Abstract
In tumorous conditions, STAT1, traditionally recognized for its anti-tumor role in immunology, exhibits pro-survival characteristics, though unclear mechanisms. Investigating STAT1 function in isogenic colorectal tumor cells with wild-type or mutant KRAS, we found that STAT1 specifically promotes tumor survival and proliferation with mutant KRAS. Gene expression profiling revealed that STAT1 promotes the expression of sterol and lipid biosynthesis genes in these cells. This effect depends on STAT1 phosphorylation at S727, which upregulates SREBP1 and SREBP2 to drive de novo lipid production. In mutant KRAS cells, STAT1 amplifies the mevalonate pathway, maintaining its S727 phosphorylation and establishing a positive feedback loop through the transcription factors YAP1 and TEAD4, further driving lipid biosynthesis and tumor growth. This STAT1-YAP1 axis promotes mutant KRAS tumor cells’ resistance to mevalonate pathway inhibitors, which can be overcome by pharmacologically targeting the YAP1-TEAD interaction. Moreover, this axis contributes to the inherent resistance of mutant KRAS colon cancer cells to EGFR-targeted therapy. Together, these findings identify the STAT1-YAP1 pathway as a critical mediator of therapy resistance and a promising therapeutic target in mutant KRAS colorectal cancer.
Similar content being viewed by others
Introduction
The RAS family of small GTPases, often mutated in about 30% of human cancers, commonly acquires activating mutations at G12, G13, or Q61 that lock the protein in its active, GTP-bound state1. Among the RAS isoforms, Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) gene is the predominant one mutated in three of the most lethal cancers globally, namely colorectal, lung, and pancreatic cancers2. Mutant KRAS accelerates proliferation and establishes crosstalk between tumors and stromal cells in the tumor microenvironment, overcoming challenges such as low nutrient availability and immune-mediated anti-tumor responses3. Additionally, mutant KRAS alters key metabolic processes, including elevating glucose, lipids, glutamine, and glucose uptake and consumption, to support biosynthetic pathways and enhance cellular redox potential3. Tumors harboring KRAS mutations exhibit dependence on these metabolic adaptations, suggesting potential novel avenues for cancer therapy3.
The signal transducer and activator of transcription 1 (STAT1) plays a crucial role in innate immunity, safeguarding the host against infections caused by viruses and other pathogens as a major mediator of interferon (IFN)4. STAT1 plays a dual role in cancer, acting as both a tumor suppressor and promoter depending on context5. It exhibits anti-tumor effects by enhancing immune responses and suppressing tumor growth in certain models, such as breast cancer5,6. However, it can also support oncogenesis by weakening immune surveillance in blood cancers5. In solid tumors, STAT1 contributes to resistance to chemotherapy and radiotherapy by inducing interferon-stimulated genes that protect against DNA damage5,7. STAT1 also enhances cell survival in response to chemotherapy by activating the phosphoinositide 3-kinase (PI3K)/mechanistic target of rapamycin (mTOR) pathway and promoting the translation of anti-apoptotic genes8,9,10.
YES-associated protein 1 (YAP1) is hyperactivated in numerous human cancers and demonstrates tumorigenicity in mouse cancer models11. YAP1 is a crucial target for inactivation by the HIPPO tumor suppressor pathway, which is modulated by diverse signals, including oncogenic and metabolic determinants12. Mutant KRAS promotes the nuclear localization and gene transactivation capacity of YAP1 through both HIPPO-dependent and independent pathways13,14,15. YAP1 displays a pro-survival role in colorectal, lung, and pancreatic cancers with KRAS mutations16,17,18,19.
Tumors often exploit alterations in metabolic pathways to generate intermediates necessary for rapid proliferation20. One such pivotal pathway in tumor proliferation is de novo lipogenesis20. While normal cells typically rely on lipid uptake from circulation for their metabolic needs, tumors respond to the heightened demand for membrane biogenesis during proliferation by activating de novo sterol and lipid biosynthetic pathways21. Sterol regulatory element-binding proteins (SREBPs) 1 and 2, transmembrane proteins located in the endoplasmic reticulum, play a crucial role in this process22. Depletion of sterols or unsaturated fatty acids triggers the trafficking of SREBPs to the Golgi apparatus, where proteolytic cleavage activates them, allowing entry into the nucleus to upregulate the expression of cholesterol and lipid biosynthetic genes22. There are three isoforms of SREBPs; the alternatively spliced forms SREBP-1a and SREBP-1c, with the latter being dominant in cultured cells, activate transcription of genes involved in fatty acid synthesis, while SREBP-2 upregulates enzymes required for cholesterol biosynthesis22.
Mutant KRAS elevates de novo lipid biosynthesis by stimulating sterol regulatory element-binding proteins (SREBPs) and fatty acid synthase (FASN) activity23,24,25. However, the specific pathways involved in de novo lipogenesis in mutant KRAS cancer cells remain partially understood. STAT1 is known for its role in cholesterol metabolism through the transcriptional upregulation of 25-hydroxycholesterol in macrophages as part of an anti-viral response induced by IFN26. Conversely, YAP1 is involved in upregulating de novo lipogenesis in non-tumorigenic tissue and diabetic mouse models27,28. However, it remains unknown whether STAT1 or YAP1 are implicated in lipogenic programs of tumor cells.
The upregulation of STAT1 in human colon adenocarcinomas, compared to normal epithelial cells29, suggests that STAT1 may contribute to colorectal cancer (CRC) growth. Using isogenic CRC cells with either wild-type or mutant KRAS, we demonstrate that STAT1 specifically promotes the survival and growth of tumor cells with mutant KRAS. The pro-survival function of STAT1 is intricately linked to its ability to enhance the expression of SREBPs and downstream sterol and lipid biosynthetic genes in mutant KRAS cells. Our findings reveal that the activation of the mevalonate pathway by STAT1 leads to increased nuclear localization and enhanced transcriptional function of YAP1. Subsequently, activated YAP1 forms a complex with the transcription factor TEAD4, thereby increasing the expression of SREBPs and lipogenic gene expression in mutant KRAS cells.
Our study underscores the establishment of a feedforward loop between STAT1 and YAP1, driving the upregulation of lipogenic pathways and conferring resistance to mevalonate pathway and EGFR inhibitors in mutant KRAS cells. This intricate interplay reveals a potential target for therapeutic intervention in the context of mutant KRAS-associated cancers.
Results
STAT1 promotes the survival of colorectal tumor cells with mutant but not wild type KRAS
In our investigation, we utilized human CRC HCT116 and DLD1 cells expressing a KRAS G13D allele, along with their isogenic counterparts HK2-8 and DKO-1 cells, which harbor wild-type (WT) KRAS30. Silencing of STAT1 using siRNAs resulted in a significant decrease in the colony-forming efficacy of HCT116 and DLD1 cells, whereas the colony-forming efficacy of HK2-8 and DKO-1 cells remained unaffected (Fig. 1a; Supplementary Fig. S1a). Immunoblotting validated the reduction of STAT1 in these isogenic colon cancer cells (Fig. 1b; Supplementary Fig. S1b). We tested the specificity of STAT1 downregulation by verifying that expression of STAT3 and STAT5 upon treatment with STAT1 siRNAs remained unimpaired (Fig. 1b). These data collectively emphasize the pro-survival function of STAT1 specifically in tumor cells with mutant KRAS, with no discernible impact on cells with wild-type (WT) KRAS.
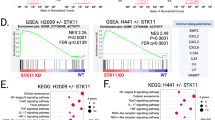
a Evaluation of colony formation efficacy of HCT116 and HK2-8 cells after treatments with either scramble siRNAs or STAT1 siRNAs. The graphs represent data obtained from 3 biological replicates, each of which included 3 technical replicates, and represent ±SEM (*) P < 0.05 (**) P < 0.01; (t-test), NS, non-significant. b Immunoblotting of indicated protein from extracts of siRNA-treated cells. Quantifications show the relative intensity of STAT1 normalized to ACTIN from 3 biological replicates and represent ±SEM * P < 0.05 (t-test). c Volcano Diagram illustrates the number of genes differentially expressed in STAT1-replete (WT; control) compared to STAT1-/- HCT116 cells. Essential genes of sterol and lipid biosynthetic pathways are indicated. Dashed horizontal and vertical lines indicate significance thresholds (|FC | > 0.5, P < 0.05). Genes are colored in gray (non-significant), blue (P-value significant), and red (both P-values and fold change are significant). A positive fold change means that the gene is upregulated by STAT1. All labeled genes exhibit statistically significant upregulation (logFC > 0.5, P < 0.0005), consistent with their role in cholesterol biosynthesis and lipid metabolism. d Bar plot of biological processes (BP) from gene ontology (ON) significantly enriched in STAT1-dependent genes in HCT116 cells. e KEGG pathways under the control of STAT1 in HCT116 cells.
STAT1 upregulates SREBPs and lipid biosynthetic genes in mutant KRAS tumor cells
To explore the mechanisms underlying STAT1 function, we conducted a comparative analysis of gene expression profiles between proficient (STAT1+/+) and STAT1-deficient (STAT1−/− via CRISPR) HCT116 cells. Verification of STAT1 deletion was accomplished through immunoblotting, both before and after treatment with IFNs, known to induce STAT1 expression (Supplementary Fig. S1c). Our analysis revealed 1922 genes that were upregulated, and 2058 genes downregulated by STAT1 (Fig. 1c, Supplementary Data 1). Gene ontology (GO) enrichment and pathway assessments unveiled the involvement of STAT1-regulated genes in diverse biosynthetic processes, with a prominent emphasis on the upregulation of steroid and lipid biosynthetic pathways (Fig. 1d, e, Supplementary Data 1).
The analysis of gene expression data revealed STAT1 involvement in the upregulation of SREBF1 and SREBF2 mRNAs, responsible for encoding SREBP-1 and SREBP-2, respectively (Fig. 1c). These transcription factors act as master regulators for cholesterol and lipid biosynthetic genes22. To confirm this, we employed STAT1 siRNAs to downregulate STAT1 and observed a reduction in SREBF1 and 2 mRNA levels in HCT116 cells carrying the KRAS G13D, but not in isogenic HK2-8 cells with WT KRAS (Fig. 2a). Further, treatment with STAT1 siRNAs resulted in decreased expression of both the full-length (FL) 125 kDa and mature (M) 70 kDa forms of SREBP-1 and 2 in HCT116 and DLD-1 cells harboring mutant KRAS, while no such effect was observed in isogenic HK2-8 and DKO-1 cells with WT KRAS (Fig. 2b). These findings support the conclusion that the STAT1-mediated upregulation of SREBPs occurs specifically in cells with mutant KRAS.
a Detection of SREBF mRNAs in colon cancer cells with intact or impaired STAT1. SREBF 1 and 2 mRNA levels were normalized to ACTIN and TUBULIN mRNAs used as internal controls. Data obtained from 3 biological replicates each of which contained 3 technical replicates and represent ±SEM ** P < 0.01: ***P < 0.001 (t-test), NS, non-significant. b Immunoblotting for SREBP1 and 2 in colon cancer cells with intact or downregulated STAT1. Quantifications show the relative intensity of proteins normalized to TUBULIN. FL, full length; M, mature form. c Schematic representation of the mevalonate pathway. Genes in red are SREBP-dependent genes. d ChIP-seq data from ENCODE (UCSC data base) indicating the binding of SREBP1 and 2 to transcriptional regulatory regions of mevalonate pathway genes. Graphs show the expression of ACAT1, HMGCR and IDI1 mRNAs by qPCR in cells treated with scrambled or STAT1 siRNAs. Gene expressions were normalized to ACTIN and TUBULIN mRNAs used as internal controls. Data were obtained from 3 independent experiments performed in triplicates and represent ±SEM *P < 0.05, **P < 0.01, *** P < 0.001 (t-test). e Immunoblotting of HCT116 protein extracts replete (control) or deplete (−/−) for STAT1 by CRISPR (cell line #1 and #2). Detection of STAT1 and rate-limiting enzymes of sterol and lipid biosynthetic pathway HMGCR and FAS, respectively.
Within the genes upregulated by STAT1, acetyl-CoA acetyltransferase (ACAT1), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), and isopentenyl-diphosphate delta-isomerase 1 (IDI1) encode pivotal enzymes in the mevalonate pathway, a critical player in the synthesis of cholesterol and isoprenoid metabolites (Fig. 2c)31. Analysis of the Encyclopedia of DNA Elements (ENCODE) revealed the presence of SREBP1 and 2 binding sites in the regulatory regions of these genes, implying an indirect role of STAT1 in their expression through the upregulation of SREBPs (Fig. 2d). Indeed, STAT1 downregulation led to a reduction in the expression of ACAT1, HMGCR, and IDI1 mRNAs in HCT116 cells, while no such effect was observed in isogenic HK2-8 cells (Fig. 2d). Immunoblotting of HCT116 cells further confirmed that the deletion of STAT1 by CRISPR reduced the expression of SREBP-dependent gene products, such as HMGCR, the rate-limiting enzyme of the mevalonate pathway31, and fatty acid synthase (FAS), the rate-limiting enzyme of fatty acid biosynthesis in CRCs32 (Fig. 2e). These findings suggest that STAT1, acting through SREBPs, fosters the expression of key enzymes in sterol and lipid biosynthetic pathways specifically in cells with mutant KRAS.
STAT1 stimulates the transcription of SREBF1 and 2 genes specifically in mutant KRAS cells
SREBF1 and 2 are transcribed from distinct promoters33. Analysis of the Encyclopedia of DNA Elements (ENCODE) database revealed two STAT1 binding sites in the SREBF1 gene and one in SREBF2 (Fig. 3a). Chromatin immunoprecipitation (ChIP) assays showed the binding of endogenous STAT1 to the regulatory regions of SREBF1 and SREBF2 in HCT116 cells compared to HK2-8 cells (Fig. 3b). As STAT1 transcriptional activity is modulated by phosphorylation at tyrosine (Y) 701 and serine (S) 72734, we investigated if phosphorylation at these sites influences the transcription of SREBF genes.
a ChIP-seq data from ENCODE (UCSC database) indicating STAT1 binding to the regulatory regions of SREBF1 and 2 genes. b ChIP assays of endogenous STAT1 bound to SREBF gene segments containing STAT1 binding sites in HCT116 and HK2-8 cells. IgG, non-specific control antibody. c Expression of GFP (control) and GFP-tagged STAT1 proteins that are either intact (wild type, WT), impaired for phosphorylation (Y701F or S727A) or S727 phosphomimetic (S727E) in HCT116 STAT1−/− cells. GFP+ cells were sorted by flow cytometry, and extracts were immunoblotted for GFP or ACTIN. d Detection of SREBF-1 and 2 mRNAs by qPCR in HCT116 STAT1−/− cells expressing either GFP or GFP-STAT1 forms. e ChIP assays of GFP-STAT1 for binding to STAT1 sites of SREBF genes in reconstituted HCT116 STAT1−/− cells using GFP antibody. b–e Data obtained from 3 biological replicates and represent ±SEM *P < 0.05, **P < 0.01, ***P < 0.001 (t-test).
To investigate this, we reconstituted HCT116 STAT1−/− cells with GFP-tagged human STAT1 variants: wild-type (WT), phosphorylation-deficient mutants Y701F and S727A, and the phosphomimetic mutant S727E35. GFP positive cells were sorted and tested for GFP-STAT1 expression via immunoblotting and expression of native SREBF1 and SREBF2 genes through qPCR. All GFP-STAT1 variants were expressed at comparable levels (Fig. 3c). Notably, SREBF1 and SREBF2 mRNA levels were elevated in cells expressing GFP-STAT1 WT or Y701F, but not in those expressing S727A or S727E (Fig. 3d). These findings indicate that the S727E phosphomimetic mutant fails to substitute for S727 phosphorylation in activating STAT1.
Subsequent ChIP assays confirmed the binding of GFP-STAT1 WT and GFP-STAT1 Y701F to the regulatory regions of SREBF1 and SREBF2 genes in the reconstituted HCT116 STAT1−/− cells (Fig. 3e). In contrast, GFP-STAT1 S727A showed no significant DNA binding compared to cells reconstituted with GFP alone (Fig. 3e). These results highlight the critical role of S727 phosphorylation in STAT1-mediated transcriptional upregulation of SREBF1/2 in mutant KRAS cells.
STAT1 promotes the nuclear localization and transcriptional function of YAP1 in mutant KRAS cells
A notable property of the mevalonate pathway is its ability to enhance the activity of the transcriptional activator YAP136, a critical pro-survival factor in mutant KRAS cancers16,17,18. We hypothesized that STAT1 may, at least partially, rely on YAP1 to exert its pro-survival effects on mutant KRAS CRC cells.
To investigate this, we examined the nuclear trafficking of YAP1 in HCT116 STAT1+/+ and STAT1−/− cells treated with either serum or lysophosphatidic acid (LPA), both of which activate YAP136. Immunofluorescence (IF) analysis demonstrated that both treatments stimulated the nuclear localization of YAP1 in HCT116 cells but not in HK2-8 cells, indicating that mutant KRAS promotes the nuclear trafficking of YAP1 (Fig. 4a). Deletion of STAT1 prevented the stimulation of YAP1 nuclear localization by serum or LPA in HCT116 cells, while in HK2-8 cells, STAT1 inactivation did not produce noticeable effects (Fig. 4a). A similar observation was made when YAP1 nuclear localization was assessed in HCT116 and HK2-8 cells using subcellular fractionation and immunoblotting. Specifically, deletion of STAT1 decreased the nuclear fraction of YAP1 in HCT116 cells but not in HK2-8 cells after serum stimulation (Fig. 4b, c).
a HCT116 and HK2-8 cells replete or deplete of STAT1 were serum-starved for 18 h (untreated; UT) and stimulated with either 10% fetal bovine serum or 25 μM LPA for 1 h. Cells were subjected to IF analyses of YAP1 (red) along with DAPI staining of DNA (blue). Graphs show the quantification of YAP1 nuclear localization in 300 cells. Scale bar: 25 μm. b, c Cells were subjected to cytoplasmic (C), and nuclear (N) fractionation followed by immunoblotting for the indicated proteins. TUBULIN or THO complex 1 (THOC1) was used as cytoplasmic or nuclear marker, respectively. Quantification in panel b is based on three biological replicates, while panel c is based on two biological replicates. d, e HCT116 STAT1+/+ and STAT1−/− cells were transfected with either pGL3-luciferase reporter plasmid (control) or 8xGTIIC plasmid containing the firefly luciferase reporter gene under the control of 8x TEAD binding sites in CTGF minimal promoter. Transfected cells were serum-starved for 18 h followed by stimulation with either 10% fetal bovine serum or 25 μM LPA for 6 h. A plasmid expressing the renilla luciferase gene was used as internal control. f, g HCT116 cells were serum starved for 18 h followed by stimulation of 10% fetal bovine serum in the absence or presence of 2.5 mM cerivastatin (panel f, g) or 10 μM ROCK kinase inhibitor Y-27632 (panel f) for 18 h. Protein extracts were subjected to immunoblotting for the indicated proteins. a, b, d, e Graphs show the quantifications from 3 biological replicates and represent ±SEM *P < 0.05, **P < 0.01, ***P < 0.001 (t-test), NS, non-significant. In c, data represent the quantification of 2 biological replicates.
We then investigated the role of STAT1 in YAP1-mediated gene transactivation. Since YAP1 does not have DNA-binding activity, its transcriptional function relies on interactions with DNA-bound transcription factors such as members of the transcription enhanced associate domain (TEAD) family37. To assess this, we conducted transient transfection assays using a luciferase reporter gene under the control of 8 binding sites of TEAD in the minimal promoter of the connective tissue growth factor (CTGF) gene (8xGTIIC), a classical YAP1/TEAD-dependent gene38. We found that YAP1-mediated transactivation of the 8xGTIIC promoter was significantly reduced in STAT1−/− compared to STAT1+/+ HCT116 cells following serum or LPA treatment (Fig. 4d, e). Taken together, these results support the interpretation that STAT1 promotes the nuclear trafficking and transactivation activity of YAP1 in mutant KRAS cells.
Activation of YAP1 by STAT1 requires the upregulation of the mevalonate pathway
We further explored whether STAT1 upregulation of the mevalonate pathway contributes to YAP1 activation in mutant KRAS cells. To this end, we tested the effects of fluvastatin and cerivastatin, which are inhibitors of the rate-limiting enzyme of the mevalonate pathway, HMGCR31, on YAP1 nuclear localization in HCT116 cells. Immunofluorescence assays revealed that serum stimulation increased YAP1 nuclear localization in HCT116 cells with native STAT1, an effect that was prevented by pre-treatment with either fluvastatin or cerivastatin (Supplementary Fig. S2a). However, YAP1 nuclear localization was not increased by serum in HCT116 STAT1−/− cells either in the absence or presence of fluvastatin or cerivastatin (Supplementary Fig. S2a). The addition of mevalonate metabolite into the media stimulated the nuclear localization of YAP1 in STAT1+/+ cells in the presence of fluvastatin or cerivastatin (Supplementary Fig. S2a). This is because addition of mevalonate can bypass the inhibition of the enzymatic activity of HMGCR by fluvastatin or cerivastatin (Fig. 2c). Addition of mevalonate also restored the nuclear localization of YAP1 in STAT1−/− cells in the absence as well as presence of fluvastatin or cerivastatin (Supplementary Fig. S2a). This data verifies that STAT1 employs the mevalonate pathway to promote the nuclear trafficking of YAP1 in mutant KRAS cells.
Because sterol biosynthesis has been linked to the activation of YAP1 through increased RHO GTPase signaling36, we investigated the effects of Y-27632, an inhibitor of RHO-associated coiled-coil kinases (ROCK)39, on the nuclear localization of YAP1. Serum stimulation of HCT116 cells promoted nuclear accumulation of YAP1, whereas treatment with Y-27632 inhibited this translocation (Supplementary Fig. S2b). Furthermore, treatment with either cerivastatin or Y-27632 blocked serum-induced phosphorylation of STAT1 at S727, without affecting ERK activation, as evidenced by unchanged ERK phosphorylation levels (Fig. 4f). Inhibition of the mevalonate pathway by cerivastatin did not restore YAP1 phosphorylation at S127 after serum treatment, a modification that suppresses YAP1 activity (Fig. 4g). Given that YAP1 S127 phosphorylation is a major inactivation mechanism mediated by the HIPPO pathway40, these findings suggest that YAP1 activation through the mevalonate pathway occurs independently of HIPPO signaling, consistent with previous studies36. Collectively, our results indicate that ROCK activation downstream of mevalonate pathway contributes to STAT1 S727 phosphorylation in mutant KRAS cells (Fig. 4f, g).
YAP1 stimulates the expression of SREBPs and lipid biosynthetic genes in mutant KRAS cells
To understand YAP1’s function, we examined the gene expression profiles of HCT116 cells either with an intact (control) or deleted YAP1 (YAP1−/−). Data analysis revealed that YAP1 promotes the expression of lipid biosynthetic genes (Fig. 5a, Supplementary Data 1). Gene ontology and KEGG pathway analyses confirmed that lipid biosynthetic processes, like sterol and fatty acid metabolism, are upregulated by YAP1 in mutant KRAS cells (Fig. 5b, Supplementary Data 1). Consistent with YAP1 role in lipid biosynthesis, the silencing of YAP1 by siRNAs decreased the mRNA expression of SREBF1 and SREBF2, as well as the expression of SREBP-dependent genes such as ACAT1, HMGCR, and IDI1, in HCT116 cells but not in HK2-8 cells (Fig. 5c; Supplementary Fig. S3).
a Volcano diagram showing the number of differentially expressed genes in YAP1-replete (WT; control) compared to YAP1−/− HCT116 cells. Dashed horizontal and vertical lines indicate significance thresholds (|FC | > 0.5, P < 0.05). A positive fold change means that the gene is upregulated by YAP1. Genes are colored in gray (non-significant), blue (P-value significant), and red (both P-values and fold change are significant). All labeled genes exhibit statistically significant upregulation (logFC > 0.5, P < 0.0005), consistent with their role in cholesterol biosynthesis and lipid metabolism. b Top KEGG pathways under the control of YAP1 in HCT116 cells. c Graphs assess the expression of SREBF1 and 2 mRNAs by qPCR in cells treated with scrambled or YAP1 siRNAs. Gene expressions were normalized to ACTIN and TUBULIN mRNAs used as internal controls. Data were obtained from 3 independent experiments performed in triplicates and represent ±SEM *P < 0.05, **P < 0.01, ***P < 0.001 (t-test). d ChIP-seq data from ENCODE indicating the binding of TEAD4 to transcriptional regulatory regions of SREBF genes. e ChIP assays of YAP1 for binding in complex with TEAD4 to SREBF genes in HCT116 cells, both in the presence and absence of STAT1 and/or YAP1. IgG, non-specific control antibody. f Immunoblotting of SREBP1 and 2 in isogenic pair colon cancer cells prior to and after YAP1 downregulation by siRNAs. FL, full length. g Immunoblotting of SREBP1, 2 and TEAD4 in isogenic pairs of colon cancer cells treated with scrambled or TEAD4 siRNAs. h Immunoblotting of SREBP1 and 2 proteins in HCT116 cells treated with TEAD inhibitor 15 μM VT104 for the indicated time points. f, g, h Quantification of proteins normalized to TUBULIN for each blot is indicated.
To investigate a possible direct effect of YAP1 on the expression of sterol biosynthetic genes, we analyzed the ChIP-seq data from the ENCODE database. We identified TEAD4 binding sites, a transcriptional partner of YAP1, in the regulatory regions of SREBF genes (Fig. 5d). Using ChIP assays, we confirmed YAP1 binding to the regulatory regions of SREBF1 and SREBF2 that contain TEAD4 binding sites (Fig. 5e). This effect was absent in HCT116 cells where YAP1, STAT1, or both proteins were deleted using the CRISPR approach (Fig. 5e). The absence of YAP1 binding to SREBF genes in STAT1-deleted cells further supports the role of STAT1 in enhancing YAP1 function in HCT116 cells.
Downregulation of YAP1 by siRNAs resulted in a decrease in SREBP1 and SREBP2 protein levels in HCT116 and DLD-1 cells with mutant KRAS, but not in HK2-8 and DKO-1 cells with WT KRAS, respectively (Fig. 5f). Similarly, siRNA-mediated downregulation of TEAD4 reduced the expression of SREBP1 and SREBP2 proteins in HCT116 cells, but not in the isogenic HK2-8 cells (Fig. 5g). Additionally, treatment of HCT116 cells with the TEAD inhibitor VT10441 reduced SREBP1 and SREBP2 levels in HCT116 cells (Fig. 5h).
We further confirmed the role of YAP1 in regulating SREBP1/2 expression using HCT116 YAP1−/− cells reconstituted with either the constitutively active YAP1 5SA or YAP1 5SA/S94A, which is unable to interact with TEAD37. YAP1 5SA restored SREBF1/2 mRNA and SREBP1/2 protein levels in YAP1-deficient cells, whereas the YAP1 5SA/S94A mutant did not show this effect (Supplementary Fig. S4)
These findings strongly support a role for YAP1-TEAD4 in transcriptionally upregulating SREBP1/2, thereby promoting the activation of downstream sterol biosynthetic genes in mutant KRAS cells.
STAT1 and YAP1 act together via the mevalonate pathway to promote the survival and growth of mutant KRAS cells
Further analysis of the gene expression profiles of proficient and deficient HCT116 cells for STAT1 and/or YAP1 revealed that 186 genes were upregulated by both STAT1 and YAP1 (Fig. 6a, Supplementary Data 1). These genes included those with roles in sterol biosynthesis and fatty acid metabolism (Fig. 6b–d, Supplementary Data 1), consistent with the ability of STAT1 and YAP1 to stimulate the expression of SREBPs at the transcriptional level. Taken together, the data indicate that STAT1 and YAP1 form a feedforward autoregulatory loop that drives the expression of SREBPs and lipid biosynthetic genes in mutant KRAS cells (Fig. 6e).
Venn diagram (a) and Volcano diagram (b) of genes that are commonly upregulated by STAT1 and YAP1 in HCT116 cells. Essential genes of sterol and lipid biosynthetic pathways are indicated in the Volcano diagram. b Dashed horizontal and vertical lines indicate significance thresholds (|FC | > 0.5, P < 0.05). A positive fold change means that the gene is upregulated by STAT1 and YAP1. Genes are colored in gray (non-significant), blue (P-value significant), and Red (both P-values and fold change are significant). All labeled genes exhibit statistically significant upregulation (logFC > 0.5, P < 0.0005), consistent with their role in cholesterol biosynthesis and lipid metabolism. c Bar plot of biological processes (BP) from gene ontology (GO) significantly enriched in the common set of genes under the control of both YAP1 and STAT1 identified by gene expression profile analysis. d KEGG pathways under the control of STAT1 and YAP1 in HCT116 cells. e This schematic illustrates the cooperative role of STAT1 and YAP1 in promoting SREBP expression and activating the mevalonate pathway in mutant KRAS CRCs. The STAT1–YAP1 axis functions as a feedforward autoregulatory loop that sustains sterol biosynthesis. STAT1, phosphorylated at S727, directly induces the transcription of SREBF1 and SREBF2 genes. Elevated SREBP levels, in turn, enhance mevalonate pathway activity, leading to the prenylation, plasma membrane anchoring and activation of RHO GTPases. This activation promotes further phosphorylation of STAT1 at S727 and stimulates YAP1 nuclear localization and activation. Although YAP1 acts downstream of STAT1, it also reinforces the loop by cooperating with TEAD4 to transcriptionally upregulate SREBF genes. Created in BioRender. Koromilas, A. (2025) https://BioRender.com/nz5wlhr.
We next investigated the implication of the STAT1-YAP1 arm in the survival and growth of the mutant KRAS CRCs. Treatment with YAP1 siRNAs notably reduced the ability of HCT116 and DLD-1 cells to form colonies, while it did not affect colony formation in HK2-8 and DKO-1 cells, respectively (Supplementary Fig. S5a). Concurrent treatment with YAP1 and STAT1 siRNAs reduced the survival of HCT116 and DLD-1 cells to a similar degree as individual treatments with either YAP1 or STAT1 siRNAs (Supplementary Fig. S5a). Conversely, treatment with YAP1 and/or STAT1 siRNAs, alone or in combination, did not impact the survival of HK2-8 and DKO-1 cells compared to treatment with control scrambled siRNAs (Supplementary Fig. S5a). Immunoblot analysis confirmed the effectiveness of siRNAs in reducing STAT1 and/or YAP1 levels in the isogenic CRCs (Supplementary Fig. S5b). These findings suggest that STAT1 and YAP1 collaborate to support the survival of mutant KRAS tumor cells.
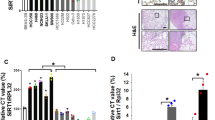
In tumor transplantation assays, we found that deletion of either STAT1 or YAP1 in HCT116 cells by CRISPR led to a similar decrease in tumor growth rate in nude mice, and this growth inhibition was not further diminished by the combined deletion of STAT1 and YAP1 (Fig. 7a). Loss of STAT1 reduced YAP1 nuclear localization and increased its cytoplasmic presence in HCT116 tumors in nude mice, as shown by immunohistochemistry (IHC) analysis, consistent with STAT1-mediated promotion of YAP1 nuclear localization (Fig. 7b). This data provides further support for the collaborative function of STAT1 and YAP1 in the stimulation of the growth of mutant KRAS CRCs.
a, b HCT116 cells with intact or depleted STAT1 and/or YAP1 expression were subcutaneously transplanted into female nu/nu mice (n = 10 per group). Tumor volume (mm³) was measured over the indicated time course. At the end of the study, tumor tissues from 3 mice were analyzed by IHC to assess H&E staining and the subcellular localization of YAP1 (b). c, d Similarly, HCT116 cells with combined YAP1 and STAT1 deletion or expression were transplanted into nu/nu mice (n = 5 per group). When tumors reached ~200 mm3, mice were treated by oral gavage with either vehicle or cerivastatin (CERI). Tumor growth was monitored over time. Red and blue arrows indicate the start point of treatment of YAP1+/+ STAT1+/+ and YAP1−/− STAT1−/− tumors, respectively. At the endpoint, tumors from 3 mice were subjected to IHC for H&E and YAP1 detection (d). a–d Data represent mean ± SEM. Statistical significance was determined by t-test: *P < 0.05, **P < 0.01, ***P < 0.001. Scale bar in panels b and d: 50 μm and 25 μm (insert image). Quantification graphs panels b and d show Histo (H)-scores for nuclear and cytoplasmic YAP1 staining. e HCT116 xenografts were established in nu/nu mice (n = 5 per group). Upon tumor growth to ~200 mm3 (red arrow), mice received either vehicle, cerivastatin (oral), afatinib (intraperitoneal), or the combination of both. Tumor volume was tracked for the indicated duration. Data represent mean ± SEM. ***P < 0.001 (t-test). f In a similar setup, mice bearing HCT116 xenografts (~200 mm3 tumors) were treated with vehicle, cerivastatin (oral), VT104 (oral), or their combination. Tumor growth was monitored throughout the experiment. Data represent mean ± SEM. **P < 0.01 (t-test). g This schematic model illustrates how the STAT1–YAP1 signaling axis enhances the mevalonate pathway, thereby supporting tumor growth and chemoresistance in mutant KRAS CRC. Inhibiting YAP1-TEAD4 activity (e.g., using VT104) disrupts the SREBP-driven feedback loop that sustains mevalonate pathway activation. Additionally, pharmacological blockade of the mevalonate pathway with statins increases tumor sensitivity to YAP1–TEAD4 inhibition in xenograft models of mutant KRAS CRC.
Colony formation and IC50 assays revealed that HCT116 cells were more sensitive than HK2-8 cells to cerivastatin and fluvastatin treatment (Supplementary Fig. S6a, b), suggesting that mutant KRAS enhances susceptibility to mevalonate pathway inhibition. Moreover, HCT116 cells lacking STAT1, YAP1, or both showed increased vulnerability to these inhibitors compared to control cells (Supplementary Fig. S6c, d). These findings indicate that STAT1 and YAP1 support cell survival in mutant KRAS cells by promoting mevalonate pathway activity.
We further assessed the significance of the mevalonate pathway in the tumorigenic effects of STAT1 and YAP1 arm in immune deficient mice. We found that treatment with cerivastatin substantially decreased the growth of control HCT116 tumors proficient for STAT1 and YAP1 (i.e., STAT1+/+ YAP1+/+ tumors) compared to vehicle treated tumors, and that this growth inhibition was at comparable level to the growth of STAT1−/− YAP1−/− tumors (Fig. 7c). Furthermore, cerivastatin treatment did not lead to additional growth reduction in STAT1−/− YAP1−/− tumors (Fig. 7c), indicating that the mevalonate pathway plays a significant, if not exclusive, role in supporting the tumorigenic function of STAT1 and YAP1 in HCT116 cells. IHC analysis revealed decreased nuclear localization of YAP1 in HCT116 tumors from nude mice treated with cerivastatin, further supporting the role of the mevalonate pathway in promoting YAP1 nuclear accumulation (Fig. 7d).
STAT1 and YAP1-mediated resistance to mevalonate pathway inhibition is linked to the efficacy of EGFR and YAP1-TEAD anti-tumor treatments
KRAS mutations in CRC play a crucial role in to the development of resistance to epidermal growth factor receptor (EGFR) inhibition via partially understood mechanisms42. Gene ontology analysis of the STAT1 and YAP1-dependent pathways in HCT116 cells indicated a positive effect of the joint action of these transcription factors on the regulation of EGFR pathway (Fig. 6c). We investigated whether the pro-survival effects of STAT1 and YAP1 influence the response of mutant KRAS cells to EGFR inhibition and whether such an effect could be mediated, at least in part, by the upregulation of the mevalonate pathway.
Examination of the responses of HCT116 cells to treatment with the EGFR inhibitor afatinib revealed that single as well as combined loss of STAT1 and YAP1 renders HCT116 cells more susceptible to treatment than control replete cells (Supplementary Fig. S7a). Further analysis of the HCT116 cell responses to combined treatments with afatinib with either cerivastatin or fluvastatin showed that mevalonate pathway inhibition synergized with EGFR inhibition to suppress HCT116 cell proliferation (Supplementary Fig. S7b). These anti-proliferative effects of the drug treatments were more pronounced in HCT116 cells with single or combined deletions of STAT1 and YAP1 compared to control HCT116 cells (Supplementary Fig. S7b).
To assess the significance of the mevalonate pathway upregulation in treating mutant KRAS tumor cells with EGFR inhibitors, we evaluated HCT116 cell growth in immunodeficient mice before and after treatment with afatinib and cerivastatin. While single treatments with either afatinib or cerivastatin at low concentrations did not yield significant anti-tumor effects, combined treatments significantly suppressed tumor growth rates (Fig. 7e). These results implicate the STAT1-YAP1 axis in the development of resistance to EGFR inhibition via the upregulation of the mevalonate pathway.
Further analysis of the STAT1-YAP1 axis in anti-tumor treatments revealed that HCT116 cells remained resistant to the pan-KRAS inhibitor BI-286543, even when STAT1 and/or YAP1 were absent (Supplementary Fig. S7c). However, combination treatments using mevalonate pathway inhibitors, afatinib, BI-2865, or the YAP1-TEAD inhibitor VT104 showed that dual inhibition of the mevalonate pathway and YAP1-TEAD produced the strongest antiproliferative effects (Supplementary Fig. S7d).
Building on these findings, we evaluated the anti-tumor efficacy of co-targeting the mevalonate pathway and YAP1-TEAD in HCT116 xenografts using immunodeficient mice. While treatment with VT104 or low dose cerivastatin alone had minimal impact, their combination produced a marked reduction in tumor growth (Fig. 7f). These results support a therapeutic strategy that disrupts SREBP feedback activation through YAP1-TEAD inhibition in conjunction with mevalonate pathway blockade, offering a promising approach to counteract STAT1-driven tumorigenesis in mutant KRAS colorectal CRCs (Fig. 7g).
Discussion
CRC is a heterogenous disease characterized by multiple gene alterations, activation of distinct pathways and metabolic remodeling involved in its pathogenesis44. Large-scale transcriptomic analysis has revealed the molecular heterogeneity of CRC and led to the classification of tumors into four consensus molecular subtypes (CMS). Among these, the CMS3 subtype, representing 32% of patients, is associated with a “metabolic” phenotype enriched in KRAS mutations45. KRAS mutations in CRC exhibit differential metabolic remodeling, which can impact disease outcomes and responses to anti-cancer treatments. In mouse models of CRC, metabolic profiling accurately stratified tissues based on various combinations of genetic alterations in APC, KRAS, and PTEN indicating that metabolic profiling can be utilized for patient stratification and the implementation of personalized therapies46.
Previous studies have shown that expression of KRASG13D in colorectal tumor cells alters their metabolic profile compared to WT KRAS, leading to increased cholesterol biosynthesis and suppression of the interferon response47. Consistent with these findings, our research indicates that STAT1 plays a crucial role in promoting the sterol and lipid biosynthetic pathways in HCT116 and DLD1 cells harboring KRASG13D, but not in isogenic tumor cell lines with WT KRAS. We observed that STAT1 stimulates the expression of SREBPs at the transcriptional level leading to the upregulation of de novo sterol and lipid biosynthetic genes. In non-tumorigenic mouse models, STAT1 has been found to inhibit energy expenditure in the liver and reduce mitochondrial biogenesis by suppressing the transcription factor PGC148. Conversely, PGC1 coactivates SREBPs, enhancing lipogenic gene expression in the mouse liver49. These observations suggest that the upregulation of SREBPs and lipid biosynthetic pathways in our study is not an inherent function of STAT1, but rather a consequence of mutant KRAS expression.
Previous research has shown that STAT3 promotes the transcriptional upregulation of SREBPs in glioblastoma cells50, and that STAT5 functions downstream of mTOR to stimulate SREBP1 expression in hepatocellular carcinoma51. However, we found that siRNA-mediated downregulation of either STAT3 or STAT5 did not affect the growth of HCT116 and DLD1 cells or the expression of SREBP1/2 in these cell lines (Supplementary Fig. S8). These results suggest a specific role for STAT1 in regulating SREBP1/2 expression in mutant KRAS CRCs.
Oncogenic KRAS can stimulate mTORC1 in breast epithelial cells, leading to SREBP activation and enhanced lipid synthesis23. Since STAT1 promotes cell survival by upregulating PI3K-mTOR signaling8, it may indirectly increase SREBP expression through this pathway. However, this mechanism alone is unlikely to explain the specificity of STAT1 role in mutant KRAS cells, as STAT1-driven PI3K-mTOR activation occurs in both non-transformed and transformed context8.
The transcriptional effect of STAT1 on the increased expression of SREBPs is independent of Y701 phosphorylation but relies on its phosphorylation at S727. Unphosphorylated STAT1 at Y701 can stimulate gene transcription by binding DNA with low affinity52. Conversely, S727 phosphorylation can enhance the transcriptional functions of STAT1, independent of Y701 phosphorylation, in cells exposed to UV, IL1 or TNF52. In Wilm’s tumors, one of the most common pediatric solid cancers, STAT1 S727 phosphorylation stimulates tumor growth and protects tumor cells from apoptosis under conditions of stress35. Our research has demonstrated that STAT1 S727 phosphorylation in the mutant KRAS CRCs is constitutively upregulated via the mevalonate pathway, necessitating the involvement of RHO GTPases. We found that pharmacological inhibition of ROCK significantly reduces STAT1 S727 phosphorylation, suggesting the involvement of this kinase or another downstream kinase in this process. While RHO GTPases have been previously shown to mediate the phosphorylation and transcriptional activation of STATs53, their role in stimulating STAT1 S727 phosphorylation in mutant KRAS CRCs represents a novel finding.
Although previous studies have identified a role for RHO GTPases in stimulating the nuclear localization and function of YAP136, our data reveal that this process is specifically controlled by STAT1 in mutant KRAS CRCs. The stimulation of YAP1 function by STAT1 is not mediated through direct physical interaction between the two proteins (Supplementary Fig. S9). Instead, it depends on the upregulation of the mevalonate pathway, forming a feedforward loop that sustains the upregulation of sterol biosynthetic genes through the direct transcriptional activation of SREBF1/2 genes by the YAP1-TEAD4 complex (Fig. 6e). In non-tumorigenic models, YAP1 upregulates serum- and glucocorticoid-regulated kinase (SGK) 1 to stimulate mTOR activity, leading to SREBP activation27, Additionally, YAP1 directly interacts with SREBPs to promote lipid biosynthetic gene transcription in hepatocytes of diabetic mice28. However, in mutant KRAS CRCs YAP1 connects with STAT1 to sustain SREBP expression and lipid biosynthesis, highlighting a unique interplay between STAT1, YAP1, and the mevalonate pathway in cancer cell metabolism and growth.
Our study demonstrates that the STAT1-YAP1 axis, through the upregulation of the mevalonate pathway, significantly enhances the growth of mutant KRAS CRC tumors. We obtained evidence that lung tumor H358 cells harboring KRAS G12C also depend on STAT1 and YAP1 for survival and the upregulation of SREBP1/2 expression (Supplementary Fig. S10).This is consistent with other research that highlights the tumorigenic role of SREBPs in mutant KRAS-driven cancers54,55and the beneficial impact of mevalonate pathway inhibition by statins in CRC prevention56. Additionally, STAT1 and YAP1 jointly confer resistance to mevalonate pathway inhibitors in mutant KRAS CRCs. Although STAT1 has been previously recognized for its role in protecting tumor cells from chemotherapeutic drugs57,58, this is the first report highlighting its involvement in mediating tumor survival against metabolic therapies targeting a mutant KRAS cancer. On the other hand, multiple studies have supported a pro-survival role for YAP1 in mutant KRAS cancers16,17,18,19. Inhibiting the transcriptional activity of YAP1 with TEAD inhibitors significantly disrupts mutant KRAS effector pathways and sensitizes tumor cells to drugs targeting mutant KRAS59,60,61.
We observed that HCT116 cells are resistant to pharmacological inhibition of mutant KRAS but highly sensitive to mevalonate pathway inhibitors especially when combined with the pharmacological inhibition of YAP1-TEAD (Fig. 7g). Thus, KRAS mutations may shift the dependency of tumors to other pathways for their growth like the mevalonate pathway. Consistent with this view, previous studies have shown that mutant KRAS cancers can undergo a switch in oncogene addiction, driven by the activation of distinct effector pathways and metabolic changes that influence their responsiveness to anti-tumor therapies62.
In CRC, mutant KRAS is recognized as a key factor influencing response to chemotherapeutic therapies42. For instance, resistance to anti-EGFR therapy is closely associated with KRAS mutations through mechanisms that are not fully understood42. Activation of YAP1 by the mevalonate pathway has been associated with resistance to EGFR inhibition in colorectal cancer63. Expression of KRAS G13D in CRC cells alters EGFR networks, reshaping the metabolic and transcriptional landscapes of tumor cells, which has significant implications for therapy47,64. Our findings indicate that the STAT1-YAP1 axis plays a role in the development of resistance to the EGFR inhibitor afatinib by upregulating the mevalonate pathway. Notably, this resistance can be overcome by inhibiting the mevalonate pathway in xenograft assays in mice.
The cooperation between STAT1 and YAP1 in promoting lipid biosynthesis may have significant implications for the establishment of anti-tumor immunity in mutant KRAS cancers. STAT1 is a key player in the IFN response triggered by the activation of the stimulator of interferon genes (STING) pathway65. In contrast, YAP1 has antagonistic effects on STING pathway activation and anti-tumor immunity66. However, research has shown that cholesterol biosynthesis can undermine STING signaling in virus-infected cells and tumor cells67,68,69. Cholesterol also promotes the retention of STING in the endoplasmic reticulum, a process that can be reversed by cholesterol depletion, thereby stimulating anti-tumor immunity when treated with immune checkpoint inhibitors70. The ability of STAT1 to collaborate with YAP1 to upregulate the mevalonate pathway may reveal a new mechanism used by mutant KRAS tumors to compromise the IFN response and impair anti-tumor immunity. This potential function of STAT1 would differ from its established role in activating the IFN response and could provide a crucial link between its tumor-intrinsic and immune regulatory functions in the treatment of mutant KRAS cancers.
Materials and methods
Cell culture and treatments
HCT116, HK2-8, DLD-1, and DKO-1 cells were cultured in Dulbecco’s Modified Eagle Medium (Wisent) supplemented with 10% fetal calf serum (Wisent) and 100 U/mL penicillin-streptomycin (Wisent). Isogenic colon cancer cell lines were authenticated for the presence of the KRAS G13D by DNA sequencing. The cells were free of mycoplasma contamination. Depletion of STAT1 and YAP1 using clustered regularly interspaced palindromic repeats (CRISPR)/CAS9 was conducted as previously described9,71; the guide RNA sequences are provided in Supplementary Table S1. Control cells were those expressing CRISPR/Cas9 vectors lacking guide RNA sequences. Transient downregulation of STAT1 or YAP1 was achieved using four different siRNAs (Horizon Discovery) with sequences listed in Supplementary Table S1. Fluvastatin, cerivastatin, afatinib and BI-2865 were purchased from MedChemExpress, VT104 was acquired from DC Chemicals, Y-27632 was sourced from Selleckhem and 4’,6-diamidino-2-phenylindole (DAPI) was obtained from Roche. Colony formation assays were conducted with 2 × 103 cells in 6-well petri dishes subjected to treatments as indicated in the Fig. legends for up to 14 days. Cells were fixed in 3.7% formaldehyde (v/v) and stained with 0.2% crystal violet (w/v). Colonies were scored using an automated cell colony counter (GelCount; Oxford Optronix).
siRNA, DNA transfections and luciferase reporter assay
Plasmid and siRNA transfections were carried out using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s specifications. The 8xGTIIC-luciferase construct was obtained from Addgene (plasmid #34615)38. Plasmids containing the green fluorescence protein (GFP)-tagged forms of wild type STAT1, STAT1 Y701F or STAT1 S727A cDNA were obtained from Addgene (plasmid #12301,12302 and 12304)35. Firefly luciferase assays were performed with the Dual-Luciferase Reporter Assay System (Promega) using Renilla luciferase reporter gene serving as an internal control72.
Chromatin immunoprecipitation (ChIP) and real-time PCR
ChIP assays were conducted following previously established protocols72. Briefly, cells were cross-linked with formaldehyde at a final concentration of 1% for 10 min followed by addition of glycine in PBS at a final concentration of 125 mM. Cells were rinsed ice cold 1XPBS twice and scraped and collected in 1 ml ice cold PBS. Cells were spun down and washed with cold PBS once, and nuclear-enriched extracts were prepared by 1 ml lysis buffer (5 mM PIPES, pH 8.0, 85 mM KCl, 0.5% NP-40) plus protease inhibitor. The lysate was sonicated with a Sonifier (Vibra Cell, Soinc & Material INS) to shear the chromatin (Output 20%, 100% duty cycle, 8×15-sec pulses), and the samples were clarified by centrifugation. Protein–DNA complexes were immunoprecipitated (IP) with anti-STATs or GFP-Trap Agarose overnight at 4°C on a rotator. Anti-STATs IP samples were incubated with protein A-agarose for 1 h at 4 °C. Anti-STATs or GFP-Trap Agarose samples were followed by one time low salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-Cl pH8.1, 150 mM NaCl), two time high salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-Cl pH8.1, 500 mM NaCl), one time LiCl buffer (250 mM LiCl, 1% NP40, 1 mM EDTA, 10 mM Tris-CL pH8.1, 1% deoxycholate). The protein-DNA complexes were eluted from the beads by addition of 1% SDS, 0.1 M NaHCO3 at 65 °C for 1 hour. The beads were removed by centrifugation, and the supernatants were incubated overnight at 65 °C to reverse the cross-linking. The DNA was extracted by the addition of RNase A (50 μg/ml, 37 °C for 30 min) and proteinase K solution (300 µg/mL, 55 °C for 2 h) and subjected to purification with a QIAquick PCR purification kit. The enriched DNA was quantified by real-time PCR using a set of primers that cover the STAT1-binding site in the SREBF1 and SREBF2 genes (Supplementary Table S1). Signals obtained from each immunoprecipitation were expressed as a percentage of the total input chromatin. Percent (%) input = 2% x 2 (C[T] 2% Input Sample-c[T] IP Sample) where C[T] = CT = threshold cycle of PCR reaction.
Total RNA was isolated using TRIzol (Invitrogen), and 1 µg of RNA was reverse transcribed (RT) using the SuperScript III Reverse Transcriptase kit (Invitrogen) with 100 µM oligo (dT) primer, following the manufacturer’s instructions. Real-time PCR was carried out using the SensiFast SYBR Lo-ROX kit (Bioline) with primers listed in Supplementary Table S1. The PCR assays included primers for GAPDH and ACTIN mRNAs as internal controls, in accordance with the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines73.
Immunoblotting
Cells were washed twice with ice-cold PBS, and proteins were extracted using an ice-cold lysis buffer containing 10 mM Tris-HCl (pH 7.5), 50 mM KCl, 2 mM MgCl2, 1% Triton X-100, 3 μg/ml aprotinin, 1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 mM dithiothreitol, 0.1 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride. The extracts were incubated on ice for 15 minutes, then centrifuged at 10,000 × g for 15 minutes at 4 °C. Supernatants were stored at −80 °C. Protein concentrations were measured using the Bradford assay (Bio-Rad). To evaluate the expression of various proteins, 50 µg of protein extracts from the same set of samples were loaded in parallel onto two identical sodium dodecyl sulfate (SDS)-polyacrylamide gels. After transferring the proteins to Immobilon-P membranes (Millipore), the blots were cut into smaller sections based on the molecular weights of the target proteins. One section was probed for the phosphorylated protein of interest, while the corresponding section was probed for the total protein. This approach bypassed the limitations caused by increased background signal following the stripping of phospho-specific antibodies, which interfered with subsequent reprobing using total antibodies. Protein extract loading was normalized to ACTIN or TUBULIN levels. The antibodies used for immunoblotting are listed in Supplementary Table S2. Protein visualization was performed using enhanced chemiluminescence (ECL) according to the manufacturer’s instructions (Amersham Biosciences). Band quantification within the linear range of exposure was carried out using ImageJ 1.51e software (NIH, Maryland, USA).
Gene expression analysis
Samples were processed using the Affymetrix Clariom S Human HT Expression platform. The data was normalized using the ‘rma’ method in the Bioconductor package ‘oligo’. The ‘rma’ method involves background subtraction, normalization with the RMA algorithm, and summarization using median-polish. The expression values were then transformed to the log2 scale. Annotation for the Affymetrix Clariom S Human HT was obtained using the ‘clariomshumanhttranscriptcluster.db’ database provided by Bioconductor. Differential expression analysis of genes in STAT1 and/or YAP1-deficient cells compared to proficient (control) cells was performed using the Bioconductor package ‘limma’74. Gene network analysis was conducted using the output of the Gene Ontology (GO) analyses with the Bioconductor package ‘DOSE’75.
Subcellular fractionation and immunofluorescence assays
Subcellular fractionation, cells were lysed on 10 cm plates using 500 µl of buffer containing 250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, and 1 mM EGTA, and were incubated on ice for 20 minutes. The nuclear pellet was obtained by centrifugation at 720 g for 5 minutes, then washed and lysed with standard RIPA lysis buffer. The supernatant, representing the cytoplasmic fraction, was collected, and sample buffer was added. THOC-1 served as the nuclear marker, while tubulin was used as the cytoplasmic marker.
For immunofluorescence staining, cells were fixed with 4% paraformaldehyde for 10 minutes, washed with PBS, permeabilized with 0.5% Triton X-100 for 5 minutes, and blocked with 5% goat serum and 5% BSA for 30 minutes. Antigen detection was carried out by incubating the cells with the primary antibody overnight at 4°C, followed by incubation with goat anti-rabbit Alexa Fluor 546 as the secondary antibody for 30 minutes at room temperature. Nuclei were counterstained with 0.5% DAPI. The primary and secondary antibodies used are listed in Supplementary Table S2.
Xenograft tumor assays
Tumor transplantation assays were conducted in 8-week-old female athymic nude mice (Charles River Inc.) following the established protocol9. Briefly, cells were suspended in a 1:1 v/v mixture of phosphate-buffered saline (PBS): Matrigel (Corning) and injected subcutaneously into the flanks of nude mice. Mice were inoculated with 5×105 cells in 200 µl PBS: Matrigel mix. Tumor growth was monitored twice per week using digital calipers, and the volume was calculated using the formula: tumor volume [mm3] = π/6 x (length [mm]) x (width [mm]) x (height [mm]). Mice were subjected to treatments with afatinib, cerivastatin or VT104 at doses reported to have minimal side effects on body toxicity59,76,77,78. Tumor growth observation ended when the tumor reached approximately 2.0 cm (20 mm) in any direction. The tumor measurements/volumes permitted by the Animal Welfare Committee of McGill University were not exceeded in any of the animal experiments. The animal studies were conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) of McGill University, and all procedures were approved by the Animal Welfare Committee of McGill University (protocol #5754). The study fully complied with all relevant ethical regulations for animal use.
Immunohistochemistry
Tumor tissues were fixed in 10% buffered formalin phosphate, paraffin embedded, and sectioned. Paraffin was removed from the sections after treatment with xylene, rehydrated in graded alcohol. Tumor samples were cut at 4-µm, placed on TOMO slides (VWR) and dried overnight at 37 °C, before IHC processing. The slides were then loaded onto the Discovery XT Autostainer (Ventana Medical System). All solutions used for automated immunohistochemistry were from Ventana Medical System (Roche Tissue Diagnostics) unless otherwise specified. Slides underwent de-paraffinization, heat-induced epitope retrieval (CC1 prediluted solution, Roche Tissue Diagnostics, 06414575001). Immunostaining for YAP1 was performed using a heat protocol. Briefly, rabbit polyclonal anti-YAP1 (CST, cat# 14074S) diluted in the antibody diluent (Roche Tissue Diagnostics, 06440002001) were manually applied for 32 min at 37 °C then followed by the appropriate detection kit (OmniMap anti-Rabbit-HRP, Roche Tissue Diagnostics, 05269679001, for 8 min, followed by ChromoMap-DAB, Roche Tissue Diagnostics, 05266645001). Slides were counterstained with Hematoxylin (Roche Tissue Diagnostics, 05266726001) for 12 minutes. Sections were scanned using the Aperio AT Turbo Scanner (Leica Biosystems). Quantification of stained sections was performed using QuPath 0.5.0.
Statistical and reproducibility
Statistical analysis was conducted using three biological replicates. Error bars represent the standard error as indicated, and significance in differences between arrays of data was determined using a two-tailed Student’s t-test.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA seq data generated in this study have been deposited in the Gene Expression Omnibus (GEO) GSE278710. Uncropped, unedited blot images and the gating strategies of FACS plots are available in Supplementary Information, while numerical source data for the graphs are included in Supplementary Data 2.
References
Simanshu, D. K., Nissley, D. V. & McCormick, F. RAS proteins and their regulators in human disease. Cell 170, 17–33 (2017).
Hobbs, G. A., Der, C. J. & Rossman, K. L. RAS isoforms and mutations in cancer at a glance. J. Cell Sci. 129, 1287–1292 (2016).
Kerk, S. A., Papagiannakopoulos, T., Shah, Y. M. & Lyssiotis, C. A. Metabolic networks in mutant KRAS-driven tumours: tissue specificities and the microenvironment. Nat. Rev. Cancer 21, 510–525 (2021).
Philips, R. L. et al. The JAK-STAT pathway at 30: much learned, much more to do. Cell 185, 3857–3876 (2022).
Meissl, K., Macho-Maschler, S., Müller, M. & Strobl, B. The good and the bad faces of STAT1 in solid tumours. Cytokine 89, 12–20 (2017).
Raven, J. F. et al. Stat1 is a suppressor of ErbB2/Neu-mediated cellular transformation and mouse mammary gland tumor formation. Cell Cycle 10, 794–804 (2011).
Cheon, H. et al. IFNbeta-dependent increases in STAT1, STAT2, and IRF9 mediate resistance to viruses and DNA damage. EMBO J. 32, 2751–2763 (2013).
Wang, S., Patsis, C. & Koromilas, A. E. Stat1 stimulates cap-independent mRNA translation to inhibit cell proliferation and promote survival in response to antitumor drugs. Proc. Natl. Acad. Sci. USA 112, E2149–E2155 (2015).
Wang, S., Darini, C., Desaubry, L. & Koromilas, A. E. STAT1 promotes KRAS colon tumor growth and susceptibility to pharmacological inhibition of translation initiation factor eIF4A. Mol. Cancer Ther. 15, 3055–3063 (2016).
Wang, S. & Koromilas, A. E. STAT1-mediated translational control in tumor suppression and antitumor therapies. Mol. Cell Oncol. 3, e1055049 (2016).
Harvey, K. F., Zhang, X. & Thomas, D. M. The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 (2013).
Piccolo, S., Panciera, T., Contessotto, P. & Cordenonsi, M. YAP/TAZ as master regulators in cancer: modulation, function and therapeutic approaches. Nat. Cancer 4, 9–26 (2023).
Reddy, B. V. V. G. & Irvine, K. D. Regulation of Hippo Signaling by EGFR-MAPK Signaling through Ajuba Family Proteins. Dev. Cell. 24, 459–471 (2013).
Hong, X. et al. Opposing activities of the Ras and Hippo pathways converge on regulation of YAP protein turnover. EMBO J. 33, 2447–2457 (2014).
Zhang, Z. et al. OTUB2 promotes cancer metastasis via hippo-independent activation of YAP and TAZ. Mol. Cell. 73, 7–21.e27 (2019).
Kapoor, A. et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell 158, 185–197 (2014).
Shao, D. D. et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 158, 171–184 (2014).
Zhang, W. et al. Downstream of mutant KRAS, the transcription regulator YAP Is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci. Signal. 7, ra42–ra42 (2014).
Johnson, C. W. & Haigis, K. M. All roads lead to Rome: YAP/TAZ activity influences efficacy of KRASG12C inhibitors. Cancer Res. 83, 4005–4007 (2023).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
Peck, B. & Schulze, A. Lipid metabolism at the nexus of diet and tumor microenvironment. Trends Cancer 5, 693–703 (2019).
Soyal, S. M., Nofziger, C., Dossena, S., Paulmichl, M. & Patsch, W. Targeting SREBPs for treatment of the metabolic syndrome. Trends Pharmacol. Sci. 36, 406–416 (2015).
Ricoult, S. J., Yecies, J. L., Ben-Sahra, I. & Manning, B. D. Oncogenic PI3K and K-Ras stimulate de novo lipid synthesis through mTORC1 and SREBP. Oncogene 35, 1250–1260 (2016).
Singh, A. et al. De novo lipogenesis represents a therapeutic target in mutant Kras non-small cell lung cancer. FASEB J. 32, fj201800204–fj201800204 (2018).
Gouw, A. M. et al. Oncogene KRAS activates fatty acid synthase, resulting in specific ERK and lipid signatures associated with lung adenocarcinoma. Proc. Natl. Acad. Sci. USA 114, 4300–4305 (2017).
Blanc, M. et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 38, 106–118 (2013).
Vaidyanathan, S. et al. YAP regulates an SGK1/mTORC1/SREBP-dependent lipogenic program to support proliferation and tissue growth. Dev. Cell. 57, 719–731.e718 (2022).
Shu, Z. et al. A functional interaction between Hippo-YAP signalling and SREBPs mediates hepatic steatosis in diabetic mice. J. Cell Mol. Med. 23, 3616–3628 (2019).
Chou, P. H. et al. A chemical probe inhibitor targeting STAT1 restricts cancer stem cell traits and angiogenesis in colorectal cancer. J. Biomed. Sci. 29, 20 (2022).
Shirasawa, S., Furuse, M., Yokoyama, N. & Sasazuki, T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science 260, 85–88 (1993).
Mullen, P. J., Yu, R., Longo, J., Archer, M. C. & Penn, L. Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer 16, 718–731 (2016).
Li, J. N., Mahmoud, M. A., Han, W. F., Ripple, M. & Pizer, E. S. Sterol regulatory element-binding protein-1 participates in the regulation of fatty acid synthase expression in colorectal neoplasia. Exp. Cell Res. 261, 159–165 (2000).
Shimomura, I., Shimano, H., Horton, J. D., Goldstein, J. L. & Brown, M. S. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J. Clin. Invest. 99, 838–845 (1997).
Stark, G. R. & Darnell, J. E. Jr. The JAK-STAT pathway at twenty. Immunity 36, 503–514 (2012).
Timofeeva, O. A. et al. Serine-phosphorylated STAT1 is a prosurvival factor in Wilms’ tumor pathogenesis. Oncogene 25, 7555–7564 (2006).
Sorrentino, G. et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 16, 357–366 (2014).
Zhao, B. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 (2008).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Ishizaki, T. et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 57, 976–983 (2000).
Zhao, B., Li, L., Tumaneng, K., Wang, C. Y. & Guan, K. L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 24, 72–85 (2010).
Tang, T. T. et al. Small molecule inhibitors of TEAD auto-palmitoylation selectively inhibit proliferation and tumor growth of NF2-deficient mesothelioma. Mol. Cancer Ther. 20, 986–998 (2021).
Knickelbein, K. & Zhang, L. Mutant KRAS as a critical determinant of the therapeutic response of colorectal cancer. Genes Dis. 2, 4–12 (2015).
Kim, D. et al. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature https://doi.org/10.1038/s41586-023-06123-3 (2023).
Yaeger, R. et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell. 33, 125–136.e123 (2018).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Vande Voorde, J. et al. Metabolic profiling stratifies colorectal cancer and reveals adenosylhomocysteinase as a therapeutic target. Nat. Metab. 5, 1303–1318 (2023).
Charitou, T. et al. Transcriptional and metabolic rewiring of colorectal cancer cells expressing the oncogenic KRAS(G13D) mutation. Br. J. Cancer 121, 37–50 (2019).
Sisler, J. D. et al. The signal transducer and activator of transcription 1 (STAT1) inhibits mitochondrial biogenesis in liver and fatty acid oxidation in adipocytes. PLoS One 10, e0144444 (2015).
Lin, J. et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell 120, 261–273 (2005).
Fan, Y. et al. STAT3 activation of SCAP-SREBP-1 signaling upregulates fatty acid synthesis to promote tumor growth. J. Biol. Chem. 300, 107351 (2024).
Li, T. et al. mTOR direct crosstalk with STAT5 promotes de novo lipid synthesis and induces hepatocellular carcinoma. Cell Death Dis. 10, 619 (2019).
Yang, J. & Stark, G. R. Roles of unphosphorylated STATs in signaling. Cell Res. 18, 443–451 (2008).
Corry, J., Mott, H. R. & Owen, D. Activation of STAT transcription factors by the Rho-family GTPases. Biochem Soc. Trans. 48, 2213–2227 (2020).
Wen, Y.-A. et al. Downregulation of SREBP inhibits tumor growth and initiation by altering cellular metabolism in colon cancer. Cell Death Dis. 9, 265 (2018).
Ruiz, C. F., Montal, E. D., Haley, J. A., Bott, A. J. & Haley, J. D. SREBP1 regulates mitochondrial metabolism in oncogenic KRAS expressing NSCLC. FASEB J. 34, 10574–10589 (2020).
Dobrzycka, M. et al. Statins and colorectal cancer—a systematic review. Exp. Clin. Endocrinol. Diabetes 128, 255–262 (2020).
Khodarev, N. N. et al. Signal transducer and activator of transcription 1 regulates both cytotoxic and prosurvival functions in tumor cells. Cancer Res. 67, 9214–9220 (2007).
Khodarev, N. N., Roizman, B. & Weichselbaum, R. R. Molecular pathways: interferon/stat1 pathway: role in the tumor resistance to genotoxic stress and aggressive growth. Clin. Cancer Res. 18, 3015–3021 (2012).
Edwards, A. C. et al. TEAD inhibition overcomes YAP1/TAZ-driven primary and acquired resistance to KRASG12C inhibitors. Cancer Res. 83, 4112–4129 (2023).
Hagenbeek, T. J. et al. An allosteric pan-TEAD inhibitor blocks oncogenic YAP/TAZ signaling and overcomes KRAS G12C inhibitor resistance. Nat. Cancer 4, 812–828 (2023).
Mukhopadhyay, S. et al. Genome-wide CRISPR screens identify multiple synthetic lethal targets that enhance KRASG12C inhibitor efficacy. Cancer Res. 83, 4095–4111 (2023).
Yuan, T. L. et al. Differential effector engagement by oncogenic KRAS. Cell Rep. 22, 1889–1902 (2018).
Liu, B. S. et al. Inhibition of YAP reverses primary resistance to EGFR inhibitors in colorectal cancer cells. Oncol. Rep. 40, 2171–2182 (2018).
Kennedy, S. A. et al. Extensive rewiring of the EGFR network in colorectal cancer cells expressing transforming levels of KRASG13D. Nat. Commun. 11, 499 (2020).
Hopfner, K.-P. & Hornung, V. Molecular mechanisms and cellular functions of cGAS–STING signalling. Nat. Rev. Mol. Cell Biol. 21, 501–521 (2020).
Zhang, Q., Zhou, R. & Xu, P. The hippo pathway in innate anti-microbial immunity and anti-tumor immunity. Front Immunol. 11, 1473 (2020).
Zhu, Y. et al. STING: a master regulator in the cancer-immunity cycle. Mol. Cancer 18, 152 (2019).
O’Neill, L. A. How low cholesterol is good for anti-viral immunity. Cell 163, 1572–1574 (2015).
York, A. G. et al. Limiting cholesterol biosynthetic flux spontaneously engages type I IFN signaling. Cell 163, 1716–1729 (2015).
Zhang, B. -c et al. Cholesterol-binding motifs in STING that control endoplasmic reticulum retention mediate anti-tumoral activity of cholesterol-lowering compounds. Nat. Commun. 15, 2760 (2024).
Shen, B. et al. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 23, 720–723 (2013).
Wang, S., Raven, J. F., Durbin, J. E. & Koromilas, A. E. Stat1 phosphorylation determines Ras oncogenicity by regulating p27 kip1. PLoS One 3, e3476 (2008).
Bustin, S. A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622 (2009).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Yu, G., Wang, L.-G., Yan, G.-R. & He, Q.-Y. DOSE: an R/Bioconductor package for disease ontology semantic and enrichment analysis. Bioinformatics 31, 608–609 (2014).
Gbelcová, H. et al. Differences in antitumor effects of various statins on human pancreatic cancer. Int. J. Cancer 122, 1214–1221 (2008).
Zhao, T. et al. AZD8055 enhances in vivo efficacy of afatinib in chordomas. J. Pathol. 255, 72–83 (2021).
Moll, H. P. et al. Afatinib restrains K-RAS-driven lung tumorigenesis. Sci. Transl. Med. 10, https://doi.org/10.1126/scitranslmed.aao2301 (2018).
Acknowledgements
We thank S. Shirasawa (Fukuoka University, Japan) for HCT116 and HK2-8 cells; M. Sudol (National University of Singapore) for the CRISPR-YAP1_gRNA construct; R. DeBose-Boyd (UT Southwestern Medical Center) for HMGCR (A9) mouse monoclonal antibody. The work was supported by funds from the Cancer Research Society Inc., the Marjorie Sheridan Innovation grant of the Canadian Cancer Society (CCSRI #701631) and Canadian Institutes of Health Research (CIHR; PJT-178173) to A.E.K.; H.K. is supported by George G. Harris Fellowship in Cancer Research from McGill university and internal scholarship award from Lady Davis Institute. J.Y.Z. is supported by a studentship from Fonds de recherche Santé Québec.
Author information
Authors and Affiliations
Contributions
S.W. collected, analyzed, interpreted the data and prepared the figures of the manuscript; S.D. performed the bioinformatics analyses of the sequencing data; H.K. assisted in transfection assays of cells; K.K.L. contributed to analysis of cell responses to drug treatments and J.Y.Z. assisted in the analysis of anti-tumor treatments in mice; A.E.K. designed the study, interpreted the data, wrote, and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Tobias Ackermann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Georgios Giamas and Johannes Stortz. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, S., Diao, S., Kim, H. et al. A feedforward loop between STAT1 and YAP1 stimulates lipid biosynthesis, accelerates tumor growth, and promotes chemotherapy resistance in mutant KRAS colorectal cancer. Commun Biol 8, 1278 (2025). https://doi.org/10.1038/s42003-025-08740-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08740-2