Abstract
White matter tracts (WMTs), which mediate information transmission in the brain, are closely associated with the pathogenesis of psychiatric disorders, yet the causality of their associations remain unclear. Thus, we employed two-sample bidirectional Mendelian randomization to explore the causality between WMTs and 10 psychiatric disorders. We found that one standard deviation changes of WMTs metrics modified risks for 8 psychiatric disorders by 2·2% to 71·4%. For example, increased fornix/stria terminalis radial diffusivities elevated PTSD risk by 8.3%, while heightened mode anisotropy reduced Tourette syndrome risk by 71.4%. Reversely, alcohol use disorder increased the risk of WMTs abnormalities. Our study provides novel insights into the potential causality between WMTs and psychiatric disorders, indicating that alterations of WMTs may serve as biomarkers for psychiatric disorders.
Similar content being viewed by others
Introduction
Psychiatric disorders impose a substantial burden on global health, as evidenced by the Global Burden of Diseases 20211, which shows that eight psychiatric disorders are among the top 25 contributors to global disease burden. Recently, a large body of literature has shown that white matter tracts (WMTs), which are responsible for information transmission, are closely related to the onset, development, and prognosis of psychiatric disorders2,3,4,5. Researchers often utilize diffusion tensor imaging (DTI) sequence of magnetic resonance imaging (MRI) to visualize WMTs and assess their integrity6,7, as well as measure potential damage to axons8, myelin8, and cell membranes8. These functional changes in DTI9,10 are characterized by five parameters/metrics: fractional anisotropy (FA), mean diffusivities (MD), axial diffusivities (AD), radial diffusivities (RD), and mode of anisotropy (MO). Different WMTs exhibit variations in both structure and function. For readers seeking further explanations, please refer to Table 1.
Research has indicated that certain characteristics of WMTs could potentially serve as diagnostic markers for psychiatric disorders11. For example, patients with major depressive disorder (MDD) who had suffered non-suicidal self-injury showed reduced WMT integrity compared to healthy controls12 and appeared to have more severe dysfunction of the kynurenine/tryptophan pathway13. Compared with MDD, the alterations of WMTs in patients with bipolar disorder (BD) were more evident14. However, many studies focus solely on the FA value to represent changes in the properties of WMTs, as summarized in Table S1, while neglecting the contributions of other parameters such as MD, RD, AD, and MO. In addition, although many studies have shown associations between WMTs and psychiatric disorders, the causality and directions of these associations are still unclear. Therefore, the lack of clarity about the causal association between WMTs and psychiatric disorders, as well as the neglection of other important parameters, has limited the application of DTI techniques in the field of psychiatric disorders9.
While causal inference in observational studies was commonly confounded by complex factors, such as the medications and environment15, Mendelian randomization (MR) studies can effectively mitigate these confounding effects by utilizing genetic polymorphisms as instrumental variables (IVs) for exposure16. The core idea of MR is to “infer causality between exposure and outcome using genetic variants as IVs, leveraging the free and random assortment of genes and the stability of genotypes against environmental influences to avoid confounding bias in traditional epidemiology effectively”17. Current MR research has explored the causal association between imaging-derived phenotype and 10 psychiatric disorders18, between brain functional networks and 12 psychiatric disorders19, between white matter hyperintensities and anxiety disorders20, between brain structure and Alzheimer’s disease21, and between cortical structure, white matter microstructure, and neurodegenerative diseases22. These studies revealed some causal associations between brain structure and function and neuropsychiatric disorders. However, these studies fail to uncover the causalities between WMTs and psychiatric disorders. Thus, we conducted bidirectional MR in this study to explore the causal associations between WMTs and psychiatric disorders. We found that the changes of WMTs metrics modified risks for 8 psychiatric disorders. Reversely, alcohol use disorder (AUD) increased the risk of WMTs abnormalities. Our study provides novel insights into the potential causality between WMTs and psychiatric disorders, indicating that alterations of WMTs may serve as biomarkers for psychiatric disorders.
Results
Overview of MR
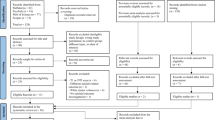
The research design is illustrated in Fig. 1. and the baseline characteristics of genome-wide association studies (GWAS) are described in Table 2. Among all included GWAS data of psychiatric disorders, only AUD and post-traumatic stress disorder (PTSD) included a small portion of UK Biobank (UKB) data, with the sample overlap being <5%. The GWAS sample size of psychiatric disorders ranged from 14,307 to 1,222,882. Further descriptions and downlaod links of GWAS data can be obtained in Supplementary Data 1 and Supplementary Data 2. All MR processes followed the Burgess23 and Strengthening the Reporting of Observational Studies in Epidemiology guidelines24 and the checklist was in Table S2.
AD axial diffusivities, ADHD attention deficit hyperactivity disorder, ASD autism disorder, AUD alcohol use disorder, BD bipolar disorder, CUD cannabis use disorder, FA fractional anisotropy, MD mean diffusivities, MDD major depression disorder, MO mode of anisotropy, MR mendelian randomization, PTSD post-traumatic stress disorder, RD radial diffusivities, SCZ schizophrenia, TS Tourette syndrome, WMTs white matter tracts, OUD opioid use disorder.
Before addressing confounding factors, we screened 1667 single nucleotide polymorphism (SNP) loci as IVs for forward MR (Supplementary Data 3) and 301 for reverse MR (Supplementary Data 4), leaving 1376 and 301, respectively, after confounders removal (Supplementary Data 5–8). Besides previously reported confounders such as socioeconomic status and education, additional non-brain structural and nonpsychiatric factors were included, such as pulse pressure (rs2645466), coronary artery disease (rs4894803), and uterine leiomyoma or ER-positive breast cancer (rs10828248), etc. (Supplementary Data 7). After outliers detection (Supplementary Data 9–10), 11,745 SNPs pairs between WMTs and psychiatric disorders were validated for forward MR analysis (Supplementary Data 11), and 30,343 SNPs pairs between psychiatric disorders and WMTs were validated for reverse MR analysis (Supplementary Data 12).
In the results of forward MR, 150 WMTs-psychiatric disorders pairs were nominally significant (Praw < 0.05). After conducting sensitivity analysis, limiting the number of SNPs and performing FDR correction, we identified significant associations between WMTs and: attention deficit hyperactivity disorder (ADHD) (1/108 tests), Tourette syndrome (TS) (1/108), BD (1/109), schizophrenia (SCZ) (3/109), MDD (1/109), opioid use disorder (OUD) (2/103), AUD (1/109), and PTSD (14/109). Additionally, we observed the presence of reverse causal associations between AUD and WMTs. The results and outlier analysis details of forward MR and reverse MR can be found in Supplementary Data 13–20. During statistical analysis, the F values of the IVs in the final results were all >20, and the statistical power was >90%. All reported associations we reported below had passed FDR correction (FDR P < 0.05; see Supplementary Data 13 and Supplementary Data 20 for full statistics). Finally, our findings indicate causal associations between WMTs and SCZ, BD, PTSD, MDD, AUD, OUD, ADHD, and TS and found reverse causal associations of AUD with four types of WMTs.
Causal effects of white matter tracts on ADHD and TS
As shown in Fig. 2 and Supplementary Data 20, we uncovered causal associations of the column and body of fornix (FX) with ADHD, as well as of the fornix (cres)/stria terminalis (FXST) with TS. Specifically, for ADHD, a one s.d. increase in MO of the FX was associated with a 31·3% increase in the odds of ADHD (OR = 1·313, 95% CI = 1·117–1·543, Praw < 9·96 × 10−4, FDR-P < 4·89 × 10−2). Conversely, for TS, a one s.d. increase in MO of the FXST was linked to a 71·4% decrease in the odds of TS (OR = 0·286, 95% CI = 0·166–0·493, Praw < 6·61 × 10−6, FDR-P < 5·44 × 10−4).
The forest plot illustrates the significant causalities. The effect estimates displayed in the figure were calculated by IVW method, representing the OR of psychiatric disorders per 1 s.d. change in WMTs. And the error bars repersent the 95% CI. Only FDR-significant (FDR p < 0.05) that passed heterogeneity and pleiotropytests, sensitivity analysis, and confounding factor adjustment were presented in the figure.
Causal effects of white matter tracts on BD and SCZ
As shown in Fig. 2 and Supplementary Data 20, we identified a causal association of FXST and BD, as well as of FXST, superior corona radiata (SCR), and retrolenticular part of internal capsule (RLIC) with SCZ. We found that a one s.d. increase in MO of the FXST was associated with a 33·4% decrease in odds of BD (OR = 0·666, 95% CI = 0·544–0·816, Praw < 8·63 × 10−5, FDR-P < 7·48 × 10−3). Specifically, one s.d. increase in FA of the FXST was associated with a 37·6% decrease in SCZ risk (OR = 0·724, 95% CI = 0·618–0·849, Praw < 6·74 × 10−5, FDR-P < 5·24 × 10−3). Conversely, one s.d. increase in FA of the SCR led to 16·0% elevation in SCZ risk (OR = 1·160, 95% CI = 1·058–1·272, Praw < 1·09 × 10−4, FDR-P < 3·72 × 10−2). Additionally, one s.d. increase in MO of RLIC was linked to a 22·5% (OR = 1·225, 95% CI = 1·105–1·357, Praw < 1·55 × 10−3, FDR-P < 5·24 × 10−3) increase in odds of SCZ.
Causal effects of white matter tracts on MDD, OUD, and AUD
As shown in Fig. 2 and Supplementary Data 20, we identified causal associations of the genu of corpus callosum (GCC) with MDD and OUD, as well as of the inferior fronto-occipital fasciculus (IFO) with AUD. For OUD, one s.d. increase in MD and RD of the GCC region was linked to 11·4% (OR = 0·886, 95% CI = 0·824–0·952, Praw < 9·30 × 10−4, FDR-P < 3·62 × 10−2) and 12·7% (OR = 0·873, 95% CI = 0·803–0·949, Praw < 1·48 × 10−3, FDR-P < 3·62 × 10−2) decreases in risk respectively. Similarly, one s.d. increase in MD was linked to 12·2% elevation in MDD risk (OR = 1·122, 95% CI = 1·054–1·195, Praw < 3·12 × 10−4, FDR-P < 3·27 × 10−2). Furthermore, one s.d. increase in AD of the IFO was associated with a 7·9% decrease in AUD risk (OR = 0·921, 95% CI = 0·883–0·961, Praw < 1·51 × 10−4, FDR-P < 5·52 × 10−3).
Causal effects of white matter tracts on PTSD
As shown in Fig. 2 and Supplementary Data 20, we uncovered causal associations between PTSD and 7 WMTs: cingulum connecting to hippocampus (CGH), FXST, posterior corona radiata (PCR), RLIC, superior fronto-occipital fasciculus (SFO), superior longitudinal fasciculus (SLF) and uncinate fasciculus (UNC). One s.d. increase in FA for PCR, SFO, SLF, and UNC were found to decrease the risk of PTSD by 2·2% (OR = 0·978, 95% CI = 0·960–0·996, Praw < 1·82 × 10−2, FDR-P < 4·79 × 10−3), 4.3% (OR = 0·957, 95% CI = 0·930–0·986, Praw < 3·16 × 10−2, FDR-P < 1·53 × 10−2), 2.3% (OR = 0·977, 95% CI = 0·959–0·995, Praw < 1·40 × 10−2, FDR-P < 3·94 × 10−3), and 3.8% (OR = 0·962, 95% CI = 0·938–0·987, Praw < 2·89 × 10−3, FDR-P < 1·47 × 10−3), respectively. Furthermore, one s.d. increases in MD of CGH (OR = 1·036, 95% CI = 1·017–1·055, Praw < 1·40 × 10−4, FDR-P < 2·21 × 10−3), SFO (OR = 1·042, 95% CI = 1·013–1·072, Praw < 4·74 × 10−3, FDR-P < 1·91 × 10−2) and UNC (OR = 1·040, 95% CI = 1·011–1·070, Praw < 2·89 × 10−3, FDR-P < 2·36 × 10−2), RD of CGH (OR = 1.050, 95% CI = 1·025–1·076, Praw < 7·94 × 10−5, FDR-P < 1·67 × 10−3), FXST(OR = 1·083, 95% CI = 1·050–1·117, Praw < 4.25 × 10−7, FDR-P < 2·67 × 10−5), PCR (OR = 1·025, 95% CI = 1·007–1·043, Praw < 2.08 × 10−2), RLIC (OR = 1·027, 95% CI = 1·009–1·045, Praw < 3·73 × 10−3, FDR-P < 1·68 × 10−2) and SLF (OR = 1·023, 95% CI = 1·006–1·041, Praw < 7·88 × 10−3, FDR-P < 2·61 × 10−2), and AD of PCR (OR = 1·047, 95% CI = 1·016–1·080, Praw < 2·97 × 10−3, FDR-P < 1·49 × 10−3) contributed to an increased risk of PTSD. Lastly, one s.d. increase in MO of the ACR was associated with a 6% decrease in the odds of PTSD (OR = 0·940, 95% CI = 0·895–0·988, Praw < 1·45 × 10−2, FDR-P < 4·03 × 10−3).
Reverse Mendelian randomization
As shown in Fig. 3. and Supplementary Data 20, we identified reverse causal associations of AUD with CGC, body of corpus callosum (BCC) and ACR. Higher risks of AUD was associated with decreased FA (IVW β = −0·246, 95% CI = −0·392 to −0·093, Praw < 1·53 × 10−3, FDR-P < 1·53 × 10−3) and increased RD (IVW β = 0·259, 95% CI = 0·111–0·406, Praw < 5·84 × 10−4, FDR-P < 5·85 × 10−4) of CGC, increased MD (IVW β = 0·236 95% CI = 0·074–0·398, Praw < 4·22 × 10−3, FDR-P < 4·22 × 10−3) and increased AD (IVW β = 0·229, 95% CI = 0·075–0·383, Puncorrected < 3·60 × 10−3, FDR-P < 3·59 × 10−3) in BCC.
The forest plot illustrates the significant causalities. The effect estimates displayed in the figure were calculated by IVW method. And the error bars repersent the 95% CI. All statistical tests were two-sided, and only significant results (FDR-P < 0.05) that passed heterogeneity and pleiotropytests, sensitivity analysis, and confounding factor adjustment were presented in the figure.
Multivariate Mendelian randomization and validation
We conducted multivariate MR analysis25 on SCZ, OUD, and PTSD, and only the association between FXST_RD and PTSD (OR = 1·063, 95% CI = 1·004–1·125, P < 3·61 × 10−2) was retained. More details can be found in Supplementary Data 21. Subsequently, we partially validated our results using GWAS data for ADHD, AUD, BD, and SCZ from the FinnGen R11 database. The causal association between the MO of FX and ADHD in the forward MR analysis was replicated (OR = 1·806, 95% CI = 1·127–2·892, P < 1·39 × 10−2). Although BD did not yield identical replication results, it produced similar results: one s.d. increase in MO of FX (OR = 1·486, 95% CI = 1·065–2·072, P < 1·97 × 10−2) and RD of FXST (OR = 1·335, 95% CI = 1·025–1·740, P < 3·21 × 10−2) were associated with increasing risk of BD, and one s.d. increase FA of FXST (OR = 0·776, 95% CI = 0·613–0·982, P < 3·50 × 10−2) was associated with decreasing risk of BD. Taking into account the close spatial relationship (with a certain degree of overlap) between FX and FXST, as well as the potential correlations among the parameters FA, RD and MO, these similar results of BD could also be considered a successful replication.
Confounding factors, outliers, and sensitivity analysis
To quantify confounding effects, we conducted parallel MR analyses using both pre-exclusion (N = 1667 SNPs) and post-exclusion (N = 1376 SNPs) IVs across all pairs. The comparative results (Supplementary Data 7 and Supplementary Data 16) revealed that only 2 associations exhibited meaningful changes of IVW estimates before/after confounder adjustment (among the 24 statistically significant associations): FX_MO-ADHD and GCC_MD-MDD transitioned from nonsignificant to significant (P > 0.05 → P < 0.05), while all others maintained consistent directionality and significance thresholds. Specifically, 1 SNP related to ascending thoracic aortic diameter was removed from FX_MO. And 6 SNPs, primarily associated with lipids, Alzheimer’s disease, aging, and glutamic-oxaloacetic transaminase levels, were removed from the IVs of GCC_MD. Crucially, no associations showed β value reversals.
Regarding outliers, among the 24 significant results, 11 were obtained after excluding outliers: FXST_FA-SCZ (3), ACR_MO-PTSD (3), FXST_RD-PTSD (1), PCR_AD-PTSD (1), PCR_RD-PTSD (1), RLIC_RD-PTSD (2), SFO_MD-PTSD (3), SLF_FA-PTSD (4), SLF_RD-PTSD (4), UNC_FA-PTSD (3), and GCC_MD-MDD (3). Importantly, all IVW β values maintained directional consistency after outlier removal, indicating that the impact of outlier removal on the results is acceptable (please see the details in Supplementary Data 9 and Supplementary Data 17).
For sensitivity analyses, MR-PRESSO global tests and MR-Egger intercepts showed no evidence of pleiotropy (all P > 0.05). While we observed directional discrepancies between IVW and MR-Egger β values for FXST_FA-SCZ, the MR-Egger estimates were nonsignificant (all P > 0.05), and the direction of IVW β values were consistent with MR-median and MR-mode methods. Furthermore, among the 24 significant WMTs-psychiatric disorder associations, 16 showed evidence of sample overlap influence (p_difference < 0.05). Crucially, all associations maintained consistent β value directionality before and after sample overlap adjustment (Supplementary Data 20). Notably, corrected effect estimates did not attenuate toward the null point; instead, effects strengthened through increased absolute β values. This directional reinforcement aligns with and further substantiates our primary findings. Finally, we observed coordinated alterations across all DTI parameters. These findings collectively confirm the reliability of our MR causal inferences (please see the details in Supplementary Data 18).
Discussion
To our knowledge, the current study represents the most comprehensive investigation to date of the causal associations between WMTs and psychiatric disorders. As summarized in Fig. 4, our findings not only establish forward causal associations between 24 WMTs-psychiatric disorder pairs but also reveal reverse causal associations between AUD and WMTs. These results indicate that WMTs may hold the potential to act as target regions for diagnosis or intervention in psychiatric disorders.
Recent MR studies have investigated the causal associations between imaging-derived phenotype (IDP) and psychiatric disorders, encompassing brain functional networks19, cortical structures21, white matter microstructure22,26, and combinations of the three19. Building upon previous research, we have incorporated additional DTI parameters, expanded the scope to include more psychiatric disorders potentially related to WMTs, and significantly increased the sample sizes of psychiatric disorders. Guo et al.18 utilized the GWAS data from Smith et al.27 to validate the causal associations between IDP (which includes WMTs) and 10 psychiatric disorders, uncovering causal associations between WMTs and SCZ, as well as anorexia nervosa. Comparing with Guo et al.18, we have incorporated additional DTI parameters (AD and RD), and expanded the scope to include more psychiatric disorders potentially related to WMTs (AUD, OUD and cannabis use disorder (CUD)). To minimize the potential bias, we excluded psychiatric disorders with a high risk of producing diagnosis bias based on our clinical experience. For example, generalized anxiety disorder and panic disorders are classified equally as anxious disorders in the database. But classifying them this way makes the different illnesses seem more similar than they really are, which makes it difficult for us to get interpretable results from data. Furthermore, we significantly increased the sample sizes (ADHD: from 53,293 to 225,534; BD: from 51,710 to 413,466; MDD: from 142,646 to 713,314; PTSD: from 146,660 to 1,222,882) of psychiatric disorders. Moreover, we implemented more specialized control measures to overcome limitations inherent in previous confounder and outlier removal methods. As previous GWAS studies had demonstrated, the genetic traits of brain structure were significantly stronger than those of brain functional networks27. Therefore, compared to brain networks, utilizing SNPs related to brain structure would be more advantageous in revealing causal associations from a genetic perspective, yielding results with greater generalizability.
Although imaging indicators are indirect proxies, current studies tend to interpret decreases in FA and increases in MD as indicators of white matter myelin integrity damage6,7 or axonal damage8. MO designates the type of anisotropy as a continuous measure, indicating differences in diffusion tensor shape ranging from planar (flattened cylinders) to linear (tubes)7, which can help to map the direction of the WMTs. When dealing with more complex fiber structures where multiple fiber orientations coexist within a single voxel, techniques such as fiber orientation distribution are employed to measure this complexity. In such cases, MO can be utilized to signify the predominant or mean orientation when numerous fiber directions are identified. Although the association between WMTs and psychiatric disorders varies according to age, gender, medication, population (e.g., twins), disease severity stratification, and imaging control methods, as our mini review shown (Supplementary Materials), the preponderance of evidence currently supported that decrease in FA, increase in MD and RD, and alteration of AD were associated with the risk of psychiatric disorders and the severity of progression. Similarly, our MR results supported this tendency and aligned closely with 3 distinct patterns of neuropathology observed in the investigation of WMTs in patients5: (1) developmental abnormalities in limbic fibers (CGH, FX, and FXST), (2) abnormal maturation in long-range association fibers (IFO), and (3) severe developmental abnormalities and accelerated aging in callosal fibers (GCC and BCC).
The first main finding of this study is the causal associations between limbic system-related fibers (FXST or FX) and psychiatric disorders (SCZ, BD, ADHD, and TS). FXST, composed of FX and ST, is a fiber bundle associated with the limbic system10: the FX connects to the hippocampus, and the ST connects to the amygdala. Thus, alterations in FXST may contribute to emotional dysregulation28,29 (in SCZ, BD, TS, related to the amygdala) or cognitive impairments28,29 (in ADHD, related to the hippocampus). We speculated that the similar results of ADHD, TS, BD and SCZ are partly coming from the overlap of their similar pathogenesis, as suggested by evidence linking WMTs integrity in these regions to both genetic influences and environmental exposures, particularly in psychiatric developmental disorders like ADHD and autism spectrum disorder (ASD)4,30, which could partly explain the similarity of some clinical symptom (such as mood instability). Furthermore, we notice that the significant results of FXST or FX are mostly related to the MO parameter, which potentially suggests that the loss of complexity in WMTs may be the underlying process of psychiatric disorders. In conjunction with the existing evidence of close associations between the limbic cortical regions, limbic fibers and psychiatric disorders10,28,29, we can conclude that both the cortex and WMTs of the limbic system are inextricably linked to the risk of ADHD, TS, BD, and SCZ.
The second main finding of this study is the results of PTSD and other psychiatric disorders. The results of PTSD suggested that it is an environmentally dependent psychiatric disorder with a relatively weaker association with genetics, which aligned with previous research31,32,33. Furthermore, we found a causal association between GCC and MDD, while GCC has connections with the frontal cortex and is involved in sensory and visuospatial processing34,35, and it is closely related to social functioning impairment and dissociative symptoms33. Besides, the associations between WMTs and AUD in forward MR and reverse MR indicated that the substance itself (alcohol) is a significant risk factor in the occurrence of substance abuse disorders, but genetic factors are also influential.
This study had several limitations. Firstly, some overlap (<5%) was unavoidable in the samples, potentially introducing bias into our results. Although we used MRlap analysis to evaluate the effect of sample overlapping, MRlap could only evaluate the aggregate effects of sample overlap on results (indicating bias direction) without enabling precise quantification of bias effect size. Given that the MRlap findings ultimately aligned with our primary results, we conclude that while sample overlap introduced detectable bias, such bias would not alter the direction or attenuate the magnitude of our effect estimates. Secondly, we could not assess the impact of population and diagnostic stratification on the research results due to the use of publicly available GWAS databases. Thirdly, imaging data are indirect evidence, which limits our ability to infer the exact neurobiological processes from them. Fourthly, although we have controlled for both confounding factors and outliers, there could still be omitted factors and also potentially overall controlled factors (which are unnecessary and likely cause false negatives). Lastly, caution should be exercised when interpreting the clinical implications of OR values estimated by MR, as it utilizes risk SNP of exposure to explore the lifelong impact of exposures on outcomes, rather than the effects of specific interventions over a period of time, and cannot be equated with RCT studies.
In summary, by leveraging a genetically informed causal inference framework within the context of established (though often correlative) links between WMT microstructure and psychiatric disorders, our findings suggested potential causal associations between WMTs characteristics and psychiatric disorders through MR, which exhibited both similarities and differences—a pattern contingent upon the clinical feature similarities among different psychiatric disorders. This genetically informed approach suggests that specific WMTs characteristics could serve as hallmarks for psychiatric disorders11, providing referential targeted regions for psychiatric disorders (diagnosis or intervention), thereby facilitating future clinical practice and scientific research.
Methods
Data sources
Data on white matter microstructure were obtained from Zhao et al.36. Zhao et al. conducted a GWAS on white matter microstructure using dMRI data from 33,292 individuals in the UKB British cohort, reporting a cumulative total of 9,023,710 SNPs (Chromosomes 1–22). The analysis method for white matter microstructure was derived from DTI models using the ENIGMA-DTI pipeline. They analyzed five primary DTI metrics (FA, MD, AD, RD, MO) across 21 brain WMTs, generating 110 DTI phenotypes for each individual. A detailed description of the GWAS can be found in Supplementary Data 1. The GWAS summary statistics are publicly available at Zenodo (https://zenodo.org/records/4549730), and the results can also be browsed through the BIG-KP knowledge portal (https://bigkp.org/).
We sourced data from public databases for psychiatric disorders, prioritizing GWAS-summary-level data when available. To minimize bias in results due to sample overlap between exposure and outcome, as well as ethnic differences, our selection criteria for psychiatric disorder GWAS data were as follows: (1) exclusion of UKB data; (2) inclusion of individuals of European descent (EUR); (3) if UKB data were included, the total sample size had to exceed 732,640 (ensuring a maximum sample overlap rate of <5%). According to Burgess et al., a 5% sample overlap in MR studies is estimated to introduce a bias of <0·15%37. The GWAS data on 10 psychiatric disorders, including ADHD2, ASD38, SCZ39, BD40, MDD41, PTSD42, AUD43, OUD44, CUD45, and TS46, were sourced from public databases. The GWAS sample size of psychiatric disorders ranged from 14,307 to 1,222,882.
Missing data processing
We performed SNP position conversion based on chromosome and position for GWAS data lacking SNP ID using the GRCh37 reference. We matched the missing effective allele frequencies (EAF) using data from the author’s tutorial or the 1000 Genomes Project. If EAF remained missing after this step, we substituted it with EAF = 0·5.
Selection of instrumental variables (IVs)
MR analysis relies on three crucial assumptions: (1) the IVs should be associated with the exposure; (2) the IVs should be independent of confounding factors; (3) the IVs should only influence the outcome through the exposure directly. These assumptions necessitate a strong correlation between IVs and exposure and independence, and the pleiotropy of IVs does not interfere with the association between the exposure and the outcome16. The first two assumptions are primarily ensured through the selection of IVs. We selected SNPs with P < 5 × 10−8 to satisfy the strong correlation requirement. To ensure the independence of SNPs, we initially remove linkage disequilibrium (LD) due to close spatial associations, with parameters set as: r2 = 0·001, window size = 1000 kb, gene reference = 1000 GENOME (EUR). Subsequently, we retrieved all traits corresponding to each SNP that passed both the P-value and LD screenings on LDlink (https://ldlink.nih.gov/?tab=home) and excluded those SNPs unrelated to brain imaging changes and psychiatric disorders, thereby controlling for confounding factors. These confounding factors include socioeconomic status, smoking, alcohol consumption, and many other factors that were not individually listed in previous MRs. Furthermore, to mitigate the impact of weak IVs, we calculated the F-statistic to measure the strength of IVs, with an F-statistic > 10 indicating a low risk of using weak instruments in MR analysis. Parameters calculated alongside the F-statistic included R2 (the proportion of variance in the exposure explained by the genetic IVs), n (the sample size of the GWAS for the exposure), and k (the number of genetic IVs for the exposure).
Bidirectional Mendelian randomization
Before conducting the MR analysis, we excluded variants with an EAF < 0·01. Furthermore, to ensure that the SNPs of the IVs originated from the same direction of the DNA strand and could be utilized in both the exposure and outcome datasets, we harmonized the exposure and outcome data and removed palindromic SNPs with EAF close to 0·5 (which could introduce potential strand flip problems).
For the forward MR, WMTs were used as exposure, and psychiatric disorders as outcome, while for the reverse MR, exposure and outcome were exchanged. The primary analytical method for MR was inverse variance weighted (IVW); supplementary analytical methods included Wald-ratio, MR weight median, MR Egger, and MR weighted mode. The statistical significance threshold for association was set at P value < 0.05 with a false discovery rate (FDR) corrected using the IVW method. Specifically, FDR correction was applied per psychiatric disorder using the Benjamini–Hochberg procedure. For each disorder, we corrected for the number of tested WMT phenotypes. The odds ratio (OR) was used to represent the magnitude of the causal effect. Leveraging the multivariate regression concept in multivariate MR25, we explored the presence of dominant traits in WMTs. To examine the generalization of the forward causal association between WMTs and psychiatric disorders, we revalidated the forward MR with the FinnGen R11 dataset47.
Sensitivity analysis and outlier screening
We employed MR-PRESSO, MR Egger intercept, Cochran’s Q statistic48,49, and leave-one-out analysis for sensitivity and pleiotropy assessments. And we used MRlap to assess the influence of sample overlap50. Initially, we used the MR-PRESSO global test to examine horizontal pleiotropy. The intercept of MR Egger represented the average pleiotropy of all IVs, with a value different from zero indicating the presence of directional pleiotropy. Heterogeneity was evaluated using Cocharan’s Q. Lastly, we performed a “leave-one-out” analysis to test whether specific SNPs drove the causal association.
Selection and interpretation of results
Consistent with Carter’s recommendations for cautious interpretation of MR findings51, we implemented a multi-layered framework to ensure biological plausibility alongside statistical significance.
First, all reported results satisfied strict statistical criteria: (1) FDR-adjusted p value < 0·05; (2) No evidence of pleiotropy (MR-PRESSO Global Test p > 0·05, MR-Egger intercept p > 0·05); and (3) Directional consistency across ≥3 of the 4 MR methods.
Second, we established neurobiological coherence criteria for DTI parameters. Given their known physiological interrelationships, causal associations were required to demonstrate directional consistency across metrics/parameters within each WMT. Specifically: (1) Decreased FA must correspond with either increased MD and/or increased RD and/or decreased AD; (2) Increased MD must align with either increased RD or decreased AD. This approach prioritized findings with greater biological plausibility by evaluating directional concordance through established neurobiological mechanisms. For example, reduced FA accompanied by increased MD and RD would consistently indicate myelin impairment. While statistically significant but biologically discordant results (e.g., isolated parameter changes lacking supporting directional patterns) would be dismissed, this tiered evaluation supplemented—rather than replaced—statistical significance thresholds.
Third, we implemented a parallel-instrument analytical approach to address potential overcorrection bias from indiscriminate exclusion of SNPs unrelated to brain imaging or psychiatric disorders. First, parallel MR analyses were conducted using both pre-exclusion and post-exclusion IVs sets. When exclusion of confounder-associated SNPs materially altered results, which was defined as both (1) reversal of β value directionality and (2) transition across the statistical significance threshold (P < 0.05), we conducted a literature search to exam whether there are other studies that could support the alteration of results between the excluded traits and target outcomes.
Statistics and reproducibility
All MR analyses were carried out in the R environment (version 4.4.1), using TwoSampleMR (version 0.6.5), MendelianRandomization (version 0.10.0) and Oneclick (version 5.1.10). All codes we used were provided at https://github.com/Yifan-xyy/Code_for_MR.git. In order to correct for multiple testing in performing forward MR and reverse MR analyses, the FDR method was used. The P value threshold for statistical significance was as follows: FDR-P < 0.05 for forward MR and reverse MR; 0.05 for multivariate MR and validation MR.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Code availability
All analyses were carried out in the R environment (version 4.4.1), using TwoSampleMR (version 0.6.5), MendelianRandomization (version 0.10.0) and Oneclick (version 5.1.10). The code utilized in this study can be obtained through email inquiry or download from link: https://github.com/Yifan-xyy/Code_for_MR.git.
References
Ferrari, Alize J. et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2133–2161 (2024).
Demontis, D. et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat. Genet. 55, 198–208 (2023).
Wong, S. A. et al. Internal capsule microstructure mediates the relationship between childhood maltreatment and PTSD following adulthood trauma exposure. Mol. Psychiatry 28, 5140–5149 (2023).
Andrews, D. S. et al. A longitudinal study of white matter development in relation to changes in autism severity across early childhood. Biol. Psychiatry 89, 424–432 (2021).
Cetin-Karayumak, S. et al. White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol. Psychiatry 25, 3208–3219 (2020).
Douaud, G. et al. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. Neuroimage 55, 880–890 (2011).
Acosta-Cabronero, J., Williams, G. B., Pengas, G. & Nestor, P. J. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain 133, 529–539 (2010).
Song, S. K. et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20, 1714–1722 (2003).
Tae, W.-S., Ham, B.-J., Pyun, S.-B., Kang, S.-H. & Kim, B.-J. Current clinical applications of diffusion-tensor imaging in neurological disorders. J. Clin. Neurol. 14. https://doi.org/10.3988/jcn.2018.14.2.129 (2018).
Mori, S. et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 40, 570–582 (2008).
Delvecchio, G., Pigoni, A., Bauer, I. E., Soares, J. C. & Brambilla, P. Disease-discordant twin structural MRI studies on affective disorders. Neurosci. Biobehav. Rev. 108, 459–471 (2020).
Hu, C. et al. Microstructural abnormalities of white matter in the cingulum bundle of adolescents with major depression and non-suicidal self-injury. Psychol. Med. 54, 1113–1121 (2024).
Wang, L. et al. Associations between the kynurenine pathway and the brain in patients with major depressive disorder-A systematic review of neuroimaging studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 121, 110675 (2023).
Comai, S. et al. Selective association of cytokine levels and kynurenine/tryptophan ratio with alterations in white matter microstructure in bipolar but not in unipolar depression. Eur. Neuropsychopharmacol. 55, 96–109 (2022).
Bouziane, C. et al. White matter by diffusion MRI following methylphenidate treatment: a randomized control trial in males with attention-deficit/hyperactivity disorder. Radiology 293, 186–192 (2019).
Zheng, J. et al. Recent developments in Mendelian randomization studies. Curr. Epidemiol. Rep. 4, 330–345 (2017).
Sanderson, E. et al. Mendelian randomization. Nat. Rev. Methods Primers 2, 6 (2022).
Guo, J. et al. Mendelian randomization analyses support causal relationships between brain imaging-derived phenotypes and risk of psychiatric disorders. Nat. Neurosci. 25, 1519–1527 (2022).
Mu, C., Dang, X. & Luo, X.-J. Mendelian randomization analyses reveal causal relationships between brain functional networks and risk of psychiatric disorders. Nat. Hum. Behav. 8, 1417–1428 (2024).
Zanoaga, M. D. et al. Brainwide Mendelian randomization study of anxiety disorders and symptoms. Biol. Psychiatry 95, 810–817 (2024).
Seyedsalehi, A. et al. Educational attainment, structural brain reserve and Alzheimer’s disease: a Mendelian randomization analysis. Brain 146, 2059–2074 (2023).
Wang, Z. et al. Bidirectional two-sample Mendelian randomization analyses support causal relationships between structural and diffusion imaging-derived phenotypes and the risk of major neurodegenerative diseases. Transl. Psychiatry 14, 215 (2024).
Burgess, S. et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 4, 186 (2019).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA 326, 1614–1621 (2021).
Burgess, S. & Thompson, S. G. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 181, 251–260 (2015).
Li, H. Q. et al. Generalized fear after acute stress is caused by change in neuronal cotransmitter identity. Science 383, 1252–1259 (2024).
Smith, S. M. et al. An expanded set of genome-wide association studies of brain imaging phenotypes in UK Biobank. Nat. Neurosci. 24, 737–745 (2021).
Hermens, D. F. et al. Sex differences in fronto-limbic white matter tracts in youth with mood disorders. Psychiatry Clin. Neurosci. 76, 481–489 (2022).
Barth, C. et al. In vivo white matter microstructure in adolescents with early-onset psychosis: a multi-site mega-analysis. Mol. Psychiatry 28, 1159–1169 (2023).
Hegarty, J. P. et al. A twin study of altered white matter heritability in youth with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 63, 65–79 (2024).
Korgaonkar, M. S. et al. White matter anisotropy and response to cognitive behavior therapy for posttraumatic stress disorder. Transl. Psychiatry 11, 14 (2021).
Dennis, E. L. et al. Altered white matter microstructural organization in posttraumatic stress disorder across 3047 adults: results from the PGC-ENIGMA PTSD consortium. Mol. Psychiatry 26, 4315–4330 (2021).
Ju, Y. et al. White matter microstructural alterations in posttraumatic stress disorder: an ROI and whole-brain based meta-analysis. J. Affect Disord. 266, 655–670 (2020).
Alexander, A. L. et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage 34, 61–73 (2007).
Pryweller, J. R. et al. White matter correlates of sensory processing in autism spectrum disorders. Neuroimage Clin. 6, 379–387 (2014).
Zhao, B. et al. Common genetic variation influencing human white matter microstructure. Science 372, eabf3736 (2021).
Burgess, S., Davies, N. M. & Thompson, S. G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 40, 597–608 (2016).
Grove, J. et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51, 431–444 (2019).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022).
Mullins, N. et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 53, 817–829 (2021).
Als, T. D. et al. Depression pathophysiology, risk prediction of recurrence and comorbid psychiatric disorders using genome-wide analyses. Nat. Med. 29, 1832–1844 (2023).
Nievergelt, C. M. et al. Genome-wide association analyses identify 95 risk loci and provide insights into the neurobiology of post-traumatic stress disorder. Nat. Genet. 56, 792–808 (2024).
Zhou, H. et al. Multi-ancestry study of the genetics of problematic alcohol use in over 1 million individuals. Nat. Med. 29, 3184–3192 (2023).
Deak, J. D. et al. Genome-wide association study in individuals of European and African ancestry and multi-trait analysis of opioid use disorder identifies 19 independent genome-wide significant risk loci. Mol. Psychiatry 27, 3970–3979 (2022).
Levey, D. F. et al. Multi-ancestry genome-wide association study of cannabis use disorder yields insight into disease biology and public health implications. Nat. Genet. 55, 2094–2103 (2023).
Yu, D. et al. Interrogating the genetic determinants of Tourette’s syndrome and other tic disorders through genome-wide association studies. Am. J. Psychiatry 176, 217–227 (2019).
Kurki, M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613, 508–518 (2023).
Bowden, J. et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int. J. Epidemiol. 48, 728–742 (2019).
Greco, M. F., Minelli, C., Sheehan, N. A. & Thompson, J. R. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 34, 2926–2940 (2015).
Mounier, N. & Kutalik, Z. Bias correction for inverse variance weighting Mendelian randomization. Genet. Epidemiol. 47, 314–331 (2023).
Carter, A. R., Fraser, A., Howe, L. D., Harris, S. & Hughes, A. Why caution should be applied when interpreting and promoting findings from Mendelian randomisation studies. Gen. Psychiatr. 36, e101047 (2023).
Hu, R., Stavish, C., Leibenluft, E. & Linke, J. O. White matter microstructure in individuals with and at risk for bipolar disorder: evidence for an endophenotype from a voxel-based meta-analysis. Biol. Psychiatry 5, 1104–1113 (2020).
Li, M., Wang, Y., Tachibana, M., Rahman, S. & Kagitani-Shimono, K. Atypical structural connectivity of language networks in autism spectrum disorder: a meta-analysis of diffusion tensor imaging studies. Autism Res. 15, 1585–1602 (2022).
Parlatini, V. et al. White matter alterations in Attention-Deficit/Hyperactivity Disorder (ADHD): a systematic review of 129 diffusion imaging studies with meta-analysis. Mol. Psychiatry 28, 4098–4123 (2023).
Aboitiz, F., Scheibel, A. B., Fisher, R. S. & Zaidel, E. Fiber composition of the human corpus callosum. Brain Res. 598, 143–153 (1992).
Acknowledgements
We appreciate the user-friendly and open-source code provided by the One Click Analyses team. We want to acknowledge the participants and investigators of the FinnGen R11 study. This work was supported by the National Key Research and Development Program of China (2023YFC2506800), the National Natural Science Foundation of China (no. 82371499) and Young Elite Scientists Sponsorship Program by CAST (2023QNRC001).
Author information
Authors and Affiliations
Contributions
Y.F. Yu, K. Yuan, and S.Z. Ai proposed the topic and main idea. Y.F. Yu, S.Z. Ai, and K. Yuan were responsible for data acquisition, analysis, or interpretation of data. Y.F. Yu wrote the initial draft. K. Yuan, S.Z. Ai, T.Y. Jia, X. Lin, S.H. Chang, Y.P. Bao, J. Sun, T. Gao, and J. Shi commented on and revised the manuscript. All authors contributed to the final draft of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary handling editor: Benjamin Bessieres. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, Y., Jia, T., Lin, X. et al. Unveiling causal relationship between white matter tracts and psychiatric disorders. Commun Biol 8, 1221 (2025). https://doi.org/10.1038/s42003-025-08669-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08669-6
This article is cited by
-
Transferosomes as Advanced Nanocarriers for Enhanced Drug Delivery in Skin Cancer Therapy: A Comprehensive Review
Applied Biochemistry and Biotechnology (2026)
-
Sol–gel synthesized BaMn0.5Ti0.5O3 perovskite: structural, dielectric, and electrical properties with temperature-dependent analysis
Journal of Sol-Gel Science and Technology (2025)
-
Eco-friendly MOF composites for effective photodegradation of ciprofloxacin in water systems
Rendiconti Lincei. Scienze Fisiche e Naturali (2025)
-
A critical review on g-C₃N₄ for environmental remediation: synthesis, properties, and pollutant photodegradation
Rendiconti Lincei. Scienze Fisiche e Naturali (2025)
-
A Comprehensive Review on g-C3N4–TiO2 Nanocomposites for Photocatalytic Removal of Organic Pollutants: Insights into Synthesis and Degradation Mechanisms
Journal of Inorganic and Organometallic Polymers and Materials (2025)