Abstract
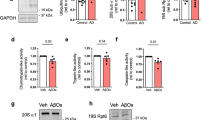
PSD-95, a major scaffolding protein, requires palmitoylation to remain at synapses where it plays critical roles in synaptic structure and function. Here, we show that PSD-95 palmitoylation is specifically reduced in the hippocampus of female Alzheimer’s disease (AD) model mice. Accordingly, these mice have significant memory deficits that are not observed in male AD model mice. Systemic injections of Palmostatin B, a depalmitoylating enzyme inhibitor (including the one acting on PSD-95), rescues memory deficits in female AD model mice and restores PSD-95 palmitoylation levels. Importantly, both synaptic structure and function are impaired in female AD model mice, and these deficits are normalized in Palmostatin B injected animals. This drug has no effects on amyloid plaques or GFAP levels, indicating that the rescue of behavioral and synaptic deficits is not due to effects on plaque or astrogliosis related AD pathology. Our data instead suggest that the sex-dependent rescue we observe is mediated by the stabilization of small, vulnerable dendritic spines. This study demonstrates that increasing PSD-95 palmitoylation might be an effective way to protect synapses from AD pathology and therefore a promising therapy for AD.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information and Supplementary Data files (all numerical Source data for graphs shown in the main Figures can be found in Supplementary Data 1–3). All other data are available from the corresponding author on reasonable request. Material requests should be sent to the corresponding author.
References
Lopez-Lee, C., Torres, E. R. S., Carling, G. & Gan, L. Mechanisms of sex differences in Alzheimer’s disease. Neuron 112, 1208–1221 (2024).
Koran, M. E. I., Wagener, M. & Hohman, T. J. & for the Alzheimer’s Neuroimaging Initiative. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 11, 205–213 (2017).
Inguanzo, A. et al. Atrophy trajectories in Alzheimer’s disease: how sex matters. Alzheimer's Res. Ther. 17, 79 (2025).
Moutinho, S. Women twice as likely to develop Alzheimer’s disease as men—but scientists do not know why. Nat. Med. 31, 704–707 (2025).
Jack, C. R. et al. Age, sex, and APOE ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurol. 72, 511 (2015).
Mifflin, M. A. et al. Sex differences in the IntelliCage and the Morris water maze in the APP/PS1 mouse model of amyloidosis. Neurobiol. Aging 101, 130–140 (2021).
Robertson, K. V. et al. Knockdown of microglial iron import gene, Slc11a2, worsens cognitive function and alters microglial transcriptional landscape in a sex-specific manner in the APP/PS1 model of Alzheimer’s disease. J. Neuroinflammation 21, 238 (2024).
Zhang, C. et al. Corticotropin-releasing factor receptor-1 antagonism mitigates beta amyloid pathology and cognitive and synaptic deficits in a mouse model of Alzheimer’s disease. Alzheimer’s Dement. 12, 527–537 (2016).
Brandt, N., Löffler, T., Fester, L. & Rune, G. M. Sex-specific features of spine densities in the hippocampus. Sci. Rep. 10, 11405 (2020).
Jabra, S. et al. Sex- and cycle-dependent changes in spine density and size in hippocampal CA2 neurons. Sci. Rep. 14, 12252 (2024).
DeKosky, S. T. & Scheff, S. W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 27, 457–464 (1990).
Selkoe, D. J. Alzheimer’s disease is a synaptic failure. Science 298, 789–791 (2002).
Boros, B. D. et al. Dendritic spines provide cognitive resilience against Alzheimer’s disease. Ann. Neurol. 82, 602–614 (2017).
Gylys, K. H. et al. Synaptic changes in Alzheimer’s disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am. J. Pathol. 165, 1809–1817 (2004).
Inestrosa, N. C. et al. Tetrahydrohyperforin prevents cognitive deficit, Aβ deposition, tau phosphorylation and synaptotoxicity in the APPswe/PSEN1ΔE9 model of Alzheimer’s disease: a possible effect on APP processing. Transl. Psychiatry 1, e20 (2011).
Shao, C. Y., Mirra, S. S., Sait, H. B. R., Sacktor, T. C. & Sigurdsson, E. M. Postsynaptic degeneration as revealed by PSD-95 reduction occurs after advanced Aβ and tau pathology in transgenic mouse models of Alzheimer’s disease. Acta Neuropathol. 122, 285–292 (2011).
Hong, J. Y. et al. High-throughput enzyme assay for screening inhibitors of the ZDHHC3/7/20 acyltransferases. ACS Chem. Biol. 16, 1318–1324 (2021).
Almeida, C. G. et al. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol. Dis. 20, 187–198 (2005).
Scheff, S. W., Ansari, M. A. & Mufson, E. J. Oxidative stress and hippocampal synaptic protein levels in elderly cognitively intact individuals with Alzheimer’s disease pathology. Neurobiol. Aging 42, 1–12 (2016).
Pham, E. et al. Progressive accumulation of amyloid-β oligomers in Alzheimer’s disease and APP transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J. 277, 3051–3067 (2010).
El-Husseini, A. E. et al. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J. Cell Biol. 148, 159–172 (2000).
Won, S. J., Cheung See Kit, M. & Martin, B. R. Protein depalmitoylases. Crit. Rev. Biochem. Mol. Biol. 53, 83–98 (2018).
Fujimoto, T. et al. P-selectin is acylated with palmitic acid and stearic acid at cysteine 766 through a thioester linkage. J. Biol. Chem. 268, 11394–11400 (1993).
Yokoi, N. et al. Identification of PSD-95 depalmitoylating enzymes. J. Neurosci. 36, 6431–6444 (2016).
Sanders, S. S. et al. Curation of the mammalian palmitoylome indicates a pivotal role for palmitoylation in diseases and disorders of the nervous system and cancers. PLoS Comput. Biol. 11, e1004405 (2015).
Petropavlovskiy, A. A., Kogut, J. A., Leekha, A., Townsend, C. A. & Sanders, S. S. A sticky situation: regulation and function of protein palmitoylation with a spotlight on the axon and axon initial segment. Neuronal Signal 5, NS20210005 (2021).
El-Husseini, A. E.-D. et al. Synaptic strength regulated by palmitate cycling on PSD-95. Cell 108, 849–863 (2002).
Schnell, E. et al. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl. Acad. Sci. USA 99, 13902–13907 (2002).
Bats, C., Groc, L. & Choquet, D. The interaction between stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53, 719–734 (2007).
Jeyifous, O. et al. Palmitoylation regulates glutamate receptor distributions in postsynaptic densities through control of PSD95 conformation and orientation. Proc. Natl. Acad. Sci. USA. 113, E8482–E8491 (2016).
Chowdhury, D. & Hell, J. W. Ca2+/calmodulin binding to PSD-95 downregulates its palmitoylation and AMPARs in long-term depression. Front. Synaptic Neurosci. 11, 6 (2019).
Shen, Z.-C. et al. APT1-mediated depalmitoylation regulates hippocampal synaptic plasticity. J. Neurosci. 42, 2662–2677 (2022).
Mao, S., Zhao, X., Wang, L., Man, Y. & Li, K. Palmitoylation-related gene ZDHHC22 as a potential diagnostic and immunomodulatory target in Alzheimer’s disease: insights from machine learning analyses and WGCNA. Eur. J. Med. Res. 30, 46 (2025).
Lin, D. T. & Conibear, E. ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization. Elife 4, e11306 (2015).
Dore, K. et al. PSD-95 protects synapses from β-amyloid. Cell Rep. 35, 109194 (2021).
Chen, S. et al. Palmitoylation-dependent activation of MC1R prevents melanomagenesis. Nature 549, 399–403 (2017).
Martin, B. R., Wang, C., Adibekian, A., Tully, S. E. & Cravatt, B. F. Global profiling of dynamic protein palmitoylation. Nat. Methods 9, 84–89 (2012).
Pavon, M. V., Navakkode, S., Wong, L.-W. & Sajikumar, S. Inhibition of nogo-A rescues synaptic plasticity and associativity in APP/PS1 animal model of Alzheimer’s disease. Semin. Cell Dev. Biol. 139, 111–120 (2023).
Viana da Silva, S. et al. Early synaptic deficits in the APP/PS1 mouse model of Alzheimer’s disease involve neuronal adenosine A2A receptors. Nat. Commun. 7, 11915 (2016).
Moolman, D. L., Vitolo, O. V., Vonsattel, J.-P. G. & Shelanski, M. L. Dendrite and dendritic spine alterations in alzheimer models. J. Neurocytol. 33, 377–387 (2004).
Knafo, S. et al. Widespread changes in dendritic spines in a model of Alzheimer’s disease. Cereb. Cortex 19, 586–592 (2009).
Chai, G., Wang, Y., Zhu, D., Yasheng, A. & Zhao, P. Activation of β2-adrenergic receptor promotes dendrite ramification and spine generation in APP/PS1 mice. Neurosci. Lett. 636, 158–164 (2017).
Xu, L. et al. Deficits in N-methyl-D-aspartate receptor function and synaptic plasticity in hippocampal CA1 in APP/PS1 mouse model of Alzheimer’s disease. Front. Aging Neurosci. 13, 772980 (2021).
Yook, Y. et al. Hyperfunction of post-synaptic density protein 95 promotes seizure response in early-stage aβ pathology. EMBO Rep. 25, 1233–1255 (2024).
Kanadome, T., Yokoi, N., Fukata, Y. & Fukata, M. Systematic screening of depalmitoylating enzymes and evaluation of their activities by the acyl-PEGyl exchange gel-shift (APEGS) assay. Methods Mol. Biol. 2009, 83–98 (2019).
Jiao, S.-S. et al. Sex dimorphism profile of Alzheimer’s disease-type pathologies in an APP/PS1 mouse model. Neurotox. Res. 29, 256–266 (2016).
Wang, J., Tanila, H., Puoliväli, J., Kadish, I. & van. Groen, T. Gender differences in the amount and deposition of amyloidβ in APPswe and PS1 double transgenic mice. Neurobiol. Dis. 14, 318–327 (2003).
Jia, Y., Du, X., Wang, Y., Song, Q. & He, L. Sex differences in luteinizing hormone aggravates Aβ deposition in APP/PS1 and Aβ1-42-induced mouse models of Alzheimer’s disease. Eur. J. Pharm. 970, 176485 (2024).
Curdt, N. et al. Search strategy analysis of Tg4-42 Alzheimer mice in the Morris water maze reveals early spatial navigation deficits. Sci. Rep. 12, 5451 (2022).
Spencer, B. et al. Anti-α-synuclein immunotherapy reduces α-synuclein propagation in the axon and degeneration in a combined viral vector and transgenic model of synucleinopathy. Acta Neuropathol. Commun. 5, 7 (2017).
Huang, X. et al. S-acylation of p62 promotes p62 droplet recruitment into autophagosomes in mammalian autophagy. Mol. Cell 83, 3485–3501 (2023).
Martínez-Martos, J. M. et al. Sexual and metabolic differences in hippocampal evolution: Alzheimer’s disease implications. Life 14, 1547 (2024).
Kamenetz, F. et al. APP processing and synaptic function. Neuron 37, 925–937 (2003).
Rostagno, A., Cabrera, E., Lashley, T. & Ghiso, J. N-terminally truncated Aβ4-x proteoforms and their relevance for Alzheimer’s pathophysiology. Transl. Neurodegener. 11, 30 (2022).
Liu, W. et al. Chemical genetic activation of the cholinergic basal forebrain hippocampal circuit rescues memory loss in Alzheimer’s disease. Alzheimer’s. Res. Ther. 14, 53 (2022).
Cai, Z., Wan, C.-Q. & Liu, Z. Astrocyte and Alzheimer’s disease. J. Neurol. 264, 2068–2074 (2017).
Kraft, A. W. et al. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J. 27, 187–198 (2013).
Shi, Q. et al. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci. Transl. Med. 9, eaaf6295 (2017).
Carter, S. F. et al. Astrocyte biomarkers in Alzheimer’s disease. Trends Mol. Med. 25, 77–95 (2019).
Kim, J. & Tsien, R. W. Synapse-specific adaptations to inactivity in hippocampal circuits achieve homeostatic gain control while dampening network reverberation. Neuron 58, 925–937 (2008).
Fernández-Pérez, E. J. et al. Changes in neuronal excitability and synaptic transmission in nucleus accumbens in a transgenic Alzheimer’s disease mouse model. Sci. Rep. 10, 19606 (2020).
Latif-Hernandez, A. et al. The two faces of synaptic failure in AppNL-G-F knock-in mice. Alzheimer’s. Res. Ther. 12, 100 (2020).
Back, M. K., Ruggieri, S., Jacobi, E. & Von Engelhardt, J. Amyloid beta-mediated changes in synaptic function and spine number of neocortical neurons depend on NMDA receptors. IJMS 22, 6298 (2021).
Liu, G., Choi, S. & Tsien, R. W. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron 22, 395–409 (1999).
Zhang, J., Yang, Y., Li, H., Cao, J. & Xu, L. Amplitude/frequency of spontaneous mEPSC correlates to the degree of long-term depression in the CA1 region of the hippocampal slice. Brain Res. 1050, 110–117 (2005).
Sala, C. et al. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron 31, 115–130 (2001).
Peng, Y.-R. et al. Coordinated changes in dendritic arborization and synaptic strength during neural circuit development. Neuron 61, 71–84 (2009).
Booker, S. A. et al. Altered dendritic spine function and integration in a mouse model of fragile X syndrome. Nat. Commun. 10, 4813 (2019).
Medel Sánchez, A. et al. Aducanumab in Alzheimer’s disease: a comparative study of its effects on dementia and mild cognitive impairment. Cureus 16, e75907 (2024).
Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 11, 1006–1012 (2012).
Valenzuela, M. J. & Sachdev, P. Brain reserve and dementia: a systematic review. Psychol. Med. 36, 441–454 (2006).
Zaręba-Kozioł, M. et al. S-Palmitoylation of synaptic proteins as a novel mechanism underlying sex-dependent differences in neuronal plasticity. Int. J. Mol. Sci. 22, 6253 (2021).
Li, R. & Singh, M. Sex differences in cognitive impairment and Alzheimer’s disease. Front. Neuroendocrinol. 35, 385–403 (2014).
Shankar, G. M. et al. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 27, 2866–2875 (2007).
Goode, L. K. et al. Examination of diurnal variation and sex differences in hippocampal neurophysiology and spatial memory. eNeuro 9, ENEURO.0124-22.2022 (2022).
Le, A. A. et al. Metabotropic NMDAR signaling contributes to sex differences in synaptic plasticity and episodic memory. J. Neurosci. 44, e0438242024 (2024).
Narattil, N. R. & Maroun, M. Differential role of NMDA receptors in hippocampal-dependent spatial memory and plasticity in juvenile male and female rats. Hippocampus 34, 564–574 (2024).
Maffioli, E. et al. Insulin and serine metabolism as sex-specific hallmarks of Alzheimer’s disease in the human hippocampus. Cell Rep. 40, 111271 (2022).
Bhattacharyya, R., Barren, C. & Kovacs, D. M. Palmitoylation of amyloid precursor protein regulates amyloidogenic processing in lipid rafts. J. Neurosci. 33, 11169–11183 (2013).
Andrew, R. J. et al. Lack of BACE1 S-palmitoylation reduces amyloid burden and mitigates memory deficits in transgenic mouse models of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 114, E9665–E9674 (2017).
Natale, F. et al. Inhibition of zDHHC7-driven protein S-palmitoylation prevents cognitive deficits in an experimental model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 121, e2402604121 (2024).
Koster, K. P. et al. Loss of depalmitoylation disrupts homeostatic plasticity of AMPARs in a mouse model of infantile neuronal ceroid lipofuscinosis. J. Neurosci. 43, 8317–8335 (2023).
Puhl, A. C. et al. Identification of new modulators and inhibitors of palmitoyl-protein thioesterase 1 for CLN1 Batten disease and cancer. ACS Omega 9, 11870–11882 (2024).
Fukata, Y. et al. Local palmitoylation cycles define activity-regulated postsynaptic subdomains. J. Cell Biol. 202, 145–161 (2013).
Xu, B. et al. Loss of thin spines and small synapses contributes to defective hippocampal function in aged mice. Neurobiol. Aging 71, 91–104 (2018).
Dumitriu, D. et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J. Neurosci. 30, 7507–7515 (2010).
Jankowsky, J. L. et al. APP processing and amyloid deposition in mice haplo-insufficient for presenilin 1. Neurobiol. Aging 25, 885–892 (2004).
Vorhees, C. V. & Williams, M. T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858 (2006).
Quehenberger, O. et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 51, 3299–3305 (2010).
Ting, J. T., Daigle, T. L., Chen, Q. & Feng, G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol. Biol. 1183, 221–242 (2014).
Acknowledgements
We would like to thank Jazmin Florio and Michael Mante for behavioral testing training and help with tissue harvesting. We thank the late Dr. Eric Smeltz for giving Ahmed Khalil access to his GC/MS instrument. We also thank Drs. Roberto Malinow, Helmut Kessels, Christina Sigurdson and Alexandra Newton for their helpful comments on the manuscript. This work was funded by the National Institute on Aging, grant AG067049 to K.D. We also acknowledge grant P30NS047101 from NINDS, which funds the UCSD School of Medicine Microscopy core.
Author information
Authors and Affiliations
Contributions
Y.D. and K.P. performed the main experiments. A.Q.P. and M.D. contributed to electrophysiology data collection and analysis. A.L. did the thioflavin-S and GFAP experiments and contributed to writing the manuscript. C.M., M.D., M.G., and M.S. helped with biochemical experiments. A.K. did all the metabolomics experimentation and analysis. R.R. provided mice and access to Morris water maze equipment. M.M., I.B., and H.K. helped with data analysis and animal work. K.D. and Y.D. designed the research and wrote the manuscript. K.D. also performed behavioral testing, acquired funding and supervised all research. All authors contributed to the finalization of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary handling editors: Christian Wozny and Rosie Bunton-Stasyshyn. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Du, Y., Prinkey, K., Pham, A.Q. et al. Sex-dependent rescue of memory and synaptic deficits in AD model mice by increasing PSD-95 palmitoylation. Commun Biol (2026). https://doi.org/10.1038/s42003-026-09702-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-026-09702-y