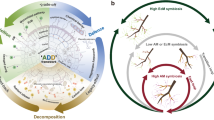
Abstract
Besides their symbiotic association with tree rootlets, ectomycorrhizal (EM) fungi have been commonly detected in nature in deadwood and plant debris of various tree species. However, their potential dual roles as symbiotrophs and saprotrophs are still debated. Here, we provide evidence from a series of experiments on the plasticity of symbiotrophic-saprotrophic lifestyles of the ectomycorrhizal fungus Piloderma croceum associated with Quercus robur L. Specifically, we find that P. croceum efficiently colonizes deadwood of oak in an experimental system without living oak. Results based on the productions of hydrolytic enzymes and corticrocin as well as the 14C content in deadwood and mycelium of P. croceum demonstrate its capability of wood decomposition and assimilation of C from the decomposing wood. Our results also show that in presence of wood pieces colonized by saprotrophic mycelium of P. croceum, the roots of oak plants develop true EM symbiosis with Hartig net formation. Collectively, our results indicate a role for mycelium growing in deadwood as an underestimated EM fungus propagule bank, suggesting that deadwood and other decomposing plant material may indirectly influence the productivity of forest ecosystems by contributing to the recruitment of mycorrhizal fungi, thereby enhancing plant nutrient acquisition.
Similar content being viewed by others
Introduction
Ectomycorrhizal fungi (EM fungi) play a central role in providing trees with important nutrients especially in boreal and temperate but also in some subtropical and tropical forest ecosystems1. The existence and functioning of EM fungal mycelia determine plant growth and productivity in the forest ecosystems and contribute to the large global pool of carbon in soils1,2. The mycelia of EM fungi extend from different individual host tree species and form common mycorrhizal networks in soils, which influence plant nutrient and carbon acquisition at ecosystem level3. These mycelia can act as propagules for EM fungi to colonize seedlings and stimulate forest regeneration3. In areas where mycorrhizal networks are not available or poorly formed, EM fungal spores in soils can be considered an important source of propagules4. EM fungal spores may persist in the soil for decades before colonizing plant roots5. Extramatrical mycelium and spores in soils are thus considered as the major propagule banks of EM fungi3, but it is currently unknown whether EM fungi colonized plant debris could also serve as propagule banks.
Recent experimental evidence, based on high-throughput sequencing, has demonstrated that deadwood harbors diverse EM fungal communities6,7,8. EM fungi colonize deadwood and use this niche, among others (litter and living roots). EM fungi have evolved on repeated independent occasions among clades of initially humus and wood saprotrophic fungi, so that EM fungal taxa are spread across the classification of Asco- and Basidiomycota, where they still neighbor some saprotrophic sister taxa within clades9,10. EM fungi can compete with saprotrophs in deadwood. Their competitiveness depends on priority effects (or community assembly history), depending on their early arrival to the substrate or the wood decay stage8,11. Although EM fungi can also be detected at early wood decay stages in some temperate and subtropical tree species, they dominate during the late decomposition stages6,8,12. Some EM fungi have been suggested to have proteolytic activity via the secretion of relevant enzymes that support the decomposition of dead biomass13,14. Several studies have demonstrated the potential of EM fungi to degrade complex plant polymers, including cellulose, hemicellulose, pectin, and lignin15,16. However, the enzymatic machinery for the decomposition of these polymers is strongly reduced in EM fungi15,17,18. Some EM fungi can oxidize organic matter, either by Fenton chemistry, such as Paxillus involutus and Piloderma croceum19, or by using peroxidases, such as Cortinarius spp.20. It has been suggested that these activities serve to obtain nitrogen from organic sources21. The conservation level of enzymes for plant polymer decomposition is particularly high in P. croceum, whose genome contains 52, 18, and 38 genes related to cellulose/hemicellulose, pectin, and lignin decomposition, respectively17. Such carbon degrading enzyme activities can serve to release glucose and other small carbon compounds and molecules from complex organic sources22,23 which can be utilized by P. croceum. Therefore, decomposing plant materials may promote ectomycorrhizal establishment and propagation of P croceum in temperate forest ecosystems24. It has been hypothesized to be a potential ectomycorrhizal propagule bank in forest ecosystems6,25. Although some EM fungi might have evolved from either saprotrophic or endophytic fungi, their evolution via saprotrophic fungi has been more common26. Decomposing plant materials including, deadwood and leaf litter are present together or in the vicinity with fine roots in the detritusphere and topsoil24,27. Closely related fungi even within the same genera have evolved differently, by specializing in these substrates17. While saprotroph fungi have evolved toward litter and deadwood materials with increasing/maintaining of plant cell wall degrading enzymes related genes (especially Carbohydrate-Active EnZymes (CAZymes)), many EM fungi displayed reduction of such genes, while maintaining and increasing symbiosis related genes such as sugar transporters, transposable elements (TEs) and small secreted proteins (SSPs)17,28. However, some EM fungi have retained substantial numbers of plant cell-wall degrading genes and have been found to have the ability to decompose plant organic matter17,29,30. Thus, deadwood and leaf litter can be considered as alternative substrates for such EM fungi, in life phases or compartments where they cannot establish ectomycorrhiza6,11,12,31. In forest ecosystem, it is also common that fine root patches of diverse trees colonize decomposing deadwood and leaf litter, from which they obtain nutrients via their EM fungal partners. When EM fungi can survive as saprotroph on decomposing plant materials (especially deadwood), they can increase their potential to find right host plants and to increase the chance of successful colonization6.
The plasticity of symbiotroph-saprotroph EM fungal lifestyles has been hypothesized for more than a decade32. A study also demonstrated that many saprotrophic fungi are able to colonize fine roots of ectomycorrhizal trees and form either incomplete or complete ectomycorrhizas, indicating the potential for such plasticity29. However, thus far there is no substantial evidence that demonstrates the efficient colonization of deadwood and its components by EM fungi and whether EM fungi-colonizing deadwood can change their lifestyles from saprotrophic to ectomycorrhizal programs. The dual role of EM fungi as a symbiotroph and a saprotroph therefore still under debate33.
In this study, Piloderma croceum associated with Quercus robur L. was used to demonstrate the plasticity of symbiotroph-saprotroph lifestyles of EM fungi. The saprotroph lifestyle can be demonstrated by the ability of Piloderma croceum to colonize and decompose dead organic matter from wood. The decomposition products (especially glucose) and nutrients released from dead organic matter have to be incorporated into the mycelium of the fungus. To demonstrate the switch from the saprotroph to the symbiotroph- lifestyle, P. croceum living in the saprotroph stage on dead wood, had to be able to colonize the living roots of Q. robur plants and exhibit a clear symbiotic apposition structure (i.e. Hartig net), as well as a benefits of EM formation, i.e. tree growth stimulation. Therefore, in this current work nine experiments were carried out to test whether (1) P. croceum can colonize a decomposing plant material, deadwood (bark, outer sapwood, inner sapwood, and the complete deadwood) of Quercus robur in the absence of living oak plants (experiments 1 to 4) and (2) such colonized decaying wood can serve as a propagule bank for forming new mycorrhizal symbiosis when faced with roots of living oaks (experiments 5 to 9). Specifically, we aimed to answer four questions. First, could P. croceum grow and persist on bark and wood substrates (experiment 1)? This would demonstrate the capacity to live or persist when not in symbiosis with roots (but not yet to establish a saprotrophic lifestyle). Second, what was the fungal enzymatic activity in wood colonized by P. croceum (experiment 2)? This would demonstrate that the fungus can release enzymes (as expected) that are capable of decomposing polymers of wood fibers, i.e., that it is truly saprotrophic. Third, was any wood carbon or related products, as measured via all-E-tetradeca-2, 4, 6, 8, 10, 12-hexaene-l,14-dioic acid (corticrocin) (experiment 3) and carbon isotope (14C) (experiment 4), detectable in vegetative tissue of the fungus? This would further demonstrate that the fungus is capable of decomposition and assimilating carbon and glucose from wood fibers and polymers, i.e., saprotrophic. Fourth, can deadwood be a source of dispersal for P. croceum to establish ectomycorrhizal roots (experiments 5 to 9)? This would show that wood can be a source of inoculants for the fungus, a key tenant of the research.
Based on plasticity of symbiotroph-saprotroph lifestyles of EM fungi, we hypothesized that P. croceum is able to grow and persist on bark and wood substrates32 (hypothesis 1). We hypothesized that based on its gene repertoire, P. croceum can secrete both hydrolytic (β-glucosidase) and oxidative enzymes (laccase and peroxidases) that are important for cellulose/hemicellulose and lignin degradation during an exclusive saprotrophic life phase17,18 (hypothesis 2). Furthermore, we expected that P. croceum would produce N-acetyl glucosaminidases and acid phosphatases to acquire N from chitin and organic P. Because of its ability to produce enzymes for C acquisition and lignin degradation/modification, we also hypothesized that the C released from deadwood could be transferred to and assimilated by P. croceum mycelium (hypothesis 3). We expected that Q. robur plants associated with P. croceum originating from deadwood would show the benefit of EM formation34 and their plasticity to outsource C with higher principal root, lateral root, and total root biomass, and stimulate plant growth with higher stem, shoot, and total plant biomass, compared with non-inoculated plants (hypothesis 4).
Results and discussion
P. croceum efficiently colonized deadwood of oaks in the experimental system without living oak, however, the rate of colonization after 4 months of incubation time varied depending on the substrate (100 in bark, 84.6 in complete wood, 40 in sapwood, and 16.7% in inner sapwood) (Fig. 1a and Supplementary Data 1, experiment 1). This finding answers our first question (hypothesis 1) and demonstrates the capacity of P. croceum to live or persist when not in symbiosis with roots of Q. robur. The earliest colonization of deadwood by P. croceum was observed after 2 weeks to 1 month in the bark and complete wood samples (Figs. 1 and S1). At this stage, white mycelium covered parts of the deadwood near the inoculated areas. After 1–2 months, the white mycelium became yellow and extended its development on deadwood (Figs. 1 and S1). After 4 months, the bark and complete wood were covered with massive amounts of P. croceum yellow mycelium, whereas the colonization on sapwood and inner sapwood was moderate. The yellow pigment synthesized by the mycelium of P. croceum has been identified as corticrocin, which is formed during colonization of mycorrhizal host roots35. We demonstrate here that the production of the yellow pigment, as indicated by the size of yellow colonies (p < 0.001) and optical density (OD) 379 (p < 0.001), was significantly influenced by the available glucose and growth of P. croceum in pure culture (Fig. 2a–c and Supplementary Data 2). Thus, corticrocin production in the mycelium of P. croceum colonizing deadwood in MMN 1/10 medium without any other soluble carbohydrate source, but with deadwood substrate, is a good indicator that P. croceum can partly decompose bark and sapwood to acquire organic carbon and glucose, which is consistent with our hypothesis 3. Substantial amount of glucose released from deadwood is required for corticrocin synthesis, thus P. croceum had to decompose deadwood over 1–2 months to reach such level, which also depended on the types of the deadwood materials. Our results show that on fresh bark fastest colonization rates are displayed by a yellow P. croceum mycelium that produces corticrocin. A recent study demonstrated that for temperate trees, the non-structural carbohydrates (NSC especially sugars and starch) are the most concentrated, in the outermost stem wood36. In contrast, the concentrations of NSC is lower, when the mycelium colonization is progressing deeper toward the pith36. We hypothesize that P. croceum takes advantage from the higher concentration of NSC at the outermost part of the stem (bark and vicinity area) to synthesize the corticrocin using the beta-glucosidase produced. Further studies are needed to confirm what supports P. croceum growth in the bark, whereby it is documented in literature that the front of mycelium colonies may recycle resources accumulated in their older part37.
Saprophytic ability a colonization success of different woody materials and b increase in Δ14C in mycelium (mean ± SE) of P. croceum that assimilated C from the introduced wood (IM = initial mycelium of P. croceum, M = 4-month mycelium of P. croceum, MW = 4-month mycelium of P. croceum growing near inner sapwood, and W = inner sapwood). Δ14C values of agar media are provided in Table S1. Different letters indicated significant differences of Δ14C (‰) among mycelial types according to one-way ANOVA with post hoc Tukey’s HSD test at p < 0.05 (n = 3). Differences of Δ14C (‰) between mycelium and wood were analyzed using a t-test at p < 0.05 (n = 3). ***p < 0.001, **p < 0.01.
a, b Give the diameter of the total and yellow (mean ± SE), respectively. Different letters indicated significant differences of the diameter of the total and yellow colony among different available glucose concentrations according to Kruskal–Wallis test with post hoc Mann–Whitney pairwise test at p < 0.05 (n = 5–7). c Gives the ratio between optical density of corticrocin extracts (OD379) and colony dry weight. Different letters indicated significant differences of the ratio between OD379 and colony dry weight among different available glucose concentrations according to Kruskal–Wallis test with post hoc Mann–Whitney pairwise test at p < 0.05 (n = 4). The test statistic H and p values from Kruskal–Wallis test are shown in the upper left-hand corner of each figure.
Hydrolytic and oxidative enzyme activities in complete wood colonized by P. croceum were measured using microplate assays38,39. The results show that P. croceum produced significant amounts of hydrolytic enzymes on deadwood (p = 0.007–0.009), including β-glucosidase (588.4 ± 54.7 nmol h−1 g dry wood−1, mean ± SE), N-acetylglucosaminidase (717.2 ± 157.5, mean ± SE), and acid phosphatase (603.7 ± 95.1, mean ± SE), whereas the activities of oxidative enzymes, including laccase and peroxidase, were undetectable (Table 1). Thus, the glucose available for corticrocin formation was apparently related to β-glucosidase activity, which enables the hydrolysis of cellulose or hemicellulose from Q. robur bark or sapwood. The found activity of N-acetyl glucosaminidase and acid phosphatase demonstrated that P. croceum was also able to recycle N via chitin degradation from part of its own mycelium, and to acquire phosphorus from the wood substrate. The chitinase activity of P. croceum is directly related to earlier observations of P. involutus. Under C starvation, P. involutus uses autolysis with the associated chitinase activity to recycle N and C from its dead fungal mycelium37. The results from enzymatic assays demonstrate that P. croceum can release hydrolytic enzymes that are capable of decomposing wood fibers, i.e., to adopt a saprotrophic lifestyle in deadwood (hypothesis 2). The absence of oxidative enzymes, including laccase and peroxidase observed in this study, may reflect either a limited potential of P. croceum to produce lignin degrading/modifying enzymes17, or a potential not to produce these enzymes as long as more readily available resources are available. Production of oxidative enzymes important for lignin modification/degradation is more energy intensive than for hydrolytic enzymes, and also requires more resources, such as copper and manganese40. These metals are limited in our experimental setup, which contains only deadwood on diluted Modified Melin-Norkrans 1/10 medium.
After we introduced the mycelia on deadwood with higher 14C content than on agar, the mycelium 14C content significantly increased from the agar value and initial mycelia (p < 0.01), further demonstrating the capability of wood decomposition and assimilation of C from the wood (hypothesis 3) (Fig. 1b and Supplementary Data 1). Taking the common definition of fungal saprotrophs as “fungi that are able to live independently from dead materials and to degrade organic residues contained therein to obtain carbon”41, all the evidence from this series of experiments demonstrates the saprophytic capacity of P. croceum when grown on deadwood as a unique organic substrate. Deadwood can be considered to be more resistant compared to other forms of plant materials such as leaf and fine root litter, as it can persist many years in temperate forest ecosystems12. Obviously, the colonization success of P. croceum on deadwood warrants its long lasting survival apart from symbiotic association. Our finding that bark appears to be preferentially colonized and decomposed by P. croceum can be explained by the higher concentration of macronutrients and mineral elements (especially N, Mg, Fe) in bark of Q. robur as compared to sapwood42. Furthermore, the concentration of non-structural carbohydrates, including sugars and starch is also higher in the outermost part of the wood than the inner part of the wood36. Such non-structural carbohydrates, macronutrients and mineral elements are crucial for microbial enzyme production and activities43,44. Furthermore, bark of Quercus spp. can be considered as an important source of secondary metabolites which may impact growth of P. croceum45.
To evaluate the role of deadwood as a propagule bank to form mycorrhizal symbiosis on the roots of living Q. robur, we introduced colonized woody material from different types into the soil in the vicinity of the roots of living oaks under controlled conditions (Figs. 3 and S1 and Supplementary Data 3). Sterile oak wood without P. croceum was used as the control. P. croceum was detected in the rhizosphere of oak plants after 14 weeks in most inoculation treatments; however, only in the bark (one out of five replicates) and complete deadwood (two out of seven replicates) treatments was yellow mycelium of P. croceum visible on the oak roots (Supplementary Data 4 and Fig. S1). The oak plants with visible P. croceum mycelium on the roots had significantly higher dry biomass of principle root (p = 0.008), lateral roots (p = 0.003), total roots (p = 0.001), stem (p < 0.001), shoot (p < 0.001), and total plant (p < 0.001) compared to other oak plants, regardless of treatment (hypothesis 4) (Fig. 3a, b and Supplementary Data 3). We did not detect P. croceum in the control treatment, where the oak plant did not benefit from EM formation, but we detected P. croceum in the oak rhizosphere of all inoculation treatments using the Illumina sequencing approach. However, we detected this fungus frequently (relative abundance >50%) only in the treatments consisting of sterile soil with deadwood containing visible yellow mycelium of P. croceum (Supplementary Data 4). Microscopic observations of root tips surrounded by P. croceum mycelia showed the formation of a Hartig net (Fig. 3g, h), indicating a typical ectomycorrhizal symbiotic interaction. These results show that in presence of wood pieces colonized by saprotrophic mycelium of P. croceum, the roots of oak plants were not only superficially colonized by yellow mycelium, but also developed true EM symbiosis with Hartig net formation. Although noteworthy because P. croceum requires a delay and a certain carrying capacity of the plant to form fully differentiated EM with a Hartig net46, this result indicates that the inoculation efficiency provided from the pre-colonized deadwood can urge full EM differentiation.
a Root dry weight (mean ± SE) and b plant growth parameters (stem, shoot, and total plant dry weight) (mean ± SE). C = control, CW = control with sterile complete oak wood, B = oak bark with P. croceum, W = oak deadwood with P. croceum, and M = oak and bark with P. croceum which formed yellow mycelium on oak root tips. Different letters indicated significant differences of the dry mass of roots and plant growth parameters according to one-way ANOVA with post-hoc Tukey’s HSD test at p < 0.05. c–h Microscopic analysis of oak roots in control and deadwood with P. croceum treatments. In contrast with the control, which did not show any fungal structures (c–e), mycelium of P. croceum was found to cover the root tips of oak (f) and the Hartig net was detectable in cross sections of paraffin-embedded root tips and stained with fluorescently labeled wheat germ agglutinin (g, h). Note the green fluorescence of outer mycelium (arrows) and Hartig net (arrowheads) in (h). Pictures were taken using a stereomicroscope (c, f), bright field (d, g), and fluorescence microscopy (e, h). Bars represent 500 µm in (c, f) and 50 µm in (d, e, g, h).
Overall, we demonstrated the plasticity of the symbiotroph-saprotroph lifestyles of ectomycorrhizal P. croceum associated with Q. robur, which is in line with the hypotheses from the earlier studies29,32. The past field observations, based on fruiting bodies, were focused on the mycorrhizal stage and not on the saprophytic stage. However, molecular tools have allowed the detection of the dual lifestyle17,18. Importantly, we demonstrated the role of deadwood as a de novo ectomycorrhizal propagule bank. We provided evidence that P. croceum survives on oak deadwood for at least 2 years (Fig. S2). Thus, deadwood can influence forest health and productivity by acting as a substrate for a pool of organisms that can optionally be symbiotrophs or saprotrophs. Future studies, especially using transcriptomics are needed to gain a better understanding of mechanisms behind the plasticity of the symbiotroph-saprotroph lifestyles of ectomycorrhizal fungi.
Materials and methods
P. croceum as saprotroph on Q. robur deadwood
Experiment 1: Could P. croceum grow and persist on bark and deadwood substrates?—Colonization success experiment (experimental design based on 74 deadwood materials).
This experiment was used to test the hypothesis 1. P. croceum J. Erikss. and Hjortst isolate DSMZ 4824; ATCC MYA-4870 was maintained under dark conditions at 20 °C on Modified Melin-Norkrans C (MMNC) agar medium with sugar and subsequently transferred to Modified Melin-Norkrans (MMN) 1/10 medium (no sugar or other carbohydrate sources) for 1 month in a Petri dish (94 mm)47,48. After 3.5 months, different woody substrates (including bark with nine replicates), outer sapwood with 15 replicates, and complete deadwood with 26 replicates) were obtained from 2 cm branches of Q. robur L., obtained from older clonal field trees DF15947 were autoclaved thrice and placed on the top of the growing colony. Additionally, we included inner sapwood (cutting the tree rings between 1955 and 1963 and autoclaving three times, 24 replicates) for this colonization success experiment. These inner sapwood samples contained a significant amount of 14C compared with recently collected normal oak wood. Colonization of P. croceum was observed over 4 months. Contamination was not detected in the control group. Percent colonization was calculated for each woody substrate and used as the indicator for colonization success.
Experiment 2: What was the enzymatic activity of wood colonized by P. croceum?—Hydrolytic and oxidative enzymes in deadwood. This experiment was used to test the hypothesis 2.
Sterile complete deadwood obtained from oak branches of DF159 trees was placed on MMN 1/10 agar with and without P. croceum (control) with the same setting as experiment one for 4 months (maintained under dark conditions at 20 °C). The wood samples were homogenized and ground into powder. Hydrolytic enzyme analyses were performed for β-glucosidase (EC 3.2.1.21), N-acetylglucosaminidase (EC 3.1.6.1), and phosphatase (EC 3.1.3.2), using 4-methylumbelliferone (MUB) as a substrate, as described in previous publications23,38,39. Oxidative enzymes (phenol oxidase (EC 1.10.3.2) and general peroxidase (EC 1.11.1.7) were analyzed using 3, 3′, 5, 5′-tetramethylbenzidine (TMB) substrate, as described in our previous study39. The experiment was conducted with 5 replicates and differences of each enzyme activity between control wood and wood with P. croceum were analyzed using a Mann-Whitney U-test.
Experiment 3: Corticrocin production and its association with available glucose—We varied the concentration of glucose in the MMN 1/10 media (0.0, 0.15, 0.6, 2.5, and 10.0 g/L) used to cultivate P. croceum J. Erikss. and Hjortst isolate DSMZ 4824; ATCC MYA-4870.
The culture plates were incubated at 20 °C for 9 weeks in dark. The dry weight and diameters of the total and yellow part of the colonies were determined. Corticrocin production was measured at the optical density OD 379. The experiment consisted of five to seven replicates for dry weight and diameter and four replicates for destructive OD measurements. Data were tested for normality and equality of group variance. Significant differences of the diameter of the total and yellow colony among different available glucose concentrations were tested using Kruskal–Wallis test incorporated with Mann–Whitney pairwise comparisons at p < 0.05. This experiment was used to test the hypothesis 3 that P. croceum can release enzymes that are capable of decomposing wood fibers and polymers into different C forms, including glucose. Thus, glucose is important for corticrocin production and strongly linked with corticrocin concentration in mycelium of P. croceum.
Experiment 4: Was any wood carbon, as measured via isotopes, detectable in vegetative tissue of the fungus?—Carbon released from deadwood, transported, and assimilated to P. croceum mycelium.
To demonstrate the uptake of wood carbon by mycelia, we used wood with higher 14C content than the MMNC and MMN 1/10 agar media (Δ14C values of agar media are provided in Table S1). This experiment was used to test the hypothesis 3. We observed wood that grew four decades ago from slices of Q. robur trunks. At this time, the Δ14C in atmospheric CO2 was ~150‰ higher than that in agar medium49. The high atmospheric Δ14C signature is imprinted in the wood produced in those years of the atmosphere. We initially cultured P. croceum mycelium on MMNC agar medium (n = 3). Then we used the initial mycelium to inoculate cultures on C-poor MMN 1/10 agar and maintained them under dark conditions at 20 °C, one set with wood pieces, and the second control set without wood. The samples were analyzed by the Keck Carbon Cycle AMS facility at the University of California, Irvine, and in the radiocarbon laboratory in Jena, Germany. Significant differences of Δ14C (‰) among mycelial types were analyzed according to one-way ANOVA at p < 0.05. Differences of Δ14C (‰) between mycelia and wood were analyzed using a t-test. Data were tested for normality and equality of group variance.
P. croceum colonized oak deadwood changes its lifestyle from saprotroph to ectomycorrhizal in Q. robur
Experiment 5: Can deadwood be a source of dispersal by P. croceum for establishing ectomycorrhizal roots?—Deadwood inoculation and evaluation of symbiotic success.
Woody materials from Experiment 1 were used in this experiment. Because of the lower colonization success of sapwood and inner sapwood, we did not include them in this experiment. This experiment consisted of four treatments with five to seven replicates (sterile soil without deadwood (five replicates), sterile soil with sterile complete deadwood, bark, and sapwood (five replicates), sterile soil with bark containing P. croceum (five replicates), and sterile soil with dead wood containing P. croceum (seven replicates) (Fig S1). The oak DF159 micro-cuttings47 were maintained for 14 weeks at 23 °C before evaluation of the symbiosis success. The root system grew in the Petri dish under dark conditions and the shoot under a 16:8 h day: night photoperiod (photosynthetic photon flux density of 180 µmol m−2 s−1). Symbiosis success was indicated by visible yellow mycelium on the roots of oak micro-cuttings. Furthermore, we confirmed the success of symbiosis by Illumina sequencing of the rhizosphere of oak micro-cuttings with and without yellow mycelium (Experiment 7) and by microscopic observation (Experiment 8).
Experiment 6: Ectomycorrhizal effects—Determination of ectomycorrhizal effects.
This experiment was used to test the hypothesis 4. We evaluated the ectomycorrhizal effects on all micro-cuttings from Experiment 5. Based on our previous publication, P. croceum is known to positively impact the dry mass of roots (principal roots, lateral roots, and total roots) and plant growth parameters (dry mass of stem, shoot, and total plant)34. Therefore, these parameters were evaluated. Plant dry weight was determined by oven-dry at 105 °C for 24 h or until constant weight. Significant differences of the dry mass of roots and plant growth parameters were analyzed using one-way ANOVA at p < 0.05. Data were tested for normality and equality of group variance.
Experiment 7: The existence of P. croceum—Molecular detection of P. croceum in the rhizosphere of Q. robur DF159.
The rhizospheres of oak micro-cuttings from the same treatments were pooled, sterile soil without deadwood, sterile soil with sterile complete deadwood, sterile soil with bark containing P. croceum (without yellow mycelium), sterile soil with bark containing P. croceum (with yellow mycelium), sterile soil with deadwood containing P. croceum (without yellow mycelium), and sterile soil with deadwood containing P. croceum (with yellow mycelium). DNA was extracted from 250 mg of the pooled rhizosphere soil and soil samples using DNeasy PowerSoil Kit (Qiagen, Hilden, Germany) with aid of a Precellys 24 tissue homogenizer (Bertin Instruments, Montigny-le-Bretonneux, France). Illumina sequencing and bioinformatics analyses were performed according to our published protocol50. Briefly, fungal amplicon libraries were constructed using the fungal primer pair fITS7 (5′-GTGARTCATCGAATCTTTG-3′) and ITS4 primer (5′-TCCTCCGCTTATTGATATGC-3′)51. Amplifications were performed using 20-µL reaction volumes with 5 × HOT FIRE Pol Blend Master Mix (Solis BioDyne, Tartu, Estonia). The products from three technical replicates were then pooled in equimolar concentrations. Paired-end sequencing (2 × 300 bp) was performed on the pooled PCR products using a MiSeq Reagent kit v3 on an Illumina MiSeq system (Illumina Inc., San Diego, CA, United States) at the Department of Soil Ecology, Helmholtz Centre for Environmental Research, Germany. Paired-end sequences were quality-trimmed, filtered for chimeras, and merged using the DADA2 package52. High-quality reads were clustered into amplicon sequence variants (ASVs). Fungal ASVs were classified against the UNITE v7.2 database53. Sequencing was performed carefully by pooling negative control of all PCR runs and used as sequencing control. Raw sequence data were deposited in the Sequence Read Archive (SRA) operated by the National Center for Biotechnology Information (NCBI) under BioProject accession number: PRJNA930471.
Experiment 8: P. croceum ectomycorrhizal structure—Observation of P. croceum structure in roots of Q. robur.
Tips from oak roots grown without P. croceum (control) and on deadwood with P. croceum (sample T3E2.2 005) were fixed with 4% (w/v) paraformaldehyde/ 0.1% (v/v) TritonX-100 in phosphate-buffered saline (PBS). Micrographs were obtained using a STEMI 2000c stereomicroscope (Zeiss GmbH, Jena, Germany) equipped with an AxioCam Mrc5 CCD camera (Zeiss). Then, the root tips were dehydrated using a graded series of ethanol and embedded in Paraplast (Sigma-Aldrich). Cross-sections of Paraplast-embedded material (5 µm thick) were mounted on poly L-lysine-coated slides, deparaffinized, and rehydrated. The sections were stained with 25 µg ml−1 wheat germ agglutinin conjugated with Alexa Fluor488 (Invitrogen) in PBS at room temperature for 20 min. After washing in PBS, micrographs were taken by bright-field and fluorescence (excitation BP 500/20, emission LP 515) microscopy using an AxioImager equipped with an AxioCam Mrc5 CCD camera (Zeiss).
Experiment 9: Can deadwood be a source of dispersal by P. croceum for establishing ectomycorrhizal roots?—Deadwood as an EM fungal Propagule Bank.
P. croceum was cultivated on MMN 1/10 medium (no sugar or other carbohydrate sources) for 1 month in a Petri-dish (94 mm). After 3.5 months, the complete deadwood (three replicates) was obtained from 2 cm branches of Q. robur L. obtained from clonal trees DF15931, which were autoclaved thrice and placed on the top of the growing colony. P. croceum was grown on MMN 1/10 medium and oak deadwood for 1 and 2 years. For the control treatment, sterile oak wood was placed on an MMN 1/10 medium. The colonies of P. croceum on MMNC agar, colonies of P. croceum on MMN 1/10 agar, and oak deadwood with and without P. croceum were placed on MMNC agar medium. Fungal growth and development of yellow mycelium of P. croceum were recorded after 2 weeks.
Statistics and reproducibility
Differences of enzyme activities between the oak deadwood with and without (control) P. croceum were analyzed using to Mann–Whitney U-test at p < 0.05 (n = 5). Differences of Δ14C (‰) among mycelial types were analyzed using one-way ANOVA with post-hoc Tukey’s HSD (Honestly Significant Difference) test at p < 0.05 (n = 3). Differences of Δ14C (‰) between mycelium and wood were analyzed using a t-test at p < 0.05 (n = 3). Differences of the diameter of the total and yellow colony among different available glucose concentrations were analyzed using Kruskal-Wallis test with post hoc Mann–Whitney pairwise test at p < 0.05 (n = 5–7). Differences of the ratio between OD379 and colony dry weight among different available glucose concentrations were analyzed using Kruskal–Wallis test with post hoc Mann–Whitney pairwise test at p < 0.05 (n = 4). Differences of the dry mass of roots and plant growth parameters were analyzed using one-way ANOVA with post hoc Tukey’s HSD test at p < 0.05 (n = 3–5). For all parametric tests (one-way ANOVA and t-test), all datasets were tested for normality and equality of group variance. All statistical analyses were performed using the PAST program (version 2.17c)54.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Raw sequences were deposited in the Sequence Read Archive (SRA) operated by the National Center for Biotechnology Information (NCBI) under BioProject accession number: PRJNA930471. Experimental design, evidence shows that P. croceum survives on oak deadwood for at least 2 years and 14C data for the agar media used in this study can be found in Supplementary information. Source data underlying graphs in the study can be found in Supplementary Data 1–3.
References
Anderson, I. C. & Cairney, J. W. G. Ectomycorrhizal fungi: exploring the mycelial frontier. FEMS Microbiol. Rev. 31, 388–406 (2007).
Allen, M. F. & Kitajima, K. Net primary production of ectomycorrhizas in a California forest. Fungal Ecol. 10, 81–90 (2014).
Okada, K. H. & Matsuda, Y. Soil spore bank communities of ectomycorrhizal fungi in Pseudotsuga japonica forests and neighboring plantations. Mycorrhiza 32, 83–93 (2022).
Glassman, S. I., Levine, C. R., DiRocco, A. M., Battles, J. J. & Bruns, T. D. Ectomycorrhizal fungal spore bank recovery after a severe forest fire: some like it hot. ISME J. 10, 1228–1239 (2016).
Glassman, S. I. et al. A continental view of pine-associated ectomycorrhizal fungal spore banks: a quiescent functional guild with a strong biogeographic pattern. New Phytol. 205, 1619–1631 (2015).
Purahong, W. et al. Characterization of unexplored deadwood mycobiome in highly diverse subtropical forests using culture-independent molecular technique. Front. Microbiol. 8, 574 (2017).
Ottosson, E. et al. Diverse ecological roles within fungal communities in decomposing logs of Picea abies. FEMS Microbiol. Ecol. 91, fiv012 (2015).
Purahong, W. et al. Increasing N deposition impacts neither diversity nor functions of deadwood-inhabiting fungal communities, but adaptation and functional redundancy ensure ecosystem function. Environ. Microbiol. 20, 1693–1710 (2018).
Hibbett, D. S., Gilbert, L. B. & Donoghue, M. J. Evolutionary instability of ectomycorrhizal symbioses in basidiomycetes. Nature 407, 506–508 (2000).
Tedersoo, L., May, T. W. & Smith, M. E. Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20, 217–263 (2010).
Rajala, T., Peltoniemi, M., Pennanen, T. & Mäkipää, R. Fungal community dynamics in relation to substrate quality of decaying Norway spruce (Picea abies [L.] Karst.) logs in boreal forests. FEMS Microbiol. Ecol. 81, 494–505 (2012).
Purahong, W., Wubet, T., Krüger, D. & Buscot, F. Molecular evidence strongly supports deadwood-inhabiting fungi exhibiting unexpected tree species preferences in temperate forests. ISME J. 12, 289–295 (2018).
Taylor, A. F. S., Martin, F. & Read, D. J. Fungal diversity in ectomycorrhizal communities of Norway Spruce [Picea abies (L.) Karst.] and Beech (Fagus sylvatica L.) along North-South transects in Europe. in Carbon and Nitrogen Cycling in European Forest Ecosystems (ed. Schulze, E.-D.) 343–365 (Springe, 2000).
Mahmood, S., Finlay, R. D., Erland, S. & Wallander, H. Solubilisation and colonisation of wood ash by ectomycorrhizal fungi isolated from a wood ash fertilised spruce forest. FEMS Microbiol. Ecol. 35, 151–161 (2001).
Kohler, A. et al. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat. Genet. 47, 410–415 (2015).
Chen, D. M., Bastias, B. A., Taylor, A. F. S. & Cairney, J. W. G. Identification of laccase-like genes in ectomycorrhizal basidiomycetes and transcriptional regulation by nitrogen in Piloderma byssinum. New Phytol. 157, 547–554 (2003).
Miyauchi, S. et al. Large-scale genome sequencing of mycorrhizal fungi provides insights into the early evolution of symbiotic traits. Nat. Commun. 11, 5125 (2020).
Pellitier, P. T. & Zak, D. R. Ectomycorrhizal fungi and the enzymatic liberation of nitrogen from soil organic matter: why evolutionary history matters. New Phytol. 217, 68–73 (2018).
Shah, F. et al. Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors. New Phytol. 209, 1705–1719 (2016).
Bödeker, I. T. M. et al. Ectomycorrhizal Cortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. New Phytol. 203, 245–256 (2014).
Rineau, F. et al. Carbon availability triggers the decomposition of plant litter and assimilation of nitrogen by an ectomycorrhizal fungus. ISME J. 7, 2010–2022 (2013).
Santos, C. A. et al. An engineered GH1 β-glucosidase displays enhanced glucose tolerance and increased sugar release from lignocellulosic materials. Sci. Rep. 9, 4903 (2019).
German, D. P. et al. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 43, 1387–1397 (2011).
Olchowik, J. et al. Effect of deadwood on ectomycorrhizal colonisation of old-growth oak forests. Forests 10, 480 (2019).
Purahong, W. et al. Potential links between wood-inhabiting and soil fungal communities: Evidence from high-throughput sequencing. MicrobiologyOpen 8, e00856 (2019).
Lewis, J. D. Mycorrhizal fungi, evolution and diversification of. in Encyclopedia of Evolutionary Biology (ed. Kliman, R. M.) 94–99 (Academic Press, 2016).
Lang, A. K., Jevon, F. V., Vietorisz, C. R., Ayres, M. P. & Hatala Matthes, J. Fine roots and mycorrhizal fungi accelerate leaf litter decomposition in a northern hardwood forest regardless of dominant tree mycorrhizal associations. New Phytol. 230, 316–326 (2021).
Khan, F. K., Sánchez-García, M., Johannesson, H. & Ryberg, M. High rate of gene family evolution in proximity to the origin of ectomycorrhizal symbiosis in Inocybaceae. New Phytol. 244, 219–234 (2024).
Smith, G. R., Finlay, R. D., Stenlid, J., Vasaitis, R. & Menkis, A. Growing evidence for facultative biotrophy in saprotrophic fungi: data from microcosm tests with 201 species of wood-decay basidiomycetes. New Phytol. 215, 747–755 (2017).
Rúa, M. A. et al. Ectomycorrhizal fungal communities and enzymatic activities vary across an ecotone between a forest and field. J. Fungi 1, 185 (2015).
Tanunchai, B. et al. Tree mycorrhizal type regulates leaf and needle microbial communities, affects microbial assembly and co-occurrence network patterns, and influences litter decomposition rates in temperate forest. Front. Plant Sci. 14, 1239600 (2023).
Koide, R. T., Sharda, J. N., Herr, J. R. & Malcolm, G. M. Ectomycorrhizal fungi and the biotrophy–saprotrophy continuum. New Phytol. 178, 230–233 (2008).
Lindahl, B. D. & Tunlid, A. Ectomycorrhizal fungi—potential organic matter decomposers, yet not saprotrophs. New Phytol. 205, 1443–1447 (2015).
Herrmann, S. et al. Endogenous rhythmic growth in oak trees is regulated by internal clocks rather than resource availability. J. Exp. Bot. 66, 7113–7127 (2015).
Schreiner, T., Hildebrandt, U., Bothe, H. & Marner, F.-J. Chemical and Biological characterization of corticrocin, a yellow pigment formed by the ectomycorrhizal fungus Piloderma croceum. Z. für Naturforsch. C. 53, 4–8 (1998).
Furze, M. E. et al. Seasonal fluctuation of nonstructural carbohydrates reveals the metabolic availability of stemwood reserves in temperate trees with contrasting wood anatomy. Tree Physiol. 40, 1355–1365 (2020).
Akroume, E. et al. First evidences that the ectomycorrhizal fungus Paxillus involutus mobilizes nitrogen and carbon from saprotrophic fungus necromass. Environ. Microbiol. 21, 197–208 (2019).
Sinsabaugh, R. L. et al. Soil microbial activity in a Liquidambar plantation unresponsive to CO2-driven increases in primary production. Appl. Soil Ecol. 24, 263–271 (2003).
Purahong, W. et al. Tree species, tree genotypes and tree genotypic diversity levels affect microbe-mediated soil ecosystem functions in a subtropical forest. Sci. Rep. 6, 36672 (2016).
Wong, D. W. S. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 157, 174–209 (2009).
Müller, K. et al. Saprotrophic and ectomycorrhizal fungi contribute differentially to organic P mobilization in beech-dominated forest ecosystems. Front. For. Glob. Change 3 (2020).
Krutul, D. et al. Influence of urban environment originated heavy metal pollution on the extractives and mineral substances content in bark and wood of oak (Queecus robur L.). Wood Res. 59, 177–190 (2014).
Prescott, L., Harley, J. & Klein, D. Microbiology (McGraw-Hill Higher Education, 1999).
Purahong, W. et al. Effects of forest management practices in temperate beech forests on bacterial and fungal communities involved in leaf litter degradation. Microb. Ecol. 69, 905–913 (2015).
Sirgedaitė-Šėžienė, V., Čėsnienė, I., Leleikaitė, G., Baliuckas, V. & Vaitiekūnaitė, D. Phenolic and antioxidant compound accumulation of Quercus robur bark diverges based on tree genotype, phenology and extraction method. Life 13, 710 (2023).
Herrmann, S., Oelmüller, R. & Buscot, F. Manipulation of the onset of ectomycorrhiza formation by indole-3-acetic acid, activated charcoal or relative humidity in the association between oak microcuttings and Piloderma croceum: influence on plant development and photosynthesis. J. Plant Physiol. 161, 509–517 (2004).
Herrmann, S., Munch, J.-C. & Buscot, F. A gnotobiotic culture system with oak microcuttings to study specific effects of mycobionts on plant morphology before, and in the early phase of, ectomycorrhiza formation by Paxillus involutus and Piloderma croceum. New Phytol. 138, 203–212 (1998).
Schubert, R. et al. Quantitative detection of agar-cultivated and rhizotron-grown Piloderma croceum Erikss. & Hjortst. by ITS1-based fluorescent PCR. Mycorrhiza 13, 159–165 (2003).
Trumbore, S. E., Sierra, C. A. & Hicks Pries, C. E. Radiocarbon nomenclature, theory, models, and interpretation: measuring age, determining cycling rates, and tracing source pools. in Radiocarbon and Climate Change: Mechanisms, Applications and Laboratory Techniques (eds Schuur, E. A. G., Druffel, E. & Trumbore, S. E.) 45–82 (Springer International Publishing, 2016).
Tanunchai, B. et al. Analysis of microbial populations in plastic–soil systems after exposure to high poly(butylene succinate-co-adipate) load using high-resolution molecular technique. Environ. Sci. Eur. 33, 105 (2021).
Ihrmark, K. et al. New primers to amplify the fungal ITS2 region—evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 82, 666–677 (2012).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Nilsson, R. H. et al. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 47, D259–D264 (2019).
Hammer, Ø, Harper, D. A. T. & Ryan, P. D. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 9 (2001).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualized project: W.P., F.B. and S.H. Developing ideas and experiment planning: W.P., B.T., S.H., M.T. and E.-D.S. Molecular lab work: B.T. and W.P. Lab work on plant growth parameters and root traits: B.T., L.J. and W.P. Measurement of hydrolytic and oxidative enzymes in deadwood: B.T., L.J. and W.P. Lab work on corticrocin production and its association with available glucose: S.H. Lab work on carbon released from deadwood, transported, and assimilated to P. croceum mycelia: Bo.H. and E.-D.S. Lab work on observation of P. croceum structure in roots of Quercus robur: Be.H. and H.S. Bioinformatics analyses: B.T., L.J. and W.P. Data analyses: W.P. and B.T. First draft writing: W.P. and B.T. All of the authors reviewed and provided comments and suggestions for the paper. All authors gave final approval for paper submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: David Favero.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Purahong, W., Tanunchai, B., Ji, L. et al. Plasticity of symbiotroph-saprotroph lifestyles of Piloderma croceum associated with Quercus robur L.. Commun Biol 8, 1344 (2025). https://doi.org/10.1038/s42003-025-08762-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08762-w