Abstract
Aging brain undergoes a structural decline over lifespan accompanied by changes in neurotransmitter levels, leading to altered functional markers. Past studies have reported human resting state brain display a remarkable preservation of coordination among neural assemblies stemming from an underlying neurocomputational principles along aging trajectories, however, the true nature of which remains unknown. Here, we identify the computational mechanisms with which neurotransmitters, such as altered GABA and glutamate concentrations, can preserve functional integration across lifespan aging, despite structural decline. We employ multiscale, biophysically grounded modeling, constrained by the empirically derived anatomical connectome of the human brain, where the neurotransmitter concentrations can be free parameters that are algorithmically adjusted to maintain regional homeostasis and optimal working point. The two estimated neurotransmitters can maintain critical firing rates in the brain region and mimic age-associated functional connectivity patterns, consistent with empirical observations. We identified invariant GABA and reduced glutamate as the principle computational mechanism that can explain the topological variation of functional connectivity along lifespan, validated using graph-theoretic metrics. The results are subsequently replicated on three distinct datasets. Thus, the study offers an operational framework that integrates brain network dynamics at macroscopic and molecular scales, to gain insight into age-associated neural disorders.

Similar content being viewed by others
Introduction
Aging effects are observed at multiple scales of brain organization, ranging from microscopic level alteration in neurotransmitter levels1,2,3,4,5,6,7, to degradation in regional gray matter volume8, and long-range white matter tracts among brain areas9,10,11. At the macroscopic level, changes in neuronal coordination cause cognitive and behavioral impairments12,13,14, or changes to the functional integration in unfolding brain networks15,16,17. Because of such multi-scale interactions, the effects of aging on neural activity become complex. For instance, some functions of the healthy aging brain deteriorate largely, such as declined processing speed, inhibitory function, long-term memory, and working memory18. In contrast, other cognitive functions, such as, implicit memory and knowledge storage, remain relatively preserved and show greater resilience to the effects of brain aging16,18,19,20,21,22. Earlier investigations have shown that despite age-associated structural decreases, functional imaging studies reveal somewhat surprising yet pervasive increases in prefrontal activation with age, reflecting the brain’s adaptive and resilient nature. This helps protect cognitive function during healthy aging, as explained by the scaffolding theory of aging and cognition18. Thus, an overarching question arises: can the age-related changes captured by neuroimaging markers be integrated into an operational framework that encompasses the myriad of complex interactions spanning multiple scales of brain organization, including large-scale neural field dynamics and neurotransmitter kinetics?
To conceptualize an operational framework for understanding age-related changes in the brain, one can delve into the structural and functional markers across the lifespan23,24. Typically, graph properties can quantify alterations in brain networks, e.g., modularity and global efficiency14,25,26,27. For instance, using a longitudinal design, Coelho et al. observed that the modularity of whole-brain structural connectivity (SC) increases with age in a moderate-sized group of human participants (N = 51)10,27. This general trend also persisted in cross-sectional data from different publicly available cohorts of large sizes (N = 174) from three independent datasets used in our study. An increasing number of studies suggest the effective use of graph properties to capture alterations in resting-state functional connectivity (FC) across lifespan aging16,23,28. Thus, graph-theoretic measures of network segregation, integration and resilience can serve as excellent validation tools to compare age-related variations and reorganization in structural and functional brain networks across cohorts .
In parallel, complexity of neural coordination in time-varying functional networks29,30,31,32 can also provide validation measures to quantify age-related network changes across cohorts. The brain-wide dynamics, involve transitions between “order"—when network nodes may display in-sync activity and “disorder" when nodes are weakly synchronized or display incoherent dynamical relationship33,34. Such transitory “in-between" dynamical state of the brain is assumed to be metastable, which does not commit to a specific attractor state, but remains perpetually prepared to internalize a stimulus that drives the transitions from one attractor state to another35. In characterizing global dynamics of the coordinated neural activity at rest, the measure of metastability has been extensively used across various research studies11,16,29,36. Being in a metastable state allows the brain to maximize information processing and to promptly react to any internal or external stimulus37,38,39. This dynamical state is often conceptualized as the optimal working point of a healthy brain30. Emergence of such neural dynamics depends on two cardinal factors: (1) mutual influences among brain-wide neural populations mediated by their interconnecting anatomical projections (i.e., SCs), and (2) neurotransmitters responsible for regional firing activity of excitatory and inhibitory populations. While the interconnected fibers serve as the structural foundation for synaptic communication across distributed cortical networks, the neurotransmitter kinetics regulate local excitation-inhibition homeostasis, together enabling the integration of neural activity that supports brain functions and cognitive processes.
Available evidence suggests that qualitative variations of SC properties play an important role in reshaping the brain dynamics differently, likely through their influence on the local ratio, or balance of excitatory and inhibitory cell activity40,41. An earlier study showed how the interplay between structural topology and key controlling parameters, such as excitatory-inhibitory (E-I) balance, played a pivotal role in determining the critical dynamics42. An example in the macaque brain showed that a biophysically-grounded computational model, simulated on its connectome, revealed both feedback projections and a heterogeneous distribution of fiber strengths among brain regions as fundamental for the emergence of ignition-like dynamics43. These emergent dynamics are thought to be a necessary condition for conscious processing44. Ideally, one could rely on the biophysically plausible nonlinear model of neural dynamics to accurately illustrate the importance of anatomical connectivity in supporting coordinated brain dynamics. For example, when simulated by constraining human interregional SC—derived from diffusion-weighted magnetic resonance imaging (MRI), the model replicates empirically observed patterns of interregional FC34. Using such a computational framework, numerous studies have demonstrated how the aging brain gradually tunes its intrinsic parameters, e.g., global coupling strength or local E-I regulations11,24,45,46,47,48. This is identified as an effective mechanism that compensates for white-matter loss and upholds its operational working point at maximal metastability. However, the computational principles unifying age-related changes in structural network properties and compensatory mechanisms involving two primary neurotransmitters, GABA and glutamate, have received less attention. Under this background, we hypothesize that healthy aging, where fluid intelligence, language, and other higher-order cognitive functions are typically preserved, must also underlie an invariance of the optimal dynamic working point, indexed by metastability, mediated by the multi-scale interactions involving neurotransmitter kinetics and E-I membrane currents at the neural field level.
To achieve this aim, we adopt the perspective of dynamical compensation underlying healthy aging. We seek to explore mechanistic insights of the adaptive nature of the brain by conceptualizing metastable dynamics as the healthy brain’s optimal working point. The analytical framework integrates the balanced homeostasis regulated by GABA-glutamate with empirical observations on SC and FC graph properties. Accordingly, we employ a biophysically inspired multiscale dynamical mean field (MDMF) model, constrained by human anatomical connectomes. Under optimal parameters, coupling strength, and excitatory and inhibitory neurotransmitters (glutamate and GABA), the model is capable of reproducing resting-state neural activity46,49. The large-scale brain dynamics are modeled by local inhibitory and excitatory neuronal populations with uniform properties across all brain regions. The assumption is based on a homogeneous distribution of the two model parameters, GABA and glutamate concentrations, which are estimated through state-of-the-art model inversion methods. The two free parameters are algorithmically adjusted while preserving desired metastable dynamics within the aging cohorts and achieve a uniform firing rate of approximately 3Hz across all brain regions (in line with experimental data50,51,52). Age-related changes in SC properties solely influence the parameter estimation, further validated through qualitative comparison between the empirical observations on FC changes and model-based FC properties. Our model simulation demonstrates that in response to the structural insults, the aging brain engages in a continuous functional reorganization primarily driven by the excitatory neurotransmitter, glutamate, playing a central role in this compensatory process.
Methods
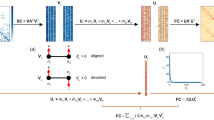
Figure 1 depicts multiscale brain organization, and workflow for model simulation and evaluation. Using two biologically inspired constraints, geared towards fitting the spatio-temporal characteristics, we undertake a model inversion to predict variations in GABA and glutamate concentrations mediated by the SC changes with age. Ideally, the static FC provides a temporal averaged snapshot of all covariations pairwise between brain areas, can be considered as a summary measure of spatial features. Thereby, the closeness between simulated and empirical FC is minimized to estimate the two model parameters. Meanwhile, the resting-state brain dynamics shows a tendency to remain at a maximally metastable state29, quantified by the dynamical measure of metastability that depicts variance in the Kuramoto order parameter53 fluctuations over time. To match the resting state activity, the measure of metastability is maximized on the two parameter plane, set as another optimization condition. Next, both the conditions are unified to estimate the model based GABA-glutamate values. Finally, we need a set of independent metrics which are not directly related to the method of parameter estimation but can be an independent validation measure for the usability of the model. To meet this end, topological properties of empirical FC computed using graph theoretic metrics can be compared against measures of integration/ segregation applied to simulated FC.
a First column displays a schematic diagram of different brain scales from microscopic to macroscopic level. Second column shows functions at different brain scales. Global dynamics quantified by metastability and E-I homeostasis are observed at macroscopic and mesoscopic scales, whereas, glutamate-GABA kinetics can be observed at microscopic level. Third column presents the working principles of the computational construct. The MDMF model placed at each node of an anatomical brain network (top tile) are optimally tuned by the two parameters (glutamate, GABA) across lifespan aging while maintaining E-I balance. b Overview of the entire pipeline for model fitting and evaluation. Empirical structural connectivity (SC) is constrained to the MDMF model that generates simulated FCs. Two conditions, i.e., maximizing metastability and minimizing FC distance between empirical and simulated FCs, are used to estimate optimal Glutamate and GABA values of for individual participants. Based on the optimal parameter values, simulated FCs are generated for individual participants, while the critical firing rates are maintained. The graph theoretic metrics of segregation and integration from the simulated FCs are measured and compared against the empirical FC properties, which provides an independent qualitative validation for the robustness of our results.
Empirical data description
Data sets for this study were obtained from three different sources. We acknowledge Berlin Centre for Advanced Imaging, Charité University Medicine, Berlin54 for data collection and sharing. We used the pre-processed data provided by the group. This dataset was collected by a 3T Siemens Tim Trio scanner with a 32-channel head coil (2 mm isotropic voxel size). The pre-processed data from the Nathan Kline Institute (NKI) Rockland was obtained from the publicly accessible UCLA Multimodal Connectivity Database (UMCD)55. We acknowledge the Cambridge Centre for aging and Neuroscience (CamCAN), University of Cambridge, UK, for data collection and sharing. They used 3T Siemens Tim trio scanner with a 32-channel head coil (voxel size 3 × 3 × 4.4 mm56). We have pre-processed randomly selected participants from similar age groups to CamCAN, Berlin and NKI datasets. Details of participants, data acquisition, and pre-processing are mentioned in the Supplementary Materials with a summary of demographics in Table1.
Dynamical measure of metastability
Recent computational brain network models30,57 have predicted (for instance) that the human brain operates at maximum metastability during the resting state. It is manifested as an optimal information processing capability and switching behavior30,58,59,60. Also, the existing cognitive aging theories are explained better with the concept of metastability16,29,60 and used to explore changes in the physiological substrate over aging16,61. We have chosen metastability as one of the optimal conditions to connect the parametric role to the functional organization. Metastability indexes the perpetual state of transition observed in brain signals without committing to a specific attractor state62,63,64 and has been used as a metric to capture the dynamic working point of the brain at rest by several researchers29,30,49. There are several advantages of using metastability such as, the metric can be used to quantify the tendencies of neural systems to operate at segregative and integrative modes64, characterize the state of criticality where any dynamical system remains on the verge of phase transition thus enhancing information processing capabilities30,65. Finally, the phenomena can be studied via generative models of neural dynamics, and hence allows the researchers to gain mechanistic insights into network mechanisms generating brain signals49,66,67. Motivated from these knowledge, we have utilized the dynamical measure off metastability to capture the brain dynamics, further we set these as one of the key constraints in our computational framework to optimize the model-based parameters.
First, the Kuramoto order parameter53, r(t) is calculated by, \(r(t)=\frac{1}{n}\left| {\sum }_{k = 1}^{n}{e}^{i{\phi }_{k}(t)}\right|\). where, ϕk(t) is the instantaneous phase of kth brain region. The value of r(t) lies between 0 ≤ r(t) ≤ 1; r(t) = 1 representing complete synchronous, r(t) = 0 for desynchrony, and (0 < r(t) < 1) representing partial synchrony among all brain regions. Hence, r(t) indexes the Global coherence among a system of oscillating brain regions.
We measure the temporal variability of r(t) to capture the fluctuations in global spatial synchrony within the whole brain over time. Variation in r(t) indicates the fluctuation among collective states (such as coherent or incoherent) over time, which is indexed by metastability. Metastability29,49,60 is used to capture the temporal fluctuation, estimating the tendency of a brain region to deviate from the coherent manifold. Thus, it could be a better measurable unit for observing spatial cohesion for a short period (maybe a few hours) and may also serve as a key constituent for observing a gradual change in lifespan brain neural dynamics on a parametric plane (e.g., GABA, glutamate plane) over a very slow time scale, such as the lifespan. Metastability (M) can be calculated by taking the standard deviation of r(t) over time:
where \(\langle.\rangle\) denotes average over time. When compared across data sets, the measure of metastability can extract information about dynamic complexity associated with healthy lifespan ageing (Fig. 2).
Preparing empirical SCs and FCs for network analysis
To obtain graph properties, we use undirected-weighted and binary network measures from the Brain Connectivity Toolbox (BCT) (http://www.brain-connectivity-toolbox.net). The structural connectivity (SC) is weighted graph, normalized by the maximum count of white-matter fibers, thus it ranges between 0 ≤ SC ≤ 1 for individual subjects obtained from diffusion weighted imaging (DWI). We derive participant-wise undirected-weighted network properties of the normalized SC. The functional connectivity (FC) is a symmetric matrix that captures the brain-wide long-term correlations between any pair of brain regions, measured by Pearson correlation coefficient ranging between −1 ≤ FC ≤ 1. We convert the original weighted FC matrix into a undirected binary graph by applying a threshold (δ) based on the p values obtained from pair-wise Pearson correlations while taking the pairwise correlation of the BOLD signals between any pair of brain regions. We average the thresholds over entire cohorts to obtain a single threshold value. Thereafter, the weighted FCs are converted into the binary network by setting the condition as, FCi,j = 1, if abs(FCi,j) > δ, where δ = 0.076; otherwise, FCi,j = 0. In other words, only the significant correlations are chosen to build the binary FC network. Next, we have derived the FC properties for various other threshold values in order to check threshold dependency on the graph theoretical metrics. In particular, the FC thresholding is defined by the minimum correlation coefficient value corresponding to the p values < 0.04 and 0.03, i.e., abs(FC) ≥ δ = 0.085 and 0.091. For the three chosen thresholds, preserved edge is between 88 to 85% of the total network’s edge (please see, Supplementary Material Fig. S3). Finally, we tested statistically significant changes in the network properties between two age groups for both brain networks (SC and FC) using independent t-tests and Mann–Whitney U test analyses for the three data sets (Fig. 3a–f).
a–f Top row presents the SC properties in two age groups (n = 37 young and n = 32 older adults). a Structural modularity is increased, and b transitivity shows a decreasing trend. It can be interpreted as declined structural segregation. Due to loss in white-matter tracts in the aging brain, the connections across modules become more sparse. Thus, segregated SC resulted in a decreased inter-regional exchange of excitation. Though c global efficiency remains invariant, d significantly decreased average characteristic path length indicates a decline in network integration, which has a direct impact on the network resilience, confirmed by e assortativity, and f node betweenness centrality comparing the two age groups. g–l Functional network properties are shown in the second row (n = 37 young and n = 32 older adults). g Functional modularity remains unaltered with age, implying an average number of functional modules remains intact. However, specific brain regions could vary within a module in the two age groups. A significant increase (p < 0.001) is observed in i global efficiency and j average characteristic path length. Increased global efficiency and average characteristic path length in the elderly group signify an increased functional network integration. No significant changes are seen in k assortativity and l node betweenness centrality between the two groups, which describe unaltered functional resilience in aging brain. To visualize alteration patterns between the age groups, we join the two means by a black line, where the standard deviations from the mean are shown in blue lines. The independent t-test analysis determines significant changes (no changes). Network properties are derived using Brain Connectivity Toolbox26.
Definitions of graph theoretical metrics
The network properties were computed using BCT26. Brain network’s segregation is captured by measuring modularity and transitivity. Integrative capabilities of a network can be captured by global efficiency and average characteristic path. Structural and functional network resilience or vulnerability is assessed using assortativity and node betweenness centrality. The definition and description of the graph theoretical metrics are:
-
(1)
Modularity25 of a network is \(Q=\frac{1}{l}{\sum }_{(i,j\in N)}[{a}_{ij}-\frac{{k}_{i}{k}_{j}}{l}]{\delta }_{{m}_{i}{m}_{j}}\), where mi is the module containing node i, and \({\delta }_{{m}_{i}{m}_{j}}=1\), if mi = mj, and 0 otherwise. a is a binary adjacency matrix. l is the number of links and N is total nodes. For a weighted graph, modularity is \({Q}^{w}=\frac{1}{{l}^{w}}{\sum }_{(i,j\in N)}[{w}_{ij}-\frac{{k}_{i}^{w}{k}_{j}^{w}}{l}]{\delta }_{{m}_{i}{m}_{j}}\), where lw is the sum of weights in the network; wij is the connectivity weight between nodes i and j; \({k}_{i}^{w}\), \({k}_{j}^{w}\) are the weighted degrees of nodes i and j, respectively. Modularity gives network resilience and adaptability, measuring the degree of segregation. Communities are subgroups of densely interconnected nodes sparsely connected with the rest of the network. In the case of a functional network, modularity signifies coherent clusters of functional modules.
-
(2)
Transitivity26,68 is a classic measure of clustering coefficient that captures the tendency of a node to cluster together. High transitivity means the network contains communities or groups of nodes that are densely connected internally. Transitivity of a network is \(T=\frac{{\sum }_{i\in N}2{t}_{i}}{{\sum }_{i\in N}{k}_{i}({k}_{i}-1)}\), where ki is the degree of ith-node. Number of triangles around a node i is ti=\(\frac{1}{2}{\sum }_{j,h\in N}{a}_{ij}{a}_{ih}{a}_{jh}\). a is the adjacency matrix (undirected binary graph). For a weighted graph, geometric mean of triangles around node i is \({t}_{i}^{w}\)=\(\frac{1}{2}{\sum }_{j,h\in N}{({w}_{ij}{w}_{ih}{w}_{jh})}^{1/3}\).
-
(3)
Global efficiency69,\(E=\frac{1}{n}{\sum }_{i\in N}\frac{{\sum }_{j\in N,j\ne i}{d}_{ij}^{-1}}{n-1}\), where N is the set of all nodes; n is the total number of nodes; dij is the shortest path length between node i and j. \({d}_{ij}={\sum }_{{a}_{uv}\in {g}_{i\leftrightarrow j}}{a}_{uv}\). gi↔j is the shortest path (geodesic). The average inverse shortest path length is the global efficiency, which may significantly contribute to integration in larger and sparser networks70. In a weighted graph, the shortest weighted path length between i and j, \({d}_{ij}^{w}={\sum }_{{w}_{uv}\in {g}_{i\leftrightarrow j}^{w}} \, f({w}_{uv})\), where f is a map (inverse) from weight to length. \({g}_{i\leftrightarrow j}^{w}\) is the shortest weighted path between node i and j.
-
(4)
Characteristic path length71,\(L=\frac{1}{n}{\sum }_{i\in N}\frac{{\sum }_{j\in N,j\ne i}{d}_{ij}}{n-1}\), where N is the set of all nodes; n is the total nodes; dij is the shortest path length between i and j. The characteristic path length for weighted graphs is an estimate of proximity. The global efficiency is the average of the inverse shortest path length. For weighted graphs, we use the shortest weighted path length \({d}_{ij}^{w}\).
-
(5)
Assortativity72, \(A=\frac{{l}^{-1}{\sum }_{(i,j\in L)}{w}_{ij}{k}_{i}{k}_{j}-{[{l}^{-1}{\sum }_{(i,j\in L)}\frac{1}{2}{w}_{ij}({k}_{i}+{k}_{j})]}^{2}}{[{l}^{-1}{\sum }_{(i,j\in L)}\frac{1}{2}{w}_{ij}({k}_{i}^{2}+{k}_{j}^{2})]-{[{l}^{-1}{\sum }_{(i,j\in L)}\frac{1}{2}{w}_{ij}({k}_{i}+{k}_{j})]}^{2}}\),where ki, kj are the weighted degrees of nodes i and j, respectively; wij is the connectivity weight between nodes i and j; L is the set of all edges within the network, and l is the total number of edges. Structural and functional network resilience is assessed utilizing the assortativity73,74 and node betweenness centrality75.
-
(6)
Node betweenness centrality26,75 of vertex i is \({b}_{i}=\frac{1}{(n-1)(n-2)}{\sum }_{hj\in N}\frac{{\rho }_{h,j}^{(i)}}{{\rho }_{hj}}\), for h ≠ j, h ≠ i, j ≠ i. The number of shortest paths between h and j is given by ρhj, and \({\rho }_{hj}^{(i)}\) is the number of shortest paths between h and j that pass through node i. Betweenness centrality is computed equivalently on weighted networks, and path lengths are computed on the weighted paths. We take (1/n)∑i∈nbi to get the average node betweenness centrality. Betweenness centrality measures the extent to which a vertex lies on paths between other vertices. Vertices with high betweenness have considerable influence within a network under their control over information passing between others. If the vertices are removed, they will cost in disrupted communication, thus depicting the network’s resilience or vulnerability.
Multi-scale dynamic mean field (MDMF) model
The dynamics of a putative brain area is governed by a stochastic nonlinear dynamical system, the multi-scale dynamic mean field (MDMF) model49 that describes the homeostatic regulation, the regional excitatory-inhibitory (E-I) neural firing activity. The large-scale brain model is constrained by the SC matrix, and generates simulated FC which is compared against empirical resting state FC to estimate the two free parameters (GABA and Glutamate) in an optimal way. Thereby, the brain-wide coordinated dynamics are emerged from the interplay between brain’s anatomical topology and neurotransmitter levels. Individual brain region is modeled by two distinct pools of excitatory and inhibitory neuronal populations having recurrent E-I, I-I, and I-E interactions, and coupled with neurotransmitters GABA and glutamate via NMDA (N-methyl-D-aspartate) and GABA synapses, respectively. The study by Destexhe et al.76 conceptualized the occurrence of neurotransmitter release into the synaptic cleft as a pulse following the arrival of an action potential at the presynaptic terminal. Accordingly, one can consider a chemical kinetic reaction as R + T ↔ TR, where T, R, and TR are the neurotransmitters released in the synaptic cleft, unbound receptors, and bound receptors of postsynaptic neuron, respectively. Subsequently, neurotransmitter release can be captured in the rate equation that describes the dynamics of probability of open channels of a specific neurotransmitter (synaptic gating variable). Detailed expressions of how the concentration of GABA and glutamate can be obtained from population-level averaging from individual neuron-level synaptic concentrations are presented in our earlier study49. The long-range connections are made between the excitatory pools of any pair of brain regions, defined as SC, which is derived from the fiber densities (interconnected fiber bundles) computed by diffusion tensor imaging data of healthy human adults. The regional excitatory pools receive following input currents: inhibitory currents from local inhibitory pools, self excitatory currents, long-range excitatory currents from the excitatory pools of other brain regions, and a constant external current. The brain-wide dynamics is described by the current-based MDMF model49 as:
where, the subscripts i, j indicate brain areas, i, j = 1, 2, … ,N. N is total number of brain regions and its value is different for different parcellations, e.g., N = 68 for Desikan-Killiany atlas77, and N = 188 for Craddock-200 atlas78. The superscripts E and I represent excitatory and inhibitory populations, respectively. \({S}_{i}^{(E,I)}(t)\) denotes the average excitatory or inhibitory synaptic gating variables of the ith region. The time-dependent gating variables are drawn by averaging the fraction of open channels of neurons.
The base model proposed in ref. 46 is identical in form for the first four Eqs. (2)–(5), and the later two Eqs. (6) and (7) are proposed by Naskar et al.49, by incorporating kinetics of the two primary neurotransmitters, glutamate (Tglu) and GABA (Tgaba) within the dynamics of the two gating variables (\({S}_{i}^{(E,I)}(t)\)). Thus, the MDMF model extends computational observations beyond large-scale neuroimaging, capturing neuromolecular dynamics that govern region-specific E-I balance and neural firing. The two free parameters, Tglu and Tgaba, capturing the neurophysiology of the excitatory and inhibitory process, are algorithmically adjusted to fit the model generated FC and empirical FC. For a fixed glutamate and GABA concentration, we select coupling strength G for which simulated and empirical rsFC distance is minimum and their correlation is maximum, while maintaining a firing rate approximately 3–4 Hz79. The fitting of G and its effects on the parameter estimation is shown in Supplementary Material Fig. S8. Stochasticity is introduced by additive uncorrelated white Gaussian noise νi in two gating variables with intensity σ for individual regions. Default parameters are given in Supplemental Materials Table S2.
The local synaptic coupling strength from inhibitory to excitatory pools is denoted by Ji. Dynamics of the inhibitory feedback is governed by Eq. (8). At the mean-field level, the biological complexity involved in the balance of E-I can be captured grossly using the mathematical implementation of the inhibitory plasticity rule80. An inhibitory plasticity rule represents changes in Ji(t) (synaptic weight) to ensure that the inhibitory current clamps to an excitatory population. Thus, homeostasis is achieved by the dynamics of local inhibitory weights Ji(t) as a function of time, such that the firing rate of the excitatory population is maintained at the target firing rate ρ = 3 Hz. The chosen target firing rate emerges when E-I balance is achieved. γ is the learning rate in sec.
We have not considered spatial variability in the neurotransmitters’ concentrations, thus GABA and Glutamate values are homogeneously distributed across all brain regions. Hence, we will get one single pair of parameter values for each independent simulation. For the optimal choice of parameters, the regional excitatory firing rates are approximately 3–4 Hz, concurrent with empirical observations50,51,52,79. Since the model is stochastic in nature, it is unlikely that the converge to a unique solution. Thereby, we have assessed the model performance by comparing simulated FC (generated for the estimated GABA-glutamate values) against empirical FC changes with age. The optimal GABA and Glutamate levels can be estimated from a model inversion approach where the whole-brain dynamics is constrained according to the following conditions: (1) while a normal healthy brain displays metastability at rest, it is maximized on the two parameter plane, and (2) the mean-square root-error between empirical and simulated FCs (mseFC) is minimized. The concept is elaborated with detailed descriptions and figures; see Fig. S4 in Supplementary Material.
Simulated BOLD activity
We calculate model generated resting state BOLD signals from the model-based neural activity of the excitatory pool firing rate \({r}_{i}^{(E)}\) in individuals brain region ith using the hemodynamic Balloon-Windkassel model in refs. 81,82. In the hemodynamic model, an increase in the firing rate \({r}_{i}^{(E)}\) increases vasodilatory signal si subjected to autoregulatory feedback. The blood flow fi in ith brain region responds in proportion to this signal with concurrent changes in blood volume vi and deoxyhemoglobin content qi.
ρ = 0.34 is the resting oxygen extraction fraction, τ = 0.98 is a time constant, and α = 0.32 is the Grubb’s exponent, represents the resistance of the veins. κ = 0.65 and γ = 0.41. The BOLD signal for ith brain region is considered as a static nonlinear function of volume vi and deoxyhemoglobin qi comprised of volume-weighted sum of extra- and intravascular signals, given by:
where k1 = 7ρi, k2 = 2, k3 = 2ρi − 0.2, and V0 = 0.2 is resting blood volume fraction. All the biophysical parameters are taken from ref. 81. To consider functionally relevant frequency range for resting-state conditions, the simulated BOLD activity has been filtered using a bandpass filter (0.001 Hz < f < 0.1 Hz). Thereafter, we detrended the simulated BOLD activity, and used Pearson correlation between any pair of brain regions to generate the simulated or model-based FCs.
Functional connectivity distance
To compare the simulated FC with empirical FC, we calculated the linear distance for each combination of Tgaba, and Tglu parameters. The FC distance for a total of n regions is calculated using mean-square root-error (mse) between the two FCs as:
Please note, to calculate the linear distance between the empirical and simulated FCs, the weighted FCs are considered, whereas the binarized FCs have been used to derive graph properties.
Model-based parameter estimation based on spatio-temporal properties
Model inversion is a key step for parameter optimization of complex nonlinear models83. The following steps are followed to extract the GABA and Glutamate on an individual participant basis: (1) Structural connectivity matrices are derived from diffusion tensor imaging data of each participant. Detailed descriptions of data acquisition and structural connectivity preparations are explained in the earlier subsection “Dynamical measure of metastability” and Supplemental Material Section 1. (2) MDMF model units are placed for each node of the structural connectivity matrix, so that simulated neural activity, e.g., firing rate of excitatory/inhibitory populations at each brain area can be generated. (3) The hemodynamic BOLD activity is then estimated by the Balloon-Windkessel algorithm, to estimate the brain dynamics at each parcel. (4) Pearson correlation is derived from the simulated BOLD time series among brain regions and referred to as simulated FC or model-FC. Simultaneously, metastability is also computed using steps outlined in “Dynamical measure of metastability”. (5) The parameter space of GABA/ Glutamate (Tgaba, Tglu) is scouted using two constraint equations addressing the issues of spatial and temporal complexity respectively. Euclidean distance between simulated and empirical FC is minimized, and the parameter distribution, where metastability is maximum, has been identified (Fig. 4a–e). The test parameter space for glutamate (Tglu) [0.2, 15] and GABA (Tgaba) [0.2, 7] are motivated from empirical observations49 varied along a set of 75 × 35 = 2625 discrete, equispaced points with concentrations in units of mMol. For each tuple (Tglu, Tgaba), we generate simulated BOLD signals and model-based FC for a given SC from one participant which we compare to the empirical FC. As a result, total number of model simulation is 456,750 (75 × 35 × 174) for the considered cohort of 174 subjects from three datasets. For each subjects total simulation time was set to 7 min (total 7 × 60 × 1000, with 0.1 msec time interval resulting in 4,200,001 iterations, where first 2 min are discarded as a transient time). One subject required approximately 22 h of simulation time on a CPU with 4 cores for parallel computations. Thus, the optimal set of \({T}_{glu}^{opt}\) and \({T}_{gaba}^{opt}\) can be conceptualized to be a simple average of the optimal values, separately obtained from spatial and temporal constraint conditions, following the relationship,
where a = 0.5, because, equal weighting is assigned to the measures of spatial and temporal complexity. This way, we obtain each pair of optimal concentrations, \(GGC={({T}_{glu},{T}_{gaba})}^{opt}\) in mMol. We calculate the GABA-glutamate ratio (GGR) by taking their ratio as \(GGR={({T}_{glu}/{T}_{gaba})}^{opt}\) for individual subjects. Participant-wise estimation steps of glutamate, GABA values using the MDMF model are presented in Supplementary Material Fig. S6. Finally, we perform a two-sample t-test to find statistical differences in the estimated GGC and GGR between the two age groups in the three datasets.
First, the SCs of individual groups are averaged (young n = 1, old n = 1). Next, 50 independent simulations are performed. a The 3-D maps of metastability and mean-square root-error between FCs (mseFC) are plotted on Tgaba − Tglu parameter plane. The blue stars, marked on the 2D parameter plane, indicate optimal Tglu, and Tgaba values. b Clouds of estimated parameters are shown for young adults and elders, produced by 50 independent simulations. Blue and red circles represent the average values in young and old groups, respectively. c Regional excitatory and inhibitory firing rates are plotted for the estimated average Tglu, and Tgaba values. d Empirical and simulated static FCs are plotted using estimated parameters, taking average over trials. e Empirical and simulated FC dynamics (FCD) are shown in time-versus-time plot. All the simulated results are produced using estimated parameter values.
Statistics and reproducibility: model performance evaluation and test-retest validation
To check the reproducibility of the model-based estimation of GABA-Glutamate values from averaged subjects, we have conducted 50 independent simulations. Clouds of estimated values are shown in Fig. 4, where blue and red circles represent the average values in young and older adults, respectively. Furthermore, to evaluate the generative power of the MDMF model we separated the individual groups in CamCAN and NKI into two cohorts, approximately 40%, labeled as training cohorts (Nyoung = 15, Nold = 10 for NKI, Nyoung = 18, Nold = 14 for CamCAN), and 60% testing (Nyoung = 23, Nold = 17 for NKI, Nyoung = 24, Nold = 22 for CamCAN) without any overlapping individuals. The training data was used for subject-by-subject estimation of \({T}_{glu}^{opt}\) and \({T}_{gaba}^{opt}\) where the input parameter was the SC of each subject. The mean of \({T}_{glu}^{opt}\) and \({T}_{gaba}^{opt}\) computed for young and elderly groups for CamCAN and NKI cohorts is considered a representative estimate of optimal GABA and Glutamate for each age group.
Simulated FC from BOLD time series, generated using MDMF model, were computed in the testing cohort where the input parameters were the SC of each subject and optimal GABA-glutamate for each age group. Subsequently, the graph theoretic metrics were applied on empirical SC and simulated FC of the test cohort and compared qualitatively with graph theoretic metrics for the entire cohort to evaluate model performance. Model performance is illustrated by measuring graph properties of the model generated FCs for two datasets, shown in “Model performance evaluation” section. Further, we verified our observations for different thresholds utilized to binarize the FCs; please refer to Supplementary Material Fig. S7. The graph theoretical metrics are calculated from empirical FCs using various thresholds (used to binarize the empirical rsFC), which are also used to check the model-generated FCs properties. Finally, to check the robustness of our observation, participant-wise GABA-Glutamate values are estimated for different coupling strengths (G), presented in Supplementary Material Fig. S8. To compare between two age groups, we have used independent t-test, or Mann–Whitney U test (for less participants).
Results
Results are separated into three parts. First, we present the outcomes of graph theoretic network analysis conducted on non-invasive neuroimaging data encompassing both structural and functional aspects derived from diffusion MRI and functional-MRI data. We account for the altered and unchanged graph theoretical metrics in young and elderly cohorts. The empirical observations were validated in three independent datasets. For clear and interpretable comparisons, we purposefully choose two extreme age groups (young adults (18–34) and elders (60–85)). This group-wise design allowed us to capture broad age-related differences in brain network dynamics at the two aging boundaries, and allows us to examine subtle changes occurring in glutamate and GABA levels over a very slow time scale (lifespan aging) that compensates for structural loss, eventually sustaining essential neural dynamics and critical firing rates. To maximize contrasts in glutamate-GABA values and their ratio, we focus on the two opposing ends of the aging spectrum, providing a straightforward observational window to test any contrast in neurotransmitter levels. Our second step of analysis is to elucidate the sensitivity of the parameter estimation process—a key step to effective modeling of empirical FC in different cohorts. We averaged SCs of all the participants within each group. To check trial-wise variability in parameter estimations, we conducted independent simulations on the two averaged SCs corresponding to the two groups, while keeping all other settings fixed. Next, we examine age-related dynamical compensatory mechanisms via computational model dependent prediction of shifts in neurotransmitter to preserve desired brain dynamics, contributing to balance homeostasis, as evidenced from the excitatory-inhibitory firing rates. Finally, we evaluate our model performance, making a qualitative comparison between graph properties of the empirical and simulated FCs.
Dynamic working point: empirical metastability
To understand changes in the neural coordination dynamics among brain areas across the lifespan, we utilize the dynamical measure of metastability, defined in Eq. (1). Figure 2a–c presents violin plots of metastability, derived from participant-wise empirical resting-state BOLD signals for the two age groups.
Violin plots of metastability of young and elderly subjects are shown in Fig. 2a–c for CamCAN, NKI and Berlin data. First, we performed a detrending on the preprocessed BOLD signals of individual brain areas for each subject. Next, the instantaneous phase of each region was determined using the Matlab function “hilbert.m”. These two steps are repeated for individual subjects. We checked statistical variations between the two age groups using an independent t-test for CamCAN data and Mann–Whitney U test for Berlin data. We observed no significant changes in the metastability between the two age groups, suggesting that despite structural changes, the whole-brain dynamical complexity measured by metastability remains unaltered in elderly subjects.
Alterations in brain networks with age
We have measured weighted network properties of structural connectivity (SC), and for resting-state functional connectivity (rsFC), we have used undirected binary network properties in order to understand the age-dependent alterations in segregation, integration, and resilience features of brain networks. The global graph properties of participant-wise SCs and rsFCs are computed using Brain Connectivity Toolbox (http://www.brain-connectivity-toolbox.net)26, presented in top row, Fig. 3a–f, and lower row Fig. 3g–l, respectively. Statistical significance in the metrics between two age groups is determined by independent t-test (for CamCAN and NKI datasets) and Mann–Whitney U test (for Berlin dataset). Changes in graph properties of SCs and FCs are observed based on segregation [see Fig. 3a, b and g, h], integration [see Fig. 3c, d and i, j] and resilience [see Fig. 3e, f and k, l] of the networks.
Segregation: modularity and transitivity
Segregation is a network property, where dense connectivity is observed within the sub-modules of a large network; however, sparse connections among the sub-modules exist25,26. To characterize network segregation, we computed modularity and transitivity (classical measure of clustering coefficient) of structural and functional networks, shown in Fig. 3a, b and g, h, respectively. Modularity signifies how the network is densely connected among the areas physically within a sub-cluster but has sparse interactions across the segregated sub-clusters. A network’s transitivity measures how nodes cluster together. Lower transitivity means the network contains sparsely connected dominant sub-modules. Thus, both measures qualify as network segregation measures.
Increased modularity14 [shown in Fig. 3a] and decreased transitivity [in Fig. 3b] along lifespan were observed while applying these measures on structural connectivity matrices. Earlier studies have shown that segregated structure is an outcome of the factors affected mainly by age, e.g., white-matter fiber tracts reductions10,24,84,85 and degradation in long-range interareal connections86, thus this is a reconfirmation of previous results across three datasets.
Next, modularity and transitivity were applied in resting state functional connectivity (rsFC) to characterize functional segregation. Invariance in modularity [Fig. 3g] computed from rsFC implies no change in the number of functional modules or clusters evolved from the resting state BOLD activity in the large-scale brain network. It is observed that the transitivity of structural network was decreased (Fig. 3b) in elder subjects, whereas transitivity of functional network increased with age (Fig. 3h). Significantly changed transitivity of FCs in elderly subjects indicates rewiring in the functional connections while preserving modularity, i.e., keeping functional modules intact. An aging brain might calibrate controlling parameters to maintain a self-sustaining pattern in brain dynamics preserving functional modules to avoid functional decline due to altered structure. However, the regions within individual functional clusters or modules may differ across subjects and age groups over time.
Integration: global efficiency and characteristic path length
Structural integration signifies that paths are sequences of distinct areas and links. It represents potential routes of information flow between pairs of brain areas26. Characteristic path length and global efficiency are measures of global connectedness, providing an estimate of how easily information can be integrated across the network87. Global efficiency, related to characteristic path length, is the average of the inverse of shortest path length. Compared to characteristic path length, global efficiency is less influenced by nodes that are relatively isolated from the network26,69. Network integration is observed using global efficiency and characteristic path length from SCs and FCs, shown in Fig. 3c, d and i, j, respectively. Structural integration26 declines with age as suggested by a decreasing trend in global efficiency14 and significantly decreased characteristic path length (Fig. 3c, d) in elderly subjects. In general, the characteristic path length is primarily influenced by long paths (infinitely long paths are an illustrative extreme), while the short paths primarily influence global efficiency26. On more extensive and sparer networks, paths between disconnected nodes are defined to have infinite length, and correspondingly zero efficiency, thus affecting long- and short-range information flows across the whole brain.
Functional integration in the brain can be interpreted as the ability to combine specialized information from distributed brain areas rapidly26. Paths in binarized functional networks represent an overall projection of sequences of statistical associations and may not correspond to information flow along anatomical connections. Precisely, a functional network’s global efficiency measures a network’s ability to transmit information at the global level88, which is the inverse of characteristic path length. Increased functional integration, as observed from an increasing trend in global efficiency and average characteristic path length in older groups (see Fig. 3i, j), might be associated with the compensatory process against the decline in structural integration (Fig. 3c, d), which enhances the communicability or the ability to combine technical information from distributed cortical circuits. The relationship between structure and functions may not necessarily be one-to-one linear mapping, as intrinsic biological parameters largely influence the emergence of collective cortical dynamics.
Resilience: assortativity and betweenness centrality
Resilience refers to the ability of a brain network to recover and preserve functions under structural degradation26. Some studies have shown that certain brain areas are more prone to overall disruption of function, but several nodes remain unaffected by random perturbations89. Networks with fewer influential nodes tend to be more resilient. The commonly used metrics of network resilience are assortativity, and node betweenness centrality26. Networks with a positive assortativity coefficient likely have a resilient core of mutually interconnected hubs. In contrast, a negative assortativity coefficient is likely to have widely distributed and consequently vulnerable hubs26.
Assortativity and node betweenness centrality of SCs and FCs are shown in Fig. 3e, f and k, l, respectively. Resilience of the structural network (Fig. 3e, f) is significantly decreased in the older group, as observed from a significant decline in assortativity and betweenness centrality in Fig. 3e, f, respectively. The assortativity coefficient is a correlation coefficient between the strengths (weighted degrees) of all nodes on two opposite ends of a link. Decreased assortativity indicates a reduced tendency to link nodes with similar strengths. It reconfirmed our previous observations on reduced integration and increased segregation in structure, which could occur due to the loss of connectivity strengths (network became sparse, weighted degrees of individual nodes were decreased) and deterioration in long-range white-matter tracts connecting distant brain regions24.
On the other hand, resilience of the functional network from rs-FC, measured at the level of individual subjects using assortativity (Fig. 3k) and node betweenness centrality (Fig. 3l), remains unaltered, essentially no significant changes (p = 0.6 and p = 0.45), between two groups. It can be interpreted as a functional compensation among nodes of aging brain against the deteriorated structural resilience (Fig. 3e, f), thus upholding functional resilience.
We validate our observations on two other data sets, NKI and Berlin data, and the results are presented in Supplemental Materials Fig. S1. We observe similar pattern of alteration and re-organization in empirical SC and rsFC properties, as seen in CamCAN data. Though no statistical significance is seen in the network properties for the Berlin data set (which could be due to comparatively fewer subjects in the two groups), it shows similar alteration patterns as seen in the other two data sets. The results on changes in FC properties are also reproduced for four different thresholds used to generate binarized FC networks, shown in Supplemental Materials Fig. S2 for a curious reader.
Summary of observations from empirical data
We employed network analysis tools on structural and functional connectivity matrices in young and aged cohorts from three different datasets and report that there are overarching changes in anatomical topology, possibly stemming from displaying changes in white matter fibers, as well as, the measures of segregative and integrative functional network are also altered in aging brain, indicating re-organized FCs. On the other hand, large-scale cortical coordinated dynamics, measured from the resting state empirical BOLD activity, remain preserved with aging, sustaining the desired working point. This led us to hypothesize that a mechanistic approach taken by the aging brain to achieve function preservation may be via changing the neurotransmitter concentrations to maintain a homeostatic equilibrium between excitatory and inhibitory current dynamics. Previous studies have proposed that a homeostatic balance can be maintained when the brain operates at maximal metastability49. Thus, the goal of the remaining results section is to identify the age-associated changes in GABA-Glutamate concentrations, using the observation of preserved metastability and aspects of functional networks as proxy measures of constancy of the dynamic working point in a healthy brain30.
Model-based observations
Simulation results: optimal parameter selection and consistency
For a discrete grid of 2625 entries, we have generated mseFC and metastability maps for each age group.
The 3D maps obtained for mseFC and metastability on GABA-glutamate plane are shown in Fig. 4a. For each set of (Tglu, Tgaba), we stored the values of metastability and mseFC measures. The optimal GABA-glutamate values are indicated by blue stars-circles, where the blue dashed lines depict projection on the GABA and glutamate axes. To check the consistency of estimated model parameters, we first averaged the SCs and FCs of the participants from the individual group. To estimate two parameters, we did 50 independent runs with random initial conditions. The scatter plot shows clouds of estimated parameters in Fig. 4b for three datasets. The parameters averaged over trials are shown in blue and red circles for younger and older participants, respectively. The optimal parameter set can vary across trials, but remains bounded within a cloud of observation. It may be due to the MDMF model’s high dimensionality, nonlinearity, and stochasticity effects that resulted in different but close estimations for independent runs. For the choice of optimal GABA and glutamate values, the excitatory and inhibitory firing rates remain around 4 Hz and 6 Hz in all brain regions, shown in Fig. 4c. The simulated FCs are then generated using the estimated parameter values, placed next to the empirical FCs for the three datasets in Fig. 4d.
Mahalanobis distance is a pairwise Euclidean distance, calculated between the dominant dynamic FC subspace of one time point and all other time points. The Mahalanobis distances are derived using the algorithms proposed in refs. 32,90, and the temporal stability matrices are plotted for empirical and simulated FC dynamics (FCD) in Fig. 4e. These results are produced using the estimated parameter values.
Due to the higher parcellation scheme used in the NKI dataset, the static FC patterns exhibit more complexity and appear lacking in features compared to FC generated from Berlin and CamCAN. However, qualitative similarity in dFC patterns across all data sets can be clearly visible in all three datasets. Overall, simulated FC and dFC when compared for each cohort CamCAN, NKI, or Berlin, using MDMF model at optimal Glutamate-GABA values showed high degree of similarity with empirical data.
The MDMF model-based glutamate-GABA concentrations
Estimated glutamate-GABA concentrations (GGC) and GABA/glutamate ratio (GGR) at the level of individual subjects from the two age cohorts are shown in Fig. 5, results from CamCAN data in Fig. 5a, b, NKI data in Fig. 5c, d and Berlin dataset in Fig. 5e, f.
Participant-by-participant basis GABA-Glutamate values and their ratio are shown in a, b for CamCAN (young n = 37, old n = 32), c, d for NKI (young n = 39, old n = 27), and e, f Berlin data (young n = 25, old n = 14). The aging brain significantly tunes the glutamate level more prominently than that of GABA level, where a significant decay in GGR can also be observed. The star * indicates p < 0.05.
Figure 5a shows glutamate and GABA concentrations in young and old groups from CamCAN. Glutamate level is decreased in older groups, where GABA remains almost unchanged. A notable change in the ratio is observed (p < 0.05) in Fig. 5b. The average values of glutamate and GABA for the younger and older group are shown in the black star and green circle, respectively. The scattered blue stars and red circles are the estimated glutamate and GABA values for individual subjects, separating into young and older groups. The three data sets found a similar pattern of altered GABA glutamate levels with age. Further, we have performed a similar analysis on the NKI data set, presented in Fig. 5c, d. Figure 5c shows glutamate and GABA concentrations in young and old groups. Glutamate level is decreased in older groups, where GABA remains almost unchanged. A notable change in the ratio is observed (p < 0.05) in Fig. 5d. We conducted similar analysis on Berlin data. Figure 5e shows violin plots of estimated concentrations of the two metabolites in two age groups, where glutamate level is decreased significantly (p < 0.05), but GABA level remains invariant. A significance decrease in GABA-glutamate ratio is observed from the model-estimated results between two age groups, see Fig. 5f. Furthermore, we illustrate the effect of varying global coupling strength (G) on GABA/glutamate estimation in Supplementary Material Fig. S8. This figure shows: (1) optimal GABA/Glutamate estimates at different G values, (2) excitatory and inhibitory firing rates obtained from a single participant, and (3) empirical and simulated FCs from both a young adult and an elderly participant. Our analysis reveals that there exists a range of G values where the simulated FC fits well with the empirical FCs. Most importantly, while the estimated glutamate/GABA values remain consistent across this range, only the excitatory and inhibitory firing rates show variation.
The altered level of glutamate concentrations and glutamate-GABA ratio can be interpreted by unaltered neural dynamics, as quantified by the dynamical measure of metastability, which is preserved across aging. Altered glutamate giving rise to the desired complex dynamics essentially reorganized functional connections as evidenced from the empirical network metrics, such as increased transitivity (clustering coefficient), increased integration and invariant resilience in functional network.
The model simulated results suggest that the aging brain alters the optimal operating point30 by manipulating the two model parameters, in particular by reducing glutamate, to compensate for age-related variability in collective dynamics and avoid functional decline. A study using empirical data has found similar decreasing trends in the concentrations of glutamate in the left hippocampus (HC) and anterior cingulate cortex (ACC) with age91. The decreasing trend in glutamate concentration suggests a link between the neurotransmitter and the possible adaptive mechanism of the aging brain to compensate for structural degradation. In our study, we observed that the re-orientation in FC network results from the re-organization in the underlying brain dynamics when the two neurotransmitters control the intrinsic compensatory processes to maintain an equilibrium in the E-I ratio at rest, where the excitatory firing rate sustains at a critical range of 3–4 Hz. We must mention that the two neurotransmitters in this study, are only the model parameters that control the dynamics of the MDMF model. We did not attempt to validate the absolute levels of the two neurotransmitters in the context of aging or compare them with the empirical data. All the results on neurotransmitters are theoretical predictions about possible parameter requirements for maintaining desired brain dynamics in a changing structural network.
Model performance evaluation: graph theoretical metrics computed from simulated FC
A test-retest validation is conducted to assess the model based observations. Consequently, our goal is to retest the model performance under estimated optimal parameter values as test condition and empirical results as true cases. In particular, we aim to evaluate the extent to which the model-based FCs, generated using averaged estimated parameters calculated by splitting the cohorts, can replicate similar patterns of changes observed in the empirical FC properties when comparing the two aging cohorts. First, the estimated optimal values of glutamate and GABA from a few subjects (see Methods section “Statistics and reproducibility: model performance evaluation and test-retest validation”) are calculated and averaged for each age group. The average values corresponding to the two datasets are: (1) CamCAN: \({T}_{glu}^{opt}=7.03\) mMol and \({T}_{gaba}^{opt}=3.08\) mMol for young; \({T}_{glu}^{opt}=6.88\) mMol and \({T}_{gaba}^{opt}=3.0\) mMol for old; (2) NKI: \({T}_{glu}^{opt}=11.53\) mMol and \({T}_{gaba}^{opt}=3.39\) mMol for young and \({T}_{glu}^{opt}=11.13\) mMol and \({T}_{gaba}^{opt}=3.49\) mMol for old. Using these parameters and subject-by-subject SC, we computed the BOLD time series using MDMF (Eq. (7)) followed by computing FC using Pearson correlation. Next, we binarized the FC and calculated graph theoretical measures at the individual level, shown in Fig. 6, two bottom rows. We generate the binarized FC network for a threshold δ = 0.081 corresponding to a p < 10−4, such that FCij = 1, if abs(FCij) > δ; otherwise FCij = 0. To compare the graph metrics estimated across age groups, we have performed the Mann–Whitney U test, the non-parametric alternative to the independent sample t-test due to fewer test subjects. The upper rows display empirical FC properties for CamCAN and NKI data, see Fig. 6, top two rows; two bottom rows show results from the CamCAN and NKI datasets, for simulated FC network properties in blue and red dots for young and older participants, respectively. The threshold for statistical significance is considered to be p < 0.05, and is not significant marked by NS. Due to a smaller number of participants in the Berlin data set within two age groups, we did not perform the predictive analysis to evaluate the model performance. We performed test-retest validation of the simulated FC network prediction for two different thresholds used to binarize the simulated FCs, presented in Supplementary Material Fig. S7. Of all measures, only the centrality of the node betweenness is not fully predicted from the test subjects of NKI data, even with varying thresholds (used to build binary FCs).
Top two rows display network properties of the empirical rsFC, compared against the observations from simulated FCs (bottom two rows), which are obtained for the optimal parameter choices. Number of test subjects are: n = 23 young and n = 22 older adults for CamCAN data, and n = 23 young and n = 17 older adults for NKI data. The variability is coming solely from individual’s SC and is mainly assessed in the test cohort. The MDMF model inversion reproduces almost similar alterations between two age groups occurring for the test cohorts, where, the modeling parameters are computed from training cohorts. Except, alterations in betweenness centrality is not fully predicted from the test subjects of NKI data. Statistical differences are assessed by Mann–Whitney U test, where p < 0.05 implies statistically significance, otherwise not significant (NS). A solid black line connecting two mean values of younger and elderly groups, is plotted to visually tracking alterations. The errorbars show standard deviations.
The graph metrics from the MDMF model for both NKI and CamCAN datasets display qualitatively similar variation across age groups, as we observed from the network properties of empirical resting-state FCs between the two groups. These results validate the predictive power of the MDMF model and give us confidence in its use to characterize how the aging brain alters its biological parameters to preserve the collective dynamics around a desired operating point, which may allow the brain to react to any external or internal stimulus flexibly. The alteration can also be interpreted as how the aging brain reorganizes its functional connectivity by re-adjusting the controlling parameters in the face of structural degradation in healthy aging.
Discussion
Several critical reviews have highlighted the urgent need for tools that can leapfrog studies of neuroscience from a scale-specific biological observation to explain ongoing behavior towards the development of comprehensible multi-scale models that can generate an understanding of the interactions among multiple organizational scales92,93,94. This study focuses on a whole-brain computational model that captures the interactions between two observational levels of neural complexity, ultra-slow BOLD-fMRI dynamics, and neurotransmitter kinetics. The precise interactions transcending the biological scales of observations allow the estimation of critical markers such as Glutamate-GABA concentrations from model inversion techniques across healthy human aging. First, we illustrate that functional segregation, integration, and resilience measures can be preserved and sometimes enhanced across the healthy aging process, even though similar metrics applied on structural network topology point out a gross degradation. Second, we identify a key invariant that captures the dynamic working point of the brain, as well as provides a link to model the interaction between neurotransmitter kinetics and emergent metastable dynamics of the local field with aging. Third, we could estimate the optimal concentration changes in GABA and glutamate associated with healthy aging by model inversion under the constraint of an optimized dynamic working point30. Finally, a test-retest validation allowed us to evaluate the predictive capability of MDMF based neurotransmitter estimation. We assessed the model performances under the estimated parameter sets, i.e., assessing the extend to which the model generated results are able to capture similar age-related changing patterns observed in the empirical FC properties. Thus, our study developed a computational microscope based on a biophysically inspired generative neural mass model linking large-scale neuroimaging data with microscopic parameters. The advantage of using this model is two-fold, reconciling observational accounts of metabolite mapping with mechanistic insights of neuronal network workings and possibly setting up future studies that can use this framework to predict the onset of neurodevelopmental and neuroinflammatory disorders where the local E/I balance is crucially perturbed. We discuss these issues in more detail in the remaining sections.
Structure-function markers of healthy aging
The most salient feature of our empirical network analysis is that the graph theoretical measures, segregation, integration, and resilience, derived from SCs and resting state FCs, showed alterations with age (Fig. 3). Our observations are in line with earlier reports on age-related structural changes17,27, and functional re-organization95,96,97. Using multiple network measures such as characteristic path length and global efficiency gives us a set of tools to capture variability in functional network’s integration across subjects98, between groups87,99,100,101,102 and are of immense practical importance in brain network study103,104, however, cautionary approach must be followed when mechanistic interpretations about engagements of neuronal populations are made, using measures of statistical dependency. In the field of aging research, tracking a gamut of network measures can examine the idea of neurocompensation105 by which the brain areas improve upon their segregative, integrative and cooperative behavior in the face of structural decline24,106,107. Being at metastable state, the system is essentially situated in a transient state where it can have more opportunities of choosing an attractor, this can also be interpreted as a state where information processing capabilities increase, leading to higher flexibility16. Interestingly, metastability calculated from empirical BOLD signals was unaltered between the young and elderly participants. This can be interpreted as the preservation of information processing capability across the aging cohorts, who, for the most part, are capable of complex cognitive functions (although showing a decrease in performance indices over age, see ref. 31) emerge from underlying compensatory mechanisms that preserves the global dynamical complexity of the brain. From the perspective of the current study, we use this observation as a constraining tool for model inversion using the MDMF model49 to estimate the Glutamate and GABA concentration changes across aging trajectories.
Insight from the model simulation
A key contribution of the present study was the development of a framework that could estimate the local synaptic GABA, Glutamate concentrations (GGC) and GABA, Glutamate ratio (GGR) from non-invasive fMRI. We observed that age negatively correlated with glutamatergic regulation, which was reduced with healthy aging, in concurrence with earlier evidence13,108,109,110. It is important to note that in the absence of brain-wide MRS data on GABA/glutamate concentrations, evaluating a goodness of fit of model estimated neurotransmitter concentrations is out of scope of the present manuscript. Rather, this work seeks to provide a deeper insight on a probable computational principle by which interaction between brain network dynamics and neurotransmitter kinetics gives rise to a complex adaptive process associated with brain aging. To gain an empirical view of neuromodulation in the aging brain, we refer to the existing literature reporting regional changes in metabolites. For example, empirical observations from healthy human adults in the posterior cingulate cortex109 and rodent brain13,108, revealed significant reduction in the glutamate level in the aging brain, aligned with the patterns of alteration observed in our model-based prediction. The reduced glutamate level has been interpreted as a key mechanism by which the aging brain adapts to maintain the desired regulation of I-E by down-regulating glutamate and also decreasing the expression of several other presynaptic markers111. Another study reported a similar negative correlation with glutamate contents in the aging brain in the motor cortex, but a positive correlation with other metabolites, for example, glutamine, N-acetyl aspartate and creatinine2. Further, the reduction of glutamate in the motor cortex is linked with the neuronal loss/shrinkage during aging2. Other empirical examples showed significant changes at 20% to 50% in the glutamate contents in the frontal cortex, hippocampus, and cerebral cortex with aging112,113. Nevertheless, a review on the critical perspective of aging and development on glutamatergic transmission had categorically shown the effects of aging based on the shreds of evidence from various in vitro and in vivo studies on rodent brains, also includes contrasting observations114. For instance, studies also reported non-significant changes in glutamate content in the dorsal prefrontal cortex, sulcal prefrontal cortex, temporal cortex, and medial prefrontal cortex during aging115,116.
One of the crucial observations that emerge from this theoretical approach is that age-related alterations are different in GABA than glutamate, even though we varied GABA-glutamate concentrations homogeneously across brain areas. The estimated glutamate content was significantly reduced, whereas GABA showed very less variability with age. Similar alteration pattern in the two metabolites was observed an earlier work in the context of aging110, and also in rodent brain that significantly influenced cortical plasticity111. However, in contrast to our observation on GABA, studies have re-ported age-dependent reduction in GABA concentration derived by magnetic resonance spectroscopy (MRS) in human subjects. In particular, reduced GABA was evidenced in the motor, visual, auditory, somatosensory areas, and the perisylvian region of the left hemisphere, which caused an abnormal information processing3,4. Our model simulation showed significant decay in GABA-glutamate ratio in older adults compared to the younger participants, consistent with the MRS-based experimental observation in the PCC/precuneus117.
Overall, our model simulations suggest that, despite structural decline, the brain-wide neural dynamics are preserved across aging trajectory through readjustments in neurotransmitter levels and their ratio, leading to unaffected E-I balance. Thereafter, the interplay between tuned metabolite levels and cortical plasticity provokes a gradual functional re-organization in the aging brain over a prolonged time (lifespan), while maintaining desired homeostasis. To this point, a critical failure of the present MDMF model is the inability to predict accurate changes in glutamate and GABA levels in individual brain regions. Thus, for future work, it will be advisable to make model-based predictions of heterogeneous distribution of metabolites in the brain which we discuss further in the following sub-section. Nonetheless, much fine-tuning of glutamatergic activity occurs via modulation of the NMDA receptors, which are invisible to MRS because of its limitation in detecting a neurochemical concentration within a localized region of tissues (typically in the order of functional pools of GABA as they might be more tightly bound to macromolecules than others, rendering 2 × 2 × 2 cm3)118. Available evidence suggests that MRS can not separate between different them less “visible” to MRS119,120. In that case, the proposed theoretical framework could be a supporting tool to look into the age-related fluctuations in the metabolites at rest and task-evoked activity, besides MRS-driven data, within a localized area and across whole-brain.
Limitations and future directions
The computational framework we have presented in this article gives a bird’s eye view of the neuromolecular mechanisms at the whole-brain level. Nonetheless, the biology of the brain is way more complex and our model also has several limitations. First, spatial heterogeneity of the two metabolites in the cortex is not considered in this study. In future, incorporating training models that consist of template MRS data from individual brain regions can be used to address the limitation of regional heterogeneity in the two metabolites. An atlas of whole brain metabolite map from future MRS studies can be used to tune the MDMF model for better predictions of resting state BOLD dynamics that can improve model fits to empirical FC properties. This would also require the implementation of a more advanced model inversion technique beyond two-parameter (GABA, Glutamate concentrations for this manuscript) fitting with optimized metastability and mseFC used here. In other words, machine learning models such as Bayesian model inversion can replace this step. Some other potential limitations are: (1) An earlier observation121 on a rat brain experiment showed alteration in GABAergic and glutamatergic behavior affected by the age were highly probable to occur in a presynaptic mechanism than in a postsynaptic mechanism. On the other hand, the MDMF model does not distinguish between the pre- or postsynaptic concentration of GABA-glutamate121,122. (2) Uptake and release of glutamate113 cannot be separated using the present method. The model does not explicitly tell about the two major sub-types of GABA, i.e., GABAA and GABAB. (3) Measure of total GABA concentration remains unclear whether the GABA signal represents cytoplasmic, vesicular, or free extracellular GABA118.
It is crucial to note that the standard deviation of instantaneous Kuramoto order parameter as a measure of metastability has a significant limitation: it is only meaningful when applied to rhythmic dynamics. To overcome this limitation, we implemented a narrow band filter (0.001 Hz to 0.1 Hz) on both the empirical and simulated BOLD signals prior to calculating metastability. This filtering process effectively enhanced the rhythmicity of the BOLD signals, thereby ensuring that our metastability measurements remained valid and interpretable throughout our analysis.
While experimental research has demonstrated that metabolite alterations aid in early Alzheimer’s disease (AD) diagnosis123 and that GABA-mediated excitation-inhibition (E-I) balance restoration can treat depression124, the in silico paradigm offers complementary mechanistic insights into disrupted neural dynamics. For instance, computational studies have revealed how noise in the left temporal lobe impairs functional connectivity in AD125, a framework further extended by recent in-silico perturbation protocols for dynamic recovery126,127. In a long run, the theoretical construct of multiscale modeling can be a useful tool in designing personalized model to identify dynamical mechanisms of imbalanced homeostasis and region-specific vulnerabilities in neural circuit dysfunction across neuropsychiatric disorders and neuropathological conditions, e.g., neural migration disorder128, Parkinson’s disease129, AD123, attention deficit hyperactivity disorder130 and autism131,132.
Data availability
Data can be downloaded for CamCAN from https://www.CamCAN.org/ and NKI Rockland from https://fcon_1000.projects.nitrc.org/indi/pro/nki.html. The Berlin data cannot be shared due to Ethical guidelines of the project. For replication purposes, numerical source data associated with Figs. 2–6 can be found in Supplementary Data 3–5, respectively.
Code availability
The codes for all the analyses to generate the figures are provided at https://bitbucket.org/cbdl/agingmdmf/src/master/.
References
Cotman, C. W., Monaghan, D. T., Ottersen, O. P. & Storm-Mathisen, J. Anatomical organization of excitatory amino acid receptors and their pathways. Trends Neurosci. 10, 273–280 (1987).
Kaiser, L., Schuff, N., Cashdollar, N. & Weiner, M. Age-related glutamate and glutamine concentration changes in normal human brain: 1h mr spectroscopy study at 4 t. Neurobiol. Aging 26, 665–672 (2005).
Rojas, D., Singel, D., Steinmetz, S., Hepburn, S. & Brown, M. Decreased left perisylvian gaba concentration in children with autism and unaffected siblings. Neuroimage 86, 28–34 (2014).
Puts, N. et al. Reduced gaba and altered somatosensory function in children with autism spectrum disorder. Autism Res. 10, 608–619 (2017).
Maes, C. et al. Age-related differences in gaba levels are driven by bulk tissue changes. Hum. Brain Mapp. 39, 3652–3662 (2018).
Roalf, D. R. et al. A quantitative meta-analysis of brain glutamate metabolites in aging. Neurobiol. Aging 95, 240–249 (2020).
Porges, E. C., Jensen, G., Foster, B., Edden, R. A. & Puts, N. A. The trajectory of cortical gaba across the lifespan, an individual participant data meta-analysis of edited mrs studies. Elife 10, e62575 (2021).
Sullivan, E. & Pfefferbaum, A. Diffusion tensor imaging and aging. Neurosci. Biobehav. Rev. 30, 749–761 (2006).
Samanez-Larkin, G. R., Kuhnen, C. M., Yoo, D. J. & Knutson, B. Variability in nucleus accumbens activity mediates age-related suboptimal financial risk taking. J. Neurosci. 30, 1426–1434 (2010).
Coelho, A. et al. Signatures of white-matter microstructure degradation during aging and its association with cognitive status. Sci. Rep. 11, 1–12 (2021).
Chakraborty, P., Saha, S., Deco, G., Banerjee, A. & Roy, D. Contributions of short-and long-range white matter tracts in dynamic compensation with aging. Cereb. Cortex 35, bhae496 (2025).
Jolles, J. Cognitive, emotional and behavioral dysfunctions in aging and dementia. Prog. Brain Res. 70, 15–39 (1986).
Benedetti, M. S., Cini, M., Fusi, R., Marrari, P. & Dostert, P. The effects of aging on mao activity and amino acid levels in rat brain. J. Neural. Transm. Suppl. 29, 259–268 (1990).
Li, X. et al. Age-related decline in the topological efficiency of the brain structural connectome and cognitive aging. Cereb. Cortex 30, 4651–4661 (2020).
Hirsiger, S. et al. Structural and functional connectivity in healthy aging: associations for cognition and motor behavior. Hum. Brain Mapp. 37, 855–867 (2016).
Naik, S., Banerjee, A., Bapi, R., Deco, G. & Roy, D. Metastability in senescence. Trends Cogn. Sci. 21, 509–521 (2017).
Luo, N. et al. Age-related structural and functional variations in 5,967 individuals across the adult lifespan. Hum. Brain Mapp. 41, 1725–1737 (2020).
Park, D. C. & Reuter-Lorenz, P. The adaptive brain: aging and neurocognitive scaffolding. Annu. Rev. Psychol. 60, 173–196 (2009).
Schaie, K. Intellectual development in adulthood. Handb. Psychol. Aging 4, 266–286 (1996).
Park, D. & Schwarz, N. Cognitive Aging: A Primer (Psychology Press, 2012).
Uddin, L. Q. Cognitive and behavioural flexibility: neural mechanisms and clinical considerations. Nat. Rev. Neurosci. 22, 167–179 (2021).
Aron, L., Zullo, J. & Yankner, B. The adaptive aging brain. Curr. Opin. Neurobiol. 72, 91–100 (2022).
Betzel, R. F. et al. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage 102, 345–357 (2014).
Petkoski, S., Ritter, P. & Jirsa, V. K. White-matter degradation and dynamical compensation support age-related functional alterations in human brain. Cereb. Cortex 33, 6241–6256 (2023).
Newman, M. E. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 103, 8577–8582 (2006).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069 (2010).
Coelho, A. et al. Reorganization of brain structural networks in aging: a longitudinal study. J. Neurosci. Res. 99, 1354–1376 (2021).
Garrett, D. D. et al. Moment-to-moment brain signal variability: a next frontier in human brain mapping? Neurosci. Biobehav. Rev. 37, 610–624 (2013).
Deco, G. & Kringelbach, M. L. Metastability and coherence: extending the communication through coherence hypothesis using a whole-brain computational perspective. Trends Neurosci. 39, 125–135 (2016).
Deco, G., Kringelbach, M., Jirsa, V. & Ritter, P. The dynamics of resting fluctuations in the brain: metastability and its dynamical cortical core. Sci. Rep. 7, 1–14 (2017).
Thuwal, K., Banerjee, A. & Roy, D. Aperiodic and periodic components of ongoing oscillatory brain dynamics link distinct functional aspects of cognition across adult lifespan. Eneuro 8, 1–18 (2021).
Sastry, N. C., Roy, D. & Banerjee, A. Stability of sensorimotor network sculpts the dynamic repertoire of resting state over lifespan. Cereb. Cortex 33, 1246–1262 (2023).
Bullmore, E. & Sporns, O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198 (2009).
Deco, G., Jirsa, V. & McIntosh, A. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 12, 43–56 (2011).
Pillai, A. S. & Jirsa, V. K. Symmetry breaking in space-time hierarchies shapes brain dynamics and behavior. Neuron 94, 1010–1026 (2017).
Kelso, J. A. S. Multistability and metastability: understanding dynamic coordination in the brain. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 367, 906–918 (2012).
Haldeman, C. & Beggs, J. Critical branching captures activity in living neural networks and maximizes the number of metastable states. Phys. Rev. Lett. 94, 058101 (2005).
Schneidman, E., Berry, M., Segev, R. & Bialek, W. Weak pairwise correlations imply strongly correlated network states in a neural population. Nature 440, 1007–1012 (2006).
Vázquez-Rodríguez, B. et al. Stochastic resonance at criticality in a network model of the human cortex. Sci. Rep. 7, 1–12 (2017).
Wang, X.-J. Macroscopic gradients of synaptic excitation and inhibition in the neocortex. Nat. Rev. Neurosci. 21, 169–178 (2020).
Deco, G. et al. Dynamical consequences of regional heterogeneity in the brain’s transcriptional landscape. Sci. Adv. 7, eabf4752 (2021).
Marder, E. & Goaillard, J.-M. Variability, compensation and homeostasis in neuron and network function. Nat. Rev. Neurosci. 7, 563–574 (2006).
Joglekar, M. R., Mejias, J. F., Yang, G. R. & Wang, X.-J. Inter-areal balanced amplification enhances signal propagation in a large-scale circuit model of the primate cortex. Neuron 98, 222–234 (2018).
Mashour, G. A., Roelfsema, P., Changeux, J.-P. & Dehaene, S. Conscious processing and the global neuronal workspace hypothesis. Neuron 105, 776–798 (2020).
Vogels, T., Sprekeler, H., Zenke, F., Clopath, C. & Gerstner, W. Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science 334, 1569–1573 (2011).
Deco, G. et al. How local excitation–inhibition ratio impacts the whole brain dynamics. J. Neurosci. 34, 7886–7898 (2014).
Vattikonda, A., Surampudi, B. R., Banerjee, A., Deco, G. & Roy, D. Does the regulation of local excitation-inhibition balance aid in recovery of functional connectivity? a computational account. NeuroImage 136, 57–67 (2016).
Lavanga, M. et al. The virtual aging brain: causal inference supports interhemispheric dedifferentiation in healthy aging. NeuroImage 283, 120403 (2023).
Naskar, A., Vattikonda, A., Deco, G., Roy, D. & Banerjee, A. Multi-scale dynamic mean field model (MDMF) relates resting-state brain dynamics with local cortical excitatory–inhibitory neurotransmitter homeostasis. Netw. Neurosci. 5, 757–782 (2021).
Burns, B. D. & Webb, A. The spontaneous activity of neurones in the cat’s cerebral cortex. Proc. R. Soc. Lond. Ser. B. Biol. Sci. 194, 211–223 (1976).
Koch, K. & Fuster, J. Unit activity in monkey parietal cortex related to haptic perception and temporary memory. Exp. Brain Res. 76, 292–306 (1989).
Softky, W. R. & Koch, C. The highly irregular firing of cortical cells is inconsistent with temporal integration of random epsps. J. Neurosci. 13, 334–350 (1993).
Kuramoto, Y. Chemical turbulence. In Chemical Oscillations, Waves, and Turbulence, 111–140 (Springer, 1984).
Schirner, M., Rothmeier, S., Jirsa, V. K., McIntosh, A. R. & Ritter, P. An automated pipeline for constructing personalized virtual brains from multimodal neuroimaging data. NeuroImage 117, 343–357 (2015).
Brown, J. et al. The ucla multimodal connectivity database: a web-based platform for brain connectivity matrix sharing and analysis. Front. Neuroinform. 6, 28 (2012).
Shafto, M. A. et al. The cambridge centre for ageing and neuroscience (cam-can) study protocol: a cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 14, 1–25 (2014).
Surampudi, S. et al. Resting state dynamics meets anatomical structure: temporal multiple kernel learning (tmkl) model. NeuroImage 184, 609–620 (2019).
Werner, G. Metastability, criticality and phase transitions in brain and its models. Biosystems 90, 496–508 (2007).
Deco, G., Rolls, E. & Romo, R. Stochastic dynamics as a principle of brain function. Prog. Neurobiol. 88, 1–16 (2009).
Shanahan, M. Metastable chimera states in community-structured oscillator networks. Chaos Int. J. Nonlinear Sci. 20, 013108 (2010).
Naik, S., Subbareddy, O., Banerjee, A., Roy, D. & Bapi, R. Metastability of cortical bold signals in maturation and senescence. In 2017 International Joint Conference on Neural Networks (IJCNN), 4564–4570 (IEEE, 2017).
Bressler, S. L. & Kelso, J. A. S. Cortical coordination dynamics and cognition. Trends Cogn. Sci. 5, 26–36 (2001).
Tognoli, E. & Kelso, J. A. S. Brain coordination dynamics: true and false faces of phase synchrony and metastability. Prog. Neurobiol. 87, 31–40 (2009).
Tognoli, E. & Kelso, J. S. The metastable brain. Neuron 81, 35–48 (2014).
Cocchi, L., Gollo, L. L., Zalesky, A. & Breakspear, M. Criticality in the brain: a synthesis of neurobiology, models and cognition. Prog. Neurobiol. 158, 132–152 (2017).
Breakspear, M. Dynamic models of large-scale brain activity. Nat. Neurosci. 20, 340–352 (2017).
Roberts, J. A. et al. Metastable brain waves. Nat. Commun. 10, 1056 (2019).
Newman, M. E. The structure and function of complex networks. SIAM Rev. 45, 167–256 (2003).
Latora, V. & Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 87, 198701 (2001).
Estrada, E. & Hatano, N. Communicability in complex networks. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 77, 036111 (2008).
Watts, D. & Strogatz, S. Collective dynamics of ‘small-world’ networks. Nature 393, 440–442 (1998).
Newman, M. E., Barabási, A.-L. E. & Watts, D. J. The Structure and Dynamics of Networks (Princeton University Press, 2006).
Newman, M. E. Mixing patterns in networks. Phys. Rev. E 67, 026126 (2003).
Newman, M. E. Assortative mixing in networks. Phys. Rev. Lett. 89, 208701 (2002).
Freeman, L. C. Centrality in social networks conceptual clarification. Social Networks 1, 215–239 (1978).
Destexhe, A., Mainen, Z. F. & Sejnowski, T. J. Synthesis of models for excitable membranes, synaptic transmission and neuromodulation using a common kinetic formalism. J. Comput. Neurosci. 1, 195–230 (1994).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
Craddock, R. C., James, G. A., Holtzheimer III, P. E., Hu, X. P. & Mayberg, H. S. A whole brain fmri atlas generated via spatially constrained spectral clustering. Hum. Brain Mapp. 33, 1914–1928 (2012).
Bittner, S. R. et al. Population activity structure of excitatory and inhibitory neurons. PLoS ONE 12, e0181773 (2017).
Hellyer, P. J., Jachs, B., Clopath, C. & Leech, R. Local inhibitory plasticity tunes macroscopic brain dynamics and allows the emergence of functional brain networks. NeuroImage 124, 85–95 (2016).
Friston, K. J., Harrison, L. & Penny, W. Dynamic causal modelling. Neuroimage 19, 1273–1302 (2003).
Cabral, J., Hugues, E., Sporns, O. & Deco, G. Role of local network oscillations in resting-state functional connectivity. Neuroimage 57, 130–139 (2011).
Sorrentino, P. et al. The virtual multiple sclerosis patient. Iscience 27, 110101 (2024).
Kristanto, D., Liu, M., Liu, X., Sommer, W. & Zhou, C. Predicting reading ability from brain anatomy and function: from areas to connections. NeuroImage 218, 116966 (2020).
Davis, S. et al. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage 46, 530–541 (2009).
Betzel, R. F. & Bassett, D. S. Specificity and robustness of long-distance connections in weighted, interareal connectomes. Proc. Natl. Acad. Sci. USA 115, E4880–E4889 (2018).
Paldino, M. J., Zhang, W., Chu, Z. D. & Golriz, F. Metrics of brain network architecture capture the impact of disease in children with epilepsy. NeuroImage Clin. 13, 201–208 (2017).
Wang, J., Zuo, X. & He, Y. Graph-based network analysis of resting-state functional MRI. Front. Syst. Neurosci. 4, 1419 (2010).
Achard, S., Salvador, R., Whitcher, B., Suckling, J. & Bullmore, E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 26, 63–72 (2006).
Hutchison, R. M. et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80, 360–378 (2013).
Hädel, S., Wirth, C., Rapp, M., Gallinat, J. & Schubert, F. Effects of age and sex on the concentrations of glutamate and glutamine in the human brain. J. Magn. Reson. Imaging 38, 1480–1487 (2013).
Rosen, R. Life, Itself: A Comprehensive Inquiry Into the Nature, Origin, and Fabrication of Life (Columbia University Press, 1991).
Frackowiak, R. & Markram, H. The future of human cerebral cartography: a novel approach. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 370, 20140171 (2015).
D’Angelo, E. & Jirsa, V. The quest for multiscale brain modeling. Trends Neurosci. 45, 777–790 (2022).
Meunier, D., Achard, S., Morcom, A. & Bullmore, E. Age-related changes in modular organization of human brain functional networks. Neuroimage 44, 715–723 (2009).
Song, J. et al. Age-related reorganizational changes in modularity and functional connectivity of human brain networks. Brain Connect. 4, 662–676 (2014).
Zhang, Y. et al. Age-associated differences of modules and hubs in brain functional networks. Front. Aging Neurosci. 12, 607445 (2021).
Shinn, M. et al. Functional brain networks reflect spatial and temporal autocorrelation. Nat. Neurosci. 26, 867–878 (2023).
Rudie, J. D. et al. Altered functional and structural brain network organization in autism. NeuroImage Clin. 2, 79–94 (2013).
Suo, X. et al. Disrupted brain network topology in pediatric posttraumatic stress disorder: a resting-state fMRI study. Hum. Brain Mapp. 36, 3677–3686 (2015).
Ye, M. et al. Changes of functional brain networks in major depressive disorder: a graph theoretical analysis of resting-state fMRI. PLoS ONE 10, e0133775 (2015).
Hadley, J. A. et al. Change in brain network topology as a function of treatment response in schizophrenia: a longitudinal resting-state fMRI study using graph theory. npj Schizophr. 2, 1–7 (2016).
Lynn, C. W. & Bassett, D. S. The physics of brain network structure, function and control. Nat. Rev. Phys. 1, 318–332 (2019).
Sporns, O. Graph theory methods: applications in brain networks. Dialogues Clin. Neurosci. 20, 111–121 (2022).
Reuter-Lorenz, P. A. & Cappell, K. A. Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci. 17, 177–182 (2008).
Daselaar, S. M. et al. Less wiring, more firing: low-performing older adults compensate for impaired white matter with greater neural activity. Cereb. Cortex 25, 983–990 (2015).
Pathak, A., Sharma, V., Roy, D. & Banerjee, A. Biophysical mechanism underlying compensatory preservation of neural synchrony over the adult lifespan. Commun. Biol. 5, 567 (2022).
Davis, J. & Himwich, W. Neurochemistry of the developing and aging mammalian brain. In Neurobiology of Aging. (eds Ordy, J. M. & Brizzee, K. R.) 329–357 (Springer, 1975).
Suri, S. et al. Effect of age and the apoe gene on metabolite concentrations in the posterior cingulate cortex. Neuroimage 152, 509–516 (2017).
Hu, Y., Chen, X., Gu, H. & Yang, Y. Resting-state glutamate and gaba concentrations predict task-induced deactivation in the default mode network. J. Neurosci. 33, 18566–18573 (2013).
Liguz-Lecznar, M. et al. Altered glutamate/gaba equilibrium in aged mice cortex influences cortical plasticity. Brain Struct. Funct. 220, 1681–1693 (2015).
Wallace, D. & Dawson, R. Effect of age and monosodium-l-glutamate (msg) treatment on neurotransmitter content in brain regions from male fischer-344 rats. Neurochem. Res. 15, 889–898 (1990).
Saransaari, P. & Oja, S. Age-related changes in the uptake and release of glutamate and aspartate in the mouse brain. Mech. Ageing Dev. 81, 61–71 (1995).
Segovia, G., Porras, A., Del Arco, A. & Mora, F. Glutamatergic neurotransmission in aging: a critical perspective. Mech. Ageing Dev. 122, 1–29 (2001).
Fornieles, F., Peinado, J. & Mora, F. Endogenous levels of amino acid neurotransmitters in different regions of frontal and temporal cortex of the rat during the normal process of aging. Neurosci. Lett. Suppl. 26, 150 (1986).
Donzanti, B. A. & Ung, A. K. Alterations in neurotransmitter amino acid content in the aging rat striatum are subregion dependent. Neurobiol. Aging 11, 159–162 (1990).
Gonen, O. M. et al. Resting-state functional connectivity and quantitation of glutamate and gaba of the pcc/precuneus by magnetic resonance spectroscopy at 7t in healthy individuals. PLoS ONE 15, e0244491 (2020).
Stagg, C., Bachtiar, V. & Johansen-Berg, H. What are we measuring with gaba magnetic resonance spectroscopy? Commun. Integr. Biol. 4, 573–575 (2011).
Floyer-Lea, A., Wylezinska, M., Kincses, T. & Matthews, P. Rapid modulation of gaba concentration in human sensorimotor cortex during motor learning. J. Neurophysiol. 95, 1639–1644 (2006).
Stagg, C. J. et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 29, 5202–5206 (2009).
Rozycka, A. et al. Glutamate, gaba, and presynaptic markers involved in neurotransmission are differently affected by age in distinct mouse brain regions. ACS Chem. Neurosci. 10, 4449–4461 (2019).
Pitler, T. & Alger, B. Differences between presynaptic and postsynaptic gabab mechanisms in rat hippocampal pyramidal cells. J. Neurophysiol. 72, 2317–2327 (1994).
Huang, D. et al. Glutamate-glutamine and gaba in brain of normal aged and patients with cognitive impairment. Eur. Radiol. 27, 2698–2705 (2017).
Myers, J., Evans, C., Kalk, N., Edden, R. & Lingford-Hughes, A. Measurement of gaba using j-difference edited 1h-mrs following modulation of synaptic gaba concentration with tiagabine. Synapse 68, 355–362 (2014).
Demirtaş, M. et al. A whole-brain computational modeling approach to explain the alterations in resting-state functional connectivity during progression of alzheimer’s disease. NeuroImage Clin. 16, 343–354 (2017).
Vohryzek, J. et al. Design of effective personalised perturbation strategies for enhancing cognitive intervention in Alzheimer’s disease. bioRxiv https://www.biorxiv.org/content/10.1101/2023.04.20.537688v1 (2023).
Perl, Y. S. et al. Model-based whole-brain perturbational landscape of neurodegenerative diseases. Elife 12, e83970 (2023).
Luhmann, H. J., Fukuda, A. & Kilb, W. Control of cortical neuronal migration by glutamate and gaba. Front. Cell. Neurosci. 9, 4 (2015).
O’Gorman Tuura, R., Baumann, C. & Baumann-Vogel, H. Beyond dopamine: Gaba, glutamate, and the axial symptoms of parkinson disease. Front. Neurol. 9, 806 (2018).
Edden, R., Crocetti, D., Zhu, H., Gilbert, D. & Mostofsky, S. Reduced gaba concentration in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 69, 750–753 (2012).
Rojas, D., Becker, K. & Wilson, L. Magnetic resonance spectroscopy studies of glutamate and gaba in autism: implications for excitation-inhibition imbalance theory. Curr. Dev. Disord. Rep. 2, 46–57 (2015).
Purkayastha, P., Malapati, A., Yogeeswari, P. & Sriram, D. A review on gaba/glutamate pathway for therapeutic intervention of asd and adhd. Curr. Med. Chem. 22, 1850–1859 (2015).
Sivagnanam, S. et al. Introducing the neuroscience gateway. 5th International Workshop on Science Gateways (IWSG 2013) 993, 1–7 (2013).
Acknowledgements
S.S. was supported by NPDF, SERB-DST, India, File ID: PDF/2021/000585. D.R. was supported by Ramalingaswami Fellowship, Department of Biotechnology (DBT), India, Award ID: BT/RLF/Re-entry/07/2014 and Department of Science and Technology (DST), India, Award ID: SR/CSRI/21/2016. A.B. was supported by award ID: F.NO.K-15015/42/2018/SP-V from The Ministry of Youth Affairs and Sports, Government of India and NBRC Flagship program, award ID: BT/MED-III/NBRC/Flagship/Flagship2019 from the Department of Biotechnology, Government of India. The authors acknowledge the Neuroscience Gateway133 for facilitating the resources required for model simulation. We acknowledge BioRender for the icons and assets used in Fig. 1 (Created in https://BioRender.com). We extend our sincere thanks to the editors and reviewers for their invaluable suggestions and thoughtful comments that helped in improving the quality of the manuscript.
Author information
Authors and Affiliations
Contributions
S.S. and A.B. conceived the study; S.S., P.C., and A.B. performed the analysis; A.N., D.R., and A.B. contributed tools; S.S. and A.B. wrote the first draft; all authors reviewed and edited the manuscript; D.R. and A.B. supervised the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Sahar Ahmad and Jasmine Pan. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Saha, S., Chakraborty, P., Naskar, A. et al. Local homeostasis preserves global neural dynamics compensating for structural loss during human lifespan aging. Commun Biol 8, 1251 (2025). https://doi.org/10.1038/s42003-025-08690-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08690-9