Abstract
The medial temporal lobes (mTL) are thought to enhance visual processing of fearful faces, yet the underlying mechanisms remain underspecified. To fill this gap, we recorded and compared event-related potentials (ERPs) and stimulus-induced gamma-band activity (GBA) from 36 patients with left- or right-hemispheric antero-medial temporal lobe resections including the amygdala (lTLR/rTLR) and 18 healthy controls. Only rTLR patients were found to lack fear-neutral differentiation in early P1 amplitudes (~100 ms) and exhibited heightened GBA for neutral faces over ipsi-resectional occipito-temporal areas (95–300 ms). lTLR patients showed strongest emotion differentiation in ERP components beyond the P1. Therefore, the right mTL, potentially particularly the amygdala, appears to support rapid attentional shifts toward fear and to coordinate fear-neutral differentiation in GBA. Conversely, the left mTL seems to down-regulate fear responses. These results reveal complementary, lateralized, and time-specific roles of the medial temporal lobes in fear processing, thereby refining models of emotional vision.
Similar content being viewed by others
Introduction
The human visual system prioritizes facial expressions as they are essential for communication and interaction1,2,3, above all when they display fear4. Medial temporal lobe (mTL) structures, particularly the amygdala, are thought to rapidly facilitate the allocation of visual attention to emotionally salient faces through bi-directional connections to the visual system5,6. Although a famous patient with bilateral amygdala destruction has been shown to be severely impaired when processing fearful faces7, some functional MRI (fMRI) studies reported preserved visual discrimination of fearful from neutral faces in individuals with unilateral8 and potentially even bilateral amygdala lesions9. This suggests the existence of multiple pathways of visual emotion processing that are not yet well-characterized10,11. Crucially, it is largely unknown when and how the mTL drives processing enhancements for fearful faces, whether the hemispheres differ in their contribution, and when emotion differentiation might occur independently of the mTL. Current models on emotional vision5,11 lack characterization of the role of the mTL across time. Such insights could also be relevant to advance clinical applications of affective neuroscience12,13,14,15.
Electrophysiological studies on patients with mTL damage or resections help delineate its role during fear perception with high temporal precision. For instance, studies on components of event-related potentials (ERPs) are well-suited to characterize the signal strength of cortical afferents and efferents over time16,17. Given that the functional significance of many ERP components is known, it is also possible to assign any disruptions to changes in perceptual and cognitive processes. However, so far, sparse small-n studies have yielded inconclusive findings: An older study associated primarily left amygdala sclerosis (six out of seven studied patients) with lacking differential responses to fearful faces in early visual attention around 100 ms (P1 component) and later, around 600 ms (P600 component), in processes associated with memory encoding and stimulus evaluation18. By contrast, a recent report19 observed absence of the often reported N1 component enhancement for fearful faces4,20 in six patients with right but not eight with left temporal lobe resections (rTLR/lTLR, respectively), suggesting an impact of rTLR on the structural encoding of fearful faces and no effect of lTLR4,21. Thus, evidence is contradictory as to when exactly the mTL, potentially particularly the amygdala, facilitates fear perception as operationalized by scalp ERPs. Furthermore, the lateralization of mTL and specifically amygdala functions has long been debated22,23,24,25, partly due to the lack of systematic comparisons between effects of right and left mTL lesions.
Oscillatory gamma-band activity (GBA, 35–90 Hz) allows insights into intra-network computations during information processing and integration. It is thought to encode how efficiently internal neural representations are adapted to certain stimulus attributes26,27,28,29. A well-adapted system results in stronger within-system GBA synchronization, indicating successful reduction of activity redundancies, so-called sparsification through predictive coding26. Fittingly, Li and Keil10 summarize evidence that assumes visual cortical sparsification as one main contributor to fear processing. Indeed, visual cortical GBA has been associated with fear discrimination30,31,32. However, although a role for GBA is sometimes implicitly assumed32, to our knowledge, there are no studies investigating the effect of mTL lesions on GBA correlates of emotional face processing.
Therefore, the present study explores the time- and laterality-specific contributions of the mTL to the emotion-sensitive ERP components, P1, N1, early posterior negativity (EPN), and late positive potential (LPP)4,18,19,33,34,35, as well as to oscillatory GBA32,36,37,38,39. ERPs and GBA are complimentary and temporally highly resolved neural signatures of emotional face processing. We derived these markers from a comparatively large and well characterized sample of patients with unilateral temporal lobe resections (18 lTLR and 18 rTLR, Fig. 1) and a case-matched healthy control group (HC). Participants were presented with a series of fearful and neutral facial expressions. Building on previous research19, we expected that rTLR patients would exhibit reduced emotion differentiation in early ERP components. Given the sparse data on emotion differentiation in lTLR patients, we did not formulate directional a priori hypotheses for this group. Nevertheless, based on previous findings of altered fear processing in individuals with left-hemispheric amygdala lesions18, we anticipated that their responses would deviate from the overall group mean. Our primary focus is on electrophysiology data, but we also assessed subjective stimulus appraisal and recognition memory for faces in the three groups of participants.
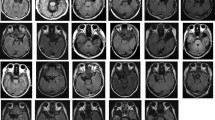
Overlap of temporal lobe resections, which consisted of anteromedial (including the hippocampus, n = 13) and apical (hippocampus sparing, n = 5) temporal lobe resections in the lTLR patients (top row) and exclusively of anteromedial temporal lobe resections (n = 18) in all rTLR patients (n = 18, bottom row). Images are centered at the mean overlap maxima of the resections. Abbreviations: lTLR left temporal lobe resection, rTLR right temporal lobe resection.
Results
Higher arousal and negative valence for fearful faces across groups
Self-reports showed higher arousal for fearful compared to neutral faces (Mfear = 4.145, SDfear = 1.370; Mneutral = 2.991, SDneutral = 0.933; β = −1.035, p = 2.67e−06). Valence ratings were significantly lower for fearful than for neutral faces (Mfear = 2.798, SDfear = 0.740; Mneutral = 3.717, SDneutral = 0.700; β = 0.900, p = 3.33e−07). Groups did not differ regarding their arousal or valence ratings (Supplementary Fig. 1a, Supplementary Tables 1 and 2).
Reduced face recognition in rTLR and general recognition bias for fearful faces
Overall recognition scores (discrimination index, DI) were worse in rTLR (DI: MrTLR = 0.132, SDrTLR = 0.150; MlTLR = 0.230, SDlTLR = 0.189; MHC = 0.227, SDHC = 0.206; βGroup = −0.065, p = 0.012). Across groups, recognition bias was higher for fearful than neutral faces (Bias: Mfearful = 0.494, SDfearful = 0.181, Mneutral = 0.365, SDneutral = 0.178; βEmotion = 0.644, p = 0.0005, Supplementary Fig. 1b, Supplementary Tables 3 and 4). There was no Group × Emotion interaction on recognition accuracy.
ERPs reveal attenuated early fear signals in rTLR and a sustained increase of fear differentiation in lTLR
Our main interest was to characterize the temporal dynamics of fear processing between and within groups. Therefore, linear mixed models (LMMs) were applied to single-trial amplitude values of four ERP components that are typically sensitive to visual and higher cognitive emotion processing. Additionally, we controlled for clinical and resection-specific factors in separate control models. Finally, we assessed associations of electrophysiological and behavioral measures. For brevity, we only report contrasts that relate to our hypotheses in the main text. These are also illustrated in Fig. 2. Supplementary Tables 5 and 6 document all model coefficients.
a Data and analysis for the P1. rTLR patients exhibited the least emotion differentiation (βEmotion × Group (rTLR) × ERP = −0.081, p = 0.025). b Data and analysis for the N1. lTLR patients exhibited larger emotion differentiation than the unweighted average across all groups (βEmotion × Group (lTLR) × ERP = −0.081, p = 0.040). c Data and analysis for the EPN. As a trend, lTLR patients exhibited larger emotion differentiation than the unweighted average across all groups (βEmotion × Group (lTLR) = −0.080, p = 0.084). d Data and analysis for the LPP. lTLR patients exhibited larger emotion differentiation than the unweighted average across all groups (βEmotion × Group (lTLR) × ERP = 0.094, p = 0.017). ERP curves depict mean amplitudes of the marked channel clusters for each group (n = 18 per group). The grey area indicates the time-window in which the ERP component was extracted from the channels marked in black. Difference topographies (fearful-neutral) are averaged across time-points within the respective time-window. Boxplots show differential (fearful-neutral) amplitudes extracted from the channel clusters, averaged across time. Whiskers indicate the interquartile range. The bold horizontal line indicates the distribution median. Dots indicate single-subject values. Black squares indicate the distribution mean. lTLR patients with apical resections (spared hippocampus, n = 5) are marked in blue. Brackets mark significant comparisons that specify significant differences and interactive effects of Group and Emotion (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001). Since significant emotion differentiation in lTLR patients was shifted forwards (βfearful - neutral = 0.106, p = 0.050, d = 0.410; Supplementary Fig. 2) and not found in the a priori defined cluster (βfearful - neutral = 0.119, p = 0.277, d = 0.185), the significance marking is depicted in brackets. Abbreviations: HC healthy controls, lTLR left temporal lobe resection, ns not significant, rTLR right temporal lobe resection.
Importantly, while we found an overall effect of Emotion (βEmotion = −0.156, p < 2e−16), this interacted with the Group and ERP component. Patient groups exhibited distinct emotion differentiation depending on the ERP component: In the P1, rTLR patients exhibited the least emotion differentiation (βEmotion × Group (rTLR) × ERP = −0.081, p = 0.025). For HC but not rTLR patients, P1 amplitudes were higher in response to fearful than neutral faces (HC: βfearful − neutral = 0.260, p = 0.018, d = 0.494; rTLR: βfearful − neutral = −0.097, p = 0.356, d = −0.234, Supplementary Table 5). For lTLR patients, higher P1 amplitudes in response to fearful than neutral faces were present in a forward-shifted electrode cluster (lTLR: βfearful − neutral = 0.106, p = 0.050, d = 0.410, Supplementary Fig. 2) but not in the a priori defined cluster (βfearful − neutral = 0.119, p = 0.277, d = 0.185). The shifted cluster was chosen based on post-hoc assessment of the differential (fearful-neutral) P1 topography in lTLR patients. No other group showed significant emotion differentiation in the shifted cluster (HC: βfearful - neutral = 0.062, p = 0.270, d = 0.334, rTLR: βfearful − neutral = −0.023, p = 0.696, d = −0.104 (Supplementary Table 6).
Beyond the P1, all groups showed an emotion differentiation in favor of fear, i.e., lower amplitudes in response to fearful than neutral faces in the N1 and EPN component and higher amplitudes in response to fearful than neutral faces in the LPP component (all ps < 0.009, all ds > 0.604; Supplementary Table 6). However, lTLR patients exhibited larger emotion differentiation than the unweighted average across all groups in the N1 as well as LPP component (N1: βEmotion × Group (lTLR) × ERP = −0.081, p = 0.040; LPP: βEmotion × Group (lTLR) × ERP = 0.094, p = 0.017). A separate LMM for the EPN (Supplementary Table 7) confirmed that this was, as a trend, also present in the EPN (βEmotion × Group (lTLR) = −0.080, p = 0.084). Control models revealed that larger LPP amplitudes in response to fearful than neutral faces in rTLR patients was associated with smaller fusiform resection volume (βGroup (rTLR) × ERP = −0.032, p = 0.026). This was not observed for the other components. The lTLR group showed no effects of resection volume on electrophysiological emotion differentiation (Supplementary Table 10).
Brain-behavior associations
For the HC and rTLR group, a larger difference of EPN amplitudes in favor of fearful faces was indicative of an enhanced memory bias for fearful relative to neutral faces (HC: βfearful-neutral = −0.177, t(35) = −1.852, p = 0.072, rTLR: βfearful-neutral = −0.161, t(35) = −2.099, p = 0.043). This was not present in lTLR patients (βfearful-neutral = 0.103, t(35) = 1.321, p = 0.195), as also reflected in the Group × EPN interaction (βGroup (lTLR) × EPN = 0.182, p = 0.011; Supplementary Fig. 4, Supplementary Table 9). Furthermore, only for rTLR patients was there an association between LPP emotion differentiation and the differential recognition bias: When the difference in LPP amplitudes between fearful and neutral faces decreased, the recognition bias increased for fearful relative to neutral faces (βGroup (rTLR) × LPP = −0.201, p = 0.030; Supplementary Fig. 4, Supplementary Table 9; rTLR: βfearful-neutral = −0.267, t(35) = −2.333, p = 0.026, HC: βfearful-neutral = 0.061, t(35) = 0.880, p = 0.385, lTLR: βfearful-neutral = 0.008, t(35) = 0.102, p = 0.920). Additionally, the analyses revealed a significant association between N1 emotion differentiation and arousal ratings across all groups: The higher the N1 amplitude difference in favor of fearful faces, the higher the difference in arousal ratings between fearful and neutral faces (βN1 = −0.874, p = 0.008). No significant effects were found for the valence ratings (all ps > 0.100).
GBA reveals altered cortical organization in rTLR
With pointwise cluster-based permutation tests of fixed-effects t-values, we identified time- and frequency-windows of GBA (35–90 Hz)40 alterations during emotion processing within and between the groups. A main effect of Emotion was found over left occipital contacts from 85 – 195 ms in a frequency range of 70-85 Hz, driven by higher GBA power in response to neutral than fearful faces (Supplementary Fig. 3). Critically, the fearful-neutral differentiation at right occipital contacts differed significantly for rTLR patients compared to the unweighted average across all groups from 95 to 300 ms in a frequency range of 60–80 Hz (summed cluster tGroup (rTLR) × Emotion = −1421.000, p = 0.0002). This difference was driven by higher posterior GBA power in response to neutral than fearful faces in rTLR patients only (βfearful-neutral = −0.048, t-ratio = −3.875, p = 0.0001, d = −0.856, Figs. 3, 4, Supplementary Table 8, Supplementary Fig. 3).
a Grand-average time-series of GBA power topographies (35–90 Hz). Data per group (n = 18 per group) are averaged across the indicated time-points and frequencies. b Grand-average time-frequency plots, averaged across the frontal and posterior half of the scalp electrodes. Face images are derived from the FACES database82 and printed with previous permission. Abbreviations: HC healthy controls, lTLR left temporal lobe resection, rTLR right temporal lobe resection.
a Distribution of t-values where fearful-neutral differentiation varied between rTLR patients and the unweighted average across all groups (summed cluster tGroup (rTLR) × Emotion = −1421.000, p = 0.0002; n = 18 per group). The topography is averaged across time-points (95–300 ms) and frequencies (60–80 Hz) of significant effects. Time-frequency plots are averaged across channels. Significant channels and time-windows are outlined or marked in black. b Distribution of differential GBA power within the cluster of the significant Group (rTLR) × Emotion interaction. c Distribution of power values per group and condition extracted from the channel clusters, averaged across time-points and frequencies within the respective cluster. Topographies depict grand-average GBA scalp distribution, averaged across time-points and frequencies of the corresponding effect. For all boxplots, whiskers indicate the interquartile range. The bold horizontal line indicates the distribution median. Dots indicate single-subject values. Black squares indicate the distribution mean. lTLR patients with apical resections (spared hippocampus, n = 5) are marked in blue. Brackets mark significant comparisons that specify interactive effects of Group and Emotion (***p ≤ 0.001). Abbreviations: GBA gamma-band activity, HC healthy controls, lTLR left temporal lobe resection, ns not significant, rTLR right temporal lobe resection.
The control analyses (Supplementary Table 11) suggested no significant effect of the control factors on the Group (rTLR) × Emotion interaction. Likewise, we found no significant associations between GBA power and behavioral responses (Supplementary Table 9). A summary of the decisive interactions in ERP components and GBA is reported in Table 1.
Discussion
Present electrophysiological data reveal distinct temporal profiles of emotional face processing in rTLR and lTLR patients. Specifically, rTLR patients exhibited reduced P1 amplitudes to fearful compared to neutral faces, while also demonstrating increased GBA in response to neutral versus fearful faces over ipsi-resectional, i.e., right occipito-temporal, areas. By contrast, lTLR patients showed enhanced fear differentiation in ERP components beyond the P1 and no distinct pattern in GBA.
The P1 results imply that rTLR patients fail to effectively tune initial perception towards fearful faces. The P1 amplitude in response to emotion has been previously associated with amygdala blood flow33 and is mostly generated by input to the visual cortex41. Thus, specifically the feedback from the right amygdala to visual cortices appears to be absent in rTLR patients5. Correspondingly, emotion differentiation in the P1 was previously reported to be preserved in patients with mTL lesions sparing the amygdala18. However, this previous study reported a lack of P1 enhancement by fearful faces in six patients with left amygdala sclerosis, whereas our larger sample indicates this to be due to absence of the right one. One reason for this discrepancy might be sclerosis-induced reorganization or functional disruptions in the previous report42. In the present sample, according to histological exams, amygdalae were structurally intact at resection. Furthermore, we found no evidence of the influence of hippocampal or fusiform resection extent on P1 emotion differentiation in rTLR patients. Thus, particularly efferents from the right amygdala to the visual cortex may be crucial to heightening the competitive strength of fearful faces in early, pre-conscious visual attention. The right amygdala likely evaluates rudimentary perceptual cues from subcortical, magnocellular pathways in a fast first-sweep of emotion detection and subsequently inputs information to the visual cortex6,43. This could also lead to absent orienting responses towards diagnostic facial features44 and impaired emotion recognition in low-intensity and ambiguous facial expressions8. Interestingly, absent emotion differentiation in the P1 in rTLR patients is seemingly not limited to facial stimuli, as it was also found for more complex emotional scenes35 and more abstract written words45. Thus, early emotion discrimination might rely on the right amygdala across stimulus modalities. This might specifically underlie rapid, low resolution thalamo-amygdalar fear cues33,46. Since descriptively, ERP difference potentials (fearful minus neutral), while statistically significant, were reduced in rTLR patients even beyond the P1, initial amygdalar feedback might also affect subsequent information processing although the role of downstream structures seems to increase. A similar pattern was found by another recent study on emotional picture processing following rTLR35. This could account for the reduced N1 emotion differentiation in rTLR as observed by Framorando and colleagues19. Of note, those authors found no emotion differentiation in the P1 in any group, which contrasts with our data. One possible explanation could be the variability of P1 emotion effects across studies that might be particularly limiting in small-n studies4,47. Generally, the largely preserved post-P1 emotion differentiation in the present rTLR patients is also consistent with the idea of an increasingly distributed network for threat processing over time5,11. Accordingly, we found an association of fusiform resection extent and emotion differentiation in the LPP (but not earlier components) in those patients. Additionally, for rTLR patients, the recognition bias for fearful relative to neutral faces was attenuated with larger LPP emotion differentiation in favor of fear. This might reflect a stronger reliance of rTLR patients on cognitive processes to support fear discrimination34,48. Future work should test such effects in more detail. Indeed, there is growing evidence emphasizing an alternative to the amygdala-centric view of emotion processing. This mechanism might be, in part, realized within the visual system itself10.
Whereas rTLR had reduced ERP responses to fearful faces at very early processing stages, lTLR exhibited a larger fearful-neutral differentiation in ERP components from the N1 onward. Heightened fear processing after lTLR might explain previous reports of emotional dysregulation in patients with left temporal lobe strokes49,50. Hypervigilance has been found to be specific to damage to the bilateral basolateral amygdala in humans51. Overall, present data suggest that resection of specifically the left-hemispheric amygdala results in a disinhibition of fear-specific signaling and a resulting exaggeration of fear responses52. In fact, data might be explained through interhemispheric cooperation and the interplay of both amygdalae in normal emotion processing. Resting-state data by Tetereva and colleagues53 demonstrate that the left amygdala exhibits the most prominent functional connectivity to the right amygdala, while the right amygdala exhibits higher interconnectivity than the left to sensory and associative areas. Gläscher and Adolphs (2003)54 argue that the left amygdala decodes arousal signals from a stimulus, while the right amygdala activates the arousal system. Hence, the left mTL, potentially particularly the amygdala, seems to regulate cortical information processing, potentially by inhibiting disproportionate activation of the right amygdala and subsequent fear processing in the cortex. Alternatively, it is possible that lTLR-driven increases in processing of facial affect are caused by a dysfunctional feedback between the left middle temporal gyrus, relevant for emotion regulation55,56, and the ipsilateral amygdala57. lTLR might also lead to re-organizational processes that affect early visual perception as reflected in the topographically shifted P1 emotion differentiation. A similar topographic shift in the P1 was found for those patients during word processing45. Furthermore, adequate memory representations in lTLR patients seemed dissociated from visual salience34,58 as shown by a missing association of EPN emotion differentiation with the memory bias. Of note, these mechanisms seemingly do not generalize to word processing45. Here, lTLR patients showed a rather consistent decrease in left-hemispheric emotion differentiation in the N1 and LPP components beyond the P1. Since word processing is thought to depend on lexical categorization in left fronto-temporal areas59, cerebral networks for emotion differentiation in abstract and learned stimuli such as written words could differ from those recruited during the processing of biologically salient emotion cues like faces60.
Turning to GBA, we propose that the right mTL is integral to a circuit-level computational process26,27 that differentiates fearful and neutral expressions in right occipito-temporal areas. Here, rTLR patients over-represented neutral faces. Hence, we tentatively propose that a right-hemispheric and mTL-dependent GBA circuit is relevant for the differentiation of fearful and neutral faces through the relative de-prioritization of non-emotional faces. Hence, disrupted structural integrity of the right mTL might not affect the emotion-specific sparsification mechanism per se10,26, but instead its competitive strength compared to the representation of neutral content. This hints towards potential qualitative differences in neural networks encoding fear and neutral content. Local prioritization of fearful faces in the right mTL might well depend on functional connections with the ipsilateral amygdala61. However, given the limited number of studies in this area, the functional network behind occipito-temporal GBA should be investigated in the future. Beyond the amygdala, it may rely on the structural integrity of the fusiform gyrus31,62,63 or the temporal pole64,65,66,67. One future approach could be to investigate frequency coupling within and between the amygdala and visual cortices.
In contrast to the electrophysiological data, behavioral data revealed no effects of TLR on verbal report of subjective emotional experience, consistent with recent constructivist ideas suggesting that the conscious categorization of emotions does not necessarily link to one specific neural correlate68. Interestingly, fear differentiation in the N1 was associated with higher arousal scores for fearful than neutral faces across all groups. Hence, subjectively tangible emotional arousal might be first differentiated during the N1 time-window69. This also supports the assumption that signals from the left amygdala determine the degree of stimulus arousal54, given that changes in emotion differentiation in lTLR patients emerged specifically with the N1 time-window. Results from the recognition memory task do confirm an involvement of the right mTL, likely the right hippocampus70, in memory for faces as rTLR patients performed more poorly. Moreover, all groups showed a recognition bias for fearful faces, which could stem from an adjustment of retrieval criteria in favor of fear-related stimuli by the frontal cortex71.
Although these data advance our understanding of the neural mechanisms underlying emotional vision, their interpretation has some limits that need to be acknowledged. First, generalizability is limited given that our sample consisted of a clinical population. While we tried to control for between-sample confounds using a case-control study, it remains difficult to deduce the function of healthy brain tissue based on the comparison of a clinical sample with a healthy control group. Second, scalp EEG cannot reliably uncover activity within deeper brain regions16. Hence, to directly investigate amygdalar activity, intracranial recordings might be best suited for such analyses39. Similarly, the neural sources of the surface effects are still unknown, which future studies could approximate with inverse source modelling. Such analyses could also estimate the role of resection-induced changes in volume conductor properties. Relatedly, large cerebrospinal fluid cavities might have led to distortions of scalp topographies72,73. Additionally, although implicitly assumed, functional connectivity, e.g., between the amygdala and visual areas, was not tested in the present study, but could be analyzed with intracranial recordings or combined EEG and neuroimaging data in the future74.
Overall, the present study provides compelling evidence for functionally distinct roles of the left and right the mTL during emotional vision. Previous models on emotional vision emphasized that the amygdala enhances the intensity of visual processing in favor of emotion5. Here, we demonstrate that this might adequately describe the contribution of the right amygdala to processes that generate early ERP components but not the complementary and potentially regulatory function of the left amygdala25. Importantly, to the best of our knowledge, robust data regarding the specific timing of those contributions were lacking. Furthermore, present data suggest that a comprehensive characterization of emotion processing necessitates the analysis of oscillatory synchronization within and potentially also between structures. These findings could inform clinical approaches to effectively regulate amygdala responses in anxiety and trauma disorders, as recently trialed by using neuromodulation14,75. Furthermore, they could have relevant implications for the surgical treatment of epilepsy in patients with comorbid psychiatric disorders. For instance, trauma symptoms were shown reduced after rTLR but heightened after lTLR76,77. Although these are only single cases and there is some conflicting data78, these studies imply that interactions of unilateral TLR with psychiatric symptoms might be, at least in part, predicted based on resection side.
Materials and methods
Sample
Data from 18 lTLR patients (eight female), 18 rTLR patients (eight female), and 18 matched healthy controls (HC, nine female) with no past or acute neurological and psychiatric disorders are included in this report (Table 2). All groups were individually matched regarding age (+/− 1 year), sex, and education (highest educational qualification), resulting in a case-control study. Originally, 20 participants per group participated in this study. Two participants were excluded because of technical issues, three due to large perspiration and movement artifacts (n = 3), and one due to a large infarction in the left ventral visual pathway. All rTLR patients and HC were right-handed, four lTLR patients were left-handed. Two of those showed typical left-sided language lateralization. Language lateralization in the remaining two was unclear. Our sample sizes are considerably larger than those reported in previous publications18,19 but we did not pre-determine sample sizes statistically. Nevertheless, data simulations indicate that the present participant and trial numbers should be sufficient to investigate the measures of interest79.
For all patients, temporal lobe resections included the amygdala and surrounding tissue as well as the hippocampus (anteromedial temporal lobe resection). In five lTLR patients, the hippocampus was spared (apical resection). Figure 1 shows the resection extents and their overlap. Patients were surgically treated at the Department of Epileptology (Krankenhaus Mara) of Bielefeld University for pharmacoresistant temporal lobe epilepsy. Testing was done at least 24 months after surgery to ensure sufficient post-operative adjustment in all patients. No patients had amygdala lesions prior to the surgery, as identified from structural imaging and post-operative histology. Patients’ surgery outcome was classified according to criteria proposed by Engel80. Thirty patients were free of disabling seizures (Engel I), two patients reported rare disabling seizures (Engel II), and four had less favorable outcomes (Engel III & IV). Eighteen patients (9 rTLR, 9 lTLR) confirmed to use anti-seizure medication at the time of testing. Participants gave written informed consent according to the Declaration of Helsinki and received a monetary reward of 100 Euros for their participation in the entire study, which consisted of EEG, fMRI, and extensive neuropsychological testing. fMRI and neuropsychology data are reported elsewhere8,81. The study was approved by the ethics committee of the German Psychological Association (DGPs). All ethical regulations relevant to human research participants were followed.
Stimuli
Images of faces were derived from the FACES database of the Max Planck Society for the Advancement of Science with prior permission82, the NimStim Set of Facial Expressions83, and the Karolinska Directed Emotional Faces database84. Only heads and shoulders, in color, were placed centrally on a white background and scaled to a uniform width and height (430 pixels, 9.28° vertical visual angle [centered]).
For each participant, from a set of 120 identities that were balanced for sex and age, 40 identities per facial expression (fearful, neutral) were pseudo-randomly chosen and presented in a passive viewing paradigm. Additionally, 20 identities per expression were shown as novel faces in a recognition experiment 24 h after incidental encoding. Face selection did not vary between groups. Additionally, six happy expressions were presented randomly across the block to reduce predictability of subsequent stimuli.
Procedure
Faces were presented centrally on a 17-inch TFT monitor with a refresh rate of 60 Hz that was positioned on a table in front of the participants. Participants were seated in a comfortable chair at about 70 cm from the center of the screen. They were instructed to attentively view randomly presented negative and neutral faces. This task was chosen to ensure implicit emotion processing without distracting task effects85. The selected stimulus set of 80 faces was divided into four blocks and presented in randomized order. Participants were instructed to remain as quiet as possible and refrain from eye and body movements during each stimulation block. Pseudo-randomly alternating with the blocked presentation of faces were same-length blocks of visual scenes35 and word stimuli45 to reduce habituation to a specific stimulus type. Self-paced breaks were offered between stimulation blocks. After a run of four blocks, the entire presentation was repeated to facilitate incidental encoding, and every face was presented twice, leading to 344 trials. Stimuli were shown for 800 ms. A blank black screen served as the inter-stimulus interval which was presented for 1500 ms ( ± 200 ms jitter). Stimulus presentation was controlled using the Presentation® Software (Version 18.1, Neurobehavioral Systems Inc., Berkeley, CA, http://www.neurobs.com) running on a DELL OptiPlex 755 PC. EEG testing took about 40 min. Twenty-four hours after the stimulus presentation, participants were presented again with 80 identities with fearful or neutral expressions out of which 20 identities per expression were already presented in the main EEG experiment. Participants were then asked to classify the faces as either “old” or “new” with a pseudo-randomly assigned button press (left and right arrow). Stimuli were shown until response or for a maximum of 2000 ms. After 2000 ms, a black screen with a central white question mark instructed the participants to respond. It was shown until a response or a maximum of 1000 ms. A black screen with a central white fixation cross served as the inter-stimulus interval and was presented for 1500 ms. Finally, participants rated both valence and arousal on a 7-point Likert scale of 10 fearful and 10 neutral faces that were randomly chosen from the pool of presented faces. Because the electrophysiological data were the main measures of interest, full data and analysis of recognition and rating tasks are reported in Supplementary Tables 1–4 and Supplementary Fig. 1).
Resection mapping
To evaluate resection extent, structural MRI images were used. MRI data were recorded using a 3T Magnetom Verio Scanner (Siemens, Erlangen, Germany) with a 12-channel head coil. High-resolution T1-weighed structural images were acquired in 192 sagittal slices (TR = 1900 ms, TE = 2.5 ms, voxel size = 0.75 × 0.75 × 0.8 mm, matrix size = 320 × 320 × 192). Resected areas were traced manually in the individual structural T1 images of the patients and converted to Montreal Neurological Institute (MNI) space by applying the deformation fields derived from normalizing procedure in the preprocessing of the respective patient to the resection mask.
EEG processing
During stimulus incidental encoding, the EEG was continuously recorded from 128 active BioSemi Ag-AgCl electrodes using the ActiView software (http://www.biosemi.com). The horizontal electrooculogram (EOG) was measured with two electrodes placed on the outer canthi of the left and right eyes. Two further electrodes for the vertical EOG were placed over and under the left eye. The sampling rate was set to 1024 Hz. Impedances were kept below 25 kΩ, in line with system recommendations. Online recording was referenced to the Cz electrode. Pre-processing of offline data was performed using the FieldTrip toolbox86 (version 20200121) in MATLAB (version 2019b, The MathWorks Inc.). Offline, data was re-referenced to the common average. A high-pass Butterworth first-order zero-phase filter of 0.1 Hz, a 49-51 Hz discrete Fourier transform line noise filter, and a low-pass first-order zero-phase Butterworth filter of 200 Hz were applied. Filtered data were cut into segments of −1500 to 2100 ms relative to stimulus onset and then down-sampled to 500 Hz. Omission of oculomotor and technical (i.e., line or channel noise) artefacts was done by omitting respective independent components. Finally, if single trials still exceeded a maximum amplitude range of 250 μV, those were excluded as well. On average, 16.11% of trials per condition were rejected. The rejection rate did not differ between conditions (t(53) = 0.084, p = 0.933), but marginally fewer trials were rejected in the rTLR data compared to HC (t(35) = 2.300, p = 0.083) and lTLR (t(35) = 2.260, p = 0.083). On average, 1.92% of electrodes were interpolated per participant. The channel interpolation did not differ between groups (all ps ≥ 0.222).
ERP analysis
To ensure methodological comparability between TLR studies18,19, we analyzed four ERP components with pre-defined temporal and spatial occurrences. These allow inferences about specific levels of sensory and higher cognitive processing of emotional value. For this analysis, an additional low-pass first-order zero-phase Butterworth filter of 40 Hz was applied to ensure accurate detection of sharp peak components87. Per channel and participant, the ERP was extracted from −200 to 800 ms relative to stimulus onset. Baseline-correction used 200 ms before stimulus onset. Data were then averaged across trials for each participant. Electrode clusters for each component were selected in line with the literature. To investigate the P1, a time-window ranging from 90 to 130 ms was segmented using two symmetrical clusters of five occipitoparietal electrodes each (Fig. 2a88,89). Two symmetrical channel clusters of eight occipitoparietal electrodes were used to analyze the N1 (140–200 ms, Fig. 2b19,35) and EPN (200 to 350 ms, Fig. 2c34,35). For the LPP, data were extracted from 400 to 800 ms at two centroparietal clusters comprising 13 channels each (Fig. 2d34,35). The midline electrodes were equally allocated to the right and left LPP cluster. ERP component extraction was performed using custom code and the FieldTrip toolbox86 (version 20200121) in MATLAB (version 2019b, The Math Works Inc.).
Additionally, post-hoc assessment of the differential P1 topography (fearful-neutral) showed a topographic shift of the emotion differentiation in lTLR patients. Therefore, we conducted a separate analysis of P1 amplitudes in a shifted electrode cluster (BioSemi channel labels: D24, D25, D31, D32, B10, B11, B14, B15, see Supplementary Fig. 2).
Spectral analysis
From −1500 ms to 2100 ms relative to stimulus onset, the time-frequency power spectrum of each single trial was calculated at every scalp channel from 30 to 90 Hz. Data were down-sampled to 200 Hz prior to analysis. We used Slepian tapers with a fixed window length of 200 ms and applied spectral smoothing through multi-tapering (three tapers) of 20 Hz for all frequencies40. This way, an equal number of time-frequency bins across the time-frequency spectrum was obtained for statistical analysis90. A baseline correction for the frequency calculations was applied by expressing the gamma-power as a relative change to the power in the baseline of −600 to −100 ms pre-stimulus interval. Finally, epochs containing residual artefacts (e.g., muscular activity, technical noise) that were not previously detected in the time domain were excluded from analysis after careful trial-by-trial visual inspection. This rejection was conducted without knowledge of the condition that a trial belonged to. However, it was not blind regarding the group allocation. Nonetheless, this did not lead to systematic differences in trial counts between groups (see paragraph “EEG processing”). Spectral analysis was performed using the custom code and the FieldTrip toolbox86 (version 20200121) in MATLAB (version 2019b, The MathWorks Inc.).
Statistics and reproducibility
General approach
For all statistical analyses, we evaluated mixed-effects linear regression models in R91 running in RStudio (version 2023.03.1)92. For electrophysiological data, we used single-trial data to account for individual trial-by-trial variability and to elegantly handle the hierarchical structure of the mixed-design data91. Factors were effect-coded as repeated a priori contrasts that compare the means of factor levels to the unweighted average93 within a linear mixed model (LMMs, lme4 v.1.1-34). This method allows direct inferences about the direction of a given effect and interaction due to a priori defined contrasts while simultaneously accounting for the variance of all factor levels. Models were fitted with by-ERP component random slopes and by-subject random intercepts to account for repeated measures and trial dependencies94. The significance of model coefficients was tested and reported with two-sided Satterthwaite t-tests. Unstandardized fixed effects coefficients are reported as effect sizes in the LMMs. Significance of beta-coefficient t-values was tested with Montecarlo permutation tests. The alpha-level was set to 0.05. Data was randomly shuffled between conditions (single trials of one condition were kept together while permuting conditions), and after each permutation step, an LMM was calculated. Permutation steps were repeated 1000 times. Empirical t-values were then compared to the distribution of permuted t-values95. The permutation distribution is a data-driven non-parametric distribution. Therefore, no degrees of freedom are given. Post-hoc comparisons were performed with the predicted marginal means using emmeans96 (v.1.8.8). With Holm corrections of the post-hoc tests, we accounted for the inflation of false positives in multiple testing per measure97. For the post-hoc tests, Cohen’s d is reported as the effect size. It is interpreted as small when d = 0.20, medium when d = 0.50, and large when d = 0.8098.
Behavioral data
Stimulus ratings, both valence and arousal, were calculated as mean ratings from a discrete 7-point Likert scale across all stimuli of the same emotion condition per subject. To determine recognition accuracy while controlling for a response bias, the corrected discrimination index (DI: Hits—false alarms) was reported according to the two-high threshold model99. The response bias (Bias) was calculated as false alarms divided by 1-recognition accuracy.
For all behavioral data, we averaged data across trials for each participant and condition to calculate the indices of interest. The models included fixed effects for the experimental factors Group (HC, lTLR, rTLR) and Emotion (fearful, neutral) and their interaction as follows:
Random effects were omitted for the analysis of average behavioral data to avoid overparameterization. Each measure was analyzed with its own statistical model.
ERP components
All ERP components were included in one LMM. The models included the fixed effects for the experimental factors ERP component (P1, N1, and LPP), Group (HC, lTLR, rTLR), Emotion (fearful, neutral), and Hemisphere (left, right) and their interaction as follows:
The contrasts compare the mean of one specific factor combination to the unweighted average across all factor combinations, facilitating a direct interpretation of the effect size in data units while accounting for all factor levels and data dependencies. Contrasts can be interpreted as follows: the beta coefficient indicates the difference in units for the specific factor level/combination compared to the unweighted average across all levels/combinations. Dependency between trials and ERP component amplitudes was accounted for by the specification of the random effects. Because of the sum-to-zero constraint in effect-coded models, no contrasts tested for effects in the EPN and HC group. A separate model that investigates interactions in the EPN is documented in Supplementary Table 7. Estimated marginal means and post hoc tests of all groups and ERP components can be found in Supplementary Table 6.
GBA
Because, to the best of our knowledge, no study investigated effects of TLR on emotion differentiation in GBA, we opted for a data-driven approach in the spectral analysis. We aimed to identify any time-frequency points of significant effects with respect to GBA power. For this, GBA was analyzed from 35 to 90 Hz40 across all scalp channels from 0 to 800 ms, corresponding to the analyzed time-window for the ERPs. We specified a model with the fixed effects for the experimental factors Group (HC, lTLR, rTLR) and Emotion (fearful, neutral) as follows:
To assess pointwise significance of LMM coefficients while effectively accounting for heightened false positives in multiple comparisons of multidimensional data, we utilized cluster-based corrections of Montecarlo permutation tests. Clustering was performed using the maximum summation approach100. The analysis code was modified based on the analysis by Méndez-Bértolo and colleagues6. T-values of each cluster were summed and the greatest sum among all clusters was entered into the permutation distribution. Significant clusters were determined based on both temporal and frequency adjacency with a cluster threshold of α = 0.01, correcting for multiple comparisons on the cluster-level. Only clusters that span more than 10 ms and 5 Hz are reported. Estimated marginal means and post-hoc tests of all groups in clusters indicating an interaction can be found in Supplementary Table 8.
Behavior—electrophysiology associations
We investigated whether emotion differentiation in electrophysiological data was associated with systematic changes in emotion differentiation in the stimulus ratings and recognition performance. To do so, we calculated the difference between responses to fearful versus neutral faces for both behavioral and electrophysiological measures. For each behavioral measure (arousal, valence recognition performance, memory bias), a linear mixed model (LMM) was calculated, testing for the effects of the differential ERP component amplitude or GBA power, group, and their interaction on behavioral emotion differentiation. Models were fitted with by-group random intercepts. Full model coefficients are detailed in Supplementary Table 9. Significant brain-behavior associations are illustrated in Supplementary Fig. 4.
Control analyses
To explore whether any effect of emotion might be moderated by clinical factors such as the age at resection, age at epilepsy onset, time since resection, and BDI scores, we calculated the fear-neutral difference and entered them as dependent variables into our control model. Beforementioned clinical variables served as predictors. Furthermore, to test whether the extent of hippocampal101 and fusiform gyrus63 (FG) resections might have contributed to altered electrophysiological emotion differentiation in patients, we investigated whether the magnitude of respective resections associated with changes in differential emotion processing in the EEG measures. Resection volume was quantified by assessing the overlap between individual lesion masks and a standardized anatomical atlas. Manually constructed lesions masks (see paragraph “resection mapping”) were first resampled to match the dimensions of the Automated Anatomical Labeling102 (AAL) atlas using nearest-neighbor interpolation. A binary lesion mask was then generated to include all nonzero voxels, representing the extent of resection. To determine the resection volume of specific brain regions, the resampled lesion mask was overlaid onto the atlas, and the number of voxels corresponding to the respective region was extracted. The proportion of region-specific voxels affected by resection was then calculated relative to the total number of region-specific voxels in the atlas. All image processing and quantifications were performed using nibabel and nilearn in Python 3.
Predictors were centered to avoid multicollinearity. Models were individually calculated for lTLR and rTLR patients to avoid overparameterization and to ensure a more detailed description of each patient sample. This approach was identical for the ERP components and GBA. For the GBA data, we modelled data within the Group x Emotion interaction clusters identified through the cluster-based LMM approach. Full model coefficients of ERP data are documented in Supplementary Table 10. Full model coefficients of GBA data are documented in Supplementary Table 11.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data needed to evaluate the conclusions are present in the paper or the supporting information. The datasets generated in this study are available under this link: https://gitlab.ub.uni-bielefeld.de/ae02/weidnertlr so are numerical source data for graphs and charts. Raw data are available from the authors upon reasonable request to the corresponding author.
Code availability
The codes generated in this study are available under this link: https://gitlab.ub.uni-bielefeld.de/ae02/weidnertlr.
References
Palermo, R. & Rhodes, G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia 45, 75–92 (2007).
Andrew, R. J. Evolution of facial expression. Science 142, 1034–1041 (1963).
Itier, R. J. & Taylor, M. J. Source analysis of the N170 to faces and objects. Neuroreport 15, 1261–1265 (2004).
Schindler, S. & Bublatzky, F. Attention and emotion: an integrative review of emotional face processing as a function of attention. Cortex 130, 362–386 (2020).
Vuilleumier, P. How brains beware: neural mechanisms of emotional attention. Trends Cogn. Sci. 9, 585–594 (2005).
Méndez-Bértolo, C. et al. A fast pathway for fear in human amygdala. Nat. Neurosci. 19, 1041–1049 (2016).
Adolphs, R., Tranel, D., Damasio, H. & Damasio, A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372, 669–672 (1994).
Reisch, L. M. et al. Face processing and efficient recognition of facial expressions are impaired following right but not left anteromedial temporal lobe resections: Behavioral and fMRI evidence. Neuropsychologia 174, 108335 (2022).
Becker, B. et al. Fear processing and social networking in the absence of a functional amygdala. Biol. Psychiatry 72, 70–77 (2012).
Li, W. & Keil, A. Sensing fear: fast and precise threat evaluation in human sensory cortex. Trends Cogn. Sci. 27, 341–352 (2023).
Pessoa, L. & Adolphs, R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat. Rev. Neurosci. 11, 773–783 (2010).
Fruchter, E. et al. Amygdala-derived-EEG-fMRI-Pattern Neurofeedback for the treatment of chronic post-traumatic stress disorder. A prospective, multicenter, multinational study evaluating clinical efficacy. Psychiatry Res. 115711; https://doi.org/10.1016/j.psychres.2023.115711 (2024).
Engdahl, B. et al. Post-traumatic stress disorder: a right temporal lobe syndrome?. J. Neural Eng. 7, 66005 (2010).
Gill, J. L. et al. A pilot study of closed-loop neuromodulation for treatment-resistant post-traumatic stress disorder. Nat. Commun. 14, 2997 (2023).
Younes, K. et al. Right temporal degeneration and socioemotional semantics: semantic behavioural variant frontotemporal dementia. Brain 145, 4080–4096 (2022).
Nunez, P. L. & Srinivasan, R. Electric fields of the brain. The neurophysics of EEG. 2nd ed. (Oxford University Press, 2006).
Luck, S. J. An introduction to the event-related potential technique (The MIT Press, Cambridge, Mass., [etc.], 2005).
Rotshtein, P. et al. Amygdala damage affects event-related potentials for fearful faces at specific time windows. Hum. Brain Mapp. 31, 1089–1105 (2010).
Framorando, D., Moses, E., Legrand, L., Seeck, M. & Pegna, A. J. Rapid processing of fearful faces relies on the right amygdala: evidence from individuals undergoing unilateral temporal lobectomy. Sci. Rep. 11, 426 (2021).
Durston, A. J. & Itier, R. J. The early processing of fearful and happy facial expressions is independent of task demands - Support from mass univariate analyses. Brain Res. 1765, 147505 (2021).
Vogel, E. K. & Luck, S. J. The visual N1 component as an index of a discrimination process. Psychophysiology 37, 190–203 (2000).
Palomero-Gallagher, N. & Amunts, K. A short review on emotion processing: a lateralized network of neuronal networks. Brain Struct. Funct. 227, 673–684 (2022).
Ocklenburg, S., Peterburs, J. & Mundorf, A. Hemispheric asymmetries in the amygdala: a comparative primer. Prog. Neurobiol. 214, 102283 (2022).
Sergerie, K., Chochol, C. & Armony, J. L. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 32, 811–830 (2008).
Xie, T. et al. The case for hemispheric lateralization of the human amygdala in fear processing. Mol. Psychiatry 30, 2252–2259 (2025).
Vinck, M. & Bosman, C. A. More gamma more predictions: gamma-synchronization as a key mechanism for efficient integration of classical receptive field inputs with surround predictions. Front. Syst. Neurosci. 10, 35 (2016).
Fries, P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu. Rev. Neurosci. 32, 209–224 (2009).
Fries, P., Scheeringa, R. & Oostenveld, R. Finding gamma. Neuron 58, 303–305 (2008).
Peter, A. et al. Surface color and predictability determine contextual modulation of V1 firing and gamma oscillations. eLife Sci. Public. Ltd (2019).
Balconi, M. & Lucchiari, C. Consciousness and arousal effects on emotional face processing as revealed by brain oscillations. A gamma band analysis. Int. J. Psychophysiol. 67, 41–46 (2008).
Janik, A., Susilo, T., Rezlescu, C. & Banissy, M. Enhancing facial emotion recognition with tACS induced gamma oscillations. J. Vis. 14, 1391 (2014).
Luo, Q., Holroyd, T., Jones, M., Hendler, T. & Blair, J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. NeuroImage 34, 839–847 (2007).
Müller-Bardorff, M. et al. Early brain responses to affective faces: a simultaneous EEG-fMRI study. NeuroImage 178, 660–667 (2018).
Schupp, H. T. et al. The facilitated processing of threatening faces: an ERP analysis. Emotion 4, 189–200 (2004).
Mielke, M. et al. Right medial temporal lobe structures particularly impact early stages of affective picture processing. Human Brain Mapping 43; https://doi.org/10.1002/hbm.25687 (2022).
Guex, R. et al. Temporal dynamics of amygdala response to emotion- and action-relevance. Sci. Rep. 10, 11138 (2020).
Keil, A., Müller, M. M., Ray, W. J., Gruber, T. & Elbert, T. Human gamma band activity and perception of a gestalt. J. Neurosci. 19, 7152–7161 (1999).
Müsch, K., Siegel, M., Engel, A. K. & Schneider, T. R. Gamma-band activity reflects attentional guidance by facial expression. NeuroImage 146, 1142–1148 (2017).
Weidner, E. M. et al. Amygdala and cortical gamma-band responses to emotional faces are modulated by attention to valence. Psychophysiology 61, e14512 (2024).
Moratti, S., Méndez-Bértolo, C., Del-Pozo, F. & Strange, B. A. Dynamic gamma frequency feedback coupling between higher and lower order visual cortices underlies perceptual completion in humans. NeuroImage 86, 470–479 (2014).
Pourtois, G., Dan, E. S., Grandjean, D., Sander, D. & Vuilleumier, P. Enhanced extrastriate visual response to bandpass spatial frequency filtered fearful faces: time course and topographic evoked-potentials mapping. Hum. Brain Mapp. 26, 65–79 (2005).
Powell, H. W. R. et al. Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsia 48, 1512–1525 (2007).
Dahlén, A. D., Schofield, A., Schiöth, H. B. & Brooks, S. J. Subliminal Emotional Faces Elicit Predominantly Right-Lateralized Amygdala Activation: A Systematic Meta-Analysis of fMRI Studies. Front. Neurosci. 16, 868366 (2022).
Gamer, M., Schmitz, A. K., Tittgemeyer, M. & Schilbach, L. The human amygdala drives reflexive orienting towards facial features. Curr. Biol. 23, R917–R918 (2013).
Kissler, J., Mielke, M., Reisch, L. M., Schindler, S. & Bien, C. G. Effects of unilateral anteromedial temporal lobe resections on event-related potentials when reading negative and neutral words. Lang., Cognit. Neurosci. 38, 1–19 (2023).
Diano, M., Celeghin, A., Bagnis, A. & Tamietto, M. Amygdala Response to Emotional Stimuli without Awareness: Facts and Interpretations. Front. Psychol. 7, 2029 (2017).
Clark, V. P., Fan, S. & Hillyard, S. A. Identification of early visual evoked potential generators by retinotopic and topographic analyses. Hum. Brain Mapp. 2, 170–187 (1994).
Fields, E. C. The P300, the LPP, context updating, and memory: What is the functional significance of the emotion-related late positive potential?. Int. J. Psychophysiol. 192, 43–52 (2023).
Carota, A., Staub, F. & Bogousslavsky, J. Emotions, behaviours and mood changes in stroke. Curr. Opin. Neurol. 15, 57–69 (2002).
House, A., Dennis, M., Molyneux, A., Warlow, C. & Hawton, K. Emotionalism after stroke. BMJ 298, 991–994 (1989).
Terburg, D. et al. Hypervigilance for fear after basolateral amygdala damage in humans. Transl. Psychiatry 2, e115 (2012).
Terburg, D. et al. The basolateral amygdala is essential for rapid escape: a human and rodent study. Cell 175, 723–735.e16 (2018).
Tetereva, A. O. et al. Asymmetry of amygdala resting-state functional connectivity in healthy human brain. Neuroreport 31, 17–21 (2020).
Gläscher, J. & Adolphs, R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J. Neurosci. 23, 10274–10282 (2003).
Wang, J. et al. The critical mediating roles of the middle temporal gyrus and ventrolateral prefrontal cortex in the dynamic processing of interpersonal emotion regulation. NeuroImage 300, 120789 (2024).
Yun, J.-Y. et al. The left middle temporal gyrus in the middle of an impaired social-affective communication network in social anxiety disorder. J. Affect. Disord. 214, 53–59 (2017).
Iidaka, T. et al. Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. J. Cogn. Neurosci. 13, 1035–1047 (2001).
Schupp, H. T. et al. Selective visual attention to emotion. J. Neurosci. 27, 1082–1089 (2007).
Gagl, B. et al. The lexical categorization model: A computational model of left ventral occipito-temporal cortex activation in visual word recognition. PLOS Comput. Biol. 18, e1009995 (2022).
Reisch, L. M., Wegrzyn, M., Woermann, F., Bien, C. G. & Kissler, J. Negative content enhances stimulus-specific cerebral activity during free viewing of pictures, faces, and words. Hum. Brain Mapp. 41, 4332–4354 (2020).
Novitskaya, Y., Schulze-Bonhage, A., David, O. & Dümpelmann, M. Intracranial EEG-based directed functional connectivity in alpha to gamma frequency range reflects local circuits of the human mesiotemporal network. Brain Topogr. 38, 10 (2024).
Palmisano, A. et al. Face pareidolia is enhanced by 40 Hz transcranial alternating current stimulation (tACS) of the face perception network. Sci. Rep. 13, 2035 (2023).
Klopp, J., Marinkovic, K., Chauvel, P., Nenov, V. & Halgren, E. Early widespread cortical distribution of coherent fusiform face selective activity. Hum. Brain Mapp. 11, 286–293 (2000).
Yang, H., Susilo, T. & Duchaine, B. The anterior temporal face area contains invariant representations of face identity that can persist despite the loss of right FFA and OFA. Cereb. Cortex 26, 1096–1107 (2016).
Liu, T.-Y., Chen, Y. S., Su, T. P., Hsieh, J. C. & Chen, L. F. Abnormal early gamma responses to emotional faces differentiate unipolar from bipolar disorder patients. BioMed. Res. Int. 2014, 1–9 (2014).
Sato, W. et al. Gamma oscillations in the temporal pole in response to eyes. PLOS ONE 11, e0162039 (2016).
Olson, I. R., Plotzker, A. & Ezzyat, Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain 130, 1718–1731 (2007).
Feldman Barrett, L. The theory of constructed emotion: an active inference account of interoception and categorization. Soc. Cogn. Affect Neurosci. 12, 1–23 (2017).
Almeida, P. R. et al. Perceived arousal of facial expressions of emotion modulates the N170, regardless of emotional category: time domain and time-frequency dynamics. Int. J. Psychophysiol. 99, 48–56 (2016).
Kapur, N., Friston, K. J., Young, A. W., Frith, C. D. & Frackowiak, R. S. Activation of human hippocampal formation during memory for faces: a PET study. Cortex 31, 99–108 (1995).
Windmann, S. & Kutas, M. Electrophysiological correlates of emotion-induced recognition bias. J. Cogn. Neurosci. 13, 577–592 (2001).
Piai, V., Oostenveld, R., Schoffelen, J. M. & Piastra, M. C. The impact of CSF-filled cavities on scalp EEG and its implications. Psychophysiology 61, e14624 (2024).
Huiskamp, G. EEG-MEG source characterization in post surgical epilepsy: the influence of large cerebrospinal fluid compartments. Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf. 2004, 4401–4404 (2004).
Salmela, V., Salo, E., Salmi, J. & Alho, K. Spatiotemporal dynamics of attention networks revealed by representational similarity analysis of EEG and fMRI. Cereb. Cortex 28, 549–560 (2018).
Paschen, E. et al. On-demand low-frequency stimulation for seizure control: efficacy and behavioural implications. Brain 147, 505–520 (2024).
Adami, P., König, P., Vetter, Z., Hausmann, A. & Conca, A. Post-traumatic stress disorder and amygdala-hippocampectomy. Acta Psychiatr. Scandinavica 113, 360–363 (2006).
Bijanki, K. R. et al. Case series: unilateral amygdala ablation ameliorates post-traumatic stress disorder symptoms and biomarkers. Neurosurgery 87, 796–802 (2020).
Haslund-Vinding, J. L., BalslevJørgensen, M., Engelmann, C. M., Ziebell, M. & Elklit, A. Right temporal lobe epilepsy surgery activates suppressed post-traumatic stress disorder 31 years after a robbery. Acta Neurochir. 164, 549–554 (2022).
Jensen, K. M. & MacDonald, J. A. Towards thoughtful planning of ERP studies: How participants, trials, and effect magnitude interact to influence statistical power across seven ERP components. Psychophysiology 60, e14245 (2023).
Engel, J. Outcome with respect to epileptic seizures. In Surgical outcomes of the epilepsies (2nd edition), edited by J. Engel. 2nd ed., pp. 609–621(Raven Press, 1993).
Reisch, L. M. et al. Effects of left and right medial temporal lobe resections on hemodynamic correlates of negative and neutral scene processing. Hum. Brain Mapp. 43, 3293–3305 (2022).
Ebner, N. C., Riediger, M. & Lindenberger, U. FACES–a database of facial expressions in young, middle-aged, and older women and men: development and validation. Behav. Res 42, 351–362 (2010).
Tottenham, N. et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 168, 242–249 (2009).
Lundqvist, D., Flykt, A. & Öhman, A. The Karolinska Directed Emotional Faces—KDEF. CD ROM from Department of Clinical Neuroscience (Psychology section, Karolinska Institutet, 1998).
Kulke, L. & Pasqualette, L. Emotional content influences eye-movements under natural but not under instructed conditions. Cognit. Emot. 36, 332–344 (2022).
Oostenveld, R., Fries, P., Maris, E. & Schoffelen, J.-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 156869 (2011).
Widmann, A., Schröger, E. & Maess, B. Digital filter design for electrophysiological data—a practical approach. J. Neurosci. Methods 250, 34–46 (2015).
Schindler, S., Bruchmann, M., Gathmann, B., Moeck, R. & Straube, T. Effects of low-level visual information and perceptual load on P1 and N170 responses to emotional expressions. Cortex 136, 14–27 (2021).
Schindler, S., Bruchmann, M., Bublatzky, F. & Straube, T. Modulation of face- and emotion-selective ERPs by the three most common types of face image manipulations. Soc. Cogn. Affect. Neurosci. 14, 493–503 (2019).
Gross, J. et al. Good practice for conducting and reporting MEG research. NeuroImage 65, 349–363 (2013).
Frömer, R., Maier, M. & Abdel Rahman, R. Group-level EEG-processing pipeline for flexible single trial-based analyses including linear mixed models. Front. Neurosci. 12, 48 (2018).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2021).
Schad, D. J., Vasishth, S., Hohenstein, S. & Kliegl, R. How to capitalize on a priori contrasts in linear (mixed) models: a tutorial. J. Mem. Lang. 110, 104038 (2020).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting Linear Mixed-Effects Models using lme4, 2014.
Raychaudhuri, S. Introduction to Monte Carlo simulation. In 2008 Winter Simulation Conference (2008).
Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. (No Title) (2018).
Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 (1979).
Cohen, J. Quantitative methods in psychology : a power primer. Psychol. Bull. 112, 1155–1159 (1992).
Snodgrass, J. G. & Corwin, J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J. Exp. Psychol. Gen. 117, 34–50 (1988).
Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data (2007).
Zheng, J. et al. Amygdala-hippocampal dynamics during salient information processing. Nat. Commun. 8, 14413 (2017).
Rolls, E. T., Huang, C.-C., Lin, C.-P., Feng, J. & Joliot, M. Automated anatomical labelling atlas 3. NeuroImage 206, 116189 (2020).
Beck, A. T., Steer, R. A. & Brown, G. K. Beck depression inventory (BDI-II) (Pearson, 1996).
Spielberger, C. D. State-Trait Anxiety Inventory. A Comparative Bibliography (1983).
Acknowledgements
This work was funded by grants from the German Research Foundation: Deutsche Forschungsgemeinschaft, Grant/Award Numbers BI1254/8-1 and KI1286/6-1. We thank Prof. Dr. Stephan Moratti, Dr. Alba Peris-Yagüe, and Dr. Manuela Costa for helping with the analysis of time-frequency data. Thank you to Dr. Sebastian Geukes for helping with the LMM pipeline. Thank you to Prof. Dr. Axel Mayer for reviewing the statistical analysis. We would also like to express our gratitude to all the participants.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
E.W. conducted participant testing and performed data processing, analysis, and visualization. M.M. and L.M.R. conducted participant recruitment and testing. L.M.R. performed structural MRI scans and processing of MRI data. C.G.B. contributed to the study design and supported patient recruitment and organization of participant testing in the hospital (Krankenhaus Mara, Bielefeld). J.K. contributed to the study design and supervised data analysis. E.W. drafted and revised the manuscript under supervision of J.K. L.M.R., M.M., and C.G.B. helped to draft and revise the manuscript. All authors read and approved of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Judith Domínguez-Borràs and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Christian Beste and Jasmine Pan. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weidner, E.M., Reisch, L.M., Mielke, M. et al. Fear perception as a function of hemisphere- and time-specific dynamics in the medial temporal lobes. Commun Biol 8, 1105 (2025). https://doi.org/10.1038/s42003-025-08542-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08542-6