Abstract
Effective interpersonal interactions necessitate constant monitoring of others’ actions, a process known as interpersonal performance monitoring, which is influenced by the dopaminergic system and characterized by specific electrocortical signatures. To examine how this process is affected in Parkinson’s Disease (PD), we assess patients with PD performing coordination tasks with a virtual partner (VP) under two conditions: on dopaminergic medication (PD ON) and after withdrawal (PD OFF). During Interactive trials, which require adaptation to the VP’s actions, PD OFF performance is impaired compared to PD ON. Electroencephalography (EEG) analysis reveals in PD OFF increased midfrontal Delta-Theta activity during Interactive trials. Multivariate EEG analysis distinguishes Interactive from Cued trials, especially in PD OFF. Our findings highlight the role of dopamine in modulating electrocortical markers of interpersonal performance monitoring, with significant implications for understanding and treating PD.
Similar content being viewed by others
Introduction
The gradual degeneration of dopaminergic neurons in the substantia nigra pars compacta, a hallmark of Parkinson’s disease (PD), affects the functioning of subcortical and cortical areas, especially frontal and cingulate regions1,2. This cascade of changes leads to motor symptoms and impairments in higher-order cognitive functions3,4. A prominent cognitive and motor feature in PD is inflexible behavior, marked by difficulties in adapting to external requirements when necessary5. Behavioral flexibility is essential for effectively coordinating actions with a partner during everyday interactions and purposeful joint actions, demanding the capacity to predict and adaptively respond to the actions of others6. Indeed, interpersonal interactions can go wrong in many ways, from communication breakdowns to conflicts and lack of reciprocity, underscoring the critical need for continuous monitoring of others’ behavior, referred to as interpersonal performance monitoring7. Examining unexpected events during joint actions thus offers valuable insights into how the performance monitoring system allocates resources to oversee others’ actions. Behavioral studies reveal that processes resembling those engaged during self-generated errors also come into play when witnessing another person’s mistakes and when interacting with others in response to their errors8. Furthermore, electrocortical signatures associated with error processing (i.e., increase in midfrontal theta power, the error-related negativity-ERN, the early and late error positivity, Pe) reflecting the neurocomputational processing of the monitoring system during individual motor tasks9,10,11,12,13,14 are also elicited when interacting with a partner in response to their errors or in conditions necessitating continuous monitoring and adaptation to their actions7,15,16. Moreover, studies using non-invasive brain stimulation have shown that facilitating the activity of midfrontal cortex or the connected network, results in better performance in interpersonal motor interactions17,18. Neurophysiological evidence highlights the crucial role of the anterior cingulate cortex (ACC) in generating midfrontal theta and error-related negativity (ERN) during performance monitoring19,20. The early Pe, instead, is suggested to originate from the anterior regions of the ACC21, and has been associated to attentional reorientation22, while the late Pe may originate from the insular cortices, signaling error awareness23.
Among these electrocortical markers associated with the activity of the performance monitoring system, ERN and midfrontal theta oscillations are proposed to be influenced by dopaminergic activity24. Notably, studies have demonstrated that the midfrontal cortex receives dense dopaminergic projections from the ventral tegmental area (VTA)25. However, the impact of dopamine on modulating interpersonal performance monitoring system’s activity through neuronal synchronization remains understudied. To address this gap, examining patients with PD under various pharmacological conditions, such as during dopaminergic medication (PD ON) and after drug withdrawal (PD OFF), allows for a direct exploration of the dopaminergic system’s contribution to interpersonal performance monitoring. A recent study revealed that patients with PD in ON exhibited the expected increase in midfrontal theta power following the observation of erroneous actions, while patients with PD in OFF did not. These findings imply that the depletion of dopamine has a significant impact on this neurophysiological marker associated with performance monitoring26.
The role of dopamine in modulating the performance monitoring system’s activity during interpersonal motor interactions remains less explored. One behavioral study27 has shown that patients with PD in OFF manifest difficulties in coordinating their actions with a virtual partner, in conditions requiring continuous monitoring adaptation to its actions. Interestingly, this ability is maintained when patients take dopaminergic medications. Nevertheless, the connection between these observed difficulties and potential alterations in the neurophysiological markers associated with interpersonal performance monitoring remains uninvestigated.
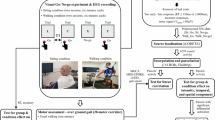
In addressing this research gap, the current investigation involved the examination of patients with PD both in the ON and OFF condition, alongside a group of healthy controls (HCs). Electroencephalography (EEG) was recorded while participants were tasked with grasping a bottle-shaped object in synchrony with a virtual partner (Fig. 1)28,29,30,31,32,33,34,35,36. This task included two distinct conditions: (i) a Cued condition, where participants had advance knowledge of their designated grasp location, and (ii) an Interactive condition, requiring participants to coordinate their action according to the virtual partner’s movement by either imitating or complementing its movement. In the Imitative trials, both the participant and the virtual partner grasped the same portion of the object (e.g., both grasped the upper part). In the Complementary trials, the participant was required to grasp the opposite part of the object compared to the virtual partner (e.g., if the virtual partner grasped the upper part, the participant grasped the lower part, and vice versa). This condition thus demanded continuous monitoring and adaptation to the virtual partner’s actions. Moreover, we included a correction factor, involving sudden changes in the virtual partner’s actions (VP’s correction), prompting participants to flexibly adapt to the movements of their virtual partner in the Interactive condition. Our hypotheses build upon prior research and anticipates replicating findings that suggest patients with PD in OFF encounter challenges in coordinating with a virtual partner, particularly in the Interactive compared to the Cued condition27. Furthermore, we hypothesize that these coordination difficulties may be paralleled by changes in electrocortical markers associated with interpersonal performance monitoring and adaptation, particularly midfrontal theta oscillations, which have been previously shown to be modulated by dopaminergic contributions26. We complement classical univariate analyses (focusing on electrocortical markers of interpersonal performance monitoring, such as the ERN, Pe, and midfrontal Delta-Theta power, as described by Moreau et al.15 and Pesci et al.37) with multivariate pattern analysis (MVPA) to describe whether different interactive conditions are characterized by different cortical processing patterns as a function of dopaminergic impairments. Specifically, we aim to: (i) determine if and when a classifier can distinguish between the Interactive and Cued conditions across the three groups based on whole-brain EEG data; (ii) assess whether the classifier accuracy varies between PD ON, PD OFF, and HCs; and (iii) evaluate whether the classifier can differentiate between the Interactive and Cued conditions before the VP’s Correction, especially in the PD OFF condition, suggesting a modulation of proactive cognitive control (i.e., the anticipation of cognitively demanding events, such as the possible VP’s Correction, before they occur) in patients with PD.
Results showed that patients with PD in OFF exhibited worse behavioral performance in the Interactive condition compared to patients with PD in ON. Univariate EEG analyses revealed higher midfrontal Delta-Theta activity in the Interactive condition in PD OFF compared to PD ON. Finally, the multivariate classification analysis demonstrated that a classifier could distinguish between Interactive and Cued trials based on whole-brain EEG patterns before the VP’s correction, with superior performance observed in the PD OFF condition. These findings: (i) underscore the distinct involvement of neural networks involved in interpersonal performance monitoring during Interactive compared to Cued trials; (ii) highlight the modulation of neural correlates associated with interpersonal performance monitoring by the dopaminergic system; and (iii) support the notion that proactive cognitive control is impaired in patients with PD38.
Results
Behavioral performance in interpersonal motor interactions is modulated by dopamine depletion
To investigate the role of the dopaminergic system during interpersonal motor interactions, we analyzed the performance of patients with PD both in the ON and OFF condition, as well as the performance of a group of HCs, in a highly-ecological and well-validated Joint-Grasping task. We used Grasping Asynchrony, i.e., the absolute time delay between the participant’s and the virtual partner’s touch-time on a bottle-shaped object (Fig. 1), to measure the success of the interaction. In a second analysis, this dependent measure was corrected by subtracting the performance in the control condition (i.e., Cued condition), which does not rely on interpersonal performance monitoring, from the performance in the Interactive condition (i.e., Grasping Asynchrony in Interactive condition minus Grasping Asynchrony in Cued condition). For control analyses on participants’ Movement Time and Reaction Times please refer to Supplementary Tables 12–15.
Behavioral (i.e., Grasping Asynchrony) results are plotted in Fig. 2. In detail, the 3 Condition (HCs/PD ON/PD OFF) × 2 Interactivity (Interactive/Cued) × 2 Correction (Correction/NoCorrection) linear mixed model on Grasping Asynchrony (marginal R2m = 0.21 and a conditional R2c = 0.63) showed a significant main effect of the Condition factor [F(2, 53.86) = 34.79, p < 0.001, bootstrap p < 0.001], with patients performing significantly worse (p < 0.0001) in the motor interaction task when they were in the OFF (M = 364.45 ms, SD = 385.14) than in the ON medication condition (M = 328.94 ms, SD = 274.84). Importantly, no significant difference emerged between patients in ON medications condition and HCs (M = 266.82 ms, SD = 223.63) (p = 0.51) nor between patients in OFF medications condition and HCs (p = 0.21). Moreover, a significant interaction (Condition x Interactivity) [F(2, 54.1) = 47.56, p < 0.001, bootstrap p < 0.001] was found (Fig. 2A). Post-hoc tests showed that in all groups performance was significantly worse in Interactive compared to Cued conditions (all ps < 0.01). Moreover, patients performed significantly worse in the Interactive task when they were in the OFF (M = 518.3 ms, SD = 295.42) than in the ON medication condition (M = 443.24 ms, SD = 295.42) (p < 0.001). No other comparison was significant (all ps > 0.25). The Condition factor did not significantly interact with any other factor. See Supplementary Tables 4–6 for all the significant results of the linear mixed model on Grasping Asynchrony and related post-hocs.
A Only in the Interactive condition patients performed significantly worse (p < 0.0001) in the motor interaction task when they were in the OFF than in the ON medication condition. No other comparison resulted as significant (all ps > 0.25). The upper asterisk highlights that, in all groups, participants’ performance was worse in the Interactive compared to the Cued condition (all ps < 0.01—Supplementary Tables 4–5). B Analysis performed on the Interactive—Cued index. Patients performed significantly worse in the motor interaction task when they were in the OFF than in the ON medication condition (p < 0.0001) and also compared to HCs (p = 0.03). Each point represents one single trial; the central line in each boxplot represents the median; box limits represent upper and lower quartiles; dark bold points represent single participant mean scores. N PD OFF = 15. N PD ON = 15. N HCs = 15.
A second analysis was run on the Interactive minus Cued condition Grasping Asynchrony index (Fig. 2B). The 3 Condition (HCs/PD ON/PD OFF) × 2 Correction (Correction/NoCorrection) linear mixed model on Grasping Asynchrony (marginal R2m = 0.09 and a conditional R2c = 0.56) showed a significant main effect of the Condition factor [F(2, 53.82) = 88.36, p < 0.001, bootstrap p < 0.001], with patients performing significantly worse (p < 0.0001) in the motor interaction task when they were in the OFF (M = 303.26 ms, SD = 267.88) than in the ON medication condition (M = 224.57 ms, SD = 232.55) and also compared to HCs (p = 0.03) (M = 143.3 ms, SD = 203.45), while no significant difference emerged between patients in ON medications condition and HCs (p = 0.49).
Interpersonal performance monitoring oscillatory power, but not ERPs’ amplitude, increases in patients with PD when OFF medication
At the neurophysiological level, we measured different electrocortical markers of interpersonal performance monitoring, such as the Pe (in the time-domain), and the midfrontal Delta-Theta power (in the time-frequency domain), as described by Moreau and colleagues15.
Starting from the analysis on the amplitude of the Pe component, the 3 Condition (HCs/PD ON/PD OFF) × 2 Interactivity (Interactive/Cued) × 2 Correction (Correction/NoCorrection), linear mixed model on Pe (marginal R2m = 0.13 and a conditional R2c = 0.26) showed no significant main effect or interaction with the factor Condition (all ps > 0.08). See Supplementary Tables 10–11 for all the significant results of linear mixed model on Pe and related post-hocs.
All time-domain results are plotted in Fig. 3.
A Grand averages of the Pe component over FCz in all experimental conditions, divided by condition (HC/ PD ON/OFF). Data are time-locked to the correction of the Virtual Partner (or the equivalent frame when no correction occurred). Shaded areas around each trace represent SEM. B Topographies of the difference between the Pe component (from 250 to 400 ms after the VP’s correction) in the Correction compared to the NoCorrection conditions, divided between Interactive and Cued conditions as well as between groups. The scale represents the amplitude of the signal (µV) over time and electrodes. N PD OFF = 15, N PD ON = 15, N HCs = 15.
Focusing on the midfrontal oscillatory activity in the Delta-Theta band (2–7 Hz) typically related to action monitoring, we extracted, separately for each group and condition (3 × 2 × 2 factorial design), the single-trial ERD/S in the Delta-Theta (2–7 Hz) band in the 200–700 ms following the correction of the VP’s movement (or the equivalent frame in NoCorrection trials).
The 3 Condition (HCs/PD ON/PD OFF) × 2 Interactivity (Interactive/Cued) × 2 Correction (Correction/NoCorrection) linear mixed model on Delta-Theta ERD/S (marginal R2m = 0.05 and a conditional R2c = 0.10) showed a significant Condition × Interactivity interaction [F(2, 55.62) = 10.52. p < 0.001, bootstrap p < 0.001]. Post-hoc tests showed that in all groups Delta-Theta ERS was higher in Interactive compared to Cued conditions (all ps < 0.01). Moreover, Delta-Theta ERS was significantly higher in the Interactive condition when patients were in the OFF (M = 1.92 Hz, SD = 2.62) than in the ON medication condition (M = 1.56 Hz, SD = 2.42) (p < 0.001). No other comparison resulted as significant (all ps > 0.50). See Supplementary Tables 7–9 for all the significant results of the linear mixed model on Delta-Theta ERD/S and related post-hocs. Figure 4 represents the ERD/ERS for each condition (HCs, PD, ON and OFF) separately in the Interactive and in the Cued blocks.
Left columns: time-frequency representations for all the Interactive conditions (collapsing Correction and NoCorrection trials). Right columns: time-frequency representations for all the Cued conditions (collapsing Correction and NoCorrection trials). The upper and right inserts in next to each time-frequency plot highlight, respectively, power changes at 5 Hz (i.e., centered on Theta) throughout the entire epoch and power changes for each frequency at the time were the error-related Delta-Theta synchronization was at its peak (i.e., 300 ms). PD OFF = 15, PD ON = 15, HCs = 15.
As control analyses, we also run the same set of statistical comparisons on ERD/ERS values in the Alpha (8–13 Hz) and Beta (14–30 Hz) band. None of these analyses revealed significant main effects or interactions (see Supplementary Tables 16–18).
A second analysis was run on the Interactive minus Cued condition Delta-Theta ERD/S index. The 3 Condition (HCs/PD ON/PD OFF) × 2 Correction (Correction/NoCorrection) linear mixed model on Delta-Theta ERD/S (marginal R2m = 0.02 and a conditional R2c = 0.10) showed a significant main effect of the Condition factor [F(2, 54.82) = 15.95, p < 0.001. bootstrap p < 0.001], with higher Delta-Theta ERS (p < 0.0001) in the motor interaction task when patients were in the OFF (M = 1.10 Hz. SD = 2.67) than in the ON medication condition (M = 0.57 Hz. SD = 2.47), while no significant difference emerged between patients in ON medications condition and HCs (p = 0.1) (M = 0.78 Hz. SD = 2.71) and between patients in OFF medications condition and HCs (p = 0.61).
See Fig. 5 for a representation of the difference in power between Interactive and Cued trials for each group (for the respective topographies, see Figs. S3–5).
A Time-frequency representations showing a main effect of the condition factor. PD OFF patients showed a stronger difference between the frontocentral synchronization in Delta-Theta, when subtracting the Cued from the Interactive condition, compared to themselves in the ON condition. B As in Fig. 2, each point in the lower plot represents the difference in the single-trial induced power in each Interactive trial minus the mean induced power in Cued trials over FCz, in the Delta-Theta frequency band (2–7 Hz) in the 200–700 ms time window. The central line in each boxplot represents the median; boxplot’s inner extremes represent second and third quartile. and boxplot’s outer extremes represent first and fourth quartiles; dark bold points represent single participant mean scores. C Results of the analysis on the interaction between midfrontal Delta-Theta ERS and behavioral performance in each condition (Interactive vs Cued). The higher the midfrontal Delta-Theta synchronization, the better participants’ behavioral performance (i.e., the lower the Grasping Asynchrony) in the Interactive condition. Shaded areas represent 95% confidence intervals. PD OFF = 15, PD ON = 15, HCs = 15.
Midfrontal Delta-Theta ERS is associated with behavioral performance in the Interactive condition
To test the influence of participants’ midfrontal Delta-Theta synchronization on the ability to perform the Joint-Grasping task, we entered single-trial data of midfrontal Delta-Theta ERS as continuous predictor in a linear mixed model, while single-trial Grasping Asynchrony scores represented the dependent variable. The model also included as categorical predictors the Interactivity (Interactive, Cued) and Condition (PD ON/PD OFF/HCs) factors. Only significant main effects or interactions with the continuous predictor Delta-Theta synchronization are reported here.
The model showed a significant interaction between Interactivity and Delta-Theta ERS (F(1, 9492.8) = 8.32 p = 0.004). Simple slope analysis showed that only the slope of the Interactive condition was significantly different from zero as a function of Delta-Theta synchronization [LCI -4.56, UCI -0.62]. More specifically the higher midfrontal Delta-Theta ERS the lower the Grasping Asynchrony in the Interactive condition (that is the better participants’ performance) (Fig. 5C).
EEG decoding highlights differences in the neural patterns related to early stages of interpersonal monitoring dependent on dopamine depletion
A MVPA was conducted to determine whether and when neurophysiological indices associated with the Interactive condition could be distinguished from those linked to the Cued condition across different groups. This analysis aimed to investigate the role of dopamine in differentially influencing neural processing related to performance monitoring based on varying levels of task interactivity. This corresponds to assessing if and how a classifier (i.e., a LDA model) would be able to distinguish the single-trial EEG patterns related to the monitoring of the VP during the Interactive compared to the Cued task, in each experimental group. The results of these analyses are shown in Fig. 6. In the lower panel, the results from the classification are shown separately for each experimental group, highlighting that Interactive and Cued trials could be decoded significantly better than chance for each group. Specifically, for all groups, the LDA performance was significantly different than chance level as early as ~1500 ms before the VP correction, both when decoding Interactive and Cued trials (significant clusters time windows: HCs Interactive [−1.61 to 1 s], Cued [−1.56 to 1 s]; PD ON Interactive [−1.45 to 1 s], Cued [−0.84 to 1 s]; PD OFF Interactive [−1.59 to 1 s], Cued [−1.78 to 1 s]—as shown by the bold dotted lines at the top and bottom of the decoding curves in Fig. 6B). The classification pattern remained sustained over time and reached its maximum peak after 0 ms (i.e., when the correction occurs).
A MVPA results highlighting the performance of the LDA classifier (i.e., classifier’s output) in distinguishing trials in the Interactive compared to the Cued condition, for each experimental group. Dotted lines at the top indicate time-windows when the decoding performance for the Cued condition significantly differed between PD OFF and PD ON (light green line), and between PD OFF and HCs (purple line). The dotted line at the bottom indicates the time-window when the decoding performance for the Interactive condition significantly differed between PD OFF and PD ON (dark green line). B Decoding performance (i.e., classifier’s output) plotted separately for each group. Dotted lines at the top and bottom highlight time-windows with significantly (p < 0.05, cluster-corrected) better than chance classification. “Mean RT” = average subject’s movement start. Shaded lines represent the performance of the classifier for each subject. N PD OFF = 15, N PD ON = 15, N HCs = 15.
In order to compare the performance of the classifier across groups, i.e., the effectiveness of the linear combination of features that allow the classification, we performed, separately for Interactive and Cued trials decoding, (1) a dependent samples t-test across time between PD ON and PD OFF, and (2) two independent samples t-tests across time between PD ON and HCs, and PD OFF and HCs with statistical significance being assessed through non-parametric cluster-based permutations (n = 1000; Maris & Oostenveld39,). We found that the performance of the classifier in decoding Cued trials was significantly better when patients were OFF compared to ON medication, in a 2.4 s time window between −1.38 and 1 s around the correction of the VP (light green dotted line on top of Fig. 6A). as well as for the HCs group compared to the PD ON one, in a much shorter 0.30 s time window between −0.22 and 0.08 s around 0 (purple dotted line on top of Fig. 6A). Moreover, with regard to Interactive trials, the performance of the classifier was significantly better when patients were OFF compared to ON medication, in a 1.7 s time window between -0.70 and 1 s around VP’s correction (dark green dotted line at the bottom of Fig. 6A).
In order to test the weight of different electrodes contributing to classification performance, we ran a searchlight analysis across time and electrodes with the same parameters implemented for the classification across time points (i.e., LDA classifier, k-fold cross-validation), this time quantifying the classifier’s performance in terms of % accuracy. These results are plotted in Fig. 7 as topographies in eight time-windows of interest (from −1 to 1 s around the timepoint of the correction, i.e., 0, in steps of 250 ms), showing that the classifier relied on a distributed cluster of electrodes to decode Interactive vs Cued trials, with a fronto-central cluster being most prominent from ∼500 ms before the VP’s correction (or the equivalent frame in NoCorrection trials) in all groups. This approach complements the results from the univariate analysis and localizes the results from the binary classification over time (Fig. 7), providing further evidence for a modulation of the performance monitoring system by the dopaminergic system.
In all groups, fronto-central sites consistently contributed to the classifier’s performance from ∼500 ms before the VP’ correction (time 0). The two dots on each topography represent electrodes Cz and FCz. The scale represents the LDA accuracy over time and electrodes. N PD OFF = 15, N PD ON = 15, N HCs = 15.
Discussion
To explore the impact of dopamine on behavioral and electrocortical signatures of interpersonal performance monitoring, we conducted an experiment involving patients with PD in two conditions, i.e., while regularly receiving dopaminergic medication (PD ON), and after the withdrawal from medication (PD OFF), alongside a group of HCs. Participants were tasked with coordinating their actions with a virtual partner under conditions that either required (Interactive condition) or did not require (Cued condition) continuous monitoring of and adaptation to the partner’s actions.
Dopamine depletion worsens behavioral performance during interpersonal motor interactions
Behavioral results showed that, after controlling for patients’ motor and cognitive ability to perform the task in Cued (control) trials, PD OFF performed worse in the Interactive trials compared to themselves in the ON condition. In keeping with Era et al.27, this confirms the role of the dopaminergic system in supporting the ability to adapt one’s movements to those of an interaction partner, particularly in situations necessitating continuous monitoring of its actions. Indeed, PD OFF exhibited interpersonal coordination difficulty in contexts requiring flexibility in adapting to others’ movements. In the Cued condition, instead, participants were already instructed on which action to perform and likely dedicated fewer cognitive resources to monitoring the partner’s behavior. This result corroborates the notion that dopaminergic medication facilitates the ability of patients with PD to successfully coordinate with a virtual partner when predictions and monitoring of its actions are required27.
Univariate analyses unveil dopamine-modulated electrocortical markers of interpersonal performance monitoring in the time-frequency domain
Concerning the electrocortical markers associated with interpersonal performance monitoring, results revealed that only midfrontal Delta-Theta activity was influenced by dopamine levels. Indeed, higher midfrontal Delta-Theta activity was observed in PD OFF compared to PD ON, aligning with the proposed role of dopamine in performance monitoring and the regulation of social predictive processes40. Interestingly, however, PD OFF showed an hyperactivation of the performance monitoring system, highlighted by increased synchronization in the frontal Delta-Theta band during the Interactive trials, compared to PD ON patients. This is in contrast with what previously found in studies investigating the role of dopamine in modulating the activity of the performance monitoring system in individual/observational tasks, showing a reduced activity of the performance monitoring system in PD OFF26. Additionally, prior studies have consistently shown that PD patients, compared to HCs, display diminished midfrontal theta activity during tasks that require cognitive control41 (see Narayanan et al.42, for a review). Although not statistically significant in univariate analysis, the pattern of results in the Cued condition mirrored those found in prior studies26 (a study in which patients with PD had simply to observe a movement after a cue but did not have to actively adjust their own movement in order to anticipate other’s actions), showing decreased synchronization in the frontal Delta-Theta band in PD OFF compared to themselves in the ON condition and HCs. Thus, dopamine may influence the activity of the performance monitoring system differently depending on the context’s interactivity level. Specifically, the performance monitoring system is more active in PD OFF when there is a greater need to monitor and adapt to others’ actions (Interactive condition) compared to situations where such monitoring and adaptation are less necessary (Cued condition) or when passively observing a movement performed by a virtual arm observed from a first-person perspective26.
Higher Delta-Theta synchronization is associated with better performance in the Interactive condition, suggesting its potential compensation function in PD OFF
While the Interactive condition was associated with overall worse behavioral performance in PD OFF, when directly examining the relationship between single-trial midfrontal Delta-Theta synchronization and patients’ ability to perform the Joint-Grasping Task, the higher the midfrontal Delta-Theta synchronization the better the behavioral performance (i.e., lower Grasping Asynchrony). This finding aligns with recent evidence indicating that cognitive dysfunction in PD is closely related to diminished midfrontal Delta-Theta power43. Specifically, PD patients with cognitive impairments show reduced midfrontal Delta-Theta activity across a variety of cognitive control tasks, and these reductions significantly correlate with clinical measures of cognitive decline.
Functional Magnetic Resonance Imaging findings indicate that behavioral adjustments trigger activations in both the ACC and the lateral prefrontal cortex (LPFC) (Kerns et al.44). The ACC is thought to oversee individuals’ actions and, when needed, communicate with the LPFC to engage cognitive control mechanisms to improve subsequent performance44. Crucially, theta oscillations are implicated in facilitating neural communication between midfrontal and frontal areas during motor adaptation45. Reduced functional connectivity between the ACC and LPFC has been shown in patients with PD, in tasks tapping on performance monitoring abilities46. This reduced connectivity may explain the poorer behavioral performance of patients with PD (especially in the OFF condition) in the Interactive condition of the present study, which requires continuous monitoring and adaptation to others’ behavior. Conversely, higher midfrontal Delta-Theta synchronization in PD OFF patients may represent an attempt to compensate for disrupted communication between the ACC, involved in monitoring the partner’s actions during interpersonal motor interactions, and the frontal regions involved in behavioral adaptation.
Dopamine depletion does not modulate ERPs associated with interpersonal performance monitoring
The present results also revealed a detectable Pe recorded over fronto-central electrodes in all groups in Correction trials. Notably, the lack of a significant difference in the Pe response among groups aligns with the suggestion of an independent generation of this component from the dopaminergic system11. This evidence aligns with a previous study indicating that dopamine depletion in patients with PD is associated with a selective modulation of midfrontal theta activity in tasks relying on performance monitoring, while leaving the Pe component unaffected26. Conversely, we did not detect any late Pe over the parietal electrodes, which aligns with previous studies suggesting that in older adults, a component shift from posterior to anterior areas may occur26,47. Surprisingly, we did not detect any ERN, an error-related marker present in previous studies investigating interpersonal performance monitoring in young adults15,16. However, the ERN was absent also in previous studies investigating older populations with tasks tapping into the activity of the performance monitoring system26,48.
EEG decoding analyses further support dopamine role in differentiating the neural patterns related to interpersonal performance monitoring before the VP’s Correction
Finally. we complemented our univariate analyses with a multivariate approach, showing that a classifier (i.e., a LDA model) was able to distinguish between Interactive and Cued trials based on the EEG patterns in all three groups, as early as 1500 ms before the VP’s Correction or the corresponding time window in No-Correction trials. Moreover, the performance of the classifier was significantly better when relying on the neural patterns recorded in patients with PD in OFF compared to themselves in the ON condition, in both the Cued and Interactive conditions (Fig. 6A). Thus, the absence of medication leads to a significantly stronger differentiation of the neural correlates distinguishing interpersonal interactions requiring continuous monitoring and adaptation to others’ actions (i.e., Interactive) to conditions not tapping on these abilities (i.e., Cued). It is noteworthy that the classifier effectively distinguished between the Interactive and Cued conditions well before the VP’s Correction, with better performance observed in PD OFF compared to PD ON. This, coupled with the finding that patients with PD, particularly in the OFF condition, showed altered behavioral performance and EEG activity in the Interactive condition regardless of whether the virtual partner performed a movement Correction, supports the notion that proactive cognitive control is impaired in patients with PD38. In fact, proactive cognitive control, which involves anticipating and preventing cognitively demanding events (the possible VP’s Correction in this case) before they occur, is a common factor in both Interactive-Correction and Interactive-NoCorrection trials.
Finally, the classifier’s better performance in the PD OFF condition compared to the ON one for both Interactive and Cued trials further supports the idea that dopamine influences neural activity across various levels of interactivity.
Interestingly, the results of the searchlight classification highlighted the involvement of fronto-central sites in distinguishing Interactive and Cued trials. Notably, this complements the analyses of midfrontal Delta-Theta synchronization, where we focused on induced, non-phase locked, power (thus, without looking at the evoked, phase-locked, patterns used by the classifier). All in all, the MVPA results: (i) further support the different involvement of the neural network involved in interpersonal performance monitoring in the Interactive compared to the Cued trials; (ii) most importantly, they highlight the modulation of the neural correlates of interpersonal performance monitoring by the dopaminergic system; and (iii) by demonstrating that the classifier effectively distinguished between the Interactive and Cued conditions well before the VP’s Correction, with better performance in the PD OFF condition, they provide evidence that proactive cognitive control is impaired in patients with PD.
In conclusion, our study underscores the crucial role of the dopaminergic system in influencing both behavioral and electrophysiological responses during interpersonal performance monitoring. The findings identify electrocortical markers modulated by dopamine, associated with performance monitoring in interactive tasks. These insights may guide the development of clinical interventions combining non-invasive brain stimulation with interpersonal motor interactions, potentially enhancing neurophysiological markers of interpersonal performance monitoring and improving motor interaction abilities in patients with PD.
Limitations
While our study offers novel insights into the dopaminergic modulation of interpersonal performance monitoring in PD, several limitations should be considered. First, the relatively small sample size (n = 15 per group), although partially mitigated by the within-subject design that enhances statistical power, inevitably limits the generalizability of our findings. Second, the demographic composition of our sample was highly unbalanced, with the PD group consisting almost entirely of male participants (14 out of 15), which restricts the applicability of our results to the broader PD population, particularly to female patients. Future studies with more balanced samples will be crucial to explore potential sex-related differences in these effects. Additionally, while PD patients completed the task in two counterbalanced sessions (ON and OFF medication), healthy control participants were tested only once, thus implying patients acquired more task familiarity compared to controls. This unbalance was mitigated by the counterbalancing of the ON and OFF sessions in the patients group. Finally, despite our efforts to implement a rigorous washout protocol, including the tapering of long-acting medications and an 18-h interval before OFF sessions, it remains possible that long-duration effects of dopamine agonists persisted. The potential influence of these residual pharmacological effects on our findings should, therefore, be acknowledged.
Furthermore, while we interpret our findings within the framework of interpersonal performance monitoring, it is important to note that the Interactive and Cued conditions differ in other aspects. Notably, only the Interactive condition requires participants to predict and respond to a partner’s actions, whereas the Cued condition does not involve interpersonal prediction or motor adaptation. Nevertheless, we observed modulations in midfrontal Delta-Theta activity, an oscillatory marker previously associated with performance monitoring, specifically in the Interactive condition. This supports the interpretation that the observed behavioral and neural changes reflect dopamine-related modulation of interpersonal performance monitoring. Moreover, although the Interactive condition also involves behavioral adaptation, particularly in Correction trials, the behavioral and electrophysiological effects reported here emerged independently of such Correction. Therefore, we interpret them as being more closely related to ongoing performance monitoring rather than to post-correction adaptation. That said, we acknowledge that additional mechanisms, such as action prediction and dynamic social adjustment, may have contributed to the observed effects. Future studies will be essential to disentangle the specific contributions of these processes.
Methods
Participants
Sixteen patients affected by Parkinson’s Disease (i.e., PD) were involved in the study. The sample size was determined through a prospective power analysis performed with the software More Power (Campbell and Thompson49,). We inserted as expected effect size the partial eta squared value (.38) observed in Pezzetta et al.26, where the electrocortical signatures associated to the activity of the performance monitoring system were studied in patients with PD. The analysis indicated that a 2 × 2 × 2 within-between design, a power of 0.80 and a partial eta squared value of 0.38 (as is computed from Pezzetta et al.26) required a sample size of 16 participants.
All participants were right-handed, had normal or corrected-to-normal vision and were naive as to the purpose of the experiment. Patients with PD were recruited for the study according to the following inclusion criteria: (i) diagnosis of idiopathic PD (United Kingdom Parkinson’s Disease Society brain bank criteria.) (ii) absence of dementia (Mini Mental State Examination, MMSE above or equal to 25); (iii) absence of other neurological and psychiatric diseases; (iv) taking daily doses of L-Dopa or a dopamine agonist (L-Dopa equivalent doses are reported in Table 1, see Supplementary Table 2 for a specification on patients’ medication). One patient was excluded from the final sample, due to excessive muscular artifacts in the EEG, leading to the exclusion of >25% of the ICA components. The final sample included 15 patients (14 males, 1 female, group average age = 67.47 ± 7.48 years; group average years of education = 14 ± 3.87; group average MMSE = 29.4 ± 0.63). Beside being based on a prospective power analyses, the sample size is the same as in previous studies investigating performance monitoring in patients with PD (the EEG study by Pezzetta et al.26, n = 15 and the behavioral study by Era et al.27n = 15). The socio-demographic and clinical characteristics of patients who participated in the study are reported in Table 1. Moreover, 15 HCs were involved in the study as a control group (13 males, 2 females, group average age = 67.2 ± 5.75 years; group average years of education = 14.67 ± 3.29; group average MMSE = 28.9 ± 0.9). Age, years of education and MMSE scores did not differ between the PD and HCs groups (Age: t = 69, p = 0.49; education: t = 0, p = 1; and MMSE score: t = 0.60, p = 0.55, respectively), as checked by means of independent samples t-tests.
HCs were recruited for the study only if the following criteria were satisfied: (i) absence of neurological and/or psychiatric diseases, (ii) absence of psychological and/or cognitive disorders, (iii) absence of medications with psychotropic action and (iv) MMSE above or equal to 25.
Patients were tested in three different experimental sessions over three different days. During the first one, they completed a neuropsychological assessment under their daily dopaminergic treatment. After this first session, patients were tested in two experimental conditions (in two different days), always at the same time of the day, with ~15 days as an intersession interval. In the OFF Condition, patients had been in drug withdrawal for 18 h, and they were tested in the morning. More specifically, in the OFF Condition, long-lasting medications, such as dopamine agonists and extended-release Levodopa were tapered down over the 3 days preceding the experimental session. In the final 2 days, standard L-Dopa therapy was progressively reduced, first in dosage, then in frequency, until the final dose, which was administered at least 18 h prior to the OFF session. In the ON Condition they were examined 60 min after their first morning therapeutic dose of levodopa and/or dopamine agonists. The condition was counterbalanced across patients so that half of them performed the first experimental session in ON Condition and the other half in OFF. HCs performed the task in a single session, after which they were administered the MMSE. Moreover, to ascertain the efficacy of the dopaminergic medication in improving extrapyramidal motor symptoms, after each session, the patients were administered the MDS-UPDRS-Part III (Motor examination50—a 27-items scale where each item is evaluated on a 5-point Likert scale, ranging from 0 to 4, where higher scores indicating higher extrapyramidal motor symptoms) and the Hoehn and Yahr51 (this scale identifies eight illness stages, indicated with the following numbers: 0-1-1.5-2-2.5-3-4-5, in which higher scores indicate higher extrapyramidal motor symptoms) scales in both ON and OFF conditions (see Table 1). The experimental protocol was approved by the ethics committee of the Fondazione Santa Lucia (Prot. CE/PROG.939) and was carried out in accordance with the ethical standards of the 1964 Declaration of Helsinki and later amendments. Participants gave their written informed consent to take part in the study. All ethical regulations relevant to human research participants were followed. For indication about the Neuropsychological assessment performed see Supplementary Information and Supplementary Table 3.
Experimental stimuli and set-up
The stimuli of the motor interaction task were the same used in previous studies28,29,30,31,32,33,34,35,36,52,53. They consisted of ten grasping movements performed by a virtual partner (five precision and five power grips). In detail, 3DS Max 2011 (Autodesk. Inc.) was employed to design the virtual scenario, where a virtual partner (created in Maya 2011 (Autodesk. Inc.) using a customized Python script (Prof. Orvalho V., Instituto de Telecomunicacoes. Porto University) was inserted. The virtual partner was created starting from the kinematics of a real person’s arm (recorded via a SMART-D motion capture system [Bioengineering Technology & Systems {B|T|S}]) that was recorded while grasping the upper part of a bottle (precision grip) or the lower part (power grip) with his right hand. Importantly, the virtual partner was displayed from the shoulders down, without the neck and head, to prevent facial expressions from exerting unwanted influences. Precision and power grip clips lasted the same amount of time (∼2000 ms). At the beginning of each stimulus, the virtual partner was still, with its hand positioned on the table. A variable amount of time (i.e., between 200 and 500 ms) after participants released the Start button, the virtual partner started moving. The time of the virtual partner’s touch on the bottle was determined using a photodiode, which was positioned on the screen displaying the virtual partner. To precisely mark the moment of contact, a small white square was inserted into the lower right corner of the video frame in which the virtual partner’s hand made contact with the bottle. The photodiode detected the sudden change in screen luminance caused by the appearance of the white square, allowing us to register the precise on-screen time of the virtual partner’s touch. This method provides a more accurate and temporally reliable measure than relying on the pre-programmed video timeline and was used as the reference point to compute Grasping Asynchrony and EEG correction-locked responses.
Experimental task
Participants were requested to engage in a controlled motor interaction ‘Joint-Grasping task’ (Fig. 1), where participants’ goal can only be achieved by predicting and monitoring the virtual partner’s movements and, therefore, adapting to them in real-time in order to grasp an object in synchrony. In detail, participants sat at a rectangular table, with a bottle-shaped object located in front of them. The object could be reached on two different parts: its lower part performing a whole-hand grasp (power grip) and its upper part performing a thumb-index precision grip (Movement Type factor). The virtual partner was presented on a monitor positioned on the table, behind the bottle-shaped object (Fig. 1). Each trial started with the participant positioning their right hand over a start-button on the table, and their index finger and thumb touching each other. In different blocks, participants were required to: (i) monitor the movement of their partner online, in order to select whether to perform a precision grip or a power grasping based on the movement of the partner, as they were asked to perform an opposite or same action compared to that of their partner (Interactive condition) or (ii) follow an auditory instruction indicating whether to perform a precision grip or a power grasp, regardless of the type of movement of their partner (Cued condition). Thus, participants were instructed to ignore whether the virtual partner grasped the upper or lower part of the object and stick to the cue throughout the trial, even when the virtual partner’s movement differed from the cued response. In the Cued condition, the virtual partner performed a grasping movement in all trials, grasping the upper or lower part of the object in equal proportions. This resulted in trials where the participant and the virtual partner performed either the same movement (imitative) or different movements (complementary), though this congruency was incidental and not task-relevant, in the sense that participants were asked to ignore whether the partner aimed at the upper or lower part of the bottle. In both conditions, participants were instructed to grasp the bottle-shaped object as synchronously as possible with their virtual partner performing complementary or imitative actions with respect to it. In the imitative condition, participants grasped the same portion of the object as the virtual partner. In the complementary condition, instead, participants grasped the bottle-shaped object on different parts (e.g., the virtual partner grasped the lower part via power grip, and the participants grasped the upper part via precision grip, or vice versa). In order to promote the need to monitor the virtual partner’s actions in the entire experiment, in 36% of trials, the virtual partner performed a movement change by switching from a power grasp to a precision grip (or vice versa) during the reaching phase of the movement (Correction factor). In the Cued condition, participants were instructed to ignore this change and continue following the auditory cue; in contrast, in the Interactive condition, they had to adapt their action to the virtual partner’s final movement. In each session, participants performed two successive 64-trial Interactive and two successive 64-trial Cued blocks (in a counterbalanced order across participants), each comprising half power grasp and half precision grips. Thus, participants performed 10 NoCorrection and 6 Correction trials in each condition of the following 2 × 2 × 2 design: 2 (Interactive/Cued) × 2 (Complementary/Imitative) × 2 (Precision/Power grip). The total number of trials, per subject, was 256, and 48 Correction and 80 NoCorrection trials per subject, separately for the Interactive and for the Cued condition, were analyzed. The imitative and complementary conditions, along with the power and precision grip conditions, were included to enhance the variability of interactions that participants had to perform, thereby making the task more engaging. However, since no specific hypotheses were formulated regarding the role of these conditions in modulating behavioral or neural outcomes, they were not included as factors in the analyses. Stimuli presentation and randomization were controlled by E-Prime2 software (Psychology Software Tools Inc., Pittsburgh, PA). The experimental protocol never lasted more than 45 min to avoid exceeding the time window of the effect of the dopaminergic therapy.
The moment in which participants touched the bottle was recorded thanks to touch-sensitive markers placed on the target spots of the object at 15 and 23 cm of the total height of the object. The trial timeline was as follows: participants heard the imitative/complementary or up/down auditory instruction, and they were presented with a still virtual partner. After its appearance, they were allowed to release the start button, and, upon releasing it, the virtual partner started its reach-to-grasp movement. Thus, the beginning of the interaction and, particularly, of the movement of the virtual partner, was constrained to participants releasing the start button. This was done to overcome patients’ difficulty in initiating movements (present in patients with PD)54 and allow them to engage in a real-time interaction with their partner. Movements were always performed with the right hand. The intertrial interval depended on the time participants took to go back from the bottle to the starting button. The experimenter manually moved to the following trial as soon as participants went back to the starting position and pressed the start button.
Behavioral data
We excluded from the analyses the trials in which participants (i) missed the touch-sensitive markers, preventing from recording their responses, (ii) did not respect their Imitative/Complementary or Up/Down instructions, (iii) behavioral values that fell 2.5 SDs above or below each individual mean for each experimental condition (outlier trials) (on average, excluded trials of Grasping Asynchrony = 14.9 ± 10.2–5.47% of the total, specifically: 14 ± 10.4 for the PD ON group; 13.2 ± 8.5 for the PD OFF group; 17.6 ± 11.6 for the HCs group). We considered as main behavioural measure Grasping Asynchrony (GAsynchr), i.e., the absolute value of time delay between the participant’s and virtual partner’s touch-time on the bottle-shaped object. We run a first analysis on raw Grasping Asynchrony data as a dependent variable. In a second analysis, we subtracted from each individual’s single data point in the Interactive conditions the corresponding individual’s means in the Cued conditions (as in Era et al.27). This way, we indexed the participants’ ability to perform the motor interaction task (requiring predicting and monitoring the virtual partner’s actions), net of their baseline ability in performing precision and power grasping actions as measured in the Cued condition (not requiring predicting and monitoring the virtual partner’s actions). Detailed analyses of reaction times and movement times are provided in the Supplementary materials.
EEG recording and preprocessing
EEG signals were recorded and amplified using a Neuroscan SynAmps RT amplifiers system (Compumedics Limited. Melbourne. Australia). These signals were acquired from 58 tin scalp electrodes embedded in a fabric cap (Electro-Cap International, Eaton, OH), arranged according to the 10–20 system. The EEG was recorded from the following channels: Fp1, Fpz, Fp2, AF3, AF4, F7, F5, F3, F1, Fz, F2, F4, F6, F8, FC5, FC3, FC1, FCz, FC2, FC4, FC6, T7, C5, C3, C1, Cz, C2, C4, C6, T8, TP7, CP5, CP3, CP1, CPz, CP2, CP4, CP6, TP8, P7, P5, P3, P1, Pz, P2, P4, P6, P8, PO7, PO3, AF7, POz, AF8, PO4, PO8, O1, Oz and O2. Horizontal electro-oculogram (HEOG) was recorded bipolarly from electrodes placed on the outer catchi of each eye and signals from the left earlobe were also recorded. All electrodes were physically referenced to an electrode placed on the right earlobe and were algebraically re-referenced off-line to the average of both earlobe electrodes. Impedance was kept below 5 KΩ for all electrodes for the whole duration of the experiment, amplifier hardware band-pass filter was 0.1–200 Hz and sampling rate was 1000 Hz. Offline, we applied a high pass filter to 0.1 and a low pass filter to 48 in order to remove slow drifts and line noise (50 Hz) from the signal. The two filters were applied separately as suggested by the latest version of EEGLAB55. Then, channels exhibiting excessive amounts of noise were detected by running the clean_rawdata function as implemented in EEGLAB55, by removing the channels which trace correlated with their direct neighbors for less than 80% of the time. After removing the above mentioned channels, we epoched the continuous data into segments of 10.000 ms—from −5.000 to 5.000 around the beginning of each trial. In order to identify and remove from the signal ocular artefacts (eye blinks and saccades) and excessive muscular noise, an Independent Component Analysis (ICA)56 was performed on the epoched data. Artefactual components were identified based on their time course and topography and removed. On average, 9 (±2.74) components were removed per recording session.
After the ICA, the bad electrodes previously removed were interpolated, with their signal being reconstructed based on the one from their surrounding neighbors with a “weighted” approach, as implemented in Fieldtrip57. Finally, EEG data were re-referenced to the average of all channels, and epochs containing remaining artefacts such as electrode jumps were removed following visual inspection (on average 14 trials (±2.2) for the HCs, 27 trials (±5.2) for the PD ON and 15 trials (±2.5) for the PD OFF were excluded).
EEG analysis
Univariate analysis
We analyzed the event-related potentials (ERPs) over fronto-central electrodes (i.e., FCz) time-locked to the correction of the VP (or the equivalent time frame in the No-Correction trials), as well as the activity in the time-frequency domain focused on midfrontal Delta-Theta.
ERPs
EEG time- series were high-pass filtered at 1 Hz to remove slow drifts from the data, hence reducing the contribution of slow potentials (in previous works using the same paradigm it was checked that grand average waveforms with different filters (i.e., 0.5 or 1 Hz) maintain the same morphology, without introducing distortions, see Moreau et al.15. Since we found no ERN in response to the VP’s correction in any condition (see “Discussion”), only the Pe component was further analyzed, being quantified as the mean amplitude in the time window between 200 and 450 ms over electrode FCz. No other components (including any trace of the late Pe over parietal sites—see Fig. S6) were found.
ERD/S
After preprocessing, as in the time-lock analysis, we high-pass filtered the segmented EEG signal at 1 Hz. Moreover, to remove the effects of ERPs in the time-frequency domain we subtracted the individual mean evoked response from each single trial, thus removing phase-locked activity58,59. Each epoch was then put in the frequency domain using a Hanning-tapered window60 with a 50 ms time resolution, obtaining single-trial power representations for frequencies in a range from 1 to 40 Hz in steps of 0.25. For visualization, the grand averages across subjects were displayed as event-related desynchronization/synchronization (ERD/ERS) with respect to a baseline period ranging from −0.2 to 0 ms before the correction occurred.
To statistically compare midfrontal Delta-Theta across experimental factors, after visually inspecting the time-frequency plots we identified a coherent pattern of activity spanning from Delta (2–4 Hz) to Theta (4–7 Hz) (Figs. 4–6). This was in line with previous studies on action monitoring in healthy subjects15 and in PD patients26. We also strengthen our choice by running a control analysis correlating the results in the Delta and Theta band (extracted separately) and running a t-test between conditions correcting for multiple comparisons with non-parametric permutation tests. Please refer to the Supplementary Materials (Figs. S1–2). Thus, we extracted the ERD/ERS for the Delta-Theta band (2–7 Hz) between 200 and 700 ms after the correction (or the corresponding frame in the NoCorrection trials) and analyzed the modulation of power over FCz (as in Moreau et al.15). The obtained single-trial induced power was then analyzed through Linear Mixed Models as for the behavioral measures.
Finally, we computed a difference in power, for each group, between single trials in the Interactive condition and average Cued trials. As a control, we also run all statistical analysis on the ERD/ERS for the Alpha (8–13 Hz) and Beta (14–30 Hz) band (see Supplementary Materials and Supplementary Tables 16–18).
Interaction between behavioural and EEG data
To test the influence of midfrontal Delta-Theta synchronization on participants’ ability to perform the Joint-Grasping task, we entered as continuous predictor single-trials data of midfrontal Delta-Theta ERS in a linear mixed model, performed with R Studio software. Single trials grasping Asynchrony scores were the dependent variable. We included Interactivity (Interactive, Cued) and Condition (PD ON, OFF, HCs) as categorical predictors. Statistical significance of fixed effects was determined using type III ANOVA test, with the lmer function from lme4 package. Estimates of slopes of the continuous predictor trend for each level of the factor were performed with the ‘Estimated Marginal Means’ R package61 (version 1.3,3) via the emtrends functions.
The continuous predictor entered in the linear mixed model was mean-centered. The model included fixed effects for Condition and Interactivity factors, the continuous predictor Delta-Theta ERS, and as random intercept the Participants and number of trials, as more complex models, including the random slope of the Interactivity factor did not converge.
Multivariate analysis
To better identify the neural patterns that were sensitive to the interactive context (i.e., Interactive vs Cued), as well as to investigate their modulation by PD and/or deficits in the levels of dopamine in the brain, we adopted a multivariate approach. Multivariate analyses are more sensitive than univariate analyses62,63, since they assume that the processing of different stimuli or tasks relies on different neural patterns to be exploited, thus they treat whole-brain sensor/source-level data as response patterns rather than investigating changes between average responses for each single sensor/source (as in the univariate approach)64. Analyses were performed using the MVPA-Light toolbox (Treder65,) and custom scripts in Matlab R2019a. Prior to every classification, data were normalized using z-scores to center and scale the training data, providing numerical stability65. Across all classification analyses, if a class had fewer trials than another, we corrected the imbalance by undersampling the over-represented condition (i.e., randomly removing trials). Then, using the preprocessing option mv_preprocess_average_samples, training data (i.e., EEG trials) from the same class (i.e., Interactive/Cued) were randomly split into 5 groups and averaged, so that the single-trial dataset was replaced by 5 averaged trials (i.e., samples). The classifications were then run using these averaged samples, as this step is known to increase the signal-to-noise ratio64,65.
We performed binary classification analyses in time (−4000 to 1000 ms around the VP’s correction), using Interactive/Cued samples, for each Condition separately (i.e., HCs, ON, and OFF). To do so, a Linear Discriminant Analysis (LDA) classifier was implemented using the MVPA-Light toolbox. For linearly separable data, an LDA classifier divides the data space into n regions, depending on the number of classes, and finds the optimal separating boundary between them using a discriminant function to find whether the data fall on the decision boundary (i.e., 50% classification accuracy) or far from it (i.e., >50% classification accuracy). A k-fold cross-validation procedure in which samples were divided into 5 folds was repeated 5 times so that each sample was either used for training or testing at least once, and the raw output of the model was extracted and averaged for each decoded class at the subject-level. The decoding curves for each condition were then compared through (1) a dependent samples t-test across time between PD ON and OFF groups, and (2) two independent samples t-tests across time between PD ON/OFF and HCs groups. Statistical significance was assessed through non-parametric cluster-based permutations39.
Moreover, to investigate what electrodes contributed most to the classification over time, we performed a searchlight analysis using two neighboring matrices for time points and electrodes, respectively. Searchlight analysis is one approach to localize multivariate effects, as it strikes a balance between localization and statistical power65,66. Thus, in this analysis each electrode/time-point and its direct neighbors acted as features for the classification, resulting in a channels × time points matrix of accuracy scores tested for statistical significance. By plotting the results from this matrix also on a spatial topography averaged in specific time windows of interest, we then visualized which electrodes carried the most weight in the temporal decoding.
Statistics and reproducibility
Linear mixed models were performed with R Studio software. Single trials grasping Asynchrony, Pe, Delta-Theta ERD/S scores were the dependent variables in separate mixed models (see Supplementary Materials for the LMM analysis with Movement Time, Reaction Times, Alpha ERD/S and Beta ERD/s as dependent variables). We included Interactivity (Interactive/Cued), Condition (PD ON/PD OFF/HCs) and Correction (Correction/NoCorrection) as categorical predictors. Models were estimated using the lmer() function from the lme4 package67; to assess statistical significance of fixed effects, we applied Type III ANOVA with Satterthwaite’s approximation for degrees of freedom using the anova() function from the lmerTest package68. This approach tests each fixed effect across all levels of the factor. Since our main hypothesis did not focus on Interaction Type (Complementary/Imitative) and Movement Type (Precision/Power), these factors were collapsed in order to have a higher number of trials for each condition. Thus, the models included as fixed effects the Condition, Interactivity and Correction factors; as random intercepts the Participants and the random slope of the Interactivity and Correction factor. For Grasping Asynchrony and Delta-Theta ERD/S, we also conducted linear mixed models on the difference between single-trial Grasping Asynchrony and Delta-Theta ERD/S scores in the Interactive condition and the respective average score in the Cued condition. Separate mixed models were used for each dependent variable. Condition (PD ON/PD OFF/HCs) and Correction (Correction/NoCorrection) were categorical predictors, with Participants as a random intercept and the random slope for the Correction factor. All tests of significance were based on an α level of 0.05. All variables were log-transformed before running the LMMs, except for Grasping Asynchrony data, to which we applied the square root transformation as it better fitted this data distribution. Post-hoc tests were performed using the Bonferroni method when appropriate via the emmeans61 packages. The mixed function of the afex package69 was used to compute robust p values with the bootstrap method when significant effects were observed. This approach enhances the reliability of statistical inferences as we resampled the data 1000 times generating an empirical distribution of the test statistic. The ggplots2 package70 was used to perform boxplots of the data, where mixed models analyses were performed. In all the analyses we report significant main effects or interactions that include the factor Condition. All other main effects and interactions are reported in Supplementary Tables 4–18.
Data availability
All data are available at https://osf.io/f93e2/71.
Code availability
All codes are made available upon request.
References
Ullsperger, M. & Von Cramon, D. Y. The role of intact frontostriatal circuits in error processing. J. Cogn. Neurosci. 18, 651–664 (2006).
Wylie, S. A. et al. Subthalamic nucleus stimulation influences expression and suppression of impulsive behaviour in Parkinson’s disease. Brain 133, 3611–3624 (2010).
Blesa, J., Foffani, G., Dehay, B., Bezard, E. & Obeso, J. A. Motor and non-motor circuit disturbances in early Parkinson disease: which happens first?. Nat. Rev. Neurosci. 23, 115–128 (2021).
Ponsi, G., Scattolin, M., Villa, R. & Aglioti, S. M. Human moral decision-making through the lens of Parkinson’s disease. NPJ Parkinson’s Dis. 7, 18 (2021).
Galea, J. M., Bestmann, S., Beigi, M., Jahanshahi, M. & Rothwell, J. C. Action reprogramming in Parkinson’s disease: response to prediction error is modulated by levels of dopamine. J. Neurosci. 32, 542–550 (2012).
Sebanz, N., Bekkering, H. & Knoblich, G. Joint action: bodies and minds moving together. Trends Cogn. Sci. 10, 70–76 (2006).
Era, V., Boukarras, S. & Candidi, M. Neural correlates of action monitoring and mutual adaptation during interpersonal motor coordination. Phys. Life Rev. 28, 43–45 (2019).
Sacheli, L. M., Musco, M. A., Zazzera, E. & Paulesu, E. Mechanisms for mutual support in motor interactions. Sci. Rep. 11, 3060 (2021).
Cohen, M. X. Error-related medial frontal theta activity predicts cingulate-related structural connectivity. NeuroImage 55, 1373–1383 (2011).
Falkenstein, M., Hohnsbein, J., Hoormann, J. & Blanke, L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr. Clin. Neurophysiol. 78, 447–455 (1991).
Falkenstein, M. et al. Action monitoring, error detection, and the basal ganglia: an ERP study. Neuroreport/Neuroreport 12, 157–161 (2001).
Luu, P., Tucker, D. M. & Makeig, S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin. Neurophysiol. 115, 1821–1835 (2004).
Fusco, G., Cristiano, A., Perazzini, A. & Aglioti, S. M. Neuromodulating the performance monitoring network during conflict and error processing in healthy populations: Insights from transcranial electric stimulation studies. Front. Integr. Neurosci. 16, 953928 (2022).
Fusco, G., Fusaro, M. & Aglioti, S. M. Midfrontal-occipital θ-tACS modulates cognitive conflicts related to bodily stimuli. Soc. Cogn. Affect. Neurosci. 17, 91–100 (2020).
Moreau, Q., Candidi, M., Era, V., Tieri, G. & Aglioti, S. M. Midline frontal and occipito-temporal activity during error monitoring in dyadic motor interactions. Cortex 127, 131–149 (2020).
Moreau, Q., Tieri, G., Era, V., Aglioti, S. M. & Candidi, M. The performance monitoring system is attuned to others’ actions during dyadic motor interactions. Cereb. Cortex 33, 222–234 (2022).
Boukarras, S. et al. Midfrontal theta transcranial alternating current stimulation facilitates motor coordination in dyadic Human–Avatar interactions. J. Cogn. Neurosci. 34, 897–915 (2022).
Sacheli, L. M. et al. Neuromodulation of the left inferior frontal cortex affects social monitoring during motor interactions. J. Cogn. Neurosci. 35, 1788–1805 (2023).
Cavanagh, J. F. & Frank, M. J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 18, 414–421 (2014).
Jocham, G. & Ullsperger, M. Neuropharmacology of performance monitoring. Neurosci. Biobehav. Rev.33, 48–60 (2009).
Holroyd, C. B. & Coles, M. G. H. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 109, 679–709 (2002).
Ridderinkhof, K. R., Ramautar, J. R. & Wijnen, J. G. To PE or not to PE: A P3-like ERP component reflecting the processing of response errors. Psychophysiology 46, 531–538 (2009).
Klein, T. A., Ullsperger, M. & Danielmeier, C. Error awareness and the insula: links to neurological and psychiatric diseases. Front. Hum. Neurosci. 7, 14 (2013).
Parker, K. L., Chen, K.-H., Kingyon, J. R., Cavanagh, J. F. & Narayanan, N. S. Medial frontal ∼4-Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. J. Neurophysiol. 114, 1310–1320 (2015).
Ullsperger, M., Danielmeier, C. & Jocham, G. Neurophysiology of performance monitoring and adaptive behavior. Physiol. Rev. 94, 35–79 (2014).
Pezzetta, R. et al. Combined EEG and immersive virtual reality unveil dopaminergic modulation of error monitoring in Parkinson’s disease. NPJ Parkinson’s Dis. 9, 3 (2023).
Era, V. et al. The dopaminergic system supports flexible and rewarding dyadic motor interactive behaviour in Parkinson’s Disease. Soc. Cogn. Affect. Neurosci. 18, nsac040 (2023).
Sacheli, L. M., Candidi, M., Era, V. & Aglioti, S. M. Causative role of left aIPS in coding shared goals during human–avatar complementary joint actions. Nat. Commun. 6, 7544 (2015).
Sacheli, L. M. et al. Prejudiced interactions: implicit racial bias reduces predictive simulation during joint action with an out-group avatar. Sci. Rep. 5, 8507 (2015).
Sacheli, L. M., Tieri, G., Aglioti, S. M. & Candidi, M. Transitory inhibition of the left anterior intraparietal sulcus impairs joint actions: a continuous theta-burst stimulation study. J. Cogn. Neurosci. 30, 737–751 (2018).
Candidi, M. et al. Come together: human–avatar on-line interactions boost joint-action performance in apraxic patients. Soc. Cogn. Affect. Neurosci. 12, 1793–1802 (2017).
Gandolfo, M., Era, V., Tieri, G., Sacheli, L. M. & Candidi, M. Interactor’s body shape does not affect visuo-motor interference effects during motor coordination. Acta Psychol. 196, 42–50 (2019).
Fini, C., Era, V., Da Rold, F., Candidi, M. & Borghi, A. M. Abstract concepts in interaction: the need of others when guessing abstract concepts smooths dyadic motor interactions. R. Soc. Open Sci. 8, 201205 (2021).
Era, V., Aglioti, S. M. & Candidi, M. Inhibitory theta burst stimulation highlights the role of left aIPS and right TPJ during Complementary and imitative human–avatar interactions in cooperative and competitive scenarios. Cereb. Cortex 30, 1677–1687 (2019).
Boukarras, S., Era, V., Aglioti, S. M. & Candidi, M. Competence-based social status and implicit preference modulate the ability to coordinate during a joint grasping task. Sci. Rep. 11, 5321 (2021).
Boukarras, S. et al. Interpersonal physiological synchrony during dyadic joint action is increased by task novelty and reduced by social anxiety. Psychophysiology 62, e70031 (2025).
Pesci, U. G., Moreau, Q., Era, V. & Candidi, M. The bodily appearance of a Virtual Partner affects the activity of the action observation and action monitoring systems in a minimally interactive task. eNeuro ENEURO.0390-24.2025 https://doi.org/10.1523/eneuro.0390-24.2025 (2025).
Kricheldorff, J., Ficke, J., Debener, S. & Witt, K. Impaired proactive cognitive control in Parkinson’s disease. Brain Commun. 5, fcad327 (2023).
Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190 (2007).
Solié, C., Girard, B., Righetti, B., Tapparel, M. & Bellone, C. VTA dopamine neuron activity encodes social interaction and promotes reinforcement learning through social prediction error. Nat. Neurosci. 25, 86–97 (2021).
Singh, A., Richardson, S. P., Narayanan, N. & Cavanagh, J. F. Mid-frontal theta activity is diminished during cognitive control in Parkinson’s disease. Neuropsychologia 117, 113–122 (2018).
Narayanan, N. S., Jourahmad, Z., Cole, R. C. & Cavanagh, J. F. Cognition falters at ~4 Hz in Parkinson’s disease. Trends Cogn. Sci. 28, 789–791 (2024).
Singh, A. et al. Evoked mid-frontal activity predicts cognitive dysfunction in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 94, 945–953 (2023).
Kerns, J. G. et al. Anterior cingulate conflict monitoring and adjustments in control. Science 303, 1023–1026 (2004).
Oehrn, C. R. et al. Neural communication patterns underlying conflict detection, resolution, and adaptation. J. Neurosci. 34, 10438–10452 (2014).
De Bondt, C. C. et al. Reduced task-related functional connectivity during a set-shifting task in unmedicated early-stage Parkinson’s disease patients. BMC Neurosci. 17, 20 (2016).
Overbeek, T. J. M., Nieuwenhuis, S. & Ridderinkhof, K. R. Dissociable components of error processing. J. Psychophysiol. 19, 319–329 (2005).
Spinelli, G., Pezzetta, R., Canzano, L., Tidoni, E. & Aglioti, S. M. Brain dynamics of action monitoring in higher-order motor control disorders: the case of apraxia. ENeuro 9, ENEURO.0334-20.2021 (2022).
Campbell, J. I. D. & Thompson, V. A. MorePower 6.0 for ANOVA with relational confidence intervals and Bayesian analysis. Behav. Res. Methods 44, 1255–1265 (2012).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Hoehn, M. M. & Yahr, M. D. Parkinsonism. Neurology 17, 427 (1967).
Era, V., Candidi, M., Gandolfo, M., Sacheli, L. M. & Aglioti, S. M. Inhibition of left anterior intraparietal sulcus shows that mutual adjustment marks dyadic joint-actions in humans. Soc. Cogn. Affect. Neurosci. 13, 492–500 (2018).
Era, V., Aglioti, S. M., Mancusi, C. & Candidi, M. Visuo-motor interference with a virtual partner is equally present in cooperative and competitive interactions. Psychol. Res. 84, 810–822 (2018).
Marsden, C. D. Slowness of movement in Parkinson’s disease. Mov. Disord. 4, S26–S37 (1989).
Delorme, A. & Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21 (2004).
Jung, T.-P. et al. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin. Neurophysiol. 111, 1745–1758 (2000).
Oostenveld, R., Fries, P., Maris, E. & Schoffelen, J.-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 156869 (2011).
Sauseng, P. et al. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience 146, 1435–1444 (2007).
Pezzetta, R., Nicolardi, V., Tidoni, E. & Aglioti, S. M. Error, rather than its probability, elicits specific electrocortical signatures: a combined EEG-immersive virtual reality study of action observation. J. Neurophysiol. 120, 1107–1118 (2018).
Cohen, M. X. Analyzing Neural Time Series Data. https://doi.org/10.7551/mitpress/9609.001.0001 (The MIT Press eBooks, 2014).
Lenth, R., Singmann, H., Love, J., Buerkner, P., & Herve, M. Package “Emmeans”. R Package Version 4.0-3. http://cran.r-project.org/package=emmeans (2018).
Haxby, J. V. et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293, 2425–2430 (2001).
Tucciarelli, R., Turella, L., Oosterhof, N. N., Weisz, N. & Lingnau, A. MEG multivariate analysis reveals early abstract action representations in the lateral occipitotemporal cortex. J. Neurosci. 35, 16034–16045 (2015).
Grootswagers, T., Wardle, S. G. & Carlson, T. A. Decoding dynamic brain patterns from evoked responses: a tutorial on multivariate pattern analysis applied to time series neuroimaging data. J. Cogn. Neurosci. 29, 677–697 (2017).
Treder, M. S. MVPA-Light: a classification and regression toolbox for multi-dimensional data. Front. Neurosci. 14, 289 (2020).
Kriegeskorte, N., Goebel, R. & Bandettini, P. Information-based functional brain mapping. Proc. Natl. Acad. Sci. 103, 3863–3868 (2006).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. LMERTest Package: Tests in Linear Mixed Effects models. J. Stat. Softw. 82, 1–26 (2017).
Singmann, H., Bolker, B. and Westfall, J. Afex: analysis of factorial experiments. R Package Version 0.15–2 (2015).
Wickham, H. Ggplot2. Use R!. https://doi.org/10.1007/978-3-319-24277-4 (2016).
Era, V. et al. Dopaminergic modulation of behavioral and electrocortical markers of interpersonal performance monitoring in Parkinson’s Disease [Data set]. OSF. https://osf.io/f93e2/ (2025).
Acknowledgements
V.E. was supported by Bando Giovani Ricercatori. Ministero italiano della Salute. GR-2021-12372923. M.C. was supported by Sapienza University grants (n. MA22117A8A97CD42, RM1221816C827130 and RG123188B4631694).
Author information
Authors and Affiliations
Contributions
V.E. Conceptualization, Methodology, Data acquisition, Data Curation, Formal analysis, Funding acquisition, Supervision, Writing–Original draft; U.P. Methodology, Data acquisition, Data Curation, Formal analysis, Visualization, Writing–Original draft; Q.M. Methodology, Data acquisition, Formal analysis, Writing–Review and editing; R.P. Conceptualization, Formal analysis, Writing–Review and editing; Z.S. Data acquisition, Data Curation, Writing–Review and editing; A.P. Data acquisition, Data Curation, Writing–Review and editing; A.C. Supervision, Writing–Review and editing; S.T. Data acquisition, Data Curation, Writing–Review and editing; M.C. Conceptualization, Funding acquisition, Writing–Review and editing; S.M.A. Conceptualization, Funding acquisition, Supervision, Writing–Review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Bronagh McCoy, Josefine Waldthaler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Christian Beste and Jasmine Pan. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Era, V., Pesci, U.G., Moreau, Q. et al. Dopaminergic modulation of behavioral and electrocortical markers of interpersonal performance monitoring in Parkinson’s Disease. Commun Biol 8, 1195 (2025). https://doi.org/10.1038/s42003-025-08638-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08638-z