Abstract
Smoking among men of childbearing age poses a significant threat to their reproductive health. Nicotine, the primary bioactive compound in tobacco, adversely affects sperm characteristics, but mechanisms underlying its effects and if these effects are reversible upon cessation are unclear. We assessed the impact of nicotine exposure and its cessation on spermatogenesis and DNA methylation. Our findings revealed that nicotine exposure reduces sperm quality and leads to testicular damage. However, these effects can be reversed to some degree following nicotine cessation. In spermatogenesis, nicotine exposure reduced the proportion of somatic cells and terminal elongating spermatids, inhibited meiosis, and impeded histone to protamine transition. Additionally, it disrupted energy metabolism by interfering with the tricarboxylic acid cycle and promoting anaerobic respiration, leading to decreased ATP levels in the testes. These metabolic changes were associated with hypoxia and oxidative stress, which can be reversed post-cessation. We further found that nicotine exposure significantly altered global sperm DNA methylation patterns, and smoking cessation effectively reversed abnormal DNA methylation. Our results from both humans and mice emphasize the potential for recovery of sperm quality and epigenetic integrity after short-term smoking cessation, which is beneficial for male reproductive function as well as potentially the health of offspring.
Similar content being viewed by others
Introduction
The ubiquity of smoking among men of reproductive age poses a significant public health challenge worldwide. According to the Global Burden of Disease Study 2019 (GBD 2019), 32.7% of males in this age group were active smokers1. In addition, the consumption of alternative tobacco products, including e-cigarettes, has to increase dramatically over the past decade, particularly among younger demographics2. Smoking presents profound consequences associated with increased risk of severe health conditions, such as lung cancer, chronic obstructive pulmonary disease (COPD), and ischemic heart disease3. Regarding male fertility, smoking has been documented to negatively impact semen quality, affecting sperm count, motility, and morphology4,5. Nicotine, the primary bioactive compound in both tobacco and e-cigarettes, is of notable interest owing to its biochemical function6,7. Previous studies focused on the roles of nicotine in addiction and neural influence during smoking8. In terms of male fertility, studies have demonstrated that nicotine exposure can markedly modify testicular histology and sperm characteristics9,10. Recently, nicotine-induced molecular alterations have been investigated, particularly the role of Nrf2 in mediating ferroptosis in the blood-testis barrier of nicotine-exposed mice11. However, the precise mechanisms underlying the detrimental effects of nicotine on male fertility remain elusive. Furthermore, studies focusing on spermatogenesis, a complex biological process regulated by multiple factors12,13, have yet to fully elucidate the microenvironmental and transcriptomic variations in the testis resulting from nicotine exposure.
DNA methylation, a pivotal epigenetic modification, is critical in regulating chromatin accessibility and transcription factor binding14. During spermatogenesis, DNA methylation has been reported to modulate the expression of numerous germ cell-specific genes, such as Lin28a and Nanos215. Moreover, X chromosome inactivation occurs physiologically in females and is limited to germ cells in the male, which plays a crucial role in spermatogenesis16. Xist has been proved to motivate this process and was regulated by DNA methylation17. Notably, transient DNA demethylation occurs at the preleptotene (PreL) and is maintained until leptotene spermatocytes18. During that time, methylation levels of the whole genome decrease by 20% and have been linked with meiotic recombination19. Studies have highlighted the impact of nicotine on sperm DNA methylation, indicating variable methylation levels at specific genetic loci or CpG islands leading to potential adverse health outcomes in offspring, such as increased spontaneous locomotor activity and attention deficits by altering methylation levels in the promoter of Dat20,21,22. Therefore, a comprehensive mapping of altered genome-wide DNA methylation patterns in male sperm following nicotine exposure is crucial.
In this study, combined with human sperm samples, a mouse model of nicotine exposure was established to elaborate on its influence on spermatogenesis and whole-genome DNA methylation. Sperm quality parameters were quantitatively assessed using Computer-Assisted Sperm Analysis (CASA). Comprehensive testicular evaluations included histological examination, single-cell RNA sequencing, and fluorescence-activated cell sorting (FACS) analysis. Testicular metabolic profiling was performed using targeted metabolomics coupled with ATP quantification. Sperm DNA methylation modifications were characterized through Whole Genome Bisulfite Sequencing (WGBS) and validated by pyrosequencing-based methylation analysis. Notably, a most recent report by the World Health Organization (WHO) on the global tobacco epidemic highlights a growing population of ex-smokers, but there are limited relevant studies concerning this group23,24. Consequently, we placed special emphasis on investigating the potential reversibility of nicotine-induced damage following cessation. Our findings demonstrated partial restoration of spermatogenesis and DNA methylation patterns after nicotine withdrawal, providing important insights into the recovery potential following smoking cessation.
Results
Nicotine exposure-induced damage to sperm quality and quantity is partially reversible
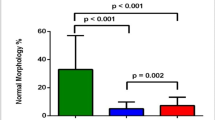
To evaluate the effect of smoking on sperm characteristics and determine the possibility of recovery post-smoking cessation, human sperm samples were collected from three groups: non-smokers (Control), smokers (Smoking), and ex-smokers (Cessation). Importantly, all ex-smokers abstained from smoking for more than one spermatogenic cycle (at least three months). There was no significant difference in age and body mass index (BMI) among the three groups. Furthermore, the Cessation group had a similar smoking history to the Smoking group before quitting (Supplementary Table 1). Although no marked alteration in semen volume was observed (Supplementary Fig. 1A), smoking reduced sperm concentration and total sperm motility rate, including progressive and non-progressive motility rates (Fig. 1A–C and Supplementary Fig. 1B). Additionally, smokers displayed a reduced proportion of normal sperm morphology and an increased DNA fragmentation ratio compared to non-smokers (Fig. 1D and Supplementary Fig. 1C). Notably, smoking cessation resulted in shifting of the aforementioned abnormal parameters to their normal values or showed considerable improvement (Fig. 1A–D and Supplementary Fig. 1B, C). These findings indicated that smoking-induced damage to human sperm quality and quantity could be partially reversible after quitting.
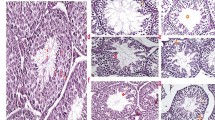
A–D Sperm characteristics in non-smokers (n = 18; Control), smokers (n = 19; Smoking), and ex-smokers (n = 14; Cessation). A Sperm concentration. B Total motility. C Progressive motility. D Rate of normal morphology. E Schematic diagram illustrating experimental design and animal group allocation. F–I Dynamical parameters of sperm samples from three mice groups (CON, NIC, and CES) were measured by Computer-assisted sperm analysis (CASA, n = 8 mice per group). F Sperm concentration. G Sperm motility. H Progressive motility. I Average path velocity (VAP). J, K testicular length (J, n = 22 testes per group) and weight (K, n = 10 mice per group) in NIC mice relative to CON and CES mice. L Hematoxylin and eosin (H&E) staining of testicular samples from three groups post-nicotine exposure. Black scale bar = 200 μm, blue scale bar = 40 μm. M, N Histological characteristics of seminiferous tubule examined by H&E staining. M Spermatogenic epithelium thickness (n = 20 tubules per group). N Seminiferous tubule diameter (n = 24 tubules per group). O Plasma concentrations of testosterone (n = 8 mice per group). Data are represented as mean ± SEM. All statistical analyses were performed using ANOVA followed by Bonferroni’s multiple comparisons test, ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns not significant.
To explore the mechanisms underlying smoking-induced male fertility impairment, a mouse model of nicotine (the primary bioactive component in tobacco) intake via drinking water was established, beginning at 3 weeks of age and continuing for 8 weeks (Fig. 1E). Consistent with the findings in human samples, the nicotine-exposed mice group (NIC) exhibited a decrease in sperm concentration, total motility and progressive motility rates, and average path velocity (VAP) compared with the control group (CON). However, in the mice group (CES) that underwent a 5-week nicotine cessation period following its exposure, these detrimental effects were largely reversed (Fig. 1F–I). The NIC group exhibited reduced testicular length and weight, which were restored in the CES group (Fig. 1J, K). Likewise, the testis to body weight ratio, which was compared within age-matched three groups of mice, followed a similar pattern, showing a decrease in the NIC group and recovery in the CES group (Supplementary Fig. 1D). Histological examination of the testicular tissue revealed a significant reduction in the thickness of the seminiferous epithelium and seminiferous tubule diameter in response to nicotine exposure, which improved after nicotine cessation with negligible differences compared to the control group (Fig. 1L–N). In addition, serum testosterone levels were low in the NIC mice; however, the hormone levels returned to baseline following nicotine cessation (Fig. 1O).
Furthermore, examination of serological markers of liver and kidney function, along with glucose metabolism, highlighted marginal variations in albumin, urea nitrogen, and glucose tolerance across the three groups. However, total protein levels were elevated in the NIC group (Supplementary Fig. 1E–I). Together, both human and mouse experimental data indicated that cessation of smoking for merely one spermatogenic cycle could partly reverse the reproductive damage caused by previous smoking exposure.
Single-cell transcriptome analysis reveals nicotine-induced adverse effects on spermatogenesis
Single-cell RNA sequencing was conducted on testicular samples from CON and NIC mice groups. Uniform manifold approximation and projection (UMAP) visualization analysis identified 18 major cell types based on their respective marker genes (Fig. 2A, B). Notably, the percentage of all somatic cell types (Sertoli, mesenchymal, immune, and Leydig cells) was reduced in NIC mice, highlighting the adverse effects of nicotine on testicular stroma (Fig. 2C). In germ cells, the proportion of initial round spermatids (rSTid_1) decreased while that of terminal spermatocytes (SCyte_lP) increased, indicating the disruptive impact of nicotine on the meiosis process. Moreover, the abundance of terminal elongating spermatids (eSTid_4) and mature sperms also declined in NIC mice (Fig. 2C). Furthermore, differentially expressed genes (DEGs) in CON and NIC mice groups were identified based on the criteria of a log2-transformed fold change (FC) exceeding 0.25 and a p-value threshold of 0.01. Major transcriptional differences between the two groups were noticed in immune cells, SCyte_lP cells, rSTid_1 cells, and eSTid_4 cells (Fig. 2D and Supplementary Data 1). Gene ontology (GO) analysis of upregulated DEGs in SCyte_lP cells demonstrated significant enrichment in respiratory chain complexes, especially complex IV, including key genes such as mt-Co1, mt-Co2, Cox5a, and Cox6c (Fig. 2E). Conversely, downregulated DEGs in SCyte_lP cells were primarily involved in spermatid development and sperm motility, including transition proteins and protamine families (Fig. 2F). Next, expression levels of genes related to respiratory chain complexes and transition proteins/protamine families across all germ cell types in CON and NIC mice revealed consistent variation patterns in most germ cells (Fig. 2G). Gene expression levels were selectively validated by performing quantitative PCR (qPCR) in testicular samples from the three mice groups (CON, NIC, and CES). Results indicated a partial reversal of nicotine-induced differential gene expression post-smoking cessation (Fig. 2H, I).
A Uniform manifold approximation and projection (UMAP) visualization of all 47,775 cells after quality control. The dot color indicates cell types. Sertoli Sertoli cells, Mes mesenchymal cells, Immune immune cells, Leydig Leydig cells, SPG spermatogonia, SCyte_LZ leptotene and zygotene spermatocytes, SCyte_eP early pachytene spermatocytes, SCyte_lP late pachytene and meiosis II spermatocytes, rSTid round spermatids, eSTid elongating spermatids, Sperm mature sperm. B Dot plot showing the expression levels of marker genes in each cell type. C The percentage of various cell types in CON and NIC mice. The dashed box represents somatic and late spermatogenic cells (eSTid_4 and sperm). D Bar plot displaying the number of upregulated and downregulated differentially expressed genes (DEGs). Gene ontology (GO) analyses of upregulated (E) and downregulated (F) DEGs in SCyte_lP cells. G Heatmap demonstrating the relative expression levels of selected genes linked to respiratory chain complexes and transition proteins/protamines in all germ cell types. Quantitative PCR-based detection of gene expression levels of upregulated (H) and downregulated (I) DEGs in testicular tissue across the three groups (n = 5 mice per group). Data are represented as mean ± SEM. All statistical analyses were performed using ANOVA followed by Bonferroni’s multiple comparisons test, ***p < 0.001; **p < 0.01; *p < 0.05; ns not significant.
Additionally, the alterations in germ cell proportion were further confirmed by employing flow cytometry (Fig. 3A). Statistical analysis revealed an elevation in meiosis II spermatocytes with a reduction in round spermatids in NIC mice (Fig. 3B, C). The expression levels of selected DEGs were further verified in meiotic phase II spermatocytes and round spermatocytes of CON and NIC mice, which were consistent with single cell RNA sequencing data (Fig. 3D, E). Considering the reduced proportion of round spermatids and low sperm concentration, apoptosis levels in nicotine-exposed testes were examined. KEGG pathway analysis of several cell types, particularly DEGs upregulated during the later stages of spermatogenesis, indicated significant enrichment in apoptosis-related pathways (Fig. 3F). Immunofluorescence (IF) studies revealed a significant increase in cleaved Caspase-3 expression levels within the seminiferous tubules of NIC mice, while apoptosis within the seminiferous tubules of CON and CES mice was not significantly activated (Fig. 3G, H). To further confirm in which cell types apoptosis occurs in nicotine-exposed testes, we conducted TUNEL staining and identified spermatids using peanut agglutinin (PNA) staining. The results showed that, except for spermatids, apoptosis also occurs in spermatocytes (Fig. 3I). Altogether, these findings provide molecular insights into the mechanisms underlying nicotine-induced abnormalities in spermatogenesis, particularly during meiosis.
A Hoechst fluorescence-based gating on individual spermatogenic populations. Before isolating spermatogenic cells, the main cell population was selected, and adherent or inactive cells were excluded. The proportions of meiosis II spermatocytes (B) and round spermatids (C) in CON and NIC mice (n = 7 mice per group). Detection of gene expression levels of DEGs using qPCR (n = 5 mice per group) in meiosis II spermatocytes (D) and round spermatids (E). F KEGG pathway analyses of upregulated DEGs in eSTid_2 and eSTid_4. Immunofluorescence images (G) and statistical analysis (H) of testicular samples stained with cleaved Caspase-3 and DAPI across the three groups (n = 8 mice per group). Scale bar = 100 μm. I Immunofluorescence images of testicular samples stained with TUNEL, PNA, and DAPI. The white arrows indicate different types of germ cells. Scale bar = 50 μm. Data are represented as mean ± SEM. All statistical analyses were performed employing a two-tailed unpaired t-test or ANOVA followed by Bonferroni’s multiple comparisons test, ***p < 0.001; **p < 0.01; *p < 0.05; ns not significant.
Nicotine exposure-induced modifications in testicular microenvironment are partially reversible
The testicular microenvironment plays a crucial role in spermatogenesis25; therefore, the microenvironment characteristics were examined using a targeted metabolomics approach. A total of 279 metabolites were identified, with 197 metabolites possessing quantitative information (Fig. 4A and Supplementary Data 2). Principal component analysis (PCA) demonstrated distinct metabolic differences between NIC and CON mice, while partial recovery of testicular metabolites was observed in CES mice (Fig. 4B). Consecutively, metabolites exhibiting significant variations were identified, characterized by an FC > 1.5 and a p-value < 0.05. Compared to CON mice, the testicular samples of NIC mice showed a decline in four metabolites, including citrate and aconitate (key components of the tricarboxylic acid (TCA) cycle). Contrastingly, 23 metabolites (including lactate) were elevated in the NIC group, indicating a shift towards anaerobic respiration (Fig. 4C). Notably, only three metabolites (citrate, dTTP, and taurodeoxycholic acid) were affected in CES mice relative to CON mice, with negligible variations observed in other metabolites (Fig. 4D). A heatmap of differentially expressed metabolites (DEMs) across the three groups demonstrated substantial recovery of the testicular microenvironment following nicotine cessation (Fig. 4E). KEGG pathway analysis of the upregulated metabolites in NIC mice revealed marked enrichment in cysteine and methionine metabolism, suggesting a potential increase in methyl donor availability (Fig. 4F).
A The classification of all metabolites with quantitative information. B Principal component analysis (PCA) plot of the three mice groups (CON, NIC, and CES) based on targeted metabolomic data (n = 4 mice per group). Volcano plots exhibiting differential metabolites in NIC (C) and CES (D) mice compared to CON mice. Metabolites with a fold change (FC) >1.5 and a p-value < 0.05 were considered differential. E Heatmap of all differentially expressed metabolites (DEMs) across the three groups (NIC and CES mice compared to CON mice). F KEGG pathway analysis of the increased metabolites in NIC mice relative to CON mice. Quantification of ATP levels (G, n = 8 mice per group) and ADP-to-ATP ratio (H, n = 4 mice per group) in the testicular samples from the mice three groups. Immunofluorescence images (I) and statistical analysis (J) of testicular samples stained with DHE probe and DAPI across the three groups (n = 6 mice per group). Scale bar = 100 μm. Immunohistochemistry images (K) and statistical analysis (L) of mice testes stained with hypoxia-inducible factor 1 alpha (HIF-1α) across the three groups (n = 6 mice per group). Scale bar = 200 μm. The red arrows indicate hypoxic somatic and germ cells. Data are represented as mean ± SEM. All statistical analyses were performed using ANOVA followed by Bonferroni’s multiple comparisons test, ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns not significant.
Next, the relative abundance of metabolites associated with the TCA cycle and glycolysis across the three groups was determined. Concerning TCA cycle components, nicotine exposure decreased the abundance of citrate, aconitate, and isocitrate in NIC mice compared to CON and CES mice (Supplementary Fig. 2A). Interestingly, all glycolytic intermediates (including lactate) were elevated in nicotine-exposed testes, which exhibited partial reversal post-nicotine cessation (Supplementary Fig. 2B). To further validate TCA cycle imbalance and activation of anaerobic respiration in the nicotine-exposed testes, ATP levels were quantified across the three groups as a measure of energy supply. A noteworthy reduction in ATP concentrations within the testes of NIC mice was observed compared to both CON and CES mice (Fig. 4G). Additionally, the ADP-to-ATP ratio, derived from peak intensity analysis of the targeted metabolomic profiling, exhibited a rise in NIC mice (Fig. 4H), indicative of a compromised ATP synthesis capacity. Upregulation of respiratory chain complexes in NIC mice could be linked to mitochondrial hyperfunction; therefore, the reactive oxygen species (ROS) levels within the testes were estimated. ROS levels were higher in NIC mice compared to CON mice, with a partial recovery noticed in CES mice (Fig. 4I, J). Considering the compromised ATP synthesis in the testicular microenvironment, nicotine exposure may hypothetically induce testicular tissue hypoxia and mitochondrial function compensation. Hence, the levels of hypoxia-inducible factor 1 alpha (HIF-1α) were assessed; NIC mice displayed increased hypoxia in their seminiferous tubules and interstitial tissue compared to CON mice, while the difference was insignificant in CES mice (Fig. 4K, L). Overall, these observations suggested that nicotine-induced metabolic and hypoxic alterations in the testicular microenvironment could be partially reversed after cessation.
Nicotine exposure-induced methylation changes in sperm DNA are partially reversible
Testicular metabolomic analysis demonstrated an elevation in methyl donors in NIC mice, indicative of DNA methylation modifications. To explore sperm-associated epigenetic characteristics following nicotine exposure and cessation, sperm DNA methylation status was analyzed using high-performance liquid chromatography (HPLC) for quantifying 5-methylcytosine (5mC). The proportion of 5mC in NIC mice was dramatically higher than that in CON and CES mice (Fig. 5A). Furthermore, whole-genome bisulfite sequencing (WGBS) was conducted to delineate sperm DNA methylation profiles with greater precision. Comparative analysis of global DNA methylation levels revealed a notable appreciation in NIC mice (86.99%) compared to CON mice (85.01%), while CES mice exhibited partial recovery to 84.92% (Fig. 5B). In particular, DNA methylation variations were significant in the intron regions (NIC: 84.96% vs. CON: 83.76%) and satellite DNA (NIC: 69.78% vs. CON: 70.82%) (Fig. 5C). The methylation dynamics during nicotine exposure and subsequent cessation were explored by analyzing differentially methylated regions (DMRs), characterized by an average methylation difference surpassing 20% and a q value < 0.05 using a 500 bp size window. A total of 393 hypermethylated and 184 hypomethylated DMRs (Supplementary Data 3) were identified, with nicotine cessation effectively reversing the aberrant DNA methylation in these regions (Fig. 5D, E). The methylation profiles of promoter regions, pivotal for transcriptional regulation, were assessed based on the methylation status at transcription start sites (TSSs) across the genome. TSSs with a methylation difference exceeding 10% and a p-value < 0.05 were considered significant for subsequent analyses. A total of 107 hypermethylated and 152 hypomethylated TSSs were identified in NIC mice compared to CON mice. Consistent with the DMR analysis, 101 hypermethylated and 145 hypomethylated TSSs in NIC mice showed varying degrees of recovery after nicotine cessation (Fig. 5F, Supplementary Data 4). GO analysis indicated significant enrichment of hypomethylated TSSs in biological processes related to spermatogenesis and cell differentiation, particularly those associated with meiosis and germ cell development (Fig. 5G). Genes integral to these processes, including Sycp3, Syce1, Hormad2, Sycp1, and Ddx4, were prominent.
A Detection of 5-methylcytosine (5-mC) proportion using HPLC (n = 5 mice per group). B Global sperm DNA methylation levels across the three groups. C DNA methylation levels in various regions of the whole sperm genome. Methylation levels of 393 hypermethylated (D) and 184 hypomethylated (E) differentially methylated regions (DMRs) across the three groups (NIC mice relative to CON mice). F Heatmap for 259 differentially methylated transcription start sites (TSSs) in the three groups (NIC mice compared to CON mice). G GO analysis of hypomethylated TSSs in NIC mice compared to CON mice. H The visualization of methylation levels in promoter regions of Irf7 and Sycp3. Pyrosequencing analysis (n = 4 mice per group) on the promoter regions of Irf7 (I) and Sycp3 (J). Pyrosequencing analysis (n = 5 per group) on the promoter regions of IRF7 (K) and SYCP3 (L) in human sperm samples. Data are represented as mean ± SEM. All statistical analyses were performed using ANOVA followed by Bonferroni’s multiple comparisons test, **p < 0.01; *p < 0.05; ns not significant.
To confirm the differential methylation status of the predicted promoters identified by TSS analysis, promoter regions of spermatogenesis-related genes were subjected to pyrosequencing, focusing on three hypermethylated (Irf7, Adam7, and Wfdc8) and three hypomethylated genes (Sycp3, Sycp1, and Syce1). Aligning with the WGBS results (Fig. 5H), nicotine cessation induced a reversal of methylation levels at several CpG sites within the promoters of these genes (Fig. 5I, J and Supplementary Fig. 3). Likewise, the methylation levels in the promoter regions of the same genes in human sperm samples were also validated. There was no difference in demographic information among patients whose sperm samples were involved in pyrosequencing (Supplementary Table 2). The results showed that following cessation, the DNA methylation changes induced by nicotine partially recovered in both hypermethylation and hypomethylation promoters (Fig. 5K, L). These findings strengthen the credibility of the conclusions derived from the mouse model, underscoring their translational significance and relevance to human studies.
Discussion
Spermatogenesis is a complex developmental process influenced by various exogenous physical and chemical factors26,27. This study contributes to the extensive body of literature documenting the adverse effects of nicotine exposure on spermatogenesis, including reduced sperm concentration, motility, morphology, and histological damage to the testes10,28. The current findings align with previous reports; however, the precise mechanisms underlying these detrimental effects remain unclear. In this study, nicotine exposure caused drastic disruptions in the testicular microenvironment and altered gene expression profiles within the testes. Single-cell RNA sequencing demonstrated a reduction in the percentage of all somatic cell types, indicating severe damage to interstitial tissue. Notably, the lower proportion of Leydig cells may correlate with the decreased serum testosterone levels in nicotine-exposed mice29. The increase in testosterone levels following short-term cessation suggests that damage to testicular function is somewhat reversible.
Nicotine exposure affected energy metabolism by unbalancing the TCA cycle and activating anaerobic respiration, leading to a significant drop in ATP concentration. This contrasts with the documented augmentation in mitochondrial activity during spermatogonia differentiation, supporting a metabolic shift from glycolysis to oxidative phosphorylation25. However, the present single-cell RNA sequencing data revealed an upregulation in the transcription of respiratory chain complexes, suggesting that mitochondrial function was not entirely impaired but rather altered in response to nicotine exposure. The presence of nicotine probably influences the generation of ROS (a byproduct of oxidative metabolism) in mitochondria, resulting in excessive mitochondrial fusion and subsequent oxidative stress30,31. Previous studies have reported nicotine-induced tissue hypoxia in the aortic wall, potentially linked to endothelial dysfunction32,33. Here, similar findings were observed in the nicotine-exposed testicular tissue, leading to hypoxia and compensatory mitochondrial dysfunction. Moreover, even though three metabolites related to TCA cycle were significantly decreased in nicotine-exposed testes, other metabolites didn’t exhibit abnormality. It meant that the effects of nicotine exposure on TCA cycle of the testes were partial and unbalanced, which was concentrated in the upstream TCA intermediates. Further study may focus on the specific internal inference of nicotine exposure on TCA cycle of testes to provide new insights into the energy metabolism of testes.
This study demonstrated substantial downregulation of transition proteins and protamines in spermatocytes and round spermatids post-nicotine exposure- key components involved in sperm chromatin condensation and male fertility. Specifically, protamines are critical in replacing transition proteins during spermatid elongation34. In previous studies, smoking has been associated with abnormal expression of protamine genes (PRM1 and PRM2) in human sperm, as well as altering the PRM1 to PRM2 ratio35,36. The present study is the first report demonstrating that nicotine exposure alone could reduce the mRNA expression levels of these vital proteins in spermatocytes and round spermatids.
Epigenetics refers to heritable modifications in gene expression that do not alter the DNA sequence; DNA methylation, histone modifications, and noncoding RNAs are the primary mechanisms of epigenetic regulation37. Among these, DNA methylation is the most extensively studied epigenetic mechanism in the male germline, crucial for regulating the plasticity of spermatogonial stem cells (SSCs). For instance, SSCs with a mutant DNA methyltransferase 3 A failed to differentiate, leading to male infertility38. A wave of DNA demethylation occurs during the initiation of meiosis in spermatogenesis, which is essential for male meiotic recombination19. Moreover, DNA methylation in the promoter regions of specific genes can impact the health of offspring in later life. For example, nicotine-exposed male mice offspring exhibited enhanced spontaneous locomotor activity with deficits in reversal learning, which was associated with decreased DNA methylation at the promoter regions of the dopamine D2 receptor20. The present WGBS data revealed a noteworthy increase in global DNA methylation levels in mice sperm post-nicotine exposure. Notably, the current identification of hypomethylated promoters related to the meiotic cell cycle and germ cell development included key genes, such as Sycp3, Ddx4, Hormad2, and Sycp1, which regulate transposon silencing or homologous chromosomal pairing and recombination39,40. These genes have previously shown sensitivity in response to DNA methylation changes41. This study further validated these findings in sperm samples from smokers, enhancing the applicability and relevance to human studies. DNA methylation is mainly regulated by methyl donors, DNA methyltransferase, and ten-eleven translocation (TET) enzyme42,43. In vivo, several metabolites in the microenvironment may influence the results of DNA methylation, such as vitamin D (by regulating DNMT1)44 and components of the methionine cycle (by regulating methyl donors)45. The abnormal DNA methylation in sperm induced by smoking and nicotine may be connected to hypoxia and increased ROS levels in the testicular microenvironment. A previous study suggested that hypoxia-induced loss of TET enzyme activity caused hypermethylation at gene promoters in tumor tissue46. Interestingly, both hypermethylated and hypomethylated regions were observed in nicotine-exposed mice sperm samples. Similar differential methylation patterns and abnormal ROS levels have been documented in the semen samples of normozoospermic patients47, although the exact mechanism remained unclear. Therefore, additional research is required to determine the association of the hypomethylated promoter regions of key genes with impaired spermatogenesis in nicotine-exposed mice or the fertility of offspring. Except for this, combined with our findings that nicotine exposure significantly reduces the expression of transition proteins and protamines, interestingly, similar variations were detected in the bisphenol A-exposed testes, with inference of histone-to-protamine transition and DNA hypermethylation in spermatozoa48. Moreover, age and DNA methylation patterns in sperm have a strong association, which allowed for the construction of epigenetic clocks and was known as sperm epigenetic age49. A recent study has reported that this phenomenon may be related to abnormalities in the extensive chromatin remodeling during the histone–protamine exchange, which indicates the potential relationship between DNA methylation and histone modification50.
In clinical practice, smokers are routinely advised to quit smoking before planning for pregnancy to protect sperm quality and offspring health. However, a large proportion of smokers remain skeptical about the potential benefits of short-term cessation, ultimately undermining their motivation to quit. This study emphasizes the findings from the cessation group, highlighting the importance of short-term smoking/nicotine cessation. Previous studies have demonstrated an improvement in sperm count and motility post-smoking cessation51, which is corroborated by the present findings in human subjects. The biological effects of nicotine cessation were studied using a mouse model; nicotine was administered through drinking water to minimize potential genetic confounding factors52. Switching to normal drinking water for an additional 5 weeks following exposure implemented nicotine cessation, with both exposure and cessation periods exceeding the duration of a single spermatogenic cycle53. This model of substance withdrawal has also been adopted in other studies54,55 to ensure consistency in exposure duration, despite the potential age-related variations. The current findings indicated that nicotine cessation could partly reverse its adverse effects on sperm parameters and testicular histology. Moreover, most abnormalities in the testicular microenvironment and germline gene expression, including metabolic alterations, apoptosis, hypoxia, and oxidative stress, showed improvement following nicotine cessation. However, the less pronounced recovery of citrate and aconitate levels, along with Prm2 expression, highlighted the limitations of short-term cessation. Taking DNA methylation, cessation effectively reversed the methylation levels in both hypermethylated and hypomethylated DMRs in nicotine-exposed mice sperm samples. A similar recovery was also evident in the promoter regions of differentially methylated genes, which was further validated in sperm samples from ex-smokers. Our study demonstrates that testicular and sperm abnormalities induced by physical or chemical exposures are reversible upon cessation of the insult. Interestingly, similar findings were observed at varicocele-associated complex methylation alterations, including global hypomethylation, specific regional methylation alterations, and potential involvement of methylases, demethylases, and oxidative stress-related mechanisms. Antioxidant therapy has been shown to improve sperm quality in varicocele-related infertility, regardless of surgical intervention. Additionally, emerging insights from nutri-epigenomics highlight how dietary factors influence DNA/RNA methylation via one-carbon metabolism, offering novel avenues for disease management56. Together, these findings underscore the remarkable plasticity of the testis in reversing epigenetic alterations. Moreover, although the potential transgenerational effects of paternal nicotine exposure on behavior and energy metabolism have been reported previously20,57, a detailed investigation is requisite to determine whether these effects can be fully mitigated following cessation.
In conclusion, this study demonstrated the detrimental effects of nicotine exposure on spermatogenesis, disrupting the testicular microenvironment and impairing the critical transition from histone to protamine in sperm chromatin. Additionally, nicotine exposure induced alterations in sperm DNA methylation patterns, likely due to increased ROS levels. Notably, these disruptions in spermatogenesis and DNA methylation seemed not permanent and could be partially reversed upon nicotine cessation. To the best of our knowledge, these findings provide new insights into the underlying mechanisms of nicotine-induced impairment of reproductive function, and underscore the clinical significance of promoting smoking cessation during the preconception period to protect both reproductive health and the well-being of future generations.
Methods
Human subjects
The human study was reviewed and approved by the institutional review board of Zhejiang Provincial People’s Hospital (2021QT130). All ethical regulations relevant to human research participants were followed. Each participant provided written informed consent, permitting their sperm sample usage for future research. After routine semen examination, the leftover sperm fractions (excess material) were collected at the Center for Reproductive Medicine, Zhejiang Provincial People’s Hospital. Participants were categorized as non-smokers, smokers, and ex-smokers according to their personal history. Pack-year refers to the number of packs smoked/day × number of smoking years, with one pack-year defined as 20 cigarettes smoked every day for 1 year. Semen parameters were analyzed in accordance with the WHO standards. Liquefied semen samples were washed with 1X PBS and centrifuged at 600 × g for 5 min; both steps were repeated thrice, and the obtained sperm pellets were stored at −80 °C for subsequent analyses.
Animal model
C57BL/6J male mice were acquired from and maintained under the specific pathogen-free (SPF) conditions at the Shanghai Model Organisms Center and the Fudan University Laboratory Animal Resources Center (Shanghai, China). The animal experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Shanghai Model Organisms Center (2022-0043). We have complied with all relevant ethical regulations for animal use. To establish the model of nicotine exposure followed by its cessation20,58, three-week-old male mice were randomly allocated to one of the three groups: (1) Control (CON) group, receiving normal drinking water; (2) Nicotine-exposed (NIC) group, receiving drinking water administered with 200 μg/mL nicotine (Sigma, N3876); (3) Nicotine cessation (CES) group, which received drinking water containing 200 μg/mL nicotine for 8 weeks, succeeded by normal drinking water without nicotine for an additional 5 weeks. After 12 h of fasting, all mice were anesthetized with pentobarbital sodium following body weight measurement and collection of blood as well as testicular samples for further analyses. Briefly, the cauda epididymis was dissected from each mouse and placed in a pre-warmed M2 medium (Sigma, M7167) before gently squeezing the caudal tissue to release sperm into the media. The sperm-containing media was then incubated at 37 °C for 30 min and later transferred to a fresh tube using a cell filter. Sperm cells were collected by centrifugation at 800 × g for 5 min, accompanied by 1X PBS washing and centrifugation step. Afterward, the obtained sperm pellets were treated with somatic lysis buffer (SLB: 0.1% SDS, 0.5% Triton-X-100 in DEPC water) on ice for 20 min and then centrifuged at 1000 × g for 5 min. The resulting sperm samples were washed twice with 1X PBS, centrifuged (4000 × g for 3 min), and flash frozen in liquid nitrogen before storage at −80 °C for ensuing analyses.
Computer-assisted sperm analysis
Computer-assisted sperm analysis (CASA) was conducted accordingly59. Prior to use, human tubal fluid (HTF) was placed in an incubator overnight at 37 °C with 5% CO2. The unilateral cauda epididymis was excised from the mice and gently squeezed to release the caudal fluid. The excised tissue was then transferred to a pre-warmed HTF medium and maintained at 37 °C for 50 min. During this period, the dish was rotated slowly to facilitate sperm extraction. Consecutively, the collected sperm suspension (100 μL) was loaded onto standard two-chamber counting slides. CASA was performed using the HTM-IVOS system (Version 10.8; Hamilton-Thorne Research) at the Shanghai Institute for Biomedical and Pharmaceutical Technologies.
Histological evaluation
Testicular morphology was assessed by hematoxylin and eosin (H&E) staining. Briefly, intact testicular tissue was fixed in medicated Bouin’s fixative (Servicebio, G1121) for 24 h. Subsequently, the tissue samples were dehydrated by being immersed in ascending ethanol concentrations and later dipped thrice in xylene. Next, the tissue was embedded in paraffin and sectioned into 4-μm thick slices, followed by H&E staining at room temperature using an H&E Staining Kit (Beyotime, C0105S). After mounting, the slides were examined under a bright field microscope. ImageJ (Version 1.54 d) was adopted for analyzing spermatogenic epithelium thickness and seminiferous tubule diameter.
Serologic testing
Evaluation of liver and kidney function by serologic biochemical testing was performed at the Fudan University Laboratory Animal Resources Center. Serum testosterone levels were quantified using the mouse testosterone enzyme-linked immunosorbent assay (ELISA) kit (MEIMIAN, MM-0569M2).
Intraperitoneal glucose tolerance test
An intraperitoneal glucose tolerance test (IPGTT)60 was conducted on the three groups of mice. Briefly, mice subjected to overnight fasting were administered a 20% glucose solution at a dose of 2 g/kg body weight via intraperitoneal injection. The blood samples were extracted from mice tails, and subsequent glucose levels were measured at intervals of 0, 15, 30, 60, 90, and 120 min post-injection with an Accu Chek Performa glucometer (Roche, Basel, Switzerland).
Single-cell RNA sequencing
Single-cell RNA sequencing was performed on the testes of mice61. Briefly, the fresh and intact bilateral testes of each mouse were sterilely isolated post-nicotine exposure and stored in 1 mL of tissue preservation solution (Singleron, 1190062) on ice. The specimens were washed thrice with Hanks Balanced Salt Solution (HBSS), succeeded by removal of the tunica albuginea and mincing of the specimens into small pieces to facilitate digestion using Dissociation Solution (Singleron, 1190062) at 37 °C for 15 min. The resulting cell suspension was then filtered through a 40 μm sterile strainer, pelleted at 800 × g for 2 min, washed, and resuspended in cold PBS for sequencing. The cell viability of each sample was evaluated employing Trypan Blue staining, with viability typically exceeding 80%.
The single-cell RNA sequencing library of testicular cells was prepared according to the manufacturer’s instructions. In brief, freshly prepared cell suspensions (10,000 cells per sample) were loaded into Chromium microfluidic chips with 3’ (v3) chemistry and barcoded with a 10x Chromium Controller (10x Genomics). RNA from the barcoded cells was subsequently reverse-transcribed, and sequencing libraries were constructed using a Chromium Single Cell 3’ (v3) reagent kit (10x Genomics, PN-1000269) following the manufacturer’s instructions. Sequencing was performed on Illumina (NovaseqX Plus PE150) as per the machine’s instructions (Illumina). Reads were analyzed using 10x Genomics Cell Ranger software (version 7.2.0) and mapped to the mouse reference genome GRCm38/mm10. Reads possessing the same cell barcode, unique molecular identifier (UMI), and gene were combined to calculate the number of UMIs per gene per cell. The resulting UMI count tables for each cellular barcode were subjected to further analyses using R (v4.3.2).
Quantitative real-time PCR
Total RNA was isolated using the RNAeasy Animal RNA Isolation Kit (Beyotime, R0026), and cDNA was synthesized using the HiScript IV RT SuperMix for qPCR (Vazyme, R423-01). Quantitative real-time PCR (qPCR) was performed with the ChamQ Universal SYBR qPCR Master Mix (Vazyme, Q711). Specific primers for each gene (Supplementary Table 3) were designed using Primer Express software (Applied Biosystems). qPCR was carried out in 384-well plates on the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems).
Flow cytometry
The flow cytometry-based isolation of mice spermatocytes was adapted from the reported protocol62. In detail, testicular tissue from both testes was immersed in 10 mL of pre-warmed Collagenase I (Worthington, LS004196, 120 U/mL) solution and shaken horizontally at 120 rpm for 10 min at 34 °C. The supernatant was then filtered through a 40 μm cell filter into a fresh tube, leaving ~1 mL of the collagenase solution with the remaining tissue. Subsequently, the tube sample was treated with 5 mL of pre-warmed Trypsin (Gibco, 15050065), 4 mL of pre-warmed HBSS (Servicebio, G4202), and 100 μL of DNase I (Sigma, DN25, 5 mg/mL), followed by shaking at 120 rpm for 8 min at 34 °C. During incubation, the mixture was periodically resuspended using a dropper. The supernatant was again filtered into a fresh new tube and then washed using DMEM (Gibco, 10569010) containing 10% fetal bovine serum (FBS, Gibco, A5669402) before cell counting. The cell pellets were resuspended to a concentration of 106 cells/mL and incubated with 5 μg/mL Hoechst dye (Sigma, 14533) along with 10 μL Dnase I at 60 rpm for 30 min at 34 °C. Immediately prior to sorting, the cells (107/mL) were stained with 10 μg/mL propidium iodide (PI, Beyotime, ST511).
The filtered cells were detected using the Cytek® Aurora CS system at IMIB, Fudan University. The sorting rate was adjusted to 5000 events per second, and a total of 100,000 events were pre-recorded before setting the gate parameters. Initially, Forward Scatter (FSC-A) and Side Scatter (SSC-A) were adjusted to define the main cell population. Next, adherent cells were excluded using FSC-A and FSC-H. Then, the PI signal was detected in the Yellow Green 3 (Comp-YG3-A) and SSC-A channels. Finally, cells at various stages of spermatogenesis, including PreL, leptotene/zygotene (L/Z), pachytene (P), diplotene (D), meiosis II spermatocytes (M II), and round spermatids (RS), were isolated in two distinct channels: Ultraviolet 8 (Comp-UV8-A) and Ultraviolet 4 (Comp-UV4-A). The populations of interest were ultimately collected into the DMEM containing 2% FBS.
Immunofluorescence and immunohistochemistry
For immunofluorescence (IF) studies, fixed testicular tissue was embedded in optimal cutting temperature (OCT) solution. Following freezing on dry ice, the slides were prepared using a cryostat (Thermo, Cryotome E). For detecting ROS by IF, slides were incubated with dihydroethidium (DHE) solution (Sigma, D7008) at 37 °C for 30 min, succeeded by counterstaining with DAPI (Beyotime, C1002) for 10 min at room temperature in the dark. For cleaved Caspase-3 IF detection, slides were incubated overnight with primary cleaved Caspase-3 antibody (Proteintech, 25128-1-AP, 1:200) at 4 °C and later treated with DAPI and Cy3 secondary antibody (Abcam, ab97075, 1:200) for 1 h in the dark. For TUNEL IF detection, slides were incubated with lectin PNA, Alexa Fluor 594 conjugate (Invitrogen, L32459) at room temperature for 1 h in the dark, and then treated with One Step TUNEL Apoptosis Assay Kit (Beyotime, C1088). After mounting, the slides were observed using an inverted microscope (Nikon, ECLIPSE Ti Series). The resulting image is then analyzed with ImageJ.
For immunohistochemistry (IHC) examination, fixed testicular tissue was sectioned into 5-μm thick slices and stained with H&E dye. Following antigen retrieval, the sections were incubated overnight at 4 °C with diluted anti-HIF-1α antibody (Abcam, ab114977, 1:200) and thereafter rinsed using PBS containing 0.1% Triton X-100 (PBST). Next, the HRP secondary antibody (Abcam, ab6721, 1:1000) was applied to the tissue slides, which were then stained with diaminobenzidine (DAB) (Beyotime, P0202). After staining with hematoxylin and mounting, the slides were visualized under a bright field microscope. ImageJ was employed to quantify the HIF-1α positive area63.
HPLC and LC-MS/MS analyses
The 5-mC content in the sperm genome was quantified by HPLC as described with slight modifications64. Briefly, 100 ng of genomic DNA was treated with 100 μg/mL RNase A (Roche, RNASEA-RO) and 5 U/mL RNase T1 (Sigma, R1003) at 37 °C for 4 h to remove RNA. For DNA degradation, the purified DNA was subjected to Nuclease P1 treatment (Sigma, N8630) at 37 °C for 4 h. Subsequently, the resulting sample was treated with 10X calf intestinal alkaline phosphatase (CIAP) buffer and 0.5 μL CIAP (TAKARA, 2250 A) at 37 °C for 4 h. Deproteinated samples were detected using an Agilent 6495 triple quadrupole mass spectrometer at the Center for Excellence in Molecular Cell Science, CAS. The global 5-mC% of each sample was determined by comparing the HPLC peak areas of cytosine (C) and 5-mC standards at 280 nm. Finally, the global 5-mC content in DNA was calculated by 5-mC% = [5-mC/(C + 5-mC)] × 100.
For metabolomic analysis65, 30 mg of testicular tissue samples were homogenized and resuspended in 600 μL of water:acetonitrile (v:v, 50:50) solution. Subsequent to sample condensation, targeted metabolomics analysis was performed on an AB SCIEX QTRAP® 6500 + LC-MS/MS system at the Institute of Metabolism & Integrative Biology (IMIB), Fudan University. The raw data was standardized relative to the protein content of each sample.
ATP quantification
ATP quantification in mice testicular samples was conducted employing the ATP Assay Kit (Beyotime, S0026). This kit was based on the demand of ATP during luciferase oxidizes luciferin and produces bioluminescent light. Fresh samples were treated with lysis buffer and homogenized. Post-centrifugation, the supernatant obtained was treated with the ATP detection reagent. Luminescence was rapidly recorded using a multimode microplate reader (TECAN, Spark®) with an integration time of 10 s per well.
Whole-genome bisulfite sequencing
Mice sperm DNA was extracted using the TIANamp Micro DNA Kit (TIANGEN, DP316). Whole-genome bisulfite sequencing (WGBS) was performed to map sperm DNA methylation. A total of 100 ng of genomic DNA was fragmented to ~350 bp using Hieff® Smearase (YEASEN, 12907ES24). Subsequently, the fragmented DNA was converted to bisulfite with EpiArt DNA Methylation Bisulfite Kit (Vazyme, EM101-01). WGBS libraries were prepared employing the EpiArt DNA Methylation Library Kit for Illumina V3 (Vazyme, NE103-01) following the manufacturer’s instructions. The libraries were purified using VAHTS DNA Clean Beads (Vazyme, N411-01). For each group, sperm WGBS libraries from 3 mice were pooled in equal proportions to generate a sequencing library, which was then sequenced on the Illumina NovaSeq 6000 in paired-end mode (PE150). Quality- and adaptor-trimmed raw sequences were aligned to the NCBI37/mm9 primary genome and deduplicated using Bismark. Enrichment analyses of marker genes were carried out using the clusterProfiler R package (version 4.10.0). EnrichGO function-based GO enrichment analysis was conducted, with GO terms considered significant having a p-value < 0.05 and a q-value < 0.2.
Pyrosequencing methylation analysis
Candidate genes identified from the WGBS data were selected for further evaluation. The differentially methylated promoter region sequence was subjected to pyrosequencing methylation analysis66. Briefly, genomic DNA was bisulfite converted using the EpiTect Bisulfite kit (QIAGEN, 59104). Primers for the target site were designed using PyroMark Assay Design 2.0 (Qiagen, Supplementary Table 4). For each PCR, 2 μL of treated DNA was added in a 50 μL reaction system. Pyrosequencing was then performed on a PyroMark Q48 Autoprep, and the degree of methylation at CpG sites was determined by pyro-Q CpG software.
Statistics and reproducibility
GraphPad Prism (version 9.4) and R (version 4.3.2) were employed for statistical analysis. Data were analyzed using a two-tailed unpaired Student’s t test or ANOVA followed by Bonferroni’s multiple comparisons test. Significance levels have been indicated as ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns not significant. Data are expressed as mean ± SEM. All animal experiments were performed at least twice using independent biological samples of mice. All data shown in figures are biological replications rather than technical replications.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Supporting data can be requested by contacting the corresponding author. The single-cell RNA sequencing data are on the Gene Expression Omnibus (GEO) of the National Center of Biotechnology Information (NCBI) with accession number GSE283769; the WGBS data are on the GEO of the NCBI with accession number GSE284004. The numerical source data for graphs and charts is shown in Numerical source data (Supplementary Data 5).
References
GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 397, 2337–2360 (2021).
Mantey, D. S., Omega-Njemnobi, O. & Montgomery, L. Flavored tobacco use is associated with dual and poly tobacco use among adolescents. Addict. Behav. 93, 269–273 (2019).
Dai, X. et al. Health effects associated with smoking: a Burden of Proof study. Nat. Med. 28, 2045–2055 (2022).
Sharma, R., Harlev, A., Agarwal, A. & Esteves, S. C. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization laboratory methods for the examination of human semen. Eur. Urol. 70, 635–645 (2016).
Tang, Q. et al. Semen quality and cigarette smoking in a cohort of healthy fertile men. Environ. Epidemiol. 3, e055 (2019).
Chen, B. et al. Gut bacteria alleviate smoking-related NASH by degrading gut nicotine. Nature 610, 562–568 (2022).
Rauthan, M. et al. MicroRNA regulation of nAChR expression and nicotine-dependent behavior in C. elegans. Cell Rep. 21, 1434–1441 (2017).
Kolodner, G., DiClemente, C. C. & Miller, M. M. Nicotine addiction: a burning issue in addiction psychiatry. Psychiatr. Clin. North Am. 45, 451–465 (2022).
Coskun, G., Ozgur, H., Doran, S. & Polat, S. Ameliorating effects of curcumin on nicotine-induced mice testes. Turk. J. Med Sci. 46, 549–560 (2016).
Aldaddou, W. A., Aljohani, A. S. M., Ahmed, I. A., Al-Wabel, N. A. & El-Ashmawy, I. M. Ameliorative effect of methanolic extract of Tribulus terrestris L. on nicotine and lead-induced degeneration of sperm quality in male rats. J. Ethnopharmacol. 295, 115337 (2022).
Zhang, Z. et al. The role of ferroptosis mediated by Bmal1/Nrf2 in nicotine -induce injury of BTB integrity. Free Radic. Biol. Med. 200, 26–35 (2023).
Griswold, M. D. Spermatogenesis: the commitment to meiosis. Physiol. Rev. 96, 1–17 (2016).
Carrageta, D. F. et al. Signatures of metabolic diseases on spermatogenesis and testicular metabolism. Nat. Rev. Urol. 21, 477–494 (2024).
Liu, X. S. et al. Editing DNA methylation in the mammalian genome. Cell 167, 233–247.e217 (2016).
Wang, J. et al. NRF1 coordinates with DNA methylation to regulate spermatogenesis. FASEB J. 31, 4959–4970 (2017).
Murat, F. et al. The molecular evolution of spermatogenesis across mammals. Nature 613, 308–316 (2023).
Lobo, J. et al. XIST-promoter demethylation as tissue biomarker for testicular germ cell tumors and spermatogenesis quality. Cancers 11, 1385 (2019).
Gaysinskaya, V. et al. Transient reduction of DNA methylation at the onset of meiosis in male mice. Epigenet. Chromatin 11, 15 (2018).
Huang, Y. et al. Single-cell multi-omics sequencing of human spermatogenesis reveals a DNA demethylation event associated with male meiotic recombination. Nat. Cell Biol. 25, 1520–1534 (2023).
McCarthy, D. M. et al. Nicotine exposure of male mice produces behavioral impairment in multiple generations of descendants. PLoS Biol. 16, e2006497 (2018).
Zhang, M. et al. Paternal nicotine exposure induces hyperactivity in next-generation via down-regulating the expression of DAT. Toxicology 431, 152367 (2020).
Schrott, R. et al. Sperm DNA methylation altered by THC and nicotine: Vulnerability of neurodevelopmental genes with bivalent chromatin. Sci. Rep. 10, 16022 (2020).
Murphy, P. J. et al. NRF2 loss recapitulates heritable impacts of paternal cigarette smoke exposure. PLoS Genet. 16, e1008756 (2020).
Gartner, C. & Hall, W. D. Mixed progress in global tobacco control. PLoS Med. 21, e1004392 (2024).
Lord, T. & Nixon, B. Metabolic changes accompanying spermatogonial stem cell differentiation. Dev. Cell 52, 399–411 (2020).
Wakayama, S. et al. Evaluating the long-term effect of space radiation on the reproductive normality of mammalian sperm preserved on the International Space Station. Sci. Adv. 7, eabg5554 (2021).
Liu, X. R. et al. Association between tea consumption and semen quality among 1385 healthy Chinese men. Chemosphere 303, 135140 (2022).
Hamed, K. A., El-Fiky, S. A., Gawish, A. M., Khalil, W. K. B. & Mohamed, H. R. H. Alleviation of nicotine-induced reproductive disorder, clastogenicity, and histopathological alterations by fenugreek saponin bulk and nanoparticles in male rats. Environ. Sci. Pollut. Res Int. 29, 47488–47501 (2022).
Zhao, X. et al. Nicotine induced autophagy of Leydig cells rather than apoptosis is the major reason of the decrease of serum testosterone. Int. J. Biochem. Cell Biol. 100, 30–41 (2018).
Scherz-Shouval, R. & Elazar, Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 17, 422–427 (2007).
Zhang, Z. et al. Nicotine induces senescence in spermatogonia stem cells by disrupting homeostasis between circadian oscillation and rhythmic mitochondrial dynamics via the SIRT6/Bmal1 pathway. Life Sci. 352, 122860 (2024).
Seo, E., Kang, P. & Seol, G. H. Trans-anethole prevents hypertension induced by chronic exposure to both restraint stress and nicotine in rats. Biomed. Pharmacother. 102, 249–253 (2018).
Kugo, H., Moriyama, T. & Zaima, N. Nicotine induces vasa vasorum stenosis in the aortic wall. Biotech. Histochem. 99, 197–203 (2024).
Sassone-Corsi, P. Unique chromatin remodeling and transcriptional regulation in spermatogenesis. Science 296, 2176–2178 (2002).
Hammadeh, M. E., Hamad, M. F., Montenarh, M. & Fischer-Hammadeh, C. Protamine contents and P1/P2 ratio in human spermatozoa from smokers and non-smokers. Hum. Reprod. 25, 2708–2720 (2010).
Yu, B. et al. Cigarette smoking is associated with abnormal histone-to-protamine transition in human sperm. Fertil. Steril. 101, 51–57.e51 (2014).
Asenius, F., Danson, A. F. & Marzi, S. J. DNA methylation in human sperm: a systematic review. Hum. Reprod. Update 26, 841–873 (2020).
Dura, M. et al. DNMT3A-dependent DNA methylation is required for spermatogonial stem cells to commit to spermatogenesis. Nat. Genet 54, 469–480 (2022).
Stallmeyer, B. et al. Inherited defects of piRNA biogenesis cause transposon de-repression, impaired spermatogenesis, and human male infertility. Nat. Commun. 15, 6637 (2024).
Biot, M. et al. Principles of chromosome organization for meiotic recombination. Mol. Cell 84, 1826–1841.e1825 (2024).
Mochizuki, K. et al. Repression of germline genes by PRC1.6 and SETDB1 in the early embryo precedes DNA methylation-mediated silencing. Nat. Commun. 12, 7020 (2021).
Mahmoud, A. M. & Ali, M. M. Methyl donor micronutrients that modify DNA methylation and cancer outcome. Nutrients 11, 608 (2019).
Davletgildeeva, A. T. & Kuznetsov, N. A. The role of DNMT methyltransferases and TET dioxygenases in the maintenance of the DNA METHYLATION LEvel. Biomolecules 14, 1117 (2024).
Liang, Y. et al. Vitamin D alleviates HFD-induced hepatic fibrosis by inhibiting DNMT1 to affect the TGFbeta1/Smad3 pathway. iScience 27, 111262 (2024).
Kang, W., Zhang, Y., Cui, W., Meng, H. & Zhang, D. Folic acid promotes peripheral nerve injury repair via regulating DNM3-AKT pathway through mediating methionine cycle metabolism. Neuromol. Med. 27, 23 (2025).
Thienpont, B. et al. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature 537, 63–68 (2016).
Darbandi, M. et al. Reactive oxygen species-induced alterations in H19-Igf2 methylation patterns, seminal plasma metabolites, and semen quality. J. Assist Reprod. Genet. 36, 241–253 (2019).
Ryu, D. Y. et al. Abnormal histone replacement following BPA exposure affects spermatogenesis and fertility sequentially. Environ. Int. 170, 107617 (2022).
Ashapkin, V., Suvorov, A., Pilsner, J. R., Krawetz, S. A. & Sergeyev, O. Age-associated epigenetic changes in mammalian sperm: implications for offspring health and development. Hum. Reprod. Update 29, 24–44 (2023).
Sawant, S. et al. Associations between sperm epigenetic age and semen parameters: an evaluation of clinical and non-clinical cohorts. Curr. Issues Mol. Biol. 46, 1567–1578 (2024).
Kulaksiz, D. et al. Sperm concentration and semen volume increase after smoking cessation in infertile men. Int. J. Impot Res. 34, 614–619 (2022).
McCarthy, D. M. et al. Transgenerational transmission of behavioral phenotypes produced by exposure of male mice to saccharin and nicotine. Sci. Rep. 10, 11974 (2020).
Oakberg, E. F. Duration of spermatogenesis in the mouse. Nature 180, 1137–1138 (1957).
Nemmar, A. et al. Waterpipe smoke-induced hypercoagulability and cardiac injury in mice: Influence of cessation of exposure. Biomed. Pharmacother. 146, 112493 (2022).
Schonfeld, M., O’Neil, M., Weinman, S. A. & Tikhanovich, I. Alcohol-induced epigenetic changes prevent fibrosis resolution after alcohol cessation in miceresolution. Hepatology 80, 119–135 (2024).
Naderi, N., Tavalaee, M. & Nasr-Esfahani, M. H. The epigenetic approach of varicocele: a focus on sperm DNA and m6A-RNA methylation. Hum. Reprod. Update 31, 81–101 (2025).
Liu, Y. et al. Effects of paternal exposure to cigarette smoke on sperm DNA methylation and long-term metabolic syndrome in offspring. Epigenet. Chromatin 15, 3 (2022).
Vallaster, M. P. et al. Paternal nicotine exposure alters hepatic xenobiotic metabolism in offspring. Elife 6, e24771 (2017).
Jin, Z. R. et al. Roles of CatSper channels in the pathogenesis of asthenozoospermia and the therapeutic effects of acupuncture-like treatment on asthenozoospermia. Theranostics 11, 2822–2844 (2021).
Qin, Y. et al. Macrophage deletion of Noc4l triggers endosomal TLR4/TRIF signal and leads to insulin resistance. Nat. Commun. 12, 6121 (2021).
Jacquelot, N. et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat. Immunol. 22, 851–864 (2021).
Gaysinskaya, V., Soh, I. Y., van der Heijden, G. W. & Bortvin, A. Optimized flow cytometry isolation of murine spermatocytes. Cytom. A 85, 556–565 (2014).
Varghese, F., Bukhari, A. B., Malhotra, R. & De, A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One 9, e96801 (2014).
Raad, G. et al. Adverse effects of paternal obesity on the motile spermatozoa quality. PLoS One 14, e0211837 (2019).
Cong, Q. et al. HCV poly U/UC sequence-induced inflammation leads to metabolic disorders in vulvar lichen sclerosis. Life Sci. Alliance 4, e202000906 (2021).
Weinberg-Shukron, A. et al. Balanced gene dosage control rather than parental origin underpins genomic imprinting. Nat. Commun. 13, 4391 (2022).
Acknowledgements
We thank He Huang (the Institute of Metabolism & Integrative Biology, Fudan University) for his guidance and assistance in the metabolomics. We thank Zhen Lin (the Center for Excellence in Molecular Cell Science, CAS) for technical assistance with flow cytometry. This work was supported by the Shanghai Frontiers Science Research Base of Reproduction and Development. This work was supported by the National Natural Science Foundation of China (92357306, 82495190, 82271722, 82301931, 82088102), National Key R&D Program of China (2022YFC2703500), Fund of Fudan University and Cao’ejiang Basic Research (24FCA14), Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01), Shanghai Clinical Research Center for Gynecological Diseases (22MC1940200), Shanghai Urogenital System Diseases Research Center (2022ZZ01012).
Author information
Authors and Affiliations
Contributions
G.D. designed the study; C.W., F.G. and J.S. collected the samples; J.C., S.L., Z.R., X.Q. and M.Z. performed the experiments; J.C., YX.Z. and Y.Z. performed data analyses; J.C. and G.D. wrote the manuscript; YX.Z., J.S., and G.D. reviewed the manuscript; Y.Z., H.H. and G.D. funded this study. All authors have discussed the results and approved the final version of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Yesim Bilmez, Savni Sawant, and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editors: Frank Avila and David Favero.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cui, J., Wang, C., Zheng, Y. et al. Mechanisms and reversibility of nicotine-induced spermatogenesis impairment and DNA methylation changes. Commun Biol 8, 1053 (2025). https://doi.org/10.1038/s42003-025-08493-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08493-y