Abstract
Maspin/SerpinB5 is an abundant and pleiotropic protein mostly expressed by epithelia. Initially described as a tumor suppressor, it has been reported as a regulator of cell adhesion, migration, and invasion. How intracellular Maspin orchestrates these processes is poorly understood. In this study, we utilized Affinity purification-Mass spectrometry (AP/MS) alongside in vitro reconstitution assays to establish that Maspin directly interacts with microtubules and microfilaments. Additionally, CRISPR/Cas9-mediated GFP tagging of endogenous Maspin, combined with immunostaining, revealed its localization at the cortical cytoskeleton and the mitotic spindle. Depletion of Maspin by RNAi and CRISPR/Cas9 in non-transformed epithelial cell lines modifies cell-cell contact and promotes cytoskeletal rearrangements. Concomitantly, we observed a modest upregulation of mesenchymal markers during interphase and abnormal cell rounding during mitosis. An evaluation of Maspin’s effect on microtubules revealed that it suppresses their growth in vitro and in cells. Collectively, these results demonstrate that Maspin acts dynamically at the interface of the cytoskeleton and adhesion sites, modulating cell shape.
Similar content being viewed by others
Introduction
The cytoskeleton and its key regulatory proteins are central components in the maintenance of epithelial homeostasis. Cytoskeleton-binding proteins comprehend a myriad of actin-binding proteins1, microtubule-associated proteins2, cytolinkers3 and scaffold proteins4. They provide structural and functional support for cytoskeleton-dependent processes such as cell adhesion, migration and division5. In epithelial cells, the cortical cytoskeleton is dynamically coupled with cell–cell junction assembly and stability6,7, which are often disrupted in cancer8. Maspin/SerpinB5 was initially identified as a tumor suppressor gene in human mammary epithelial cells9. It belongs to the serpin (serine protease inhibitor) family due to structural homology10, but compiled data showed it does not inhibit proteases11. Maspin is abundant in epithelial cells, it is found in different subcellular compartments and it is extracellularly secreted12,13,14. It has pleiotropic biological activities including inhibition of cell proliferation15, migration16,17,18,19, invasion16,20 and metastasis21, increase of apoptosis susceptibility22,23, control of gene transcription24,25 and oxidative stress response26. Most studies done in tumor cell lines or animal models report that Maspin has a tumor suppressor activity9,27,28,29. However, it is becoming well accepted that Maspin has a dual nature, sometimes restraining cancer progression, other times promoting it14,20. The mechanisms by which Maspin regulates cellular processes, and how alterations in these mechanisms contribute to either pro-tumor or anti-tumor behavior, remain largely unknown.
We and others have shown that addition of recombinant Maspin to the cell medium results in modulation of cell adhesion and motility16,30,31. This process depends on the interaction between Maspin and the uPA/uPAR/β1-integrin complex at the cell surface19,31,32,33. In support of these findings, Maspin has been detected in the extracellular space13. Because this complex acts as an essential component of adhesion sites, integrating extracellular cues to the cytoskeleton, it has been proposed that extracellular Maspin indirectly reorganizes the cytoskeleton by acting at the cell surface18,31,34. Despite these observations, it is well known that Maspin is predominantly an intracellular protein both in normal and tumor cells12,35. The role of intracellular Maspin has been investigated mostly by Maspin ectopic gene expression in tumor cells that lost its expression9,27,36,37,38. Intriguingly, Maspin re-expression mirrored/phenocopied most of the observations done in tumor cells exposed to extracellular recombinant Maspin16,17,31,34; that is, enhancement of cell adhesion, inhibition of migration, and promotion of actin cytoskeleton reorganization. Other studies proposed that intracellular Maspin could indirectly modulate key cytoskeletal components either transcriptionally39 or post-transcriptionally40. Altogether, these data suggest a potential interplay between extracellular and intracellular Maspin in regulating cytoskeleton-dependent processes such as adhesion and migration. Whether intracellular Maspin directly interacts with and regulates the cytoskeleton remains completely unknown.
Here, we report that intracellular Maspin dynamically localizes to cytoskeleton-enriched structures such as the cortical cytoskeleton, lamellipodia and mitotic spindle. Maspin directly binds to both microfilaments and microtubules, regulating the dynamics of the latter in vitro and in epithelial cells. These findings further our understanding on the role of intracellular Maspin and may provide mechanistic insights on how it regulates adhesion, migration and morphological changes through the direct association with the cytoskeleton.
Results
AP/MS analysis and TIRF-based in vitro reconstitution assays reveal that Maspin interacts with the cytoskeleton
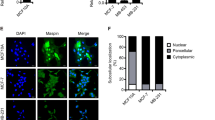
We initially selected non-transformed epithelial cell lines in order to study the function of the underexplored endogenous, intracellular Maspin. Among them, immortalized human and mouse mammary epithelial cells (MCF-10A and EpH4, respectively) and immortalized human keratinocyte cells (HaCaT) express endogenous Maspin. Opposingly, MDA-MB-231 breast cancer cells do not express Maspin and were used as a negative control for protein expression, as observed by Western blot using an anti-Maspin monoclonal antibody (Fig. 1a). We have previously used IP/MS to capture endogenous Maspin interacting proteins from MCF-10A cell lysates. In our analysis, Maspin consistently interacts with α-tubulin and actin-binding proteins41. Given the essential role of microtubules and microfilaments throughout the cell cycle, we explored Maspin’s possible interacting partners during interphase and cell division. To this end, we arrested MCF-10A cells at G2/M transition using nocodazole (Fig. 1b), which could be confirmed by rounded morphology compared to DMSO-treated cells (Fig. 1c). Cell lysates were incubated with recombinant GST-Maspin and GST baits and further purified. The eluates were separated by SDS-PAGE and visualized by silver staining. GST-Maspin and GST were promptly detected (Fig. 1d, arrows) as well as different co-eluted material. Proteomics analysis identified interacting proteins exclusively found or enriched in GST-Maspin baits as represented by heatmap according to LFQ Fold Change values (Fig. 1e and Supplementary Data 1). String database and Gene Ontology integration further indicated that most of the captured interacting proteins are cytoskeleton components (Fig. 1f). Among them, we identified Plectin (PLEC), a plakin family member that links the three main components of the cytoskeleton to adhesion sites42; α-actinin-4 (ACTN4), an actin cross-linking protein43, and subunits of the non-muscle myosin II (MYH9 and MYL6), which controls actomyosin contractility44. For both conditions (DMSO vs. Nocodazole), we consistently detected two interacting proteins - Tubulin Alpha 1c (TBA1C) and Actin Gamma (ACTG) - suggesting that Maspin potentially interacts with cytoskeleton subunits as well. We further confirmed Maspin-actin and Maspin-tubulin interactions in non-arrested condition (no Nocodazole) using pull-down followed by Western blot for actin and tubulin (Fig. 1g). Interestingly, recombinant Maspin captured endogenous Maspin, indicative of possible self-association, as previously observed45,46.
a Maspin protein expression in HaCaT, MCF-10A, EpH4 and MDA-MB-231 cells analyzed by Western blot with an anti-Maspin antibody. Anti-GAPDH antibody was used as a loading control; (b) Workflow of final pull-down eluates submitted to proteomic analysis. MCF-10A cell lysates previously treated with Nocodazole or DMSO were incubated with recombinant GST-Maspin or GST-only negative control as baits; (c) Bright-field image of Nocodazole-arrested (rounded morphology) or DMSO-treated (flatten morphology) MCF-10A cells. Scale bar: 25 µm; (d) GST- and GST-Maspin pull-down eluates were analyzed by SDS-PAGE followed by silver staining. Eluates were submitted to proteomic analysis; (e) Heatmap for interacting proteins exclusively identified or enriched in GST-Maspin samples over GST-only negative control according to normalized Label Free Quantification (LFQ) Fold Change values. Maspin is not depicted (see Supplementary Data 1); (f) Protein-protein interaction network generated by String for all hits found in (e). Black edges represent reported physical interactions and nodes were colored according to protein class. Top 10 Molecular Function terms ranked by FDR score in Gene Ontology analysis (lower); (g) GST-pull-down (same as in d), followed by Western blot against α-tubulin, β-actin and Maspin (two upper membranes). GST-only and GST-Maspin baits were visualized by Ponceau staining prior to immunoblot (lower membrane); (h) Maspin complexed to αβ-tubulin heterodimer (upper) or with β-actin homodimer (lower) according to AlphaFold 3. Maspin RCL (Reactive Center Loop) region is highlighted in cyan; (i) Schematic representation of F-actin in vitro reconstitution assay (upper). Pre-assembled AlexaFluor-labeled F-actin in the presence of purified GFP-Maspin (100 nM) was visualized by TIRF microscopy (lower); (j) Schematic representation of Taxol-stabilized microtubules in vitro reconstitution assay (upper). Pre-assembled TAMRA-labeled microtubules in the presence of purified GFP-Maspin (250 nM) were visualized by TIRF microscopy (lower). Scale bar: 10 μm.
Next, we checked for a presumed direct binding of Maspin to actin and tubulin using AlphaFold 347 (Fig. 1h). Modeling predictions suggested that Maspin interacts mostly through its Reactive Center Loop motif (RCL, aa 334-345) with the surface of α-tubulin (aa 401-450) on the αβ-tubulin heterodimer (ipTM = 0.5). The RCL region was also predicted to interact with a region on the β-actin homodimer (aa 348-355) (ipTM = 0.17). We then validated the direct interaction between Maspin and microfilaments and microtubules using total internal reflection fluorescence (TIRF) microscopy-based in vitro reconstitution assays. To this end, we generated an N-terminal fluorescently-tagged recombinant Maspin and purified it along with the corresponding control empty vector encoding for GFP using a His-StrepII double-tag purification strategy48 (Supplementary Fig. 1a). Tagged Maspin or the control GFP-only were added to pre-assembled F-actin and Taxol-stabilized microtubules and analyzed by TIRF microscopy. Purified recombinant Maspin, but not the control GFP-only (Supplementary Fig. 1b), promptly bound to F-actin (Fig. 1i) and to stabilized microtubules (Fig. 1j) in the nanomolar range in vitro. Collectively, these data indicate that Maspin directly interacts with the cytoskeleton.
Maspin dynamically associates with the cytoskeleton of epithelial cells
The cytoskeleton enables the communication between the cell and extracellular environment49. Given that Maspin was previously observed at the cell cortex and interacts with actin filaments in vitro (Fig. 1i), we hypothesized that intracellular Maspin may be part of the adhesion complexes which allows this communication. In our proteomic assay, we failed to capture classical cell adhesion molecules such as E-cadherin or even β1-integrin, which is a known Maspin interacting protein30. To substantiate our hypothesis, we integrated our AP/MS-identified interacting proteins to an Adhesome dataset, manually curated from different large-scale proteomic studies (Supplementary Fig. 1c). This Adhesome is mainly composed of hemidesmosomes (Integrin-mediated), adherens junctions and desmosomes (E-cadherin and Desmoplakin) and cortical cytoskeleton components (Supplementary Data 2). We found that Adhesome and Maspin share 11 interacting proteins (Supplementary Fig. 1d). They were classified mainly as adhesion-cytoskeleton related terms by GO molecular function analysis (Supplementary Fig. 1e). Interestingly, Maspin was repeatedly captured as part of different adhesion plaques in most of these studies, co-occurring with plakins such as Plectin (Supplementary Fig. 1d), which was captured in our AP/MS analysis (Fig. 1e) and is an integrin interactor50.
In order to track Maspin spatiotemporal distribution in live MCF-10A cells, we fluorescently tagged the C-terminal and N-terminal regions of endogenous Maspin through a CRISPR/Cas9-mediated knock-in strategy (Supplementary Fig. 1f). Western blot analysis for all knock-in stable pools with anti-GFP and anti-Maspin antibodies indicated the expected molecular weight bands for fusion proteins (Supplementary Fig. 1g). As previously observed by immunostaining51,52, endogenously-tagged Maspin is highly abundant and ubiquitously distributed in a diffuse pattern, it locates mostly to the cytoplasm of all three knock-in cells, as observed by fluorescence live cell imaging (Supplementary Fig. 1h). Given that we did not observe substantial changes in Maspin subcellular localization among all three knock-in pools and due to a higher editing efficiency observed for the N1-terminus-tagged Maspin (Supplementary Fig. 1g), we selected this cell population to perform our downstream assays (hereafter named GFP-Puro-N1-Maspin).
In monolayer cell culture, MCF-10A cells are known to spontaneously shift their arrangement according to confluency. At low to medium confluency, they pack into clusters, exhibiting discreet lamellipodial protrusions, whereas typical cobblestone epithelial organization is adopted at high confluency53. To evaluate GFP-Puro-N1-Maspin subcellular localization in cells, we monitored the fluorescence within 24 h post-seeding by live imaging. Notably, Maspin localizes to the leading edge of lamellipodia of collectively migrating cells (Supplementary Video 1). As confluency increases, it becomes mostly confined to cytoplasm, adopting a diffuse pattern (Supplementary Video 1).
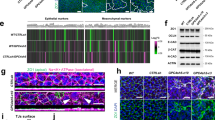
Due to Maspin’s high abundance in cells and the challenges posed by epifluorescence in accurately visualizing its subcellular localization, we co-stained F-actin and α-tubulin in our GFP-Puro-N1-Maspin cells, then analyzed the specimens using confocal microscopy, which provides better spatial resolution. Under standard culture conditions, Maspin is barely detected at the cell cortex (Fig. 2a, left panel, Control), which led us to hypothesize that it dynamically associates with the cortical cytoskeleton depending on the cell density, as observed for some cytoskeleton-binding proteins54. We thus performed a calcium switch assay to monitor Maspin localization during the dynamic re-establishment of cell–cell contacts. To this end, extracellular calcium was depleted from confluent cells with EGTA (Supplementary Fig. 2f) and cells were allowed to recover in complete medium for 1 h. Strikingly, GFP-Puro-N1-Maspin was detected at the cell cortex during the re-establishment of cell–cell contact (Fig. 2a, left panel, Recovery), a process that is highly dependent on the rearrangement of the cortical actin cytoskeleton55. In non-treated cells, we observed Maspin at the lamellipodia of clustered cells at low to medium confluency (Fig. 2b, left panel) and, unexpectedly, at the mitotic spindle (Fig. 2c, left panel). Finally, transient expression of GFP-Maspin in non-transformed RPE-1 cells, which lack endogenous Maspin (Supplementary Fig. 2a), further confirmed its localization at the cell cortex and lamellipodia of individualized cells (Supplementary Fig. 2b).
a Maspin detected at the cell cortex during cell–cell contact re-establishment. Confluent GFP-Puro-N1-Maspin knock-in cells (left panel) were treated with 4 mM EGTA for 45 min, followed by recovery in complete medium for 1 h, fixed and co-stained for F-actin and α-tubulin. WT versus KO cells were treated identically and co-stained for Maspin, F-actin and α-tubulin (middle and right panels); (b) Maspin detected at the lamellipodia. Clustered GFP-Puro-N1-Maspin knock-in cells exhibiting discreet lamellipodial protrusions were fixed and co-stained for F-actin and α-tubulin (left panel). Clustered WT and KO cells were co-stained for Maspin, F-actin and α-tubulin (middle and right panels); (c) Maspin detected at the mitotic spindle. Asynchronous mitotic GFP-Puro-N1-Maspin knock-in cells were fixed and co-stained for F-actin and α-tubulin (left panel). WT versus KO cells were co-stained for Maspin, F-actin and α-tubulin (middle and right panels). Nuclei were stained with Hoechst. Individual Z-slices are depicted in the figure. Scale bars: 10 µm.
We and others56 have observed a heterogeneous staining pattern for different commercially available anti-Maspin antibodies, which may be related to different protein domains used for raising the antibody or Maspin self-assembly45. To confirm Maspin subcellular localization observed in our GFP-Puro-N1-Maspin cells, we employed a monoclonal antibody that often detects the endogenous protein at cytoskeleton-enriched structures. Co-staining of Maspin, F-actin and α-tubulin of MCF-10A WT cells confirmed that the protein locates at the cell cortex in calcium switch assays (Fig. 2a, middle panel and Supplementary Fig. 2g), at the lamellipodia of spreading cells (Fig. 2b, middle panel) and at the mitotic spindle (Fig. 2c, middle panel). To validate this antibody, we established Maspin knock-out (KO) clones using CRISPR/Cas9-genome editing in MCF-10A and HaCaT cells. Sequencing analysis of edited regions confirmed the presence of several insertions and deletions (Supplementary Fig. 2c), which were quantitatively described by Inference of CRISPR Edits (ICE) algorithm57 (Supplementary Fig. 2d). Subsequently, Western blot analysis verified the complete absence of the protein in all KO clones. (Supplementary Fig. 2e). Regardless of the condition, Maspin staining patterns reported above were validated in at least one of our MCF-10A KO clones (Fig. 2a–c, right panels and Supplementary Fig. 2h). Maspin localization at mitotic spindle was further validated in our HaCaT KO clone (Supplementary Fig. 2i). Taken together, these data demonstrate that intracellular Maspin dynamically associates with the cytoskeleton in distinct model systems and under different conditions.
Loss of Maspin induces spatial reorganization of the cytoskeleton
The contribution of Maspin to in vivo lethality has been addressed by different studies. Although divergent data brought controversy to the field58, it became established that Maspin is essential to embryogenesis and tumor suppression59,60,61,62. Downregulation of endogenous Maspin in epithelial cells has been typically carried out using RNAi13,20 and, to our knowledge, Maspin knock-out clones have not been previously reported. Thus, we investigated how F-actin and microtubules are spatially distributed in our MCF-10A KO clones (C10 and D9). Confocal Z-stack sections indicated that cortical F-actin is reduced in KO compared to WT cells (Fig. 3a, b). To rule out the possibility that phenotypic changes associated to Maspin depletion were due to clonal artifacts as previously speculated63, we also transiently knocked-down Maspin in MCF-10A cells using commercially available siRNA sequences with low (siMaspin #60) and intermediate efficiencies (siMaspin #95) (Supplementary Fig. 3a, b) and in EpH4 cells stably expressing shMaspin (Supplementary Fig. 3d, e). Consistent with our KO cells, we observed a similar reduction in cortical F-actin for both MCF-10A siMaspin and EpH4 cells stably expressing shMaspin (Supplementary Fig. 3c and f, respectively). As for microtubules, they appear to become more interspaced and disorganized (Fig. 3a, b), although we could not quantitatively describe these modifications.
a Maximum intensity projections for MCF-10A KO versus MCF-10A WT cells during interphase co-stained for F-actin and α-tubulin. Insets represent reduced cortical F-actin for KO as compared to WT cells. Scale bar: 10 µm; (b) Cortical F-actin fluorescence intensity for MCF-10A WT (n = 59), KO C10 (n = 41 cells) and KO D9 (n = 56 cells); c Live imaging of MCF-10A WT versus KO cells upon mitotic entry. Black arrows indicate retraction tails. Scale bar: 25 µm; (d) Cell area prior to mitotic rounding WT (n = 49 cells), KO C10 (n = 49 cells), KO D9 (n = 49 cells); (e) Maximal tail length for WT (n = 50 cells), KO C10 (n = 48 cells) and KO D9 (n = 50 cells) cells during mitotic progression; (f) Mitotic duration for WT (n = 50 cells), KO C10 (n = 48 cells) and KO D9 (n = 50 cells). Cells were imaged at interval frames of 5 min; (g) MCF-10A cells released after nocodazole blockage and co-stained for F-actin, α-tubulin and nuclei. Heatmaps indicate distribution of F-actin fluorescence intensity in both MCF-10A WT and KO cells. Scale bar: 10 µm and 5 µm (inset); (h) Cell roundness was measured for MCF-10A WT (n = 59 cells), KO C10 (n = 53 cells) and KO D9 (n = 53 cells) at metaphase; (i) Representation of mitotic measurements (d1 and d2); j Spindle length (d2) was measured for WT (n = 43 cells), KO C10 (n = 48 cells) and KO D9 (n = 58 cells); (k) Cortical distance along spindle axis (d1) measured for WT (n = 43 cells), KO C10 (n = 52 cells), KO D9 (n = 52 cells) cells at metaphase; l Cortical distance along spindle axis to spindle length ratio (d1/d2) measured for WT (n = 43 cells), KO C10 (n = 52 cells) and KO D9 (n = 50 cells) at metaphase. n.s. = not significant.
Upon mitotic entry, cells partially lose adhesion to the substrate and acquire a rounded morphology64. Since Maspin was detected at the spindle zone (Fig. 2c, left and middle panels) and it interacts with actin and tubulin in nocodazole-arrested cells (Fig. 1e), we assessed the morphology of rounded cells by bright-field live imaging (Fig. 3c). Interestingly, KO cells occupy a larger area prior to retraction (Fig. 3d) and longer retraction tails that remain connected to the neighboring cells upon mitotic entry (Fig. 3e, Supplementary Video 2). These abnormal retraction tails are usually bipolar, but asymmetric in length (Fig. 3c and e). These changes, however, do not result in modifications in mitotic duration (Fig. 3f) or cell death following mitosis (Supplementary Video 2). Next, we arrested MCF-10A KO cells with nocodazole at mitotic entry and monitored their progression after release from nocodazole-block. F-actin and microtubule staining indicated substantial morphological changes in mitotic cells at metaphase (Fig. 3g). Notably, metaphasic MCF-10A KO cells display reduced roundness (Fig. 3h), with F-actin often randomly distributed at the cell cortex. We then measured mitotic parameters (Fig. 3i) and observed that Maspin KO cells display decreased spindle length (Fig. 3j), which could also be seen in asynchronous MCF-10A-siMaspin cells (Supplementary Fig. 3g) and in thymidine-synchronized EpH4-shMaspin cells (Supplementary Fig. 3h). Because the spindle distance scales according to cell size65 and our synchronized KO cells exhibit reduced size, we measured the cortical distance along the spindle axis (Fig. 3k, d1) to check if reduction in spindle length (Fig. 3j, d2) was due to the reduced cell size. This is true for KO C10 cells, but not for KO D9 cells (Fig. 3l, d1/d2), suggesting that additional factors may contribute to the reduction in spindle length in Maspin KO cells. As Maspin interacts with both microfilaments and microtubules, our KO model system cannot distinguish whether these phenotypes are a consequence of Maspin acting on either one or the other.
In addition to the morphological changes observed for our mitotic KO cells (Fig. 3c–l), these cells partially lose their ability to arrange as regular packed clusters during interphase, occasionally exhibiting an elongated shape (Supplementary Fig. 3i). To gauge for potential cell–cell contact changes, we stained our KO cells for E-cadherin. Notably, we found a mixed pattern for E-cadherin labeling in MCF-10A KO cells, which appeared either as spiky extensions resembling filopodia projections or as cytoplasmic puncta. On the other hand, MCF-10A WT cells exhibit continuous, linear E-cadherin, at cell–cell junctions (Supplementary Fig. 3j). In addition, a slight decrease in E-cadherin intensity at the cell cortex was observed for these KO cells (Supplementary Fig. 3k). Next, we quantified protein levels of epithelial-to-mesenchymal transition (EMT) markers, as the aforementioned modifications were indicative of EMT activation66. We noticed a modest upregulation of β-catenin, Slug and Zeb, but not of Vimentin in Maspin KO cells (Supplementary Fig. 3l). Collectively, these results point towards a scenario where depletion of Maspin in non-transformed MCF-10A cells concurrently occurs with changes that resemble the early stages of EMT.
Maspin negatively regulates microtubule growth in vitro and in cells
Although evidence suggested that Maspin regulates the actin cytoskeleton34, no previous report addressed its role on microtubules. We have previously detected α-tubulin as a major Maspin-binding partner in IP/MS analysis41. In our immunofluorescence analysis, we could not quantitatively describe the microtubule reorganization pattern observed in Maspin-depleted cells (Fig. 3a and Supplementary Fig. 3a–f). Therefore, we decided to investigate whether Maspin regulates microtubule dynamics in cells. For this purpose, cells were initially treated with nocodazole to induce microtubule depolymerization, and microtubule recovery after nocodazole washout was subsequently compared between WT and Maspin-deficient cells. Surprisingly, microtubule reassembly was consistently faster in MCF-10A siMaspin cells (Supplementary Fig. 4a) and in EpH4 shMaspin cells (Supplementary Fig. 4b) after nocodazole washout, as observed by α-tubulin intensity at MTOCs. In order to confirm that this phenotype was a direct consequence of decreased Maspin levels in these cells, the same experiment was repeated in KO C10 cells transiently transfected with GFP-only or GFP-Maspin. Although the control GFP-only slightly interfered with microtubule regrowth pattern, GFP-Maspin re-expression in KO cells suppressed α-tubulin intensity at levels below those observed in KO C10 or WT cells transfected with GFP-only (Supplementary Fig. 4c, d).
Next, we assessed microtubule dynamics by transducing MCF-10A Maspin KO cells with a lentiviral construct encoding EB1-mCherry67, a microtubule-plus-end-binding tracker (Fig. 4a, b). In these cells, we observed an increase in microtubule growth rates as compared to MCF-10A WT cells (Fig. 4c). Re-expression of GFP-Maspin in KO cells, but not GFP-only, restored microtubule growth rate at similar levels to those observed in WT cells (Fig. 4c). Taken together, these data suggest that Maspin specifically suppresses microtubule growth and spatially reorganizes the microtubules of epithelial cells.
a Western blot analysis for Maspin in MCF-10A WT, KO C10 and KO D9 cells stably expressing EB1-mCherry. Cells were transiently transfected with GFP-Maspin or the control GFP-only for 24 h. Cell lysates were sequentially probed using an anti-GFP antibody followed by an anti-Maspin antibody. Anti-GAPDH antibody was used as a loading control; (b) Live imaging of MCF-10A WT, KO C10 and KO D9 cells stably expressing EB1-mCherry and transiently expressing GFP-Maspin or the control GFP-only alone; (c) Microtubule mean growth rates (µm/min) in WT (n = 148 comets, 11 cells), KO C10 (n = 135 comets, 9 cells), KO C10 + GFP-only (n = 100 comets, 8 cells), KO C10 + GFP-Maspin (n = 146 comets, 14 cells), KO D9 (n = 142 comets, 7 cells), KO D9 + GFP-only (n = 110 comets, 9 cells) and KO D9 + GFP-Maspin (n = 119 comets, 10 cells); (d) Schematic representation of dynamic microtubules using an in vitro reconstitution assay (upper). Pre-assembled TAMRA-labeled GMPCPP-seeds in the presence of Atto633-tubulin and purified GFP-Maspin at different concentrations were visualized by TIRF microscopy (lower). Scale bar: 10 µm; (e) Representative kymographs extracted from dynamic microtubules in (d). Scale bar: 5 µm (horizontal), time: 5 min (vertical); (f) Plot of microtubule growth rates as a function of GFP-Maspin concentration with 11 µM tubulin. Control (0 nM; n = 20 microtubules), GFP-Maspin (50 nM; n = 15 microtubules) and GFP-Maspin (500 nM; n = 17 microtubules); (g) Fluorescence intensity over time for GMPCPP-seeds exposed to GFP-Maspin (500 nM). Maspin binds to the seeds and tubulin is increasingly recruited to them (black arrows, (d)).
As we have shown that Maspin directly interacts with stabilized microtubules (Fig. 1j), we probed for the direct effect of Maspin on microtubule growth using a TIRF-based in vitro reconstitution assay. To this end, we assembled dynamic microtubules in the presence of stable GMPCPP-seeds and purified recombinant Maspin or the corresponding control GFP-only. Interestingly, we noticed a significant suppression of microtubule growth when Maspin was added at low concentration (50 nM) (Fig. 4d–f). This suppressive effect increases at high concentration (500 nM) (Fig. 4d–f), where Maspin also readily decorates and recruits free tubulin to GMPCPP-seeds (Fig. 4d, black arrows, and g). We could not observe, however, the binding of Maspin to dynamic microtubules. Oppositely, a control GFP-only at high concentration (500 nM) did not suppress microtubule growth or bind to the seeds (Supplementary Fig. 4e–g). Therefore, we conclude that the effect of growth suppression in cells is a direct effect of Maspin on microtubule growth.
Discussion
Our findings reveal that intracellular Maspin directly interacts with the cytoskeleton of epithelial cells and modulates their shape. Previous studies have determined a heterogeneous set of Maspin-binding partners using different experimental approaches such as yeast-two-hybrid and co-immunoprecipitation. These include Histone Deacetylase 1 (HDAC1)25, Interferon Regulatory Factor 6 (IRF6)24, uPAR/β1-integrin18,30,31,33 and Desmoplakin68. Here, we employed affinity purification using recombinant Maspin as a bait to capture its interactors from asynchronous and synchronized MCF-10A cells. We found novel cytoskeleton-binding proteins (Plectin, α-actinin-4 and myosin II subunits) and cytoskeleton subunits (actin and tubulin) among Maspin interactors. The interaction of Maspin with the cytoskeleton was further validated using TIRF-based in vitro reconstitution assays of microfilaments and microtubules.
In epithelial cells, cytoskeleton-binding proteins synergistically act at the interface between the membrane-associated cell adhesion molecules and the cortical actomyosin cytoskeleton69. Previous data suggested that Maspin locates at the cell cortex and is associated with the insoluble cytoskeleton fraction of MCF-10A cells30. Our proteomic results, large-scale proteomic data mining and imaging assays demonstrate that Maspin is an underappreciated protein component acting at this interface. In agreement with these observations, we detected intracellular Maspin at cytoskeleton-enriched structures during dynamic cellular processes, including cell–cell contact establishment (cell cortex), cell migration (lamellipodia) and cell division (mitotic spindle). Maspin accumulation at the mitotic spindle has not been previously reported and highlights its versatility in regulating not only the actin cytoskeleton, but also the microtubules of epithelial cells. This dynamic association may explain why this interaction has been previously missed, since we observed that intracellular Maspin adopts a more diffuse distribution as cell density increases. Indeed, other cytoskeleton-binding proteins have been reported to rely on cell density in order to partition at the cell cortex54.
Different studies have observed that Maspin can indirectly modulate the actin-cytoskeleton architecture by interacting at the cell surface with the urokinase plasminogen uPA/uPAR/β1-integrin complex19,30,31,33. For instance, ectopic expression and recombinant Maspin extracellularly delivered to tumor cells devoid of endogenous Maspin resulted in changes on the actin organization that are compatible with a less motile phenotype and ultimately impact cell shape34. These modifications include a thicker cortical F-actin, decrease in lamellipodia and stress fiber number, and a more regular cell shape. They are consistent with Maspin’s role in maintaining the epithelial morphology and agree with our F-actin imaging in MCF-10A KO cells, in which we observed a reduced cortical pattern and irregular cell shape. Importantly, in these studies Maspin’s effect on microfilaments results from β1-integrin activation19,31 and modulation of small GTPases of the Rho family70. In contrast to these previous studies, our results clearly demonstrate that Maspin directly interacts with microfilaments, suggesting that it may regulate its dynamics and/or architecture. These data indicate that Maspin modulates F-actin architecture by two different mechanisms - on the cell surface interacting with integrins which then convey mechanical forces to the actin cytoskeleton across the plasma membrane, and inside the cell, directly interacting with F-actin. Our results are further supported by the recently described interaction between Maspin and Desmoplakin in human keratinocytes68. Intracellular Desmoplakin is a central component of desmosomes and distributes at the cell cortex as well. Interestingly, we captured another plakin family member (Plectin) in our AP/MS analysis, which suggests a redundant association of intracellular Maspin with plakins at the intersection of different cell junctions and the cortical cytoskeleton of epithelial cells71. Future studies should uncouple the contributions of extracellular and intracellular Maspin in regulating the actin cytoskeleton of cells.
As an inhibitor of cell motility, invasion and metastasis, Maspin has been linked to epithelial-to-mesenchymal transition (EMT) in both normal and tumor contexts20,72,73. EMT is a stepwise process that involves changes in cell adhesion, cytoskeleton and gene transcription66,74. It has been proposed that Maspin controls epithelial plasticity by interacting with a diverse set of partners in a context-dependent manner. In prostate cells, it behaves as an endogenous inhibitor of HDAC1, controlling a subset of genes related to epithelial differentiation39. In keratinocytes, it acts in concert with Desmoplakin to regulate Dolichol-Phosphate Mannosyl transferase (DPM1)-dependent control of epidermal differentiation68. In mammary cells, it is part of an interplay with IRF6 to prevent a mesenchymal phenotype24. This poses a complex scenario whereby different Maspin-related binding partners may contribute to EMT activation, progression or maintenance. Here, we report modifications that are compatible with the early activation steps of an EMT state in non-transformed MCF-10A Maspin KO cells66,74. We observed an increased cell area, decrease in cortical F-actin and increased expression of the EMT transcription factors Zeb1 and Slug. These changes were accompanied by a slight downregulation of E-cadherin at the cell–cell junction, but not cell–cell contact disruption. Most strikingly, we observed increased microtubule growth rates in Maspin KO cells, which were rescued by transient re-expression of GFP-Maspin. Although cytoskeleton remodeling is often regarded as a consequence rather than causative of EMT activation75,76, cumulative data suggest that modulation of cytoskeleton-binding proteins may trigger EMT-related phenotypes76,77,78. Indeed, we found Maspin directly interacting with microtubules in vitro and regulating their dynamics in cells. The effect appears to be direct, as growth suppression in the presence of Maspin was also observed in in vitro dynamic microtubule assay. However, we could not ascertain whether it interacts with the growing microtubule end or lattice during interphase. Likewise, a strong interaction of Maspin with the growing microtubule lattice was not observed in our in vitro dynamic microtubule assay. Growth suppression may therefore result from transient interactions of Maspin with the growing microtubule end. This mechanism is supported by the interaction of Maspin with GMPCPP-stabilized microtubules, which is a proxy for the GTP cap at microtubule ends79. If Maspin regulates microtubule dynamics at the epithelial cell cortex in tune with other MAPs77 and plakins80,81 to prevent EMT has yet to be determined. In addition to cell density dependency, we believe that the abundant, ubiquitously and punctiform rather than filamentous staining observed for endogenous Maspin in non-dividing cells imposes challenges in determining its subcellular localization accurately. It is thus possible that high-resolution imaging will be necessary to confirm this transient association during interphase in the future.
In contrast to what we observed for non-dividing cells, we detected Maspin decorating the mitotic spindle during mitosis. Mitotic rounding occurs at the onset of mitosis and it is an essential process for chromosome capture, segregation and cytokinesis82,83. In this process, stress fibers are disassembled and rearranged into a rigid actomyosin cytoskeleton, which, coordinated by specific actin cross-linkers, retracts the plasma membrane and promotes loss of cell adhesion83,84. Among them, Plectin84, the most abundant Maspin-binding partner identified in our asynchronous cells, along with α-actinin-444,85, myosin II subunits44 and annexin A286,87, are key regulators in actin organization during mitosis. We hypothesize that our KO cells exhibit abnormal retraction tails and reduced roundness due to disruption of Maspin interaction with these binding partners, ultimately interfering with mitotic rounding and reducing the spindle length. It should be noted, however, that Maspin regulates microtubule dynamics in non-dividing cells. Therefore, further studies are necessary to define if Maspin also controls spindle length by regulating microtubule dynamics during mitosis, as proposed for other cytoskeleton-binding proteins67,87.
Materials and methods
Cell culture and treatments
Immortalized human mammary epithelial cells (MCF-10A) (BCRJ - Banco de Células do Rio de Janeiro) were cultured in DMEM/F12 (12500062, Gibco) containing 5% donor horse serum (26050088, Gibco), 20 ng/mL EGF (PHG0311, Thermo Fisher Scientific), 0.5 mg/mL hydrocortisone (H0888, Sigma-Aldrich), 100 ng/mL cholera toxin (C8052, Sigma-Aldrich), 10 µg/mL insulin (I0516, Sigma-Aldrich) and 1x penicillin-streptomycin (15070063, Gibco) (i.e. “complete medium”). Immortalized mouse mammary epithelial cells (EpH4) (a kind gift from Dr. Alexandre Bruni-Cardoso, Department of Biochemistry, University of Sao Paulo) were cultured in DMEM/F12 supplemented with 5 µg/mL insulin, 50 µg/mL gentamicin (15750078, Gibco), 1 µg/mL puromycin (P9620, Sigma-Aldrich) and 2% fetal bovine serum (Cultilab). Immortalized human keratinocyte cells (HaCaT) (BCRJ - Banco de Células do Rio de Janeiro) and human embryonic kidney cells (HEK293T) were cultured in DMEM (12100061, Gibco) supplemented with 10% fetal bovine serum and 1x penicillin-streptomycin. Human retinal pigment epithelial cells (hTERT RPE-1) were a gift from Arnold Hayer (McGill University) and were cultured in DMEM/F12 supplemented with 10% fetal bovine serum, 10 µg/mL hygromycin B and 1x penicillin-streptomycin. All cell lines were kept at 37 °C and 5% CO2 in a humidified incubator (Thermo Fisher Scientific) and routinely trypsinized in 0.05% Trypsin-EDTA (25300054, Gibco).
Experiments with nocodazole: in order to enrich for mitotic cells (G2/M arrest and release), cells were treated with 50 ng/mL of nocodazole (sc-3518, Santa Cruz Biotech.) for 16 h, followed by two washes with warm PBS and release into fresh medium without nocodazole for 30 min. For microtubule regrowth assays, cells were kept in a complete medium containing 1 µg/mL of nocodazole for 1 h, then washed twice with warm PBS and recovered in drug-free fresh medium at 37 oC.
Calcium switch assay: to deplete extracellular calcium, confluent cells were treated with 4 mM EGTA in complete medium for 45 min. Subsequently, cells were washed twice with complete medium at 37 °C and allowed to recover in fresh complete medium without EGTA for 1 h at 37 °C.
Overexpression
For microtubule regrowth assays, MCF-10A cells were seeded at 80% confluency on top of poly-D-lysine pre-treated glass coverslips in a p6-multiwell. Cells were transiently transfected with 2500 ng of GFP-Maspin or GFP-only constructs using 7.5 µL of Lipofectamine 3000 for 24 h according to manufacturer’s instructions and treated with nocodazole as previously mentioned. For EB1 comets assay, MCF-10A cells stably expressing EB1-mCherry construct were seeded at 60% confluency in a 35 mm glass bottom dish (#150680, Thermo Fisher Scientific) and transiently transfected with 2000 ng of GFP-Maspin or GFP constructs using 6.0 µL of Lipofectamine 3000 for 24 h according to manufacturer’s instructions.
A lentiviral construct encoding EB1-mCherry was kindly gifted by Dr. Takanari Inoue67 and packaged in HEK293T cells. Briefly, 5.5 × 105 cells were plated in a p6-multiwell and transfected with 2500 ng of plasmid DNA at 3:2:1 ratio [1250 ng EB1-mCherry:833 ng pPAX2 (#12260 Addgene):416 ng pMD2.G (#12259 Addgene)] using 7.5 µL of Lipofectamine 3000 according to manufacturer’s instructions. Medium was replaced by DMEM supplemented with 10% fetal bovine serum without antibiotics following 6 h of transfection. After 48 h, the supernatants were harvested, passed through a 0.45 µm PES filter and stored at 4 oC. Fresh medium was added to the cells and an additional lentiviral supernatant was harvested after 24 h, filtered and pooled with the previous one. Lentiviral supernatants were concentrated for 2 h using the Lenti-X-Concentrator (Takara Bio) at 4 oC and centrifuged according to manufacturer’s instructions. Finally, lentiviral particles were resuspended in 200 uL of DMEM/F12 and delivered to 1.5 × 105 MCF-10A cells cultured in complete medium containing 8 µg/mL of polybrene (TR-1003-G, Sigma-Aldrich) in a p6-multiwell. After 40 h, medium was changed and EB1-expressing cells were selected with puromycin (1.0 µg/mL) for 1 week.
RNAi-mediated knockdowns
For transient knockdown, MCF-10A cells were cultured in complete medium in a p6-multiwell plate for 24 h. For siRNA transfection, 150 µL of Opti-MEM I (31985062, Gibco) and 8 µL of Lipofectamine RNAiMAX (13778150, Invitrogen) were added to tubes. siRNAs (120 pmol, stock solution 20 nmol) were added on top of each individual tube and incubated for 10 min at room temperature. Cell medium was changed shortly before transfection and final mixtures were added dropwise and cells were incubated for 72 h. The following commercially available siRNA sequences were used: Stealth siRNAs targeting Maspin mRNA sequence - HSS107960 (siMaspin #60) and HSS182295 (siMaspin #95) (Thermo Fisher Scientific) - along with the negative control Stealth Negative Control Low GC duplex (siNC) (452002, Thermo Fisher Scientific). The corresponding sequences are shown in Table 1.
For stable knockdown, EpH4 cells were cultured in complete medium in a p24-multiwell plate 24 h prior to transductions at 70% confluency. Next, fresh medium containing 8 µg/mL of polybrene (TR-1003-G, Sigma-Aldrich) was added to each well and commercially available lentiviral particles (SHCLNV-NM_009257, MISSION® Lentiviral Transduction Particles, Sigma-Aldrich) containing the lentiviral vector pLKO.1-puro (SHC001, Sigma-Aldrich) were added dropwise on top of cells at a MOI (Multiplicity of infection) value of 1.3. After 24 h, medium was changed and cells allowed to recover for an additional 24 h. Next, puromycin selection (2.5 µg/mL) was carried out for 2 weeks and cells were further cultured in the presence of 1.0 µg/mL of puromycin. The shRNA sequences targeting Maspin mRNA along with the negative control (SHC001V, Sigma-Aldrich) are described in Table 2.
All Maspin knockdowns were confirmed by Western blot and immunofluorescence with anti-Maspin antibodies.
Generation of Maspin knock-in and knock-out cells by CRISPR/Cas9
CRISPR/Cas9 knock-in was performed using Cas9 Nuclease 2-NLS (Synthego), sgRNAs targeting Maspin gene sequence (N1/N2-terminal and C-terminal) and a template DNA generated by PCR containing a GFP and Puromycin resistance sequence (A42992, Invitrogen). All primers used are described in Table 3.
PCR mixes containing 1 U of Q5 polymerase (M0491S, New England Biolabs), 1x reaction buffer, 10 mM dNTPs, 0.5 µL of each template (GFP-Puro), 10 µM primers and nuclease free H2O (up to 50 µL) were ran on a DNA Engine TETRAD2 Peltier Thermal cycler (MJ 426 Research) with the following program: initial denaturation at 98 °C for 10 min followed by 30 cycles of denaturation at 98 °C for 30 s, annealing at 56 °C for 25 s and elongation at 72 °C for 1 min, final elongation at 72 °C for 7 min and a 4 °C hold. PCR products were visualized on a 1% agarose gel (ChemiDoc MP Imaging System, Bio-Rad) and the amplicons were further purified (T1030L, New England Biolabs) prior to mixing with RNP complexes.
CRISPR/Cas9 knock-out was performed using Cas9 Nuclease 2-NLS (Synthego) and a mix of 3 different sgRNAs (Multi-guide system, Synthego) targeting exon 2 of Maspin with the following sequences: sgRNA1 (AAGCCGAATTTGCTAGTTGC), sgRNA2 (GAGAAGAGGACATTGCCCAG) and sgRNA3 (CACTGCAAATGAAATTGGAC).
For both knock-in and knock-out, RNP complexes were formed in Nucleofector solution at a 6:1 ratio (sgRNA:Cas9) and immediately transfected by Nucleofection into MCF-10A or HaCaT cells using the Amaxa SF Cell Line 4D-Nucleofector X kit S (Lonza, PBC2-00675) and the program DS-138 (MCF-10A and HaCaT) on the 4D-Nucleofector X unit. Cells were harvested and 4 × 105 cells were added to the RNP complex mix in a 16-well Nucleocuvette. After nucleofection, the transfected cells were resuspended in conditioned medium DMEM/F12 (MCF-10A) or DMEM (HaCaT), plated in p12-multiwell plates, and 24 h later cells were fed with conditioned media. This conditioned media was previously obtained by pooling and filtering the supernatants of MCF-10A or HaCaT confluent cells after 72 h and 96 h of subculturing, and by mixing them with fresh complete medium at a 1:1 ratio.
After nucleofection, knock-in cells were cultured in conditioned media, allowed to reach 70% confluency and split into three wells of a p12-multiwell plate. Puromycin selection was carried out after 24 h at different doses (0.5 µg/mL; 0.75 µg/mL and 1 µg/mL) for 2 weeks and fluorescence signal monitored using Incucyte (Sartorius). MCF-10A knock-ins were then scaled up and routinely cultured in the presence of puromycin (0.5 µg/mL for C-terminal tagged; 1 µg/mL for N1/N2-terminal tagged).
After nucleofection, knock-out cells were allowed to reach 90% confluency and the edited pool was sequenced to confirm knock-out efficiency. Single-cell cloning was then performed using DispenCell Single-Cell Dispenser (Molecular Devices) according to manufacturer’s instructions and cells were individually seeded in a 96-multiwell plate containing conditioned media at 37 oC. The plate was monitored weekly for colony formation until 80% confluency was reached (around 2 weeks). Single cell colonies were then expanded in 24-multiwell plates containing fresh media and further scaled up. Genomic DNA was extracted (T3010L, New England Biolabs) from at least 5 different clones and PCR was conducted with primers targeting a region on exon 2 for human Maspin (F′-ACAGAAAGTTCCTTGTTTCTTCTGA; R′-GCCTGTCCCAGATGTGAGTT). PCR mixes containing 1 U of PfuX7 polymerase, 1x reaction buffer, 10 mM dNTPs, 50 ng of genomic DNA, 10 µM primers and nuclease free H2O (up to 50 µL) were ran on a DNA Engine TETRAD2 Peltier Thermal cycler (MJ Research) with the following program: initial denaturation at 98 °C for 10 min followed by 30 cycles of denaturation at 98 °C for 30 s, annealing at 54 °C for 25 s and elongation at 72 °C for 1 min, final elongation at 72 °C for 7 min and a 4 °C hold. PCR products were visualized on a 1% agarose gel (ChemiDoc MP Imaging System, Bio-Rad), purified (T1030L, New England Biolabs) and sent for Sanger Sequencing (Génome Québec) with a nested sequencing primer (TTCCTTGTTTCTTCTGAGATCAATTACTTT). Sequencing data was analyzed with Synthego’s ICE software, available at: https://www.synthego.com/products/bioinformatics/crispr-analysis.
Molecular cloning, protein expression and purification
The coding sequence for full-length human Maspin protein was PCR amplified from previously described GFP-Maspin construct88 using PfuX7 polymerase and the following primers: (F′ GGGGAGCUATGGATGCCCTGCAACTAG;R′GGGGAACUAGGAGAACAGAATTTGCCAAAG). This amplicon was inserted into a modified pHAT-HGUS vector containing an N-terminal 6xHis-tag followed by EGFP, and a C-terminal Strep-tag II using the USER enzyme cloning system89.
His-GFP-Maspin-StrepII protein was purified as previously described48. E. coli BL21 (DE3) transformed with protein expression vectors were grown to O.D. 0.6 at 37 °C, and expression was induced using 0.5 mM Isopropyl β-D-1- thiogalactopyranoside (IPTG) at 18 °C for 16 h. Bacterial pellets were harvested by centrifugation and resuspended in Buffer A (50 mM Na2HPO4, 300 mM NaCl, 4 mM imidazole, pH 7.8). Cells were lysed using a French press (EmulsiFlex-C5, Avestin). Constructs containing both His and Strep tags were purified using gravity flow columns containing His60 Ni-NTA resin (Clontech) followed by Streptactin affinity chromatography (IBA Lifesciences, Germany). Purified Maspin was eluted with BRB80 (80 mM PIPES-KOH, pH 6.85, 1 mM EGTA, 1 mM MgCl2) or Tris-HCl (100 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA) containing 2.5 mM desthiobiotin and 10% glycerol. Purified Maspin was always used fresh for each experiment. Protein purity was analyzed by SDS-PAGE gel stained with Coomassie blue. Protein concentration was determined by absorbance at 510 nm with a DS-11 FX spectrophotometer (DeNovix, Inc)48.
Tubulin was purified from bovine brains as previously described90, using Fractogel EMD SO3- (M) resin (Sigma-Aldrich) instead of phosphocellulose. Tubulin was labeled using Atto-633 NHS-Ester (ATTO-TEC) and tetramethylrhodamine (TAMRA, Invitrogen) as described91. An additional cycle of polymerization/depolymerization was performed before use. Protein concentrations were determined using a DS-11 FX spectrophotometer (DeNovix, Inc).
The pGEX-4T1 and pGEX-Maspin constructs were previously described30 and used to express control GST-only and GST-Maspin bait proteins, respectively. E. coli BL21(DE3) transformed with protein expression vectors were grown to O.D. 0.6 at 37 °C, and expression was induced using 0.8 mM IPTG at 37 °C for 4 h. Bacterial pellets were harvested by centrifugation and resuspended in Buffer T1 (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 5 mM MgCl2, 1 mM PMSF, and 10 μg/mL leupeptin and aprotinin). Cells were lysed on ice by sonication (10 cycles of 30 s each with intervals of 1 min, 70% amplitude) (Vibra-Cell VC 505 sonicator (SONICS, Newtown, CT, USA)). The material was centrifuged at 14,000 rpm at 4 °C for 30 min, and the soluble fractions containing the GST-only or GST-Maspin proteins were collected, frozen at -80 oC and used for further affinity purification experiments92. An SDS-PAGE followed by Coomassie Brilliant Blue R-250 (Bio-Rad, Hercules, CA, USA) staining was used to assess protein purity after affinity purification. GST-fusion protein concentration was estimated based on a standard curve using bovine serum albumin (BSA) at different known concentrations in the same gel. This gel was scanned with an Odyssey Imager (LI-COR, Lincoln, NE, USA) operated with dedicated Odyssey V 3.0 software (LI-COR, Lincoln, NE, USA), which was used for band quantification in ImageJ.
Pull-down and Western blot
For pull-down experiments, bacterial lysates were separately incubated with glutathione agarose (16100, Thermo Fisher Scientific) and left rotating for 1.5 h at 4 °C. The beads were washed 6x with buffer T2 (50 mM Tris pH 7.5, 0.5% Triton X-100, 150 mM NaCl, 5 mM MgCl2 and 1x protease inhibitor cocktail) with intercalated centrifugations at 800 × g for 3 min at 4 °C. Supernatants from the final washes were removed and freshly harvested MCF-10A cell lysates were added to the beads. The reaction mixtures were allowed to rotate at 4 °C for 1 h and then centrifuged at 800 × g for 5 min at 4 °C. The beads were washed three times with buffer I (Tris 25 mM pH 7.3, 150 mM NaCl) and eluted with buffer G (reduced glutathione 10 mM, 150 mM NaCl, 1 mM DTT, 50 mM Tris-HCl pH 8.5) under constant rotation at 4 °C. An aliquot of 5 µL of eluates were run on an SDS-PAGE gel followed by silver staining carried out to confirm recombinant protein elution. For pull-down validations, 10 µL of each eluate were denatured in 3 x Laemmli buffer and immunoblot performed with specific primary antibodies. MCF-10A cell lysates were obtained by scraping cells in modified buffer I (Tris 25 mM pH 7.3, 150 mM NaCl, 0.1% IGEPAL, 1 x protease cocktail inhibitor, 1 mM NaF, 1 mM Na3VO4) and thoroughly pipetting to avoid aggregates. Then, a sonication step was done on ice (3 cycles, 7 s each with 30 s intervals and 20% amplitude) followed by a final centrifugation at 14,000 rpm 4 °C for 10 min.
Western blot analysis was performed as previously described41 and quantified by densitometry using ImageJ. Primary antibodies were diluted in TBS-T only: mouse anti-Maspin (1:2000, clone 5C6.2, MABC603, Millipore), mouse anti-Maspin (1:20,000, clone AT2N6, MAB8227, Abnova); mouse anti-GAPDH (1:1000, GA1R, MA5-15738, Invitrogen), mouse anti-α-tubulin (1:1000, clone B7, sc-5286, Santa Cruz Biotech.), mouse anti-E-cadherin (1:1000, HECD-1, 13-1700, Invitrogen), mouse anti-β-catenin (1:1000, sc-7963 Santa Cruz Biotech.), mouse anti-Vimentin (1:1000, #CBL202, Chemicon), mouse anti-Slug (1:1000, A-7, sc-166476, Santa Cruz Biotech.), mouse anti-Zeb1 (1:1000, H-3, sc-515797, Santa Cruz Biotech.), mouse anti-GFP (1:1000, GF28R, MA5-15256, Invitrogen). Secondary antibodies were diluted in 5% skim milk in TBS-T: goat anti-mouse HRP (1:10.000, 1721011 Bio-Rad).
Proteomics, gene ontology and interactomes
Samples were digested and cleaned as previously described93. Briefly, eluates were resuspended in 400 μL of ice-cold 100 mM Tris-HCl buffer at pH 8.5. For protein precipitation, 100 μL of cold trichloroacetic acid (TCA) was added and samples were incubated overnight at 4 °C. After centrifugation at 14,000 rpm for 30 min at 4 °C, pellets were washed twice with ice-cold acetone and allowed to dry at room temperature. Protein concentration was quantified using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) following manufacturer’s recommendations. For protein digestion, 100 μg of total protein were resuspended in 30 μL of a solution containing 8 M urea, 75 mM NaCl, and 50 mM Tris at pH 8.2. Proteins were reduced with 5 mM DTT for 30 min at room temperature, followed by alkylation with 14 mM iodoacetamide in the dark for 30 min. Subsequently, protein extracts were digested overnight with 1 μg of trypsin (Promega, Madison, Wisconsin) in a solution containing 10 mM Tris-HCl at pH 8.0 and 2 mM CaCl2, maintained at 37 °C. Digestion reactions were halted by the addition of 5% formic acid, and the samples were vacuum dried. After protein digestion, the samples were redissolved in 400 μL of 0.1% TFA and desalted using the Sep-Pak Light tC18 column (Waters, Milford, Massachusetts). The desalted samples were then dried and prepared for mass spectrometry analysis.
Peptides were resuspended in 0.1% formic acid and injected into a custom-made 5 cm reversed-phase pre-column (inner diameter 100 μm, filled with 10 μm C18 Jupiter resins - Phenomenex, Torrance, California) connected to a nano HPLC system (NanoLC-1DPlus, Proxeon, Thermo Fisher Scientific, Waltham, Massachusetts). Peptide fractionation was performed on a custom 10 cm reversed-phase capillary emitter column (inner diameter 75 μm, filled with 5 μm C18 Aqua resins - Phenomenex) using a gradient of 3–35% acetonitrile in 0.1% formic acid over 45 min, followed by a gradient of 35–95% for 5 min, maintained at 95% for 8 min and, 95–5% for 2 min at a flow rate of 300 nL/min. The eluted peptides were directly analyzed using an LTQ-Orbitrap Velos mass spectrometer (Thermo Fisher Scientific). Source voltage and capillary temperature were set to 1.9 kV and 200 °C, respectively. The mass spectrometer operated in a data-dependent acquisition mode, automatically switching between one Orbitrap full scan and ten ion trap tandem mass spectra. FT scans were acquired from m/z 200 to 2000 with a mass resolution of 30,000. MS/MS spectra were obtained at a normalized collision energy of 35%. Singly charged and charge-unassigned precursor ions were excluded, with dynamic exclusion set to 45 s for exclusion duration, 500 for exclusion list size, and 30 s for repeat duration.
Raw data were processed using MaxQuant software version 1.6.10.43 and the Andromeda search engine. Proteins were identified by searching against the complete database sequence of Homo sapiens (downloaded from UniProt - UP000005640- 82,518 entries) along with a set of commonly observed contaminants. Carbamidomethylation (C) was set as a fixed modification, while oxidation (M) and acetylation (N-terminal) were set as variable modifications. The maximum number of modifications per peptide was set to 5, with a maximum of 2 missed cleavages. The MS1 tolerance was 6 ppm and the MS2 tolerance was 0.5 Da. The maximum false discovery rates for peptides and proteins were set to 0.01. For matching between runs, a time window of 2 min was used. Bioinformatics analysis was performed using Perseus software (http://www.perseus-framework.org/). Proteins matching the reverse (or contaminants) database, or identified only by modified peptides, were filtered out. Proteins were identified by at least one unique peptide. Relative protein quantification was carried out using the LFQ algorithm of MaxQuant, with a minimum ratio count of two. Protein quantification was based on LFQ values of “razor and unique peptides” of unmodified peptides, using the Proteingroups.txt. We kept proteins exclusively found or enriched (LFQ > 1.5-fold) in at least 2 biological replicates from GST-Maspin baits compared to GST-only baits. An average for LFQ values corresponding to each protein hit was taken and a final normalization factor was set for all hits not found based on the lowest LFQ value found for a hit within each group (GST- or GST-Maspin). Fold change was then calculated by taking the mean values of GST-Maspin hits divided by the corresponding mean values of GST-only control hits (Supplementary Data 1). A final heatmap was plotted with the adjusted fold change values using GraphPad Prism 8.0.
GO enrichment analysis was performed by combining proteins identified in DMSO-treated samples and Nocodazole-treated samples (gene list) and submitting them to the Shiny GO 0.80 platform94. An FDR of 0.05 was set as a cutoff to select the top 10 most significant molecular functions (Homo sapiens). Interactomes were generated using String database (Homo sapiens), medium confidence (0.400) and FDR of 0.05. For the Adhesome dataset, protein lists from each study were downloaded and manually organized (Supplementary Data 2). Venn diagrams were generated by intersection of protein hits using https://bioinformatics.psb.ugent.be/webtools/Venn/. All networks were imported into Cytoscape and nodes and edges customized according to protein classes and direct physical interactions, respectively. The latter was complemented by manual annotations of previous studies where interactions were found, but not reported by the String database.
In silico protein-protein interaction prediction
Protein complexes were generated by inputting the human sequences of SerpinB5 (P36952), TBA1A (Q71U36), TBB1 (Q9H4B7) and ACTB (P60709) into AlphaFold 347. Complexes were colored and analyzed using ChimeraX v. 1.8.
F-actin binding assay
In vitro Maspin-F-actin interaction assay was performed using rabbit skeletal muscle actin (Cytoskeleton, #AKL99). Briefly, purified actin was reconstituted in General Actin buffer (5 mM Tris-HCl pH 8.0, 0.2 mM CaCl2) according to manufacturer’s instructions. Actin was incubated on ice for 30 min and centrifuged at 14,000 rpm for 15 min at 4 °C. The supernatant (200 µL) was transferred to a new tube and 20 µL of Actin Polymerization buffer (500 mM KCl, 20 mM MgCl2, 10 mM ATP) were added along with 1 µL of labeled actin. The reaction mixture was incubated at 30 °C for 30 min and centrifuged at 100,000 × g in an Airfuge (Beckman-Coulter) for 10 min. For TIRF experiments, flow channels were first incubated with a polyclonal rabbit anti-actin antibody (Biomedical Technologies Inc., BT-560) diluted in 1x KMEI buffer (50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM imidazole pH 7.0) at 1:50 and then blocked with 5% Pluronic F-127. Channels were washed twice with BRB80 and polymerized F-actin diluted in TIRF imaging buffer (1x KMEI, 100 mM DTT, 0.2 mM ATP, 15 mM glucose, 0.5% methyl cellulose, 0.01 mg/mL catalase, 0.05 mg/mL glucose oxidase, 0.1% BSA) and loaded into the channels. Images were taken prior to flowing in recombinant Maspin through the channel95.
Paclitaxel-stabilized microtubules and dynamic microtubule assays
For paclitaxel-stabilized microtubules, a polymerization mixture was prepared with BRB80 + 32 μM tubulin + 1 mM GTP + 4 mM MgCl2 + 5% DMSO. The mixture was incubated on ice for 5 min, followed by incubation at 37 °C for 30 min. The polymerized microtubules were diluted into prewarmed BRB80 + 10 μM paclitaxel, centrifuged at 110,000 rpm (199,000 × g) in an Airfuge (Beckman-Coulter), and resuspended in BRB80 + 10 μM paclitaxel (99.5 + %, GoldBio).
To visualize dynamic microtubules, we reconstituted microtubule growth from GMPCPP double stabilized microtubule ‘seeds’96. In short, cover glass was cleaned in acetone, sonicated in 50% methanol, sonicated in 0.5 M KOH, exposed to air plasma (Plasma Etch) for 3 min, then silanized by soaking in 0.2% dichlorodimethylsilane in n-heptane. 5 μl flow channels were constructed using two pieces of silanized cover glasses (22 × 22 and 18 × 18 mm) held together with double-sided tape and mounted into custom-machined coverslip holders. GMPCPP seeds were prepared by polymerizing a 1:4 molar ratio of TAMRA labeled:unlabeled tubulin in the presence of GMPCPP (Jena Biosciences) in two cycles, as described previously96. Channels were first incubated with anti-TAMRA antibodies (Invitrogen) and then blocked with 5% Pluronic F-127. Flow channels were washed three times with BRB80 before incubating with GMPCPP seeds. On each day of experiments, tubes of unlabeled and Atto-633 labeled tubulin were thawed and mixed at a 1:17 molar ratio, subaliquoted and refrozen in liquid nitrogen. For consistency in microtubule growth dynamics, one subaliquot of tubulin was used for each experiment. Microtubule growth from GMPCPP seeds was achieved by incubating flow channels with 11 µM tubulin in imaging buffer: BRB80, 1 mM GTP, 0.1 mg/mL bovine serum albumin (BSA), 10 mM dithiothreitol, 250 nM glucose oxidase, 64 nM catalase, and 40 mM D-glucose.
Live cell imaging
Cells were seeded in a p12-multiwell plate and monitored using the Incucyte Live-Cell Analysis System (Sartorius) in a humidified incubator at 5% CO2 and 37 °C. Acquisition time was set at 5 min time intervals for asynchronous cell population or at 2 h time interval for knock-in cells over a total period of 24 h. At least 25 different fields per well were imaged using a 20x objective (bright field or 488 nm wavelength for knock-in cells).
Immunofluorescence
Cells were cultured on Poly-D-Lysine-coated glass coverslips (1 mg/mL for MCF-10A, P6407, Sigma-Aldrich and 100 µg/mL for EpH4, A3890401, Gibco). Cells were fixed with PFA 4% in PBS for 10 min, washed twice with PBS and permeabilized with 0.5% Triton X-100 in PBS for 10 min at room temperature. Next, a blocking solution of 10% goat serum (G9023, Sigma-Aldrich) in PBS was added for 1 h at room temperature. Primary antibodies were diluted in blocking buffer and incubated overnight at 4 °C in a humidified chamber. Secondary antibodies were diluted in PBS and incubated for 1 h at room temperature in the dark. Nuclei were stained with Hoechst diluted in PBS (1:15,000) for 5 min. Glass coverslips were washed with PBS (10 min) and mounted with FluorSave (345789, Millipore) onto glass slides. Primary antibodies diluted in blocking solution were: mouse anti-α-tubulin (1:100, clone B7, sc-5286, Santa Cruz Biotech.), mouse anti-E-cadherin (1:100, HECD-1, 13-1700, Invitrogen), mouse anti-Maspin (1:100, clone AT2N6, MAB8227, Abnova), rabbit anti-TUBA4A (1:100, SAB2102603, Sigma-Aldrich). Secondary antibodies diluted in PBS were: goat anti-mouse Alexa Fluor 488 (1:500, A28175, Invitrogen), goat anti-rabbit Alexa Fluor 647 (1:500, A32733, Invitrogen). Rhodamine Phalloidin (R415, Invitrogen) was incubated along with secondary antibodies at 1:500 dilution.
Image acquisition and quantification
Data were acquired with a customized Zeiss Axio Observer seven equipped with a Laser TIRF III and 405/488/561/757-760 nm lasers, Alpha Plan-Apo 100x/1.46 Oil DIC M27, and Objective Heater 25.5/33 S1. Images on both systems were recorded on a Prime 95B CMOS camera (Photometrics) with a pixel size of 107 nm. For in vitro microtubule dynamics assay, images were taken at 10 s time intervals for at least 16 min. For EB1 comets tracking, cells were imaged at 500 ms time intervals for 2 min on an inverted Zeiss Axio Observer 7 widefield microscope equipped with Zeiss digital camera Axiocam 208 and Plan-Apochromat 100x/1.40 NA Oil objective lens. Experiments were carried out in a 5% CO2 atmosphere and 37 °C humidified environment. Microscope control was executed using ZenBlue (Zeiss).
Confocal images were acquired on an inverted Leica TCS SP8 confocal microscope equipped with a HC PL APO CS2 63x/1.4 NA or 100x/1.4 NA objective lens. Image sets were acquired under the same gain and exposure time conditions within each experiment. Microscope control was executed using LasX software (Leica Microsystems).
All images were processed and analyzed using Fiji (ImageJ). Representative images were generated using the maximum intensity projection (Fiji/ImageJ) for Fig. 3a and c and for Supplementary Figs. 3b, e, g, h and j, 4a–c; and the Sum of slices for Supplementary Fig. 2b. Single Z-slices (0.6 µm each) are represented in Fig. 2a–c and in Supplementary Fig. 2f–h, i.
For EB1 comets tracking, we selected only MCF-10A cells stably expressing similar levels of EB1-mCherry for comparison analysis. EB1 comets were tracked using the MTrackJ plug-in97. For this purpose, a 1-frame value was set for time step size and the maximum intensity was selected for the snap feature. Comets were manually tracked for at least 3 consecutive frames using the selection tool. For microtubule regrowth assays, fluorescence intensity was measured based on a fixed circular ROI drawn and equally set for all MTOCs.
Fluorescence intensity was measured manually drawing a 3-width segmented line around the cell boundaries (E-cadherin) or at the cell cortex (F-actin) using the segmented line tool. To this end, 4 Z-slice sections of 0.6 µm each from intermediate slices (total of 10 Z-sections/field) were analyzed using the maximum intensity projection.
The cell area was measured by drawing a 3-width segmented line around the boundaries of interphase cells in a time frame of 5 min before undergoing retraction, which was visually accompanied by bright-field imaging. Cell roundness was measured using ImageJ and spindle length was manually measured drawing a line along the pole-to-pole distance of metaphasic mitotic cells in imageJ.
Statistics and reproducibility
All statistical analyses and data plotting were performed using GraphPad Prism 8 (www.graphpad.com). In the scatter dot plots bars, dots represent independent measurements grouped from at least two independent experiments. Mean and standard deviation were represented below each graph. For numerical data, Shapiro–Wilk normality tests were applied and unpaired two-tailed Student’s t-tests were used where two independent groups were analyzed. Multiple comparisons were done using One-Way ANOVA followed by Dunnett’s Multiple Comparison test. A p-value less than or equal to 0.05 was considered statistically significant.
Figure design and schematics
Figures were assembled using Adobe Illustrator and illustrations were created using BioRender (Fig.1b—license number BB28J81SCP; Fig.1i—VF28J829YZ; Fig.1j—ZT28J82GUZ; Fig.3e—XP28J833L8; Fig.3i—XG28J80LUZ; Fig.4d—NE28J810PA; Supplementary Fig. 1f—EX28J81F7U).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data are available in the published article and its online supplemental material. Mass spectrometry proteomics data underlying Fig. 1 and the Supplementary Data 1 have been deposited to the ProteomeXchange Consortium via the PRIDE repository with a reference number: PXD057847. Supplementary videos and data, numerical data underlying the graphs and raw data for western blot can be found at: https://doi.org/10.6084/m9.figshare.27107311.v1.
References
Pollard, T. D. Actin and actin-binding proteins. Cold Spring Harb. Perspect. Biol. 8, a018226 (2016).
Goodson, H. V. & Jonasson, E. M. Microtubules and microtubule-associated proteins. Cold Spring Harb. Perspect. Biol. 10, a022608 (2018).
Hu, L., Huang, Z., Wu, Z., Ali, A. & Qian, A. Mammalian plakins, giant cytolinkers: versatile biological functions and roles in cancer. Int. J. Mol. Sci. 19, 974 (2018).
DiRusso, C. J., Dashtiahangar, M. & Gilmore, T. D. Scaffold proteins as dynamic integrators of biological processes. J. Biol. Chem. 298, 102628 (2022).
Hohmann, T. & Dehghani, F. The cytoskeleton—a complex interacting meshwork. Cells 8, 362 (2019).
Cavey, M. & Lecuit, T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb. Perspect. Biol. 1, a002998 (2009).
Shigetomi, K. & Ikenouchi, J. Cell adhesion structures in epithelial cells are formed in dynamic and cooperative ways. BioEssays 41, 1800227 (2019).
Le Bras, G. F., Taubenslag, K. J. & Andl, C. D. The regulation of cell-cell adhesion during epithelial-mesenchymal transition, motility and tumor progression. Cell Adh. Migr. 6, 365–373 (2012).
Zou, Z. et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science 263, 526–529 (1994).
Law, R. H. P. et al. An overview of the serpin superfamily. J. Biol. Chem. 280, 22356–22364 (2005).
Pemberton, P. A. et al. The tumor suppressor maspin does not undergo the stressed to relaxed transition or inhibit trypsin-like serine proteases: evidence that maspin is not a protease inhibitory serpin. J. Biol. Chem. 270, 15832–15837 (1995).
Pemberton, P. A. et al. Maspin is an intracellular serpin that partitions into secretory vesicles and is present at the cell surface. J. Histochem. Cytochem. 45, 1697–1706 (1997).
Dean, I. et al. The secretion and biological function of tumor suppressor maspin as an exosome cargo protein. Oncotarget 8, 8043–8056 (2017).
Tian, C. et al. Cancer cell–derived matrisome proteins promote metastasis in pancreatic ductal adenocarcinoma. Cancer Res. 80, 1461–1474 (2020).
Machowska, M., Wachowicz, K., Sopel, M. & Rzepecki, R. Nuclear location of tumor suppressor protein maspin inhibits proliferation of breast cancer cells without affecting proliferation of normal epithelial cells. BMC Cancer 14, 142 (2014).
Sheng, S. et al. Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc. Natl. Acad. Sci. USA 93, 11669–11674 (1996).
Bass, R., Moreno Fernández, A. M. & Ellis, V. Maspin inhibits cell migration in the absence of protease inhibitory activity. J. Biol. Chem. 277, 46845–46848 (2002).
Qin, L. & Zhang, M. Maspin regulates endothelial cell adhesion and migration through an integrin signaling pathway. J. Biol. Chem. 285, 32360–32369 (2010).
Ravenhill, L., Wagstaff, L., Edwards, D. R., Ellis, V. & Bass, R. G-helix of maspin mediates effects on cell migration and adhesion. J. Biol. Chem. 285, 36285–36292 (2010).
Sakabe, T., Wakahara, M., Shiota, G. & Umekita, Y. Role of cytoplasmic localization of maspin in promoting cell invasion in breast cancer with aggressive phenotype. Sci. Rep. 11, 11321 (2021).
Goulet, B., Chan, G., Chambers, A. F. & Lewis, J. D. An emerging role for the nuclear localization of maspin in the suppression of tumor progression and metastasis. Biochem. Cell Biol. 90, 22–38 (2012).
Liu, J., Yin, S., Reddy, N., Spencer, C. & Sheng, S. Bax mediates the apoptosis-sensitizing effect of maspin. Cancer Res 64, 1703–1711 (2004).
Mahajan, N. et al. Maspin binds to cardiolipin in mitochondria and triggers apoptosis. FASEB J. 33, 6354–6364 (2019).
Bailey, C. M. et al. Mammary serine protease inhibitor (maspin) binds directly to interferon regulatory factor 6: Identification of a novel serpin partnership. J. Biol. Chem. 280, 34210–34217 (2005).
Li, X. et al. HDAC1 inhibition by maspin abrogates epigenetic silencing of glutathione S-transferase Pi in prostate carcinoma cells. Cancer Res 66, 9323–9329 (2006).
Mahajan, N., Shi, H. Y., Lukas, T. J. & Zhang, M. Tumor-suppressive maspin functions as a reactive oxygen species scavenger: Importance of cysteine residues. J. Biol. Chem. 288, 11611–11620 (2013).
Abraham, S., Zhang, W., Greenberg, N. & Zhang, M. Maspin functions as a tumor suppressor by increasing cell adhesion to extracellular matrix in prostate tumor cells. J. Urol. 169, 1157–1161 (2003).
Shao, L. J., Shi, H. Y., Ayala, G., Rowley, D. & Zhang, M. Haploinsufficiency of the maspin tumor suppressor gene leads to hyperplastic lesions in prostate. Cancer Res 68, 5143–5151 (2008).
Beltran, A. et al. Re-activation of a dormant tumor suppressor gene maspin by designed transcription factors. Oncogene 26, 2791–2798 (2007).
Cella, N. et al. Maspin is physically associated with β1 integrin regulating cell adhesion in mammary epithelial cells. FASEB J. 20, 1510–1512 (2006).
Bass, R., Wagstaff, L., Ravenhill, L. & Ellis, V. Binding of extracellular maspin to β1 integrins inhibits vascular smooth muscle cell migration. J. Biol. Chem. 284, 27712–27720 (2009).
Yin, S. et al. Maspin retards cell detachment via a novel interaction with the urokinase-type plasminogen activator/urokinase-type plasminogen activator receptor system. Cancer Res. 66, 4173–4181 (2006).
Endsley, M. P. et al. Maspin, the molecular bridge between the plasminogen activator system and β1 integrin that facilitates cell adhesion. J. Biol. Chem. 286, 24599–24607 (2011).
Al-Mamun, M. et al. Effects of noninhibitory serpin maspin on the actin cytoskeleton: a quantitative image modeling approach. Microsc. Microanal. 22, 394–409 (2016).
Thul, P. J. et al. A subcellular map of the human proteome. Science 356, eaal3321 (2017).
Jiang, N., Meng, Y., Zhang, S., Mensah-Osman, E. & Sheng, S. Maspin sensitizes breast carcinoma cells to induced apoptosis. Oncogene 21, 4089–4098 (2002).
Latha, K., Zhang, W., Cella, N., Shi, H. Y. & Zhang, M. Maspin mediates increased tumor cell apoptosis upon induction of the mitochondrial permeability transition. Mol. Cell Biol. 25, 1737–1748 (2005).
Zhang, W., Shi, H. Y. & Zhang, M. Maspin overexpression modulates tumor cell apoptosis through the regulation of Bcl-2 family proteins. BMC Cancer 5, 50 (2005).
Bernardo, M. M. et al. Maspin reprograms the gene expression profile of prostate carcinoma cells for differentiation. Genes Cancer 11, 1009–1022 (2011).
Chen, E. I. et al. Maspin alters the carcinoma proteome. FASEB J. 19, 1123–1124 (2005).
Longhi, M. T. et al. PI3K-AKT, JAK2-STAT3 pathways and cell–cell contact regulate maspin subcellular localization. Cell Commun. Signal. 19, 86 (2021).
Jefferson, J. J., Leung, C. L. & Liem, R. K. H. Plakins: Goliaths that link cell junctions and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 5, 542–553 (2004).
Sjöblom, B., Salmazo, A. & Djinović-Carugo, K. α-Actinin structure and regulation. Cell. Mol. Life Sci. 65, 2688–2701 (2008).
Rosenblatt, J., Cramer, L. P., Baum, B. & McGee, K. M. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell 117, 361–372 (2004).
Liu, T., Pemberton, P. A. & Robertson, A. D. Three-state unfolding and self-association of maspin, a tumor- suppressing serpin. J. Biol. Chem. 274, 29628–29632 (1999).
Al-Ayyoubi, M., Gettins, P. G. W. & Volz, K. Crystal structure of human maspin, a serpin with antitumor properties. J. Biol. Chem. 279, 55540–55544 (2004).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
McAlear, T. S. & Bechstedt, S. The mitotic spindle protein CKAP2 potently increases formation and stability of microtubules. Elife 11, e72202 (2022).
Charras, G. & Yap, A. S. Tensile forces and mechanotransduction at cell–cell junctions. Curr. Biol. 28, R445–R457 (2018).
Koster, J., Van Wilpe, S., Kuikman, I., Litjens, S. H. M. & Sonnenberg, A. Role of binding of plectin to the integrin β4 subunit in the assembly of hemidesmosomes. Mol. Biol. Cell 15, 1211–1223 (2004).
Bodenstine, T. M. et al. Internalization by multiple endocytic pathways and lysosomal processing impact maspin-based therapeutics. Mol. Cancer Res. 12, 1480–1491 (2014).
Longhi, M. T., Magalhães, M., Reina, J., Freitas, V. M. & Cella, N. EGFR signaling regulates maspin/serpinb5 phosphorylation and nuclear localization in mammary epithelial cells. PLoS ONE 11, e0159856 (2016).
Debnath, J., Muthuswamy, S. K. & Brugge, J. S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256–268 (2003).
Cohen, D. et al. Borg5 restricts contractility and motility in epithelial MDCK cells. J. Cell. Sci 137, jcs261705 (2024).
Vasioukhin, V., Bauer, C., Yin, M. & Fuchs, E. Directed actin polymerization is the driving force for epithelial cell- cell adhesion. Cell 100, 209–219 (2000).
Wakahara, M. et al. Clinical significance of subcellular localization of maspin in breast carcinoma: an immunohistochemical study using two different antibodies. Yonago Acta Med 66, 19–23 (2023).
Conant, D. et al. Inference of CRISPR edits from sanger trace data. CRISPR J. 5, 123–130 (2022).
Teoh, S. S. Y. et al. Maspin is not required for embryonic development or tumour suppression. Nat. Commun. 5, 3164 (2014).
Gao, F., Shi, H. Y., Daughty, C., Cella, N. & Zhang, M. Maspin plays an essential role in early embryonic development. Development 131, 1479–1489 (2004).
Whisstock, J. C. & Bird, P. I. An essential role of maspin in embryogenesis and tumor suppression — letter. Cancer Res 77, 5207 (2017).
Dzinic, S. H. et al. An essential role of maspin in embryogenesis and tumor suppression. Cancer Res 77, 886–896 (2017).
Zhang, M., Hendrix, M. J. C., Pemberton, P. A., Sakr, W. A. & Sheng, S. An essential role of maspin in embryogenesis and tumor suppression — response. Cancer Res 77, 5208–5210 (2017).
Teoh, S. S., Whisstock, J. C. & Bird, P. I. Maspin (SERPINB5) is an obligate intracellular serpin. J. Biol. Chem. 285, 10862–10869 (2010).
Jones, M. C., Zha, J. & Humphries, M. J. Connections between the cell cycle, cell adhesion and the cytoskeleton. Philos. Trans. R. Soc. B Biol. Sci. 374, 20180227 (2019).
Krüger, L. K. & Tran, P. T. Spindle scaling mechanisms. Essays Biochem 64, 383–396 (2020).
Yang, J. et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 21, 341–352 (2020).
Nihongaki, Y., Matsubayashi, H. T. & Inoue, T. A molecular trap inside microtubules probes luminal access by soluble proteins. Nat. Chem. Biol. 17, 888–895 (2021).
Rathod, M. et al. DPM1 modulates desmosomal adhesion and epidermal differentiation through SERPINB5. J. Cell Biol. 223, e202305006 (2024).
Chugh, P. & Paluch, E. K. The actin cortex at a glance. J. Cell Sci. 131, jcs186254 (2018).
Shi, H. Y., Stafford, L. J., Liu, Z., Liu, M. & Zhang, M. Maspin controls mammary tumor cell migration through inhibiting Rac1 and Cdc42, but not the RhoA GTPase. Cell Motil. Cytoskel 64, 338–346 (2007).
Valiron, O. et al. Desmoplakin expression and organization at human umbilical vein endothelial cell-to-cell junctions. J. Cell Sci. 109, 2141–2149 (1996).
Wang, N. & Chang, L. L. Maspin suppresses cell invasion and migration in gastric cancer through inhibiting EMT and angiogenesis via ITGB1/FAK pathway. Hum. Cell 33, 663–675 (2020).
Liu, B. X. et al. SERPINB5 promotes colorectal cancer invasion and migration by promoting EMT and angiogenesis via the TNF-α/NF-κB pathway. Int. Immunopharmacol. 131, 111759 (2024).
Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 (2014).
Datta, A. et al. Cytoskeletal dynamics in epithelial-mesenchymal transition: Insights into therapeutic targets for cancer metastasis. Cancers 13, 1882 (2021).
Tanaka, H. et al. siRNA gelsolin knockdown induces epithelial-mesenchymal transition with a cadherin switch in human mammary epithelial cells. Int. J. Cancer 118, 1680–1691 (2006).
Pongrakhananon, V., Wattanathamsan, O., Takeichi, M., Chetprayoon, P. & Chanvorachote, P. Loss of CAMSAP3 promotes EMT via the modification of microtubule–Akt machinery. J. Cell Sci. 131, jcs216168 (2018).
Geay Plakins are involved in the regulation of centrosome position in polarized epithelial cells. Biol. Cell 116, e2400048 (2024).
Roostalu, J. et al. The speed of GTP hydrolysis determines GTP cap size and controls microtubule stability. Elife 9, e51992 (2020).
Noordstra, I. et al. Control of apico-basal epithelial polarity by the microtubule minus-end-binding protein CAMSAP3 and spectraplakin ACF7. J. Cell Sci. 129, 4278–4288 (2016).
Ning, W. et al. The CAMSAP3-ACF7 complex couples noncentrosomal microtubules with actin filaments to coordinate their dynamics. Dev. Cell 39, 61–74 (2016).
Richard Mcintosh, J. M. Mitosis. Cold Spring Harb Perspect. Biol. 8, a023218 (2016).
Taubenberger, A. V., Baum, B. & Matthews, H. K. The mechanics of mitotic cell rounding. Front. Cell Dev. Biol. 8, 687 (2020).
Vadnjal, N. et al. Proteomic analysis of the actin cortex in interphase and mitosis. J. Cell Sci. 135, jcs259993 (2022).
Hosseini, K. et al. Binding dynamics of α-actinin-4 in dependence of actin cortex tension. Biophys. J. 119, 1091–1107 (2020).
Pascal, A. et al. Annexin A2 and Ahnak control cortical NuMA–dynein localization and mitotic spindle orientation. J. Cell Sci. 135, jcs259344 (2022).
Goshima, G. & Scholey, J. M. Control of mitotic spindle length. Annu. Rev. Cell Dev. Biol. 26, 21–57 (2010).
Reina, J., Zhou, L., Fontes, M. R. M., Panté, N. & Cella, N. Identification of a putative nuclear localization signal in the tumor suppressor maspin sheds light on its nuclear import regulation. FEBS Open Bio. 9, 1174–1183 (2019).
Geu-Flores, F., Nour-Eldin, H. H., Nielsen, M. T. & Halkier, B. A. USER fusion: a rapid and efficient method for simultaneous fusion and cloning of multiple PCR products. Nucleic Acids Res. 35, e55 (2007).
Ashford, A. J. & Hyman, A. A. Chapter 22 - preparation of tubulin from porcine brain A2 - celis. In Cell Biology (Third Edition) (ed. Julio E.) 155–160 (2006).
Hyman, A. et al. Preparation of modified tubulins. Methods Enzymol. 196, 478–485 (1991).
Silva, L. E. et al. Proteomic and interactome approaches reveal PAK4, PHB-2, and 14-3-3ν as targets of overactivated cdc42 in cellular responses to genomic instability. J. Proteome Res. 18, 3597–3614 (2019).
De Jesus, T. C. L. et al. Chromatin proteomics reveals variable histone modifications during the life cycle of trypanosoma cruzi. J. Prot. Res. 15, 2039–2051 (2016).
Ge, S. X., Jung, D., Jung, D. & Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 36, 2628–2629 (2020).
Jiang, Y. & Huang, S. Direct visualization and quantification of the actin nucleation and elongation events in vitro by TIRF Microscopy. Bio. Protoc. 7, e2146 (2017).
Gell, C. et al. Microtubule dynamics reconstituted in vitro and imaged by single-molecule fluorescence microscopy. Methods Cell Biol. 95, 221–245 (2010).
Kapoor, V. et al. MTrack: Automated detection, tracking, and analysis of dynamic microtubules. Sci. Rep. 9, 3794 (2019).
Acknowledgements
We would like to thank Mr. Waldir Caldeira (MSc.) (Center for Image Acquisition and Microscopy at the Institute of Biosciences) and to laboratory specialist Adriana Yamaguti Matsukuma (MSc.) (Analytical Center at the Institute of Chemistry) of the University of São Paulo for assistance with imaging using the confocal microscope. We also thank Dr. Vanessa Morais Freitas and Dr. Marilene Hohmuth Lopes (Institute of Biomedical Sciences/University of Sao Paulo, Brazil) for contributing with reagents and equipment. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (www.fapesp.br) Grant Numbers: 2021/12268-4 (NC), 18/15553-9 (JPCC), the Canadian Institutes of Health Research (PJT-156193, SB) and the Natural Sciences and Engineering Research Council of Canada (NSERC; RGPIN-2017-04649, SB). LES is supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (https://www.gov.br/capes/pt-br) Doctoral scholarships (88887.506347/2020-00, 88887.816589/2023-00 and 88887.835982/2023-00). LMGP is supported by an NSERC Postdoctoral Fellowship. APJM is supported by a FAPESP Postdoctoral Fellowship (23/08391-0). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
(LES)—Conceptualization, investigation, data curation, formal analysis, methodology, visualization, writing—original draft, writing—review and editing. (LMGP)—Methodology, supervision, writing—review and editing. (TL)—Investigation, methodology. (APJM)—Investigation, formal analysis, validation, visualization. (JPCC)—Conceptualization, funding acquisition, methodology, formal analysis, validation, visualization. (SB)—Conceptualization, funding acquisition, investigation, methodology, supervision, formal analysis, validation, visualization, writing—review and editing. (NC)—Conceptualization, funding acquisition, methodology, project administration, supervision, writing - original draft, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Gregory Emery, Srikanth Budnar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Kaliya Georgieva.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
da Silva, L.E., Paim, L.M.G., Lyalina, T. et al. Maspin/SerpinB5 is a cytoskeleton-binding protein that regulates epithelial cell shape. Commun Biol 8, 1262 (2025). https://doi.org/10.1038/s42003-025-08688-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08688-3