Abstract
Water and its dissociated species at the solid‒liquid interface play critical roles in catalytic science; e.g., functions of oxygen species from water dissociation are gradually being recognized. Herein, the relationship between oxide identity (PtOHads, PtOads, and PtO2) and electrocatalytic activity of platinum for ethanol electrooxidation was obtained in perchlorate acidic solution over a wide potential range with an upper potential of 1.5 V (reversible hydrogen electrode, RHE). PtOHads and α-PtO2, rather than PtOads, act as catalytic centers promoting ethanol electrooxidation. This relationship was corroborated on Pt(111), Pt(110), and Pt(100) electrodes, respectively. A reaction mechanism of ethanol electrooxidation was developed with DFT calculations, in which platinum oxides-mediated dehydrogenation and hydrated reaction intermediate, geminal diol, can perfectly explain experimental results, including pH dependence of product selectivity and more active α-PtO2 than PtOHads. This work can be generalized to the oxidation of other substances on other metal/alloy electrodes in energy conversion and electrochemical syntheses.
Similar content being viewed by others
Introduction
In-depth exploration of the dynamic mechanism at the water–solid interface advances the development and utility of heterogenous catalysis in electrochemical1,2,3,4, chemical5,6,7,8,9, and photochemical reactions10. The key role of interfacial water for charge transfer at the water–solid interface is recognized in disparate systems11. For heterogeneous oxidation reactions, the promotion5,6,7,8,12 and inhibition9 effects of interfacial water on the catalyst surface were reported. In electrochemical reactions, the structures, orientations, and dissociation of interfacial waters depend on the applied potential, electrode composition, and solutes in the aqueous solution13,14,15. The H and O atoms of interfacial water point toward the surface at different potentials14, which aids H- and O-related reactions. Intuitively, H2O acts as a source of H/O adsorbates and promotes oxidation16 and reduction17 of substrates. However, adsorbed oxygenated species inhibit reactions in many cases18.
Platinum is known as the most active element for electrochemical reactions and remains the benchmark for electrocatalyst materials3. Potential-dependent Pt oxides and their structures were identified on single-crystal and polycrystalline electrodes by many investigators19,20,21,22,23,24,25,26, which is believed as one of the great achievements in basic electrochemistry in the past 30 years. Different analytical methods, such as voltammetric methods19,20,21, X-ray photoelectron spectroscopy (XPS)21, X-ray absorption spectroscopy (XAS)22, X-ray reflection/diffraction /scattering22,23,24,25, and Raman spectroscopy26, have been used to identify surface Pt oxides (PtOHads, PtOads, and PtO2) and their dependence on potential. Electrosorption of OHads on Pt at potentials lower than 0.95 V (vs. standard hydrogen electrode, SHE) constitutes the initial stage of Pt electrooxidation21,22, in which the “butterfly” region of CVs for Pt(111) in HClO4 solution contains random adsorption in the broad region followed by the formation of a transient p (2 × 2) adsorbate phase and a subsequent phase transition to a p (1 × 1) adlayer at the sharp peak24. The second stage proceeds with the appearance of PtOads at approximately 0.95 V, followed by the place-exchange (PE) process23,25, in which a Pt surface atom leaves its lattice site and oxygen penetrates the metal lattice; this occurs at approximately 1.05 V. After formation of place-exchanged atoms up to the physical limit at approximately 1.15 V20, other surface atoms are oxidized and leave Pt surface lattice sites, this leads to 2D Pt oxides19, which ultimately grow to form three-dimensional Pt oxides in higher potential22,26.
The function of PtOHads in electrochemical oxidations of small organic molecules (such as CO27, methanol28,29, and ethanol30,31,32,33) and other compounds (such as thiosulfate and thiourea34) has been studied in previous reports. However, the function and mechanism of Pt oxides at higher potentials, e.g., PtOads (0.95–1.15 V) and PtO2 (>1.15 V) in electrocatalytic oxidations, have rarely been studied. In this work, we systematically explored the corresponding relationship between Pt oxides and the electrooxidation activity of ethanol using online electrochemical-HPLC and verified the promoting effect of Pt oxides with DFT calculations, which gave an atom-scale mechanistic description of oxide function on ethanol oxidation. At the same time, two points are considered here. First, the relationship between oxide identity and electrooxidation activity of ethanol will not be the focus below 0.5 V (RHE); even the PtOHads can be found on Pt(100) and Pt(111) and polycrystalline Pt in the low potentials35,36,37, the reason of which is that PtOHads below 0.5 V (RHE) can be displaced by CO produced by partial C-C bond cleavage of ethanol on P(110), Pt(100), and polycrystalline Pt during repeatedly round-trip scan of applied potential30,32, resulting in CO adsorption-induced surface deactivation and this adsorbed CO can be oxidized to CO2 only above 0.6 V29. Second, we will mainly carry out the experimental study and mechanistic analysis involving the oxidation of ethanol and acetaldehyde since monobasic Pt catalyst shows quietly low CO2 selectivity38.
Results and discussion
Potential-dependent oxide identity on Pt nanoparticles in HClO4 solutions
Figure 1a shows the cyclic voltammogram (CV) for oxidation of Pt nanoparticles in 0.5 M HClO4 solution between 0.05 V and 1.5 V, in which the potential-dependent oxidation processes of Pt are identified based on previous works22,23,39,40,41,42. Desorption and adsorption of hydrogen at potentials within the range 0.05–0.40 V were followed by the electrical double layer region between 0.40 and 0.60 V. Potentials above 0.60 V constitute the Pt oxide region, where the initial stage of surface oxidation leads to the formation and adsorption of OHads39,40. The OHads coverage in region A increases with potential and begins to convert into Oads at approximately 0.95 V40, which corresponds to the start of region B indicated by the place-exchange (PE) process, i.e., a surface Pt atom leaves its lattice site and exchanges with an oxygen species23,41. Between 0.95 and 1.15 V, PtOHads gradually transforms into PtOads species22. At higher potentials, i.e., region C shown in Fig. 1a, higher-order oxides (such as α-PtO2) are formed41,42.
a Cyclic voltammogram of Pt nanoparticles in 0.5 M HClO4 solution at potentials between 0.05 V and 1.5 V with a scan rate of 50 mV s−1, in which Region A, B, and C represent the potential regions forming PtOHads, PtOads, and α-PtO2, respectively. b The variations of Raman intensity for the PtOHads and α-PtO2 along the positive voltammetry curve in 0.1 M HClO4 electrolyte with a scan rate of 50 mV s−1. The black, red, and blue solid lines represent the voltammetry curve of Pt, Raman-intensity curves for the PtOHads and α-PtO2, corresponding to the black, red, and blue Y-axes, respectively.
Figure 1b shows the correspondence between voltammetry data and the Raman spectra. The bands arising at the indicated potentials from 0.65 to 1.45 V are shown in Supplementary Fig. 1 of the Supplementary Information (SI). The band at about 935 cm−1 corresponds to the symmetric stretching mode of ClO4−43, which can be regarded as an indirect indicator for the formation of OHads adlayer26 because ClO4− is located in the compact part of the interfacial double layer according to the Stark effect44. At a potential of 1.15 V, a band begins to appear at ca. 590 cm−1; this has been assigned to amorphous α-PtO226. The Raman peak intensity for PtOHads···ClO4− shows a maximum near the maximum current generated at ca. 0.8 V and decreases with increasing potential, which indicates the formation and coverage of OHads on the surface of Pt. Additionally, the Raman intensity of the α-PtO2 signal emerges at a potential of 1.15 V and increases with potential.
The intensities of the Raman bands qualitatively followed the current for Pt oxidation at potentials higher than 0.65 V, which represented the formation and growth of Pt oxides in this region. The PtOHads species began to form above 0.60 V, the coverage of which increased with current density. The Raman intensity for PtOHads decreased with decreasing current density due to the transformation of PtOHads to PtOads. At a higher potential of approximately 1.15 V, the intensity of the PtOHads band was severely reduced as current density increased, and the appearance and increased intensity of α-PtO2 were caused by the consumption of PtOHads, as shown in Fig. 1b.
Electrocatalytic activity of Pt oxides for ethanol oxidation on Pt nanoparticles
Figure 2 shows the CV curves for ethanol oxidation on Pt nanoparticles in 0.5 M perchloric acid with different concentrations of ethanol. The current peak M (~0.7 V), beginning from region A related to PtOHads in the blank solution (black line), shifted to more positive potentials (~0.85 V) with the addition of ethanol, which might be ascribed to competitive adsorption on OHads with ethanol45 and intermediates such as CO32. Peak N (~1.3 V), which originated from α-PtO2 in the blank solution (black line), was fixed at the same potential upon the addition of ethanol. The current densities of both peak M and peak N increased upon the addition of ethanol, indicating that OHads and α-PtO2 were catalytic active centers. However, the increase in the current density at a potential of 1.05 V located in the valley between peak M and peak N was far smaller than those of peak M and peak N; i.e., PtOads did not generate the corresponding peak of current density, indicating that PtOads is an inactive center.
Further corroboration of oxide activity on three basal crystal planes of Pt in ethanol oxidation
The experimental results described above demonstrate that peak M and peak N for ethanol electrooxidation coincided with the formation and growth of Pt oxides in perchloric acid, indicating that PtOHads and α-PtO2 promote ethanol oxidation at peak M and peak N, respectively; moreover, PtOads showed almost no corresponding current peak of electrocatalysis in the ethanol oxidation. The atomic arrangement of the polycrystalline electrode is composed of basic single-crystal domains, and the surface of the Pt nanoparticle electrode was characterized by the adsorption of bismuth and germanium, as shown in Supplementary Fig. 2. Calculation results are summarized in Table 1, the Pt nanoparticles consist of 11.69% Pt(100), 16.52% Pt(111), and 71.79% Pt(110) domains. Therefore, the sum of the experimental CV curves with the ratio for three basal crystal planes agreed well with the results for Pt polycrystalline nanoparticles, as shown in Supplementary Fig. 3.
To testify the generality of catalytic function for Pt oxides, electrooxidation of ethanol on Pt(110), Pt(100), and Pt(111) was observed as shown in Fig. 3. Figure 3a, b shows the comparison of voltammetric profiles generated for Pt(110) in 0.1 M perchloric acid (Fig. 3a) with different concentrations of ethanol (Fig. 3b), and the regions for PtOHads, PtOads, and α-PtO2 are marked in Fig. 3b. Peak M was observed above 0.60 V, which corresponded to the PtOHads potential region, and the current density was increased by adding ethanol into the solution. Peak N (~1.20 –1.40 V), which corresponded to α-PtO2, showed increased intensity upon the addition of ethanol. However, the valley bottom between peak M and peak N corresponded to the current peak for PtOads in a blank solution, indicating that PtOads does not act as the active catalytic center. The oxidation behavior of ethanol on Pt(100) and Pt(111), as shown in Fig. 3c–f, was consistent with the situation of Pt(110), which further verified the relationship between Pt oxides and the activity for the electrooxidation of ethanol.
Potential-dependent product distribution
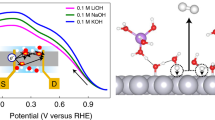
To further study the correspondence between Pt oxides and the activity of ethanol electrooxidation, chronoamperometry combined with HPLC was employed to monitor the voltammetry curves and product concentrations at different pH values, i.e., potential-dependent distribution of products for the electrooxidation of 0.1 M ethanol at different pH values are shown in Fig. 4. Both the concentrations of acetaldehyde and acetic acid approximately correspond to the current peaks, i.e., ethanol electrooxidation in peak M and peak N is derived from PtOHads and α-PtO2, respectively.
Notably, the height and area of current peak N were higher than those of peak M at all pH values, as shown in the voltammetry profiles of Fig. 4, indicating the surface PtO2 is a more powerful active center than PtOHads. For every same pH, the concentration of acetaldehyde was higher than that of acetic acid at peak M; meanwhile, the concentration of acetic acid was higher than that of acetaldehyde at peak N, demonstrating that PtOHads and amorphous α-PtO2 particles are more likely to form acetaldehyde and acetic acid, respectively. With the increase of pH, current values in both peaks increased, the reason for which is that high pH favors the formation of Pt oxides. The acetaldehyde concentration at peak M was increased with the increase in pH, and conversely, the concentration of acetic acid decreased. However, non-monotonic pH dependence of the concentration of acetaldehyde and acetic acid at peak N was observed in an acidic medium, as shown in Fig. 4. In previous reports, PtO2 nanoparticles were shown to oxidize ethanol46 and favor complete oxidation to carbon dioxide47; this non-monotonic pH dependence possibly results from other products, such as carbon dioxide, from sufficient and more powerful PtO2 active centers in high pH.
Atomic-level understanding and experimental explanation
To explain the catalytic functions of the Pt oxides (PtOHads and PtO2), the energy profiles for ethanol electrooxidation on the Pt surface were calculated, as shown in Fig. 5 and Supplementary Tables 1–3 of the SI, in which the adsorption is represented by star (*). For PtOHads, region A of the CV (Fig. 1a) corresponded to peak M for ethanol electrooxidation (Fig. 2), in which the hydroxyl groups were formed and adsorbed on the Pt surface (R1)30.
a, b The comparisons of energy profiles for the formation of the acetaldehyde and acetic acid on Pt with (red path) and without (blue path) co-adsorbed OH. c, d Energy profiles for the formation of acetaldehyde and acetic acid on Pt with α-PtO2*, respectively. Surface structures of transition states (TS1-9) can be found in the above plots. Blue, gray, red, and white balls stand for Pt, C, O, and H atoms, respectively.
It is well known that the interaction of ethanol with the surface of the Pt electrode is weak, and the presence of OHads would enable ethanol to enter the electrical double layer and adsorb on Pt. The adsorption energy (△Eads) of ethanol with co-adsorbed OHads is −0.81 eV, which is more advantageous than the energy without co-adsorbed OH (−0.30 eV), suggesting that OHads greatly enhanced ethanol adsorption on the Pt surface. Sheng et al.48 proposed that the OHads on a metal electrode, rather than the metal itself, provide the active centers and that concerted-like dehydrogenation caused by OHads acts as the dominant pathway for ethanol electrooxidation. This mechanistic pathway is used here for two reasons as follows. First, even that work48 calculated the role of water and adsorbed Hydroxyl in an alkaline medium, R1 for adsorbed hydroxyl universally accepted in acidic medium20,21,22, so this dehydrogenation mode can also be used to explain our experimental work about the active effect of PtOHads on ethanol electrooxidation in acidic solution (peak M in Fig. 2). Second, since PtOHads is distributed in large surface area (0.50 ML)49 and it can move in the surface through water exchange33, concerted-like dehydrogenation of substrate by PtOHads on surface to different products is used here as a favorable path for the full utilization of PtOHads and synergistic attraction of dihydrogen of ethanol with multiple PtOHads48, although sequence dehydrogenation33 is also possible path. However, atomic-scale direct observation is needed for tracking surface dehydrogenation. For simple, feasible computation, two OHads sites were introduced into our model (marked as OH* in the energy profiles). A comparison of the energy profiles as well as the transition states for the formation of acetaldehyde on Pt with (red path) and without (blue path) co-adsorbed OH-induced dehydrogenation of ethanol is shown in Fig. 5a, and the corresponding thermodynamic data are shown in Supplementary Table 1. Ethanol undergoes dehydrogenation to form acetaldehyde, and the route depicted in blue lines represents ethanol dehydrogenation directly onto the Pt surface, which breaks the C-H bond and O-H bond (TS1, the activation barrier Ea = 0.57 eV) to form CH3CHOads and Hads. The Hads combines with adjacent OHads generating H2Oads. The red route represents ethanol dehydrogenation using co-adsorbed OHads, in which adsorbed ethanol undergoes concerted-like C-H bond breaking and O-H bond breaking to form acetaldehyde (TS0, Ea = 0.42 eV). The lower activation barrier suggests that OHads is beneficial for the dehydrogenation of ethanol (R2).
Acetaldehyde is quite active and easily hydrated to form the geminal diol (CH3CH(OH)2) in aqueous solution, with an equilibrium constant of 1.3, as shown in reaction (R3); this is accelerated dramatically by H+ cation in acidic medium or OH− anion in alkaline medium, respectively50.
A comparison of the energy profiles, as well as the transition states for the formation of acetic acid on Pt via the dehydrogenation of geminal diol with (red path) and without (blue path) facilitation of co-adsorbed OHads, are shown in Fig. 5b, and the corresponding thermodynamic data are shown in Supplementary Table 2. The adsorption energy (△Eads) of geminal diol with co-adsorbed OHads is −0.57 eV, which is higher than the energy without co-adsorbed OHads (−0.16 eV), suggesting that OHads enhanced the adsorption of geminal diol on the Pt surface. According to previous work43, geminal diol can be dehydrogenated by PtOHads to form acetic acid, as shown in Fig. 5b (red path), for which the activation barrier was calculated to be 0.39 eV (TS4). Further, the activation barrier for the formation of acetic acid without co-adsorbed OH-induced dehydrogenation was calculated to be 0.92 eV (TS5). Therefore, the activation barrier of red path is lower than that of blue path, clarifying the catalytic function of PtOHads (R4).
The reaction rate of R3 for producing geminal diol depends on pH50. According to (R2-R4), when pH increases in acidic solution, slower reaction R3 causes higher selectivity of acetaldehyde over acetic acid, which agrees with experimental results of peak M in Fig. 4, i.e., the favorable dehydrogenation path of geminal diol (R4) in low pH medium can be experimentally supported. According to the above calculation results, mechanisms for ethanol oxidation at PtOHads active centers were defined (R1-R4). On the one hand, OHads strengthens the adsorption of ethanol on the Pt surface. On the other hand, the presence of OHads reduces the activation barrier of ethanol electrooxidation, thereby facilitating the dehydrogenation of ethanol. The comparison of the two paths can better explain the essential role of PtOHads as active center28,29.
When the applied potential in Region B increased to the range from 0.95 to 1.05 V, PtOHads was further oxidized to PtOads, as shown in Fig. 1a, through reaction (R5)51. PtOads showed no peak of current and product for ethanol electrooxidation, as shown in Figs. 2–4, due to the structural stability from the shorter bond between Oads and Pt22 and PE-induced subsurface oxygen (Osub)23, causing this oxide to be inactive.
α-PtO2 formed in region C (Fig. 1a and Fig. 3) above 1.15 V (R6-R7)26,42, which resulted in a corresponding peak for ethanol electrocatalytic oxidation, as indicated by Peak N in Figs. 2–4.
Here, the active role of α-PtO2 was explored with the catalysis model shown in Supplementary Fig. 4; the locally covered α-PtO2 micro-particle was used for ethanol electrooxidation52,53, since approximately 50% of the Pt atoms formed disordered oxides, 20% of the surface atoms were located on place-exchange sites, and 30% remained in regular surface sites during the Pt oxidation process54.
The energy profiles for ethanol oxidation in the presence of α-PtO2 are shown in Fig. 5c, d, and the calculation results are shown in Supplementary Table 3. The IS (initial state) of ethanol oxidation in the presence of α-PtO2 with an adsorption energy of −1.08 eV (−0.81 eV with OHads) indicates that α-PtO2 more greatly strengthens the adsorption of ethanol on the Pt surface than PtOHads. The black path in Fig. 5c shows the process of concerted-like ethanol dehydrogenation (the O-H and C-H band cleavage) by α-PtO2 to form acetaldehyde, of which the activation barrier (TS8, red line) is 0.37 eV. In Fig. 5d, the adsorption energy of geminal diol in the presence of α-PtO2 is −1.29 eV, much higher than that in the presence of PtOHads (−0.57 eV), suggesting that α-PtO2 results stronger adsorption of geminal diol on the Pt surface than PtOHads. Then geminal diol undergoes concerted-like dehydrogenation of C-H and O-H bonds with an energy barrier of 0.19 eV (TS9) to form CH3COOHads. The activation barrier of ethanol dehydrogenation to acetaldehyde and acetic acid by α-PtO2 is lower (0.37 and 0.19 eV) than that by OHads (0.42 and 0.39 eV), indicating that the α-PtO2 is a more active center than PtOHads to benefit the oxidation of ethanol to high concentration of acetic acid, which can explain that high selectivity of acetic acid at peak N in high potential range (Fig. 4). When pH increase, more PtO2 particles on Pt surface would form, and excess PtO2 could oxidize ethanol to other carbon products of high oxidation state such as carbon dioxide42, resulting in non-monotonic pH dependence of the concentration of acetaldehyde and acetic acid at peak N as shown in Fig. 4.
In conclusion, the relationship between Pt oxides and ethanol electrooxidation activity in perchlorate acid was obtained, i.e., surface PtOHads and α-PtO2, as active centers, catalyze ethanol oxidation; in contrast, surface PtOads is inactive and does not generate corresponding peaks for current density and product concentration. A Pt oxides-mediated reaction mechanism for ethanol oxidation on Pt catalyst was developed with the help of DFT computation, in which Pt oxide-mediated dehydrogenation and hydrated reaction intermediate, i.e., geminal diol, can perfectly explain the experimental results such as pH dependence of product selectivity and more active α-PtO2 than PtOHads. This work distinguished the functions of different Pt oxides in ethanol electrochemical oxidation and was not limited to consideration of surface PtOHads as in past studies (the maximum applied potential in most previous work did not exceed 1.10 V)28,29,30,31,32,33. These results can be extended to other electrodes, such as metal oxides16, alloys55, MOFs56, and perovskites57, and oxidations of other substrates.
Methods
Electrochemical measurements
All solutions were prepared with ultrapure water (Milli-Q system, 18.2 MΩ•cm). The chemicals used for solution preparation were perchloric acid (Suprapur, Merck) and sodium perchlorate (Suprapur, Merck). All other chemicals were of the highest commercially available purity (ethanol, acetaldehyde, and acetic acid) and were used without further purification. All solutions in the electrochemical experiments were deoxygenated with N2 (99.999%). Before each measurement, all equipment in contact with the electrolyte and electrodes was rinsed with distilled water, treated with diluted KMnO4 solution for at least 12 h, and diluted H2O2 solution for 8 h. Finally, they were thoroughly rinsed with deionized water.
The working electrode was prepared by depositing 4 µL of an aqueous solution containing 2 mg mL−1 polyoriented Pt nanoparticles (Premetek) onto a glassy carbon electrode (2.0 mm in diameter) embedded in a Teflon insulator and 2 µL of a 5 wt.% Nafion (Sigma–Aldrich) dispersion in ethanol was added to guarantee powder adhesion. The parameters, TEM images, and the X-ray diffraction pattern (XRD) of commercial Pt nanoparticles are shown in Supplementary Table 4, Supplementary Figs. 5 and 6. The single-crystal electrodes were annealed in an N-butane flame and cooled in a hydrogen and argon atmosphere. Before the experiments, cyclic voltammograms from 0.05 to 1.50 V with a scan rate of 50 mV s−1 in a nitrogen-purged supporting electrolyte were performed to ensure the cleanliness of the systems. The electrochemically active area of Pt was measured using the charge involved in the so-called hydrogen adsorption/desorption region (between 0.05 and 0.4 V) from the cyclic voltammograms. In order to quantify the proportion of Pt(100), Pt(111), and Pt (110) domains on the Pt nanoparticles, irreversible adsorption of Germanium (IV) and Bismuth (III) was performed by spontaneous deposition from a 10−2 M solution of GeO2 (Aladdin 99.999%) in 1.0 M NaOH and a saturated solution of Bi2O3 (Aladdin 99.999%) in 0.5 M H2SO4, respectivly58. After deposition, the electrode surface was rinsed with water and immersed in the electrochemical cell at 0.1 V with the droplet attached for voltammetry scanning. In Supplementary Fig. 2a, Bismuth (III) adsorbed on (111) sites shows a redox peak (R8) at around 0.63 V, whose charge is proportional to the number of (111) sites, as shown in Eq. (E1)59:
In which q represents charge density, the area (A) of Pt(111) can be obtained by (E2):
In Supplementary Fig. 2b, Germanium (IV) adsorbed on (100) sites shows a redox peak (R9) at around 0.48 V, whose charge is proportional to the number of (100) sites, as shown in Eq. (E3)60:
The area (A) of Pt(100) can be obtained by (E4):
The proportion of Pt(100) and Pt(111) domains was obtained by the ratio of (100) and (111) area to the total electrode area, respectively; the rest proportion is Pt(110) domain61.
All experiments were conducted at 25 °C using a computer-controlled electrochemical workstation (Bio-Logic VSP-300) in a conventional three-electrode glass cell deaerated by nitrogen (99.999%), including a 4.0 cm diameter platinum ring counter electrode (CE) placed 1.5 cm below the WE and a J-shaped reversible hydrogen electrode (RHE) filled with 0.50 M HClO4 or H2SO4 solution that served as the reference electrode. The RHE was placed in a fixed position relative to the WE surface, and the electrolyte solution volume was 50 mL.
In situ SERS experiments
Raman spectra were recorded with an HR-800 (Jobin Yvon-Horiba, France) spectrometer integrated with a confocal microscope. A schematic of the in situ surface-enhanced Raman spectroscopy is shown in Supplementary Fig. 7a. The spectra were obtained by excitation with an air-cooled frequency-doubled ND YAG laser emitting a wavelength of 532 nm. The working electrode was prepared by depositing 4 µL of a 2 mg mL−1 platinum nanoparticle water suspension onto a polycrystalline platinum disk electrode (2.0 mm in diameter) embedded in a Teflon insulator. The suspension was deposited with four subsequent aliquots of 1 µL. The WE was then mounted on a 20-mL electrochemical polytetrafluoroethylene (PTFE) cell designed to acquire in situ Raman spectra. A fused silica window separated the microscope objective from the 0.5 M HClO4 electrolytic solution. All potentials referred to the RHE electrode and the potential was held constant for approximately 15 min during each Raman measurement.
Chronoamperometry combined with HPLC
These experiments were performed in an electrochemical cell with 80 mL of 0.5 M solution containing HClO4 and NaClO4 and 0.1 M ethanol deaerated with nitrogen. The working electrode was prepared by depositing 30 µL of 2 mg mL−1 Pt nanoparticle suspension onto a glassy carbon electrode (6.0 mm in diameter) embedded in a Teflon insulator. The suspension was deposited into six subsequent aliquots of 5 µL, and 10 µL of 5 wt.% Nafion dispersion was added. Before sampling, the WE was immersed in 0.5 M HClO4 and NaClO4 solution with 0.1 M ethanol under potentiostatic control at different potentials for 20 min to ensure sufficient reaction. Then, the applied potential was increased to a specific value for the next sampling. The products on the interface were almost completely removed during sampling. Hence, pH was changed slightly even weak-buffering HClO4–NaClO4 or H2SO4–Na2SO4 solutions were used, e.g., the pH of a solution changed from 6.16 to 6.02 after one day of reaction, i.e., sampling shows the function of pH-buffering. To compare the voltammograms with current densities (j) for different applied potentials (E) with chronoamperometry, the j-t and j-E plots at the sampling potentials are shown in Supplementary Fig. 8. The trend for the j-E curve in Supplementary Fig. 8b was consistent with the CV curves for ethanol electrooxidation (Fig. 2), indicating that it is feasible to sample by chronoamperometry and determine the relationships between the CV curve and product concentrations. A micrometer-sized sampling tip (diameter, 0.3 mm) was placed close (10 μm) to the surface of the WE for rapid sampling (with an auxiliary pump, Dionex™). In the case of ethanol electrooxidation, the optimal flow rate was found to be 0.1 mL min−1. After collection, all samples were analyzed by HPLC (Agilent 1260) with a Phenomenex FMH-1138-KONU column (300 × 7.8 mm, 10 μm) and a refractive index detector (RID). The samples were acquired by injecting 5 μL of the solution into the HPLC system. The mobile phase of the ethanol system was 5 mM H2SO4. The temperature of the column was maintained at 60 °C in a column oven, and the separated compounds were detected at 35 °C. Detailed implementation for the combination of fraction collection from the electrode surface and subsequent sample analysis by HPLC is provided elsewhere62. A schematic of the online sample collection with the AXP pump combined with the HPLC system is shown in Supplementary Fig. 7b.
Computational methods
The calculations with spin-polarized DFT were performed with the Vienna Ab-Initio Simulation Package (VASP)63. The Kohn-Sham wave functions were expanded in a plane-wave basis set. The cutoff energy was set to 400 eV. The projector-augmented wave (PAW) method and PBE potential for the exchange-correlation function were used64. A (3 × 3 × 1) Monkhorst-Pack k-point sampling method was used65. All atoms were allowed to relax until the forces and energies fell below 0.02 eV Å−1 and 10−5 eV, respectively. We employed a Pt(111) surface with a p(4×4) periodic slab of four atomic layers including 64 atoms for OHads on Pt surface, and another Pt(111) surface with a p(4 × 4) periodic slab of two atomic layers including 32 atoms for α-PtO2 on Pt surface referring to previous work52,53, where the bottom two layers of atoms were fixed in the slab while the atoms of the rest were relaxed during all optimization processes. A vacuum region of 15 Å was created to ensure negligible interactions between mirror images. Climbing image nudged elastic band (CINEB) and Dimer were used for studying the transition state. The convergence of forces was set to 0.03 eV Å−1 using a modified VASP. The structures of ethanol, acetaldehyde, acetic acid, water, and other molecules were optimized in a 15 Å vacuum square box.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Resasco, J. et al. Enhancing the connection between computation and experiments in electrocatalysis. Nat. Catal. 5, 374–381 (2022).
Stamenkovic, V. R., Strmcnik, D., Lopes, P. P. & Markovic, N. M. Energy and fuels from electrochemical interfaces. Nat. Mater. 16, 57–69 (2016).
Yang, Y. et al. Electrocatalysis in alkaline media and alkaline membrane-based energy technologies. Chem. Rev. 122, 6117–6321 (2022).
Farnesi Camellone, M., Negreiros Ribeiro, F., Szabova, L., Tateyama, Y. & Fabris, S. Catalytic proton dynamics at the water/solid interface of ceria-supported Pt clusters. J. Am. Chem. Soc. 138, 11560–11567 (2016).
Zope, B. N., Hibbitts, D. D., Neurock, M. & Davis, R. J. Reactivity of the gold/water interface during selective oxidation catalysis. Science 330, 74–78 (2010).
Saavedra, J., Doan, H. A., Pursell, C. J., Grabow, L. C. & Chandler, B. D. The critical role of water at the gold-titania interface in catalytic CO oxidation. Science 345, 1599–1602 (2014).
Liu, Z. et al. Water-promoted interfacial pathways in methane oxidation to methanol on a CeO2-Cu2O catalyst. Science 368, 513–517 (2020).
Fang, W. et al. Physical mixing of a catalyst and a hydrophobic polymer promotes CO hydrogenation through dehydration. Science 377, 406–410 (2022).
Ping, Y., Goddard, W. A. III & Galli, G. A. Energetics and solvation effects at the photoanode/catalyst interface: Ohmic contact versus Schottky barrier. J. Am. Chem. Soc. 137, 5264–5267 (2015).
Gonella, G. et al. Water at charged interfaces. Nat. Rev. Chem. 5, 466–485 (2021).
Merte, L. R. et al. Water-mediated proton hopping on an iron oxide surface. Science 336, 889–893 (2012).
Toney, M. F. et al. Voltage-dependent ordering of water molecules at an electrode-electrolyte interface. Nature 368, 444–446 (1994).
Garcia-Araez, N., Climent, V. & Feliu, J. M. Evidence of water reorientation on model electrocatalytic surfaces from nanosecond-laser-pulsed experiments. J. Am. Chem. Soc. 130, 3824–3833 (2008).
Wang, Y. H. et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 600, 81–85 (2021).
Geng, S.-K. et al. Nickel ferrocyanide as a high-performance urea oxidation electrocatalyst. Nat. Energy 6, 904–912 (2021).
Auer, A. et al. Interfacial water structure as a descriptor for its electro-reduction on Ni(OH)2-modified Cu(111). ACS Catal. 11, 10324–10332 (2021).
Chung, D. Y., Lee, K.-J. & Sung, Y.-E. Methanol electro-oxidation on the Pt surface: revisiting the cyclic voltammetry interpretation. J. Phys. Chem. C 120, 9028–9035 (2016).
Conway, B. E. Electrochemical oxide film formation at noble metals as a surface-chemical process. Prog. Surf. Sci. 49, 331–452 (1995).
Gómez-Marín, A. M., Clavilier, J. & Feliu, J. M. Sequential Pt(111) oxide formation in perchloric acid: an electrochemical study of surface species inter-conversion. J. Electroanal. Chem. 688, 360–370 (2013).
Wakisaka, M., Suzuki, H., Mitsui, S., Uchida, H. & Watanabe, M. Identification and quantification of oxygen species adsorbed on Pt(111) single-crystal and polycrystalline Pt electrodes by photoelectron spectroscopy. Langmuir 25, 1897–1900 (2009).
Imai, H. et al. In situ and real-time monitoring of oxide growth in a few monolayers at surfaces of platinum nanoparticles in aqueous media. J. Am. Chem. Soc. 131, 6293–6300 (2009).
You, H., Zurawski, D. J., Nagy, Z. & Yonco, R. M. In situ X-ray reflectivity study of incipient oxidation of Pt(111) surface in electrolyte solutions. J. Chem. Phys. 100, 4699–4702 (1994).
Kondo, T., Masuda, T., Aoki, N. & Uosaki, K. Potential-dependent structures and potential-induced structure changes at Pt(111) single-crystal electrode/sulfuric and perchloric acid interfaces in the potential region between hydrogen underpotential deposition and surface oxide formation by in situ surface X-ray scattering. J. Phys. Chem. C 120, 16118–16131 (2016).
Magnussen, O. M. & Gross, A. Toward an atomic-scale understanding of electrochemical interface structure and dynamics. J. Am. Chem. Soc. 141, 4777–4790 (2019).
Huang, Y. F., Kooyman, P. J. & Koper, M. T. Intermediate stages of electrochemical oxidation of single-crystalline platinum revealed by in situ Raman spectroscopy. Nat. Commun. 7, 12440 (2016).
Gilman, S. The mechanism of electrochemical oxidation of carbon monoxide and methanol on platinum. J. Phys. Chem. 68, 70–80 (1963).
Rizo, R., Arán-Ais, R. M. & Herrero, E. On the oxidation mechanism of C1-C2 organic molecules on platinum. A comparative analysis. Curr. Opin. Electrochem. 25, 100648 (2021).
Mekazni, D. S., Arán-Ais, R. M., Ferre-Vilaplana, A. & Herrero, E. Why methanol electro-oxidation on platinum in water takes place only in the presence of adsorbed OH. ACS Catal. 12, 1965–1970 (2022).
Flórez-Montaño, J. et al. Mechanism of ethanol electrooxidation on mesoporous Pt electrode in acidic medium studied by a novel electrochemical mass spectrometry set-up. Electrochim. Acta 209, 121–131 (2016).
Melke, J. et al. Electrooxidation of ethanol on Pt. An in situ and time-resolved XANES study. J. Phys. Chem. C 116, 2838–2849 (2012).
Colmati, F. et al. Surface structure effects on the electrochemical oxidation of ethanol on platinum single crystal electrodes. Faraday Discuss. 140, 379–397 (2008).
Rizo, R., Ferre-Vilaplana, A., Herrero, E. & Feliu, J. M. Ethanol electro-oxidation reaction selectivity on platinum in aqueous media. ACS Sustainable Chem. Eng. 11, 4960–4968 (2022).
Zhang, W., Pan, C. & Gao, Q. Chemisorbed oxygen-species-mediated electrocatalytic oxidation of thiourea and thiosulfate. J. Phys. Chem. C 122, 24150–24157 (2018).
Garcia-Araez, N., Climent, V. & Feliu, J. M. Analysis of temperature effects on hydrogen and OH adsorption on Pt(111), Pt(100) and Pt(110) by means of Gibbs thermodynamics. J. Electroanal. Chem. 649, 69–82 (2010).
McCrum, I. T., Schwarz, K. A., Janik, M. J. & Koper, M. T. M. On the presence of surface bound hydroxyl species on polycrystalline Pt electrodes in the “hydrogen potential region” (0–0.4 V-RHE). J. Catal. 367, 332–337 (2018).
Rizo, R. et al. Investigating the presence of adsorbed species on Pt steps at low potentials. Nat. Commun. 13, 2550 (2022).
Chang, Q. et al. Enhancing CC bond scission for efficient ethanol oxidation using PtIr nanocube electrocatalysts. ACS Catal. 9, 7618–7625 (2019).
Tilak, B. V., Conway, B. E. & Angerstein-Kozlowska, H. The real condition of oxidized Pt electrodes. Part 3. Kinetic theory of formation and reduction of surface oxides. J. Electroanal. Chem. 48, 1–23 (1973).
Merte, L. R. et al. Electrochemical oxidation of size-selected Pt nanoparticles studied using in situ high-energy-resolution X-ray absorption spectroscopy. ACS Catal. 2, 2371–2376 (2012).
Drnec, J. et al. Initial stages of Pt(111) electrooxidation: dynamic and structural studies by surface X-ray diffraction. Electrochim. Acta 224, 220–227 (2017).
Kusano, S., Matsumura, D., Ishii, K., Tanaka, H. & Mizuki, J. I. Electrochemical adsorption on Pt nanoparticles in alkaline solution observed using in situ high energy resolution X-ray absorption spectroscopy. Nanomaterials 9, 642 (2019).
Alves, W. A., Téllez S, C. A., Sala, O., Santos, P. S. & Faria, R. B. Dissociation and rate of proton transfer of HXO3 (X = Cl, Br) in aqueous solution determined by Raman spectroscopy. J. Raman Spectrosc. 32, 1032–1036 (2001).
Wasileski, S. A., Koper, M. T. M. & Weaver, M. J. Field-dependent electrode–chemisorbate bonding: sensitivity of vibrational Stark effect and binding energetics to nature of surface coordination. J. Am. Chem. Soc. 124, 2796–2805 (2002).
Santiago, P. V. B. et al. Oxide formation as probe to investigate the competition between water and alcohol molecules for OH species adsorbed on platinum. Electrochim. Acta 317, 694–700 (2019).
Jin, Z. et al. Catalytic behavior of nanoparticle α-PtO2 for ethanol oxidation. J. Mol. Catal. A 191, 61–66 (2003).
Bingwa, N. et al. Synergistic effect of mesoporous metal oxides and PtO2 nanoparticles in aerobic oxidation of ethanol and ionic liquid induced acetaldehyde selectivity. Mol. Catal. 492, 110978 (2020).
Sheng, T., Lin, W. F., Hardacre, C. & Hu, P. Role of water and adsorbed hydroxyls on ethanol electrochemistry on Pd: new mechanism, active centers, and energetics for direct ethanol fuel cell running in alkaline medium. J. Phys. Chem. C 118, 5762–5772 (2014).
Drnec, J., Harrington, D. A. & Magnussen, O. M. Electrooxidation of Pt(111) in acid solution. Curr. Opin. Electrochem. 4, 69–75 (2021).
Bell, R. P. & Darwent, B. D. The kinetics of the hydration of acetaldehyde. Trans. Faraday Soc. 46, 34–41 (1950).
Gómez-Marín, A. M. & Feliu, J. M. Oxide growth dynamics at Pt(111) in absence of specific adsorption: a mechanistic study. Electrochim. Acta 104, 367–377 (2013).
Li, W. X. & Hammer, B. Reactivity of a gas/metal/metal-oxide three-phase boundary: CO oxidation at the Pt(111)–C(4 × 2)-2CO/α-PtO2 phase boundary. Chem. Phys. Lett. 409, 1–7 (2005).
Shimazaki, T., Suzuki, T. & Kubo, M. Density functional theory study on quasi-three-dimensional oxidized platinum surface: phase transition between α-PtO2-like and β-PtO2-like structures. Theor. Chem. Acc. 130, 1031–1038 (2011).
Jacobse, L. et al. Electrochemical oxidation of Pt(111) beyond the place-exchange model. Electrochim. Acta 407, 139881 (2022).
Strmcnik, D. et al. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 5, 300–306 (2013).
Xia, W., Mahmood, A., Zou, R. & Xu, Q. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 8, 1837–1866 (2015).
Hwang, J. et al. Perovskites in catalysis and electrocatalysis. Science 358, 751–756 (2017).
Solla-Gullon, J., Rodrguez, P., Herrero, E., Aldaz, A. & Feliu, J. M. Surface characterization of platinum electrodes. Phys. Chem. Chem. Phys. 10, 1349–1472 (2008).
Rodriguez, P. et al. Determination of (111) ordered domains on platinum electrodes by irreversible adsorption of bismuth. Anal. Chem. 77, 5317–5323 (2005).
Rodríguez, P. et al. Specific surface reactions for identification of platinum surface domains. Electrochim. Acta 50, 4308–4317 (2005).
Perroni, P. B., Del Colle, V., Tremiliosi-Filho, G. & Varela, H. Electro-oxidation of methanol and glucose on preferentially oriented platinum surfaces: the role of oscillatory kinetics. React. Kinet. Mech. Catal. 135, 1335–1348 (2022).
Santasalo-Aarnio, A. et al. Comparison of methanol, ethanol and iso-propanol oxidation on Pt and Pd electrodes in alkaline media studied by HPLC. Electrochem. Commun. 13, 466–469 (2011).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22120102001), the Natural Science Foundation of Jiangsu Province (Grant No. BK20211242), and the Fundamental Research Funds for the Central Universities (grant no. 2019XKQYMS77). All DFT computations were performed on the HPC Simulation Platform with support from the “Basic and emerging interdisciplinary discipline” project from the China University of Mining and Technology (CUMT). All TEM tests were provided by the Advanced Analysis and Computation Center of CUMT. The authors acknowledge the important comment of Enrique Herrero on the manuscript and thank Lichang Wang and Jichang Wang for their useful discussions.
Author information
Authors and Affiliations
Contributions
Q.G., C.P., and J.Z. conceived and designed the experiments. X.Y., Y.Q., Y.C., X.S., and C.J. performed the measurements of electrochemistry, in situ Raman, online HPLC, and basic crystal-face contents relating to Pt NP electrode. V.D.C. and X.Y. performed the electrochemical measurements on three basic single-crystal Pt electrodes. J.H. and G.W. performed the DFT calculations. Q.G., C.P., J.Z., X.Y., and J.H. analyzed experimental and theoretical data. X.Y., J.H., Q.G., C.P., and J.Z. wrote the manuscript. V.D.C. contributed to manuscript editing. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
You, X., Han, J., Del Colle, V. et al. Relationship between oxide identity and electrocatalytic activity of platinum for ethanol electrooxidation in perchlorate acidic solution. Commun Chem 6, 101 (2023). https://doi.org/10.1038/s42004-023-00908-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-023-00908-3
This article is cited by
-
Impact of pH on ethanol electro-oxidation in seawater-like electrolytes: implications for ocean-based mitigation strategies
Journal of Solid State Electrochemistry (2025)
-
Carbonate-carbonate coupling on platinum surface promotes electrochemical water oxidation to hydrogen peroxide
Nature Communications (2024)