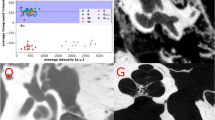
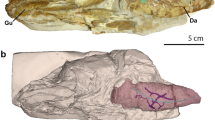
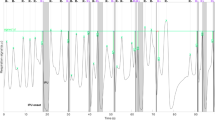
Abstract
Since the discovery of Latimeria chalumnae, coelacanths have provided a critical comparative framework for reconstructing ancestral sarcopterygian anatomy. However, the function of several anatomical features in both extant and fossil coelacanths remains unresolved. Among these, the presence of large ossified chambers in the body cavity of fossil coelacanths has remained enigmatic, with different studies proposing respiratory or auditory functions. Here, we examine lung and inner ear anatomy based on new observations from synchrotron phase-contrast microCT scans of two 240-million-year-old latimerioid coelacanths, alongside multiple developmental stages of the extant L. chalumnae. These data, combined with archival histological sections of L. chalumnae and 3D reconstructions of a Devonian coelacanth, suggest that extinct coelacanths possessed an ossified lung capable of transmitting sound pressure to auditory sensory epithelia in the inner ear via a perilymphatic system. We propose that the lung of extinct coelacanths supported both respiratory and auditory functions.
Similar content being viewed by others
Data availability
The synchrotron scan files of Graulia branchiodonta (MHNG-GEPI-V5787, holotype and MHNG-GEPI-V5787, referred specimen) are available from the ESRF Paleontology Database (https://paleo.esrf.fr/datasets/2015882168 ; https://paleo.esrf.fr/datasets/2015882170)41,42. The synchrotron scan files of Loreleia eucingulata (MHNG-GEPI-V5789, holotype) will be publicly available from the ESRF Paleontology Database (https://paleo.esrf.fr/) along with surface files of the individual bones. The fossil material housed in the Natural History Museum of Geneva is available for study upon request.
References
Brito, P. M., Meunier, F., Clément, G. & Geffard-Kuriyama, D. The histological structure of the calcified lung of the fossil coelacanth Axelrodichthys araripensis (Actinistia: Mawsoniidae). Palaeontology 53, 1281–1290 (2010).
Clément, A. M. A Late Devonian coelacanth reconfigures actinistian phylogeny, disparity and evolutionary dynamics. Nat. Commun. 15, 7529 (2024).
Woodward, A. S. Catalogue of the Fossil Fishes in the British Museum (Natural History). Part II. 567 (British Museum, 1891).
Cupello, C. Allometric growth in the extant coelacanth lung during ontogenetic development. Nat. Commun. 6, 8222 (2015).
Cupello, C. The long-time adaptation of coelacanths to moderate deep water: reviewing the evidence. Bull. Kitakyushu Mus. Nat. Hist. Hum. Hist. A 17, 29–35 (2019).
Cupello, C. Lung evolution in vertebrates and the water-to-land transition. eLife 11, e74160 (2022).
Cupello, C., Clément, G., Herbin, M., Meunier, F. J. & Brito, P. M. Pulmonary arteries in coelacanths shed light on the vasculature evolution of air-breathing organs in vertebrates. Sci. Rep. 14, 10624 (2024).
Cupello, C. The homology and function of the lung plates in extant and fossil coelacanths. Sci. Rep. 7, 9242 (2017).
Christensen, C. B., Christensen-Dalsgaard, J. & Madsen, P. T. Hearing of the African lungfish (Protopterus annectens) suggests underwater pressure detection and rudimentary aerial hearing in early tetrapods. J. Exp. Biol. 218, 381–387 (2015).
Schellart, N. A. & Popper, A. N. Functional aspects of the evolution of the auditory system of actinopterygian fish. In The Evolutionary Biology of Hearing (eds. Webster, D. B., Fay, R. R. & Popper, A. N.) 295–322 (Springer, 1992).
Rosen, D. E. & Greenwood, P. H. Origin of the Weberian apparatus and the relationships of the ostariophysan and gonorhynchiform fishes. Am. Mus. Novit. 2428, 1–25 (1970).
Millot, J. & Anthony, J. Anatomie de Latimeria chalumnae. Vol. 2: Système nerveux et organes des sens. (CNRS, 1965).
Fritzsch, B. Inner ear of the coelacanth fish Latimeria has tetrapod affinities. Nature 327, 153–154 (1987).
Fritzsch, B., Schultze, H.-P. & Elliott, K. L. The evolution of the various structures required for hearing in Latimeria and tetrapods. IBRO Neurosci. Rep. 14, 325–341 (2023).
Manuelli, L., Mondéjar Fernández, J., Dollman, K. N., Jakata, K. & Cavin, L. The most detailed anatomical reconstruction of a Mesozoic coelacanth. PLOS ONE 19, e0312026 (2024).
Reis, O. M. Die Coelacanthinen, mit besonderer Berücksichtigung der im Weissen Jura Bayerns vorkommenden Gattungen. Palaeontographica 35, 1–96 (1888).
Nulens, R., Scott, L. & Herbin, M. An Updated Inventory of All Known Specimens of the Coelacanth, Latimeria spp. (South African Institute for Aquatic Biodiversity, 2011).
Fritzsch, B. & Elliott, K. L. Fish hearing revealed: Do we understand hearing in critical fishes and marine tetrapods?. J. Acoust. Soc. Am. 154, 3019–3026 (2023).
Kasumyan, A. Structure and function of the auditory system in fishes. J. Ichthyol. 45, 223–270 (2005).
Bernstein, P. The ear region of Latimeria chalumnae: functional and evolutionary implications. Zoology 106, 233–242 (2003).
Bjerring, H. C. The inner ear of crossopterygians. In Evolutionary Biology of Primitive Fishes (eds. Foreman, R. E., Gorbman, A., Dodd, J. M. & Olsson, R.) (Springer, 1985).
Fritzsch, B. The inner ear of vertebrates: phylogenetic patterns and evolutionary significance. In The Evolutionary Biology of Hearing (eds. Webster, D. B., Fay, R. R. & Popper, A. N.) (Springer, 1992).
Fritzsch, B. & Wake, M. H. The inner ear of gymnophione amphibians and its nerve supply: a comparative study of regressive events in a complex sensory system. Zoomorphology 108, 201–217 (1988).
Forey, P. L. History of the Coelacanth Fishes. (Chapman & Hall, 1998).
Stensiö, E. On the Devonian coelacanthids of Germany with special reference to the dermal skeleton. K. Sven. Vetenskapsakad. Handl. 16, 1–67 (1937).
Bjerring, H. C. The nervus rarus in coelacanthiform phylogeny. Zool. Scr. 1, 57–68 (1972).
Smotherman, M. & Narins, P. Evolution of the amphibian ear. In Evolution of the Vertebrate Auditory System (eds. Manley, G. A., Popper, A. N. & Fay, R. R.) 164–199 (Springer, 2004).
Witschi, E. The larval ear of the frog and its transformation during metamorphosis. Z. Naturforsch. 4, 230–242 (1949).
Horowitz, S. S., Chapman, J. A., Kaya, U. & Simmons, A. M. Metamorphic development of the bronchial columella of the larval bullfrog (Rana catesbeiana). Hear. Res. 154, 12–25 (2001).
Bi, X. et al. Tracing the genetic footprints of vertebrate landing in non-teleost ray-finned fishes. Cell 184, 1377–1391 (2021).
Perry, S. F., Wilson, R. J. A., Straus, C., Harris, M. B. & Remmers, J. E. Which came first, the lung or the breath?. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 129, 37–47 (2001).
Cavin, L. & Guinot, G. Coelacanths as “almost living fossils. Front. Ecol. Evol. 2, 49 (2014).
Dutel, H. et al. Neurocranial development of the coelacanth and the evolution of the sarcopterygian head. Nature 569, 556–559 (2019).
Dutel, H. & Tafforeau, P. Latimeria chalumnae—extant coelacanth fetus and pups (Version 1) [Dataset]. European Synchrotron Radiation Facility https://doi.org/10.15151/ESRF-DC-1634387693 (2024).
Lauridsen, H., Pedersen, J. M. H., Ringgaard, S. & Møller, P. R. Buoyancy and hydrostatic balance in a West Indian Ocean coelacanth Latimeria chalumnae. BMC Biol. 20, 180 (2022).
Mirone, A., Brun, E., Gouillart, E., Tafforeau, P. & Kieffer, J. The PyHST2 hybrid distributed code for high-speed tomographic reconstruction. Nucl. Instrum. Methods Phys. Res. B 324, 41–48 (2014).
Paganin, D., Mayo, S. C., Gureyev, T. E., Miller, P. R. & Wilkins, S. W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 206, 33–40 (2002).
Manuelli, L., Covain, R. & Cavin, L. A 3D reconstruction of the skull of the West Indian Ocean coelacanth Latimeria chalumnae [Dataset]. MorphoMuseuM e211 https://doi.org/10.18563/journal.m3.211 (2023).
Ferrante, C. & Cavin, L. A deep dive into coelacanth phylogeny. PLoS One 20.6, e0320214 (2025).
Swofford, D. L. PAUP: Phylogenetic Analysis Using Parsimony and Other Methods (Sinauer Associates, 2003).
Manuelli, L., Mondéjar Fernández, J., Dollman, K. N., Jakata, K. & Cavin, L. Graulia branchiodonta gen. et sp. nov., complete holotype MHNG_GEPI_V5787 characterized using propagation phase-contrast synchrotron X-ray micro-computed tomography (Version 1) [Dataset]. European Synchrotron Radiation Facility https://doi.org/10.15151/ESRF-DC-2014789926 (2025).
Manuelli, L., Mondéjar Fernández, J., Dollman, K. N., Jakata, K. & Cavin, L. Graulia branchiodonta gen. et sp. nov., referred specimen MHNG_GEPI_V5788 characterized using propagation phase-contrast synchrotron X-ray micro-computed tomography (Version 1) [Dataset]. European Synchrotron Radiation Facility https://doi.org/10.15151/ESRF-DC-2014789934 (2025).
Acknowledgements
We thank N. Alvarez for support with the grant application; K. Jakata for performing synchrotron imaging at the ESRF; R. J. Thoni from the AMNH for providing scans of the historical histological sections of L. chalumnae; H. Dutel for collecting synchrotron data of L. chalumnae; J. M. Fernandez and C. Ferrante for discussions; F. Goussard for technical support; the Swiss National Science Foundation for providing funding (https://data.snf.ch/grants/grant/207903).
Author information
Authors and Affiliations
Contributions
L.C. and L.M. conceived and designed the study. L.M. and K.D. developed the methodology. L.M. and L.C. conducted the investigation. L.M. performed data visualization. L.C. acquired funding, administered the project and supervised the study. L.C. and L.M. wrote the original manuscript. L.M., L.C., G.C., M.H., B.F., P.E.A. and K.D. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Alice Clement and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Dennis Higgs and Michele Repetto.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manuelli, L., Clément, G., Herbin, M. et al. A dual respiratory and auditory function for the coelacanth lung. Commun Biol (2026). https://doi.org/10.1038/s42003-026-09708-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-026-09708-6