Abstract
Microglia play significant roles in spinal cord injury (SCI) progression. Previous studies have suggested that ferroptosis plays a crucial role in exacerbating neuronal death following SCI; however, the role of microglial ferroptosis in SCI and the underlying mechanisms remain elusive. Here, we elucidate that lipid droplets accumulate in microglia to facilitate microglial ferroptosis after SCI. Notably, microglial ferroptosis peaks at 3 days post-injury, after which it decreases. Microglial Period 2 (Per2) expression is elevated after SCI in vivo; this change is highly synchronized with the changes in microglial ferroptosis. Microglia-specific Per2 knockout promoted neurological function recovery by suppressing microglial ferroptosis. In vitro, Per2 overexpression and deficiency amplified and mitigated microglial ferroptosis, respectively. RNA-seq indicated that Gpx4 was downregulated by Per2. Coimmunoprecipitation demonstrated that Per2 directly interacted with PPARα. Overall, our results indicate that Per2 determines the susceptibility of microglial ferroptosis via the PPARα-Gpx4 axis after SCI.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) is a devastating form of central nervous system (CNS) trauma with high mortality and disability rates, but effective therapeutics are lacking1. The pathogenesis of SCI involves primary and secondary injury. The initial insult in SCI causes inevitable neuron loss and triggers a secondary cascade, leading to a self-destructive series of events that establishes an injury-related microenvironment. This microenvironment is characterized by severe demyelination and neurodegeneration, posing a formidable obstacle to the process of neural regeneration2,3. Inflammation is the most significant secondary injury process and directly or indirectly affects the outcome of SCI4. Microglia, which originate from primitive yolk sac progenitors, are CNS-resident immune cells and are among the earliest and most essential participants in inflammation5. Recent findings underscore the role of microglia in coordinating cellular interactions and contributing to the formation of a neuroprotective scar after SCI, thereby bolstering the protection of neural tissue6,7. Nonetheless, further investigations are needed to elucidate the role of microglia in the injury-related microenvironment after SCI.
Ferroptosis, an iron-dependent form of cell death characterized by phospholipid peroxidation, is distinct from apoptosis and necrosis8. The involvement of ferroptosis in the pathogenesis of hepatic ischemia9, neurodegenerative diseases10, and nerve injury11 has been established. SCI induces the generation of reactive oxygen species (ROS), and increased ROS levels initiate lipid peroxidation, leading to lipid membrane rupture and subsequent cell death12. Previous studies have demonstrated that ferroptosis commences at an early stage after SCI13, and emerging evidence indicates that early interventions targeting ferroptosis are beneficial for promoting motor function recovery14. Notably, among all cell types in the CNS, microglia exhibit one of the highest storage iron capacities, as well as robust transcriptional response to iron dysregulation15,16, rendering them susceptible to ferroptosis. However, the current literature concentrates more on neuronal ferroptosis than on microglial ferroptosis, and the role of microglial ferroptosis in SCI has received minimal attention.
Numerous factors influence the process of ferroptosis. A pivotal association between the circadian clock and ferroptosis has been investigated in recent years17. Previously research has shown that the autophagic degradation of specific circadian genes contributes to the promotion of ferroptotic cell death18, which indicates that ferroptosis might be regulated by circadian genes. Period 2 (Per2), an indispensable component of the circadian clock, is not only important in regulating the circadian rhythms and the expression of other circadian genes19, but also as a tumor suppressor gene that inhibits glioma development in our research20,21. Previous studies depicted the participation of Per2 in the progression of nonalcoholic steatohepatitis by promoting ferroptosis22. Meanwhile, Ahmed et al. described the contribution of the upregulation of Per2 to the inhibition of ferroptosis in cardiac disease23. Despite these advancements, whether Per2 participates in microglial ferroptosis after SCI and its underlying mechanisms remain elusive.
In the present study, we elucidate the regulatory role of Per2 in microglial ferroptosis, both in vivo and in vitro. We show that Per2 deficiency attenuates microglial ferroptosis, thus decreasing neural cell death and improving motor function after SCI. We further demonstrate that Per2 regulates microglial ferroptosis through the PPARα-GPX4 axis. Our results reveal that Per2 may be emerging as a potential therapeutic target for SCI treatment.
Results
Microglia undergo ferroptosis after SCI
Following CNS injury, blood is released from microvessels, causing erythrocyte accumulation, and local iron overload occurs when these cells are broken24. Excess iron damages neurons, contributing to impaired intracellular iron metabolism and the generation of ROS25, which are prerequisites for ferroptosis. Thus, we induced contusion SCI in wild-type C57BL/6J mice, and basso mouse scale (BMS) scores were used to assess behavioral function after SCI. The results revealed that the BMS score promptly decreased from 9 to 0 following SCI, and gradually increased over time, albeit remaining lower than normal scores (Supplementary Fig. 1a), consistent with a previous study26, which indicated the successful establishment of the SCI model. Malondialdehyde (MDA), iron (Fe), glutathione (GSH), and glutathione Peroxidase (GSH-Px) levels are all regarded as cytological symbols of ferroptosis27,28,29. Then, we evaluated ferroptosis by measuring the levels of MDA, Fe, GSH, and GSH-Px at 0.25 day, 1 day, 3 days, and 7 days post-injury (dpi). As expected, the levels of MDA and Fe significantly increased at 1 dpi after SCI peaked at 3 dpi, and then decreased at 7 dpi (Supplementary Fig. 1b, c). Concurrently, the activities of GSH and GSH-Px markedly decreased 1 dpi post-SCI, reached the lowest level at 3 dpi, and subsequently gradually increased (Supplementary Fig. 1d, e). These results demonstrated that SCI triggers ferroptosis, which is consistent with previous studies30. The ferroptosis was most pronounced at 3 dpi, which might be the optimal period for investigating ferroptosis after SCI.
However, the majority of studies have focused on neuronal ferroptosis, with limited research on ferroptosis of other types and their roles in the pathology of SCI. To address this issue, transmission electron microscopy (TEM) was used to identify the cell types undergoing ferroptosis after SCI. The most notable characteristic of ferroptotic cells is a change in mitochondrial morphology. As shown in Supplementary Fig. 1f, abnormal mitochondrial morphology, specifically the disappearance of mitochondrial cristae and an increased mitochondrial membrane density, which is consistent with prior reports31, was observed in the SCI group. Moreover, we noted that cells undergoing ferroptosis presented distinctive features, including small size, irregular nuclear morphology, high electron density in the cytoplasm, and a discernible heterochromatin pattern, which aligned with the attributes of microglia32. Together, these results indicate that microglia undergo ferroptosis following SCI.
Microglia with lipid droplet accumulation are susceptible to ferroptosis and undergo ferroptosis in a unique spatiotemporal manner after SCI
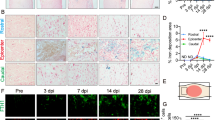
Ferroptosis is regulated by lipid metabolism33. Lipid droplets are organelles that store fatty acids, including polyunsaturated fatty acids containing phospholipids, which are particularly susceptible to peroxidation34. Cells degrade the intracellular lipid droplets via autophagy to produce lipid peroxides35 and secrete more ROS36, increasing their sensitivity to ferroptosis. Stress and inflammation result in the production of lipid droplets by macrophages36. Stress and inflammation are common features of the injury microenvironment after SCI37; thus, we wondered whether SCI induces lipid droplets to accumulate in microglia. To answer this question, costaining for Hexb and BODIPY, a dye that is commonly used to detect lipid droplets, was performed at 3 d post-SCI because ferroptosis we found that was most pronounced at 3 dpi (Supplementary Fig. 1b–e). In the lesion epicenter, the number of lipid droplets was notably greater in the SCI group than in the sham group, and the percentage of Hexb+ BODIPY+ microglia in the SCI group was significantly greater than that in the sham group (Fig. 1a–c). Thus, our results show that SCI induces abnormal lipid droplet accumulation in microglia, which may be the main reason that microglia are susceptible to ferroptosis after SCI.
a Spinal cord from sham and SCI mice lesion epicenter stained for BODIPY+ (lipid droplets) and Hexb+ (microglia). Arrows indicate lipid droplets. Scale bar, 20 μm or 10 μm. b, c Quantification of BODIPY+ lipid droplet numbers, and percent BODIPY+/Hexb+ cells in the spinal cord. (n = 5 mice per group). d Double immunostaining images of spinal cord sections, showing the microglia distribution and the spatiotemporal characteristics of ferroptosis in uninjured C57BL/6J mice, as well as in the lesion epicenter at 0.25, 1, 3, 7, and 14 dpi. Scale bar, 100 μm or 20 μm. e, f Quantification of the number of microglia and the Hexb+/Acsl4+ of the total Hexb+ microglia under different stages after SCI (n = 5 animals per group). All data are presented as the mean ± SD. P values were determined by two-tailed unpaired Student’s t-test (b, c), one-way ANOVA with Tukey’s multiple-comparisons test (e, f). ****P < 0.0001; ns not significant.
It is clear that microglia undergo cell death in the form of apoptosis38 and pyroptosis39 after SCI, to further confirm the occurrence of microglial ferroptosis and evaluate the degree of microglial ferroptosis at different stages after SCI, costaining for Acsl4 and Hexb, which are marker proteins of ferroptosis and microglia, respectively40,41, was performed. The results revealed that microglia exhibited significant ferroptosis at 1 dpi and that microglial ferroptosis was the most pronounced at 3 dpi, subsequently diminished at 7 dpi, and was restored to nearly normal levels by 14 dpi (Fig. 1d, f). Second injury following SCI inevitably results in microglia death, in addition to neuronal death. Thus, we wondered how microglial ferroptosis affects the number of microglia after SCI. To answer this question, we immunolabelled cells with Hexb and quantified the number of microglia at different stages after SCI. In the sham group, an average of 127 ± 25 microglia per mm2 were present. Only 67 ± 24 and 34 ± 24 microglia remained in the lesion site at 0.25 dpi and 1 dpi after SCI, respectively, indicating rapid cellular death (Fig.1d, e). However, the number of microglia increased significantly at 3 dpi, reaching 737 ± 170 per mm2, and then gradually decreased at 7 and 14 dpi. Under normal physiological conditions, a stable population of mature microglia is maintained in the brain through a delicate balance of cell death and proliferation42. We hypothesized that the increase in the number of microglia resulted from the proliferation of microglia under pathological conditions following SCI. To confirm this, we immunolabelled cells with Hexb and Ki-67 to detect proliferating microglia. Despite the initial decline in the number of microglia, ~81% of the microglia in the lesion site expressed Ki-67 at 1 dpi (Supplementary Fig. 2a, b). Consequently, the number of microglia near the lesion continued to increase over time. These findings indicate that microglia respond to SCI by swiftly proliferating and encircling the injury site, which is consistent with previous reports7. Then, microglial ferroptosis peaks at 3 dpi, leading to a decrease in the number of microglia.
Our results showed that SCI induces lipid droplet accumulation in microglia, increasing their susceptibility to ferroptosis. Microglia at the lesion epicenter undergo both proliferation and ferroptosis after SCI. In the initial stage, microglia undergo rapid proliferation to replenish cell numbers, resulting in a swift increase in microglia numbers. These microglia at the lesion epicenter undergo ferroptosis within 1 dpi, and microglial ferroptosis peaks at 3 dpi and gradually decreases after the acute phase. All these processes induce dynamic changes in the number of microglia. Together, these results indicate that after SCI, microglia undergo ferroptosis in a unique spatiotemporal manner.
Per2 is upregulated via the MAPK signaling pathway during microglial ferroptosis
To systematically screen and identify genes governing microglial ferroptosis, we utilized a previously described protocol to obtain reproducible and expandable adult-like microglia43. The purity of the primary microglia was validated by Iba1 immunostaining (Supplementary Fig. 3a, b).
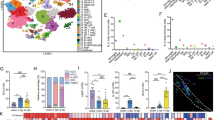
We induced ferroptosis in primary microglia with 1600 μM iron and 1 μM RSL316, and RNA sequencing was subsequently performed. As shown in Fig. 2a, the differentially expressed genes (DEGs) were notably enriched in ferroptosis and circadian rhythms pathways. The volcano plots show the significant upregulation of Per2 expression during microglial ferroptosis (Fig. 2b). Το confirm the results of RNA sequencing, qRT-PCR, and western blotting were performed. Compared with that in the DMSO control group, the mRNA expression of Acsl4 and Ptgs2, two indicators of ferroptosis, in the iron and RSL3-treated groups significantly increased (Fig. 2c), suggesting that iron and RSL3 induced ferroptosis in microglia. In addition, Per2 mRNA expression and protein levels significantly increased during microglial ferroptosis (Fig. 2c, d).
a The differentially expressed genes (DEGs) between primary microglia treated with DMSO and 1600 μM iron + 1 μM RSL3 were identified with the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. Rich factor (%) was the ratio of the number of DEGs annotated in a pathway (as indicated in the x-axis) to the number of all genes annotated in the pathway. b Volcano plot showing fold changes in genes in primary microglia treated with DMSO or 1600 μM iron + 1 μM RSL3. c qRT-PCR was performed to detect 2 ferroptosis-related genes and to verify the expression of Per2 in primary microglia treated with DMSO and 1600 μM iron + 1 μM RSL3 for 6 h (n = 3 biological repeats for each group). d Representative immunoblots of the phosphorylation status of P38 and the expression of Per2 at different time points after ferroptosis induction (the repeated immunoblots are shown in Supplementary Fig. 3c–e). e, f Quantification of the phosphorylation status of P38 and the expression of Per2 at different time points after ferroptosis induction (n = 4 biological repeats for each group). g, h Representative immunoblots (the repeated immunoblots are shown in Supplementary Fig. 3f) and quantification of the phosphorylation status of P38 after treatment with P38 MAPK inhibitor (n = 6 biological repeats for each group). i, j Representative immunoblots (the repeated immunoblots are shown in Supplementary Fig. 3g) and quantification of the expression of Per2 after treatment with P38 MAPK inhibitor (n = 6 biological repeats for each group). All data are presented as the mean ± SD. P values were determined by two-tailed unpaired Student’s t-test (c), one-way ANOVA with Tukey’s multiple-comparisons test (e, f, h, j). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant.
A previous study indicated that activating the MAPK signaling pathway can induce EGR1-mediated Per2 expression44. DEGs were found to be enriched in the MAPK signaling pathway during microglial ferroptosis in our study (Fig. 2a). Thus, to explore the mechanism by which Per2 is upregulated during microglial ferroptosis, we first assessed the phosphorylation status of P38 at different time points after ferroptosis induction. P38 phosphorylation was most significantly increased at 1 h after treatment with a ferroptosis inducer (Fig. 2d–e and Supplementary Fig. 3c–e), which indicated that the MAPK pathway plays a role in the regulation of microglial ferroptosis. Furthermore, a significant increase in Per2 protein expression was observed 8 h after treatment with the ferroptosis inducers (Fig. 2d, f, and Supplementary Fig. 3c–e). To further confirm the relationship between Per2 and the MAPK signaling pathway during microglial ferroptosis, primary microglia were pre-treated with PD169316, a selective P38 MAPK inhibitor45, which effectively reduced the level of p-P38 MAPK at 1 h (Fig. 2g, h, and Supplementary Fig. 3f) and subsequently inhibited the protein expression of Per2 at 8 h (Fig. 2i, j, and Supplementary Fig. 3g) after treatment with the ferroptosis inducers. Together, these results indicate the potential role of Per2, a pivotal component of the circadian gene network, in the regulation of microglial ferroptosis.
Per2 expression is elevated, and the changes in Per2 expression occur simultaneously with alterations in microglial ferroptosis in C57BL/6J mice after SCI
Since we found Per2 that was upregulated during microglial ferroptosis in vitro, we hypothesized that Per2 expression is elevated after SCI in vivo and that the spatiotemporal patterns in Per2 expression are similar to those observed in microglial ferroptosis. To confirm this hypothesis, we obtained continuous sections of the wild-type C57BL/6J mice spinal cord at 0.25 day, 1 day, 3 days, 7 days, and 14 days after SCI and performed costaining for Hexb and Acsl4 and Hexb and Per2 to explore the colocalization of Hexb, Per2, and Acsl4. Per2 expression was significantly increased at 1 d in the SCI group compared to the sham group, peaked at 3 d after SCI, subsequently decreased at 7 d, and was nearly restored to normal levels by 14 days (Fig. 3a, b), which indicates that SCI stimulated Per2 expression in vivo, as expected. Notably, when Per2 expression significantly increased, the expression of Acsl4 also markedly increased. When Per2 expression reached its peak, Acsl4 expression also peaked, and subsequently, both Per2 expression and Acsl4 expression show a downwards trend (Supplementary Fig. 3h); this demonstrates that the expression pattern of Per2 is spatiotemporally consistent with the pattern of microglial ferroptosis. Together, these results indicate the synchronicity between Per2 expression and microglial ferroptosis, suggesting that Per2 may be involved in regulating microglial ferroptosis during SCI.
Given the `circadian rhythms of Per2 levels, all specimens were collected between 2–4 PM. a Immunofluorescence of representative spinal cord section showing the synchronized spatiotemporal characteristics of microglial ferroptosis and Per2 expression in an uninjured C57BL/6J mouse, as well as in the lesion epicenter at 0.25, 1, 3, 7, and 14 dpi. Scale bar, 20 μm. b Quantification of the Hexb+/Per2+ of total Hexb+ microglia at different stages after SCI (n = 4–5 animals per group). All data are presented as the mean ± SD. P values were determined by one-way ANOVA with Tukey’s multiple-comparisons test (b). ****P < 0.0001; ns not significant.
Per2 deficiency in microglia alleviates extremity motor function in mice after SCI
On the basis of the aforementioned results, we hypothesized that Per2 deficiency may be beneficial for alleviating microglial ferroptosis and restoring motor function after SCI. To confirm this, we induced contusion SCI in global Per2 knockout (Per2GKO) mice, as in wild-type (WT) mice. Compared with those of WT mice, the BMS scores of Per2GKO mice were significantly greater (Supplementary Fig. 4d). The degree of microglial ferroptosis was markedly decreased in Per2GKO mice, as expected (Supplementary Fig. 4e–g).
To investigate the effect of Per2 deficiency during SCI more precisely, we crossed Hexb-CreERT2 mice with Per2flox/flox mice to specifically delete Per2 in microglia (denoted Per2CKO mice hereafter). Hexb is stably expressed in microglia core and is employed for tamoxifen-inducible Cre-mediated gene manipulation in microglia but not in CNS-associated macrophages41. Tamoxifen was injected for 5 consecutive days to induce recombination. Immunofluorescence demonstrated that Per2 was knocked out in the microglia of the spinal cord (Supplementary Fig. 5a). Both Per2CKO and Per2flox/flox mice presented maximum BMS scores of 9 (Fig. 4a). Moreover, no significant difference in the proliferation of microglia in the spinal cord was detected between Per2CKO and Per2flox/flox mice (Supplementary Fig. 5b, c). Collectively, these results indicate that we successfully generated mice with conditional knockout of Per2 in microglia and that Per2 deficiency did not alter the normal development of microglia or motor function in these mice.
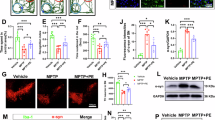
a BMS scores during 42 days of recovery after SCI in Per2flox/flox and Per2CKO mice (n = 10 animals per group). b Representative images of footprint analysis at 42 d after SCI. c, d Quantification analysis of stride length and width (n = 10 animals per group). e Immunofluorescence images of Hexb and Acsl4 at the lesion epicenter, showing decreased Acsl4 expression in microglia of the spinal cord in Per2CKO mice at 3 dpi than in Per2flox/flox mice. Scale bars, 100 μm or 20 μm. f Quantification of the Hexb+/Acsl4+ of the total Hexb+ microglia (n = 5 animals per group). g Representative TEM images of the microglia of Per2flox/flox and Per2CKO mice at 3 dpi. Red arrow: damaged mitochondria. Yellow arrow: relatively mild damaged mitochondria. Scale bars, 5 μm or 1 μm. h Nissl staining to observe the number of neurons in spinal cord tissues of the different groups at 42 dpi. Arrows indicate neurons containing Nissl bodies. Scale bars, 100 μm or 20 μm. i Quantification of the Nissl bodies (n = 6 animals per group). j Representative immunofluorescence images of NeuN in spinal cord tissues of the different groups at 42 dpi. Scale bars, 200 μm. k Quantification of the number of NeuN+ neurons (n = 6 animals per group). l The levels of IGF-1 in the serum of mice at 3 dpi (n = 10 animals per group) were detected by ELISA kits. All data are presented as the mean ± SD. P values were determined by two-way ANOVA with Tukey’s test for multiple comparisons (a), two-tailed unpaired Student’s t-test (c, d, f, i, k), and one-way ANOVA with Tukey’s multiple-comparisons test (l). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns not significant.
Limb function following SCI was also assessed via BMS scores, and the results revealed a significant increase in the BMS score of Per2CKO mice compared with that of Per2flox/flox mice, beginning from the 2nd-week post-injury (Fig. 4a). Similarly, footprint analysis also revealed that Per2CKO mice had better gait recovery and motor coordination than Per2flox/flox mice did (Fig. 4b–d). Next, we conducted Hexb and Acsl4 costaining at 3 dpi to evaluate microglial ferroptosis in Per2CKO mice. As shown in Fig. 4e, f and Supplementary Fig. 5d, e, the degree of microglial ferroptosis was significantly decreased in Per2CKO mice than in Per2flox/flox mice at 3 dpi. TEM was performed to assess mitochondrial morphology, and the results revealed that, compared with those in Per2flox/flox mice, the microglia in Per2CKO mice presented abundant mitochondrial cristae and a lower mitochondrial membrane density (Fig. 4g). Thus, our results indicate that knockout of microglial Per2 alleviates microglial ferroptosis after SCI in vivo, which is consistent with the results obtained for Per2GKO mice (Supplementary Fig. 4e–g).
Given the ability of microglia to prevent neuronal and oligodendrocyte death during SCI7 and the fact that Per2 knockout reduced microglial ferroptosis in our study, we hypothesized that more neurons may remain after SCI in Per2CKO mice. To test this hypothesis, Nissl staining and immunofluorescence staining for NeuN were performed at 42 dpi. As expected, Per2CKO mice presented more Nissl bodies and higher NeuN protein expression than Per2flox/flox mice did, indicating that more neurons were preserved around the lesion core in Per2CKO mice after SCI (Fig. 4h–k). Similar results were obtained for Per2GKO mice (Supplementary Fig. 4h–k).
The recovery of neurological function after SCI relies on sufficient reactive astrocytes46. A previous study demonstrated that microglia-mediated IGF-1 secretion triggers astrocyte proliferation and facilitates scar formation after SCI7. Interestingly, activated, proliferating microglia are an important source of IGF-147. Therefore, we wondered whether Per2CKO mice secrete more IGF-1, which may facilitate the recovery of motor function. To test this hypothesis, serum IGF-1 levels were measured via ELISA in mice at 3 dpi. As shown in Fig. 4l, the level of IGF-1 in Per2CKO mice was significantly greater than that in Per2flox/flox mice, indicating that the neuroprotective effect of Per2 conditional knockout in the mice was partially attributed to the secretion of IGF-1 by more surviving microglia. Consistent results were obtained for Per2GKO mice (Supplementary Fig. 4l).
Together, these results indicate that microglial death is reduced in Per2CKO mice due to the inhibition of ferroptosis and that the surviving and activated microglia secrete more IGF-1 to exert neuroprotective effects.
Per2 facilitates microglial ferroptosis in vitro
Since Per2 deficiency alleviated microglial ferroptosis in vivo, to further examine whether Per2 regulates microglial ferroptosis directly, in vitro experiments were subsequently performed. We generated primary microglia overexpressing Per2 (Per2OE) through lentivirus transfection (Fig. 5a, b) and isolated primary microglia from Per2GKO mice. As shown in Fig. 5c, after treatment with 1600 μM iron and 1 μM RSL3, the survival rate of Per2OE microglia was significantly lower than that of control microglia. Furthermore, immunofluorescence revealed that, compared with that in control microglia, the protein level of Acsl4 in Per2OE microglia significantly increased after treatment with the ferroptosis inducer (Fig. 5d, e), indicating that Per2OE facilitates microglial ferroptosis in vitro. Conversely, compared with WT microglia, Per2GKO microglia presented an increased survival rate and decreased Acsl4 expression after exposure to a ferroptosis inducer (Fig. 5f–h), indicating that Per2GKO microglia can increase the ability of microglia to resist ferroptosis. Additionally, we measured the level of IGF-1 in the microglial culture medium after treatment with 1600 μM iron and 1 μM RSL3. As shown in Fig. 5i, compared with WT microglia, Per2GKO microglia secreted more IGF-1, which is consistent with the in vivo results. Together, these results indicate that Per2 exacerbates microglial ferroptosis in vitro.
a The expression of Per2 was measured by western blotting after overexpressing by RNA lentivirus. b Quantification of relative levels of Per2 protein (n = 3 biological repeats for each group). c, f Ctrl or Per2OE microglia and WT or Per2GKO microglia were treated with DMSO or 1600 μM iron + 1 μM RSL3 for 24 h, and the cell viability was determined by CCK-8 assay (n = 6 biological repeats for each group). d Immunofluorescence image showing expression of ferroptosis marker Acsl4 in ctrl or Per2OE microglia after treatment with DMSO or 1600 μM iron + 1 μM RSL3 for 18 h. Scale bar, 20 μm. e Quantification of the relative fluorescence intensity of Acsl4 (n = 3 biological repeats for each group). g Immunofluorescence image showing expression of ferroptosis marker Acsl4 in WT or Per2GKO microglia after treatment with DMSO or 1600 μM iron + 1 μM RSL3 for 18 h. Scale bar, 20 μm. h Quantification of the relative fluorescence intensity of Acsl4 (n = 4–5 biological repeats for each group). i The levels of IGF-1 in the microglia culture medium (n = 5 biological repeats for each group) were detected by ELISA kits. All data are presented as the mean ± SD. P values were determined by two-tailed unpaired Student’s t-test (b, i), one-way ANOVA with Tukey’s multiple-comparisons test (c, e, f, h). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Per2 promotes microglial ferroptosis via inhibition of Gpx4
To determine the mechanism through which Per2 regulates microglial ferroptosis, we performed transcriptomic analysis via high-throughput RNA sequencing of primary microglia transfected with a control vector or Per2-overexpression vector. The DEGs were notably enriched in lipid metabolism-related pathophysiological processes, such as axonal degeneration and Parkinson’s disease (Fig. 6a), indicating that Per2 plays a vital role in regulating lipid metabolism. The volcano plot shows the downregulation of Gpx4 expression in Per2OE microglia (Fig. 6b). qRT-PCR and western blotting verified that the expression of Gpx4 in Per2OE microglia significantly decreased at both the mRNA and protein levels (Fig. 4c–e and Supplementary Fig. 6a). These results demonstrated that Per2 may regulate microglial ferroptosis through Gpx4, a key mediator of ferroptosis48
a The DEGs between the control and Per2OE microglia were identified with KEGG pathway enrichment analysis. b Volcano plot showing fold changes in genes in the control and Per2OE microglia. c, d The expression of Gpx4 was measured by qRT-PCR (n = 4 biological repeats for each group) and western blotting (the repeated immunoblot is shown in Supplementary Fig. 6a) after overexpressing by lentivirus. e Quantification of relative levels of Gpx4 protein (n = 6 biological repeats for each group). f, g Following a 24-h incubation with 500 nM selenium, a Gpx4 agonist, ctrl, and Per2OE microglia were exposed to DMSO or 1600 μM iron + 1 μM RSL3. Then, the cell viability was determined by CCK-8 assay (n = 3 biological repeats for each group), and the expression of ferroptosis marker Acsl4 was measured by immunofluorescence staining. Scale bar, 20 μm. h Quantification of the relative fluorescence intensity of Acsl4 (n = 3 biological repeats for each group). All data are presented as the mean ± SD. P values were determined by two-tailed unpaired Student’s t-test (c, e), one-way ANOVA with Tukey’s multiple-comparisons test (f, h). ***P < 0.01, ****P < 0.0001.
Aiming to further validate the aforementioned findings, we hypothesized that Gpx4 agonists can mitigate ferroptosis exacerbated by Per2OE. Previous studies have reported that selenium can mitigate ferroptosis by promoting Gpx4 expression, thereby contributing to the amelioration of stroke, and serves as an effective Gpx4 agonist49. Therefore, following a 24-h incubation with 500 nM selenium50, microglia were exposed to 1600 μM iron and 1 μM RSL3. As shown in Fig. 6f, treatment with selenium significantly increased the percentage of surviving microglia. Furthermore, immunofluorescence demonstrated that the expression of Acls4 in the group treated with selenium was significantly decreased (Fig. 6g, h), indicating that supplementation with Gpx4 can reverse ferroptosis induced by Per2OE. Together, these results demonstrated that Per2 promotes microglial ferroptosis via the inhibition of Gpx4.
Per2 relies on PPARα to modulate Gpx4 expression in microglia
Our findings have demonstrated that Per2 is upregulated in microglia after SCI, thus enhancing microglial sensitivity to ferroptosis by inhibiting the expression of Gpx4. We subsequently investigated the underlying mechanism through which Per2 suppresses Gpx4 expression. Considering that lipid droplets accumulated in microglia after SCI, we hypothesized that genes related to lipid metabolism may play a role in the regulation of Gpx4 by Per2. Previous studies have shown that PPARα, a gene associated with lipid metabolism and peroxisome proliferation51, increases the transcription of Gpx4, mitigating ferroptosis in the mouse liver52. However, whether Per2 directly interacts with PPARα to exert biological effects on microglia remains unclear. To address this issue, we used Co-IP to determine whether PPARα binds Per2. As shown in Fig. 7a and Supplementary Fig. 6b, c, endogenous Per2 coprecipitated with PPARα. The reverse experiment also confirmed that Per2 was precipitated by PPARα (Fig. 7b and Supplementary Fig. 6d, e). These results indicate that Per2 directly interacts with PPARα. Next, we further clarified whether Per2 relies on PPARα to regulate Gpx4 expression. Following a 24-h incubation with 10 μM GW647153, a potent PPARα antagonist, to inhibit PPARα expression, the expression of Gpx4 in Per2OE microglia was measured. qRT-PCR and western blotting revealed that the expression of Gpx4, which was downregulated by Per2, significantly increased at the mRNA and protein levels when PPARα was blocked (Fig. 7c–e). Together, these results indicate that Per2 regulates Gpx4 expression by directly interacting with PPARα.
a Endogenous protein interactions were assessed in whole cell lysates from microglia by immunoprecipitation with anti-Per2 or anti-IgG, and examined by immunoblotting with anti-PPARα (n = 3 independent experiments) (the repeated immunoblot is shown in Supplementary Fig. 6b, c). b Endogenous protein interactions were confirmed in whole cell lysates from microglia by immunoprecipitation with anti-PPARα or anti-IgG, and examined by immunoblotting with anti-Per2 (n = 3 independent experiments) (the repeated immunoblot is shown in Supplementary Fig. 6d, e). c Per2OE microglia following a 24-h incubation with 10 μM GW6471, a potent PPARα antagonist, to inhibit PPARα expression. The expression of Gpx4 was measured by western blotting. d Quantification of relative levels of Gpx4 protein (n = 3 biological repeats for each group). e The expression of Gpx4 was measured by qRT-PCR (n = 4 biological repeats for each group). f We proposed a model of Per2 regulating the microglial ferroptosis. Lipid peroxidation induces Per2 expression via the MAPK signaling pathway. Per2 subsequently inhibits the expression of Gpx4 by directly interacting with PPARα, which promotes microglia ferroptosis. All data are presented as the mean ± SD. P values were determined by one-way ANOVA with Tukey’s multiple-comparisons test (d, e). *P < 0.05; **P < 0.01; ns not significant.
Within 3 dpi may be the optimal time window for alleviating microglial ferroptosis
Since primary injury is inevitable after SCI, effectively reducing the damage caused by secondary injury has become a top priority. Sufficient microglia are essential for the response to injury and ensuring the survival of neurons and oligodendrocytes following an insult7. Ferroptosis significantly reduces the number of microglia, necessitating the early identification of a therapeutic time window for targeting microglial ferroptosis. Here, we found that microglia underwent ferroptosis at 1 dpi and that microglial ferroptosis peaked at 3 dpi, and gradually decreased after 7 dpi (Fig. 1a). Thus, we hypothesize that intervention is imperative in the first 3 days following SCI. To confirm this, ferrostatin-1 (Fer-1), a classic ferroptosis inhibitor54, was administered on days 0, 1, and 2 post-SCI. Fer-1 significantly decreased the protein expression of Acsl4 in microglia at 3 dpi (Fig. 8a, c). Furthermore, we quantified the number of microglia at 7 dpi and found that, compared with the control treatment, Fer-1 effectively prevented the death of microglia (Fig. 8b, d). Together, these results demonstrate that reducing ferroptosis within 3 days post-SCI is a feasible and effective strategy to prevent microglial death and thus allow microglia to exert neuroprotective effects. Within 3 days post-SCI may represent the optimal therapeutic window for alleviating microglial ferroptosis.
a, b Immunofluorescence image showing expression of Hexb and Acsl4 at the lesion epicenter in SCI mice and SCI + Fer-1 mice at 3 d and 7 d after SCI. Scale bar, 100 μm or 20 μm. c, d Quantification of the Hexb+/Acsl4+ of the total Hexb+ microglia and the number of microglia (n = 5 animals per group). All data are presented as the mean ± SD. P values were determined by two-tailed unpaired Student’s t-test (c, d). ***P < 0.001.
Taken together, our results indicate that lipid peroxidation induces Per2 expression via the MAPK signaling pathway. Per2 subsequently inhibits the expression of Gpx4 by directly interacting with PPARα, thereby increasing the susceptibility of microglia to ferroptosis. Microglial ferroptosis impairs the protective effect of microglia on the spinal cord after SCI, which potentially contributes to the poor prognosis after SCI.
Discussion
The pathological process that occurs after SCI has not yet been fully elucidated, contributing to the challenge of developing therapeutic interventions for such injuries. In our study, Per2 was found to be upregulated in microglia in the spinal cord after SCI and to promote microglial ferroptosis. In vivo, microglia-specific knockout of Per2 inhibited microglial ferroptosis, increasing neural cell survival and promoting functional recovery after SCI. In contrast, overexpression of Per2 via a lentivirus in vitro facilitated microglial ferroptosis. Through RNA-Seq analysis, we revealed that Gpx4, a key ferroptosis-related protein, was downregulated by Per2. Co-IP analysis identified PPARα, a lipid metabolism regulation-associated protein, as a binding partner and potential intracellular target of Per2. Per2 was found to aggravate microglial ferroptosis after SCI by interacting with PPARα and further reducing the expression of Gpx4. Thus, we provide evidence that Per2 may be a promising therapeutic target for SCI (Fig. 7f).
In neonatal mice, SCI initiates a scar-free healing process orchestrated by microglia. Depletion of microglia disrupts this reparative process, impeding axonal regrowth55, underscoring the crucial role of microglia in SCI. In the early stages of SCI, activated and extensively proliferating microglia actively contribute to the formation of a glial scar. This scar reduces the infiltration of immune cells into the parenchyma thereby diminishing the death of neurons and oligodendrocytes and promoting locomotor recovery7. SCI triggers the recruitment of monocytes, which differentiate into monocyte-derived macrophages (MDMs)56. The absence of microglia delays the recruitment of MDMs to the injured spinal cord. Instead, these obstructed cells invade adjacent white matter, leading to a series of inflammatory events6. These findings underscore the importance of preserving a sufficient number of microglia at the site of injury. In agreement with a previous report7, our results revealed that microglia at the injury site undergo immediate cell death after SCI, followed by robust proliferation to counteract the homeostatic imbalance caused by injury. However, it is worth further investigating why the number of microglia peaks at 3 d and then gradually decreases.
In the present study, we collected spinal cord tissues at 0.25 day, 1 day, 3 days, and 7 dpi to measure the levels of biomarkers of ferroptosis. The results revealed that the changes in the levels of ferroptosis indicators were most significant at 3 d after SCI, confirming that SCI induces ferroptosis in the injured spinal cord. Compared with neurons and astrocytes, microglia exhibit a higher capacity for iron storage15,57, and display the earliest and most prominent response to iron overload16. This heightened susceptibility renders microglia particularly prone to ferroptosis. In a diabetes model, high glucose was found to result in the accumulation of lipid droplets in microglia, which subsequently exacerbates neuroinflammation in the hippocampus by surrounding the inflammatory amplification factor TREM 1 within cells58. In addition, microglia with abnormal lipid droplet aggregation exhibit impaired lipid metabolism and secrete more ROS36, increasing the sensitivity of the cells to ferroptosis. In the present study, we observed the accumulation of lipid droplets in microglia after SCI, which may be a novel mechanism contributing to microglial ferroptosis following SCI. Previous reports have suggested that myelination failure due to recurrent microvascular ischemia in ageing individuals with white matter injury results in the production of a large amount of myelin debris, which is rich in iron and lipid, and the pathological accumulation of myelin debris in microglia increases susceptibility to lipid peroxidation injury and ferroptosis, which appears to be the major mechanism of white matter injury in Alzheimer’s disease and vascular dementia59. In our study, the microglia at the lesion epicenter underwent ferroptosis on the first day following SCI, and microglial ferroptosis peaked on the third day and subsequently exhibited a declining trend, exhibiting a unique spatiotemporal pattern. Microglial ferroptosis plays a significant role in regulating microglial populations. In the initial stages after SCI, microglial proliferation supersedes ferroptosis, increasing the number of microglia. Nevertheless, as ferroptosis becomes predominant, it markedly reduces the microglial number. This phenomenon could explain the rapid decline in microglial number observed at 7 days after SCI. However, the involvement of other cell death modalities in this process was not further investigated in our study, and the activated microglia consist of M1 and M2 phenotypes, which express different major markers, and they can polarize their functions in a continuum between two extremes as they receive and integrate environmental signals60,61, thus, the observed changes in microglia cell number could be partly due to altered expression of the marker protein, which are limitations of our study.
Circadian rhythms exert a dominant influence on mammalian metabolism and physiology, with disruptions leading to various metabolic disorders and heightened susceptibility to cancer62. Emerging evidence underscores intriguing interactions between circadian rhythms and lipid metabolism. The proliferation and differentiation of oligodendroglia are time-of-day dependent, and crucial for myelin formation. The absence of Bmal1 results in dysregulated oligodendroglia proliferation and morphology, contributing to aberrant myelination and subsequently impacting cognitive and motor functions63. Per2, acting as a negative feedback regulator of Bmal1, plays an essential role in regulating circadian rhythms and participates in lipid metabolism. Previous studies have suggested that Per2 reduces the expression of SERBP1c, inhibits lipid synthesis in glioblastoma, and suppresses glioblastoma proliferation64. Grimaldi et al. found that Per2-deficient mice present altered lipid metabolism, with drastic reductions in total triacylglycerol and non-esterified fatty acid levels65. Here, we knocked out Per2 specifically in microglia to explore the role of Per2 in ferroptosis. On the one hand, Per2-deficiency in microglia significantly reduced the ferroptosis after SCI. On the other hand, surviving and activated microglia secreted IGF-1 to stimulate astrocytes to generate glial scars, which corral inflammatory cells within areas of damaged tissue and protect adjacent viable neural tissue66. Furthermore, Per2OE and Per2GKO amplified and mitigated microglial ferroptosis in vitro, respectively. Consequently, our study reveals that Per2 exacerbates microglial ferroptosis after SCI.
Ferroptosis is regulated through numerous known pathways, however, Gpx4, an essential regulator of lipid peroxidation, serves as the core regulator of ferroptosis, with its activity determining the outcome of ferroptosis-related disease67. In our study, RNA-Seq revealed that Per2OE microglia inhibited the expression of Gpx4, increasing microglial susceptibility to ferroptosis. The exacerbation of ferroptosis induced by Per2OE was mitigated by increasing the level of Gpx4. We next asked how Per2 modulates the expression of Gpx4. PPARα, a member of the PPAR family, which also includes PPARβ and PPARγ68, regulates the expression of genes associated with lipid metabolism and peroxisome proliferation51. It has been reported that PPARα directly induces Gpx4 expression by binding to a PPRE within intron 3, suppressing ferroptosis in the mouse liver52. Moreover, Per2, which acts as a coactivator of PPARα, has been shown to promote the expression of uncoupling protein 1 in brown adipose tissue69. These findings reveal the potential regulatory relationships among Per2, PPARα, and Gpx4. To verify this finding, we performed Co-IP and identified PPARα as a putative Per2-interacting protein in microglia. Furthermore, following the administration of a PPARα antagonist, the reduction in Gpx4 expression induced by Per2OE was reversed. Thus, Per2 may regulate Gpx4 expression by directly interacting with PPARα, which may be an underlying mechanism of microglial ferroptosis during SCI.
Microglia play a pivotal role in orchestrating cellular interactions with astrocytes and MDMs, contributing to scar formation after SCI and protecting uninjured neurons and oligodendrocytes from inflammation-mediated tissue damage6,7. However, microglial ferroptosis promotes pathological processes in SCI by diminishing the microglial number. Consequently, the identification of an optimal time window for the initiation of treatments targeting microglial ferroptosis is needed. During the subacute phase post-injury, inflammation and cell death reduce the survival of peripheral nerve cells70, and the formation of a mature glial scar in the chronic phase inhibits axon regeneration after SCI71, which emphasizes that SCI should be treated in the acute phase without delay. Because microglial ferroptosis after SCI exhibits unique spatiotemporal patterns, we administered ferroptosis inhibitors within 3 dpi. The results indicated that this intervention significantly reduced the degree of microglial ferroptosis at 3 dpi and prevented the death of a substantial number of microglia at 7 dpi. Thus, our findings suggest that the initial 3 dpi may represent a critical therapeutic window for alleviating microglial ferroptosis.
In conclusion, our findings suggest that microglia Per2, through the PPARα-Gpx4 axis, facilitates microglial ferroptosis after SCI, which may adversely affect prognosis. These findings support the promising therapeutic potential of Per2 antagonists for ameliorating microglial ferroptosis following SCI. Interestingly, KL044, a stabilizer of the clock protein cryptochrome (CRY), leads to the extension of the circadian period and repression of Per2 activity. Although further experiments are needed for validation, this might be a potential strategy for pharmacologically targeting Per2 in SCI72.
Materials and methods
Animals
Male mice were employed for this study. Per2flox/-, Hexb-CreERT2 mice and global Per2 knockout mice (C57B/6J background) were procured from Shanghai Model Organisms Center, Inc., while all mice were bred at the Laboratory Animal Center of Ningxia Medical University. Wild-type C57BL/6J mice (8–10 weeks of age) were sourced from the Laboratory Animal Center of Ningxia Medical University. The inducible HexbCreERT/+ mice were crossed with Per2flox/flox mice to obtain microglia-specific knockout of Per241, in which tamoxifen-induced Cre expression results in chronic recombination in microglia. The obtained Per2flox/flox: HexbCreERT/+ (Per2CKO) and Per2flox/flox: HexbCreERT/- (Per2flox/flox) mice were used for experiments at 8–10 weeks of age. All mice were housed under controlled conditions of constant temperature (21 ± 3 °C) and humidity (50% ± 5%) with a 12-h light/dark cycle. Access to food and water was provided ad libitum. Animal experiments were conducted in accordance with protocols approved by the Laboratory Animal Ethical and Welfare Committee of the Laboratory Animal Center of Ningxia Medical University (Approval number: IACUC-NYLAC-2023-015), and we have complied with all relevant ethical regulations for animal use.
Tamoxifen preparation and treatment
Tamoxifen (Sigma Aldrich, USA) was dissolved in corn oil (Aladdin, China) at a concentration of 20 mg/ml by shaking overnight at 37 °C. To induce Cre recombinase, 7-week-old male mice received daily intraperitoneal injections of tamoxifen at 75 mg/Kg body weight for 5 days73. There is a 7-day waiting period between the final injection and subsequent experiments were conducted.
SCI model and drug treatment
Depending on the experiment performed, mice were randomly assigned to various groups. To establish the SCI model, mice underwent anesthesia with isoflurane (RWD, China) and underwent laminectomy at vertebral level T9-10, corresponding to spinal segment T10-11. A spinal cord impactor (RWD, China) with a 1.5 mm diameter was employed to impact the spinal cord by dropping a rod (weight 5 g, height 6.5 cm)26. The sham group underwent the same laminectomy without rod strikes. Following the procedure, the overlying muscular layers were sutured, and the cutaneous layers were stapled. The heating pad was used to maintain the temperature of the mice until they were fully awake. Postoperatively, the mice’s bladders were manually emptied twice daily to prevent urinary tract infections and hydronephrosis until bladder function recovery. For pain management, buprenorphine (0.05 mg/kg) was administered subcutaneously every 8 h for 3 consecutive days after establishing the SCI model. The ferrostatin-1 (Fer-1), a classic ferroptosis inhibitor, was administered intraperitoneal injection at 10 mg/kg body weight74 on days 0, 1, and 2 post-SCI. Fer-1 was dissolved in 2% DMSO + 40% PEG 300 + 2% Tween80 + saline. All the above reagents were purchased from Med Chem Express, USA. Exclude animals that either died or were in severely compromised condition following the experiment. Depending on the experiment performed, mice were killed by transcardiac perfusion at 1, 3, 7, 14, 35, and 42 days post-contusion. Given the circadian rhythms of Per2 levels, all specimens were collected between 2–4 PM.
Measurement of ferroptosis
Ferroptosis indicators include malondialdehyde (MDA), iron (Fe), glutathione (GSH), and glutathione Peroxidase (GSH-Px). MDA, a typical lipid peroxidation product, directly reflects the level of lipid peroxidation. GSH serves as a reliable indicator of the cellular redox state, while GSH-Px plays a crucial role in the antioxidant defense system of living organisms. The levels of Fe, GSH, and GSH-Px were quantified using corresponding kits (Elabscience, China). MDA levels were detected using an available MDA assay kit (Solarbio, China). All assays were performed following the manufacturer’s instructions.
Behavioral evaluation
The Basso Mouse Scale (BMS), a method developed by Basso and colleagues, was employed to evaluate the recovery of locomotor function after SCI75. Pre-operation and on days 1, 3, 7, 14, 28, 35, and 42 post-surgical, all mice were allowed to crawl freely and evaluated by two trained investigators who were blind to the experiment design. Footprint analysis was used to evaluate the gait recovery and motor coordination as described elsewhere26. The forelimbs and hindlimbs of the mice were colored with blue and red dyes, respectively, to record stride lengths and widths 42 days after injury.
Transmission electron microscopy (TEM)
Mice were sacrificed 3 days post-SCI, and 1 mm3 fragments containing the lesion site were collected from spinal cord tissue. The tissues were fixed, dehydrated, permeated, embedded, and polymerized before being sectioned into ultrathin sections (60–80 nm). These sections were stained with uranyl acetate and lead citrate before observation of the ultrastructure using transmission electron microscopy (Hitachi, Japan).
Primary microglia isolation and culture
Adult-like microglia were obtained using a previously described method43. Briefly, the head neuroepithelial layer (NEL) was isolated from mice at embryonic stage 13.5 days (E13.5), which contained microglia progenitors and neuroepithelial cells. The NEL was cultured for 14–21 days, with neuroepithelial cells acting as feeders for microglia proliferation and maturation. The cultured NEL was incubated with microbeads-coupled anti-CD11b mAb (Miltenyi Biotec, Germany) at 4 °C for 15 min. CD11b-positive cells (adult-like microglia) were isolated by magnetic-activated cell sorting (MACS) system (Miltenyi Biotec, Germany). The microglia were cultured in DMEM (Gibco, USA) containing 10% FBS (Procell, China), 1% penicillin: streptomycin (Solarbio, China), and 0.1% GlutaMAX (Gibco, USA) at 37 °C and 5% CO2. The purity of the primary microglia was validated by Iba1 immunostaining: mouse anti-Iba 1 (1:100, Abcam, ab283319).
Under the circumstances that intervention was required, primary microglia were exposed to 1600 μM iron (Ammonium iron citrate, MedChemExpress, USA) and 1 μM RSL3 (MedChemExpress, USA) to induce ferroptosis. Primary microglia were pre-treated with PD169316 (Med Chem Express, USA), a selective p38 MAPK inhibitor, for 1 h at the final concentration of 10 μM76, or with 500 nM selenium (Sigma-Aldrich, USA), an effective Gpx4 agonist, for 24 h before ferroptosis inducers exposure. Per2OE primary microglia pre-treated with GW6471 (Med Chem Express, USA), a potent PPARα antagonist, for 24 h at the final concentration of 10 μM before ferroptosis inducers exposure. The RSL3, PD169316, and GW6471 were dissolved in dimethyl sulfoxide (DMSO, Med Chem Express, USA). Iron and selenium were dissolved in DMEM (Gibco, USA).
Lentivirus transfection
Primary microglia were plated at a density of 1.4 × 105 cells per well in a 6-well plate and cultured for 24 h. The cells were transfected with Per2 overexpression or empty vector lentiviral particles (Genechem Biotechnology, China) at a multiplicity of infection (MOI) of 50. The medium was replaced 16 h post-transfection, and Per2 levels were assessed by western blotting 72 h post-transfection.
RNA sequencing
Total RNA was extracted following the TRIzol reagent instruction manual (Life Technologies, USA). RNA concentration and purity were measured with NanoDrop 2000 (Thermo Fisher Scientific, USA). RNA integrity was evaluated using the RNA Nano 6000 Assay Kit on the Agilent Bioanalyzer 2100 system (Agilent Technologies, USA). Subsequently, sequencing libraries were generated using the Hieff NGS Ultima Dual-mode mRNA Library Prep Kit for Illumina (Yeasen Biotechnology (China) Co., Ltd.) as per the manufacturer’s recommendations. The Illumina NovaSeq platform generated 150 bp paired-end reads, and raw reads underwent further processing using the BMKCloud bioinformatic pipeline tool, available on the www.Biocloud.net online platform. Log2 |foldchange| > 2 with a false discovery rate (FDR) < 0.01 were considered significant.
Immunofluorescence staining
Mice spinal cord segments containing the lesion site collected at 0.25, 1, 3, 7, and 14 days post-SCI were preserved in 4% paraformaldehyde (PFA). Paraffin-embedded sections underwent dewaxing, dehydration, antigen retrieval, and blocking. Subsequently, sections were incubated overnight at 4 °C with the following primary antibodies: rabbit anti-Hexb (1:200, Absin, abs133376), rabbit anti-Acsl4 (1:200, Abcam, ab155282), rabbit anti-Per2 (1:200, Thermo Fisher Scientific, PA5-100107), rabbit anti-Ki67 (1:200, Servicebo, GB111141-100), and rabbit anti-Neun (1:300, Servicebo, GB11138). Fluorescent-labeled secondary antibodies (red: 1:300, Servicebio, GB21301; green: 1:400, Servicebio, GB25303) were applied for 50 min, and DAPI (Servicebio, G1012) was used for nuclear counterstaining. Imaging was performed on an ortho-fluorescence microscope (Nikon Eclipse C1, Japan). Primary microglia exposed to 1600 μM iron and 1 μM RSL3 for 18 h underwent immunofluorescence staining. Cells were incubated overnight at 4 °C with the rabbit anti-Acsl4 (1:200, Abcam, ab155282).
To detect the lipid droplets of microglia, the spinal cords were extracted, fixed in 4% paraformaldehyde for 48 h, cryoprotected in 30% sucrose, and then sectioned using a freezing microtome (Thermo Fisher Scientific). Sections were stored at −20 °C in cryoprotectant solution (ethylene glycol, glycerol, 0.1 M phosphate buffer pH 7.4, 1:1:2 by volume) until used for immunohistochemistry imaging. Free-floating sections were washed three times in PBS, followed by 0.5 h blocking with 3% BSA (Servicebio, GC305010). Sections were incubated overnight at 4 °C with primary antibodies: rabbit anti-Hexb (1:200, Absin, abs133376). After the primary antibody incubation, sections were washed three times with PBS, and fluorescent-labeled secondary antibodies (red: 1:300, Servicebio, GB21301) were applied for 50 min at room temperature (RT). Sections were washed once in PBS and incubated in PBS with BODIPY™ 493/503 (1:1000, Thermo Fisher Scientific, D3922) to stain lipid droplets for 20 min at RT. Then, DAPI (Servicebio, G1012) was used for nuclear counterstaining.
Images were acquired using a fluorescence microscope (Olympus, Japan). All the measurements’ location was taken from the lesion epicenter, and quantitative analysis was performed using Image J software.
Cell viability assays
For cell viability assays, primary microglia were seeded at 1 × 104 cells/well in 96-well plates. Ferroptosis was induced by supplementing the culture medium with 1600 μM iron + 1 μM RSL3 for 24 h. CCK-8 reagent (10 μl; US Everbright, USA) was added to each well for 1 h at 37 °C, and cell viability was detected using a Multimode plate reader (PerkinElmer, USA) at 450 nm.
Real-time quantitative PCR
Total RNA extraction used TRIzol reagent (Life Technologies, USA), with cDNA synthesis by HiScript® III All-in-one RT SuperMix Perfect for qPCR (Vazyme, China). Then qRT-PCR was performed on the IQ5 PCR System (Bio-Rad, USA) with a ChamQ Universal SYBR qPCR Master Mix (Vazyme, China). Relative expression of target mRNA was normalized to β-actin expression. The expression of the gene was quantified with a modification of the 2−ΔΔCt method. The primer sequences are available in the Supplementary Table 1.
Western blot
The cells underwent lysis utilizing a comprehensive whole-cell lysis assay kit (Keygen Biotech, China). Subsequently, the supernatant was utilized for protein concentration determination through a BCA protein assay kit (Keygen Biotech, China). Equivalent protein quantities were separated using SDS-PAGE, transferred onto PVDF membranes, and blocked with 5% skim milk. Following this, the membranes were subjected to overnight incubation at 4 °C with the following primary antibodies: rabbit anti-Per2 (1:1000, Thermo Fisher Scientific, PA5-100107), rabbit anti-Gpx4(1:5000, Abcam, ab125066), mouse anti-PPARα (1:1000, Thermo Fisher Scientific, MA1-822), rabbit anti-P38 (1:1000, Proteintech, 14064-1-AP), rabbit anti-p-P38 (1:1000, Proteintech, 28796-1-AP), and mouse anti-β-actin (1:40000, Proteintech, 81115-1-RR). After three washes with TBST, the membranes were exposed to a secondary antibody (1:6000, Proteintech, SA00001-1; 1:6000, Proteintech, SA00001-2) at room temperature for 1 h. Visualization of all bands occurred on a gel imaging system (Amersham, USA) using an ECL kit (Seven Biotech, China). Subsequent quantitative analysis was conducted using Image J software.
Co-immunoprecipitation (Co-IP)
Co-IP was conducted using the PierceTM Classic Magnetic IP/CO-IP Kit (Thermo Fisher Scientific, 88804). Protein extraction from microglia employed IP lysis buffer containing protease inhibitor (Thermo Fisher Scientific, 78425), and the resulting supernatant was employed for protein concentration determination via the BCA protein assay kit (Keygen Biotech, China). Protein incubation with rabbit anti-Per2 (5 μg, Novus, NB100-125)/mouse anti-PPARα (5 μg, Thermo Fisher Scientific, MA1-822) or IgG Isotype control (5 μg, Thermo Fisher Scientific, 10500C)/IgG2b Isotype control (5 μg, Thermo Fisher Scientific, MA1-10427) occurred at 4 °C overnight, forming an antigen-antibody complex. The next day, this complex was incubated with Pierce protein A/G magnetic beads at room temperature for 1 h. Subsequent steps involved the removal of the supernatant, elution of magnetic beads with elution buffer, and magnetic bead separation using a magnetic rack (Vazyme, China). This process left the supernatant containing the target antigen. The western blot followed the previously mentioned procedure. Membranes were incubated with mouse anti-PPARα (1:1000, Thermo Fisher Scientific, MA1-822)/rabbit anti-Per2 (1:1000, Novus, NB100-125), followed by incubation with a secondary antibody (1:10000, Proteintech, SA00001-1)/antibody (1:10000, Proteintech, SA00001-2). Pulled-down protein was visualized on a gel imaging system as described earlier.
Enzyme-linked immunosorbent assay (ELISA)
Primary microglia were seeded at 2 × 105 cells/well in 6-well plates, treated with 1600 μM iron + 1 μM RSL3 for 24 h, and the supernatant was collected for analysis. Whole blood from WT and Per2GKO mice or Per2CKO and Per2flox/flox mice was centrifuged to obtain serum. IGF-1 concentration was measured using mouse IGF-1 ELISA kits (Elabscience, China) following the manufacturer’s instructions.
Nissl staining
Tissue sections prepared for immunofluorescence staining were used for Nissl staining as well. Sections underwent dewaxing, dehydration, staining with Nissl staining solution (Servicebio, China), differentiation, and washing. Images were captured using a microscope.
Statistics and reproducibility
The reproducibility of our findings was confirmed by at least three independent experiments. Data are presented as mean ± standard deviation (SD). P values were calculated using one-way ANOVA for multiple comparisons involving a single variable, a two-tailed Student’s t-test for pairwise comparisons, and a two-way ANOVA for multiple comparisons involving two variables. The images were analyzed and quantified using Image J. Details of statistical analyses and N values are found in the Figure legends. N indicates biological replicates. GraphPad Prism (v 9.1.1) facilitated data visualization and statistical analyses, with a p < 0.05 considered statistically significant. The numerical source data is presented in Supplementary Data 1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Supplementary Data 1 contains the source data for the graphs in the main figures. Supplementary Data 2 contains the original uncropped blot images of the main figures. The other data supporting the findings of this study are available from the corresponding author upon reasonable request. The RNA sequencing data generated in this study have been deposited in the NCBI Gene Expression Omnibus (GEO) under accession number GSE303249, and GSE303325.
References
McDonald, J. W. & Sadowsky, C. Spinal-cord injury. Lancet 359, 417–425 (2002).
Li, C. et al. Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Sig Transduct. Target. Ther. 7, 65 (2022).
Han, D. et al. Plasma Hemopexin ameliorates murine spinal cord injury by switching microglia from the M1 state to the M2 state. Cell Death Dis. 9, 181 (2018).
Ren, Y., Zhou, X. & He, X. Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen. Res. 9, 1787 (2014).
Kierdorf, K. et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013).
Brennan, F. H. et al. Microglia coordinate cellular interactions during spinal cord repair in mice. Nat. Commun. 13, 4096 (2022).
Bellver-Landete, V. et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 10, 518 (2019).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic. Cell Death. Cell 149, 1060–1072 (2012).
Yamada, N. et al. Iron overload as a risk factor for hepatic ischemia-reperfusion injury in liver transplantation: potential role of ferroptosis. Am. J. Transpl. 20, 1606–1618 (2020).
Wang, C. et al. Forsythoside A mitigates Alzheimer’s-like pathology by inhibiting ferroptosis-mediated neuroinflammation via Nrf2/GPX4 axis activation. Int. J. Biol. Sci. 18, 2075–2090 (2022).
Zhu, K. et al. Glycyrrhizin attenuates hypoxic-ischemic brain damage by inhibiting ferroptosis and neuroinflammation in neonatal rats via the HMGB1/GPX4 pathway. Oxid. Med. Cell. Longev. 2022, 8438528 (2022).
Xie, Y. et al. Ferroptosis: process and function. Cell Death Differ. 23, 369–379 (2016).
Feng, S.-Q. et al. Deferoxamine promotes recovery of traumatic spinal cord injury by inhibiting ferroptosis. Neural Regen. Res. 14, 532 (2019).
Ge, M. et al. Zinc attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury by activating Nrf2/GPX4 defense pathway. CNS Neurosci. Ther. 27, 1023–1040 (2021).
Song, N., Wang, J., Jiang, H. & Xie, J. Astroglial and microglial contributions to iron metabolism disturbance in Parkinson’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 967–973 (2018).
Ryan, S. K. et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 26, 12–26 (2022).
García-Montero, C. et al. Abnormal histopathological expression of klotho, ferroptosis, and circadian clock regulators in pancreatic ductal adenocarcinoma: prognostic implications and correlation analyses. Biomolecules 14, 947 (2024).
Liu, J., Yang, M., Kang, R., Klionsky, D. J. & Tang, D. Autophagic degradation of the circadian clock regulator promotes ferroptosis. Autophagy 15, 2033–2035 (2019).
Sujino, M., Nagano, M., Fujioka, A., Shigeyoshi, Y. & Inouye, S. T. Temporal profile of circadian clock gene expression in a transplanted suprachiasmatic nucleus and peripheral tissues. Eur. J. Neurosci. 26, 2731–2738 (2007).
Ma, D. et al. PER2 inhibits proliferation and stemness of glioma stem cells via the Wnt/β-catenin signaling pathway. Oncol. Rep. 44, 533–542 (2020).
Zhang, Y. et al. Circadian Period 2 (Per2) downregulate inhibitor of differentiation 3 (Id3) expression via PTEN/AKT/Smad5 axis to inhibits glioma cell proliferation. Bioengineered 13, 12350–12364 (2022).
Shu, Y. et al. Attenuation by time-restricted feeding of high-fat and high-fructose diet-induced NASH in mice is related to Per2 and ferroptosis. Oxid. Med. Cell. Longev. 2022, 1–20 (2022).
Ahmed, S. A. & Abdel-Rahman, A. Estrogen replacement restores period 2 mediated inhibition of ferroptosis and mitigates cardiac dysfunction in estrogen deficient rats. J. Pharmacol. Exp. Ther. 392, 103385 (2024).
Yu, Q. et al. Glutathione-modified macrophage-derived cell membranes encapsulated metformin nanogels for the treatment of spinal cord injury. Biomater. Adv. 133, 112668 (2022).
Zhang, Y. et al. Modes of brain cell death following intracerebral hemorrhage. Front. Cell. Neurosci. 16, 799753 (2022).
Jiang, T. et al. SIRT1 attenuates blood-spinal cord barrier disruption after spinal cord injury by deacetylating p66Shc. Redox Biol. 60, 102615 (2023).
Yang, Y. et al. Tungsten-based polyoxometalate nanoclusters as ferroptosis inhibitors modulating S100A8/A9-mediated iron metabolism pathway for managing intracerebral haemorrhage. J. Nanobiotechnol. 23, 122 (2025).
Cao, W. et al. FOXP3 promote the progression of glioblastoma via inhibiting ferroptosis mediated by linc00857/miR-1290/GPX4 axis. Cell Death Dis. 15, 239 (2024).
Can, C. et al. Exosomal circ_0006896 promotes AML progression via interaction with HDAC1 and restriction of antitumor immunity. Mol. Cancer 24, 4 (2025).
Yao, S. et al. Mesenchymal stem cell attenuates spinal cord injury by inhibiting mitochondrial quality control-associated neuronal ferroptosis. Redox Biol. 67, 102871 (2023).
Gao, M. et al. Role of mitochondria in ferroptosis. Mol. Cell 73, 354–363.e3 (2019).
Gao, S. et al. Cepharanthine attenuates early brain injury after subarachnoid hemorrhage in mice via inhibiting 15-lipoxygenase-1-mediated microglia and endothelial cell ferroptosis. Oxid. Med. Cell. Longev. 2022, 4295208 (2022).
Lee, H. et al. Cell cycle arrest induces lipid droplet formation and confers ferroptosis resistance. Nat. Commun. 15, 79 (2024).
Olzmann, J. A. & Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 20, 137–155 (2018).
Bai, Y. et al. Lipid storage and lipophagy regulates ferroptosis. Biochem. Biophys. Res. Commun. 508, 997–1003 (2019).
Marschallinger, J. et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 23, 194–208 (2020).
Ahuja, C. S. et al. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 3, 17018 (2017).
BEATTIE, M. S., FAROOQUI, A. A. & BRESNAHAN, J. C. Review of current evidence for apoptosis after spinal cord injury. J. Neurotrauma 17, 915–925 (2000).
Xiong, W. et al. Treg cell-derived exosomes miR-709 attenuates microglia pyroptosis and promotes motor function recovery after spinal cord injury. J. Nanobiotechnol. 20, 529 (2022).
Hadian, K. & Stockwell, B. R. SnapShot: ferroptosis. Cell 181, 1188–1188.e1 (2020).
Masuda, T. et al. Novel Hexb-based tools for studying microglia in the CNS. Nat. Immunol. 21, 802–815 (2020).
Askew, K. et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 18, 391–405 (2017).
You, M.-J., Rim, C., Kang, Y.-J. & Kwon, M.-S. A new method for obtaining bankable and expandable adult-like microglia in mice. J. Neuroinflammation 18, 294 (2021).
Yeo, H. et al. Transcription factor EGR1 regulates the expression of the clock gene PER2 under IL-4 stimulation in human keratinocytes. J. Investig. Dermatol. 142, 2677–2686 (2022).
He, Y. et al. Nanoporous titanium implant surface promotes osteogenesis by suppressing osteoclastogenesis via integrin β1/FAKpY397/MAPK pathway. Bioact. Mater. 8, 109–123 (2022).
Faulkner, J. R. et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 24, 2143–2155 (2004).
Lalancette-Hébert, M., Gowing, G., Simard, A., Weng, Y. C. & Kriz, J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 27, 2596–2605 (2007).
Stockwell, B. R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401–2421 (2022).
Alim, I. et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177, 1262–1279 (2019).
Puppa, L. D., Savaskan, N. E., Brauer, A. U., Behne, D. & Kyriakopoulos, A. The role of selenite on microglial migration. Ann. N. Y. Acad. Sci. 1096, 179–183 (2007).
Paumelle, R. et al. Hepatic PPARα is critical in the metabolic adaptation to sepsis. J. Hepatol. 70, 963–973 (2019).
Xing, G. et al. PPARα alleviates iron overload-induced ferroptosis in mouse liver. EMBO Rep. 23, e52280 (2022).
Chen, J. et al. Sulfuretin exerts diversified functions in the processing of amyloid precursor protein. Genes. Dis. 8, 867–881 (2020).
Yan, H. et al. Ferroptosis: mechanisms and links with diseases. Sig Transduct. Target. Ther. 6, 49 (2021).
Li, Y. et al. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature 587, 613–618 (2020).
Donnelly, D. J. & Popovich, P. G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 209, 378–388 (2008).
Absinta, M. et al. A lymphocyte–microglia–astrocyte axis in chronic active multiple sclerosis. Nature 597, 709–714 (2021).
Li, Q. et al. Impaired lipophagy induced-microglial lipid droplets accumulation contributes to the buildup of TREM1 in diabetes-associated cognitive impairment. Autophagy 19, 2639–2656 (2023).
Adeniyi, P. A. et al. Ferroptosis of microglia in aging human white matter injury. Ann. Neurol. 94, 1048–1066 (2023).
Murray, P. J. Macrophage polarization. Annu. Rev. Physiol. 79, 541–566 (2016).
Hu, X. et al. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 11, 56–64 (2014).
Sahar, S. & Sassone-Corsi, P. Metabolism and cancer: the circadian clock connection. Nat. Rev. Cancer 9, 886–896 (2009).
Rojo, D. et al. BMAL1 loss in oligodendroglia contributes to abnormal myelination and sleep. Neuron 111, 3604–3618 (2023).
Yao, J. et al. Lycium barbarum glycopeptide targets PER2 to inhibit lipogenesis in glioblastoma by downregulating SREBP1c. Cancer Gene Ther. 30, 1084–1093 (2023).
Grimaldi, B. et al. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 12, 509–520 (2010).
O’Shea, T. M., Burda, J. E. & Sofroniew, M. V. Cell biology of spinal cord injury and repair. J. Clin. Invest. 127, 3259–3270 (2017).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Tontonoz, P., Hu, E., Graves, R. A., Budavari, A. I. & Spiegelman, B. M. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 8, 1224–1234 (1994).
Chappuis, S. et al. Role of the circadian clock gene Per2 in adaptation to cold temperature. Mol. Metab. 2, 184–193 (2013).
He, X. et al. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: mechanisms and therapeutic opportunities. Cell Prolif. 55, e13275 (2022).
Silver, J. & Miller, J. H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156 (2004).
Lee, J. W. et al. Development of small-molecule cryptochrome stabilizer derivatives as modulators of the circadian clock. ChemMedChem 10, 1489–1497 (2015).
Castranio, E. L. et al. Microglial INPP5D limits plaque formation and glial reactivity in the PSAPP mouse model of Alzheimer’s disease. Alzheimers Dement. 19, 2239–2252 (2022).
Ding, Z. et al. Inhibition of spinal ferroptosis-like cell death alleviates hyperalgesia and spontaneous pain in a mouse model of bone cancer pain. Redox Biol. 62, 102700 (2023).
Basso, D. M. et al. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 23, 635–659 (2006).
Wang, H. et al. TNF-α derived from arsenite-induced microglia activation mediated neuronal necroptosis. Ecotoxicol. Environ. Saf. 236, 113468 (2022).
Acknowledgements
We would like to thank the Ningxia Key Laboratory of Stem Cell and Regenerative Medicine, Institute of Medical Sciences and Laboratory Animal Center of Ningxia Medical University for providing the platform and technical support. This study was supported by the National Natural Science Foundation of China (NO. 82260256 and 82060120); Key Research and Development Program of Ningxia Hui Autonomous Region (NO. 2022BEG01004 and No. 2020BFG02009); Central Leading Local Science and Technology Development Special Project (NO. 2022FRD05032) and Program of Ningxia Science and Technology Leading Talent (NO. 2024GKLRLX07).
Author information
Authors and Affiliations
Contributions
Hechun Xia and Heng Fan conceived of and designed the study. Pengfei Bie conducted the data analysis and wrote the manuscript. Pengfei Bie, Dongpo Su, Yang Gao, Liang Wu, Zhanfeng Niu, Yanbin Zhao, He He, Zhanfeng Jiang, Zhong Zeng and Yaolin Zhang acquired experimental data. All authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Shao-Ming Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Ivo Lieberam and Benjamin Bessieres. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bie, P., Su, D., Gao, Y. et al. Per2 deficiency in microglia alleviates motor dysfunction by inhibiting ferroptosis in spinal cord injury. Commun Biol 8, 1234 (2025). https://doi.org/10.1038/s42003-025-08664-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08664-x
This article is cited by
-
Ferroptosis-associated transcriptional factors in neurological diseases: molecular mechanisms and therapeutic prospects
Experimental & Molecular Medicine (2025)