Abstract
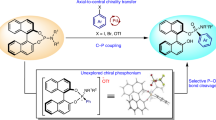
Chiral phosphorous-containing compounds are playing a more and more significant role in several different research fields. Here, we show a chiral phosphoric acid-catalyzed enantioselective phosphinylation of 3,4-dihydroisoquinolines with diarylphosphine oxides for the efficient and practical construction of a family of chiral α-amino diarylphosphine oxides with a diverse range of functional groups. The phosphine products are suitable for transforming to several kinds of chiral (thio)ureas, which might be employed as chiral ligands or catalysts with potential applications in asymmetric catalysis. Control and NMR tracking experiments show that the reaction proceeds via the tert-butyl 1-(tert-butoxy)-3,4-dihydroiso-quinoline-2(1H)-carboxylate intermediate, followed by C-P bond formation. Furthermore, computational studies elucidated that the hydrogen bonding strength between the phosphonate and isoquinolinium determines the stereoselectivity of the phosphinylation reaction.
Similar content being viewed by others
Introduction
Chiral phosphorous-containing compounds have attracted more and more attention due to their prevalent applications as chiral ligands and organocatalysts for asymmetric reactions1,2,3,4,5,6, potential biological activity7,8,9,10,11 and utilities in the field of material science12,13,14. Chiral α-amino diarylphosphine oxide represents one of the most significant organophosphorous compounds, which was always directly functioned as organocatalysts15,16 or utilized as the key building blocks in chiral ligand synthesis. What’s more, recent research also revealed that chiral α-amino diarylphosphine oxides can serve as surrogates for α-amino phosphonic acids and their phosphonate esters which might lead to important biological activity discoveries17,18. Chiral 1,2,3,4- tetrahydroisoquinoline (THIQ) is one of the most important “privileged scaffolds” present in natural products19,20,21,22, bioactive molecules23,24,25,26, and chiral catalysts27,28,29,30,31,32. Therefore, combining the substructure of α-amino diarylphosphine oxides and THIQ into a single molecule would be of great utility, no matter in discovery of new compounds with potential biological activities or development of new ligands and organocatalysts.
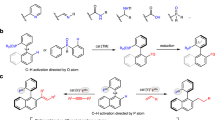
Catalytic enantioselective phosphinylation of imines with diarylphosphine oxides is the most straightforward approach to chiral α-amino diarylphosphine oxides (Fig. 1a). To the best of our knowledge, there are only three reports on the enantioselective synthesis of α-amino diarylphosphine oxides up to now. The first example of a heterobimetallic lanthanoid complex being employed to catalyze the asymmetric addition of diphenylphosphine oxide to cyclic imines was reported by Shibasaki et al. Under such catalytic conditions, high yields and good to excellent enantioselectivities were well achieved33. After this pioneering work, Antilla et al. reported the chiral magnesium BINOL phosphate-catalyzed enantioselective phosphinylation of substituted acyclic imines with diphenylphosphine to give products with up to 96% ee34. Shortly afterward, Gong and co-workers reported the first enantioselective phosphination of acyclic imines with diphenylphosphine oxide using chiral squaramide as a hydrogen bonding organocatalyst, giving structurally diverse α-amnio phosphine oxides with good to excellent enantioselectivities35. Therefore, it is highly desirable for synthetic chemists to develop new enantioselective protocols for construction of α-amino diarylphosphine oxides.
Moreover, the enantioselective protocol for constructing chiral phosphine moiety at C1 position of THIQ was rarely explored. The only work in the field was reported by Mukherjee et al. who applied chiral thiourea as the catalysts in the asymmetric synthesis of 1,2,3,4-tetrahydroisoquinoline-1-ylphosphonates (Fig. 1b)36. However, this method required harsh reaction conditions (−80 °C) and preformed silyl phosphite, and only four examples with moderate to good enantioselectivities. In our very recent work, we successfully realized enantioselective phosphonation of isoquinolines for construction of chiral α-aminodiarylphosphine oxide via chiral phosphoric acid-catalyzed dearomatization of isoquinolines37. This protocol was found to have obvious limitations owing to the difficulties lying in the further transformation of the products ascribed to its facile racemization via thermodynamically favored aromatization process. To the best of our knowledge, there is no report of asymmetric phosphinylation of 3,4-dihydroisoquinolines that utilizes diarylphosphine oxides as the nucleophiles.
It is well known that chiral phosphoric acids (CPAs) play significant roles in the research field of organocatalysis38,39,40,41,42,43,44. We are keeping interests in for its application in new asymmetric reactions and useful synthesis45,46,47. Herein, we report on the chiral phosphoric acid catalyzed asymmetric phosphinylation of 3,4-dihydroisoquinolines using secondary phosphine oxides to construct the scaffold of chiral THIQ with phosphine oxide at C1 position (Fig. 1c). This catalytic system shows quite good functional group tolerance and wide substrate scope. The applicability of this protocol was well elucidated via gram-scale synthesis and derivatization of the products with the formation of different substituted chiral (thio)ureas that could possibly function as chiral organocatalysts or ligands in asymmetric catalysis. Furthermore, the catalytic cycle was well depicted on the basis of experimental results and DFT calculations.
Results and discussions
As our continuous research interests for developing methodology on constructing chiral phosphine-containing compounds, we explored the enantioselective phosphinylation of cyclic imines to chiral α-amino phosphine oxide. Readily available 3,4-dihydroisoquinoline (1a) and diphenylphosphine oxide (2a) were selected as the model substrates. After initial trials, it was found that the title phosphinylation reaction took place smoothly when 3,4-dihydroisoquinoline was firstly treated with Boc2O. In order to achieve satisfying enantiomeric excess, 73 chiral phosphoric acids (CPA) were systematically investigated (see Supplementary Table 1 for details) and part of the results were summarized in Table 1. Firstly, a series of chiral phosphoric acids based on BINOL skeleton (1-6) were evaluated using toluene as the solvent at room temperature for 24 h (Table 1, entries 1-6), which all afforded good to excellent yields, but only with 27% ee being the best enantioselectivity (entry 6). After that, several 8H-BINOL-derived chiral phosphoric acids 7-9 were tested to be the reaction catalysts, among which 9-anthracenyl substituted phosphoric acid 7 gave the title product in 90% yield with 44% ee (entry 7). After that, the spirocyclic SPINOL-phosphoric acids were also evaluated as the catalysts on the enantioselective phosphinylation, but without getting further improvement in enantiocontrol (entries 10–12). Then, a series of solvents were screened in order to improve the enantioselectivity using 7 as the catalyst. When benzene was employed as the solvent instead of toluene, ee was sharply increased to 63%, albeit with only 81% yield (entry 13). Chloro-containing solvents such as CCl4 and dichloromethane didn’t show positive effect on this reaction, affording the desired product with only 2% and 16% ee, respectively (entries 14-15). Aprotic solvents such as EtOAc, MeCN, acetone, ether, 1,4-dioxane and THF all gave inferior enantiocontrol results (entries 16–21). To our surprise, methyl tert-butyl ether (MTBE) when being used as the solvent provided the best enantioselectivity (entry 22, 79% ee). Then, four different types of molecular sieves were introduced as the additive to the catalytic system (entries 23–26), among which 4 Å MS showed the best efficiency, providing 99% yield and 91% ee (entry 24). Finally, additional efforts at reaction optimization (temperature, catalyst loading, concentration, and activating reagents,) provided the similar levels of selectivity (for details, see Supplementary Tables 1-3). It is noted that the same level of enantioselectivity was observed by using benzene as solvent instead of MTBE (entry 27).
After the optimal conditions was set down, we then turned our attention to exploring the generality of this catalytic reaction (see Supplementary Methods). 3,4-Dihydroisoquinoline bearing substituents at different sites on the phenyl ring was firstly investigated. The results indicated that 5-substituted substrates bearing whatever electron-withdrawing or electron-donating groups were all readily phosphorylated with good to excellent yields and enantioselectivities (Fig. 2, 4baa-4faa). Sensitive functional groups like CN, Cl, Br and I (1a-e) were quite well tolerated under the catalytic conditions and above 93% ee was provided. Then, different types of functional groups at C-6 position of 3,4-dihydroisoquinoline were also studied. Electron-withdrawing groups (CN, F, Cl, Br) were all showed no negative effect on this asymmetric phosphinylation, providing the desired α-amino phosphine oxide in excellent yields and enantioselectivities (Fig. 2, 4gaa-4iaa). Substrates bearing methyl and iso-propyl groups at C-6 position both showed inferior reactivity and enantioselectivity (Fig. 2, 4kaa and 4laa). After that, substituents at C-7 position were also investigated, among which phosphinylation of substrates 1m, 1n, 1o, 1p and 1r all readily took place under the optimal conditions, offering the corresponding α-amino phosphine oxide products with good to excellent yields (76~92%) and excellent enantioselectivities (90~95%, ee), except 4qaa (56% yield and 77% ee). What’s noteworthy, substituents at C-8 position seemingly made a huge resistance for the title catalytic phosphinylation. Reaction of substrate 1s was sluggish, giving the desired product (4saa) in 53% yield with only 3% ee. Moreover, no conversion to the desired product occurred with 1t as substrate under the optimal conditions. These results might provide clues for understanding the catalytic cycle and even the enantiocontrol mechanism.
Then, the scope of nucleophilic secondary phosphine oxides was also studied. Firstly, the effects of substituents electron-property of substituents on the phenyl ring of secondary phosphine oxide were investigated, of which bis(4-(trifluoromethyl)phenyl)phosphine oxide (2b) and bis(4-(tert-butyl)phenyl)phosphine oxide (2e) both reacted readily with 6-Cl-3,4-dihydroisoquinoline (1i) with 86% ee and 93% ee respectively (Fig. 3, 4iba and 4iea). When 3,4-dihydroisoquinoline was introduced as the substrate instead of 6-Cl-3,4-dihydroisoquinoline, the conversion and enantioselectivity were both slightly lower (Fig. 3, 4aba and 4aea). After that, di([1,1’-biphenyl]-4-yl)phosphine oxide (2c) and di-p-tolylphosphine oxide (2d) were also subjected to standard catalytic conditions reacting with 6-Cl-3,4-dihydroisoquinoline (1i) and 3,4-dihydroisoquinoline (1a) to provide the title products all with excellent enantiocontrol as listed in Fig. 3 (4ica and 4ida; 4aca and 4ada). However, the strong electron-donating methoxy group (OMe) markedly decreased yield (40%) while maintaining good enantioselectivity (86% ee). What’s noteworthy, when we changed the solvent into benzene, the yield significantly increased from 40% to 80% with the same enantioselectivity (83% ee) (Fig. 3, 4ifa). The bis(naphthalen-1-yl)phosphine oxide (2f) and bis(naphthalen-2-yl)phosphine oxide (2g) were both proper nucleophiles to realize the phosphinylation of 6-Cl-3,4-dihydroisoquinoline with 91% ee and 96% ee respectively (Fig. 3, 4ifa and 4iga). Then, the steric effect of ortho-substituents was tested, product 4iia could be readily obtained with 88% yield and 90% ee when bis(2-methylphenyl)phosphine oxide (2j) was selected as the substrate, while methoxyl group played a negative role on this reaction, providing the desired product only with 35% yield and 86% ee (Fig. 3, 4ija). Fortunately, the yield could also be increased to 78% by using benzene as the solvent. Product 3ika and 3ila were both smoothly yielded with 94% ee and 96% ee, respectively when steric hindered nucleophiles bis(3,5-dimethylphenyl)phosphine oxide (2k) and bis(3,5-di-tBu-phenyl)phosphine (2l) oxide were employed under the standard conditions. Unfortunately, more sterically hindered bis(2,4,6-trimethylphenyl)phosphine oxide (2m) turned out to be unreactive under such catalytic conditions. This result might be attributed to the steric hindrance effect of the double ortho-substituents.
aReaction conditions: 1 (0.2 mmol), Boc2O 3a (0.3 mmol) and CPA 7 (5 mol%) at 50 °C for 0.5 h. 4 Å MS (50 mg), MTBE (2 mL) and 2a (0.24 mmol) was added, and the mixture was stirred at room temperature for 24 h. Isolated yields were given here and ee value was determined by HPLC (Chiralcel OD-RH). [b]Benzene was used instead of MTBE.
To further demonstrate the practicability of our protocol, the enantioselective phosphinylation was carried out on a gram scale, offering the product 4aaa with 95% yield and 91% ee under the optimal conditions (Fig. 4). After one recrystallization, the ee value increased to 99%. Under TFA/DCM or TMSCl/MeOH conditions, the Boc group was easily removed to produce 5aaa in nearly quantitative yield with slight erosion of enantioselectivity. However, 5aaa is unstable and easily racemizes in solution. Fortunately, racemization of Zinc salt 5aaa’ (solution) could be completely inhibited via removing the Boc group of 4aaa with ZnBr2, followed by condensation with iso(thio)cyanate compounds, providing divergent access to a wide spectrum of structurally diverse chiral THIQ derivatives that bear phosphine oxide and (thio)carbamide moieties (Fig. 4b). Among that, 6aaa was obtained in 85% yield and 98% ee via condensation of freshly prepared 5aaa’ solution with isothiocyanatobenzene. After that, isocyanatoadmantane was also added into the 5aaa’ solution, providing 6aab in 88% yield with 99% ee. Note that, enantiopure 1-isothiocyanatoethylbenzene and 1-isocyanatoethylbenzene were both smoothly transformed into 6aac and 6aad containing chiral scaffolds containing both phosphine oxide and (thio)carbamide moieties. Quinine-derived isothiocyanate was also verified to be effective for reaction with 5aaa’, yielding product 6aae in 86% yield and 99% ee. These chiral THIQ derivatives possibly functioned as chiral ligands or organocatalysts with potential applications in the field of asymmetric organocatalysis and Lewis base catalysis.
a Scale-up synthesis, [a]Reaction conditions: 4aa (1 mmol), ZnBr2 (1.2 mmol) in DCM (10 mL) at room temperature for 12 h, see Supplementary Notes. No further post-processing required; b Synthetic transformations, b-fRelated iso(thio)cyanate compounds (1.2 mmol) were added, and the mixture was stirred at room temperature for 24 h. Isolated yields were given here and ee value was determined by HPLC.
To provide insight into the catalytic mechanism of this reaction, several control experiments and NMR tracking experiments were carried out (Fig. 5, see Supplementary Figs. 1–7 for details). Firstly, treatment of 2,3-dihydroisoquinoline with Boc2O for 30 min at 50 °C formed a new species, whose structure got verified to be tert-butyl 1-(tert-butoxy)-3,4-dihydroisoquinoline-2(1H)-carboxylate (1a’) by its 1H NMR47,48. After that, compound 1a’ was subjected to the standard catalytic conditions in C6D6 as the solvent, of which the title product 4aaa was formed in 99% yield with 90% ee, which indicates the possible intermediacy of compound 1a’ in the catalytic cycle (Fig. 5a). Then, substrate 1a was treated with diphenyl phosphine oxide under the catalysis of CPA 7 in C6D6 at room temperature. After 12 h, the formation of diphenyl(1,2,3,4-tetrahydroisoquinolin-1-yl)phosphine oxide (1b’) was observed. Then, treatment of isoquinoline (1b’) with Boc2O in the presence of CPA 7 formed racemic title product 4aaa in 99% yield (Fig. 5b). These results show that the asymmetric reaction process does not go through via the intermediate 1b’. Finally, the experiment that 1a directly reacted with diphenyl phosphine oxide in the presence of Boc2O under the catalysis of phosphoric acid was carried out, of which the desired product 4aaa was offered in 99% yield along with only 40% ee (Fig. 5c). The result indicated that the phosphinylation reaction occurred in a stepwise manner and the clean formation of 1a’ played pivotal role in the step of enantiocontrol.
DFT calculations were also conducted to understand the mechanism and the origin of the enantioselectivity of this reaction. The catalyst CPA 7 and substrate 1a were considered in our DFT calculations. On the basis of the mechanistic discussion above, we calculated the energy profile for the pathway shown in Fig. 5a. As shown in Fig. 6, the mechanistic cycle consists of three major stages. Stage 1 (1a → 1a-1) is related to the electrophilic addition of (Boc)2O to 1a with an energy barrier of 16.6 kcal/mol. In stage 2, decarboxylation of 1a-1 to give 1a’ is rate-determining for the whole pathway, with an overall activation free-energy barrier of 30.4 kcal/mol (the energy of TS3 related to 1a’). Stage 3 (1a’→4aaa) corresponds to the enantioselective phosphinylation of 1a’ with a stepwise nucleophilic substitution mechanism via an isoquinolinium intermediate involved. In stage 3, the bonding of diphenylphosphine oxide with CPA 7 increases the acidity of CPA 7. The diphenylphosphine-bonded CPA 7 then protonates 1a’ and releases a molecular or tBuOH to give the isoquinolinium 1a’-1. The phosphinylation of the isoquinolinium is enantio-determining step. This catalytic mode is consistent with previous closely related work49,50,51. The energy difference between the enantiodetermining transition states TS-5(S) and TS-5(R) was calculated to be 2.9 kcal/mol (98% ee), which correctly reproduces the enantiomeric preference observed experimentally (91% ee for S-4aaa).
By carefully checking the structures of TS5(R) and TS5(S) (Fig. 7), we found that the CPA 7-phosphonate has stronger hydrogen bonding interaction with isoquinolinium in the favorable transition state TS5(S) than that in the TS5(R). The hydrogen at C-8 position of isoquinoline is involved in the hydrogen bonding interactions with the distance of C8-H and CPA 7-phosphonate in TS5(S) at 2.31 Å which is shorter than that in TS5(R) (2.45 Å). This finding suggests that the aryl hydrogen at C-8 position also plays a role in the determination of the stereoselectivity of this reaction, consistent with the experimental observations shown in Fig. 2 that substituents at C-8 position (4saa) give poor enantioselectivity.
Conclusions
In summary, we have developed an efficient and mild methodology for the asymmetric phosphinylation of 3,4-dihydroisoquinoline (up to 99% yield and 97% ee) using secondary phosphine oxide for the first time. Applicability of this protocol was well demonstrated via gram-scale synthesis and preparing several potential chiral organocatalysts or ligands through the derivatization of the products. Experimental studies and DFT calculations support the intermediacy of tert-butyl 1-(tert-butoxy)-3,4-dihydroisoquinoline-2(1H)-carboxylate. Also, experimental studies and DFT calculations suggest that hydrogen bonding strength between the phosphonate and isoquinolinium plays pivotal role in the determination of the stereoselectivity in this reaction.
Methods
General procedure for CPA-catalyzed Phosphinylation of 3,4-Dihydroisoquinolines
A mixture of 3,4-dihydroisoquinoline (0.2 mmol), CPA catalyst (5 mol%) and (Boc)2O (0.3 mmol) was stirred at 50 °C for 0.5 h. Then 4 Å MS (50 mg), MTBE or benzene (2 mL) and diarylphosphine oxide (0.24 mmol) was added, and the reaction was stirred at room temperature for 24 h. The reaction mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography with PE/EA (2/1) to obtain tert-butyl-1-(diphenylphosphoryl)-3,4- dihydroisoquinoline-2(1H)-carboxylates.
Data availability
All data generated during this study are included in this article and Supplementary Information. Experimental procedure, conditions optimization, control and tracking experiments, product characterization and DFT calculations are provided in the Supplementary Information. The NMR spectra of all compounds are available in Supplementary Data 1. HPLC chromatograms of the chiral products are available in Supplementary Data 2. The Cartesian coordination of the key structures are provided in Supplementary Data 3. It can be declared that all the relevant data are provided in the article and its Supplementary Information files.
References
Tang, W.-J. & Zhang, X.-M. New chiral phosphorus ligands for enantioselective hydrogenation. Chem. Rev. 103, 3029–3070 (2003).
Hayashi, T. Chiral monodentate phosphine ligand MOP for transition-metal-catalyzed asymmetric reactions. Acc. Chem. Res. 33, 354–362 (2000).
Ni, H., Chan, W.-L. A. & Lu, Y. Phosphine-catalyzed asymmetric organic reactions. Chem. Rev. 118, 9344–9411 (2018).
Wan, F. A. & Tang, W.-J. Phosphorus ligands from the Zhang lab: Design, asymmetric hydrogenation, and industrial applications. Chin. J. Chem. 39, 954–968 (2021).
Carmona, J. A., Rodríguez-Franco, C., Fernández, R., Hornillos, V. & Lassaletta, J. M. Atroposelective transformation of axially chiral (hetero)biaryls. From desymmetrization to modern resolution strategies. Chem. Soc. Rev. 50, 2968–2983 (2021).
Liu, L. et al. Copper-Catalyzed Intermolecular Enantioselective Radical Oxidative C(sp3)−H/C(sp)−H Cross-Coupling with Rationally Designed Oxazoline-Derived N,N,P(O)-Ligands. Angew. Chem. Int. Ed. 133, 26914–26717 (2021).
Engel, R. Phosphonates as analogues of natural phosphates. Chem. Rev. 77, 349–367 (1977).
Romanenko, V. D. & Kukhar, V. P. Fluorinated phosphonates: Synthesis and biomedical application. Chem. Rev. 106, 3868–3935 (2006).
Galezowska, J. & Gumienna-Kontecka, E. Phosphonates, their complexes and bio-applications: A spectrum of surprising diversity. Coord. Chem. Rev. 256, 105–124 (2012).
Horsman, G. P. & Zechel, D. L. Phosphonate biochemistry. Chem. Rev. 117, 5704–5783 (2017).
Parkinson, E. I., Erb, A., Eliot, A. C., Ju, K.-S. & Metcalf, W. W. Fosmidomycin biosynthesis diverges from related phosphonate natural products. Nat. Chem. Biol. 15, 1049–1056 (2019).
Baumgartner, T. & Réau, R. Organophosphorus π-conjugated materials. Chem. Rev. 106, 4681–4727 (2006).
Mallesham, G. et al. Phosphine oxide functionalized pyrenes as efficient blue light emitting multifunctional materials for organic light emitting diodes. J. Mater. Chem. C. 3, 1208 (2015).
Zhang, S.-A. et al. Highly efficient removal of uranium from highly acidic media achieved using a phosphine oxide and amino functionalized superparamagnetic composite polymer adsorbent. J. Mater. Chem. 8, 10925–10934 (2020).
Liu, X.-W. et al. An efficient synthesis of chiral phosphinyl oxide pyrrolidines and their application to asymmetric direct aldol reactions. Org. Biomol. Chem. 6, 3997–4003 (2008).
Morris, D. J. et al. Asymmetric organocatalysis of the addition of acetone to 2-nitrostyrene using N-diphenylphosphinyl-1, 2-diphenylethane-1, 2-diamine (PODPEN). Tetrahedron Lett. 51, 209–212 (2010).
Turkbey, B., Hoyt, R. F., Agarwal, H. K., Bernardo, M. & Sankineni, S. Magnetic resonance sentinel lymph node imaging of the prostate with gadofosveset trisodium–albumin: Preliminary Results in a Canine Model. Acad. Radiol. 22, 646–652 (2015).
Reddy, K. R. et al. Pradefovir: a prodrug that targets adefovir to the liver for the treatment of hepatitis B. J. Med. Chem. 51, 666–676 (2008).
Lane, J. W., Chen, Y.-Y. & Williams, R. M. Asymmetric Total Syntheses of (−)-Jorumycin, (−)-Renieramycin G, 3-epi-Jorumycin, and 3-epi-Renieramycin G. J. Am. Chem. Soc. 127, 12684–12690 (2005).
Vincent, G. & Williams, R. M. Asymmetric Total Synthesis of (−)‐Cribrostatin 4 (Renieramycin H). Angew. Chem. Int. Ed. 46, 1517 (2007).
Zhu, R.-H. et al. Chin. J. Chem. 32, 1039–1542 (2014).
Chrzanowska, M., Grajewska, A. & Rozwadowska, M. D. Asymmetric synthesis of isoquinoline alkaloids: 2004–2015. Chem. Rev. 116, 12369–12465 (2016).
Crestey, F. et al. Design, synthesis, and biological evaluation of Erythrina alkaloid analogues as neuronal nicotinic acetylcholine receptor antagonists. J. Med. Chem. 56, 9673 (2013).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals: miniperspective. J. Med. Chem. 57, 10257–10274 (2014).
Zhang, X.-L. et al. Structure-aided identification and optimization of tetrahydro-isoquinolines as novel PDE4 inhibitors leading to discovery of an effective antipsoriasis agent. J. Med. Chem. 62, 5579–5593 (2019).
Sharma, U. K., Ranjan, P., Eycken, E. V. & You, S.-L. Sequential and direct multicomponent reaction (MCR)-based dearomatization strategies. Chem. Soc. Rev. 49, 8721–8748 (2020).
Chakka, S. K., Andersson, G., Maguire, G. E. M., Kruger, H. G. & Govender, T. Synthesis and Screening of C1‐Substituted Tetrahydroisoquinoline Derivatives for Asymmetric Transfer Hydrogenation Reactions. Eur. J. Org. Chem. 5, 972–980 (2010).
Peters, B. et al. Novel tetrahydroisoquinoline based organocatalysts for asymmetric Diels–Alder reactions: insight into the catalytic mode using ROESY NMR and DFT studies. Tetrahedron.: Asymmetry. 21, 2859 (2010).
Naicker, T., Arvidsson, P. I., Kruger, H. G., Maguire, G. E. M. & Govender, T. Tetrahydroisoquinoline‐Based N‐Oxides as Chiral Organocatalysts for the Asymmetric Allylation of Aldehydes. Eur. J. Org. Chem. 34, 6923 (2011).
Kawthekar, R. B. et al. Synthesis of tetrahydroisoquinoline (TIQ)–oxazoline ligands and their application in enantioselective Henry reactions. Tetrahedron.: Asymmetry. 21, 846 (2010).
Cele, Z. E. D. et al. Catalytic asymmetric carbon–carbon bond forming reactions catalyzed by tetrahydroisoquinoline (TIQ) N, N′-dioxide ligands. Tetrahedron.: Asymmetry. 24, 191–195 (2013).
Liu, W.-S., Liu, S.-S., Jin, R.-W., Guo, H. & Zhao, J.-B. Novel strategies for catalytic asymmetric synthesis of C1-chiral 1, 2, 3, 4-tetrahydroisoquinolines and 3, 4-dihydrotetrahydroisoquinolines. Org. Chem. Front. 2, 288–299 (2015).
Yamakoshi, K., Harwood, S. J., Kanai, M. & Shibasaki, M. Catalytic asymmetric addition of diphenylphosphine oxide to cyclic imines. Tetrahedron Lett. 40, 2565–2568 (1999).
Ingle, G. K. et al. Chiral magnesium BINOL phosphate-catalyzed phosphination of imines: access to enantioenriched α-amino phosphine oxides. Org. Lett. 13, 2054–2057 (2011).
Kong, L.-P. et al. Highly enantioselective phosphination and hydrophosphonylation of azomethine imines: using chiral squaramide as a hydrogen bonding organocatalyst. Org. Biomol. Chem. 12, 8656–8670 (2014).
Ray, C. A. & Mukherjee, S. Enantioselective dearomatization of isoquinolines by anion-binding catalysis en route to cyclic α-aminophosphonates. Chem. Sci. 7, 6940–6945 (2016).
Gao, Z. & Guo, Y. Enantioselective phosphonation of isoquinolines via chiral phosphoric acid-catalyzed dearomatization. Chem. Commun. 58, 9393–9396 (2022).
Akiyama, T., Itoh, J., Yokota, K. & Fuchibe, K. Enantioselective Mannich‐type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Ed. 43, 1566–1594 (2004).
Uraguchi, D. & Terada, M. Chiral Brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 126, 5356–5357 (2004).
Xu, F. et al. SPINOL-derived phosphoric acids: synthesis and application in enantioselective Friedel−Crafts reaction of indoles with imines. J. Org. Chem. 75, 8677–8680 (2010).
Wang, L., Zhong, J. & Lin, X. Atroposelective Phosphoric Acid Catalyzed Three‐Component Cascade Reaction: Enantioselective Synthesis of Axially Chiral N‐Arylindoles. Angew. Chem. Int. Ed. 44, 15824–15828 (2019).
Akiyama, T. Stronger brønsted acids. Chem. Rev. 107, 5744–5758 (2007).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete Field Guide to Asymmetric BINOL Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev. 114, 9047–9153 (2014).
Lin, X., Wang, L., Han, Z. & Chen, Z. Chiral spirocyclic phosphoric acids and their growing applications. Chin. J. Chem. 39, 802–824 (2021).
Guo, Y. et al. Enantioselective Biginelli Reaction of Aliphatic Aldehydes Catalyzed by a Chiral Phosphoric Acid: A Key Step in the Synthesis of the Bicyclic Guanidine Core of Crambescin A and Batzelladine A. Synthesis. 12, 2394–2406 (2018).
Guo, Y. et al. Chiral Spirocyclic Phosphoric Acid-Catalyzed Synthesis of 4-Alkyl-3,4-dihydropyrimidin-2(1H)-one Derivatives by Asymmetric Biginelli Reactions. Asian J. Org. Chem. 9, 626–630 (2020).
Guo, Y. et al. Practical catalytic enantioselective synthesis of 2, 3-dihydroquin-azolinones by chiral brønsted acid catalysis. Org. Biomol. Chem. 19, 4146–4151 (2021).
Laconsay, C. J., Seguin, T. J. & Wheeler, S. E. Modulating Stereoselectivity through Electrostatic Interactions in a SPINOL-Phosphoric Acid-Catalyzed Synthesis of 2, 3-Dihydroquinazolinones. ACS Catal. 10, 12292–12299 (2020).
Sasamoto, N., Dubs, C., Hamashima, Y. & Sodeoka, M. Pd (II)-catalyzed asymmetric addition of malonates to dihydroisoquinolines. J. Am. Chem. Soc. 128, 14010–14011 (2006).
Michael, J., Rishel, M. J., Amarasinghe, K. K. D., Dinn, S. R. & Johnson, B. F. Asymmetric synthesis of tetrabenazine and dihydrotetrabenazine. J. Org. Chem. 74, 4001–4004 (2009).
Zhang, M. et al. Enantioselective dearomative arylation of Isoquinolines. ACS Catal. 6, 5290–5294 (2016).
Acknowledgements
Y-N.D., acknowledges the financial support from Chemistry and Chemical Engineering Guangdong Laboratory (Grant nos. 2011006 and 2132013) and the Special Fund for the Sci-tech Innovation Strategy of Guangdong Province (no. 210730166882026).
Author information
Authors and Affiliations
Contributions
Y.G., Y-N.D., and J.X. conceived and directed the project. Y.G., Z.G., N.L., and J.L. performed the experiments. Y-N.D. performed the theoretical calculations. X.B., and Z.G. analyzed the results. Y.G. and J.X. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, Y., Li, N., Li, J. et al. Chiral phosphoric acid-catalyzed enantioselective phosphinylation of 3,4-dihydroisoquinolines with diarylphosphine oxides. Commun Chem 6, 26 (2023). https://doi.org/10.1038/s42004-023-00826-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-023-00826-4
This article is cited by
-
Asymmetric dearomative single-atom skeletal editing of indoles and pyrroles
Nature Chemistry (2025)