Abstract
There is growing interest in peptide or small protein based drugs targeting G protein-coupled receptors (GPCRs) for improved subtype selectivity over small molecules. Naturally occurring toxins represent rich sources of such ligands. AdTx1 (ρ-Da1a), a three-finger toxin (3FTx) from the green Mamba Snake, selectively binds and antagonizes α-adrenoceptors. Here, we present the cryo-electron microscopy structure of α1A-adrenoceptor in complex with AdTx1. The structure reveals the molecular mechanism of the subtype selectivity and antagonist activity of AdTx1 for α1A-adrenoceptor, which is different from those revealed by the only 3FTx-GPCR structure reported so far, the Muscarinic toxins 7 (MT7) - Muscarinic acetylcholine receptor 1 (M1AChR) structure. Based on the structural information, we further engineered the AdTx1 and enhanced its antagonist activity by introducing three mutations. The results highlight the potential of developing potent toxin drugs towards GPCRs based on the 3FTx scaffold and structural information.
Similar content being viewed by others
Introduction
G protein-coupled receptors (GPCRs) represent the largest family of transmembrane receptors and regulate a variety of physiological responses. Due to their important physiological roles, GPCRs account for the targets of about one-third of the FDA-approved small-molecule drugs1. Drug innovation targeting GPCRs draws extensive attention from both academia and industry. One major challenge in GPCR drug development is to achieve high subtype selectivity because many pharmaceutically important GPCRs have closely related subtypes with high sequence similarity, for example, the nine subtypes of adrenergic receptors in the human genome. Small-molecule drugs have limited interaction interfaces and are difficult to achieve superior subtype selectivity. Peptide or small protein ligands bind to the receptors through larger interaction interfaces and thus have greater potential to be subtype-selective.
One rich source of small protein ligands comes from the venoms produced by toxic animals, such as snakes, spiders, or scorpions2. Among these venoms, some exhibit lethal and debilitating effects as a consequence of neurotoxic, cardiotoxic, and tissue-necrotizing effects. However, other venoms exhibit various pharmacological effects with a lower order of toxicity3. Many pharmacologically active molecules extracted from these venoms show potential for developing therapeutic agents4. For example, bradykinin-potentiating peptides have been used as inhibitors of angiotensin-converting enzyme5,6, eptifibatide has been used to inhibit platelet aggregation7,8, tirofiban and ancrod are used to reduce blood fibrinogen levels9. Venom toxins also have extensive applications as research tools10. For example, α-bungarotoxin has been used to characterize the fundamental mechanisms involved in neuromuscular transmission11,12.
AdTx1 is a 65-amino-acid peptide toxin isolated from the venom of the green Mamba Snake Dendrosapis angusticeps13. It functions as an antagonist of the α1A-adrenoceptor (α1AAR) with high selectivity and high affinity14. AdTx1 belongs to the 3-finger toxin (3FTx) family, which is characterized by the structures of three-finger loops connected to a central core containing four conserved disulfide bonds15. The α1AAR represents one of the nine adrenoceptor subtypes, and its activation mediates smooth muscle contraction. The α1AAR-selective agonist oxymetazoline is clinically used for the treatment of nasal obstruction16. The α1AAR antagonists are also clinically used to treat several diseases. For example, tamsulosin and silodosin are used to treat Benign Prostate Hyperplasia (BPH)17,18. Doxazosin is used to treat BPH and hypertension19,20, and prazosin is used to treat overall post-traumatic stress disorder (PTSD)21. We have recently solved the structures of the α1AAR bound with oxymetazoline, tamsulosin, or noradrenaline22. However, the detailed interaction pattern between the AdTx1 and α1AAR remains unknown. Exploring the molecular mechanism of how they interact with each other may provide valuable information to guide future peptide drug development. To achieve the research goal, we determined the structure of the AdTx1-α1AAR complex. The structure reveals the molecular mechanism of AdTx1’s antagonist activity and provides guidance to engineer AdTx1 based on the structural information. As a proof of concept, we improved the affinity of AdTx1 towards the α1AAR by introducing three mutations to the toxin. The result highlights the possibility of developing and optimizing toxin ligands for GPCRs based on structures.
Results
Complex formation and structure determination of the AdTx1-α1AAR-Nb6
Inspired by the previous work on MT7-M1AChR structures23, we tried to recombinantly express AdTx1 in Hi5 insect cells. N-terminal Maltose-binding protein (MBP) fusion was applied to improve the expression level, while MBP was digested and removed during purification (Fig. S1). The purified AdTx1 exhibits high affinity towards the α1AAR as shown by the radioligand competition binding assay. AdTx1 also shows high selectivity towards the α1AAR compared to the α1BAR and α1DAR (Fig. 1a). The results are consistent with previous reports13. To further analyze the antagonist activity of AdTx1, we established a cAMP-based GloSensor assay by co-expressing α1AAR with an engineered Gsq protein, which was constructed by replacing 15 residues at the C-terminus of Gαs with those of Gαq24. The Gsq protein could be activated by Gq-coupled GPCRs like α1AAR and triggers downstream Gs signaling pathway, such as stimulating cAMP production. As shown in Fig. S3, only HEK293T cells transfected with α1AAR resulted in oxymetazoline-induced cAMP accumulation in the GloSensor cAMP assay, indicating that the response is mediated by the transfected α1AAR receptor, but not the endogenous receptors from the HEK293T cells (Fig. S3). Compared to oxymetazoline, norepinephrine shows a certain level of activation effects on the endogenous receptors of HEK293T. As a result, we chose oxymetazoline in the subsequent functional experiments. The purified AdTx1 shows antagonist activity in inhibiting oxymetazoline-induced cAMP generation (Fig. 2b). Taken together, the recombinantly expressed and purified AdTx1 is functional and suitable for structural studies.
a AdTx1 is selective towards the α1AAR over the other subtypes of α1ARs and β2AR in a radioligand competition binding assay. Data are given as means ± SEM of 6 independent samples. b AdTx1 binds to the extracellular side of the α1AAR, with finger loop 1 interacting with the extracellular end of TM5 and TM6 (red square), finger loop 2 binds to the central cavity (green square), and finger loop 3 contacts the ECL1 (cyan square). c Sequence alignment of the extracellular regions of α1ARs. The α1AAR residues that interact with AdTx1 are colored in green. The residues on the α1BAR or α1DAR are also colored in green if they are identical to those of the α1AAR, non-conserved residues are colored in cyan.
a Structure comparison of the AdTx1-bound α1AAR structure (orange) with the tamsulosin-bound inactive state α1AAR structure (gray, PDB code: 7YMJ) and the oxymetazoline-bound active state α1AAR structure (green, PDB code: 7YM8). Finger loop 1 and finger loop 2 of AdTx1 prevent the inward movement of TM6 and TM7, which are required for the α1AAR activation (orange rectangle). AdTx1 binding causes a more outward conformation of TM7 (orange arrow) and stabilizes the PIF motif at the inactive conformation (cyan rectangle). b Mutating finger loop 1 (K7A, S8A, and I9A) has the largest effect on AdTx1 function in a 3H prazosin competition binding assay, mutating finger loop 2 (Y30A, V32A, and K34A) also reduced AdTx1 function, while mutating finger loop 3 (Y52A and S54A) does not have much effect on AdTx1 function. Data are given as means ± SEM of 6 independent samples. c Compared to tamsulosin-bound inactive α1AAR, conformational changes of F3087.35, F3127.39 are observed upon AdTx1 binding. d K34 at the finger loop 2 of AdTx1 directly interacts with D1063.32 and distorts the conformation of D1063.32, thus altering the shape of the orthosteric pocket of α1AAR. V32 at the finger loop 2 of AdTx1 extrudes the F3087.35, leading to the outward movement of TMs 6 and 7 of α1AAR. Compared to the oxymetazoline-bound active α1AAR, conformational changes of F2886.51, F3087.35, F3127.39, and W2856.48 are observed upon AdTx1 binding. e K34A mutation impairs the function of AdTx1 in a 3H prazosin competition binding assay. Data are given as means ± SEM of 6 independent samples.
We initially prepared the AdTx1-α1AAR complex and collected cryo-electron microscopy (cryo-EM) data on it. However, cryo-EM data processing was not successful due to the small size and the lack of soluble domains of the complex. We then tried to add fiducial markers to the samples to help particle alignment in cryo-EM data processing. After several trials, we succeeded in applying the previously reported nanobody-6 (Nb6) strategy to help structure determination25,26. In brief, we replaced an intracellular loop 3 (ICL3) of α1AAR (E5.68 to K6.31, superscripts indicate Ballesteros-Weinstein numbering for GPCRs27) with that of the κ-opioid receptor (κOR) (V2565.68 to R2706.31). The resulting α1AAR construct can form a complex with Nb6 through the engineered ICL3. The AdTx1-α1AAR-Nb6 complex was prepared as described in the “Methods” section. The structure of the AdTx1-α1AAR-Nb6 complex was determined by single-particle cryo-EM to a resolution of 2.87 Å (Fig. S4, Table 1). The electron densities are clear enough to model most of the receptor, AdTx1 and Nb6, except for the N-terminal region (M1 to I23) and C-terminal region (Q344 to Q370) of the α1AAR, as well as the C-terminal region of Nb6 (120–125) due to poor densities that are likely resulted from flexibility.
Overall structure
AdTx1 binds to the extracellular side of the α1AAR while Nb6 binds to the engineered ICL3 as designed (Fig. S5a). Since the Nb6 binding site is far away from the AdTx1 binding site, it was omitted from the main figures for clarity. As mentioned earlier, the AdTx1 is composed of 6 beta-strains that form 3-finger loops. The finger loop 2 penetrates deep into the central cavity of the receptor which is also the path towards the orthosteric pocket, while finger loop 1 interacts with transmembrane helices (TMs) 5 and 6 and finger loop 3 interacts with extracellular loops (ECLs) 1 and 2 (Fig. 1b). The structure is in agreement with previous mutagenesis studies, where mutations of orthosteric residues F862.64A, E872.65A, D1063.32A, F2886.51A, and F3127.39A significantly affect the affinity between AdTx1 and the α1AAR2,14. Of the 15 residues that form the AdTx1 binding site on the α1AAR, 9 of them are different in the α1BAR and 11 are different in α1DAR (Fig. 1c). According to the radioligand competition results and previous publications13, AdTx1 is selective towards the α1AAR over the other subtypes of α1ARs. (Fig. 1a). The interface differences may explain the selectivity of AdTx1 towards the different α1ARs.
Molecular mechanism of antagonist activity of AdTx1
In order to understand the molecular mechanism of AdTx1’s antagonist activity towards the α1AAR, we compared the AdTx1-bound α1AAR structure with both the oxymetazoline-bound active state of α1AAR and the tamsulosin-bound inactive state of α1AAR (Fig. 2a)22. A hallmark of GPCR activation is the outward displacement of the intracellular side of TM6. The AdTx1-bound α1AAR is in inactive conformation as expected (Fig. 2a). The conformation of TM6 is similar to that of the tamsulosin-bound α1AAR structure. A close analysis of the extracellular side of the receptor conformation reveals that the extracellular sides of the TM6 and TM7 display inward movement upon receptor activation (Fig. 2a, orange rectangle), which then induces conformational change through the PIF motif (Fig. 2a, blue rectangle) and result in the outward displacement of the intracellular side of TM622. The finger loop 1 of AdTx1 inserts between TM5 and TM6, while the finger loop 2 of AdTx1 inserts between TM7 and ECL2 (Fig. 2a, orange rectangle), thus preventing the inward movement of TM6 and TM7 and locking the receptor in an inactive conformation. Actually, in the AdTx1-bound structure, the extracellular part of TM7 is stabilized in an even more outward conformation by the finger loop 2 compared to the tamsulosin-bound structure (Fig. 2a, orange rectangle). The hydrophobic residue V32 of AdTx1 extrudes F3087.35, leading to the outward movement of TMs 6 and 7 of α1AAR (Fig. 2c, d). When compared with the tamsulosin-bound inactive α1AAR structure, F3087.35 and F3127.39 are in a more outward conformation, while tamsulosin induces an open-lid conformation of F3127.39 (Fig. 2c). Compared to the oxymetazoline-bound active α1AAR, conformational changes of F2886.51, F3087.35, F3127.39, as well as the toggle-switch W2856.48, are observed upon AdTx1 binding (Fig. 2d).
Previous mutagenesis studies have pinpointed key residues on each of the three-finger loops that play a critical role in the interaction between the toxin and the receptor2,28. These findings are consistent with the structural data. For instance, K7 from finger loop 1 is shown to engage in an interaction with E181ECL2 (Fig. 1b), the K7A mutation leads to a 5-fold decrease in binding affinity compared to wild-type toxin2. An intriguing observation was made with F10 also on Finger loop 1. Despite not having direct contact with the receptor, the F10A mutation resulted in an even more dramatic loss of affinity—approximately 18-fold lower than the native toxin2. This suggests that maintaining a hydrophobic microenvironment around finger loop 1 and its proximity to TM5/6 are essential for the stability of the toxin-receptor complex. This highlights the indirect yet significant role played by non-contacting residues in stabilizing the overall structure and orientation of the toxin, thereby influencing its binding efficiency. The structural data indicates that S8 interacts with the main chain carbonyl oxygen of F2976.60, while the side chain of I9 interacts with the benzene ring of F2976.60 (Fig. 1b), a combination of K7A/S8A/I9A triple mutation leads to a dramatic decrease in the toxin’s affinity for the receptor, estimated to be approximately 2000-fold lower than that of the wild-type toxin (Fig. 2b). This is noteworthy when compared to the relatively less severe impact of the single K7A mutation which only caused a reduction in affinity by around 5-fold2,28. Y36 from finger loop 2 forms extensive interactions with ECL2 residues I175ECL2 and Q177ECL2 (Fig. 1b), the Y36A mutation results in a dramatic 1900-fold decrease in Ki value, highlighting the importance of the interaction2,28. Interestingly, in finger loop 3, only the sidechains of Y52 and S54 are within 4 Å distance of the receptor, while the Y52A/S54A double mutation has similar affinity towards the receptor as the wild-type AdTx1 (Fig. 2b). On the contrary, the D53A single mutation reduced the affinity by 53-fold2,28. Structure information suggests D53, H29, and E3057.32 form a hydrogen bond network (Fig. S7). The results indicate that the direct interactions between finger loop 3 of the toxin and the receptor itself do not significantly contribute to the functional potency of the toxin. Instead, finger loop 3 plays a crucial role in maintaining the structural integrity and proper conformation of finger loop 2.
The finger loop 2 penetrates deep into the orthosteric pocket and forms a salt bridge with D1063.32 through K34 (Fig. 2c). D1063.32 is a highly conserved residue among the aminergic receptors, and its negatively charged side chain is important for aminergic ligands binding29,30, including adrenergic receptors, dopamine receptors, and serotonin receptors31,32,33,34. Previous active and inactive α1AAR structures reveal that both the agonists (norepinephrine and oxymetazoline) and antagonist (tamsulosin) form polar interactions with D1063.32 22. Similar polar interactions also exist in other aminergic receptors (Fig. S8). The interaction between K34 and D1063.32 distorts the conformation of D1063.32 and alters the shape of the orthosteric pocket of α1AAR (Fig. 1b, green rectangle), thus affecting the interaction between α1AAR and its orthosteric ligands (Fig. 2c). Previous studies suggest the D1063.32A mutation resulted in reduced AdTx1 affinity14. Notably, the K34A single mutation also reduces AdTx1’s affinity towards the α1AAR (Fig. 2e), as well as its antagonist activity in inhibiting oxymetazoline-induced cAMP generation in the α1AAR-Gsq GloSensor cAMP assay (Fig. S6).
Structure-based engineering improves the affinity of AdTx1 to α1AAR
We further explored whether it’s possible to improve the antagonist activity of AdTx1 based on the structural information. As described before, the salt bridge between K34 of AdTx1 and D1063.32 of a1AAR affects the shape of the orthosteric pocket and thus affects orthosteric ligand binding (Fig. 2c). We replaced K34 with arginine and found the K34R mutant shows enhanced activity compared to the wild-type AdTx1 (Fig. S9). Similarly, we mutated H29 on finger loop 2 into arginine (H29R) to enhance its interaction with E3057.32 and mutated S54 on finger loop 3 into glutamine (S54Q) or asparagine (S54N), to form hydrogen bonds between R28 of AdTx1 and D172ECL2 of α1AAR (Fig. 3a). The H29R and S54Q mutants also enhance inhibitory function compared to the wild-type AdTx1 (Fig. S9), while S54N shows impaired function, most likely suggesting that the asparagine side chain is not long enough to induce the proper hydrogen bonds formation between R28 and D172ECL2 (Fig. S9). We further generated a triple mutation of AdTx1 (AdTx1-3mut) by combining all three mutations H29R, K34R, and S54Q. While the AdTx1-3mut only shows 5-fold higher affinity than the WT AdTx1 in radioligand competition binding assay (Fig. 3b), it exhibits 12-fold higher potency in inhibiting oxymetazoline-induced receptor activation (Fig. 3c). The results highlight the possibility to engineer and improve toxin activity based on the structural information.
a H29 and K34 are chosen for mutation to increase their interactions with E3057.32 and D1063.32. S54 is chosen to be mutated to either N or Q to bridge the interaction between R28 and D172ECl2. b The AdTx1-3mut (H29R, K34R and S54Q) show higher affinity than AdTx1 in a 3H prazosin competition binding assay. Data are given as means ± SEM of 8 independent samples. c The AdTx1-3mut shows increased activity in an α1AAR-Gsq cAMP accumulation assay compared to AdTx1. Data as given as means ± SEM of 4 independent samples.
Comparison of the AdTx1- α1AAR structure with the MT7- M1AChR structure
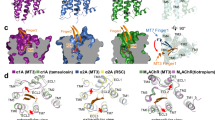
Previously, only one GPCR-snake toxin complex was reported, which is the MT7-M1AChR complex structure23. MT7 and AdTx1 share 68% sequence identity and the 3FTx scaffold13. We then compared the binding mode of MT7 for M1AChR against that of AdTx1 for α1AAR (Fig. S10). The most obvious difference is that AdTx1 binds to a deeper position than the MT7 (Fig. 4a–c). Consistent with the binding mode difference, MT7 functions as a negative allosteric modulator for the M1AChR activation and does not directly compete with orthosteric ligands23. In contrast, AdTx1 distorts the orthosteric ligand binding by the salt bridge between K34 and D1063.32 (Fig. 4c). Besides, the binding poses of MT7 and AdTx1 are shifted by around 50 degrees (Fig. 4d). While finger loop 2 of both toxins insert to the extracellular cavities of the receptors formed by ECL2, ECL3 and TM7, the finger loop 1 and 3 of AdTx1 bind to different regions of the receptor compared to the MT7. The finger loop 1 of MT7 is inserted between TM4 and TM5 of M1AChR, while finger loop 1 of AdTx1 is inserted between TM5 and TM6 of α1AAR. Finger loops 3 of MT7 interacts with TM7 of M1AChR, while finger loop 3 of AdTx1 interacts with ECL1 of α1AAR (Fig. 4d). However, even though AdTx1 inserts deeper than MT7, our radioligand competition assay shows that AdTx1 cannot fully displace prazosin, even at a concentration up to ten-thousand fold higher than that of prazosin (Fig. S11). This suggests the possible existence of an alternative state in which AdTx1 and prazosin may coexist, indicating a more complex binding mode than captured by our structure. These findings highlight the diversity of possible interaction patterns between 3FTxs and GPCRs.
a, b AdTx1 binds to a deeper position in the α1AAR (a) compared to MT7 in the M1AChR (PDB code: 6WJC) (b). White and yellow sticks indicate tamsulosin from inactive α1AAR (PDB code: 7YMJ) and atropine from MT7-M1AChR (PDB code: 6WJC). c K34 of AdTx1 directly interacts with D1063.32 of the α1AAR while R34 of MT7 is far away from D1053.32 of the M1AChR. d A top view comparison of the two structures reveals that the binding poses of MT7 and AdTx1 are shifted by around 50 degrees.
Discussion
Snakes, particularly those that rely on venom to capture prey, exhibit remarkable ecological adaptability and a diverse range of hunting strategies. These efficient predators can target a wide array of species, ranging from small mammals to birds and even other reptiles, using their venom to swiftly subdue their prey, thereby ensuring effective energy acquisition. Our high-resolution cryo-EM structure elucidates the detailed mechanism of one such venom component, AdTx1, targeting the α1AAR. Despite variations in the receptor protein sequences across different species, the AdTx1 binding site remains highly conserved, from small mammals such as mice and rabbits, through birds like the wall lizard (Podarcis muralis), to other reptiles including the pied flycatcher (Ficedula albicollis) (Fig. S12). This conservation highlights the effectiveness of AdTx1 in targeting multiple species. The highly conserved AdTx1 binding site on the receptor also indicates a conserved evolutionary pressure acting upon this binding site.
The structural complementarity of AdTx1 to the α1AAR’s extracellular vestibule is remarkable, suggesting an evolutionary fit for these toxins to their targets. This fit is not merely a consequence of size but also the spatial arrangement of key residues that mediate the antagonistic effect. The finger loops of the 3FTx scaffold, particularly loop 2, appear to be tuned for effective receptor binding and inhibition, as demonstrated by our mutagenesis experiments. The comparison between the AdTx1-α1AAR complex with the MT7-M1AChR structure reveals the adaptability of the 3FTx scaffold. It shows how subtle shifts in the binding pose can result in diverse mechanisms of receptor modulation, from negative allosteric modulation to direct competitive inhibition. One interesting observation is that the positively charged residues on finger loop 2, K34 in AdTx1 and R34 in MT7, play a key role in interacting with the receptor. This interaction highlights the importance of electrostatic complementarity between the toxin and the receptor. The specific positioning of these charged residues not only facilitates the initial encounter but also stabilizes the toxin-receptor complex, contributing to the high affinity and selectivity observed.
A comparison of the structures of AdTx1 bound to the α1AAR and cyclazocine bound to the α1BAR reveals overall similar conformation but differences in the extracellular regions of the receptors (Fig. S13). The TM2 displays more outward displacement in the α1BAR compared to α1AAR. In addition, the distinct orientations of ECL1 and ECL2 in the α1AAR versus the α1BAR contribute to the varying affinities of AdTx1 for these two receptors (Fig. S13). This observation aligns with the lower affinity of AdTx1 for the α1BAR, as demonstrated experimentally (Fig. 1a).
Through the strategic introduction of specific mutations, our research has successfully enhanced the antagonist activity of AdTx1, effectively creating a blueprint for the design and development of optimized toxin-based pharmaceuticals. The triple mutant variant, AdTx1-3mut, serves as a testament to this strategy’s feasibility, showcasing improved potency in its interaction with the α1AAR. The strategy is likely generally applicable to other GPCRs. By refining the structure and function of venom-derived peptides, we can potentially develop targeted therapies that more precisely modulate GPCR activity, thereby offering safer and more effective treatments for patients. Looking ahead, the high-resolution structural data obtained from our study provides a robust platform for computational screening and rational design of peptide libraries. This strategy has the potential to significantly expedite the identification of novel ligands with exceptional specificity and affinity for G protein-coupled receptors (GPCRs) that are of clinical importance.
The pursuit of achieving high specificity and selectivity in drug discovery, particularly within the realm of GPCR-targeted therapeutics, remains an arduous task. Our research makes a stride forward in this area by unraveling the inhibitory and selective interaction mechanism through which the Mamba snake toxin AdTx1 interacts with the human α1AAR. Peptides or small proteins like AdTx1 exhibit an inherent structural advantage due to their conformational specificity, which empowers them with an enhanced ability to discriminate between GPCR subtypes. This property is particularly advantageous when aiming at receptors that have closely related variants, where conventional small-molecule drugs often lack the necessary precision.
It is noteworthy that AdTx1 has been characterized as an insurmountable antagonist, meaning that once bound, it cannot be displaced by agonists or other antagonists. This characteristic is closely tied to its mode of action, which involves a tight and stable interaction with the receptor. The insurmountable nature of AdTx1 suggests that its binding involves covalent or highly stable non-covalent interactions that prevent displacement. This property is significant because it ensures that the receptor remains inactive for extended periods. The detailed mechanism of the insurmountable nature of AdTx1, however, is not revealed with the current structure and requires further study. In conclusion, the structural and functional data presented here not only shed light on the evolutionary adaptations of AdTx1 but also highlight the potential of 3FTx scaffolds as a platform for the development of novel, highly selective GPCR-targeted therapeutics. The insights gained from studying AdTx1’s interaction with the α1AAR may pave the way for innovative drug designs aimed at improving therapeutic outcomes while minimizing side effects.
Materials and methods
AdTx1 construct design
The AdTx1 gene was synthesized by GENEWIZ. The final expression construct contains a N-terminal GP64 signal peptide, followed in order by 6 × His tag, MBP, GS-linker, HRV 3C site, GS-linker, and AdTx1 (Fig. S1a). The construct was cloned into the multiple cloning sites of the pFastBac vector. AdTx1 mutants were generated using site-directed mutagenesis.
AdTx1 expression and purification
The plasmids encoding the WT AdTx1 and AdTx1 mutants were transformed into DH10Bac competent cells, and viruses were prepared following the manual of the Bac-to-Bac® TOPO® Expression System (Invitrogen). Protein expression was performed by adding 30 mL P2 virus to 1 liter of Hi5 cells (Thermo Fisher) with a density of 4 × 106 cells/mL. After 48 h, the transfected Hi5 cells were centrifuged at 4 °C, 4000 rpm for 20 min, and the supernatant was harvested for protein purification. After adding 50 mM Tris-HCl pH = 7.5, 1 mM NiCl2, 5 mM CaCl2, 160 μg/mL benzamidine, and 100 μg/mL leupeptin to the supernatant, the mixture was stirred at room temperature for 1 h and then centrifuged at 14,000 rpm for 30 min at 4 °C to remove metal chelators in the cell culture medium. The resulting supernatant was then applied for Ni-NTA purification. The eluate from the first Ni-NTA column was digested by 3C-protease at room temperature for 1 h followed by 4 °C overnight. The digested protein was then applied to a second Ni-NTA column. The flowthrough of the second Ni-NTA column was collected, concentrated, and purified with a size exclusion chromatography (SEC) Superdex 75 column (Cytica) in a SEC buffer (20 mM HEPES, pH = 7.5, 100 mM NaCl) (Fig. S1c). The SEC peak was checked by SDS-PAGE, and the targeted AdTx1 fraction was collected, concentrated and stored at -80 °C for further use. The expression construct sequence is shown in Fig. S1b. The protein concentration was measured using a Nanodrop (Thermo).
α1AAR construct design
The glycosylation sites (N7, N13, N22) of human α1AAR were mutated to glutamine. A hemagglutinin signal peptide and a FLAG tag were added to the N-terminal of the receptor, and the C-terminus of α1AAR was truncated at residue 370 (full length 466) followed by an 8 × His tag. The ICL3 of the α1AAR (E5.68 to K6.31) was replaced with that of the κOR (V2565.68 to R2706.31) to create a Nb6 recognition site26. In addition, two mutations S113R3.39 and M115W3.41, which are reported to stabilize the inactive state or improve receptor expression for other GPCRs22,35,36,37, are introduced to the α1AAR. This construct was subcloned to the pFastBac vector.
α1AAR expression and purification
The baculoviruses encoding the α1AAR expression construct were prepared in the same way as previously described for AdTx1 expression. The α1AAR expression was performed by adding 30 mL P2 viruses to each liter of sf9 cells (Expression Systems) with a density of 4 × 106 cells/mL. After 48 h, the transfected sf9 cells were harvested by centrifuging at 4 °C, 4000 rpm for 20 min.
The cell pellets were lysed in lysis buffer containing 20 mM Tris-HCl pH = 7.5, 1 mM EDTA, 160 μg/ml benzamidine, and 100 μg/ml leupeptin for 15 min at 4 °C. After centrifuging at 18,000 rpm for 10 min at 4 °C, the pellet was solubilized in solubilization buffer containing 20 mM HEPES pH = 7.5, 0.03% cholesterol hemisuccinate (CHS), 1% dodecyl maltoside (DDM), 750 mM NaCl, and 30% glycerol for 2 h. 2 mg/ml iodoacetamide was added to the solubilization buffer. 1 mL/L Ni-NTA beads were added to the mixture. After stirring for 2 h at 4 °C, the receptors were eluted with elution buffer containing 20 mM HEPES pH = 7.5, 0.03% CHS, 0.1% DDM, 750 mM NaCl and 200 mM imidazole. The eluate was supplemented with 2 mM CaCl2 and loaded onto the M1 column. The detergents were gradually changed from DDM to 0.01% Lauryl Maltose Neopentyl Glycol (LMNG) on the M1 column. Finally, the α1AAR receptors were eluted from M1 beads with elution buffer containing 20 mM HEPES pH = 7.5, 0.01% MNG, 0.001% CHS, 100 mM NaCl, 0.2 mg/ml Flag peptide, and 5 mM EDTA. The M1 column eluate was then concentrated and purified with a size exclusion chromatography (SEC) Superdex 200 increase column (Cytica) in SEC buffer comprised of 20 mM HEPES pH =7 .5, 0.01% MNG, 0.001% CHS, and 100 mM NaCl (Fig. S2). The SEC peak containing the receptor was then concentrated, flash-frozen in liquid nitrogen, and stored at −80 °C for further use.
Nb6 expression and purification
The Nb6 gene was synthesized by GENEWIZ and subcloned into the periplasmic expression vector pET22b. The expression construct contains a N-terminal signal peptide and a C-terminal 8 × His tag. The expression plasmids were transformed into BL21(DE3) E. coli. Protein expression was induced with 1 mM IPTG when the cells were grown to OD = 0.8, and cell pellets were harvested after expression for 20 h at 20 °C. Nb6 purification was performed as previously described. Briefly, pellets were resuspended in SET buffer (200 mM Tris, pH 8.0, 500 mM sucrose, 0.5 mM EDTA) and rotated at 4 °C for 1 h. Then, a 2 × volume of SET/4 buffer (50 mM Tris, pH 8.0, 125 mM sucrose, 0.125 mM EDTA) was added, and the mixture was stirred at room temperature for another hour. After centrifugation at 18,000 rpm, the supernatant was loaded onto Ni-NTA beads. Following washes with high salt buffer (20 mM Tris, pH 8.0, 500 mM NaCl, 20 mM imidazole) and low salt buffer (20 mM Tris, pH 8.0, 100 mM NaCl, 20 mM imidazole), the Nb6 was eluted with elution buffer (20 mM Tris, pH 8.0, 100 mM NaCl, 200 mM imidazole). The eluted Nb6 was further purified using a size exclusion chromatography (SEC) Superdex 75 increase column (Cytica) equilibrated with 20 mM HEPES-NaOH, pH 7.50, and 100 mM NaCl. The purified nanobodies were stored at −80 °C for further use (Fig. S2).
AdTx1-α1AAR-Nb6 complex formation
The purified α1AAR, AdTx1, and Nb6 were mixed in a molar ratio of 1:1.2:1.2 and incubated on ice for 1 h, and then purified by M1 affinity resin. The complex was eluted from the M1 resin with an elution buffer containing 20 mM HEPES pH = 7.5, 0.002% MNG, 0.0002% CHS and 100 mM NaCl, 0.2 mg/ml Flag peptide, and 5 mM EDTA. The eluate was concentrated and purified with a size exclusion chromatography (SEC) Superdex 200 increase column (Cytica) in SEC buffer comprised of 20 mM HEPES pH = 7.5, 0.002% MNG, 0.0002% CHS, and 100 mM NaCl (Fig. S2). The fraction containing the complex was concentrated to 5 mg/mL and used for cryo-EM sample preparation.
Cryo-EM sample preparation and data collection
4 μL of the purified AdTx1-α1AAR-Nb6 complex was added to glow-discharged holey carbon grids (Quantifoil Au R1.2/1.3, 300 mesh). The grids were blotted for 3.0 s and flash-frozen in liquid ethane cooled by liquid nitrogen with Vitrobot (Mark IV, Thermo Fisher Scientific).
The cryo-EM data were collected at the National Protein Science Research (Beijing) Facility, Tsinghua Base. The grids were transferred to a 300 kV Titan Krios G3i microscope equipped with Gatan K3 Summit detector and a GIF Quantum energy filter (slit width 20 eV). 2,049 movie stacks were collected by using the AutoEMation program38, with a defocus range from −1.3 μm to −1.8 μm. Each stack contained 32 frames and was exposed for 2.56 s. The total dose of each stack was about 50 e−/Å2. All 32 frames in each stack were aligned and summed using the whole-image motion correction program MotionCor2 and binned to a pixel size of 1.083 Å39.
Cryo-EM data processing
The following processes were performed using cryoSPARC 3.240. 2,049 dose-weighted micrographs were imported into cryoSPARC, and CTF parameters were estimated by using patch-CTF. Micrographs with a CTF fitting resolution worse than 4 Å were deleted, resulting in 2025 micrographs for further data processing. 4,578,926 particles were picked by the template picker and extracted. After four rounds of 2D classification, 115,477 particles showed clear features of Nb6, α1AAR, and AdTx1. These particles were selected to generate the initial model by Ab-Initio Reconstruction. By using this initial model, the full set of particles was subjected to 3 rounds of heterogeneous refinement, resulting in a 3.45 Å map reconstructed by 616,710 particles. Followed by non-uniform refinement and local refinement, the final map reached a resolution of 2.87 Å.
The atomic coordinates of the AdTx1-α1AAR-Nb6 complex were generated by combining homology modeling (PDB code: 4IYE) and the previous α1AAR-Nb6 model (PDB code: 7YMJ). The cryo-EM model was docked with the density map through UCSF Chimera41. The structure model was then refined through iterative manual building and refinement in PHENIX42 and COOT43. Figures were prepared using the PyMOL Molecular Graphics System v.2.4.1 (Schrödinger, LLC), UCSF Chimera, and UCSF Chimera X1.344.
Radioligand competition binding assay
The wild-type α1AAR, α1BAR, α1DAR, and β2AR genes were cloned and expressed in the same way as described for AdTx1 previously. Cell membrane extraction and radioligand binding were performed as previously reported45,46. In brief, 50 mL of cells expressing the corresponding receptors were harvested and resuspended in 8 mL of lysis buffer containing 20 mM Tris pH 7.5, 1 mM EDTA, 100 μg/ml leupeptin, and 160 μg/ml benzamidine. Cell lysates were then centrifuged at 800 rpm for 10 min at 4 °C. The supernatant containing the membrane was centrifuged again at 18,000 rpm for 30 min at 4 °C to collect the membrane pellets. Membrane pellets were resuspended in 2 mL of binding buffer containing 20 mM Tris pH 7.5, 100 mM NaCl, 100 μg/ml leupeptin, and 160 μg/ml benzamidine. The purified membrane was then aliquoted into 100 μL/tube, flash-frozen with liquid nitrogen, and stored at −80 °C before further use.
In the competition binding assay, the membrane suspension was diluted to appropriate concentration with binding buffer containing 20 mM Tris, pH 7.5, 100 mM NaCl, and 0.5 mg/mL BSA, and incubated with radioligand (1 nM 3H prazosin or 2 nM 3H DHA) as well as different concentration of AdTx1 or AdTx1 mutants or other cold ligands at room temperature.
After shaking at 150 rpm for 2 h at room temperature, the membrane was collected by filtration and washed 3–4 times with ice-cold binding buffer using a 96-well Brandel harvester. The membrane-containing filter paper was then incubated with solid scintillation mix for 5 min at 85 °C. Radioactivity results were counted by a Microbeta2 scintillation counter.
GloSensor cAMP assay
A GloSensor cAMP kit from Promega (Cat. No. E1291) was used in this study. In brief, the pGloSensor™-22F plasmid, receptor plasmid, and Gsq plasmid were transfected into HEK293T cells (ATCC). As mentioned earlier, the α1AAR couples to Gq, which could not induce cAMP accumulation; thus, we used an engineered Gsq protein constructed by replacing 15 residues at the C-terminus of Gs with those of Gq. The Gsq protein could be activated by the α1AAR and stimulate cAMP generation.
24 h after transfection, the cells were switched into CO2-Independent Medium (Gibco). Generally, the cells from one well of a 6-well plate were resuspended in 8 mL medium, and incubated with 6 mg GloSensorTM cAMP Reagent. The mixture was then transferred to a 96-well plate with 80 μL of cell suspension per well. The 96-well plate is placed at 37 °C in the dark for 1 h, then placed at room temperature in the dark for 1 h before use.
The following procedures were performed at room temperature. To measure the inhibition profile of the toxin or toxin mutants, 10 μL of the diluted AdTx1 or AdTx1 mutants were added to the well plate. After 15 min of incubation, 10 μL of the small-molecule agonists (norepinephrine or oxymetazoline) were added to the system. The chemoluminescence signal was measured around 10–15 min after the addition of the agonist.
Statistics and reproducibility
The competition binding assay and Glosensor cAMP assay curves were presented as means ± SEM, and calculated and fitted by non-linear regression using GraphPad Prism 8. Statistical comparisons were performed by an additional sum of squares F-test, and P < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The coordinates of the AdTx1-α1AAR-Nb6 structure have been deposited in the Protein Data Bank (PDB) under accession number 8HN1. The cryo-EM density map of the AdTx1-α1AAR-Nb6 structure has been deposited in the Electron Microscopy Data Bank under accession code EMD-34906.
References
Saikia, S., Bordoloi, M. & Sarmah, R. Established and In-trial GPCR Families in Clinical Trials: A Review for Target Selection. Curr. Drug Targets 20, 522–539 (2019).
Kini, R. & Doley, R. Structure, function and evolution of three-finger toxins: mini proteins with multiple targets. Toxicon 56, 855–867 (2010).
Utkin, Y. N. Animal venom studies: current benefits and future developments. World J. Biol. Chem. 6, 28 (2015).
Ianzer, D. et al. Identification of five new bradykinin potentiating peptides (BPPs) from Bothrops jararaca crude venom by using electrospray ionization tandem mass spectrometry after a two-step liquid chromatography. Peptides 25, 1085–1092 (2004).
Frangieh, J. et al. Snake venom components: tools and cures to target cardiovascular diseases. Molecules 26, 2223 (2021).
Marcinkiewicz, C. Functional characteristic of snake venom disintegrins: potential therapeutic implication. Curr. Pharm. Des. 11, 815–827 (2005).
Tcheng, J. E. & O’Shea, J. C. Eptifibatide: a potent inhibitor of the platelet receptor integrin glycoprotein IIb/IIIa. Expert Opin. Pharmacother. 3, 1199–1210 (2002).
Sherman, D. G. Ancrod. Curr. Med. Res. Opin. 18, S48 (2002).
Hannan, S., Mortensen, M. & Smart, T. G. Snake neurotoxin α-bungarotoxin is an antagonist at native GABAA receptors. Neuropharmacology 93, 28–40 (2015).
Quik, M. & Geertsen, S. Neuronal nicotinic α-bungarotoxin sites. Can. J. Physiol. Pharmacol. 66, 971–979 (1988).
Harel, M. et al. The binding site of acetylcholine receptor as visualized in the X-ray structure of a complex between α-bungarotoxin and a mimotope peptide. Neuron 32, 265–275 (2001).
Quinton, L. et al. Isolation and pharmacological characterization of AdTx1, a natural peptide displaying specific insurmountable antagonism of the alpha1A-adrenoceptor. Br. J. Pharmacol. 159, 316–325 (2010).
Maïga, A. et al. Orthosteric binding of ρ-Da1a, a natural peptide of snake venom interacting selectively with the α1A-adrenoceptor. PLoS ONE 8, e68841 (2013).
Endo, T., Tamiya, N. & Harvey, A. Snake toxins. 165–222 (Pergamon Press, New-York, 1991).
Haenisch, B. et al. Alpha-adrenoceptor agonistic activity of oxymetazoline and xylometazoline. Fundam. Clin. Pharmacol. 24, 729–739 (2010).
Roehrborn, C. G. & Schwinn, D. A. α1-Adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J. Urol. 171, 1029–1035 (2004).
Rossi, M. & Roumeguère, T. Silodosin in the treatment of benign prostatic hyperplasia. Drug Des. Dev. Ther. 4, 291–297 (2010).
Fawzy, A. et al. Doxazosin in the treatment of benign prostatic hyperplasia in normotensive patients: a multicenter study. J. Urol. 154, 105–109 (1995).
Fulton, B., Wagstaff, A. J. & Sorkin, E. M. Doxazosin. Drugs 49, 295–320 (1995).
Green, B. Prazosin in the treatment of PTSD. J. Psychiatr. Pract. 20, 253–259 (2014).
Toyoda, Y. et al. Structural basis of α1A-adrenergic receptor activation and recognition by an extracellular nanobody. Nat. Commun. 14, 3655 (2023).
Maeda, S. et al. Structure and selectivity engineering of the M muscarinic receptor toxin complex. Science 369, 161–167 (2020).
Nehmé, R. et al. Mini-G proteins: Novel tools for studying GPCRs in their active conformation. PLoS ONE 12, e0175642 (2017).
Che, T. et al. Nanobody-enabled monitoring of kappa opioid receptor states. Nat. Commun. 11, 1–12 (2020).
Robertson, M. J. et al. Structure determination of inactive-state GPCRs with a universal nanobody. Nat. Struct. Mol. Biol. https://doi.org/10.1038/s41594-022-00859-8 (2022).
Ballesteros, J. A. & Weinstein, H. in Methods in Neurosciences Vol. 25 366–428 (Elsevier, 1995).
Van Baelen, A.-C. et al. Structural and functional diversity of animal toxins interacting with GPCRs. Front. Mol. Biosci. 9, 811365 (2022).
Shi, L. & Javitch, J. A. The binding site of aminergic G protein-coupled receptors: the transmembrane segments and second extracellular loop. Annu. Rev. Pharmacol. Toxicol. 42, 437 (2002).
Surgand, J. S., Rodrigo, J., Kellenberger, E. & Rognan, D. A chemogenomic analysis of the transmembrane binding cavity of human G-protein-coupled receptors. Proteins Struct. Funct. Bioinform. 62, 509–538 (2006).
Xu, X. et al. Binding pathway determines norepinephrine selectivity for the human β1AR over β2AR. Cell Res. 31, 569–579 (2021).
Ring, A. M. et al. Adrenaline-activated structure of β2-adrenoceptor stabilized by an engineered nanobody. Nature 502, 575–579 (2013).
Xu, P. et al. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 592, 469–473 (2021).
Xiao, P. et al. Ligand recognition and allosteric regulation of DRD1-Gs signaling complexes. Cell 184, 943–956.e918 (2021).
Im, D. et al. Structure of the dopamine D2 receptor in complex with the antipsychotic drug spiperone. Nat. Commun. 11, 1–11 (2020).
Yasuda, S. et al. Hot-spot residues to be mutated common in G protein-coupled receptors of class A: identification of thermostabilizing mutations followed by determination of three-dimensional structures for two example receptors. J. Phys. Chem. B 121, 6341–6350 (2017).
Lei, J. & Frank, J. Automated acquisition of cryo-electron micrographs for single particle reconstruction on an FEI Tecnai electron microscope. J. Struct. Biol. 150, 69–80 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput Chem. 25, 1605–1612 (2004).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. Sect. D Struct. Biol. 74, 531–544 (2018).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. Sect. D: Biol. Crystallogr. 60, 2126–2132 (2004).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Kruse, A. C. et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504, 101–106 (2013).
Swaminath, G., Steenhuis, J., Kobilka, B. & Lee, T. Allosteric modulation of beta2-adrenergic receptor by Zn(2+). Mol. Pharmacol. 61, 65–72 (2002).
Acknowledgements
We are grateful for the support of the National Protein Science Research (Beijing) Facility, Tsinghua Base, and the Radioisotope Laboratory of the Center for Biomedical Analysis, Tsinghua University. This work was supported by Beijing Frontier Research Center for Biological Structure, Tsinghua University (M.S., S.Z., A.Z., Y.T., X.S., X.X., C.Y., and X.L.), Tsinghua University-Peking University Joint Center for Life Sciences (CLS) (C.Y. and X.L.), by Beijing Municipal Natural Science Foundation (Grant 5232008 to X.L.), National Natural Science Foundation of China (Grant 32122041 to X.L. and Grant 32100968 to X.X.), China Postdoctoral Science Foundation 2021M691809 (X.X.) and Tsinghua University Initiative Scientific Research Program (X.L.).
Author information
Authors and Affiliations
Contributions
M.S. performed AdTx1, α1AAR, and Nb6 expression, purification, and complex formation with help from Y.T., X.S., and X.X. S.Z., A.Z., and F.K. performed cryo-EM data processing and structure refinement. M.S. characterized the pharmacological properties of AdTx1 and mutants. S.Z. completed the preparation of cryo-EM samples, and A.Z. performed data collection and processing. C.Y. oversaw the cryo-EM data processing and structure refinement. X.L., X.S., and X.X. coordinated the experiments and oversaw the overall study. M.S., S.Z., and X.L. wrote the manuscript. All authors contributed to the editing of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Richard Lewis, Nicolas Gilles, and the other anonymous reviewer for their contribution to the peer review of this work. Primary Handling Editors: Ross Bathgate and Laura Rodríguez Pérez.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shi, M., Zhang, S., Zhu, A. et al. Molecular mechanism of human α1A-adrenoceptor inhibition by Mamba snake toxin AdTx1. Commun Biol 8, 1055 (2025). https://doi.org/10.1038/s42003-025-08405-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08405-0