Abstract
Marine biofouling caused by barnacle gregarious settlement poses significant challenges to various industries and ecosystems, such as increased drag on ship hulls, elevated fuel consumption, and heightened maintenance costs. While natural chemical cues are instrumental in driving barnacle settlement, the underlying mechanisms remain incompletely understood. In this work, we investigated the effects of adenosine (Ado), a settlement pheromone of Amphibalanus amphitrite cyprids, on cyprid exploration behavior, nano-mechanical properties of footprints, and gene expression using atomic force microscopy (AFM) and omics analysis. Results indicate that Ado significantly increases the settlement rate and exploration frequency of cyprids, and enhances the expression of the settlement-inducing protein complex (SIPC, which attracts other cyprids to settle in a gregarious manner). AFM results reveal that Ado-treated cyprids exhibit enhanced adhesion, self-healing, elasticity, and mechanical strength in their footprints, which may help them resist the shear forces from seawater. Transcriptome analysis suggests that Ado triggers the up-regulation of the transcription factors FTZ-F1 and Hr39, which may activate the 20E hormonal signaling pathway and promote the settlement process. Furthermore, Ado up-regulates the cement protein genes of CP19K-like4 and CP100K, which are involved in the initial adhesion process. These findings provide valuable insights into the role of pheromones in promoting barnacle settlement and offer a deeper understanding of the mechanisms driving this behavior.
Similar content being viewed by others
Introduction
Barnacles are ubiquitous in marine ecosystems, with some species living in a gregarious manner and causing biofouling, which poses substantial economic and environmental challenges1. During their larval phase, barnacle cyprids actively explore and assess potential substrates for settlement2. A barnacle cyprid has a pair of exploratory antennules and six pairs of swimming legs. The third segment of the exploratory antennules is specialized as an attachment disc, densely covered with villi, and the fourth segment bears arrays of long terminal setae and short subterminal setae, which can sense the physical and chemical characteristics of surfaces3. The cyprids secrete a proteinaceous, temporary adhesive known as “footprints” during their surface exploration, enabling them to attach temporarily and reversibly to substrates4. Once a suitable site is identified, the cyprid will permanently attach and metamorphose into an adult barnacle5. The “footprints” exhibit extraordinary underwater adhesion alongside notable resilience and elasticity, and such unique attributes have captured the attention of diverse disciplines, encompassing bionics and materials science6. Simultaneously, understanding the intricate adhesion mechanism of footprints is crucial for effectively preventing barnacle fouling.
The minuscule secretion of footprints by cyprids (temporary adhesive protein) complicates their isolation, necessitating initial studies on adhesive proteins primarily from adult barnacles (known as cement protein)7,8. Five component cement proteins, with molecular masses of 19, 20, 52, 68, and 100 kDa, alongside their homology proteins, were progressively characterized9,10,11. To date, the 20 and 100 kDa proteins have been successfully identified in both adult and cyprid cement glands12,13,14,15, emphasizing their fundamental role in barnacle adhesives across multiple life stages. While the composition of the footprint has been studied as described above, it remains unclear how its mechanical properties are affected by environmental factors.
The functionality of the footprint is inherently linked to its mechanical properties4. Atomic force microscopy (AFM) has enabled high-resolution visualization of footprint micro-morphology and nano-mechanical property characterization. Footprints typically exhibit an elliptical shape, with a central protein-deficient region and dimensions of ~20–40 μm16. AFM imaging reveals that the footprints form a thin film with a fibrous structure17. Furthermore, through repetitive stretching of the footprints using an AFM probe, Vancso et al. demonstrated that footprints have remarkable self-healing ability and exhibit excellent nano-mechanical properties to withstand seawater shear forces4. The results highlight the unique adaptability of footprints in the barnacle settlement process.
Cyprid settlement, critical for the reproduction of sessile barnacles, is influenced by chemical cues. For instance, barnacles associated with corals can detect host-specific chemical signals, leading strict specialists to immediate settlement upon host contact, thereby bypassing the exploratory walk phase18. The fire coral-associated barnacles retain their exploratory walks but can control the activity of nematocysts of their host, facilitating successful settlement and symbiosis19. These diverse settlement behaviors highlight the intricate strategies barnacles have evolved to survive in varied marine environments. It is well-known that cyprids exhibit a strong preference for settling near conspecific individuals, a preference mediated by chemical cues as emphasized by the pioneering work of Knight-Jones and Crisp20. A notable surface-associated cue is the settlement-inducing protein complex (SIPC), present in the cyprid footprints and responsible for attracting other larvae to settle21,22. Given SIPC’s localized effect upon substrate contact, the hypothesis of water-borne pheromones released by adults to remotely attract cyprids emerged23. Our preliminary investigations identified adenosine (Ado) as the barnacle water-borne settlement pheromone (BWSP) in Balanus albicostatus, significantly promoting cyprid settlement24, though the underlying mechanisms remain unclear.
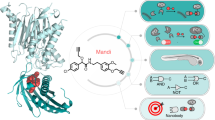
In this study, the mechanism underlying Ado-induced settlement of barnacle cyprids is investigated using AFM and transcriptomic analysis (Fig. 1). The globally distributed barnacle species, Amphibalanus amphitrite, a common fouling organism with well-established genomic resources25,26, serves as the model organism. Initially, it is confirmed that adults of A. amphitrite release Ado as a BWSP. The effects of Ado on cyprid exploratory behavior and the nano-mechanical properties of footprints are then examined using AFM. Transcriptomics analysis is also employed to elucidate the molecular mechanisms underlying Ado-induced settlement. This work aims to provide insights into the mechanics of pheromone-driven settlement of barnacle cyprids from nano-mechanical and molecular biological perspectives.
This figure summarizes the experimental framework and key findings of the study on Ado-induced settlement of A. amphitrite cyprids. The effects of Ado on cyprid exploratory behavior and the nanomechanical properties of footprints are examined using AFM. Transcriptomic analysis is further employed to uncover the molecular mechanisms underlying Ado-induced settlement.
Results and discussion
Ado as a BWSP in A. amphitrite
In marine ecosystems, chemical cues play indispensable roles in numerous biological and ecological activities. Barnacles typically exhibit gregarious settlement behavior, a crucial aspect of their reproductive strategy, and chemical cues are vital in their communication for gregarious settlement. The presence of BWSP has been substantiated through bioassays utilizing adult-conditioned seawater (ACS), which has been demonstrated for its capacity to induce the settlement of cyprids27,28. At first, the impact of ACS on the settlement of A. amphitrite cyprids was confirmed in the present work, which exhibited a significantly enhanced settlement rate of 43.7%, with a 3.2-fold increase compared to the control (Fig. 2A), suggesting the significant inducing effect of ACS on the settlement of A. amphitrite cyprids.
A Induction of A. amphitrite larval settlement by ACS (adult-conditioned seawater). The control group was treated with artificial seawater (n = 3). B Determination of the presence of Ado and Ino in A. amphitrite ACS through LC-MS. C Effects of Ado and Ino treatments on cyprid settlement (n = 3). D Ado induces cyprid settlement on NH2-modified substrates (n = 3).
Previous investigations have proposed the hypothesis that BWSP may be a molecule compound(s) with a molecular weight less than 1 kDa29. Consistently, we found the presence of purine compounds (<1 kDa) in the ACS of B. albicostatus exhibited significant inducing activity for cyprid settlement. In order to validate the presence of purine compounds in A. amphitrite ACS, liquid chromatography-mass spectrometry (LC-MS) was used here. The purine compounds Ado and inosine (Ino) were detected in A. amphitrite ACS (Fig. 2B and Supplementary Data 1). Subsequently, the settlement bioassays were performed to investigate the impact of Ado and Ino on the settlement of cyprids and to verify their potential as BWSP. The results demonstrated that Ino had no significant effect on larval settlement (Fig. 2C). In contrast, Ado exhibited significant inducing activity (Fig. 2C), indicating its potential as a BWSP, which is consistent with our preliminary experiments in B. albicostatus24.
Here, NH2-terminated glass Petri dishes, with the properties to attract cyprid settlement and facilitate the detection of footprints using AFM, were utilized to investigate the impact of Ado on cyprid footprints30. Since 0.2 μg/mL of Ado had the most significant inducing activity at the assay concentration, all subsequent experiments used a concentration of 0.2 μg/mL of Ado (Fig. 2C). The results showed that Ado remained significantly effective in inducing cyprid settlement on the NH2-modified substrates (Fig. 2D).
Effect of Ado on cyprid exploration behavior
Attachment occurs during the cyprid stage, therefore, a detailed study of cyprid exploration behavior is crucial for understanding the settling process in barnacles. The exploration and walking processes of barnacle cyprids treated with Ado and untreated cyprids were recorded (see Supplementary movie 1). Cyprids utilize their antennules to walk on the surface in a bipedal fashion, enabling surface examination to find attachment sites31. As shown in Fig. 3A, the process begins with the cyprid extending one antennule forward while keeping another antennular disc anchored to the surface by secreting footprints. The extended antennule acts as a probing and sensory organ, allowing the cyprid to select suitable attachment sites. Once the extended antennule completes its exploration, the cyprid anchors it and then extends another antennule forward, which is called one step. The previously anchored antennule then assumes the role of the probe, while the previously extended antennule becomes the anchored one. Figure 3A displays typical surface walking images of Ado-treated and control group cyprids. An Ado-treated cyprid took 1.68 s to walk two steps, while a control group cyprid took 8.42 s in comparison. To further quantify the exploration behavior, the number of steps that a cyprid explores within a 10 s period is defined as the exploration frequency. The box plot depicted in Fig. 3B shows a significant increase in exploration frequency among Ado-treated cyprids, with a mean exploration frequency of 4 ± 2 steps/10 s (Mean ± S.D.), compared to 2 ± 1 steps/10 s in the control group. As the exploration frequency accelerates, the distribution of footprints also increases.
A Typical images are showcasing the surface walking and exploration of Ado-treated and control group cyprids, containing two consecutive steps. The circular markers highlight the antennular attachment discs, with the red, yellow, and green circles representing the anchoring sites of the initial, first, and second steps, respectively. The arrows indicate the walking direction of the cyprids. B Statistical analysis of exploration frequency in Ado-treated and control group cyprids (n = 14, 13). Shown here are the mean values (red cross), the median value (gray line), the lower and upper quartiles (boxes), and the minimum and maximum (whiskers) obtained from 6 cyprids treated with Ado and 10 cyprids as controls. C Comparison of SIPC gene expression fold-change between Ado-treated cyprids and control cyprids, as determined by qRT-PCR analyses (n = 3).
The SIPC in these footprints serves as a contact pheromone to attract cyprids settlement22. Therefore, we further investigated the effect of Ado treatment on SIPC gene expression. The result of qRT-PCR demonstrated a significant up-regulation of the SIPC gene following Ado induction (Fig. 3C). This suggests that Ado not only stimulates cyprid exploratory behavior but also triggers an increase in gene expression and secretion of SIPC, thereby facilitating the gregarious settlement of conspecific individuals.
Effect of Ado on adhesion of footprints
AFM was utilized to assess the adhesion force of footprints in situ. As illustrated in Fig. 4A, the barnacle cyprid was placed on an NH2-coated glass Petri dish, and liquid AFM was performed to obtain the morphology and mechanical properties of footprints in a seawater environment. To further investigate the adhesion of footprints deposited on amino glass substrates, the adhesion force, defined as the maximum value of the retraction curve, was measured by analyzing the interaction between the NH2-modified probes and the footprints. Typical force curves of footprints from Ado-treated and untreated cyprids are displayed in Fig. 4B, C, respectively. It shows that the footprints secreted from Ado-treated cyprids exhibit a higher adhesion force. The violin plot from further statistical analysis is shown in Fig. 4D, indicating that after Ado treatment, the average adhesion force of footprints significantly increased (3.1 ± 1.1 nN) compared to the control group (0.8 ± 0.6 nN).
A The footprint of cyprid left on the NH2-coated surface was first imaged by AFM, and then the nano-mechanical properties were studied. The typical force curves of footprints from cyprids treated with Ado (B) and control (C). The gray curves represent the approach and the pink lines are the retraction curves. The maximum force magnitude during withdrawal with respect to the zero-force baseline is defined as the adhesion force. D Violin plot showing a significant increase in the adhesion force of footprints after Ado treatment (n = 57), compared to the control group (n = 52) (p < 0.001 by independent samples t test). The lines in the box represent the medians; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles; polygons represent density estimates of data and extend to extreme values.
Effect of Ado on dynamics of footprints
In the intertidal zone, barnacle cyprids searching for settlement sites are inevitably hit by ocean waves. The cyclical nature of the waves can allow time for the reformation of sacrificial bonds within the footprints before structural failure occurs4. Sacrificial bonds, which are non-covalent interactions, play a critical role in dissipating mechanical energy upon rupture. This energy dissipation mechanism prevents the molecular skeleton from fracturing and endows biomaterials with self-healing properties. A comprehensive discussion of sacrificial bonds and the concept of dissipated energy can be found in the Supplementary Methods 1.
To mimic the behavior of footprints subjected to real-world stress-relaxation cycles, the footprints were repeatedly stretched with different delay times. Figure 5A displays a series of force-extension curves with varying times of delay for the stretched footprint secreted by Ado-treated cyprids, where the recovery of sacrificial bonds can be observed, as indicated by the enhanced “saw-tooth” feature and hysteresis with increasing delay time. Additionally, the maximum stretching distance also increases with a longer delay time. Even after several cycles of stretching, these “saw-tooth” peaks do not disappear, implying that the sacrificial bonds have the opportunity to reform during the longer intervals, leading to enhanced energy dissipation and the preservation of the protein’s mechanical integrity. Similar results were observed in the collagen protein and cyprid footprints, where longer delays were found to provide more time for sacrificial bond reformation, allowing for more energy dissipation in the next stretch cycle4,32. To compare the impact of Ado on the nano-mechanical properties of footprints, repeated stretching experiments on footprints secreted by Ado-treated and untreated cyprids were conducted. The analysis focused on hysteresis length and energy dissipation, as illustrated in Fig. 5B, C. Hysteresis length represents the final position of all sacrificial bonds ruptured during stretching. In the Ado-treated group, the average hysteresis lengths were 947 ± 683 nm, 721 ± 583 nm, and 1291 ± 664 nm for delay times of 0 s, 0.5 s, and 3 s, respectively. Energy dissipation values ranged from 5 ± 8 × 10−16 J (delay time 0 s) to 2 ± 2 × 10−16 J (delay time 0.5 s) and then to 8 ± 10 × 10−16 J (delay time 3 s). Compared to the first stretch, the hysteresis length and energy dissipation decreased in the second stretch (delay time 0 s) and third stretch (delay time 0.5 s). However, in the fourth stretch (delay time 3 s), both hysteresis length and energy dissipation returned to the level of the first stretch, indicating a recovery time of ~3 s for the sacrificial bonds. In contrast, for the control group, it was found that a delay time of 3 s did not allow the sacrificial bonds to recover. Therefore, to determine the recovery time for the sacrificial bonds in the control group, we performed four stretches with delay times of 0, 3, and 10 s. Compared to the Ado-treated group, the average hysteresis length and energy dissipation of the untreated cyprid's footprints were significantly lower. Specifically, the average hysteresis lengths for delay times of 0, 3, and 10 s were 396 ± 218 nm, 409 ± 192 nm, and 453 ± 105 nm, respectively. The average energy dissipation values were only 0.7 ± 0.4 × 10−16 J, 0.8 ± 0.4 × 10−16 J, and 1.0 ± 0.7 × 10−16 J for delay times of 0, 3, and 10 s. Stretching with delay times of 0 and 3 s resulted in reduced hysteresis length and energy dissipation, but the values recovered when a delay time of 10 s was employed. It can be concluded that the sacrificial bonds of Ado-treated cyprid footprints could recover and reform within 3 s, whereas the untreated group required about 10 s for recovery. This result highlights the superior self-healing ability of the footprints secreted by Ado-treated cyprids.
A Force-extension curves of Ado-treated cyprid footprints repeatedly stretched under different delay times (from 0 to 3 s) were recorded, with only the retraction curves displayed. The shaded areas represent energy dissipation. B Hysteresis length (n = 7) and C energy dissipation (n = 6) calculated from the force-extension curves at different delay times (0, 0.5, and 3 s for the Ado-treated group, and 0.5, 3, and 10 s for the control group).
In order to obtain more precise information on the nano-mechanical properties of the footprints, the worm-like chain (WLC) model was utilized to fit the various peaks of the force-extension curve (see the WLC model subsection in the Methods). The WLC fitting can obtain the persistence length (lp), which indicates the inherent stiffness of the molecular chain33. The frequency distribution histograms of the lp for the Ado-treated and control groups are shown in Fig. 6A, B, respectively. Within the Ado-treated group, 85% of the lp values fell within the range of 0–50 pm, with 10% falling within the range of 50–100 pm. Conversely, the lp values in the control group displayed a broader distribution, spanning from 0 to 400 pm. The percentage of data within the range of 0–50 pm decreased to 58% in the control group, while the percentages within the ranges of 50–100 pm and 100–150 pm increased to 21% and 9%, respectively. As compared to the control group, the footprints in the Ado-treated group exhibited a significant reduction in persistence length (p < 0.05), suggesting a relatively lower molecular chain stiffness and higher elasticity. The increased elasticity of Ado-induced footprints suggests a higher degree of sacrificial bond breaking and reintegration, facilitating more excellent deformability and faster self-healing abilities34.
Frequency distribution histograms of persistence length for A Ado-treated and B control groups. Persistence length was obtained by fitting the peaks from force-extension curves using the WLC model. Frequency distribution histograms of peak force of the teeth for C Ado-treated and D control groups. All histograms represent the analysis of 342 peaks from 37 force-extension curves in the Ado-treated group and 375 peaks from 40 curves in the control group.
Furthermore, our analysis also encompassed the peak force values, which represent the magnitude of force required to rupture sacrificial bonds, serving as an indicator of the strength of footprints. The frequency distribution histograms of the peak force for the Ado-treated and control groups are shown in Fig. 6C, D, respectively. The peak force values within the Ado-treated group were found to primarily concentrate in the range of 0–3 nN, while the peak force values within the control group were confined to the 0–1.5 nN range, with 71% of the data concentrated in the range of 0–0.5 nN. Compared to the control group, the footprints secreted by the Ado-treated cyprids show a significant increase in peak force (p < 0.05 by nonparametric tests), indicative of enhanced mechanical strength. The increased peak force indicates a more robust and more stable presence of sacrificial bonds or intermolecular interactions within the Ado-treated footprints35, which potentially contributes to enhanced adhesion. The successful surface exploration and settlement of barnacle cyprids in the intertidal zone greatly depend on their ability to withstand shear forces from wave and tidal movements. Our study demonstrates that footprints secreted by Ado-treated cyprid exhibit significantly enhanced mechanical properties, including improved self-healing ability, elasticity, strength, and adhesive properties, which may provide cyprids with an advantage in tolerating high shear forces from waves and other environmental stressors.
Differences in gene expression induced by Ado
To better understand the molecular mechanisms underlying Ado induction in cyprids, the RNA-seq analysis on both the Ado-treated and control groups was conducted. In total, 1898 differentially expressed genes (DEGs) were identified in the Ado treatments, including 1299 (68.4%) upregulated genes and 599 down-regulated genes (Supplementary Data 2). These DEGs between the two groups were enriched in 14 Gene Ontology terms, including structural constituent of cuticle, heme binding, serine-type endopeptidase activity, and chitin binding (Fig. 7A). Notably, the structural constituent of cuticle term encompasses cuticle-related proteins such as cuticle protein, larval cuticle protein, and resilin, with 74 genes significantly upregulated (Fig. 7B). This suggests active expression of cuticle protein genes during the Ado-induced settlement process, which is essential for cuticle synthesis.
A GO terms enriched with differentially expressed genes between Ado treatment group and control group. B Volcano plots of differentially expressed genes in the structural constituent of cuticle. C KEGG enrichment of differentially expressed genes (DEGs) between Ado-treated and control groups of cyprids. D Volcano plot of DEGs enriched in the transcription factor pathway. E Volcano plot of DEGs enriched in the transporter pathway.
In the Kyoto Encyclopedia of Genes and Genomes pathway analysis (Fig. 7C), two transcription factor genes associated with cuticle formation—FTZ-F1 (nuclear hormone receptor fushi tarazu factor 1) and Hr39 (hormone receptor 39)—were identified as significantly upregulated in the Ado-treated cyprids (Fig. 7D). FTZ-F1 contains a conserved DNA-binding domain that interacts with gene promoters to regulate the expression of cuticle proteins36. Hr39 has been shown to be indispensable for the molting process in locusts, and its knockdown results in abnormal morphologies, including wing curling and delayed eclosion37. Both FTZ-F1 and Hr39 are involved in the 20-hydroxyecdysone (20E) signaling pathway, which regulates molting and metamorphosis in arthropods37,38. Previous study has proved the presence of 20E in barnacle cyprid extracts, and 20E is active in inducing cyprid settlement and metamorphosis39. Given the significant upregulation of these transcription factor genes, coupled with the active expression of cuticle protein genes, it is reasonable to hypothesize that Ado may trigger an early activation of the 20E signaling pathway, which could play a crucial role in inducing settlement.
Apart from the transcription factor pathways, the enriched transport pathway (K02000) also revealed significant findings, including a marked upregulation in the expression of organic cation transporters (OCTs) (Fig. 7E). OCTs are known to play a crucial role in facilitating the translocation of organic cations (such as 5-hydroxytryptamine (5-HT), histamine, dopamine, and norepinephrine) across the cell membrane40. Previous investigations have established the significance of 5-HT and histamine in inducing cyprid settlement41,42. Furthermore, dopamine and norepinephrine have been identified as stimulants that prompt the cement gland of the cyprid to secrete cement proteins43. Here, the increased gene expression level of OCTs in Ado-treated cyprid may help the transport of these organic cations, which in turn facilitate cyprid settlement.
Ado-induced expression of cyprid footprints and their structural insights
The analysis of the gene expression patterns of cyprid footprints was performed to obtain a deeper understanding of the effect of Ado on footprints. Notably, CP19K-like4 and CP100K genes exhibited pronounced expression, 17–430-fold higher compared to other cement protein genes in cyprids (Fig. 8A). After Ado induction, the expression of the CP19K-like4 and CP100K genes was significantly up-regulated (Fig. 8B). Given the intricate link between protein function and structure, AlphaFold2 was utilized to predict the protein structures of CP19K-like4 and CP100K (Supplementary Methods 3). As shown in Fig. 8C and Supplementary Fig. S4, CP19K-like4 exhibits a widespread presence of β-sheets, which are arranged in a reverse parallel manner. This cross-β structure is similar to the structure of A. amphitrite BSF protein with underwater adhesive capability26, and resembles adhesive proteins reported in polychaetes and sea cucumbers44,45, which can resist shear forces and give rise to force transitions46. CP19K-like4 is a homologous gene of CP19K, and it has been reported that recombinant CP19K can self-assemble into robust nanofibers that retain strong adhesion even in alkaline and high-salinity seawater, exhibiting remarkable resistance to harsh conditions47. Furthermore, the CP19K-like4 protein includes two consecutive CP19K-like domains10, potentially enabling CP19K-like4 with enhanced adhesion and resistance. Different from CP19K-like4, the structure of CP100K is characterized by a significant abundance of α-helices, which leads to a spherical conformation (Fig. 8C and Supplementary Fig. S5). CP100K contains a high content of hydrophobic amino acids (44.3%), as indicated by a positive GRAVY index (0.147) (Fig. 8D), indicating its hydrophobic nature, which may play a role in displacing interfacial water. Conversely, the GRAVY index of other cement proteins falls within the range of −1.504 to −0.061, indicating their hydrophilic properties, which may play a role in the replacement of the hydrated layer with the substrate surface and participate in the cross-linking with the substrate48. Moreover, CP100K contains a relatively high proportion of cationic amino acids (Arg and Lys, accounting for 10.9%) and aromatic amino acids (Phe and Tyr, accounting for 8.5%)49. These components may enhance the cohesion of the adhesion proteins through the formation of hydrophobic solid interactions and cation–π interactions50. Previous studies have reported the presence of CP19K-like4 in cyprid footprints15 and CP100K in cyprid cement glands12. Therefore, the observed improvements in adhesion, elasticity, and strength in Ado-treated cyprid footprints may be attributed to the significant upregulation of the CP19K-like4 and CP100K genes.
A Expression analysis of cement protein genes in A. amphitrite cyprid from the control group (n = 3). B Expression fold changes of CP19K-like4 and CP100K genes in Ado-induced cyprid compared to control groups (n = 3). C Ribbon drawings showing the three-dimensional structures of CP19K-like4 and CP100K proteins. D The predicted GRAVY index of cement proteins.
Methods
Barnacle collection and preparation of adult-conditioned seawater
A. amphitrite (=Balanus Amphitrite) individuals, each with an approximate base width of 1.2 cm, were collected from Xiamen Bay, China. To prepare barnacle ACS, individuals were subjected to scrubbing using a toothbrush to eliminate epifaunal organisms and subsequently rinsed with filtered (0.22 μm) artificial seawater (ASW)51. Subsequently, 20 individuals were placed in a 500 mL glass beaker containing 200 mL of ASW and kept at 26 °C for 12 h. The obtained ACS was then filtered through a 0.22 μm membrane prior to conducting the bioassays.
Cyprid culture and settlement assays
Barnacles were shade-dried for 24 h and subsequently immersed in seawater to procure nauplii. The nauplii were fed with Platymonas subcordiformis and cultivated into cyprids in 5 days. Cyprids were collected and preserved at 4 °C until use. To investigate the efficacy of A. amphitrite ACS in promoting cyprid settlement, we conducted experiments using 6-well plates, each containing 10 mL of the ACS solution and 30 cyprids. The bioassays were performed in triplicate, and ASW served as the control. After 12 h, the numbers of settled cyprids were enumerated using a stereomicroscope. According to the procedures described above, the two purines Ado, and Ino detected in the ACS (see “Results”) were also examined for their activity on larval settlement at concentrations of 0.1, 0.2, 0.5, and 1 μg/mL in ASW.
Liquid chromatography-mass spectrometry
To confirm the presence of purine compounds in A. amphitrite ACS, a LC-MS analysis was performed in accordance with the subsequently established protocol. The ACS underwent assessment utilizing a Q-Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific) in combination with an Ultimate 3000 HPLC system (Thermo Fisher Scientific). For the liquid chromatography step, 20 μL of the ACS was separated using a C18 SB-AQ column (250 mm × 4.6 mm, 5 μm) and eluted with a flow rate of 1 mL/min, employing 10 mM ammonium acetate (A phase) and methanol (B phase). The elution process commenced with 98% A phase for 10 min, followed by a gradient transition to 20% A phase over 20 min and an additional 10-min retention at 20% A phase. Throughout, the column temperature was held at 30 °C, and UV absorption was tracked at 254 nm. The presence of hypoxanthine, Ado, and Ino within the ACS was verified based on m/z values (Supplementary Data 1).
Ado treatment of cyprid and substrate preparation
For Ado treatment, a distinct group of cyprids was cultured in ASW supplemented with an additional Ado solution for 12 h at room temperature. The Ado solution, prepared by dissolving Ado in dimethyl sulfoxide (DMSO) at a concentration of 0.08 mg/mL, was added to attain a final Ado concentration of 0.2 μg/mL in the seawater.
The NH2-terminated glass Petri dishes were utilized to investigate the impact of Ado on cyprid footprints. The NH2-terminated surfaces were prepared as follows: firstly, the glass Petri dishes (35 mm diameter) were immersed in ethanol and subjected to ultrasonic cleaning for 10 min. The surfaces were then thoroughly rinsed with deionized water and dried using nitrogen gas. Subsequently, the Petri dishes were exposed to oxygen plasma at 120 W for 90 s to remove potential organic contamination and to create hydroxyl-rich surfaces. To further amino-functionalize the Petri dishes, 3-aminopropyl trimethoxysilane (APTMS) vapor deposition was employed. Specifically, the Petri dishes and a beaker containing APTMS solution (20 μL) were placed inside a desiccator (around 20 cm diameter). The desiccator was evacuated and then heated in a drying oven at 60 °C overnight. Subsequently, the desiccator was opened and further heated to 90 °C for 30 min.
Cyprid exploration
The exploratory behavior of cyprids was recorded to investigate the effect of Ado. Cyprids from both the Ado-treated group and the control group were placed in NH2-coated Petri dishes filled with seawater, and their exploration behaviors were recorded using an inverted optical microscope equipped with a digital video camera (Eclipse Ti2, Nikon, Japan). Individual frames extracted from the videos were analyzed using ImageJ software (V1.53a, National Institutes of Health, USA). The exploration frequency, defined as the number of temporary attachments within 10 s, was statistically quantified.
Characterization of footprints by AFM
All AFM experiments were conducted using a NanoWizard 4 instrument (JPK NanoWizard 4 XP, Bruker, Germany) equipped with an inverted microscope (Eclipse Ti2, Nikon, Japan). To obtain the nano-mechanical properties of the cyprid footprint in situ, an actively exploring cyprid was transferred to an amino-functionalized Petri dish installed on the AFM sample stage using a micropipette. The exploration behavior was observed under ×40 magnification. During cyprid surface exploration, regions with footprint deposits were marked on the screen, and then the cyprids were carefully removed. The explored surface was gently rinsed with fresh seawater to remove impurities, and the Petri dish was filled with additional seawater. Subsequently, the AFM scan head was assembled, and the silicon nitride cantilever (Scanasyst-fluid, Bruker, Germany) was positioned at the previously marked footprint areas. Every footprint was obtained from a different individual animal. The spring constant of the cantilevers was calibrated before and after the experiments using the thermal noise method, yielding values ranging from 0.6 to 0.8 N/m. To precisely locate the footprints, the footprint was initially imaged using the quantitative imaging mode in seawater. AFM-based force spectroscopy was employed to understand the nano-mechanical properties of the footprints. The cantilever was immersed in the footprints, and after a 2 s dwell time, the cantilever with attached footprint molecules was retracted from the surface. Moreover, to measure the adhesive force of the footprints with the surface, the NH2-functionalized probe (Nano World, PNP-TR-20) was utilized. The amino-functionalization of the probe was carried out following the procedure described previously for substrate preparation, and the spring constant of the cantilever was calibrated using the thermal noise method (0.1–0.3 N/m). Similarly, the footprints of Ado treatment cyprids were characterized in the same way.
Worm-like chain (WLC) model
The elasticity of the footprints was described by the WLC model, in which the extension length x and the stretching force F(x) satisfy the following equation52:
where lp is the persistence length of the molecule, L the contour length, kB the Boltzmann constant, and T the absolute temperature.
RNA-seq analysis
To investigate the molecular mechanism of Ado-induced cyprid settlement, we conducted a transcriptomic experiment. The samples utilized in this study represent a subset of those employed in our recently published article26. The assay and analytical procedures were conducted as previously described, with a brief overview of the steps provided below. The settlement assay was conducted in 24-well plates, with each well containing 1 mL of seawater and ~30 cyprids. The experiment for transcriptomic sequencing was carried out in cell culture plates with a diameter of 10 cm, each containing 30 mL of seawater and ~900 cyprids. After 16 h, we observed significant differences in the settlement rate between the Ado-induced group (0.2 µg/mL of Ado) and the control group (0.25% DMSO) in the 24-well plates. Subsequently, we collected ~200 unsettled cyprids from each cell culture plate, rinsed them with PBS, and then froze them in liquid nitrogen for further analysis.
Total RNAs were extracted using the Trizol method, and the quality of the RNA was assessed using a NanophotometerR spectrophotometer (Implen, Westlake Village, CA, USA) along with the RNA Nano 6000 Assay Kit on the Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA). Subsequently, high-quality RNA libraries were constructed and subjected to sequencing via the Illumina HiSeq NovaSeq 6000 system. The raw RNA-seq data were initially trimmed utilizing Trimmomatic v0.36 (with parameters SLIDINGWINDOW:5:20, MINLEN:50). The resultant high-quality dataset was then aligned to the reference genome using HISAT2 software v2.1.0. Estimation of transcript abundance was carried out using StringTie v1.3.4. DEGs were identified using the DESeq2 package, and the clusters and enrichments of these DEGs were analyzed using clusterProfiler v4.0.
Comparative quantitative PCR analysis
Total RNA was reverse-transcribed into first-strand cDNA using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara, RR047B). Four sets of specific primers were designed for realtime PCR analysis (β-actin: forward primer, 5′-GAAGATGACCCAGATCATGTTCGA-3′, reverse primer, 5′-TGGCGTGAGGCAGAGCGTA-3′; CP100K: forward primer, 5′-GACCCAACTACGAGACCAAT-3′, reverse primer, 5′-AGATGTGAAGACGGAAGGAC-3′; CP19K-like4: forward primer, 5′-TCACCCACCTTCAACAACTCG-3′, reverse primer, 5′-CACCTTCAGACCGTCCTTCG-3′; SIPC: forward primer, 5′- GACCAATCGTCGTCAAACC-3′, reverse primer, 5′- CGACTTAATGAGACCTCCCTC-3′). Primer's specificity was evaluated through melt-curve analysis, and the PCR products were further validated by sequencing (Supplementary Methods 2 and Supplementary Figs. S1–S3). The reaction mixture (20 μL) consisted of 1 μL of cDNA, 10 μL of 2× FastStart Universal SYBR Green Master (Rox) (Roche, 4913914001), 1 μL of each primer (10 μM), and 7 μL of nuclease-free water. Real-time PCR was performed using the QuantStudio™ 6 Flex Real-Time PCR System (Life Technologies, USA) with the following thermal cycling conditions: an initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s and 56 °C for 1 min. All reactions were performed in triplicate, and the Ct values were automatically calculated by the accompanying software. Relative gene expression levels were quantified using the 2−∆∆CT method, with β-actin serving as an internal control. Fold changes were calculated by dividing the mean expression level of the Ado-treated group by the mean expression level of the control group. Statistical significance was assessed using a two-tailed Student’s t test, with a threshold of p < 0.05.
Statistical analysis
Statistical comparisons were performed using SPSS software (V22.0, IBM, USA). Data of Ado treatment on the cyprid settlement, exploration frequency, and adhesion force were analyzed with an independent samples t-test. For all comparisons, p < 0.05 were considered as statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA-seq raw data of Ado-induced (SRX17497572, SRX17497573, SRX17497574) and DMSO-treated control (SRX17497559, SRX17497560, SRX17497561) cyprids were deposited in the SRA database under accession: PRJNA878556. LC-MC raw data is available in the Supplementary Data 1. Numerical source data for graphs are available in the Supplementary Data 3.
References
Callow, J. A. & Callow, M. E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2, 244 (2011).
Alsaab, A., Aldred, N. & Clare, A. S. Automated tracking and classification of the settlement behaviour of barnacle cyprids. J. R. Soc. Interface 14, 20160957 (2017).
Al-Yahya, H., Chen, H.-N., Chan, B. K., Kado, R. & Høeg, J. T. Morphology of cyprid attachment organs compared across disparate barnacle taxa: does it relate to habitat? Biol. Bull. 231, 120–129 (2016).
Phang, I. Y. et al. Atomic force microscopy of the morphology and mechanical behaviour of barnacle cyprid footprint proteins at the nanoscale. J. R. Soc. Interface 7, 285–296 (2009).
Phang, I. Y., Aldred, N., Clare, A. S., Callow, J. A. & Vancso, G. J. An in situ study of the nanomechanical properties of barnacle (Balanus amphitrite) cyprid cement using atomic force microscopy (AFM). Biofouling 22, 245–250 (2006).
Yuk, H. et al. Rapid and coagulation-independent haemostatic sealing by a paste inspired by barnacle glue. Nat. Biomed. Eng. 5, 1131–1142 (2021).
Kamino, K. et al. Barnacle cement proteins: importance of disulfide bonds in their insolubility. J. Biol. Chem. 275, 27360–27365 (2000).
Kamino, K., Odo, S. & Maruyama, T. Cement proteins of the acorn barnacle, Megabalanus rosa. Biol. Bull. 190, 403–409 (1996).
Lin, H. C., Wong, Y. H., Sung, C. H. & Chan, B. K. K. Histology and transcriptomic analyses of barnacles with different base materials and habitats shed lights on the duplication and chemical diversification of barnacle cement proteins. BMC Genom. 22, 783 (2021).
So, C. R. et al. Sequence basis of barnacle cement nanostructure is defined by proteins with silk homology. Sci. Rep. 6, 36219 (2016).
So, C. R. et al. Molecular recognition of structures is key in the polymerization of patterned barnacle adhesive sequences. ACS Nano 13, 5172–5183 (2019).
He, L. S., Zhang, G., Wang, Y., Yan, G. Y. & Qian, P. Y. Toward understanding barnacle cementing by characterization of one cement protein-100kDa in Amphibalanus amphitrite. Biochem. Biophys. Res. Commun. 495, 969–975 (2018).
Dominguez-Perez, D. et al. The quantitative proteome of the cement and adhesive gland of the pedunculate barnacle, Pollicipes pollicipes. Int. J. Mol. Sci. 21, 2524 (2020).
He, L. S., Zhang, G. & Qian, P. Y. Characterization of two 20kDa-cement protein (cp20k) homologues in Amphibalanus amphitrite. PLoS ONE 8, e64130 (2013).
Raine, J. Investigations of the Temporary Adhesive of Acorn Barnacle Cypris Larvae (Newcastle University, 2021).
Guo, S. et al. Barnacle larvae exploring surfaces with variable hydrophilicity: influence of morphology and adhesion of “footprint” proteins by AFM. ACS Appl. Mater. Interfaces 6, 13667–13676 (2014).
Phang, I. Y. et al. Marine biofouling field tests, settlement assay and footprint micromorphology of cyprid larvae of Balanus amphitrite on model surfaces. Biofouling 25, 139–147 (2009).
Yap, F.-C., Chen, H.-N. & Chan, B. K. Host specificity and adaptive evolution in settlement behaviour of coral-associated barnacle larvae (Cirripedia: Pyrgomatidae). Sci. Rep. 13, 9668 (2023).
Yap, F. C., Høeg, J. T. & Chan, B. K. Living on fire: deactivating fire coral polyps for larval settlement and symbiosis in the fire coral-associated barnacle Wanella milleporae (Thoracicalcarea: Wanellinae). Ecol. Evol. 12, e9057 (2022).
Knight-Jones, E. W. & Crisp, D. J. Gregariousness in barnacles in relation to the fouling of ships and to anti-fouling research. Nature 171, 1109–1110 (1953).
Petrone, L. et al. Chemistry-specific surface adsorption of the barnacle settlement-inducing protein complex. Interface Focus 5, 20140047 (2015).
Kotsiri, M. et al. Should I stay or should I go? The settlement-inducing protein complex guides barnacle settlement decisions. J. Exp. Biol. 221, jeb185348 (2018).
Abramova, A., Lind, U., Blomberg, A. & Rosenblad, M. A. The complex barnacle perfume: identification of waterborne pheromone homologues in Balanus improvisus and their differential expression during settlement. Biofouling 35, 416–428 (2019).
Wu, Z. et al. Impacts of ocean acidification and warming on the release and activity of the barnacle waterborne settlement pheromone, adenosine. Mar. Pollut. Bull. 199, 115971 (2024).
Chen, H.-N., Tsang, L. M., Chong, V. C. & Chan, B. K. Worldwide genetic differentiation in the common fouling barnacle, Amphibalanus amphitrite. Biofouling 30, 1067–1078 (2014).
Han, Z. et al. New genes helped acorn barnacles adapt to a sessile lifestyle. Nat. Genet. 56, 970–981 (2024).
Elbourne, P. D. & Clare, A. S. Ecological relevance of a conspecific, waterborne settlement cue in Balanus amphitrite (Cirripedia). J. Exp. Mar. Biol. Ecol. 392, 99–106 (2010).
Elbourne, P. D., Veater, R. A. & Clare, A. S. Interaction of conspecific cues in Balanus amphitrite Darwin (Cirripedia) settlement assays: continued argument for the single-larva assay. Biofouling 24, 87–96 (2008).
Elbourne, P. D. Ecological Role of an Adult-derived, Waterborne Cue in Cyprid Settlement in the barnacle Balanus amphitrite Darwin (University of Newcastle Upon Tyne, 2008).
Phang, I. Y., Aldred, N., Clare, A. S. & Vancso, G. J. Towards a nanomechanical basis for temporary adhesion in barnacle cyprids (Semibalanus balanoides). J. R. Soc. Interface 5, 397–402 (2008).
Maruzzo, D., Conlan, S., Aldred, N., Clare, A. S. & Hoeg, J. T. Video observation of surface exploration in cyprids of Balanus amphitrite: the movements of antennular sensory setae. Biofouling 27, 225–239 (2011).
Thompson, J. B. et al. Bone indentation recovery time correlates with bond reforming time. Nature 414, 773–776 (2001).
Bouchiat, C. et al. Estimating the persistence length of a worm-like chain molecule from force-extension measurements. Biophys. J. 76, 409–413 (1999).
Ducrot, E., Chen, Y., Bulters, M., Sijbesma, R. P. & Creton, C. Toughening elastomers with sacrificial bonds and watching them break. Science 344, 186–189 (2014).
Elbanna, A. E. & Carlson, J. M. Dynamics of polymer molecules with sacrificial bond and hidden length systems: towards a physically-based mesoscopic constitutive law. PLoS ONE 8, e56118 (2013).
Wang, H.-B., Nita, M., Iwanaga, M. & Kawasaki, H. βFTZ-F1 and Broad-Complex positively regulate the transcription of the wing cuticle protein gene, BMWCP5, in wing discs of Bombyx mori. Insect Biochem. Mol. Biol. 39, 624–633 (2009).
Zhao, X. M. et al. Nuclear receptor hormone receptor 39 is required for locust moulting by regulating the chitinase and carboxypeptidase genes. Insect Mol. Biol. 28, 537–549 (2019).
Li, K. L. et al. Role of nuclear receptors NlHR3 and NlFTZ-F1 in regulating molting and reproduction in Nilaparvata lugens (stal). Front. Physiol. 14, 1123583 (2023).
Yamamoto, H., Kawaii, S., Yoshimura, E., Tachibana, A. & Fusetani, N. 20-hydroxyecdysone regulates larval metamorphosis of the barnacle, Balanus amphitrite. Zool. Sci. 14, 887–892 (1997).
Selo, M. A., Sake, J. A., Ehrhardt, C. & Salomon, J. J. Organic cation transporters in the lung-current and emerging (patho) physiological and pharmacological concepts. Int. J. Mol. Sci. 21, 9168 (2020).
Jiang, Z. X., Ping, S. S., Jin, C. L., Tu, C. D. & Zhou, X. J. Transcriptome analysis provides insights into a molecular mechanism of histamine response in the cyprid larvae of Amphibalanus amphitrite. Mar. Ecol. Prog. Ser. 681, 1–12 (2022).
Yamamoto, H., Tachibana, A., Kawaii, S., Matsumura, K. & Fusetani, N. Serotonin involvement in larval settlement of the barnacle, Balanus amphitrite. J. Exp. Zool. 275, 339–345 (1996).
Gohad, N. V. et al. Observations on the settlement and cementation of barnacle (Balanus amphitrite) cyprid larvae after artificial exposure to noradrenaline and the locations of adrenergic-like receptors. J. Exp. Mar. Biol. Ecol. 416, 153–161 (2012).
Chen, T. et al. The Holothuria leucospilota genome elucidates sacrificial organ expulsion and bioadhesive trap enriched with amyloid-patterned proteins. Proc. Natl. Acad. Sci. USA 120, e2213512120 (2023).
Hennebert, E., Maldonado, B., Ladurner, P., Flammang, P. & Santos, R. Experimental strategies for the identification and characterization of adhesive proteins in animals: a review. Interface Focus 5, 20140064 (2015).
Sullan, R. M. A. et al. Nanoscale structures and mechanics of barnacle cement. Biofouling 25, 263–275 (2009).
Liang, C. et al. Self-assembled nanofibers for strong underwater adhesion: the trick of barnacles. ACS Appl. Mater. Interfaces 10, 25017–25025 (2018).
Liang, C. et al. Biochemistry of barnacle adhesion: an updated review. Front. Mar. Sci. 6, 565 (2019).
Gan, K. S. et al. Adhesive materials inspired by barnacle underwater adhesion: biological principles and biomimetic designs. Front. Bioeng. Biotechnol. 10, 870445 (2022).
Fan, H. L., Wang, J. H. & Gong, J. P. Barnacle cement proteins-inspired tough hydrogels with robust, long-lasting, and repeatable underwater adhesion. Adv. Funct. Mater. 31, 2009334 (2021).
Berges, J. A., Franklin, D. J. & Harrison, P. J. Evolution of an artificial seawater medium: improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 37, 1138–1145 (2001).
Kellermayer, M. S. Z. Visualizing and manipulating individual protein molecules. Physiol. Meas. 26, R119–R153 (2005).
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant 52071332, U2133213, 42376090 and 42216704, the Department of Science and Technology of Guangdong Province under Grant 2019QN01H430 and Grant 2019TQ05Z654, Fujian Provincial Natural Science Foundation of China under grant 2024J010003, the Project of the High-quality Development of Marine and Fishery Industry of Fujian under grant FJHYF-L-2023-14, the Guangdong Basic and Applied Basic Research Foundation under grant 2023B1515120090, the Natural Science Foundation of Guangdong Province under grant 2023B1515040008, the Science and Technology Innovation Commission of Shenzhen under Grant JCYJ20180507182239617 and Grant ZDSYS20190902093209795.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.G. and D.F. Methodology: X.X., Z.W., Y.H.W. and X.J.L. Investigation: X.X., Z.W. and Z.H. Supervision: S.G. and D.F. Manuscript preparation: X.X. and Z.W. Manuscript review: All authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Skarlatos Dedos and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Michele Repetto.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, X., Wang, Z., Wong, Y.H. et al. Exogenous adenosine promotes barnacle (Amphibalanus amphitrite) cyprid settlement through molecular signaling and improved adhesive mechanics. Commun Biol 8, 1296 (2025). https://doi.org/10.1038/s42003-025-08558-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08558-y