air dry for 10 min at RT and resuspended in 20 µL of nuclease-free water (129117, Qiagen). Extracted RNA was analyzed to determine its quality and concentration using a Nanodrop 2000 spectrophotometer. RNA concentration was normalized to 0.2 μg/μL per sample by dilution with Milli-Q water, followed by adding master mix (2 μl buffer, Deoxynucleoside triphosphate 0.8 μl, random primers 2 μl, and multiscribe 1 μl). Samples were then reverse transcribed using a Veriti™ 95 Thermal Cycler (Applied Biosystems, Foster City, California, USA) using the following protocol: 25 °C for 10 min, 48 °C for 30 min, 95 °C for 5, and 4. The resulting cDNA was frozen at _20 °C until required. Quantitative real time PCR (qRTPCR) was conducted to measure gene expression. Prior to plating, cDNA was diluted to a final concentration of 5 ng/µl by adding molecular-grade to each sample. The cDNA (10 ng) was then transferred into a 384 well MicroAmp Opti-Plate (Applied Biosystems, Life Technologies, Mount Waverly, Australia). Subsequently, the master-mix was prepared by mixing SYBR® Green Real-Time PCR solution (Life Technologies), molecular grade water, and both forward (5’) and reverse (3’) primers for the gene of interest. The master-mix was then transferred into each well of a 384 well plate containing cDNA for each given sample, sealed with optical film (Life Technologies), and centrifuged at 1000 x g for 3 min to eliminate air bubbles. The plate was then placed in a Quant Studio 7 Flex Real-Time PCR system (Applied Biosystem) for gene amplification. In order to compare the abundance of target genes, cycle thresholds (Ct) determined by qRTPCR were analyzed via the ΔΔCt method. All genes of interest were normalized to the housekeeper gene 18 s and expressed as a fold of the control. Human-specific primers were generated from sequences in GenBank in the table below,
Genes | Human primer sequences (5’-3’) | |
|---|---|---|
Forward primer | Reverse primer | |
FPR1 | ACCCAGAGCAAGACCACAGC | TCCATCTTGTCTGCTCCTGGA |
FPR2 | GCCTTTTGGCTGGTTCCTGTG | CCAGACTGGATGCAGGACACA |
ANXA1 | GGCCTTGGAACTGATGAAGA | GTTGTGGATAGCTTCTGGTG |
Statistical analysis
All analyses were performed with GraphPad Prism 9.0.1 (GraphPad, San Diego, CA). All the data are presented as mean ± SEM. The arterial BP data were analyzed using a two-factor split-plot analysis of variance (ANOVA) with repeated measures. Differences were considered significant when P < 0.05, after adjustment using the Greenhouse-Geisser co-efficient and the Bonferroni post-hoc test. Other data were analyzed with one-way and multivariate ANOVA (MANOVA), followed by a Bonferroni post-hoc test to assess significance between groups. P < 0.05 was deemed significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data generated or analyzed during this study are included in this published article (and its supplementary information files) or available from public data repositories. Source data for Figs. 1–6 are provided in Supplementary dataset 1. Proteomic data (RAW and processed/search files) for each tissue region (TA, LV) are available from the Proteome Xchange Consortium via the PRIDE partner repository with the dataset identifier PXD035034. For proteomics analyses, the Human Protein Atlas (https://www.proteinatlas.org/ humanproteome/ tissue) and functional enrichment annotations using g: Profiler (https://biit.cs.ut.ee/gprofiler/) were used. Further pathway enrichment map analysis was performed using Cytoscape (v3.7.1)25, Reactome25, and DAVID functional annotation25 software. Protein-protein interaction networks were described using STRINGApp incorporated into Cytoscape (v3.7.1)25. Hierarchical clustering was performed in Perseus using Euclidean distance and average linkage clustering, with missing values imputed at z-score 0. R was also used for data analysis and data visualization (ggplot2 and ggpubr packages). The supplementary data sheets and files are available via the private sharing link (https://figshare.com/s/899f2f02da0c4a6ac576) and the public sharing DOI (https://doi.org/10.6084/m9.figshare.28236392) on Figshare.
References
Gillis, E. E. & Sullivan, J. C. Sex differences in hypertension: recent advances. Hypertension 68, 1322–1327 (2016).
Ji, H. et al. Sex differences in blood pressure associations with cardiovascular outcomes. Circulation 143, 761–763 (2021).
Drummond, G. R., Vinh, A., Guzik, T. J. & Sobey, C. G. Immune mechanisms of hypertension. Nat. Rev. Immunol. 19, 517–532 (2019).
Snyder, E. C., Abdelbary, M., El-Marakby, A. & Sullivan, J. C. Treatment of male and female spontaneously hypertensive rats with TNF-alpha inhibitor etanercept increases markers of renal injury independent of an effect on blood pressure. Biol. Sex. Differ. 13, 17 (2022).
Ji, H. et al. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 64, 573–582 (2014).
Dinh, Q. N., Drummond, G. R., Sobey, C. G. & Chrissobolis, S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed. Res. Int. 2014, 406960 (2014).
Fu, T. et al. Therapeutic potential of lipoxin A4 in chronic inflammation: focus on cardiometabolic disease. ACS Pharm. Transl. Sci. 3, 43–55 (2020).
Qin, C. X. et al. Formylpeptide receptor 2: nomenclature, structure, signalling and translational perspectives: IUPHAR review 35. Br. J. Pharm. 179, 4617–4639 (2022).
Qin, C. X. et al. Endogenous annexin-A1 regulates haematopoietic stem cell mobilisation and inflammatory response post myocardial infarction in mice in vivo. Sci. Rep. 7, 16615 (2017).
Perretti, M. & Dalli, J. Exploiting the annexin A1 pathway for the development of novel anti-inflammatory therapeutics. Br. J. Pharm. 158, 936–946 (2009).
Qin, C. et al. Cardioprotective potential of annexin-A1 mimetics in myocardial infarction. Pharm. Ther. 148, 47–65 (2015).
Migeotte, I., Communi, D. & Parmentier, M. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 17, 501–519 (2006).
Pan, B. et al. A novel anti-inflammatory mechanism of high density lipoprotein through up-regulating annexin A1 in vascular endothelial cells. Biochim. Biophys. Acta 1861, 501–512 (2016).
Paravicini, T. M., Yogi, A., Mazur, A. & Touyz, R. M. Dysregulation of vascular TRPM7 and annexin-1 is associated with endothelial dysfunction in inherited hypomagnesemia. Hypertension 53, 423–429 (2009).
Yang, Y., Hutchinson, P. & Morand, E. F. Inhibitory effect of annexin I on synovial inflammation in rat adjuvant arthritis. Arthritis Rheum. 42, 1538–1544 (1999).
Vong, L. et al. Up-regulation of annexin-A1 and lipoxin A(4) in individuals with ulcerative colitis may promote mucosal homeostasis. Plos ONE 7, e39244 (2012).
Pruzanski, W. et al. Circulating group II phospholipase A2 activity and antilipocortin antibodies in systemic lupus erythematosus. Correlative study with disease activity. J. Rheumatol. 21, 252–257 (1994).
Cheuk, B. L. & Cheng, S. W. Annexin A1 expression in atherosclerotic carotid plaques and its relationship with plaque characteristics. Eur. J. Vasc. Endovasc. Surg. 41, 364–371 (2011).
Caso, V. M. et al. Regulation of inflammation and oxidative stress by formyl peptide receptors in cardiovascular disease progression. Life 11, 243 (2021).
Singh, J. et al. Novel formylpeptide receptor 1/2 agonist limits hypertension-induced cardiovascular damage. Cardiovasc. Res. 120, 1336–1350 (2024).
Jelinic, M. et al. Annexin-A1 deficiency exacerbates pathological remodelling of the mesenteric vasculature in insulin-resistant, but not insulin-deficient, mice. Br. J. Pharm. 177, 1677–1691 (2020).
Zhou, C. et al. Anxa1 in smooth muscle cells protects against acute aortic dissection. Cardiovasc. Res. 118, 1564–1582 (2022).
Ouyang, Y. et al. Transcriptome analysis reveals therapeutic potential of NAMPT in protecting against abdominal aortic aneurysm in human and mouse. Bioact. Mater. 34, 17–36 (2024).
Tham, Y. K. et al. Estrogen receptor alpha deficiency in cardiomyocytes reprograms the heart-derived extracellular vesicle proteome and induces obesity in female mice. Nat. Cardiovasc. Res. 2, 268–289 (2023).
Chen, Y. C. et al. Quantitative proteomic landscape of unstable atherosclerosis identifies molecular signatures and therapeutic targets for plaque stabilization. Commun. Biol. 6, 265 (2023).
Walsh-Wilkinson, E. et al. Age and sex hormones modulate left ventricle regional response to angiotensin II in male and female mice. Am. J. Physiol. Heart Circ. Physiol. 323, H643–H658 (2022).
Xue, B., Pamidimukkala, J. & Hay, M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am. J. Physiol. Heart Circ. Physiol. 288, H2177–H2184 (2005).
Mirabito, K. M., Hilliard, L. M., Head, G. A., Widdop, R. E. & Denton, K. M. Pressor responsiveness to angiotensin II in female mice is enhanced with age: role of the angiotensin type 2 receptor. Biol. Sex. Differ. 5, 13 (2014).
Imanishi, M. et al. Smooth muscle cell-specific Hif-1alpha deficiency suppresses angiotensin II-induced vascular remodelling in mice. Cardiovasc. Res. 102, 460–468 (2014).
Moore, J. P. et al. M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am. J. Physiol. Heart Circ. Physiol. 309, H906–H917 (2015).
Qin, C. X. et al. Cardioprotective actions of the annexin-A1 N-terminal peptide, Ac2-26, against myocardial infarction. Front. Pharm. 10, 269 (2019).
Chen, J. et al. Annexin A1 attenuates cardiac diastolic dysfunction in mice with inflammatory arthritis. Proc. Natl. Acad. Sci. USA 118, e2020385118 (2021).
Lu, S. et al. Cardiac-specific NRAP overexpression causes right ventricular dysfunction in mice. Exp. Cell Res. 317, 1226–1237 (2011).
Subbaiah, K. C. V., Wu, J., Tang, W. H. W. & Yao, P. FAM114A1 influences cardiac pathological remodeling by regulating angiotensin II signaling. JCI Insight 7, e152783 (2022).
Ramkumar, N. et al. Loss of soluble (pro)renin receptor attenuates angiotensin-II induced hypertension and renal injury. Circ. Res. 129, 50–62 (2021).
Eberson, L. S. et al. Effect of lysyl oxidase inhibition on angiotensin II-induced arterial hypertension, remodeling, and stiffness. Plos ONE 10, e0124013 (2015).
Ohtsuka, S., Kakihana, M., Watanabe, H. & Sugishita, Y. Chronically decreased aortic distensibility causes deterioration of coronary perfusion during increased left ventricular contraction. J. Am. Coll. Cardiol. 24, 1406–1414 (1994).
Park, S. & Lakatta, E. G. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med. J. 53, 258–261 (2012).
Marek, I. et al. Sex differences in the development of vascular and renal lesions in mice with a simultaneous deficiency of Apoe and the integrin chain Itga8. Biol. Sex. Differ. 8, 19 (2017).
Fashandi, A. Z. et al. Female mice exhibit abdominal aortic aneurysm protection in an established rupture model. J. Surg. Res. 247, 387–396 (2020).
Liu, T. et al. Oleic acid attenuates Ang II (angiotensin II)-induced cardiac remodeling by inhibiting fgf23 (fibroblast growth factor 23) expression in mice. Hypertension 75, 680–692 (2020).
Li, X. C., Zhou, X. & Zhuo, J. L. Evidence for a physiological mitochondrial angiotensin ii system in the kidney proximal tubules: novel roles of mitochondrial Ang II/AT(1a)/O(2)(-) and Ang II/AT(2)/NO signaling. Hypertension 76, 121–132 (2020).
Laine, H. et al. Myocardial oxygen consumption is unchanged but efficiency is reduced in patients with essential hypertension and left ventricular hypertrophy. Circulation 100, 2425–2430 (1999).
How, O. J. et al. Increased myocardial oxygen consumption reduces cardiac efficiency in diabetic mice. Diabetes 55, 466–473 (2006).
Viering, D. et al. Higher SBP in female patients with mitochondrial disease. J. Hypertens. 40, 940–946 (2022).
Muller, F. L. et al. High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates. Biochem. J. 409, 491–499 (2008).
Drose, S. Differential effects of complex II on mitochondrial ROS production and their relation to cardioprotective pre- and postconditioning. Biochim. Biophys. Acta 1827, 578–587 (2013).
Kang, B. Y. et al. Genomics of cardiac remodeling in angiotensin II-treated wild-type and LOX-1-deficient mice. Physiol. Genom. 42, 42–54 (2010).
Ren, J. et al. The molecular mechanism of Ang II induced-AAA models based on proteomics analysis in ApoE−/− and CD57BL/6J mice. J. Proteom. 268, 104702 (2022).
Nadkarni, S., Cooper, D., Brancaleone, V., Bena, S. & Perretti, M. Activation of the annexin A1 pathway underlies the protective effects exerted by estrogen in polymorphonuclear leukocytes. Arterioscler. Thromb. Vasc. Biol. 31, 2749–2759 (2011).
Castro-Caldas, M., Duarte, C. B., Carvalho, A. R. & Lopes, M. C. 17beta-estradiol promotes the synthesis and the secretion of annexin I in the CCRF-CEM human cell line. Mediators Inflamm. 10, 245–251 (2001).
Singh, J. et al. The pro-resolving mediator, annexin A1 regulates blood pressure, and age-associated changes in cardiovascular function and remodeling. FASEB J. 38, e23457 (2024).
Hughes, E. L., Cover, P. O., Buckingham, J. C. & Gavins, F. N. Role and interactions of annexin A1 and oestrogens in the manifestation of sexual dimorphisms in cerebral and systemic inflammation. Br. J. Pharm. 169, 539–553 (2013).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18, e3000410 (2020).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Berg, S. et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
Lozano, J. et al. Scalable generation of nanovesicles from human-induced pluripotent stem cells for cardiac repair. Int. J. Mol. Sci. 23, 14334 (2022).
Rai, A., Fang, H., Claridge, B., Simpson, R. J. & Greening, D. W. Proteomic dissection of large extracellular vesicle surfaceome unravels interactive surface platform. J. Extracell. Vesicles 10, e12164 (2021).
Tyanova, S., Temu, T. & Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 11, 2301–2319 (2016).
Cox, J. et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 (2011).
Evans, J., Hutchison, J., Salamonsen, L. A. & Greening, D. W. Proteomic insights into endometrial receptivity and embryo-endometrial epithelium interaction for implantation reveal critical determinants of fertility. Proteomics 20, e1900250 (2020).
Tyanova, S. & Cox, J. Perseus: A bioinformatics platform for integrative analysis of proteomics data in cancer research. Methods Mol. Biol. 1711, 133–148 (2018).
Doncheva, N. T. et al. Cytoscape stringApp 2.0: Analysis and visualization of heterogeneous biological networks. J. Proteome Res. 22, 637–646 (2023).
Flower, R. J. & Rothwell, N. J. Lipocortin-1: cellular mechanisms and clinical relevance. Trends Pharmacol. Sci. 15, 71–76 (1994).
Acknowledgements
This work was partly funded by a Vanguard grant (APP102885) from the National Heart Foundation of Australia (NHF) and Diabetes Australia Research Program (DARP) grant (Y22G-QINC) (to C.X.Q., K.L.J., and R.H.R.) and the Victorian Government of Australia’s Operational Infrastructure Support Program. D.W.G is supported by the Amelia Hains Foundation and National Health and Medical Research Council funding (MRF2015523, MRF2024039, APP2020251, APP2013158). C.X.Q. is supported by an NHF Future Leader Fellowship. K.L.J. is supported by an NHMRC ECR Fellowship. J.S. is supported by the Monash Graduate Scholarship. The authors acknowledge the technical assistance of the Monash Histology Platform, Department of Anatomy and Developmental Biology, Monash University; and the facilities and scientific and technical assistance of the National Imaging Facility (NIF), a National Collaborative Research Infrastructure Strategy (NCRIS) capability at Monash Biomedical Imaging (MBI), a Technology Research Platform at Monash University).
Author information
Authors and Affiliations
Contributions
K.L.J., G.A.H., R.H.R., and C.X.Q. study conception and design; J.S., K.L.J., H.F., H.C., A.P., C.G., F.S.T., D.W.G., and C.X.Q. performed experiments; J.S., H.K., H.F., E.S., D.W.G., and C.J.N., analyzed data; J.S., K.L.J., H.F., R.H.R., G.A.H., O.L.W., D.W.G., and C.X.Q. interpreted results; J.S., and H.K. Image acquisition; C.X.Q. Funding acquisition, J.S., and H.F. prepared figures, J.S. drafted the manuscript; J.S., K.L.J., H.K., C.G., H.K., E.S., R.H.R., O.L.W., G.A.H., D.W.G., and C.X.Q. Edited and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Dario Ummarino. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Singh, J., Jackson, K.L., Fang, H. et al. Annexin-A1 deficiency uncovers female-specific pathways in blood pressure control and cardiovascular remodeling in mice. Commun Biol 8, 955 (2025). https://doi.org/10.1038/s42003-025-08291-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08291-6