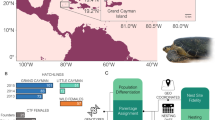
Abstract
Recent work has shown testudines can escape actuarial senescence for extended periods. However, understanding how the interplay between somatic aging and reproductive investment in highly fecund, long-lived ectotherms align with broader phylogenetic patterns remains a critical knowledge gap. Here, we present a comprehensive demographic analysis of age-specific changes in reproduction and mortality using a unique dataset on captive, known-aged green sea turtles Chelonia mydas. Despite substantial intraspecific variation, cumulative egg production showed no decline, increasing linearly for over two decades. However, mortality patterns followed the Gompertz Law, increasing exponentially with age. These results demonstrate a significant decoupling between sustained reproductive performance and age-specific mortality, building on a body of work that challenges the notion of uniformly arrested senescence. Nevertheless, life history strategies across testudines, including sea turtles, produce similar aging rates and remain low relative to endothermic tetrapods, reflecting conserved life-history patterns.
Similar content being viewed by others
Introduction
The remarkable diversity of aging rates across the tree of life has long fascinated biologists, offering a rich comparative landscape to explore factors shaping life-history evolution1,2. While senescence, characterized by a progressive decline in physiological function with age after maturity, was once considered a near-universal feature of multicellular life, an increasing body of evidence challenges this notion1. Of particular significance are testudines – turtles and tortoises – which are frequently celebrated as paragons of longevity. Many species within this ancient order exhibit exceptionally slow aging rates, a hallmark of negligible actuarial [survival] senescence, often living for several decades, and in some cases, well over a century – far exceeding similarly-sized ectothermic and endothermic tetrapods3,4. Such slow aging rates, consistently observed across diverse ecological settings, establish testudines as crucial model organisms for exploring the complex factors that delay or decouple physiological deterioration from chronological age. However, there are still unanswered questions about whether general aging patterns extend across all closely related testudine species and all fitness components.
Within the broader context of testudine life histories, sea turtles, an ancient lineage of globally threatened marine reptiles, were notably absent from recent comparative work3,4, presenting a critical, yet largely unexplored, frontier in the study of aging5. As the only extant testudines fully adapted to an oceanic existence, they occupy a unique ecological niche that has shaped a distinctive suite of life-history characteristics6. As evident in endotherms7, radiation into the marine environment could have triggered similar adaptive shifts toward a slower pace of life in sea turtles. Their delayed maturity, extensive juvenile dispersal, and long-distance reproductive migrations8, however, make them inherently challenging subjects for long-term demographic studies. Consequently, despite their unique oceanic adaptations and conservation importance, we know surprisingly little about how sea turtles age, significantly limiting our understanding of their life history. Here, we begin to address this critical gap by jointly analyzing age-specific mortality and reproductive rates in captive green turtles over a 41-year period at the Cayman Turtle Center (CTC). To our knowledge, this constitutes the only long-term capture database for known-aged sea turtles that integrates both reproduction and survival data, offering a rare glimpse into the role of aging in these ancient mariners.
To unravel broad principles governing senescence in such long-lived organisms, it is essential to consider why organisms age9. Evolutionary theories typically frame senescence as a non-adaptive consequence of trade-offs and constraints10,11,12. The declining force of natural selection with age can fail to counteract the accumulation of deleterious late-acting mutations, potentially leading to catastrophic genetic failures13. Moreover, life-history optimality proposes that aging arises from evolutionary10 and physiological9 trade-offs between early and late life fitness. These evolutionary pressures generally align aging rates with a species’ overall life strategy along a slow-fast continuum14, where a “slower” pace of life often favors delayed maturity, extended longevity, and greater future reproductive opportunities4. Thus, the rate of aging is not fixed but rather evolves as part of a species’ overall life strategy, reflecting inherited traits that can either predispose taxa to, or shield them from, senescence. In particular, the protective phenotype hypothesis may explain when we would expect life histories to deviate from expectations.
The protective phenotype hypothesis posits that traits substantially reducing extrinsic mortality risk can favor the evolution of negligible senescence – the absence of both age-specific declines in reproduction and age-specific increases in mortality. These protective traits include physical15 or chemical protection16 and modes-of-life such as volancy (flight) and fossoriality (burrowing)17. The advantage of a protective phenotype, combined with the potential for continued growth after sexual maturity (as often seen in testudines), may yield a greater benefit to life expectancy when reproductive success positively correlates with size and mortality risk is size-dependent18. Larger, more fecund phenotypes may experience reduced reproductive costs or have more efficient genetic repair mechanisms to slow the accumulation of DNA and protein damage.
Despite having hallmarks of “slow” life histories, such as delayed sexual maturity19, sea turtles exhibit the largest reproductive output among all oviparous reptiles. This seemingly paradoxical combination of life-history traits contextualizes a compelling puzzle: how do these long-lived marine specialists balance such a massive reproductive investment while maintaining physiological function? Answering this question requires examining the interplay between both survival and reproduction. Otherwise, sustained reproduction may obscure changes in survival and vice versa. Decoupling such intricately linked traits requires longitudinal data that encompass key aspects of an organism’s life cycle1. While our ultimate interest lies in deciphering aging rates in nature, the confounding effects of disease and predation20,21 underscore the importance of establishing a baseline understanding of intrinsic aging under controlled conditions22. Thus, the following sections are structured in two main parts. First, we present a demographic assessment of senescence by analyzing reproductive and survival patterns in captive green turtles. Given similarities with other testudines, we predict that aging rates, computed as the slope parameter (\({{{\rm{\beta }}}}_{1}\)) in the mortality function, are not significantly different from zero (negligible actuarial senescence) and cumulative reproductive output (number of eggs) increases linearly with age (negligible reproductive senescence). We then compare our findings with the limited information available on wild green turtles. Second, we conducted a comparative analysis to position captive green turtles within the broad array of testudine life history strategies. This approach also allowed us to determine whether aging rates and reproductive biomass production (hereafter reproductive mass) in captive and wild testudines represent recurring evolutionary outcomes or fall along a slow-fast continuum of life histories.
Results and Discussion
Reproductive output does not decline with age in captive green turtles
At the population level, cumulative reproductive output increased by 13% of its current value per unit time (θ = 0.13; 95% CI: 0.12–0.14), which approximated an almost linear increase in reproduction from age 17 to 38 years of age (Fig. 1A; SI Appendix, Fig. S1). This reproductive pattern is consistent with other testudines, such as Blanding’s turtle Emydoidea blandingii23 and painted turtles Chrysemys picta24, which tend not to show age-related declines in clutch size and reproductive frequency (but see ref. 25). Longitudinal data on Mediterranean green turtles suggest a similar pattern, where the cumulative number of clutches increases linearly with time since their first breeding season26. Unfortunately, the animals used in their study were of unknown age, complicating direct comparisons with our results.
A Solid line represents the average change in cumulative reproductive output (number of eggs) with age and associated 95% CI (dashed line). Horizontal dashed line indicates the equilibrium value, \({{\rm{\kappa }}}\). B Solid line shows age-specific changes in mortality and associated 95% CI (shaded area). Vertical dashed line represents the minimum age at first reproduction (AFR).
During a typical breeding season, female green turtles laid an average of 393 ± 238 eggs, distributed across 4.5 ± 2.0 clutches, though some individuals produced over 1000 eggs per season. Over their lifetime, females averaged a total reproductive output of 2602 eggs, with this distribution highly right-skewed and heavy-tailed. Importantly, new clutches were not clumped over time (Fig. 1A). As a result, the Poisson distribution provided a better fit to the reproductive data over competing models (\(\Delta {{{\rm{SIC}}}}_{{{\rm{GP}}}-{{\rm{Poisson}}}}=2253.4\); \(\Delta {{{\rm{SIC}}}}_{{{\rm{NB}}}-{{\rm{Poisson}}}}=2.2\)). A major factor affecting the temporal spread of reproduction is the duration between breeding seasons. In the wild, it is uncommon for females to reproduce in consecutive years27, but green turtles at the CTC have an average inter-breeding interval of 1.7 years (wild inter-breeding intervals: 2.9 and 3.2 years28,29). Factors associated with captivity – ad libitum feeding, higher quality resources, and access to suitable mates – may significantly reduce the physiological costs of reproduction but accelerate reproductive schedules. For instance, captive green turtles have a younger age at first reproduction (AFR) but reach maturity at a similar body size (measured via curved carapace length; CCL)30 to wild populations (CTC: CCL = 98.5 cm, AFR = 12.01 years; Australia (wild): CCL = 102.1 cm, AFR = 32 years31; Hawaii (wild): CCL = 94.0 cm, AFR = 27.3 years32). Only 29 of the 118 captive green turtles in Fig. 1 had an AFR less than 10 years old (range: 7–32).
At the population-level, we did not find evidence that a higher allocation to reproduction per year or a younger age at maturity33 led to an earlier deceleration in cumulative egg production. These findings are broadly consistent with Dynamic Energy Budget (DEB) models for Australian green turtles after the removal of resource limitation34. The main difference between captive and wild green turtles appears to be reproductive frequency, whereby predator-free, resource-rich captive conditions support the upper limit of their reproductive potential. However, such accelerated reproductive rates, in combination with accelerated growth rates30 could have contributed to a lower expected longevity post-maturity35 (see mortality results), which aligns with a well-supported prediction by Williams10,36. Rapid development is correlated with rapid senescence. On the other hand, the removal of environmental constraints may dampen or decouple trade-offs expected under natural conditions37,38, such as the correlation between longevity and aging rate39 or the covariance between lifespan, growth, and reproduction40,41. As such, any comparison with wild green turtle populations is currently speculative.
Observed differences in reproductive trajectories among captive turtles were mostly a result of individual heterogeneity (SI Appendix, Fig. S2). For example, four turtles laying over 10,000 eggs averaged two more clutches per year and returned to breed half a year sooner. While some individuals maintain high reproductive functioning at older ages, this may ultimately be curtailed by a rising probability of catastrophic death rather than a gradual decline in fitness42. In such cases, their reproductive lifespan is effectively truncated by mortality. The contribution of older turtles to the next generation could also decrease if developmental problems are more prevalent in elderly animals23,25. Thus, the actual number of offspring produced may decline with increasing egg production40,43. Unfortunately, we were unable to relate egg output to offspring production because hatchling data collection and egg incubation conditions were not consistent over the study period. Investigating the link between age-specific changes in egg production and offspring fitness is a critical area for future research.
Beyond 60 years old, average egg production was projected to stop, reaching a lifetime plateau at about \(\kappa =\) 13,545 eggs (Fig. 1A; SI Appendix, Table S1). Yet, it is unknown if captive green turtles will live this long. Other environmental threats that are an indirect result of aging, such as increased susceptibility to disease44, may induce mortality in elderly females. Conversely, females reproducing into old age may be a result of the selective disappearance of low-quality individuals, i.e., selective disappearance effects45. Mortality selection alters the composition of older age classes by removing frailer individuals from the population. The strength of this mortality filter depends on the overall risk of death46. When the risk of death is high, fitter individuals disproportionately represent the oldest age classes. In captivity, mortality filters are generally weak, extending the lifespan of every individual40. However, a filter for reproductive lifespan could mirror a mortality filter in which the oldest individuals reproducing represent a specific subpopulation remaining after selective disappearance47. As natural selection weakens with age, it creates a window of optimal fertility, which varies in duration across individuals48. As a result, infertility in late life may arise from complex hormonal changes48, irrespective of increases in longevity.
Females experiencing a single reproductive season (Fig. 2), hereafter termed one-time nesters (OTN), are not an artifact of captivity but are also regularly observed in wild populations49. For instance, 35% of 1770 female loggerhead turtles Caretta caretta nesting on the South-Eastern US had only one observed reproductive event50. In our study, OTN accounted for 24% of the 156 females. Compared to multi-season nesters (MSN), OTN did not differ in age at first reproduction (∆MSN-OTN = 0.5 years, t59.8 = 0.7, p = 0.5). Notably, their single reproductive event incurred no detectable survival cost. OTN experienced a reproductive lifespan 52% shorter than MSN (see survival results below) but laid significantly fewer eggs in their first breeding season (∆MSN-OTN = 76.9 eggs, t72.1 = −2.5, p = 0.02) – a difference equivalent to nearly one full clutch. The fate of short-lived OTN may further point to a selective filter favoring an intrinsic physiological resilience needed for sustained reproduction, a capacity that remains crucial even in captive conditions.
A Points represent observed annual reproductive output. Since each female only had one observed breeding season, the associated ages for each point represents age at first reproduction (AFR). Individuals that laid less than the average clutch size of 100 eggs are below the dashed line. B Solid lines show age-specific changes in mortality and associated 95% CI (shaded area). Vertical dashed line represents the minimum AFR.
Mortality rates in captive green turtles increase exponentially with age
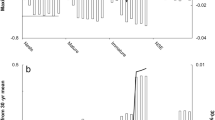
Survival patterns for captive green turtles follow the Gompertz law, which posits that adult mortality rises exponentially – or linearly on the log-scale – with age after maturity51. This mortality pattern has also been observed in captive3 and wild4 testudines. As expected, captive green turtles have a low initial mortality rate (\({{{\rm{\beta }}}}_{0}\): 0.01 – 0.04) and a slow aging rate (\({{{\rm{\beta }}}}_{1}\): 0.09–0.10), similar to other testudine species3 and orders of magnitude lower than rates typical for birds and mammals (See Fig. 1 in refs. 4,40). However, aging rates for both groups [OTN and MSN] were strictly positive (Fig. 3) and significantly different from zero (p < 0.001), revealing green turtles can experience actuarial senescence even in protected environments. This may relate to growth slowing to an asymptotic size rather than continuing indefinitely throughout life. Female green turtles at the CTC reach 95% of their maximum mass and 85% of their maximum length at AFR30, limiting any potential benefits from increasing size on fecundity.
Shaded bars represent either 95% credible intervals (non-sea turtle species3,4) or 95% confidence intervals (sea turtle species). Uncertainty estimates were computed using either Bayesian survival trajectory analysis (BaSTA) or the Delta Method. Tree tip color indicates family, and shapes and colors for aging rates and life expectancies show environment (i.e., captive (blue circles) versus wild (red squares)).
Differences in reproductive dynamics between OTN and MSN translated into distinct mortality functions (Figs. 1B and 2B). This distinction was captured by including a covariate for reproductive status (SI Appendix, Table S2), which provided a better fit to the data over assuming additional unobserved heterogeneity (via a Gamma-Makeham frailty model52,53). Initial mortality rates, which were 25% lower for MSN, shaped these differences. Both life expectancy (KLD = 1.0) and lifespan inequality (KLD = 1.0) were significantly different between OTN and MSN (SI Appendix, Fig. S3 and Table S3–4), with OTN having a 52% shorter reproductive lifespan (\({e}_{x}^{{OTN}}\) = 10.2; \({e}_{x}^{{MSN}}\) = 19.6) and greater variance in age at death (\({H}_{x}^{{OTN}}\) = 0.6; \({H}_{x}^{{MSN}}\) = 0.4). We estimated 5% of captive green turtles will survive beyond 23.4 (OTN) and 35.5 (MSN) years old, and 1% will live past 27.6 (OTN) to 39.6 (MSN) years old. Their respective aging rates correspond to a mortality doubling time of 6.7 years for OTN and 7.4 years for MSN. Whether mortality and reproductive estimates for MSN and OTN reflect individual variation in response to captivity remains unclear40. However, since individuals in the study primarily reflect the original, diverse genetic founder stock, our results are unlikely a result of inbreeding depression54.
The most common age at death (modal age; estimation based on method in ref. 55) was 10 years for OTN and 24 years for MSN. This earlier modal death age for OTN, coupled with a significantly greater variance (see \({H}_{x}^{{OTN}}\) above) suggests that deaths occur earlier on average but are more dispersed. In contrast, the narrower age range of death for MSN may indicate a more consistent timing of when late-life physiological decline leads to mortality. The earlier modal age for OTN aligns with an earlier onset of actuarial senescence post-maturity, although we could not attribute this to reproductive costs. Contrary to predictions about the timing of peak mortality36, significant increases did not begin shortly after sexual maturity for MSN but occurred approximately 12 years post-maturity (at 24 years of age), indicating a substantially delayed period before peak mortality risks manifested. This phenomenon of deferred actuarial senescence in MSN, where the primary escalation of mortality risk is postponed, has also been reported in endotherms56 and ectotherms3,4.
To explore the interplay between survival and reproduction further, we focused solely on MSN, as these individuals not only actualized the most common reproductive strategy of green turtles but also allowed us to evaluate how reproductive costs impact survival over successive breeding seasons. Recalling that cumulative reproductive output increased linearly through much of early to mid-adulthood and given that their peak mortality (modal age at death) occurs at around 24 years, the later inflection point in their reproductive trajectory (27 years; Fig. 1) suggests that mortality risk begins to accelerate before any observable decline in reproductive output. While we found no evidence that a higher annual reproductive rate or earlier maturity led to an earlier decline in egg production, the mortality pattern in MSN points to a potential trade-off between current reproduction and future survival.
Comparing mortality and reproductive patterns between captive and wild green turtles
Surprisingly, our estimate for reproductive longevity for captive green turtles (19.6 years post-maturity) closely matches those reported for wild Australian green turtle populations (18–19 years)57. Differences in longevity between captive and wild populations seem to be related to maturity time. Although green turtles may remain reproductively active for ~19 years, the waiting time to first reproduction is reduced by around 32% in captivity. Wild green turtles spend almost half their lives gathering the necessary resources to reach a minimum body size needed to meet the requirements for long-distance migration and reproduction, which is related to environmental, developmental, and physiological constraints58. Once mature, differences in reproductive rates relate to the time required to gather resources for a subsequent breeding season. In captivity, turtles do not need to migrate thousands of kilometers between breeding and foraging grounds, significantly reducing their energetic costs and increasing their reproductive rate. However, in wild leatherback turtles the stress of reproducing at a higher frequency had negative physiological effects59, suggesting a trade-off between current and future reproduction that influences lifetime reproductive success60.
Limited resources compounded by extensive migrations result in wild green turtles having fewer reproductive seasons compared to those in captivity. If wild populations follow a similar reproductive trajectory as shown in Fig. 1A, we project a 20-year reproductive lifespan with an inter-breeding interval of two and four years would result in a lifetime output of between 1324 and 4080 eggs – a figure close to the estimated lifetime reproductive output in wild Australian green turtles ( ~ 2000 eggs61). Yet, such projections from captive populations are speculative. Data from known-aged sea turtles would greatly enhance comparative analyses. Despite the hundreds of global projects monitoring sea turtle populations, several operational since the early 1960s, no long-term longitudinal data exist for known-age sea turtles of any species, rendering the estimation of age-specific mortality and reproductive rates particularly challenging62. The inability to accurately determine the absolute chronological age of living wild sea turtles remains the single most significant impediment to precisely understanding their life history63. This limitation impedes the employment of typical demographic tools64 to understand phenotypic variation and plasticity in wild populations experiencing vastly differing ecological pressures.
Alternative approaches have emerged that circumvent the need for aging live animals and allow for the construction of age- and size-specific reaction norms65. While undoubtedly valuable, such methods cannot translate into direct, individual-level changes in reproductive output and survival over a full lifespan, nor can they fully capture the dynamic interplay of environmental factors. For instance, if aging rates are environmentally modulated, as observed in other testudines3 rather than an underlying change in juvenile mortality as seen in primates66, wild populations of sea turtles may experience mortality rates that vary with local selective pressures, such as foraging ground productivity67, predation, disease, temperature regimes, and climate change68. These factors are known to strongly influence species-specific vital rates across testudines. In painted turtles, aging rates vary between and within wild and captive populations (captivity: 0.0919, 0.103; wild: 0.044, 0.014, 0.1025). Comparable results have been reported in pond sliders Trachemys scripta3,4, as well as European pond turtles Emys orbicularis4. This environmental sensitivity has important implications in understanding selection for lifespan-extending mechanisms in long-lived species, which is expected to be strong. When extrinsic mortality is low and aging-related mortality is high, as is the case in certain birds and mammals42,69, extending lifespan further would likely come at a high evolutionary cost. Life extending mechanisms may be less costly in terms of evolutionary fitness for testudines when actuarial senescence is not the primary source of mortality42, but this needs to be evaluated with empirical data. While direct longitudinal studies of known-aged individuals in the wild remain paramount, at present, research on the paradoxical life history of sea turtles can be most effectively advanced by tracking individuals from their first breeding season, employing breeding age rather than absolute age.
Comparing mortality and reproductive patterns across captive and wild testudines
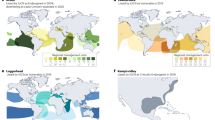
To situate our findings on captive green turtles within the broader evolutionary landscape, we conducted a comparative analysis across testudines. Captive green turtles exhibit aging rates similar to those of other testudines (Fig. 3), and the relationships among fitness-related traits in testudines (including sea turtles) generally align with established life-history patterns3,4,19, irrespective of environment. For instance, an earlier age at first reproduction is linked to a shorter lifespan and body mass is positively correlated with life expectancy (SI Appendix, Fig. S4A, B). A notable divergence, however, emerges in their reproductive investment: green turtles produce a disproportionately heavier reproductive mass relative to their aging rate, life expectancy, and AFR (Fig. 4A–C). Despite this difference, the allometric relationship between reproductive mass and body mass in green turtles matches theoretical expectations (Fig. 4D), a pattern that also holds across wild sea turtle species70. Extensive migrations between foraging and breeding grounds impose strict physiological constraints, making the large body size of sea turtles an evolutionary prerequisite for their highly migratory, oceanic lifestyle71. In general, a larger body size offers significant adaptive advantages, including reduced predation risk, a lower mass-specific metabolic rate, and greater starvation tolerance. However, these benefits are balanced by higher resource requirements, a longer maturation period, and increased susceptibility to extinction due to longer generation times.
Across testudines, we found aging rates do not strongly vary along a slow-fast continuum as typically defined by other life-history traits. Notably, the relationship between aging rates and body mass, AFR, and annual fecundity are substantially weaker than in other reptiles4 (Fig. 5A–C). Collectively, these traits explained only 8% of the variation in aging rates (Table 1A). While the direction of these relationships is similar to other reptiles (excluding testudines), the effect sizes are trivial, yielding nearly flat lines across taxa with different fecundities (slope: testudines = 0.01, reptiles = 0.11), ages at first reproduction (slope: testudines = −0.01, reptiles = −0.18), and body masses (slope: testudines = 0.00, reptiles = −0.03). Furthermore, while wild populations in our dataset exhibit slightly faster aging rates, this difference was not statistically significant (Table 1A, Fig. 5).
Solid lines indicate the fitted model, and shaded areas are 95% confidence intervals. Predictions for aging rates from Reinke et al. 4 including (teal dashed line) and excluding (teal solid line) their testudine data. Estimates for captive green turtles in plots (A–C) are shown by the green turtle silhouette, sourced from PhyloPic. AFR denotes age at first reproduction.
Consistently slow aging rates across testudines, despite substantial differences in body size, environmental conditions, and habitat use, likely stem from a weakening of life-history trade-offs. Shared protective phenotypes (e.g., shells) and physiological adaptations, such as the ability to endure food scarcity while keeping maintenance costs low relative to energy storage72, may buffer testudines against selective pressures that typically drive diverse aging trajectories in other groups. The stronger relationships reported by Reinke et al.4 appear predominantly driven by high variation in squamates, with crocodilians and tuataras showing aging patterns more aligned with testudines. Similarly, the correlation between mortality and AFR in Shine and Iverson73 arises from methodological distinctions between age-specific mortality and instantaneous adult mortality (\(-{\mathrm{ln}}({{\rm{S}}})\), where S is a point estimate for adult survival probability). Our results suggest that, within testudines, the expected covariance among life-history traits with aging rates along a slow-fast continuum is not evident because they collectively occupy the slow extreme of this broader spectrum (see also Fig. 4 in ref. 4).
While variation in reproductive mass does not directly align with the slow-fast continuum, it correlates strongly with body mass – a trait that itself is indicative of a species’ position on that continuum (SI Appendix, Fig. S4A, B). Female body mass in testudines explained 81% of the variation in annual reproductive mass (Fig. 4D). Other life-history traits, such as life expectancy, AFR, aging rate, and environment, did not explain the residual variation, and their effect actually became trivial once body mass was prioritized in a sequential decomposition of variance (Type I ANOVA). The dominant role of body size is anticipated, given its fundamental importance to organismal fitness, and it is both heritable74 and exhibits plastic responses to changing environments75, particularly during ontogeny30. In line with scaling laws76, which predict reproductive mass should follow a power function of the form \(\alpha \cdot {M}_{F}^{\beta }\), we estimate the scaling exponent (\(\beta\)) to be 0.89 (95% CI: 0.77–1.02), suggesting hypoallometric scaling. This result aligns closely with findings across sea turtles70 and testudines in general77, whereby larger species produce relatively lighter clutches (slope ≈ 0.77 in ref. 77). Interestingly, among individuals of the same species, this pattern is reversed (hyperallometric scaling)70,78.
Although inter-specific hypoallometry might imply reproduction carries a proportionally higher allometric cost for smaller species, we found no detectable direct effect of reproductive mass itself on aging rates (p = 0.87), AFR (p = 0.86), or life expectancy (p = 0.21). Reproductive mass was slightly heavier in captivity (Table 1B and Fig. 4), but this difference was not significant (∆captive-wild = 0.05; p = 0.77). Although univariate tests detected a strong phylogenetic signal for both reproductive mass and body mass (Pagel’s λ; SI Appendix, Fig. S5 and Table S5), evolutionary relatedness did not explain the residual variance for reproductive mass, nor aging rates in the PGLS models (SI Appendix, Table S6). Instead, life-history trade-offs associated with the magnitude of reproductive investment primarily manifest through adjustments in other life-history components (SI Appendix, Fig. S4), such as age at first reproduction and overall life expectancy (as suggested by correlations with body mass). For instance, turtles and tortoises are constrained by the size of their body cavity, which is affected by their overall body plan. As such, there is a trade-off between the number of eggs and egg size79. Egg size is expected to be subject to stronger directional selection than clutch size, as each egg must provision all essential resources required for hatchling survival. Empirical data support this hypothesis, finding that clutch size shows greater phenotypic variance relative to egg size80. Patterns of reproductive allocation may largely reflect these allometric and anatomical constraints, especially as both clutch and egg size generally increase with body size across testudines81, rather than being strongly lineage-specific72.
Conclusion
Our comprehensive demographic analysis of captive green turtles reveals several key insights. We found that: (1) captive green turtles maintain high, albeit variable, reproductive output well into advanced ages; (2) their mortality rates increased exponentially with age; and (3) aging rates across diverse testudines suggest evolutionarily conserved strategies, reflecting their shared position at the slow end of the life-history continuum, rather than being strongly lineage-specific. On the other hand, (4) annual reproductive mass was primarily dictated by allometric scaling with body mass, which indirectly positions testudines along a slow-fast continuum because of the relationship between body mass and other traits, such as life expectancy and AFR. A pivotal finding for this captive population of green turtles is the decoupling of reproductive and somatic aging: while reproductive output showed no age-related declines (negligible reproductive senescence), this did not coincide with negligible actuarial senescence, as mortality rates clearly increased with age. Although captive conditions may buffer against selective pressures in natural systems, thereby revealing intrinsic aging patterns, they can also accelerate reproductive schedules. The degree to which these accelerated rates in captivity impact overall longevity remains a key question. Nevertheless, given the profound lack of long-term, age-specific demographic data for any wild sea turtle species, our findings provide a crucial baseline, generating testable hypotheses for future field studies and comparative research. While protective phenotypes in testudines contribute to their renowned low baseline mortality rates – a trait clearly shared by captive green turtles – our study reaffirms that slow actuarial aging is not equivalent to a complete absence of aging40.
Methods
Data
The reproductive dataset for green turtles at the CTC consisted of 156 known-age adult females for a period of 41 years (1977–2018). The CTC is an ideal system to test theories of aging in sea turtles because of the high detection probability of nesting females in combination with the large number of records per individual. Of the 156 females, 38 turtles had only one observed reproductive season. These females were labelled OTN, whereas the remaining 118 individuals were MSN with 2 to 26 breeding seasons. Since monitoring coverage is not perfect and some clutches are missed, we estimated the missing clutch size (number of eggs per clutch) data (SI Appendix, S5 and Fig. S6), which only accounted for 8% of observations (n = 316 of 3624 clutches). We computed cumulative reproductive output by summing the number of eggs laid by a female over the course of her life. This value incorporates a female’s clutch size, or the number of eggs per clutch, and clutch frequency, or the number of clutches per reproductive season. In addition to data collection on reproductively active females, a population census was conducted annually from 1974 – 2018. This provided us with a multifaceted dataset, allowing us to determine whether an individual was alive but reproductively quiescent. The survival data had capture histories for the 118 MSN, as well as the 38 OTN. Of the 156 animals used in the analysis, females were born between 1966 and 2002, and year of death was recorded for 22% of individuals (n = 34) whereas the remaining 78% either stayed alive, escaped or died during Hurricane Michelle in 2001 (21% of the 78%), or disappeared (no recorded death or escape). Mortality estimates were not significantly affected by the removal of those 50 individuals during Hurricane Michelle in 2001 (SI Appendix, S3 and Fig. S7). For additional information on data collection, see SI Appendix, S1. We have complied with all relevant ethical regulations for animal use.
Derivation of the stochastic pure-birth reproductive model
To model reproductive dynamics, we used a stochastic birth process with a modified Gompertz skeleton \(m\left(\cdot \right)\) following the approach of Tavaré et al. 82. The expected one-step change in cumulative reproductive output for individual \(i\) in breeding season \(t\) was given by
where the statistical equilibrium \(\kappa\) determines maximum cumulative reproductive output (units: eggs) and the speed of equilibration \(\theta\), measured in units of 1/time, controls the rate at which current cumulative reproductive output approaches \({{\rm{\kappa }}}\) and is modulated by the waiting time (in years) between breeding seasons, denoted \({{{\rm{z}}}}_{{{\rm{t}}}}\) (remigration interval in wild populations). Predicted annual reproductive output \({y}_{i,t}^{A* }\) can then be computed as the difference between \(m({y}_{i,t}^{C})\) and \(m({y}_{i,t-1}^{C})\), i.e.,
We used each female’s reproductive output during their first breeding season as the initial value of the reproductive process, \({y}_{0}=m\left({y}_{1}^{C}\right)={y}_{1}^{C}\) (SI Appendix, S2). We then altered Eq. 1.1 to include environmental noise and temporal variation in vital rates. This new model mirrors that commonly applied to population dynamics, whereby we explicitly account for demographic stochasticity with added environmental variability. The latent estimate for annual reproductive output \({\mu }_{i,t}\) followed a lognormal distribution with variance \({\sigma }^{2}\),
We then assessed if Poisson, generalized Poisson, or negative binomial sampling error in Eq. 1.5 (PMF) best fit the observed data for annual reproductive output, \({{{\rm{y}}}}_{{{\rm{i}}},{{\rm{t}}}}^{{{\rm{A}}}}\). Our approach combines several important biological processes in a flexible hierarchical framework to model reproductive trajectories. In particular, Eq. 1.1 assumes that the rate of cumulative egg production is lower in early life, which is typical in sea turtles61, before increasing linearly. Late life is then characterized by a plateauing in reproduction, which is unknown and presented us with a testable hypothesis. In biological terms, our model ascribes the time-dependence acting on a female’s current reproductive effort to an exhaustible resource that changes with age. To determine ages along the population average reproductive trajectory where the slope significantly changes and the age range where the population average reproductive trajectory increases linearly, we fit a piecewise regression with an upper and lower breakpoint (Fig. S1). Model selection was done using the Geyer-Thompson algorithm83 and data cloning was used to assess parameter identifiability (see SI Appendix, S8 and Fig. S8). Additional relationships among reproductive parameters were assessed with t-tests. Throughout the text, ∆ represents the difference between two quantities and, unless otherwise stated, values represent trait averages along with their associated standard deviations. For more information, we refer the reader to the supplementary material (SI Appendix, S2).
Estimation of age-specific mortality
We used the R package BaSTA84 to assess whether changes in reproduction had associated changes in survival. Models were built at the population level, and by reproductive status (a grouping with two levels: OTN and MSN). This approach allowed us to account for individual heterogeneity. For instance, turtles that have only one observed breeding season may also have discernable differences in age-specific mortality patterns. Model selection was used to determine (1) the best mortality function, (2) whether there was an effect of time-dependent recapture probability, and (3) if including a covariate for reproductive status improved the fit over a population-level model (SI Appendix, S3). Models were fit using highly diffuse priors (SI Appendix, Fig. S9; SI Data, S1–3) because testing revealed default BaSTA priors may impose strong and, in some cases, biologically unrealistic assumptions (SI Appendix, S7 and Tables S3, 4; also see ref. 85). We performed maximum likelihood estimation via data cloning86 on the best-fitting model to assess parameter identifiability (SI Appendix, Figs. S10, 11) and resolve the dependency of the results on imposed priors. Model fit was assessed by plotting the predicted mortality curve against an unconstrained model derived from life tables (SI Appendix, Fig. S12). We then computed confidence intervals (CI) for adult age-specific mortality rate, life expectancy after first reproduction (\({e}_{x}\)), lifespan inequality after first reproduction (\({{{\rm{H}}}}_{{{\rm{x}}}}\)), and adult aging rate (\({{{\rm{\mu }}}}_{{{\rm{AR}}}}\), slope of the age-specific mortality rate computed as \({{{\rm{\beta }}}}_{1}\) in the Gompertz model). We then evaluated whether \({{{\rm{\mu }}}}_{{{\rm{AR}}}}\) was significantly different from zero using a two-sided z-test.
Exploring the relation between life history strategies and reproductive mass
We assessed whether (i) aging rates and (ii) reproductive mass reflect position along a slow-fast continuum of life histories. Our final dataset contained 56 testudine species from 66 populations, encompassing 71% of testudine families (10 of 14) and 43% of genera (40 of 93), with representatives from freshwater, marine, and terrestrial habitats, sourced from both captive (n = 47) and wild (n = 18) environments. We constructed phylogenetic least squares (PGLS) regression models for aging rate, including covariates for annual fecundity, AFR, and environment (\({{\rm{ENV}}}\)), categorized as either captive or wild. We then assessed how annual reproductive mass (RM) varied with AFR, aging rate, average life expectancy from AFR (\({e}_{x}\)), female mass (\({{{\rm{M}}}}_{{{\rm{F}}}}\)), and environment. \({{\rm{RM}}}\) was computed as \(({{\rm{CS}}}\cdot {{\rm{CF}}})\cdot {{{\rm{M}}}}_{{{\rm{E}}}}\), where CS is clutch size and CF denotes clutch frequency. \({{{\rm{M}}}}_{{{\rm{E}}}}\) is egg mass and \({{{\rm{M}}}}_{{{\rm{F}}}}\) is female body mass measured in kilograms. Mortality parameters, such as life expectancy and rate of aging, for non-sea turtle species were extracted from da Silva et al. 3 and Reinke et al. 4, and trait data were sourced from meta-analyses, or the published literature when necessary (SI Data, S4). We also compared the PGLS models to models with no covariance structure, i.e., phylogenetic independence. Before running the PGLS regression, we used univariate tests to characterize phylogenetic signal for aging rate, life expectancy, AFR, female body mass, and \({{\rm{RM}}}\) using Pagel’s \({{\rm{\lambda }}}\)87 in phytools88. For plotting purposes, predictions were made after holding the other variables in the model at the mean value within their respective environments. This approach better reflects systematic, real-world differences between wild and captive systems, allowing us to evaluate characteristics typical of each group. For more information, see SI Appendix, S4.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Raw data are not publicly available due to third-party proprietary restrictions established under agreement with the Cayman Turtle Center (owners of the data). Access requests should be directed to Walter Mustin (wgmustin@turtle.ky) via the Center’s research application process, which outlines conditions for data use and protection. All data needed to reproduce the conclusions of the PGLS regressions are present in the main text or the supplementary materials and mortality estimates for other testudines included in this study are available at3,4. All R code used in this paper is available at89.
References
Jones, O. R. et al. Diversity of ageing across the tree of life. Nature 505, 169–173 (2013).
Healy, K., Ezard, T. H. G., Jones, O. R., Salguero-Gómez, R. & Buckley, Y. M. Animal life history is shaped by the pace of life and the distribution of age-specific mortality and reproduction. Nat. Ecol. Evol. 3, 1217–1224 (2019).
da Silva, R., Conde, D. A., Baudisch, A. & Colchero, F. Slow and negligible senescence among testudines challenges evolutionary theories of senescence. Science 376, 1466–1470 (2022).
Reinke, B. A. et al. Diverse aging rates in ectothermic tetrapods provide insights for the evolution of aging and longevity. Science 376, 1459–1466 (2022).
Hoekstra, L. A., Schwartz, T. S., Sparkman, A. M., Miller, D. A. W. & Bronikowski, A. M. The untapped potential of reptile biodiversity for understanding how and why animals age. Funct. Ecol. 34, 38–54 (2020).
Stott, I. et al. Life histories are not just fast or slow. Trends Ecol. Evolution 39, 830–840 (2024).
Sol, D. et al. Adaptations to marine environments and the evolution of slow-paced life histories in endotherms. Nat. Commun. 16, 1–12 (2025).
Van Buskirk, J. & Crowder, L. B. Life-history variation in marine turtles. Copeia 1994, 66–81 (1994).
Kirkwood, T. B. L. & Austad, S. N. Why do we age? Nature 408, 233–238 (2000).
Williams, G. C. Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution 11, 398 (1957).
Hamilton, W. D. The moulding of senescence by natural selection. J. Theor. Biol. 12, 12–45 (1966).
Kirkwood, T. B. L. The Evolution of Ageing. Nature 267, 192–193 (1977).
Medawar, P. B. An Unsolved Problem in Biology. (London: Lewis, 1952).
Jones, O. R. et al. Senescence rates are determined by ranking on the fast-slow life-history continuum. Ecol. Lett. 11, 664–673 (2008).
Munshi-South, J. & Wilkinson, G. S. Bats and birds: Exceptional longevity despite high metabolic rates. Ageing Res. Rev. 9, 12–19 (2010).
Blanco, M. A. & Sherman, P. W. Maximum longevities of chemically protected and non-protected fishes, reptiles, and amphibians support evolutionary hypotheses of aging. Mechanisms Ageing Dev. 126, 794–803 (2005).
Healy, K. et al. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc. R. Soc. B: Biol. Sci. 281, 20140298 (2014).
Vaupel, J. W., Baudisch, A., Dölling, M., Roach, D. A. & Gampe, J. The case for negative senescence. Theor. Popul. Biol. 65, 339–351 (2004).
Ricklefs, R. E. Life-history connections to rates of aging in terrestrial vertebrates. Proc. Natl Acad. Sci. 107, 10314–10319 (2010).
Rose, M. R. Evolutionary Biology of Aging. (Oxford University Press, Oxford, 1991).
Comfort, A. The Biology of Senescence. (Edinburgh: Churchill Livingstone, 1979).
Rose, M. R. et al. A Hamiltonian Demography of Life History. in The Evolution of Senescence in the Tree of Life 40–55 (2017).
Congdon, J. D., Nagle, R. D., Kinney, O. M. & Van Loben Sels, R. C. Hypotheses of aging in a long-lived vertebrate, Blanding’s turtle (Emydoidea blandingii). Exp. Gerontol. 36, 813–827 (2001).
Congdon, J. D. et al. Testing hypotheses of aging in long-lived painted turtles (Chrysemys picta). Exp. Gerontol. 38, 765–772 (2003).
Warner, D. A., Miller, D. A. W., Bronikowski, A. M. & Janzen, F. J. Decades of field data reveal that turtles senesce in the wild. Proc. Natl Acad. Sci. USA 113, 6502–6507 (2016).
Rickwood, M. L. et al. Individual plasticity in response to rising sea temperatures contributes to an advancement in green turtle nesting phenology. Proc. R. Soc. B: Biol. Sci. 292, 20241809 (2025).
Solow, A. R., Bjorndal, K. A. & Bolten, A. B. Annual variation in nesting numbers of marine turtles: The effect of sea surface temperature on re-migration intervals. Ecol. Lett. 5, 742–746 (2002).
Troëng, S. & Chaloupka, M. Variation in adult annual survival probability and remigration intervals of sea turtles. Mar. Biol. 151, 1721–1730 (2007).
Omeyer, L. C. M. et al. Investigating differences in population recovery rates of two sympatrically nesting sea turtle species. Anim. Conserv. 24, 832–846 (2021).
Bjorndal, K. A., Parsons, J., Mustin, W. & Bolten, A. B. Threshold to maturity in a long-lived reptile: Interactions of age, size, and growth. Mar. Biol. 160, 607–616 (2013).
Limpus, C. J., Parmenter, C. J. & Chaloupka, M. Monitoring of Coastal Sea Turtles: Gap Analysis 2. Green Turtles, Chelonia Mydas, in the Port Curtis and Port Alma Region. Report produced for the Ecosystem Research and Monitoring Program Advisory Panel as part of Gladstone Ports Corporation’s Ecosystem Research and Monitoring Program. (2013).
Murakawa, S. K. K. & Snover, M. L. The impact of exceptional growth rates on estimations of life stage duration in Hawaiian green sea turtles. Endanger. Species Res. 35, 181–193 (2018).
Roff, D. A. Trade-offs between growth and reproduction: an analysis of the quantitative genetic evidence. J. Evolut. Biol. 13, 434–445 (2000).
Stubbs, J. L. et al. A full life cycle Dynamic Energy Budget (DEB) model for the green sea turtle (Chelonia mydas) fitted to data on embryonic development. J. Sea Res. 143, 78–88 (2019).
Metcalfe, N. B. & Monaghan, P. Growth versus lifespan: Perspectives from evolutionary ecology. Exp. Gerontol. 38, 935–940 (2003).
Gaillard, J. M. & Lemaître, J. F. The Williams’ legacy: A critical reappraisal of his nine predictions about the evolution of senescence. Evolution 71, 2768–2785 (2017).
Ricklefs, R. E. & Cadena, C. D. Lifespan is unrelated to investment in reproduction in populations of mammals and birds in captivity. Ecol. Lett. 10, 867–872 (2007).
Landes, J., Henry, P. Y., Hardy, I., Perret, M. & Pavard, S. Female reproduction bears no survival cost in captivity for gray mouse lemurs. Ecol. Evolution 9, 6189–6198 (2019).
Péron, G., Lemaître, J. F., Ronget, V., Tidière, M. & Gaillard, J. M. Variation in actuarial senescence does not reflect life span variation across mammals. PLoS Biol. 17, 1–15 (2019).
Austad, S. N. & Finch, C. E. How ubiquitous is aging in vertebrates?. Science 376, 1384–1385 (2022).
van Noordwijk, A. J. & de Jong, G. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Naturalist 128, 137–142 (1986).
Ricklefs, R. E. Insights from comparative analyses of aging in birds and mammals. Aging Cell 9, 273–284 (2010).
Schroedera, J., Nakagawa, S., Rees, M., Mannarelli, M. E. & Burke, T. Reduced fitness in progeny from old parents in a natural population. Proc. Natl Acad. Sci. USA 112, 4021–4025 (2015).
Bronikowski, A. M. et al. Sex-specific innate immunity and ageing in long-lived fresh water turtles (Kinosternon flavescens: Kinosternidae). Immun. Ageing 20, 1–12 (2023).
Bouwhuis, S., Sheldon, B. C., Verhulst, S. & Charmantier, A. Great tits growing old: Selective disappearance and the partitioning of senescence to stages within the breeding cycle. Proc. R. Soc. B: Biol. Sci. 276, 2769–2777 (2009).
Hawkes, K., Smith, K. R. & Blevins, J. K. Human actuarial aging increases faster when background death rates are lower: A consequence of differential heterogeneity? Evolution 66, 103–114 (2012).
Forsythe, A. B., Day, T. & Nelson, W. A. Demystifying individual heterogeneity. Ecol. Lett. 24, 2282–2297 (2021).
Finch, C. E. & Holmes, D. J. Ovarian aging in developmental and evolutionary contexts. Ann. N. Y. Acad. Sci. 1204, 82–94 (2010).
Thorson, J. T., Punt, A. E. & Nel, R. Evaluating population recovery for sea turtles under nesting beach protection while accounting for nesting behaviours and changes in availability. J. Appl. Ecol. 49, 601–610 (2012).
Shamblin, B. M. et al. In search of the “missing majority” of nesting loggerhead turtles: improved inter-seasonal recapture rates through subpopulation-scale genetic tagging. Mar. Biol. 168, 1–14 (2021).
Finch, C. E. Longevity, Senescence, and the Genome. (University of Chicago Press, Chicago., 1990).
Vaupel, J. W., Manton, K. G. & Stallard, E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography 16, 439–454 (1979).
Beard, R. E. Note on some mathematical mortality models. in Ciba Foundation Colloquium on Ageing 302–311 (1959).
Barbanti, A. et al. How many came home? Evaluating ex situ conservation of green turtles in the Cayman Islands. Mol. Ecol. 28, 1637–1651 (2019).
Missov, T. I., Lenart, A., Nemeth, L., Canudas-Romo, V. & Vaupel, J. W. The gompertz force of mortality in terms of the modal age at death. Demographic Res. 32, 1031–1048 (2015).
Lemaître, J. F. et al. Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proc. Natl Acad. Sci. USA 117, 8546–8553 (2020).
Chaloupka, M. & Limpus, C. Estimates of sex- and age-class-specific survival probabilities for a southern Great Barrier Reef green sea turtle population. Mar. Biol. 146, 1251–1261 (2005).
Berrigan, D. & Charnov, E. L. Reaction Norms for Age and Size at Maturity in Response to Temperature: A Puzzle for Life Historians. Oikos 70, 474 (1994).
Plot, V., Criscuolo, F., Zahn, S. & Georges, J. Y. Telomeres, age and reproduction in a long-lived reptile. PLoS ONE 7, 1–6 (2012).
Rivalan, P. et al. Trade-off between current reproductive effort and delay to next reproduction in the leatherback sea turtle. Oecologia 145, 564–574 (2005).
Hamann, M., Limpus, C. J. & Owens, D. Wm. Reprod. Cycles Males. Females Biol. Sea Turt., Vol. II 2, 135–161 (2003).
Mazaris, A. D., Broder, B. & Matsinos, Y. G. An individual based model of a sea turtle population to analyze effects of age dependent mortality. Ecol. Model. 198, 174–182 (2006).
Morales-Mérida, B. A., Pilcher, N. J. & Girondot, M. How Old Is a Turtle? Challenges in Interpreting Age Information in Sea Turtles. Ecologies 5, 502–511 (2024).
Salguero-Gómez, R. & Gamelon, M. Demographic Methods across the Tree of Life. (Oxford university press, 2021).
Girondot, M., Mourrain, B., Chevallier, D. & Godfrey, M. H. Maturity of a giant: age and size reaction norm for sexual maturity for Atlantic leatherback turtles. Mar. Ecol. 42, 1–11 (2021).
Aburto, J. M., Villavicencio, F., Basellini, U., Kjærgaard, S. & Vaupel, J. W. Dynamics of life expectancy and life span equality. Proc. Natl Acad. Sci. USA 117, 5250–5259 (2020).
Vander Zanden, H. B. et al. Foraging areas differentially affect reproductive output and interpretation of trends in abundance of loggerhead turtles. Mar. Biol. 161, 585–598 (2014).
Maurer, A. S. et al. Population Viability of Sea Turtles in the Context of Global Warming. BioScience 71, 790–804 (2021).
Ricklefs, R. E. Evolutionary theories of aging: Confirmation of a fundamental prediction, with implications for the genetic basis and evolution of life span. Am. Naturalist 152, 24–44 (1998).
Wu, N. C., Uzair, M., Annette, R. & Barneche, D. R. Size scaling of sea turtle reproduction may reconcile fundamental ecology and conservation strategies at the global scale. Glob. Ecol. Biogeogr. 31, 1277–1289 (2022).
Hendrickson, J. R. The ecological strategies of sea turtles. Integr. Comp. Biol. 20, 597–608 (1980).
Marn, N. & Kooijman, S. A. L. M. The comparative energetics of the turtles and crocodiles. Ecol. Evolution 12, 1–22 (2022).
Shine, R. & Iverson, J. B. Patterns of Survival, Growth and Maturation in Turtles. Oikos 72, 343–348 (1995).
Noordwijk, A. J. V., Balen, J. H. V. & Scharloo, W. Heritability of body size in a natural population of the Great Tit (Parus major) and its relation to age and environmental conditions during growth. Genetical Res. 51, 149–162 (1988).
Stillwell, R. C. & Fox, C. W. Geographic variation in body size, sexual size dimorphism and fitness components of a seed beetle: Local adaptation versus phenotypic plasticity. Oikos 118, 703–712 (2009).
Sibly, R. M., Brown, J. H. & Kodric-Brown, A. Metabolic Ecology: A Scaling Approach. (John Wiley & Sons, 2012).
Iverson, J. B. Correlates of Reproductive Output in Turtles (Order Testudines). Herpetological Monogr. 6, 25–42 (1992).
Marshall, D. J. & White, C. R. Have We Outgrown the Existing Models of Growth?. Trends Ecol. Evolution 34, 102–111 (2019).
Gatto, C. R., Robinson, N. J., Spotila, J. R., Paladino, F. V. & Santidrián Tomillo, P. Body size constrains maternal investment in a small sea turtle species. Mar. Biol. 167, 1–11 (2020).
Bjorndal, K. A. & Carr, A. Variation in Clutch Size and Egg Size in the Green Turtle Nesting Population at Tortuguero. Costa Rica. Herpetological 45, 181–189 (1989).
Miller, J. D. & Dinkelacker, S. A. Reproductive structures and strategies of turtles. in Biology of turtles: from structures to strategies of life (eds. Wyneken, J., Godfrey, M. H. & Bels, V.) 239–292 (CRC Press, 2007).
Tavaré, S., Marshall, C. R., Will, O., Soligo, C. & Martin, R. D. Using the fossil record to estimate the age of the last common ancestor of extant primates. Nature 416, 726–729 (2002).
Ponciano, J. M., Taper, M. L., Dennis, B. & Lele, S. R. Hierarchical models in ecology: confidence intervals, hypothesis testing, and model selection using data cloning. Ecology 90, 356–362 (2009).
Colchero, F., Jones, O. R. & Rebke, M. BaSTA: An R package for Bayesian estimation of age-specific survival from incomplete mark-recapture/recovery data with covariates. Methods Ecol. Evol. 3, 466–470 (2012).
Gao, J., May, M. R., Rannala, B. & Moore, B. R. Model misspecification misleads inference of the spatial dynamics of disease outbreaks. Proc. Natl Acad. Sci. 120, 1–9 (2023).
Lele, S. R., Dennis, B. & Lutscher, F. Data cloning: Easy maximum likelihood estimation for complex ecological models using Bayesian Markov chain Monte Carlo methods. Ecol. Lett. 10, 551–563 (2007).
Pagel, M. Inferring evolutionary processes from phylogenies. Zoologica Scr. 26, 331–348 (1997).
Revell, L. J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evolution 3, 217–223 (2012).
Glen, C. G. et al. Code for Aging dynamics in captive sea turtles reflect conserved life-history patterns across the testudine phylogeny. github.com/constantin-george/GlenEtAl2025_AgingSeaTurtles.git (2025).
Acknowledgements
The authors thank the Cayman Turtle Center for access to their long-term reproductive and capture-mark-recapture datasets, Jean-Michel Gaillard for comments on an earlier draft, J. Gordon Burleigh for advice on the comparative phylogenetic analysis, and three anonymous reviewers. C.G.G. was supported by the Archie Carr Center for Sea Turtle Research through the Jeff and Monette Fitzsimmons Fund. This work was part of C.G.G.’s PhD dissertation. All animal care was conducted in compliance with the Government of the Cayman Islands.
Author information
Authors and Affiliations
Contributions
Conceptualization: C.G.G., A.B.B., K.A.B.; Methodology: C.G.G., M.T.S., J.M.P., J.F.G.; Investigation: C.G.G., M.T.S., J.M.P., J.F.G., W.M., A.B.B., K.A.B.; Visualization C.G.G.; Funding acquisition: C.G.G., A.B.B., K.A.B.; Project administration: C.G.G., A.B.B., K.A.B.; Supervision: C.G.G., A.B.B., K.A.B.; Writing – original draft: C.G.G.; Writing – review & editing: C.G.G., M.T.S., J.M.P., J.F.G., W.M., A.B.B., K.A.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Nina Marn, Feng Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Dr Madhava Meegaskumbura and Dr Ophelia Bu. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Glen, C.G., Ponciano, J.M., Gillooly, J.F. et al. Aging dynamics in captive sea turtles reflect conserved life-history patterns across the testudine phylogeny. Commun Biol 8, 1297 (2025). https://doi.org/10.1038/s42003-025-08717-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08717-1
This article is cited by
-
Aging dynamics in captive sea turtles reflect conserved life-history patterns across the testudine phylogeny
Communications Biology (2025)