Abstract
Human skin plays a crucial role in protecting the body against environmental stressors. The skin cells involved in photoprotection are melanocytes responsible for the synthesis of melanin and contributing to the pigmentation and photoprotection of the body, but also susceptible to oxidative stress and its consequences. This paper provides a comprehensive overview of the unique functions of melanocytes, highlighting their vulnerability to oxidative stress. There is a wide spectrum of natural compounds that can support antioxidant effectiveness and, consequently, melanocytes redox balance. Essential vitamins, such as E, A and C, as well as polyphenols and melatonin contribute to the neutralization of ROS and support cellular repair mechanisms. Phytocannabinoids demonstrate anti-inflammatory/antioxidative effects based on specific receptors activation. These natural compounds offer potential protective strategies to maintain the redox homeostasis of melanocytes in conditions of potential pathologies, preventing the development of diseases, including cancer, but also therapeutic effects in pathological conditions.
Similar content being viewed by others
Introduction
Human skin is an extensive and complex organ responsible for the numerous functions essential for human functioning, including thermoregulation, water management, but also receiving stimuli from the surrounding environment, as well as immune reactions and protection against harmful xenobiotics and external physical factors1. One of the most common and unavoidable physical stressors for the skin is ultraviolet radiation (UV)—especially UVA and UVB contained in solar radiation2. UV has complex and mixed effects on human health: it mediates natural synthesis of vitamin D and endorphins3, being at the same time the most ubiquitous physical carcinogen in our environment4. It is both a mutagen due to its ability to initiate and promote cancer development, as well as a nonspecific damaging factor5. Moreover, the increase in UV radiation on earth due to stratospheric depletion remains a major environmental threat to the human organism, increasing its risk of damage due to oxidative stress6. UV radiation is highly genotoxic but is also known as a significant prooxidant factor, which induces reactive oxygen species (ROS) generation by affecting cellular components directly or by means of photosensitization mechanisms7 (Table 1). A comparative analysis of UVA, UVB, and UVC reveals substantial differences in energy efficiency and cellular outcomes across various skin conditions. UVB (290–320 nm) is significantly more energy-efficient in inducing direct DNA damage, primarily through cyclobutane pyrimidine dimers (CPDs) and 6–4 photoproducts (6–4 PPs), which are strongly associated with carcinogenesis, especially squamous cell carcinoma and basal cell carcinoma8,9. UVA (320–400 nm), though less energetic, penetrates deeper into the dermis and causes oxidative DNA lesions such as 8-oxoG and thymine glycol, often via photosensitized ROS production8,10. Compared to UVB, UVA requires around 1000-fold higher doses to elicit similar mutagenic effects9. Despite this lower efficiency, UVA is implicated in melanoma development, particularly via oxidative stress and chronic damage mechanisms8,9. UVB primarily targets the epidermis, inducing acute inflammation, keratinocyte proliferation, significant melanocyte activation and morphological changes10,11. Conversely, UVA leads to collagen degradation and apoptosis in dermal fibroblasts, with limited direct effects on melanocyte number or morphology10,12. UVC, although not naturally encountered due to atmospheric filtration, is highly energetic and has demonstrated DNA oxidation potential in vitro, particularly guanine oxidation, though it is less effective than UVB in generating hydrogen peroxide or 8-oxoG in skin contexts8,13. Importantly, darker skin phototypes exhibit greater resistance to UVB-induced cytotoxicity due to higher eumelanin levels, while UVA-induced effects may be influenced by the phototoxic potential of pheomelanin in lighter skin13. These spectral differences have significant implications for photoprotection, skin disease modeling, and personalized therapeutic strategies.
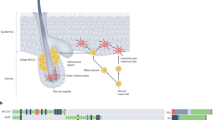
UV can only indirectly affect the internal organs, because for the most part, it does not penetrate the body any deeper than the skin. Therefore, in connection with continuous exposure to sunlight, the human skin is well-adapted to UV stress14,15. The basic adaptation can be found already in the skin structure, which is layered and includes the epidermis—the outermost layer with tightly arranged in layers keratinocytes; the dermis—rich in the intercellular matrix produced by numerous fibroblasts; and the subcutaneous layer—created by the fatty-rich adipocytes 11. Moreover, this structure is enriched with pigment cells located in the epidermis - melanocytes, whose main role is to other skin respond to UV radiation (Fig. 1). However, melanocytes’ excessive exposure to UV may cause disturbances in their functioning, thus carrying a profound risk of pigmentary changes, atrophy and even malignancy16. In addition to inducing oxidative stress, UV radiation triggers complex cellular and immunological adaptations in the skin. One of them is a phenomenon known as refractoriness, which refers to a reduced or altered responsiveness of skin cells upon repeated UV exposure. For example, it has been shown that melanocyte-stimulating factors, such as α-MSH, ET-1, or ACTH, can remain upregulated for several months to years following repeated UV exposure, long after visible pigmentation has faded17. UV radiation also alters the number and function of Langerhans cells in the epidermis, promoting their migration to lymph nodes and the induction of regulatory T cells (Tregs), which contribute to a state of local immunosuppression18,19. At the cellular level, UVB induces keratinocytes to release endothelin-1, which leads to a downregulation of E-cadherin expression in melanocytes, thereby disrupting their adhesion and intercellular communication20,21. These cumulative effects—reduced responsiveness, immune modulation, and disturbed melanocyte–keratinocyte interactions—contribute to an increased susceptibility of melanocytes to UV-induced oxidative damage and highlight the need for targeted protective strategies.
Scheme showing the depth of penetration of different types of UV radiation into the cells, creating various skin layers and the main changes in cell functioning caused by this radiation29. Figure created with BioRender.com.
Therefore, the aim of this review was to describe the oxidative damage caused in melanocytes mainly by UV radiation, as well as the possibilities and mechanisms of protecting these cells from the negative effects of UV-induced oxidative stress.
Melanocytes—morphology and function
Although present in the skin, melanocytes account for a relatively small percentage of its cellular constituents. They are localized in the epidermis, which is composed mostly of keratinocytes (~95%), and melanocytes are less than 5% of the epidermal layer22. The life cycle of skin melanocytes consists of several steps, including lineage specification from embryonic neural crest cells (melanoblasts), migration and proliferation of melanoblasts, differentiation of melanoblasts into melanocytes with dendritic morphology23. The maturation of melanocytes leads to melanin production in specific organelles—melanosomes and transport of melanosomes with melanin to keratinocytes24 (Fig. 2).
Melanocytes molecularly are recognizable by identification of melanocyte-specific proteins as tyrosinase (TYR), tyrosinase-related protein 1 and 2 (TRP1, TRP2/DCT), melanosomal matrix proteins (PMEL17, MART-1), microphthalmia transcription factor (MITF)25,26. However, melanocyte precursors, known as melanoblasts, are more difficult to identify since they don’t produce melanin and therefore don’t usually express those markers, although occasionally DCT and/or KIT are detectable as specific markers27.
The microscopic analysis indicates that mature melanocytes are thin, elongated cells with branched structures, consisting of a central cell body and long, numerous branches, or dendrites through which they interact with the adjacent keratinocytes of the basal epidermal layer28. Melanocytes are free of fibrillar material such as tonofibrils, desmosomes, and tonofilaments, typical for the keratinocyte. The intercellular bridges between them and the other cells of the epidermis are absent29. The nucleus of a melanocyte is smaller and more deeply basophilic than that of a basal keratinocyte. Dendrites, abundant mitochondria, and microfibrils are unique to melanocytes. The Golgi apparatus is usually prominent, and the endoplasmic reticulum is well developed30. The most characteristic organelle for melanocytes are melanosomes. They are produced intracellularly in several stages: stages I and II include unmelanized immature premelanosomes in the middle of the melanocyte, whereas stages III and IV contain melanized melanosomes migrating to the outer edge of the cell through the dendrites31. It is estimated that one melanocyte, through numerous dendritic extensions, interacts with ~36 keratinocytes. Although this value is similar independently of the human race, it varies between body areas32. In the epidermal basal layer, the ratio of melanocytes to keratinocytes is established at 1:1024. Moreover, this equilibrium persists throughout an individual’s lifespan, albeit the precise regulatory mechanisms remain undisclosed. Regardless of an individual’s racial background, approximately 1200 melanocytes are uniformly present per square millimeter of the skin28. The interaction between the dendritic extensions of differentiated melanocytes and keratinocytes is imperative for the transfer of melanin into keratinocytes, a process that governs skin color and contributes to the photoprotection of skin cells33. Adhesion molecules, namely E- and P-cadherins, play a crucial role in these cell-cell interactions34. Melanin granules accumulate above the nucleus of keratinocytes and are subsequently expelled with the exfoliation of epidermal cells. The precise molecular mechanisms underlying the transfer of melanosomes from melanocytes to keratinocytes remain a subject of ongoing investigation.
Melanocytes are a diverse group of cells in the human body that produce melanin35. They mostly reside in the epidermis, but large numbers of them can be found in hair follicles and in the eyes, where they produce dye for hair and eye pigmentation, respectively. However, the spectrum of their functions in the human body reaches far beyond that. For instance, these pigment-producing cells are found inside the inner ear, where they contribute to the hearing function, and in the heart, where they are involved in the electrical conductivity and support the stiffness of cardiac valves27. In the skin, melanin serves as a protective mechanism by absorbing UV radiation to shield keratinocytes from potential DNA damage. The composition and volume of melanin within an organism’s body are contingent upon several factors, including the differentiated state of melanocytes, the population of melanocytes, the extent of melanogenesis, dendritic characteristics, and various environmental elements such as the local tissue microenvironment, vascular supply, exposure to UV radiation, and ionizing radiation36. The whole process occurs inside melanosomes and begins with the translocation of the enzyme tyrosinase, which catalyzes the oxidation of tyrosine into dihydroxyphenylalanine (DOPA) and subsequently into dopaquinone (DQ)37 (Fig. 3). Further modifications, in conjunction with the creation of protein complexes, ultimately lead to the deposition of melanin into a fibrillar scaffold, primarily composed of altered PMEL17 (also known as gp100) molecules. Notably, PMEL17 serves as a significant marker for melanocytes38.
Melanocytes synthesize two distinct types of melanin: eumelanin, which is responsible for brown-black pigmentation, and pheomelanin, which imparts a red-yellow hue39. Despite originating from a shared metabolic pathway in which DQ plays a pivotal role as an intermediate, eumelanin and pheomelanin exhibit variations in their molecular size and overall characteristics40. Pheomelanin primarily comprises sulfur-containing benzothiazine and benzothiazole derivatives. The primary source of sulfur for pheomelanin synthesis is L-cysteine, making it an essential component of this process. In contrast, eumelanin is a highly diverse polymer made up of units such as 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA). Eumelanin effectively absorbs the energy of UV radiation; therefore, it is more photoprotective than pheomelanin. However, it has been shown that eumelanin induces ROS generation both dependently and independently of UV41.
Redox balance in melanocytes
ROS in melanocytes
ROS, which include both free radicals and their non-radical intermediates, are produced primarily by mitochondria and peroxisomes during normal cellular metabolic processes or because of exogenous prooxidant exposure42 (Fig. 4). In the case of an imbalance between the production of ROS in cells and the biological systems’ ability to detoxify these reactive products, oxidative stress occurs43. Moreover, it can be accentuated under pathological conditions, such as cancer or inflammation, as well as through exposure to external factors like chemicals or UV radiation. Therefore, the localization of melanocytes places them in constant contact with pro-oxidative factors, and excessive exposure to these factors means that melanocytes might be more exposed to oxidative stress than other cells17.
AP-1 activating protein-1, HIF-1α hypoxia-inducible factor-1α, NF-κB nuclear factor κ-light-chain-enhancer of activated B, NOX nicotinamide adenine dinucleotide phosphate oxidase, Nrf2 nuclear factor erythroid 2 related factor 2, PPARγ peroxisome proliferator-activated receptor γ, ROS reactive oxygen species, XO xanthine oxidase. Figure created with BioRender.com.
The term “ROS” encompasses a diverse array of oxidant molecules with varying properties and biological functions, ranging from cell signaling to causing cellular damage, as observed in the case of free radicals44. Free radicals, characterized by one or more unpaired electrons, exhibit high reactivity, and their generation in biological systems predominantly involves oxygen and nitrogen species, including hydrogen peroxide (H₂O₂), organic hydroperoxides, nitric oxide, superoxide, and hydroxyl radicals45. Under physiological conditions, ROS are primarily generated through a combination of enzymatic and non-enzymatic reactions46. Non-enzymatic production of ROS in melanocytes mainly occurs during normal cellular processes, such as mitochondrial respiration, where the electron transport chain plays a crucial role. As electrons are transferred along the respiratory chain complexes, small amounts of these electrons can prematurely interact with molecular oxygen, particularly at complexes I and III, leading to the formation of superoxide anion. Although this process is not fully efficient—it typically results in around 1–2% of consumed oxygen being converted into ROS, rather than being completely reduced to water47,48.
Beyond mitochondria, also cytosolic/membrane enzymes are important sources of ROS. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX family) are membrane-bound enzymes that generate superoxide by transferring electrons from NADPH to molecular oxygen. Depending on their localization, this process can occur on the plasma membrane or within intracellular organelles, contributing to ROS presence both in specific cellular compartments and throughout the cytoplasm49. Xanthine oxidase (XO), which participates in purine metabolism, is another key enzymatic source of ROS, producing both superoxide and H₂O₂ during the conversion of hypoxanthine to xanthine and xanthine to uric acid50.
Additionally, the skin cells neighboring melanocytes, mainly keratinocytes, have also been identified as a source of ROS via transferring H₂O₂ to melanocytes51. However, the most specific origin of ROS for melanocytes is processes related to melanin synthesis/metabolism52. As mentioned before, TYR converts tyrosine into DOPA, and subsequently DOPA to DQ—a distinctive orthoquinone that exhibits reactivity towards nucleophilic moieties, including thiol and amino groups. This catalytic cascade culminates in the production of superoxide anions. The DQ undergoes a transformative process to yield dopachrome via a redox-mediated exchange. Following spontaneous decarboxylation, dopachrome further divides its fate, giving rise to DHI, which undergoes oxidation to form indole quinone. Alternatively, it engenders DHICA following tautomerization mediated by TRP2. Subsequently, DHICA undergoes conversion into the corresponding quinone species53. However, TRP2 not only facilitates these biochemical conversions but also provides protection against oxidative stress by elevating intracellular glutathione (GSH) levels and mitigating the deleterious effects of quinones, thereby averting DNA damage initiated by free radicals.
Collectively, these sources of ROS are vital for maintaining cellular homeostasis, as they are involved in signaling pathways that regulate processes like cell growth, differentiation, and immune responses45. While ROS generation is a natural part of cellular metabolism, the balance between ROS production and antioxidant defenses is key to preventing oxidative stress and ensuring proper cellular function54. It is noteworthy that the production of ROS in melanocytes can be amplified under pathological circumstances, exemplified by instances of inflammation and cancer55. Furthermore, exposure to extraneous factors such as UV radiation or various chemical agents may also contribute to this augmented ROS generation. Epidermal melanocytes, owing to their specialized role in melanin synthesis, exhibit a heightened susceptibility to elevated ROS production, a phenomenon accentuated by solar exposure during the tanning process and instances of inflammation, subsequently culminating in post-inflammatory hyperpigmentation17. Consequently, oxidative stress has the potential to disrupt the equilibrium within melanocytes, causing significant metabolic disorders, including cells viability decrease or even their malignant metamorphosis56.
Intracellular antioxidant system of melanocytes
Due to the high risk of melanocytes exposure to harmful and prooxidant factors of the external environment, these cells are characterized by naturally well-developed complex antioxidant systems, including enzymatic and non-enzymatic antioxidants (Fig. 5). Enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) play crucial roles in detoxifying ROS and maintaining redox balance within melanocytes46. SOD catalyzes the dismutation of the superoxide radical (O₂⁻) into H₂O₂ and oxygen57,58. This H₂O₂, though less reactive, is still potentially harmful and is further detoxified by CAT and GSH-Px, which break it down into water and oxygen, reducing its potential for cellular damage. In melanocytes, CAT primarily manages H₂O₂ degradation, providing a critical defense against oxidative stress57.
Basic antioxidant system of skin melanocytes. CAT catalase, ERK extracellular signal-regulated kinase, GSH glutathione, GSH-Px glutathione peroxidase, GSSG-R glutathione reductase, JNK c-Jun N-terminal kinase, MSH melanocyte-stimulating hormone, Nrf2 nuclear factor erythroid 2 related factor 2, ROS reactive oxygen species, SOD superoxide dismutase, Trx thioredoxin, Trx-R thioredoxin reductase. Figure created with BioRender.com.
Non-enzymatic antioxidants like ascorbic acid, GSH, and α-tocopherol, complement enzymatic systems in protecting against oxidative damage46. GSH, a critical thiol-based antioxidant, is synthesized by glutamate cysteine ligase (GCL) and glutathione synthase. Recycling of oxidized GSH (GSSG) back to its reduced form is facilitated by glutathione reductase (GSR) using NADPH from the pentose phosphate pathway. Glutathione-S-transferases also contribute to detoxification by conjugating harmful compounds with GSH, which supports melanocyte survival under oxidative stress59. The thioredoxin (Trx)-based system is another crucial antioxidant pathway in melanocytes, composed of Trx, thioredoxin reductase (Trx-R), and NADPH. This system balances redox status by transferring electrons from NADPH to Trx-R, which then reduces disulfide bonds in Trx, allowing it to restore functional thiol groups on various target proteins60,61. Trx-R, a homodimeric enzyme with a regulatory selenocysteine residue, efficiently reduces H₂O₂ and other peroxides, thus protecting melanocytes from oxidative damage. The Trx-based system also regulates redox-sensitive transcription factors such as p53, which, when activated by oxidative stress, supports cell survival mechanisms in conditions with high ROS. Calcium-mediated allosteric control further modulates Trx activity, enabling dynamic responses to redox changes62. Moreover, melanin itself provides some antioxidant protection by acting as a free radical scavenger63.
The level of antioxidative proteins depends largely on the degree of gene expression, which in turn is inextricably linked to the activity of transcription factors. One of the central regulatory pathway in redox balance is the Kelch-like ECH-Associating protein 1 (Keap1)—nuclear factor erythroid 2 related factor 2 (Nrf2)—antioxidant response element (ARE) pathway, which upregulates biosynthesis of antioxidant proteins, including antioxidant enzymes such as heme oxygenase-1 (HO-1), glutathione S-transferase, NAD(P)H:quinone oxidoreductase 1 (NQO1), glutathione reductase (GR), Trx-R, CAT, and SOD, but also nonenzymatic antioxidant proteins, e.g., Trx or anti-apoptotic proteins from Bcl-2 family64. Under non-stress conditions, Nrf2 remains tethered to Keap1, which promotes its ubiquitination and degradation, keeping Nrf2 activity low65,66. During oxidative stress, modifications in cysteine residues on Keap1 release Nrf2, allowing it to translocate into the nucleus, bind to AREs in target genes, and initiate transcription of essential antioxidant genes67. The transcriptional activity of Nrf2 is also based on the level of this molecule phosphorylation68. In melanocytes, depending on the type of kinase and cell line, so far it has been proven that kinases play a dual role in Nrf2 activity regulation, e.g., increased activity of JNK (c-Jun N-terminal kinase) enhances Nrf2 expression in melanocytes line PIG1 but reducing its activation PIG3V cells69. This highlights how dysregulated JNK activity can impair oxidative stress defense mechanisms, potentially contributing to pathologic conditions like vitiligo70. Similarly, ERK (extracellular signal-regulated kinase) and its involvement in melanin synthesis via the ERK/CREB (cAMP response element binding protein) pathway demonstrate how kinases contribute not only to antioxidant defense but also to pigmentation regulation by upregulating MITF and TRP169,71. Moreover, also HO-1, a downstream target of Nrf2, is also essential for antioxidative defense in melanocytes. Enhanced JNK/Nrf2/HO-1 pathway mitigates oxidative stress by promoting melanin synthesis69.
Additionally, another transcription factor, AP-1 (activating protein-1) is significantly involved in the cellular stress response of melanocytes. Activated by ROS, AP-1 regulates gene expression related to oxidative stress and inflammation72. Consisting of dimeric transcription factors such as Jun and Fos, AP-1 binds to DNA motifs, such as TPA-responsive element (TRE) and cAMP-responsive element (CRE), initiating transcription of genes critical for cell survival and stress adaptation73,74. Moreover, apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE-1/Ref. 1) further supports this response by upregulating AP-1, enhancing melanocyte resilience to oxidative stress75.
In addition to these mechanisms, oxidative stress activates multiple MAPK pathways, including p38 MAPK, JNK, and ERK1/2. Most of them can phosphorylate Nrf2, leading to its activation. However, JNK and p38 MAPK in melanocytes might also promote mitochondrial dysfunction and apoptosis under ROS exposure76,77. Interestingly, PKCβ (protein kinase C beta) has been identified as a contributor to oxidative stress response and melanin biosynthesis in melanocytes, enhancing ROS production via mitochondrial pathways. Its role in UV-induced ROS generation underscores its contribution to both pigmentation and oxidative damage. However, in melanoma, PKCβ downregulation limits oxidative stress, providing a survival advantage under such conditions78.
On the other hand, paracrine factors also play an important role in melanocyte defense. Endothelin-1 (ET-1), produced by keratinocytes, reduces the generation of H₂O₂ in melanocytes, protecting them from UV-induced oxidative stress17. Additionally, melanocortins such as α-melanocyte-stimulating hormone (α-MSH), produced by both keratinocytes and melanocytes, activate the melanocortin-1 receptor (MC1R) on melanocytes, further enhancing their antioxidant capacity. This activation reduces H₂O₂ levels, increases CAT activity, and upregulates transcription of antioxidant genes, including HO-1, which help maintain redox homeostasis17.
Moreover, α-MSH enhances DNA repair mechanisms by increasing the activity of base excision repair enzymes like OGG1 and APE-1, both of which are crucial for addressing oxidative DNA damage17. The transcription factor p53 also plays a dual role in melanocyte defense by upregulating tyrosinase, a key enzyme in melanogenesis, and enhancing the antioxidant response via the Nrf2 pathway17, but also it can stop the melanocyte cell cycle, even leading to apoptosis79.
To summarize, melanocytes employ a multifaceted antioxidant defense system, involving both intrinsic enzymes and external signaling pathways, to protect themselves from oxidative stress. This balance is crucial for maintaining their function and survival in the face of ongoing oxidative challenges17,80,81. However, in some extreme cases, this is not enough, and human skin cells require additional external protection.
Effects of chronic oxidative stress on melanocytes
Extensive research during the past two decades has revealed that continued oxidative stress can lead to chronic inflammation, which mediates most chronic diseases, including cancer, diabetes, cardiovascular, neurological, and pulmonary diseases82. As a result of pro-oxidative conditions, a variety of transcription factors, including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), AP-1, p53, hypoxia-inducible factor-1 (HIF-1α), peroxisome proliferator-activated receptor gamma (PPARγ), β-catenin/Wnt, and Nrf2, are activated83,84,85,86 (Fig. 4). The stimulation of these transcription factors can lead to the expression of over 500 different genes, including those for growth factors, inflammatory cytokines, chemokines, cell cycle regulatory molecules, and anti-inflammatory molecules. As a result, oxidative stress activates inflammatory pathways, leading to the transformation of a normal cell into a tumor cell, increasing tumor cell survival, proliferation, chemoresistance/radioresistance, invasion, and angiogenesis82.
Simultaneously, oxidative stress increases lipid metabolism and lipid peroxidation, leading to oxidative fragmentation and cyclization of lipid hydrocarbon chains54. Reactive intermediates react mainly with polyunsaturated fatty acids (PUFAs), which leads to lipoperoxyl radicals’ generation, that further interact with lipids, forming lipid radicals and lipid hydroperoxides. These hydroperoxides are unstable and can produce new radicals, resulting in a cascade of oxidative reactions87. Finally, as products of lipid peroxidation appear reactive aldehydes (such as malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE)) and as well as cyclisation products, including isoprostanes, that have garnered attention as significant signaling molecules88. Mentioned reactive aldehydes, as well as isoprostanes, particularly F2-isoprostanes, are bioactive compounds formed via nonenzymatic peroxidation of arachidonic acid (AA), serving as reliable biomarkers for assessing oxidative stress status in various clinical conditions89. The processes of lipid peroxidation leading to the generation of lipid peroxidation products can disrupt membrane integrity, fluidity and permeability, potentially leading to inflammatory responses and cell dysfunction90.
Oxidative conditions also promote the activation of enzymes involved in lipid metabolism, leading to the generation of lipid mediators significant for cell functioning91. As a result of ROS-induced phospholipase A2 (PLA2), AA is released from phospholipids92. AA is a basic precursor of numerous lipid-based signaling molecules and can be further transformed by cyclooxygenases (COX), lipoxygenases (LOX) and cytochrome p450 (CYP450) to form eicosanoids, further influencing redox balance and inflammation by engaging specific receptors93. Additionally, phospholipids are metabolized by enzymes such as N-acyltransferase (NAT), diacylglycerol lipase (DAGL), and N-acylphosphatidylethanolamine phospholipase D (NAPE-PLD) into endocannabinoids94. Received under oxidative stress, eicosanoids and endocannabinoids are known mainly as modulators of inflammation and apoptosis in human skin cells, including melanocytes95,96,97.
In addition to lipids, proteins are also susceptible to oxidative modifications due to ROS and reactive nitrogen species (RNS). Oxidation of specific amino acid residues can lead to various forms of modifications, such as sulfonylation, nitration, or even glutathionylation. These modifications can change the conformation of proteins, resulting in dysfunction and the accumulation of misfolded proteins in the cytoplasm, which are implicated in cellular aging, apoptosis, and necrosis89. For instance, nitrotyrosine, a stable biomarker of oxidative stress, forms through a two-step nitration process involving tyrosine residues and is associated with several inflammatory diseases89. In melanocytes, nonspecific sulfonylation induced by oxidative stress disrupts cells adhesion and interactions with neighboring keratinocytes98. On the other hand, UVB-induced tyrosine nitration in melanocytes stimulates main photosensitizers, including methylene blue and pheomelanin, to reversely enhance ROS generation99. Additionally, protein can also be modified by the products of lipid peroxidation, including 4-HNE and MDA100. In melanocytes 4-HNE, through adduct formation, alters protein function, contributing to oxidative stress-induced cellular dysfunction, which mainly concerns on the functioning of cellular antioxidant system, acceleration of protein degradation, inhibition of melanocyte proliferation, photoaging processes, but also disruption of the intercellular interactions between melanocytes and keratinocytes101,102,103.
Furthermore, oxidative stress can induce damage to DNA, which is particularly concerning in the context of carcinogenesis. The generation of oxidative DNA lesions, such as 8-oxoguanine (8-oxoG), is linked to the development of various cancers, including melanoma, while the accumulation of these lesions can compromise genomic stability, leading to mutations and the eventual transformation of normal cells into malignant ones53. Cancer initiation and progression have been linked to oxidative stress through mechanisms that increase DNA mutations or induce DNA damage, genome instability, and cell proliferation82.
Non-coding RNAs as cytoprotective agents in the UV-induced stress response of melanocytes
Exposure of melanocytes to UV radiation triggers a complex regulatory network involving various non-coding RNAs (ncRNAs), including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), which play crucial roles in cytoprotection against oxidative stress. Different UV wavelengths (UVA, UVB, and UVC) elicit distinct ncRNA expression patterns, reflecting tailored responses in melanocytes compared to other skin cells104. UVA predominantly alters lncRNAs linked to TGF-β signaling and collagen metabolism in fibroblasts, implicating pathways that may also support melanocyte survival through melanocyte–keratinocyte crosstalk104. UVB, especially narrow-band UVB (NB-UVB), modulates key miRNAs such as miR-16, miR-145, miR-146a, and miR-155, downregulating pro-inflammatory and cell cycle arrest signals to promote melanocyte proliferation and repigmentation, as used therapeutically in vitiligo104. miRNAs like miR-148a and miR-340 regulate pigmentation by modulating MITF and dendrite formation, while miR-21 enhances anti-apoptotic and proliferative pathways in melanocytes post-UVA exposure, indicating multifaceted roles in pigmentation and survival104,105. Moreover, miRNAs are also involved in intercellular communication, with keratinocyte-derived exosomal miR-203 and miR-3196 influencing melanogenesis, and melanocyte-derived exosomal miRNAs participating in stress response and potentially melanoma initiation104,106.
UVC exposure predominantly activates lncRNAs that cooperate with p53 to regulate DNA damage response, apoptosis, and cell cycle arrest, vital for maintaining melanocyte genome integrity under severe UV stress104. Among lncRNAs, MALAT1, lincRNA-p21, TUG1, and H19 have been identified as key modulators: MALAT1 associates with UVB-induced senescence and photoaging in dermal fibroblasts and may have analogous roles in melanocytes, while lincRNA-p21 mediates p53-dependent apoptosis and cell cycle arrest to prevent malignant transformation107. TUG1 suppresses UVB-induced inflammation and melanogenesis by downregulating IL-6 and TNF-α, balancing pigmentation and inflammatory responses104. H19 is downregulated after UVB exposure, leading to enhanced melanogenesis via paracrine signaling, illustrating the dynamic regulation of pigmentation104. Furthermore, circRNAs like ciRS-7 act as miRNA sponges, facilitating melanogenic signaling through the STAT3–AKT–FGF2 axis, thereby integrating ncRNA networks in pigmentation and cytoprotection104,108.
UV radiation-induced ncRNAs also regulate cellular senescence and autophagy, which is critical for mitigating oxidative damage. For example, miR-23a inhibits autophagy to promote senescence but also protects from DNA damage and apoptosis, highlighting a dual cytoprotective role109. Other miRNAs, like miR-15, miR-20a/b, or miR-101 modulate cell cycle and senescence pathways, often targeting epigenetic regulators like EZH2, thus contributing to UV-induced aging and tumor suppression mechanisms109. Importantly, many ncRNAs act by sponging or modulating each other’s availability, creating regulatory networks that fine-tune responses to oxidative stress in melanocytes and neighboring skin cells104,109.
Taken together, these studies underscore that UV radiation-driven activation of ncRNAs forms a multilayered cytoprotective system in melanocytes, orchestrating pigmentation, inflammation control, DNA repair, and survival signaling. This network helps melanocytes cope with oxidative stress while preserving skin homeostasis and preventing malignant transformation104,107,109,110. Understanding the precise roles of ncRNAs in this context opens avenues for targeted therapies in pigmentation disorders and melanoma prevention.
Oxidative stress-induced disorders in melanocytes
Oxidative stress plays a pivotal role in the pathogenesis of various diseases resulting from disturbed melanocyte metabolism. The cellular and molecular changes triggered by ROS, as previously discussed, disrupt melanocyte homeostasis and contribute to pathological conditions111. These alterations can lead to a spectrum of disorders, ranging from pigmentary abnormalities like vitiligo to malignancies such as melanoma (Fig. 4). This chapter explores the diseases associated with oxidative stress in melanocytes, highlighting their underlying mechanisms and clinical implications.
Vitiligo
Vitiligo is an acquired depigmentation disorder characterized by distinct patches of skin where melanocytes, the pigment-producing cells, are destroyed due to autoimmune mechanisms112. Oxidative stress plays a central role in this melanocyte destruction, with elevated levels of ROS, such as H₂O₂, and deficiencies in key antioxidant enzymes creating a toxic environment for these cells. In vitiligo patients, melanocytes are particularly sensitive to oxidative damage, which disrupts TYR function, inhibits eumelanin synthesis, and ultimately impairs pigmentation46. A reduction in CAT and other antioxidants, such as GSH-Px activity, contributes to this stress by limiting the melanocytes’ ability to neutralize H₂O₂, which accumulates in the skin and further damages cellular systems17. High levels of H₂O₂ inactivate protective peptides like ACTH and α-MSH, while decreasing enzyme activities like methionine sulfoxide reductase, consequently intensifying oxidative damage and accelerating melanocyte death46.
Disruption of key proteins due to oxidative stress leads to melanocyte detachment from keratinocytes, which is essential for their survival, with disrupted cell adhesion proteins such as E-cadherin exacerbating melanocyte loss and disease progression113. ROS damage cellular lipids, proteins, and DNA, also triggering further immune responses that target melanocytes. In response to elevated ROS, melanocytes display reduced repair and antioxidative capacities through dysfunction of Nrf2-based pathways, diminishing their defense against oxidative damage56. Deficiencies in the Nrf2 factor not only increase susceptibility to oxidative stress but also prevent melanocytes from maintaining proper redox balance, leading to sustained ROS production and greater melanocyte vulnerability113.
Under continuous oxidative stress, cellular organelles like mitochondria and the endoplasmic reticulum (ER) are damaged. As a result of mitochondrial membrane disruption, proapoptotic factors, such as cytochrome C, are released, leading to the caspase-3 activation and apoptosis induction114. Similarly, ROS-induced ER stress triggers the unfolded protein response (UPR), which leads to further cell apoptosis and the immune system targeting melanocytes56. Other pathways implicated, explaining the development of vitiligo, include autophagy and ferroptosis, where oxidative stress impacts the cells’ ability to clear damaged components, eventually leading to cell death and depigmentation17,46.
Furthermore, oxidative stress influences immune pathways in vitiligo by promoting the release of melanocyte autoantigens that are detected by local dendritic cells. This triggers adaptive immune responses involving melanocyte-specific T-cell infiltration, amplifying melanocyte destruction115. Consequently, oxidative stress acts not only as an initial trigger in vitiligo but also as a catalyst that, by generating autoantigens and inflammatory cytokines, perpetuates autoimmune responses and drives disease progression17,46.
Melanoma
Melanoma is a highly aggressive skin cancer originating from melanocytes115,116. Characterized by its potential to metastasize and adapt to various environmental conditions, melanoma poses significant treatment challenges, often evading immune responses and developing resistance to pharmacological interventions117. A key factor in the formation and progression of melanoma are ROS and oxidative stress, primarily induced by UV radiation exposure.
The relationship between ROS and melanoma is multifaceted. UV radiation, especially UVA and UVB, triggers ROS production in melanocytes, leading to oxidative DNA damage that can initiate oncogenic mutations. Cells with genetic predispositions, such as those with mutations in the MC1R gene or lower melanin levels, exhibit impaired DNA repair mechanisms, increasing their susceptibility to melanoma development54. Although eumelanin can provide some protective effects against UV-induced damage, pheomelanin may exacerbate ROS production, leading to further oxidative stress and DNA damage in melanocytes, particularly under UV exposure118. This oxidative damage is evidenced by elevated levels of 8-hydroxydeoxyguanosine, indicating compromised DNA integrity that facilitates mutations in melanoma-related genes like BRAF and loss of tumor-suppressor functions119.
Moreover, chronic oxidative stress can lead to an inflammatory environment that enhances tumor growth and metastasis. Immune cells, such as macrophages, are recruited to the site of tumor growth, and their activity further increases ROS production, contributing to a positive feedback loop that sustains inflammation and supports melanoma cell survival71. Additionally, the remodeling of the extracellular matrix (ECM) facilitated by ROS enhances tumor invasion, allowing melanoma cells to disseminate more effectively56.
As melanoma develops, it exhibits adaptive responses to the high levels of ROS. Melanoma cells upregulate their antioxidant defenses to maintain ROS at levels that support survival and proliferation while avoiding cell death. This balance is crucial, as moderate ROS levels can activate pro-tumorigenic signaling pathways, including the PI3K/AKT and NF-κB pathways, promoting cell survival, proliferation, and invasion119,120. Interestingly, although ROS can drive tumor progression, they also have the potential to induce apoptosis when levels become excessively high, highlighting the dual nature of ROS in melanoma pathophysiology121,122. Additionally, the resistance of melanoma cells to therapies is also closely tied to their redox status. Treatment options, including chemotherapy and radiotherapy, often fail due to the upregulation of antioxidant responses in melanoma cells, allowing them to survive at elevated ROS levels. Therefore, therapeutic strategies that manipulate ROS levels, either by increasing oxidative stress to promote cell death or by targeting antioxidant pathways to inhibit tumor growth, are being explored118.
In conclusion, the interplay between ROS and oxidative stress is central to the formation, progression, and treatment resistance of melanoma. As research continues to unravel the complexities of ROS in melanoma biology, it becomes increasingly clear that understanding and targeting these pathways could hold the key to improving treatment outcomes for this formidable cancer.
Melasma
Oxidative stress that occurs in melanocytes also significantly contributes to the development of melasma, a skin disorder characterized by hyperpigmentation on sun-exposed areas of the skin123. UV radiation increases the production of ROS in melanocytes, leading to oxidative stress that activates melanogenesis through the stimulation of molecules like α-MSH124. This stress also disrupts ciliogenesis, impairing primary cilia on melanocytes, which normally regulate pigmentation125. The downregulation of Nrf2, a key antioxidant regulator, reduces the cell’s ability to manage oxidative stress, further promoting melanogenesis125. Additionally, oxidative stress increases TYR activity and enhances melanosome transfer to keratinocytes, which further intensifies melanin synthesis and skin pigmentation124,125. In summary, oxidative stress triggers increased melanogenesis and hyperpigmentation in melasma by disrupting antioxidant defenses, ciliogenesis, and pigmentation pathways.
Skin inflammation and post-inflammatory hyperpigmentation
Overproduction of ROS in melanocytes is also responsible for activation of signaling pathways such as NF-κB, MAPK, and JAK/STAT, leading to increased expression of pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α. These cytokines disrupt skin homeostasis, promoting keratinocyte hyperproliferation, vascular permeability, and immune cell infiltration, which are hallmarks of inflammatory skin conditions124,125. Moreover, oxidative damage to mitochondrial DNA (mtDNA) results in the release of mtDNA into the cytosol, where it triggers Toll-like receptor 9 (TLR9) and inflammasome activation, leading to further production of pro-inflammatory cytokines and recruitment of immune cells67. Additionally, oxidative stress-induced lipid peroxidation compromises membrane integrity, increasing cellular permeability, and perpetuating inflammatory signaling124. Furthermore, protease-activated receptor 2 (PAR2) signaling in melanocytes amplifies oxidative and inflammatory responses through the Akt/NF-κB axis, elevating cytokine production and reducing antioxidant defenses, based on CAT and SOD activity, exacerbating skin inflammation126. In addition, dysregulated mitochondrial biogenesis and excessive ATP release under oxidative conditions act as damage-associated molecular patterns (DAMPs), activating inflammasomes and enhancing chemotactic signals for immune cells like CD8 + T cells67.
These processes collectively disrupt the skin barrier, impair pigmentation regulation, and contribute to chronic inflammatory conditions, emphasizing the interplay between oxidative stress and inflammation in melanocytes127,128. Addressing these pathways through antioxidant interventions offers potential therapeutic avenues for mitigating oxidative stress-induced inflammation in skin disorders.
Post-inflammatory hyperpigmentation (PIH) is an acquired condition resulting from overproduction or abnormal release of melanin following inflammation or injury, commonly affecting individuals with Fitzpatrick skin types IV–VI due to heightened melanocyte reactivity129. Oxidative stress plays a critical role in its pathogenesis, as ROS and RNS produced during inflammation stimulate melanocyte proliferation and melanin synthesis, primarily via upregulation of TYR activity130. This leads to increased production and transfer of melanosomes to keratinocytes, with inflammatory mediators like leukotrienes and prostaglandins further exacerbating the condition129. PIH can present as tan to dark brown lesions in the epidermis or blue-gray discoloration in the dermis, with UV exposure worsening its severity by promoting melanogenic activity131.
Natural protection of melanocytes functioning
Currently, to ensure the proper functioning of melanocytes, several natural compounds to eliminate the effects of oxidative stress, UV irradiation, and cell aging are used in cosmetology and dermatology130,131,132. These compounds can be classified as antioxidants, such as polyphenols, vitamins, either phytocannabinoids or even melatonin (Fig. 6). Antioxidants play a pivotal role in neutralizing ROS and preventing oxidative damage to melanocytes, which is crucial for maintaining skin pigmentation and reducing the risk of disorders like vitiligo or melanoma (Table 2). In this section, we will explore the mechanisms through which these natural antioxidants protect melanocytes, focusing on their ability to mitigate cellular damage, enhance melanocyte survival, and promote a balanced oxidative environment. While this review focuses on the protective effects of natural compounds on melanocytes under oxidative stress, it is worth noting that many of these molecules also play broader roles in maintaining overall skin health. For instance, flavonoids, carotenoids, and certain plant-derived polyphenols exert antioxidant and anti-inflammatory effects not only in pigment cells but also in keratinocytes and fibroblasts. Such actions contribute to improved skin barrier function, enhanced wound healing, and delayed skin aging123,133. Moreover, some small drug-like molecules and non-coding RNAs modulate skin rejuvenation pathways, suggesting their potential application in promoting skin longevity beyond pigmentary regulation134,135,136.
AP-1 activating protein-1, CAT catalase, CB cannabinoid receptors, GSH glutathione, GSH-Px glutathione peroxidase, GSSG-R glutathione reductase, NF-κB nuclear factor κ-light-chain-enhancer of activated B, Nrf2 nuclear factor erythroid 2 related factor 2, Ref. 1 redox effector factor 1, ROS reactive oxygen species, SOD superoxide dismutase, Trx thioredoxin, Trx-R thioredoxin reductase. Figure created with BioRender.com.
Polyphenols
Polyphenols, a diverse group of secondary plant metabolites, are essential in neutralizing oxidative stress in melanocytes, cells particularly prone to oxidative damage due to their role in melanin synthesis137,138,139. These compounds are characterized by aromatic rings and hydroxyl groups, which allow them to scavenge ROS and stabilize free radicals, thereby protecting DNA, proteins, and lipids from oxidative damage140,141. Flavonoids, a prominent subclass of polyphenols, exhibit additional protective mechanisms. For example, quercetin and luteolin act as metal ion chelators, binding Fe²⁺ and Cu²⁺, which catalyze ROS production, thereby reducing oxidative stress within melanocytes141. These polyphenols also enhance melanocyte pigmentation by promoting TYR activity, a key enzyme in melanin biosynthesis, while simultaneously mitigating UV-induced damage142. Specific polyphenols, such as epicatechin and gallic acid, provide anti-inflammatory benefits by suppressing pro-inflammatory enzymes and cytokine synthesis, offering further protection for melanocytes137. Green tea catechins like epigallocatechin-3-gallate (EGCG) and grape seed proanthocyanidins (GSPs) exhibit photoprotective properties by absorbing UVA and UVB radiation, reducing DNA damage, and inhibiting UVB-induced cyclooxygenase-2 (COX-2) expression, which is critical in the development of skin tumors139.
In addition to their direct antioxidant properties, flavonoids like kaempferol and hyperoside demonstrate specific actions in melanocyte preservation. Kaempferol reduces ROS generation by upregulating HO-1, protecting melanogenic enzymes and enhancing the MITF pathway, essential for melanin synthesis143. Hyperoside stabilizes mitochondrial function and prevents apoptosis by activating the PI3K/AKT pathway, further supporting melanocyte viability under oxidative stress144. Quercetin-3-glucoside (isoquercetin), a glycosylated derivative of quercetin, enhances antioxidative defenses by activating Nrf2 and mitigating UVB-induced damage, making it relevant for managing both pigmentation disorders and melanoma145,146.
Polyphenols have dual roles depending on context: under non-stress conditions, they act as antioxidants; however, in melanoma cells, they can exert prooxidant effects. For instance, quercetin and luteolin at higher concentrations promote apoptosis and reduce proliferation in melanoma cells by generating ROS, thus potentially inhibiting tumor progression138,147,148. They also activate pathways such as Keap1/Nrf2/ARE, reinforcing cellular antioxidant defenses without direct ROS neutralization149. In vitiligo, flavonoids like luteolin, wogonin, and quercetin enhance melanocyte survival, reduce inflammation, and promote melanin production. Moreover, by targeting inflammatory pathways such as NF-κB and HIF-1, and reducing pro-inflammatory cytokines, mainly IL-6 and TNF-α, these compounds protect against melanocyte loss characteristic of this disorder150,151. Similarly, in melanoma prevention, EGCG and related polyphenols reduce immune suppression and inflammation by inhibiting STAT3, NF-κB, and nitric oxide synthesis, pathways linked to melanoma progression148.
The epigenetic and antiproliferative effects of polyphenols further enhance their therapeutic potential. Compounds such as EGCG and apigenin promote apoptosis in melanoma cells by modulating pathways like MAPK and PI3K-AKT, while green tea polyphenols inhibit histone deacetylase (HDAC) and activate histone acetyltransferase (HAT), reactivating tumor-suppressor genes silenced during melanoma progression138,148. Moreover, compounds like EGCG derivatives (e.g., AcEGCG) offer enhanced stability and therapeutic efficacy, particularly in reducing oxidative damage and inflammation in vitiligo models152. Additionally, EGCG, through endonuclease ERCC1 inhibition, decreases the DNA repair in human cancer cells, through synergizes the therapeutic effect of anticancer drugs (e.g., cisplatin, irinotecan)153,154. This action can be supported independently through direct DNA damage by the EGCG155, which is described as highly specific because it does not occur in non-cancer cells, as well as EGCG can reduce oxidative DNA damage in human leukocytes156. However, these studies include only a small group of cells, so it is difficult to ensure the cancer-selective effect of this polyphenol. On the other hand, similar DNA protective action has been found also in the case of other polyphenols, e.g., quercetin, that decreased the levels of 8-hydroxydeoxyguanosine in kidney or liver cells following their exposure to oxidative stress157,158,159.
Advances in nanotechnology have also improved flavonoid delivery to melanoma cells, enhancing their stability and effectiveness in preclinical models, though regulatory challenges remain for clinical applications148. Collectively, these properties position polyphenols and flavonoids as key therapeutic agents for oxidative stress-related skin conditions like vitiligo and melanoma, emphasizing the need for further clinical validation148.
Phytocannabinoids
Phytocannabinoids, are a group of naturally occuring compounds obtained from the Cannabis plant, that effectively influence the functioning of the human endocannabinoid system, which is also well developed in skin cells160. The functioning of this system is based on the interaction of ligands (including endogenous endocannabinoids, e.g., anandamide, but also exogenous plant derivatives - phytocannabinoids) with specific endocannabinoid receptors, CB1/2. Depending on the type of interaction, various cellular effects might be observed, from the activation of the antioxidant system to the initiation of the biosynthesis of pro-inflammatory proteins161.
In the context of skin melanocytes, particularly cannabidiol (CBD) and cannabigerol (CBG) exert significant modulatory effects on the cellular redox state, primarily through antioxidant, receptor-mediated, and lipid-regulating mechanisms, which may influence melanoma development and oxidative stress mitigation162,163,164. Both compounds share a phenolic hydroxyl group in their structure, enabling them to directly neutralize ROS and interrupt chain reactions that propagate oxidative damage. Moreover, their hydrophobic alkyl side chains enhance cell membrane affinity, facilitating interactions with intracellular signaling pathways165.
CBD and CBG interact with the endocannabinoid system, targeting CB2 receptors expressed on melanocytes, which mediate antioxidant and anti-inflammatory effects. Activation of CB2 reduces ROS levels and inhibits pro-inflammatory cytokines, contrasting with CB1 receptor activation, which can exacerbate oxidative stress166. Moreover, through receptor-independent actions, CBD and CBG activate Nrf2, a transcription factor that upregulates antioxidant defenses such as SOD, CAT, and GSH-Px. This enhances the cell’s intrinsic capacity to counteract oxidative damage while preserving GSH, a crucial non-enzymatic antioxidant165.
Phytocannabinoids also influence TRP channels in melanocytes, particularly TRPV1 and TRPA1. CBD functions as a TRPV1 agonist, desensitizing the receptor and mitigating ROS-mediated overactivation of TRP channels, which could otherwise disrupt calcium signaling and contribute to oxidative stress167. TRPV1 modulation also indirectly influences melanogenesis and the production of protective pigments like melanin, which can shield against UV-induced oxidative damage160. Moreover, through PPARγ activation, both cannabinoids inhibit NF-κB-mediated inflammation and enhance Nrf2-coordinated antioxidant responses. This synergy between transcription factors reinforces cellular redox balance, counteracts oxidative DNA damage, and inhibits melanoma cell proliferation160.
Additionally, CBD and CBG regulate lipid peroxidation and phospholipid profiles altered by UVA exposure. They mitigate the oxidative damage to membrane phospholipids, such as phosphatidylcholine (PC) and sphingomyelin (SM), and restore ceramide (CER) synthesis, which promotes apoptotic pathways in melanoma cells without harming healthy melanocytes162. CBD further enhances phosphatidylserine (PS) internalization, reducing immunosuppressive effects and metastatic potential associated with oxidative damage168. CBD also has therapeutic effects based on its specific action against cancer cells. It has been found that CBD inhibits oral squamous cell carcinoma cells' growth, causing direct DNA damage167, simultaneously reducing UVB-induced DNA damage in HaCaT keratinocytes visible as decreased level of cyclobutane pyrimidine dimers and DNA double-strand breaks168. Collectively, the intricate molecular actions of CBD and CBG underscore their therapeutic potential in managing oxidative stress and melanocyte-related disorders, including melanoma, by targeting both receptor-dependent and independent pathways while preserving cellular integrity165,166.
Vitamins
Vitamin C
Vitamin C, also known as ascorbic acid, is an essential nutrient with significant antioxidative potential that plays a crucial role in skin health. It neutralizes oxidants from environmental pollutants and UV radiation, particularly within the epidermis, and works alongside other enzymatic and non-enzymatic antioxidants169. Vitamin C is primarily accumulated from the bloodstream and is found in high concentrations within skin cells, where it exists intracellularly170. This antioxidant not only helps to reduce oxidative stress from various sources but also inhibits melanin synthesis by reducing TYR activity, making it effective for treating hyperpigmentation conditions171. Interestingly, vitamin C can decrease baseline levels of lipid peroxidation (measured as malonaldehyde) while reducing GSH levels in the skin, suggesting a complex interplay of antioxidants within skin cells172. Additionally, optimal dosing of vitamin C is critical, as its antioxidant properties can vary with concentration, influencing its effectiveness173.
In vitro studies indicate that due to its antioxidative properties vitamin C protects melanocytes from oxidative stress-induced membrane degradation and apoptosis, as well as increases the cell survival rate174. Moreover, vitamin C increases synthesis and secretion of melanin175 and by the improvement of antioxidant defense capacity of melanocytes significantly contributes to melanogenesis prevention46. However, regardless of the positive effect of vitamin C on healthy melanocytes, in the case of neoplastic transformed cells, vitamin C at physiological levels promotes the development and metastasis of melanoma176.
Vitamin E
Vitamin E, specifically α-tocopherol, plays a vital role in protecting human melanocytes from oxidative damage. It has a molecular structure that allows it to act as a scavenger of free radicals produced during lipid peroxidation, thereby preventing oxidative damage to unsaturated fatty acids in cell membranes. This antioxidative potential arises from its ability to neutralize free radicals, particularly when regenerated by co-antioxidants like vitamin C and glutathione, which stabilize its effects and prevent the loss of its antioxidative capacity177.
It has been found that vitamin E in skin enhances the stability and uptake of carotenoids, such as lycopene and β-carotene and protects melanocytes from oxidative stress induced by ROS and UVA radiation178. When combined with carotenoids and vitamin C, vitamin E helps to protect melanocytes from UVA-induced DNA strand breaks, a form of oxidative damage that can lead to mitochondrial DNA mutations. This protective function is particularly significant as oxidative stress can inhibit cell growth, and the combination of vitamin E with carotenoids prevents the growth inhibition typically caused by increased melanin production178.
Additionally, α-tocopheryl ferulate (α-TF), a derivative of vitamin E, has been shown to effectively inhibit melanin production in both melanoma cells and normal melanocytes, offering a protective effect against UV-induced hyperpigmentation. Moreover, α-TF prevents oxidative DNA damage such as the formation of 8-hydroxy-2-deoxyguanosine (8-OHdG), highlighting its role in reducing oxidative stress in the skin177. Therefore, vitamin E has a strong antioxidative effect in human melanocytes by scavenging free radicals, stabilizing carotenoids, and protecting against UVA-induced oxidative damage. This ensures cellular growth, inhibits excessive melanin production, and helps to prevent DNA damage caused by oxidative stress177,178.
Vitamin A
Vitamin A, in its active form as all-trans-retinoic acid (ATRA), plays a crucial role in antioxidant defense mechanisms in the skin, particularly through its impact on melanocytes and modulation of oxidative stress. Although ATRA itself doesn’t act directly as an antioxidant, it functions as a potent transcriptional regulator that influences genes critical for oxidative stress responses. For example, ATRA upregulates the expression of Trx and its downstream target methionine sulfoxide reductase A (MSRA), both of which are essential for repairing oxidatively damaged proteins and maintaining cellular redox balance179,180. Moreover, ATRA activates the Nrf2-based pathway, as well as enhances skin resilience to oxidative stress by preventing Nrf2 degradation and modulating genes involved in antioxidant defense, such as SOD and CAT181.
Furthermore, retinyl esters stored in the epidermis provide a local reservoir of vitamin A, which supports skin health by countering UV-induced oxidative stress181. Under UV exposure, ATRA reduces the expression of proteins like E3 ligase Hrd1, sustaining Nrf2 activity and contributing to photoprotection and repair mechanisms essential for melanocytes. This mechanism highlights ATRA’s role in stabilizing skin health and cellular regeneration despite oxidative challenges182. Thus, while ATRA isn’t a direct antioxidant, its gene regulatory actions are vital in mitigating oxidative stress and supporting melanocyte function within the skin.
Vitamin B3
Nicotinamide (NAM), the amide form of vitamin B3, is a precursor of NAD⁺, a crucial coenzyme involved in maintaining redox balance, regulating energy metabolism, and facilitating DNA repair183. In melanocytes, NAM functions as a potent antioxidant that protects cells from oxidative stress, ultraviolet (UV) irradiation, and senescence. It neutralizes reactive species such as singlet oxygen and prevents lipid and protein oxidation, demonstrating stronger protective effects than nicotinic acid86. Furthermore, NAM enhances the activity of endogenous antioxidant enzymes, stabilizes cellular membranes, and inhibits apoptosis under oxidative conditions184. It also supports DNA repair in UV-irradiated melanocytes by upregulating essential repair genes such as SIRT1 and p53, while simultaneously activating the Nrf2 signaling pathway184,185. By restoring intracellular levels of NAD⁺ and ATP, NAM facilitates energy-dependent repair mechanisms, including nucleotide excision repair and PARP-1–mediated repair of UV-induced DNA damage, such as cyclobutane pyrimidine dimers (CPDs) and 8-oxoG185.
In aging melanocytes, their derivative nicotinamide mononucleotide (NMN), a direct biosynthetic precursor of NAD⁺, has been shown to increase NAD⁺ levels and reduce intracellular ROS levels. This effect is mediated through the downregulation of oxidative and melanogenic signaling pathways, including the cAMP and Wnt pathways, thereby contributing to the restoration of redox homeostasis186. Collectively, NAM supports melanocyte function by limiting both exogenous and endogenous oxidative insults while promoting cellular repair capacity and metabolic resilience.
Carotenoids
Carotenoids are liposoluble pigments found in plants, algae, and animals, classified into carotenes and xanthophylls, which exhibit diverse structures influencing their biological functions187. These pigments possess a polyene backbone with conjugated double bonds, giving them strong antioxidant capabilities by quenching singlet oxygen and scavenging free radicals188. Their antioxidative properties stem from neutralizing ROS via direct radical scavenging or by enhancing endogenous antioxidant defense mechanisms, such as the Nrf2 pathway, reducing oxidative cellular damage189. In the skin, carotenoids accumulate in membranes, contributing to photoprotection and cellular stabilization against UV-induced oxidative stress, working synergistically with vitamins C and E to enhance antioxidative capacity and protect against chronic oxidative damage189,190.
Furthermore, carotenoids not only mitigate ROS but also modulate cellular pathways like NF-κB, involved in inflammation and cancer development191. Their antioxidative and anti-inflammatory actions are beneficial in skin conditions such as vitiligo, where carotenoids, along with vitamin E, may prevent melanocyte destruction by reducing oxidative stress and inflammation. Carotenoids also increase skin resistance to UVB in erythema, potentially reducing phototherapy-induced erythema and stimulating repigmentation in sun-exposed areas, especially when paired with vitamin E191. In melanoma prevention and treatment, carotenoids from microalgae demonstrate cytotoxic, antiproliferative, and pro-apoptotic effects against melanoma in vitro and in vivo, including the inhibition of melanoma cell growth through caspase activation, modulation of pro-inflammatory signaling, and induction of cell cycle arrest187. Specific carotenoids like fucoxanthin and astaxanthin have shown promise in inducing apoptosis and reducing cell migration, highlighting their potential as adjuvants in melanoma therapy187,192. Moreover, in zeaxanthin-treated melanoma cells DNA internucleosomal fragmentation leading to cell cycle arrest and apoptosis were observed187, without impairing the cell viability of non-cancer melanocytes193, in relation to which the mentioned carotenoids have a photoprotective effect194. Despite these promising effects, caution is warranted regarding carotenoid supplementation, as compounds like β-carotene may increase cancer risks in certain populations, suggesting the need for further research into safe usage and formulations195.
Melatonin
Melatonin, a hormone synthesized primarily by the pineal gland and locally in skin cells, plays a critical role in protecting melanocytes from oxidative stress by acting as a potent antioxidant and modulating cellular defense mechanisms196. It directly scavenges ROS, including H₂O₂, superoxide anion, and nitric oxide, thus reducing oxidative damage197,198. Through its metabolites, such as AFMK and 6-OHM, melatonin enhances GSH levels, a key antioxidant, and mitigates UVB-induced GSH depletion198. It also activates the Nrf2 pathway, which upregulates antioxidant enzymes like CAT, SOD2, and HO-1, strengthening the cellular response to oxidative stress197,199.
Melatonin indirectly reduces oxidative stress in melanocytes by decreasing mitochondrial energy metabolism and oxidative phosphorylation via keratinocyte-mediated effects. Additionally, it regulates the MAPK signaling pathway by inhibiting the phosphorylation of ERK, JNK, and p38, further reducing ROS production200. Beyond its receptor-mediated effects, melatonin metabolites exhibit antioxidative properties independent of melatonin’s traditional pathways, adding to its protective capabilities200. This multifaceted antioxidant activity underscores melatonin’s potential for mitigating oxidative damage in melanocytes, particularly in the context of UV exposure and pigmentation disorders.
Conclusion
Oxidative stress, driving a cascade of molecular and cellular changes, emerges as a critical factor disrupting the function and integrity of melanocytes. The antioxidant defense system of melanocytes, when exposed to daily external prooxidant factors, including UV, has difficulty maintaining redox balance, which indicates the need for additional protective strategies. Natural antioxidants show promising potential in reducing oxidative conditions and consequently maintaining the physiology of melanocytes. Understanding these mechanisms broadens the understanding of the consequences of pigmentation disorders but also paves the way for innovative therapeutic approaches aimed at reducing oxidative stress in melanocytes.
Data availability
No datasets were generated or analysed during the current study.
References
Dąbrowska, A. K. et al. The relationship between skin function, barrier properties, and body-dependent factors. Ski. Res. Technol. https://doi.org/10.1111/srt.12424 (2018).
Mullenders, L. H. F. Solar UV damage to cellular DNA: from mechanisms to biological effects. Photochem. Photobio. Sci. https://doi.org/10.1039/c8pp00182k (2018).
Hart, P. H., Gorman, S. & Finlay-Jones, J. J. Modulation of the immune system by UV radiation: more than just the effects of vitamin D?. Nat. Rev. Immunol. https://doi.org/10.1038/nri3045 (2011).
de Gruijl, F. R. Skin cancer and solar UV radiation. Eur. J. Cancer https://doi.org/10.1016/s0959-8049(99)00283-x (1999).
D’Orazio, J., Jarrett, S., Amaro-Ortiz, A. & Scott, T. UV radiation and the skin. Int. J. Mol. Sci. https://doi.org/10.3390/ijms140612222 (2013).
Wenk, J. et al. UV-induced oxidative stress and photoaging. Curr. Probl. Dermatol. https://doi.org/10.1159/000060656 (2001).
Chen, J., Liu, Y., Zhao, Z. & Qiu, J. Oxidative stress in the skin: impact and related protection. Int. J. Cosmet. Sci. 43, 495–509 (2001). 2021.
Cadet, J., Douki, T. & Ravanat, J. L. Oxidatively generated damage to cellular DNA by UVB and UVA radiation. Photochem. Photobio. https://doi.org/10.1111/php.12368 (2015).
Kappes, U. P., Luo, D., Potter, M., Schulmeister, K. & Rünger, T. M. Short- and long-wave UV light (UVB and UVA) induce similar mutations in human skin cells. J. Invest. Dermatol. https://doi.org/10.1038/sj.jid.5700093 (2006).
Wang, P. W. et al. Comparison of the biological impact of UVA and UVB upon the skin with functional proteomics and immunohistochemistry. Antioxidants https://doi.org/10.3390/antiox8120569 (2019).
Blair, M. J., Jones, J. D., Woessner, A. E. & Quinn, K. P. Skin structure-function relationships and the wound healing response to intrinsic aging. Adv. Wound Care https://doi.org/10.1089/wound.2019.1021 (2020).
Abdel-Naser, M. B., Krasagakis, K., Garbe, C. & Eberle, J. Direct effects on proliferation, antigen expression and melanin synthesis of cultured normal human melanocytes in response to UVB and UVA light. Photodermatol. Photoimmunol. Photomed. https://doi.org/10.1034/j.1600-0781.2003.00034.x (2003).
De Leeuw, S. M. et al. Melanin content of cultured human melanocytes and UV-induced cytotoxicity. J. Photochem. Photobio. B https://doi.org/10.1016/s1011-1344(01)00168-3 (2001).
Fahradyan, A. et al. Updates on the management of non-melanoma skin cancer (NMSC). Healthcare https://doi.org/10.3390/healthcare5040082 (2017).
de Gruijl, F. R. UV adaptation: pigmentation and protection against overexposure. Exp. Dermatol. https://doi.org/10.1111/exd.13332 (2017).
Mohania, D. et al. Ultraviolet radiations: skin defense-damage mechanism. Adv. Exp. Med. Biol. https://doi.org/10.1007/978-3-319-56017-5-7 (2017).
Denat, L., Kadekaro, A. L., Marrot, L., Leachman, S. A. & Abdel-Malek, Z. A. Melanocytes as instigators and victims of oxidative stress. J. Invest. Dermatol. https://doi.org/10.1038/jid.2014.65 (2014).
Schwarz, T. Mechanisms of UV-induced immunosuppression. Keio J. Med. https://doi.org/10.2302/kjm.54.165 (2005).
Loser, K. & Beissert, S. Regulatory T cells: banned cells for decades. J. Invest. Dermatol. https://doi.org/10.1038/jid.2011.375 (2012).
Jamal, S. & Schneider, R. J. UV-induction of keratinocyte endothelin-1 downregulates E-cadherin in melanocytes and melanoma cells. J. Clin. Invest. https://doi.org/10.1172/JCI13729 (2002).
Keswell, D., Kidson, S. H. & Davids, L. M. Melanocyte migration is influenced by E-cadherin-dependent adhesion of keratinocytes in both two- and three-dimensional in vitro wound models. Cell Biol. Int. https://doi.org/10.1002/cbin.10350 (2015).
Thingnes, J., Lavelle, T. J., Hovig, E. & Omholt, S. W. Understanding the melanocyte distribution in human epidermis: an agent-based computational model approach. PLoS ONE https://doi.org/10.1371/journal.pone.0040377 (2012).
Mirea, M. A., Eckensperger, S., Hengstschläger, M. & Mikula, M. Insights into differentiation of melanocytes from human stem cells and their relevance for melanoma treatment. Cancers https://doi.org/10.3390/cancers12092508 (2020).
Yamaguchi, Y., Brenner, M. & Hearing, V. J. The regulation of skin pigmentation. J. Biol. Chem. https://doi.org/10.1074/jbc.R700026200 (2007).
Cichorek, M., Wachulska, M., Stasiewicz, A. & Tymińska, A. Skin melanocytes: biology and development. Postepy Dermatol. Alergol. https://doi.org/10.5114/pdia.2013.33376 (2013).
Plonka, P. M. et al. What are melanocytes really doing all day long…?. Exp. Dermatol. https://doi.org/10.1111/j.1600-0625.2009.00912.x (2009).
Shi, X. et al. Increased melanin induces aberrant keratinocyte - melanocyte - basal - fibroblast cell communication and fibrogenesis by inducing iron overload and ferroptosis resistance in keloids. Cell Commun. Signal https://doi.org/10.1186/s12964-025-02116-z (2025).
Ali, S. S. & Naaz I. Muscle Cell and Tissue (InTech, 2015).
Le, L., Sirés-Campos, J., Raposo, G., Delevoye, C. & Marks, M. S. Melanosome biogenesis in the pigmentation of mammalian skin. Integr. Comp. Biol. https://doi.org/10.1093/icb/icab078 (2021).
Seiberg, M. Keratinocyte-melanocyte interactions during melanosome transfer. Pigment Cell Res. https://doi.org/10.1034/j.1600-0749.2001.140402.x (2001).
Rachmin, I., Ostrowski, S. M., Weng, Q. Y. & Fisher, D. E. Topical treatment strategies to manipulate human skin pigmentation. Adv. Drug Deliv. Rev. https://doi.org/10.1016/j.addr.2020.02.002 (2020).
Moreiras, H., Seabra, M. C. & Barral, D. C. Melanin transfer in the epidermis: the pursuit of skin pigmentation control mechanisms. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22094466 (2021).
Hirobe, T. Keratinocytes regulate the function of melanocytes. Dermatol. Sin. 32, 200–204 (2014).
Lee, U. J. et al. Light-triggered in situ biosynthesis of artificial melanin for skin protection. Adv. Sci. https://doi.org/10.1002/advs.202103503 (2022).
Nasti, T. H. & Timares, L. M. C. 1R, eumelanin and pheomelanin: their role in determining the susceptibility to skin cancer. Photochem. Photobiol. https://doi.org/10.1111/php.12335 (2015).
Dean, D. N. & Lee, J. C. Linking Parkinson’s disease and melanoma: interplay between α-synuclein and Pmel17 amyloid formation. Mov. Disord. https://doi.org/10.1002/mds.28655 (2021).
Singh, I. K., Espinosa, M. L., Lim, H. W. & Mohammad, T. F. A review of therapies for hyperpigmentation modulating the synthesis of eumelanin to pheomelanin. Arch. Dermatol. Res. https://doi.org/10.1007/s00403-024-03411-4 (2024).
Simon, J. D., Peles, D., Wakamatsu, K. & Ito, S. Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. https://doi.org/10.1111/j.1755-148X.2009.00610.x (2009).
Videria, I. F. S., Moura, D. F. L. & Magina, S. Mechanisms regulating melanogenesis. Bras. Dermatol. https://doi.org/10.1590/S0365-05962013000100009 (2013).
Kim, K. S., Lee, D., Song, C. G. & Kang, P. M. Reactive oxygen species-activated nanomaterials as theranostic agents. Nanomedicine https://doi.org/10.2217/nnm.15.108 (2015).
Pizzino, G. et al. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell Longev. https://doi.org/10.1155/2017/8416763 (2017).
Sies, H., Berndt, C. & Jones, D. P. Oxidative stress. Annu. Rev. Biochem. https://doi.org/10.1146/annurev-biochem-061516-045037 (2017).
Rahman, T., Hosen, I., Islam, M. T. & Shekhar, H. U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 3, 997–1019 (2012).
Diehl, C. Melanocytes and oxidative stress. Pigmentary Disord. https://doi.org/10.4172/JPD.1000127 (2014).
Burton, G. J. & Jauniaux, E. Oxidative stress. Best. Pr. Res Clin. Obstet. Gynaecol. https://doi.org/10.1016/j.bpobgyn.2010.10.016 (2011).
Mazat, J. P., Devin, A. & Ransac, S. Modelling mitochondrial ROS production by the respiratory chain. Cell Mol. Life Sci. https://doi.org/10.1007/s00018-019-03381-1 (2020).
Krause, K. H. Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp. Gerontol. https://doi.org/10.1016/j.exger.2006.10.011 (2007).
Xing, X., Dan, Y., Xu, Z. & Xiang, L. Implications of oxidative stress in the pathogenesis and treatment of hyperpigmentation disorders. Oxid. Med. Cell Longev. https://doi.org/10.1155/2022/7881717 (2022).
Pelle, E., Mammone, T., Maes, D. & Frenkel, K. Keratinocytes act as a source of reactive oxygen species by transferring hydrogen peroxide to melanocytes. J. Invest. Dermatol. https://doi.org/10.1111/j.0022-202X.2005.23661.x (2005).
Jenkins, N. C. & Grossman, D. Role of melanin in melanocyte dysregulation of reactive oxygen species. Biomed. Res. Int. https://doi.org/10.1155/2013/908797 (2013).
Snyman, M., Walsdorf, R. E., Wix, S. N. & Gill, J. G. The metabolism of melanin synthesis—from melanocytes to melanoma. Pigment Cell Melanoma Res. https://doi.org/10.1111/pcmr.13165 (2024).
Obrador, E. et al. Oxidative stress and antioxidants in the pathophysiology of malignant melanoma. Biol. Chem. https://doi.org/10.1515/hsz-2018-0327 (2019).
Battelli, M. G., Polito, L., Bortolotti, M. & Bolognesi, A. Xanthine oxidoreductase-derived reactive species: physiological and pathological effects. Oxid. Med. Cell Longev. https://doi.org/10.1155/2016/3527579 (2016).
Xuan, Y., Yang, Y., Xiang, L. & Zhang, C. The role of oxidative stress in the pathogenesis of vitiligo: a culprit for melanocyte death. Oxid. Med. Cell Longev. https://doi.org/10.1155/2022/8498472 (2022).
Wrześniok, D., Beberok, A., Otręba, M. & Buszman, E. Modulation of melanogenesis and antioxidant defense system in melanocytes by amikacin. Toxicol. Vitr. https://doi.org/10.1016/j.tiv.2013.02.002 (2013).
Demirci-Çekiç, S. et al. Biomarkers of oxidative stress and antioxidant defense. J. Pharm. Biomed. Anal. https://doi.org/10.1016/j.jpba.2021.114477 (2022).
Carpenter, E. L., Becker, A. L. & Indra, A. K. NRF2 and key transcriptional targets in melanoma redox manipulation. Cancers https://doi.org/10.3390/cancers14061531 (2022).
Carpenter, E. L. et al. Thioredoxin reductase 1 modulates pigmentation and photobiology of murine melanocytes in vivo. J. Invest. Dermatol. https://doi.org/10.1016/j.jid.2021.11.030 (2022).
Schallreuter, K. U. & Wood, J. M. Thioredoxin reductase - its role in epidermal redox status. J. Photochem. Photobiol. B https://doi.org/10.1016/s1011-1344(01)00235-4 (2001).
Lu, J. & Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. https://doi.org/10.1016/j.freeradbiomed.2013.07.036 (2014).
Rok, J. et al. Modulation of melanogenesis and antioxidant status of melanocytes in response to phototoxic action of doxycycline. Photochem. Photobiol. https://doi.org/10.1111/php.12497 (2015).
Gęgotek, A. & Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. https://doi.org/10.1007/s00403-015-1554-2 (2015).
Hseu, Y. C. et al. The in vitro and in vivo depigmenting activity of pterostilbene through induction of autophagy in melanocytes and inhibition of UVA-irradiated α-MSH in keratinocytes via Nrf2-mediated antioxidant pathways. Redox Biol. https://doi.org/10.1016/j.redox.2021.102007 (2021).
Jaramillo, M. C. & Zhang, D. D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. https://doi.org/10.1101/gad.225680.113 (2013).
Lin, Y. et al. The underestimated role of mitochondria in vitiligo: From oxidative stress to inflammation and cell death. Exp. Dermatol https://doi.org/10.1111/exd.14856 (2024).
Liu, T., Lv, Y. F., Zhao, J. L., You, Q. D. & Jiang, Z. Y. Regulation of Nrf2 by phosphorylation: consequences for biological function and therapeutic implications. Free Radic. Biol. Med. https://doi.org/10.1016/j.freeradbiomed.2021.03.034 (2021).
Yuan, J. et al. Paeoniflorin resists H2O2-induced oxidative stress in melanocytes by JNK/Nrf2/HO-1 pathway. Front. Pharm. https://doi.org/10.3389/fphar.2020.00536 (2020).
Becatti, M. et al. SIRT1 regulates MAPK pathways in vitiligo skin: insight into the molecular pathways of cell survival. J. Cell Mol. Med. https://doi.org/10.1111/jcmm.12206 (2014).
Peng, H. Y., Lin, C. C., Wang, H. Y., Shih, Y. & Chou, S. T. The melanogenesis alteration effects of Achillea millefolium L. essential oil and linalyl acetate: involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. PLoS ONE https://doi.org/10.1371/journal.pone.0095186 (2014).
Hayes, J. D., Dinkova-Kostova, A. T. & Tew, K. D. Oxidative stress in cancer. Cancer Cell https://doi.org/10.1016/j.ccell.2020.06.001 (2020).
Bejjani, F., Evanno, E., Zibara, K., Piechaczyk, M. & Jariel-Encontre, I. The AP-1 transcriptional complex: local switch or remote command?. Biochim Biophys. Acta Rev. Cancer https://doi.org/10.1016/j.bbcan.2019.04.003 (2019).
Karin, M., Liu, Z. G. & Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. https://doi.org/10.1016/s0955-0674(97)80068-3 (1997).
Sahakian, L. et al. APE1/Ref-1 as a therapeutic target for inflammatory bowel disease. Biomolecules https://doi.org/10.3390/biom13111569 (2023).
Lin, M. et al. Apigenin attenuates dopamine-induced apoptosis in melanocytes via oxidative stress-related p38, c-Jun NH2-terminal kinase and Akt signaling. J. Dermatol. Sci. https://doi.org/10.1016/j.jdermsci.2011.03.007 (2011).
Jin, R., Hu, W., Zhou, M., Lin, F. & Xu, A. Caffeic acid derivative WSY6 protects melanocytes from oxidative stress by reducing ROS production and MAPK activation. Heliyon https://doi.org/10.1016/j.heliyon.2024.e24843 (2024).
Voris, J. P. et al. Functional alterations in protein kinase C beta II expression in melanoma. Pigment Cell Melanoma Res. https://doi.org/10.1111/j.1755-148X.2009.00664.x (2010).
Choi, S. Y. et al. Exposure of human melanocytes to UVB twice and subsequent incubation leads to cellular senescence and senescence-associated pigmentation through the prolonged p53 expression. J. Dermatol Sci. https://doi.org/10.1016/j.jdermsci.2018.02.016 (2018).
Pinnell, S. R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. https://doi.org/10.1067/mjd.2003.16 (2003).
Otreba, M., Wrześniok, D., Beberok, A., Rok, J. & Buszman, E. Melanogenesis and antioxidant defense system in normal human melanocytes cultured in the presence of chlorpromazine. Toxicol. Vitr. https://doi.org/10.1016/j.tiv.2014.10.016 (2015).
Reuter, S., Gupta, S. C., Chaturvedi, M. M. & Aggarwal, B. B. Oxidative stress, inflammation, and cancer: how are they linked?. Free Radic. Biol. Med. https://doi.org/10.1016/j.freeradbiomed.2010.09.006 (2010).
Jeayeng, S. et al. Nrf2 in keratinocytes modulates UVB-induced DNA damage and apoptosis in melanocytes through MAPK signaling. Free Radic. Biol. Med. https://doi.org/10.1016/j.freeradbiomed.2017.05.009 (2017).
Zhang, B. et al. Apigenin protects human melanocytes against oxidative damage by activation of the Nrf2 pathway. Cell Stress Chaperones https://doi.org/10.1007/s12192-020-01071-7 (2020).
Kamiński, K., Kazimierczak, U. & Kolenda, T. Oxidative stress in melanogenesis and melanoma development. Contemp. Oncol. https://doi.org/10.5114/wo.2021.112447 (2022).
Papaccio, F. et al. A possible modulator of vitiligo metabolic impairment: rethinking a PPARγ agonist. Cells https://doi.org/10.3390/cells11223583 (2022).
Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. https://doi.org/10.5402/2012/137289 (2012).
Ramana, K. V., Srivastava, S. & Singhal, S. S. Lipid peroxidation products in human health and disease. Oxid. Med. Cell Longev. https://doi.org/10.1155/2019/7147235 (2019).
Edae, C. K. & Tofik, E. Biomarkers of oxidative stress and its role in atherosclerosis development. Biomed. J. Sci. Tech. Res. 32, 24659–24669 (2020).
Van der Paal, J., Neyts, E. C., Verlackt, C. C. W. & Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. https://doi.org/10.1039/c5sc02311d (2016).
Kendall, A. C. & Nicolaou, A. Bioactive lipid mediators in skin inflammation and immunity. Prog. Lipid Res. https://doi.org/10.1016/j.plipres.2012.10.003 (2013).
Dhall, S. et al. Arachidonic acid-derived signaling lipids and functions in impaired healing. Wound Repair Regen. https://doi.org/10.1111/wrr.12337 (2015).
Calder, P. C. Eicosanoids. Essays Biochem. https://doi.org/10.1042/EBC20190083 (2020).
Simard, M. et al. Biosynthesis and metabolism of endocannabinoids and their congeners from the monoacylglycerol and N-acyl-ethanolamine families. Biochem. Pharm. https://doi.org/10.1016/j.bcp.2022.115261 (2022).
Jarocka-Karpowicz, I., Dobrzyńska, I., Stasiewicz, A. & Skrzydlewska, E. 3-O-Ethyl ascorbic acid and cannabigerol in modulating the phospholipid metabolism of keratinocytes. Antioxidants https://doi.org/10.3390/antiox13111285 (2024).
Scheau, C. et al. Cannabinoids in the pathophysiology of skin inflammation. Molecules https://doi.org/10.3390/molecules25030652 (2020).
Pucci, M. et al. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J. Biol. Chem. https://doi.org/10.1074/jbc.M111.314880 (2012).
Sobiepanek, A., Baran, J., Milner-Krawczyk, M. & Kobiela, T. Different types of surface modification used for improving the adhesion and interactions of skin cells. Open Access J. Biomed. Sci. 2, 275–278 (2020).
Mariano, A. et al. Pheomelanin effect on UVB radiation-induced oxidation/nitration of l-tyrosine. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23010267 (2021).
Gęgotek, A. & Skrzydlewska, E. Biological effect of protein modifications by lipid peroxidation products. Chem. Phys. Lipids https://doi.org/10.1016/j.chemphyslip.2019.03.011 (2019).
Kostyuk, V. A. et al. Dysfunction of glutathione S-transferase leads to excess 4-hydroxy-2-nonenal and H2O2 and impaired cytokine pattern in cultured keratinocytes and blood of vitiligo patients. Antioxid. Redox Signal https://doi.org/10.1089/ars.2009.2976 (2010).
Wang, H. T., Choi, B. & Tang, M. S. Melanocytes are deficient in repair of oxidative DNA damage and UV-induced photoproducts. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1005244107 (2010).
Pizzimenti, S. et al. The inclusion complex of 4-hydroxynonenal with a polymeric derivative of β-cyclodextrin enhances the antitumoral efficacy of the aldehyde in several tumor cell lines and in a three-dimensional human melanoma model. Free Radic. Biol. Med. https://doi.org/10.1016/j.freeradbiomed.2013.06.035 (2013).
Liang, X., Zhang, C., Shen, L., Ding, L. & Guo, H. Role of non-coding RNAs in UV-induced radiation effects (Review). Exp. Ther. Med. https://doi.org/10.3892/etm.2024.12550 (2024).
Zhu, Z. et al. miR-148a-3p inhibits alpaca melanocyte pigmentation by targeting MITF. Small Rumin. Res. 177, 44–49 (2019).
Hushcha, Y. et al. microRNAs in the regulation of melanogenesis. Int. J. Mol. Sci. 22, 6104 (2021).
Kim, K. H., Kim, H. J. & Lee, T. R. Epidermal long non-coding RNAs are regulated by ultraviolet irradiation. Gene 637, 196–202 (2017).
Ouyang, Y. et al. UVB-induced ciRS-7 activates melanogenesis by paracrine effects. DNA Cell Biol. 40, 523–531 (2021).
Soheilifar, M. H., Masoudi-Khoram, N., Shirkavand, A. & Ghorbanifar, S. Non-coding RNAs in photoaging-related mechanisms: a new paradigm in skin health. Biogerontology 23, 289–306 (2022).
Li, K. et al. The role of exosomal lncRNAs in mediating apoptosis and inflammation in UV-induced skin photoaging. Front. Cell Dev. Biol. 13, 1538197 (2025).
Manga, P. & Choudhury, N. The unfolded protein and integrated stress response in melanoma and vitiligo. Pigment Cell Melanoma Res. https://doi.org/10.1111/pcmr.12947 (2021).
Lyu, C. & Sun, Y. Immunometabolism in the pathogenesis of vitiligo. Front. Immunol. https://doi.org/10.3389/fimmu.2022.1055958 (2022).
Chang, W. L. & Ko, C. H. The role of oxidative stress in vitiligo: an ppdate on its pathogenesis and therapeutic implications. Cells https://doi.org/10.3390/cells12060936 (2023).
Tian, J. et al. The formation of melanocyte apoptotic bodies in vitiligo and the relocation of vitiligo autoantigens under oxidative stress. Oxid. Med. Cell Longev. https://doi.org/10.1155/2021/7617839 (2021).
He, S., Xu, J. & Wu, J. The promising role of chemokines in vitiligo: from oxidative stress to the autoimmune response. Oxid. Med. Cell Longev. https://doi.org/10.1155/2022/8796735 (2022).
Centeno, P. P., Pavet, V. & Marais, R. The journey from melanocytes to melanoma. Nat. Rev. Cancer https://doi.org/10.1038/s41568-023-00565-7 (2023).
Bertrand, J. U. et al. Melanoma risk and melanocyte biology. Acta Derm. Venereol. https://doi.org/10.2340/00015555-3494 (2020).
Sample, A. & He, Y. Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. https://doi.org/10.1111/phpp.12329 (2018).
Venza, M. et al. Cellular mechanisms of oxidative stress and action in melanoma. Oxid. Med. Cell Longev. https://doi.org/10.1155/2015/481782 (2015).
Remigante, A. et al. Oxidative Stress and Immune Response in Melanoma: Ion Channels as Targets of Therapy. Int J. Mol. Sci. https://doi.org/10.3390/ijms24010887 (2023).
Meierjohann, S. Oxidative stress in melanocyte senescence and melanoma transformation. Eur. J. Cell Biol. https://doi.org/10.1016/j.ejcb.2013.11.005 (2014).
Cannavò, S. P. et al. The role of oxidative stress in the biology of melanoma: a systematic review. Pathol. Res Pr. https://doi.org/10.1016/j.prp.2018.11.020 (2019).
Sarkar, R., Devadasan, S., Choubey, V. & Goswami, B. Melatonin and oxidative stress in melasma - an unexplored territory; a prospective study. Int. J. Dermatol. https://doi.org/10.1111/ijd.14827 (2020).
Liu, H. M. et al. Possible mechanisms of oxidative stress-induced skin cellular senescence, inflammation, and cancer and the therapeutic potential of plant polyphenols. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24043755 (2023).
Kim, N. H. & Lee, A. Y. Oxidative stress induces skin pigmentation in melasma by inhibiting hedgehog signaling. Antioxidants https://doi.org/10.3390/antiox12111969 (2023).
Bang, E., Kim, D. H. & Chung, H. Y. Protease-activated receptor 2 induces ROS-mediated inflammation through Akt-mediated NF-κB and FoxO6 modulation during skin photoaging. Redox Biol. https://doi.org/10.1016/j.redox.2021.102022 (2021).
Christman, L., De Benedetto, A., Johnson, E., Khoo, C. & Gu, L. Polyphenol-rich cranberry beverage positively affected skin health, skin lipids, skin microbiome, inflammation, and oxidative stress in women in a randomized controlled trial. Nutrients https://doi.org/10.3390/nu16183126 (2024).
Albrecht, S. et al. Skin type differences in solar-simulated radiation-induced oxidative stress. Br. J. Dermatol. https://doi.org/10.1111/bjd.17129 (2019).
Kaufman, B. P., Aman, T. & Alexis, A. F. Postinflammatory hyperpigmentation: epidemiology, clinical presentation, pathogenesis and treatment. Am. J. Clin. Dermatol. https://doi.org/10.1007/s40257-017-0333-6 (2018).
Widelski, J. et al. Extracts from European propolises as potent tyrosinase Iinhibitors. Molecules https://doi.org/10.3390/molecules28010055 (2022).
Chaowattanapanit, S., Silpa-Archa, N., Kohli, I., Lim, H. W. & Hamzavi, I. Postinflammatory hyperpigmentation: a comprehensive overview: treatment options and prevention. J. Am. Acad. Dermatol. https://doi.org/10.1016/j.jaad.2017.01.036 (2017).
Azam, U. A new era in cosmetology. Eur. J. Biomed. Pharm. Sci. 11, 91–102 (2024).
Rajnochová Svobodová, A. et al. Effect of the flavonoids quercetin and taxifolin on UVA-induced damage to human primary skin keratinocytes and fibroblasts. Photochem Photobiol. Sci. https://doi.org/10.1007/s43630-021-00140-9 (2022).
Bassino, E., Gasparri, F. & Munaron, L. Natural dietary antioxidants containing flavonoids modulate keratinocytes physiology: in vitro tri-culture models. J. Ethnopharmacol. https://doi.org/10.1016/j.jep.2019.111844 (2019).
Kim, S. LncRNA–miRNA–mRNA regulatory networks in skin aging and therapeutic potentials. Front. Physiol. https://doi.org/10.3389/fphys.2023.1303151 (2023).
Li, K. J. et al. Recent advances in exosomal non-coding RNA-based therapeutic approaches for photoaging. Skin Res. Technol. https://doi.org/10.1111/srt.13463 (2023).
de Lima Cherubim, D. J., Buzanello Martins, C. V., Oliveira Fariña, L. & da Silva de Lucca, R. A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. https://doi.org/10.1111/jocd.13093 (2020).
Liu-Smith, F. & Meyskens, F. L. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. https://doi.org/10.1002/mnfr.201500822 (2016).
Nichols, J. A. & Katiyar, S. K. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol Res. https://doi.org/10.1007/s00403-009-1001-3 (2010).
Slika, H. et al. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. https://doi.org/10.1016/j.biopha.2021.112442 (2022).
Kopustinskiene, D. M., Jakstas, V., Savickas, A. & Bernatoniene, J. Flavonoids as anticancer agents. Nutrients https://doi.org/10.3390/nu12020457 (2020).
Takekoshi, S., Nagata, H. & Kitatani, K. Flavonoids enhance melanogenesis in human melanoma cells. Tokai J. Exp. Clin. Med 39, 116–121 (2014).
Xie, Y., Mei, X. & Shi, W. Kaempferol promotes melanogenesis and reduces oxidative stress in PIG1 normal human skin melanocytes. J. Cell Mol. Med. https://doi.org/10.1111/jcmm.17711 (2023).
Yang, B., Yang, Q., Yang, X., Yan, H. B. & Lu, Q. P. Hyperoside protects human primary melanocytes against H2O2-induced oxidative damage. Mol. Med. Rep. https://doi.org/10.3892/mmr.2016.5107 (2016).
Ha, A. T. et al. Anti-inflammatory, antioxidant, moisturizing, and antimelanogenesis effects of quercetin 3-O-β-D-glucuronide in human keratinocytes and melanoma cells via activation of NF-κB and AP-1 pathways. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23010433 (2021).
Jeong, Y. M. et al. Cytoprotective effect of green tea extract and quercetin against hydrogen peroxide-induced oxidative stress. Arch. Pharm. Res. https://doi.org/10.1007/BF02978208 (2005).
Tong, L. X. & Young, L. C. Nutrition: the future of melanoma prevention?. J. Am. Acad. Dermatol. https://doi.org/10.1016/j.jaad.2014.01.910 (2014).
Karampinis, E. et al. Non-melanoma skin cancer and vitamin D: the “lost sunlight” paradox and the oxidative stress explanation. Antioxidants https://doi.org/10.3390/antiox12051107 (2023).
Russo, G. L., Tedesco, I., Spagnuolo, C. & Russo, M. Antioxidant polyphenols in cancer treatment: friend, foe or foil?. Semin. Cancer Biol. https://doi.org/10.1016/j.semcancer.2017.05.005 (2017).
Čizmárová, B., Hubková, B., Tomečková, V. & Birková, A. Flavonoids as promising natural compounds in the prevention and treatment of selected skin diseases. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24076324 (2023).
Li, X. et al. Combining network pharmacology, molecular docking, molecular dynamics simulation, and experimental verification to examine the efficacy and immunoregulation mechanism of FHB granules on vitiligo. Front. Immunol. https://doi.org/10.3389/fimmu.2023.1194823 (2023).
Ning, W. et al. Potent effects of peracetylated (-)-epigallocatechin-3-gallate against hydrogen peroxide-induced damage in human epidermal melanocytes via attenuation of oxidative stress and apoptosis. Clin. Exp. Dermatol. https://doi.org/10.1111/ced.12855 (2016).
Heyza, J. R. et al. Targeting the DNA repair endonuclease ERCC1-XPF with green tea polyphenol epigallocatechin-3-gallate (EGCG) and its prodrug to enhance cisplatin efficacy in human cancer cells. Nutrients https://doi.org/10.3390/nu10111644 (2018).
Wu, W. et al. EGCG synergizes the therapeutic effect of irinotecan through enhanced DNA damage in human colorectal cancer cells. J. Cell Mol. Med. https://doi.org/10.1111/jcmm.16718 (2021).
Furukawa, A., Oikawa, S., Murata, M., Hiraku, Y. & Kawanishi, S. (-)-Epigallocatechin gallate causes oxidative damage to isolated and cellular DNA. Biochem. Pharm. https://doi.org/10.1016/s0006-2952(03)00541-0 (2003).
Glei, M. & Pool-Zobel, B. L. The main catechin of green tea, (-)-epigallocatechin-3-gallate (EGCG), reduces bleomycin-induced DNA damage in human leucocytes. Toxicol. Vitr. https://doi.org/10.1016/j.tiv.2005.08.002 (2006).
Hussain, Y. et al. Role of quercetin in DNA repair: possible target to combat drug resistance in diabetes. Curr. Drug Targets https://doi.org/10.2174/0113894501302098240430164446 (2024).
Barcelos, G. R. et al. Quercetin protects human-derived liver cells against mercury-induced DNA-damage and alterations of the redox status. Mutat. Res. https://doi.org/10.1016/j.mrgentox.2011.05.011 (2011).
Özyurt, H. et al. Quercetin protects radiation-induced DNA damage and apoptosis in kidney and bladder tissues of rats. Free Radic. Res. https://doi.org/10.3109/10715762.2014.945925 (2014).
Martinelli, G. et al. Cannabis sativa and skin health: dissecting the role of phytocannabinoids. Planta Med. https://doi.org/10.1055/a-1420-5780 (2022).
Lu, H. C. & Mackie, K. Review of the endocannabinoid system. Biol. Psychiatry Cogn. Neurosci. Neuroimag. https://doi.org/10.1016/j.bpsc.2020.07.016 (2021).
Łuczaj, W., Dobrzyńska, I. & Skrzydlewska, E. Differences in the phospholipid profile of melanocytes and melanoma cells irradiated with UVA and treated with cannabigerol and cannabidiol. Sci. Rep. https://doi.org/10.1038/s41598-023-43363-9 (2023).
Goenka, S. Comparative study of ∆9-tetrahydrocannabinol and cannabidiol on melanogenesis in human epidermal melanocytes from different pigmentation phototypes: a pilot study. J. Xenobiot. https://doi.org/10.3390/jox12020012 (2022).
Perez, E. et al. In vitro and clinical evaluation of cannabigerol (CBG) produced via yeast biosynthesis: a cannabinoid with a broad range of anti-inflammatory and skin health-boosting properties. Molecules https://doi.org/10.3390/molecules27020491 (2022).
Atalay, S., Jarocka-Karpowicz, I. & Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants https://doi.org/10.3390/antiox9010021 (2019).
Wroński, A., Jarocka-Karpowicz, I., Stasiewicz, A. & Skrzydlewska, E. Phytocannabinoids in the pharmacotherapy of psoriasis. Molecules https://doi.org/10.3390/molecules28031192 (2023).
Gaweł-Bęben, K., Czech, K. & Luca, S. V. Cannabidiol and minor phytocannabinoids: a preliminary study to assess their anti-melanoma, anti-melanogenic, and anti-tyrosinase properties. Pharmaceuticals https://doi.org/10.3390/ph16050648 (2023).
Gohad, P. et al. Novel cannabidiol sunscreen protects keratinocytes and melanocytes against ultraviolet B radiation. J. Cosmet. Dermatol. https://doi.org/10.1111/jocd.13693 (2020).
Billi, M. et al. DNA damage and cell death in human oral squamous cell carcinoma cells: the potential biological effects of cannabidiol. Arch. Oral. Biol. https://doi.org/10.1016/j.archoralbio.2024.106110 (2025).
Li, Y. et al. Photoprotective effects of cannabidiol against ultraviolet-B-induced DNA damage and autophagy in human keratinocyte cells and mouse skin tissue. Molecules https://doi.org/10.3390/molecules27196740 (2022).
Pullar, J. M., Carr, A. C. & Vissers, M. C. M. The roles of vitamin C in skin health. Nutrients https://doi.org/10.3390/nu9080866 (2017).
Boo, Y. C. Ascorbic acid (Vitamin C) as a cosmeceutical to increase dermal collagen for skin antiaging purposes: emerging combination therapies. Antioxidants https://doi.org/10.3390/antiox11091663 (2022).
Chen, X. Y. et al. Vitamin C induces human melanoma A375 cell apoptosis via Bax- and Bcl-2-mediated mitochondrial pathways. Oncol. Lett. https://doi.org/10.3892/ol.2019.10686 (2019).
McArdle, F. et al. UVR-induced oxidative stress in human skin in vivo: effects of oral vitamin C supplementation. Free Radic. Biol. Med. https://doi.org/10.1016/s0891-5849(02)01042-0 (2002).
Padayatty, S. J. et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J. Am. Coll. Nutr. https://doi.org/10.1080/07315724.2003.10719272 (2003).
Niu, J., Hu, W., Zhang, Q. & Zhao, G. In vitro effects of ascorbic acid on H2O2-induced oxidative injury in cultured melanocytes. Chin. J. Dermatol. 12, 39–43 (2017).
Choi, J. Y., Ha, J. W. & Boo, Y. C. Multifaceted effects of L-cysteine, L-ascorbic acid, and their derivatives on the viability and melanin synthesis of B16/F10 cells under different conditions. Antioxidants https://doi.org/10.3390/antiox13030330 (2024).
Miles, S. L., Fischer, A. P., Joshi, S. J. & Niles, R. M. Ascorbic acid and ascorbate-2-phosphate decrease HIF activity and malignant properties of human melanoma cells. BMC Cancer https://doi.org/10.1186/s12885-015-1878-5 (2015).
Funasaka, Y., Komoto, M. & Ichihashi, M. Depigmenting effect of alpha-tocopheryl ferulate on normal human melanocytes. Pigment Cell Res. https://doi.org/10.1111/j.0893-5785.2000.130830.x (2000).
Smit, N., Vicanova, J., Cramers, P., Vrolijk, H. & Pavel, S. The combined effects of extracts containing carotenoids and vitamins E and C on growth and pigmentation of cultured human melanocytes. Skin Pharm. Physiol. https://doi.org/10.1159/000080217 (2004).
Blaner, W. S., Shmarakov, I. O. & Traber, M. G. Vitamin A and vitamin E: will the real antioxidant please stand up?. Annu. Rev. Nutr. https://doi.org/10.1146/annurev-nutr-082018-124228 (2021).
Szymański Ł. et al. Retinoic acid and its derivatives in skin. Cells https://doi.org/10.3390/cells9122660 (2020).
Sorg, O., Didierjean, L. & Saurat, J. H. Metabolism of topical retinaldehyde. Dermatology https://doi.org/10.1159/000051372 (1999).
Bohn, T. et al. Carotenoids in health as studied by omics-related endpoints. Adv. Nutr. https://doi.org/10.1016/j.advnut.2023.09.002 (2023).
Scatozza, F. et al. Nicotinamide inhibits melanoma in vitro and in vivo. J. Exp. Clin. Cancer Res. 39, 211 (2020).
Boo, Y. C. Mechanistic basis and clinical evidence for the applications of nicotinamide (niacinamide) to control skin aging and pigmentation. Antioxidants https://doi.org/10.3390/antiox10081315 (2021).
Thompson, B. C., Surjana, D., Halliday, G. M. & Damian, D. L. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in primary melanocytes. Exp. Dermatol. https://doi.org/10.1111/exd.12430 (2014).
Brito, S. et al. Nicotinamide mononucleotide reduces melanin production in aged melanocytes by inhibiting cAMP/Wnt signaling. J. Dermatol. Sci. https://doi.org/10.1016/j.jdermsci.2022.05.002 (2022).
Ferraz, C. A. A., Grougnet, R., Nicolau, E., Picot, L. & de Oliveira Junior, R. G. Carotenoids from marine microalgae as antimelanoma agents. Mar. Drugs https://doi.org/10.3390/md20100618 (2022).
Genç, Y. et al. Oxidative stress and marine carotenoids: application by using nanoformulations. Mar. Drugs https://doi.org/10.3390/md18080423 (2020).
Gammone, M. A., Riccioni, G. & D’Orazio, N. Marine carotenoids against oxidative stress: effects on human health. Mar. Drugs https://doi.org/10.3390/md13106226 (2015).
Godic, A., Poljšak, B., Adamic, M. & Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell Longev. https://doi.org/10.1155/2014/860479 (2014).
Colucci, R. et al. Evaluation of an oral supplement containing Phyllanthus emblica fruit extracts, vitamin E, and carotenoids in vitiligo treatment. Dermatol. Ther. https://doi.org/10.1111/dth.12172 (2015).
Asgari, M. M., Brasky, T. M. & White, E. Association of vitamin A and carotenoid intake with melanoma risk in a large prospective cohort. J. Invest. Dermatol. https://doi.org/10.1038/jid.2012.21 (2012).
Bi, M. C. et al. Zeaxanthin induces apoptosis in human uveal melanoma cells through Bcl-2 family proteins and intrinsic apoptosis pathway. Evid. Based Complement Altern. Med. https://doi.org/10.1155/2013/205082 (2013).
Catanzaro, E., Bishayee, A. & Fimognari, C. On a beam of light: photoprotective activities of the marine carotenoids astaxanthin and fucoxanthin in suppression of inflammation and cancer. Mar. Drugs https://doi.org/10.3390/md18110544 (2020).
Kashif, M. et al. ROS-lowering doses of vitamins C and A accelerate malignant melanoma metastasis. Redox Biol. https://doi.org/10.1016/j.redox.2023.102619 (2023).
Sevilla, A., Chéret, J., Slominski, R. M., Slominski, A. T. & Paus, R. Revisiting the role of melatonin in human melanocyte physiology: a skin context perspective. J. Pineal Res. https://doi.org/10.1111/jpi.12790 (2022).
Janjetovic, Z. et al. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci. Rep. https://doi.org/10.1038/s41598-017-01305-2 (2017).
Kleszczynski, K. & Fischer, T. W. Melatonin and human skin aging. Dermatoendocrinol. https://doi.org/10.4161/derm.22344 (2012).
Yang, S. et al. Melatonin reduces melanogenesis by inhibiting the paracrine effects of keratinocytes. Exp. Dermatol. https://doi.org/10.1111/exd.14743 (2023).
Slominski, A., Wortsman, J. & Tobin, D. J. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. https://doi.org/10.1096/fj.04-2079rev (2005).
Andrés, C. M. C., Pérez de la Lastra, J. M., Andrés Juan, C., Plou, F. J. & Pérez-Lebeña, E. Superoxide anion chemistry-its role at the core of the innate immunity. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24031841 (2023).
Eskandari, M. et al. Polyphenol-hydrogen peroxide reactions in skin: in vitro model relevant to study ROS reactions at inflammation. Anal. Chim. Acta https://doi.org/10.1016/j.aca.2019.05.032 (2019).
Poljšak, B. & Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pr. https://doi.org/10.1155/2012/135206 (2012).
Al-Nu’airat, J., Oluwoye, I., Zeinali, N., Altarawneh, M. & Dlugogorski, B. Z. Review of chemical reactivity of singlet oxygen with organic fuels and contaminants. Chem. Rec. https://doi.org/10.1002/tcr.202000143 (2021).
Cals-Grierson, M. M. & Ormerod, A. D. Nitric oxide function in the skin. Nitric Oxide https://doi.org/10.1016/j.niox.2004.04.005 (2004).
Pacher, P., Beckman, J. S. & Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. https://doi.org/10.1152/physrev.00029.2006 (2007).
Huang, J. et al. Particulate matter, nitrogen dioxide, and sulfur dioxide and their associations with allergic skin diseases: A systematic review and meta-analysis. Atmos. Pollut. Res. https://doi.org/10.1016/j.apr.2023.101804 (2023).
Author information
Authors and Affiliations
Contributions
M.M., E.S., and A.G. contributed to the conception or design of the work; M.M. and A.G. drafted the work; E.S. revised it critically for important intellectual content. All authors approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Muhammad Farrukh Nisar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Dr Joao Valente and Dr Ophelia Bu.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mucha, M., Skrzydlewska, E. & Gęgotek, A. Natural protection against oxidative stress in human skin melanocytes. Commun Biol 8, 1283 (2025). https://doi.org/10.1038/s42003-025-08725-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08725-1