Abstract
Gallium-rich supported catalytically active liquid metal solutions (SCALMS) were recently introduced as a new way towards heterogeneous single atom catalysis. SCALMS were demonstrated to exhibit a certain resistance against coking during the dehydrogenation of alkanes using Ga-rich alloys of noble metals. Here, the conceptual catalytic application of SCALMS in dry reforming of methane (DRM) is tested with non-noble metal (Co, Cu, Fe, Ni) atoms in the gallium-rich liquid alloy. This study introduces SCALMS to high-temperature applications and an oxidative reaction environment. Most catalysts were shown to undergo severe oxidation during DRM, while Ga-Ni SCALMS retained a certain level of activity. This observation is explained by a kinetically controlled redox process, namely oxidation to gallium oxide species and re-reduction via H2 activation over Ni. Consequentially, this redox process can be shifted to the metallic side when using increasing concentrations of Ni in Ga, which strongly suppresses coke formation. Density-functional theory (DFT) based ab initio molecular dynamics (AIMD) simulations were performed to confirm the increased availability of Ni at the liquid alloy-gas interface. However, leaching of gallium via the formation of volatile oxidic species during the hypothesised redox cycles was identified indicating a critical instability of Ga-Ni SCALMS for prolonged test durations.
Similar content being viewed by others
Introduction
Dry reforming of methane (DRM, Eq. 1) oxidatively converts CH4 with CO2 to synthesis gas, which makes this process a promising alternative for a sustainable valorisation of methane. The integration of CO2 in the production of H2 and CO is ecologically highly attractive and a first step towards a circular economy1,2,3,4,5. The strong C–H bonds in CH4 in combination with the mildly oxidising CO2 make DRM a highly endothermic process. The operation temperatures for DRM are in the range of 700–1000 °C3, which induces rapid catalyst deactivation via coking, oxidation of the active metallic phase, and sintering4. In fact, long-term stability is one of the major challenges in the commercialisation of DRM. Hence, recent advances in catalyst research for DRM mostly focus on concepts to prevent excessive sintering and carbon deposition to improve the stability and long-term performance5,6,7,8,9.
Sintering of the active phase and coke formation are classical deactivation pathways for heterogeneous catalysts10. Sintering of Ni-based catalysts during DRM is often discussed in the literature5,11,12,13,14,15,16. Several recent studies focus on novel reactor and catalyst concepts to suppress (excessive) carbon deposition in order to enable long-term operability5,17,18,19. Two innovative approaches employing liquid alloys as the catalytically active phase in a bubble column reactor demonstrated the successful elimination of both deactivation mechanisms for DRM18. However, the approach with liquid alloys in a bubble column reactor suffers of practical issues, because of the highly corrosive nature of the liquid metals and large required quantities resulting in high reactor costs.
Such limitations in the applicability may be overcome by combining supported liquid phase (SLP) catalysis with liquid alloys. In general, SLP catalysis merges advantages from classical heterogeneous and homogeneous catalysis, such as easy handling of the catalyst and well-defined active sites, respectively20. However, the application of SLP catalysts with supported organic liquids, ionic liquids, or molten salts is limited due to the restricted thermal stability of these liquid phases21,22,23,24. Recently, we introduced a new class of SLP catalysts, namely gallium-rich supported catalytically active liquid metal solutions (SCALMS), which may extend the application of SLP catalysis to high-temperature processes due to the low metal vapour pressures (boiling point of Ga: 2400 °C) and high thermal stability of liquid metals20,25. Application of SCALMS materials in the dehydrogenation of propane resulted in suppression of carbon deposition26,27,28,29, which enables extended dehydrogenation cycle times in potential commercial applications29,30. SCALMS materials are composed of supported droplets of a liquid alloy consisting of a catalytically active metal and an excess of a low melting metal20,25,26,28,29. The insolubility of reactants and products in liquid metals confines the catalytic reaction over SCALMS to the liquid metal/gas interface20,26,31,32,33,34,35. The active sites in SCALMS have been described as single atoms of the secondary metal in a Ga matrix20,26,33,34,35.
Herein, we report the first application of SCALMS in a high-temperature process exceeding 700 °C. The performance of Ga-rich SCALMS using mesoporous SiC as carrier material was conceptually evaluated during dry reforming of methane (DRM) in the temperature range of 700–1000 °C3. Furthermore, DRM over SCALMS also represents the first application of this SLP catalysis concept for oxidative reaction mixtures. This is an essential difference due to the high oxophilicity of Ga. In our study, the evaluation of the catalytic performance of Ga-rich SCALMS with secondary metals that are potential candidates for catalysing DRM (Co, Cu, Fe, Ni) is accompanied by microscopic characterisation of the materials prepared. Moreover, the SCALMS materials are thoroughly analysed after their catalytic application to understand the mechanisms at play.
Results and discussion
Catalyst characterisation
Four abundant metals, namely Co, Cu, Fe and Ni, were selected as potential candidates for SCALMS dry reforming of methane (DRM) catalysts. Gallium-based SCALMS were prepared with these secondary metals via impregnation of pre-synthesised gallium-decorated mesoporous β-SiC (BET surface area of 23 m2 g-1; Figs. S1–S3). Analysis of the metal content resulted in Ga loadings of 4.5-4.9 wt.% and Ga/metal ratios close to the targeted value of 50 (Table 1). According to the corresponding bimetallic phase diagrams36,37,38,39, all of these Ga-rich alloys are expected to be present in the liquid state at temperatures exceeding 700 °C. Furthermore, passivation of the prepared SCALMS by a thin layer of GaxO species has been previously reported but may be easily reduced during catalysis by in situ formed reductants, such as H226,28,29. This observation has been assigned to H2 activation by the secondary metal allowing reduction of GaxO species at low temperatures.
The as-prepared SCALMS materials were characterised by means of scanning electron microscopy with elemental mapping via energy-dispersive X-ray spectroscopy (SEM-EDX) to evaluate the morphology of the Ga-rich droplets on the external surface of the β-SiC support material and the distribution of the active metal (Fig. 1; Figs. S4–S5). The Ga-droplet decoration in SCALMS was previously achieved via impregnation followed by the thermal decomposition of gallane complexes (Et3N)GaH320,26,28,29. When using ultrasound emulsification, the Ga droplets are sonochemically produced from elemental Ga, dispersed in a solvent, and can be directly deposited on the substrate40. Even though the rough outer surface of the mesoporous support may enhance physical stabilisation, the droplets showed a certain degree of clustering. A wide size distribution of the droplets was observed, ranging from less than 0.1 up to 3 μm. The low concentration of the secondary metals (<0.09 wt.%) resulted in low signal-to-noise ratios for the SEM-EDX elemental maps. Nevertheless, the acquired maps suggest a successful deposition of Ni onto the Ga phase (Fig. S5).
Catalyst testing
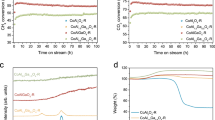
The performance of the four SCALMS systems during DRM was evaluated at 900 °C and atmospheric pressure in a quartz tube fixed-bed reactor with an equimolar feed of 3:1:1 Ar:CH4:CO2. The blank quartz tube reactor displayed negligible conversion levels (<1% for CH4 and below detection limit for CO2; Fig. S6). Of the four metals studied, the presence of Ni within the liquid Ga matrix in the Ga45Ni/SiC SCALMS resulted in the highest conversion of CH4 and CO2 (Fig. 2). No increase of activity was observed when comparing the Ga44Fe/SiC SCALMS catalyst with the Ga/SiC benchmark without any secondary metal (Fig. S7). The initial conversion of CH4 and CO2 was the highest for Ga52Cu/SiC SCALMS (Fig. 2). However, this catalyst rapidly deactivated and displayed the activity level of the Ga/SiC reference after 2 h time on stream (TOS). Aside from the Ga-Ni SCALMS, the Ga43Co/SiC SCALMS was the only other system displaying a certain level of DRM activity. However, the conversion levels of this Co-based SCALMS system rapidly decreased from an initial conversion of CH4 of 14% to 7% after 5 h TOS, close to the conversion of the Ga/SiC reference. While the initial conversion of CH4 was only marginally improved over the Ga45Ni/SiC SCALMS, an initial activation period of one hour was observed obtaining conversion levels of CH4 and CO2 of 24 and 34%, respectively (Fig. 2). Subsequently, the Ga-Ni SCALMS slowly deactivated over 20 h TOS to 8 and 11% conversion of CH4 and CO2, respectively.
a Conversion of CH4 and b CO2 using various active metals in gallium-rich alloys employing a mesoporous SiC support. Metal loadings: 4.85 wt.% Ga and 0.09 wt.% Co for Ga47Co/SiC, 4.91 wt.% Ga and 0.09 wt.% Cu for Ga52Cu/SiC, 4.53 wt.% Ga and 0.08 wt.% Fe for Ga44Fe/SiC, 4.52 wt.% Ga and 0.08 wt.% Ni for Ga45Ni/SiC. Reaction conditions: 900 °C, 1 bar, 1 g SCALMS, CH4:CO2:Ar = 1:1:3, 3 LN gcat-1 h-1.
Interestingly, CO2 conversion in the Ga-Ni and Ga-Co SCALMS catalysed DRM is 50% higher than CH4 conversion throughout the experiment (Fig. 2; Fig. S8). The Ga-Cu and Ga-Fe SCALMS also result in an initially enhanced conversion of CO2 prior to deactivation within the first hours of TOS. This distinct deviation from the equimolar conversion of the reactants suggests an additional conversion pathway for CO2, which ceases in the case of strongly deactivating Ga52Cu/SiC or barely active Ga44Fe/SiC within 5 h TOS (Fig. S8).
Proposed reaction network
Activation of CH4 during thermocatalytic DRM can be described by the formation of H2 alongside adsorbed CHx or monoatomic carbon (Fig. 3, reaction 1)41. In a simplified reaction network, monoatomic carbon is then gasified via the Boudouard reaction yielding CO (Fig. 3, reaction 2). The formation of CO is typically increased to the sacrifice of H2 due to the additional conversion of CO2 via the reverse water gas shift (RWGS) reaction (Fig. 3, reaction 3). Hence, synthesis gas from DRM is typically rich in CO and the compositions reported in the literature vary in H2/CO ratios from 0.8 to equimolarity42,43. Herein, the high reaction temperature of 900 °C allows for enhanced conversion of CO2 via the RWGS reaction when compared to lower temperatures (Fig. S9). This was confirmed in dedicated experiments studying the extent of RWGS over Ga-Ni SCALMS and the SiC support material (Fig. S10). While, the bare support material resulted in low conversion levels, almost equilibrium conversion was achieved for CO2 over the Ga45Ni/SiC SCALMS at 900 °C. Hence, the RWGS reaction is expected to play an important role as a side reaction during DRM and increases the conversion of CO2. However, additional conversion pathways for CO2 are at play as the obtained H2/CO ratio during DRM over Ga45Ni/SiC stabilises at a value of 0.55 after 1 h TOS (Fig. S8).
Gallium is highly oxophilic with a low standard Gibbs free energy (ΔGrxn0) of the oxidation reaction with O2 forming Ga2O3 of -1996.0 kJ mol-1 (Fig. S12a), which is more than twice as high as for full oxidation of pyrophoric metallic Co to Co3O4 (–802.1 kJ mol-1). Expectedly, oxidation of liquid Ga0 by mildly oxidative CO2 (Fig. 3, reaction 4) is also feasible with a Gibbs free energy at reaction temperature (900 °C) of –160.7 kJ mol-1 (Fig. S12b). In addition, partial oxidation of Ga0 is even more likely than full oxidation to Ga2O3 in the present system. Nevertheless, bulk oxidation of Ga0 is unlikely as passivation by a thin GaxO layer has been previously identified after exposure to the ambient atmosphere during the preparation of SCALMS26,28,29. Hence, we hypothesise additional conversion of CO2 to CO during DRM via partial oxidation of metallic liquid Ga (Fig. 3, reaction 4).
The presence of H2 may enable the reduction of surface oxidic gallium species even though the direct reduction of pure Ga2O3 is thermodynamically restricted at 900 °C. However, the reduction of GaxO has recently been monitored during the dehydrogenation of propane over Ga-Rh and Ga-Pt SCALMS at 500 °C by means of in situ high-resolution thermogravimetric analysis coupled with mass spectrometry (HRTGA-MS). This observation was linked to the presence of the secondary metal for the activation of H2 and for the reduction of the gallium-oxygen binding strength28,29. Hence, the presence of a dissolved metal at the GaxO interface, such as Ni, changing the gallium-oxygen binding energies and being capable of H2 activation can be expected to allow for the reduction of adjacent GaxO species (Fig. 3, reaction 5), which has recently been observed under mild conditions (300 °C) by our group for Ga-Ni SCALMS44. Reasonably, the mechanism may be similar to H2 spillover from promoters to the active metal in conventional heterogeneous catalysts45, i.e., H2 is adsorbed and dissociated on the active secondary metal providing adjacent GaxO species with activated hydrogen. Hence, the presence of the secondary metal at the interface will also strongly affect the physico-chemical properties, while the partially oxidised GaxO species are generally expected to deviate from the thermodynamically stable Ga2O3. Lastly, the reverse reaction, that is oxidation of liquid Ga0 by H2O, has been reported to be kinetically hindered on some metals due to the high stability of hydroxyl groups46,47,48,49.
Monoatomic carbon from CH4 activation may also serve as a reducing agent for GaxO species (Fig. 3, reaction 6). Thermodynamic calculations reveal a strong temperature dependency of the reduction of Ga2O3 by graphite to Ga0 and CO indicating a small Gibbs free energy at reaction conditions (900 °C) of 56.8 kJ mol–1 (Fig. S12b). Nevertheless, reduction via this pathway may become feasible for thermodynamically less stable, highly reactive monoatomic carbon in combination with partially oxidised GaxO species (Fig. 3, reaction 6)50. Hence, the closed redox cycle with reduction by monoatomic carbon potentially allows for the Boudouard reaction via a Mars-van Krevelen-type mechanism with oxidation of Ga by CO2 filling the oxygen vacancies formed by reduction with carbon. However, the impact of this reaction on the kinetically controlled Ga0-GaxO redox process may be limited when compared to the reduction pathway via activated H2 (Fig. 3, reaction 5). Furthermore, only oxidation of Ga0 by CO2 in combination with a preferential reduction of GaxO species via H2 activation on the secondary metal (Fig. 3, reaction 3) results in an enhanced conversion of CO2 when compared to CH4.
Similar redox cycles of the active metal phase during DRM have been hypothesised in the literature. Ruckenstein et al. reported a solid solution of CoO in MgO forming small clusters, which hindered sintering during DRM51. Further, the strong interaction suppressed excessive coking. The authors hypothesised that the active Co atoms are constantly oxidised by CO2 and H2O and reduced by CH4 and H2 during DRM at 900 °C, which was key to achieving high stability and catalytic activity for a 12 wt.% Co/MgO catalyst. More recently, McFarland et al. performed combined dry reforming and pyrolysis of CH4 in a molten metal bubble column reactor using a Ni1.86In alloy18. Continuous redox cycles were hypothesised for the metallic In phase. In line with early studies by Otsuka et al.52, cyclic oxidation of In and reduction of In2O3 allowed for the conversion of CO2 in the molten metal bubble column reactor, while the oxidic intermediate serves as an oxygen shuttle between CO2 and CH418.
Role of nickel concentration
A Ga12Ni/SiC SCALMS with an increased concentration of Ni (Table 1) was prepared to study the hypothesised promotional effect of active metal atoms on the kinetically controlled Ga0-GaxO redox reaction and consequentially the activity and long-term stability during DRM. This alloy composition is expected to have a liquidus line at approx. 600 °C39. The increased concentration of Ni in the SCALMS enabled elemental mapping of the active metal using SEM-EDX (Fig. 4; Fig. S13), while the general morphology of the supported Ga phase resembles the as-prepared Ga45Ni/SiC SCALMS (Fig. 1). The analysis confirms co-location of Ni and Ga, which is a prerequisite for the formation of SCALMS upon in situ reduction of the GaxO passivation layer and calcined Ni precursors. Similar to the Ga-Ni SCALMS with a lower concentration of Ni, such an activation within the first hour of DRM at 900 °C was also observed for the Ga12Ni/SiC SCALMS (Fig. 5). The maximum CH4 conversion level after this activation period was significantly increased from 24 up to 59%. Subsequently, the Ni-enriched SCALMS also deactivated to a CH4 conversion of 26%. However, contrary to the Ga45Ni/SiC SCALMS that underwent a steady deactivation, the conversion over the Ga12Ni/SiC SCALMS stabilised after 15 h TOS and was demonstrated to be stable over 100 h TOS in a dedicated long-term experiment (Fig. S14). Note, that the specific activity of Ni of approx. 0.1 molCH4 h-1 gNi-1 is inferior when compared to classical catalyst concepts, which may reach up to 100 times higher specific conversion rates53. The stabilisation at higher conversion levels may indeed be caused by enhanced activation of H2 over Ni atoms. This promotes the reduction of GaxO species (Fig. 3, reaction 5) and in consequence, shifts the kinetically controlled Ga0-GaxO redox process to the metallic side allowing for enhanced DRM over atomically dispersed Ni atoms in a matrix of Ga atoms. To further investigate this kinetically controlled redox process, the present Ga12Ni/SiC SCALMS was also tested with a 2:1 CH4:CO2 inlet ratio to compare the performance during DRM under a more reducing environment (Fig. S15). As expected, the equilibrated conversion of CH4 and CO2 lies above the one of DRM with equimolar feed as the concentration of H2 form is increased.
Conversion of a CH4 and b CO2, as well as c the obtained H2/CO ratio in the product gas during dry reforming of methane over Ga-Ni SCALMS with different concentrations of the active metal Ni employing a mesoporous SiC support. Metal loadings: 4.52 wt.% Ga and 0.08 wt.% Ni for Ga45Ni/SiC, 5.44 wt.% Ga and 0.38 wt.% Ni for Ga12Ni/SiC. Reaction conditions: 900 °C, 1 bar, 1 g SCALMS, CH4:CO2:Ar = 1:1:3, 3 LN gcat-1 h-1.
The generally increased conversion levels may be explained by the almost five times higher loading of Ni in the Ga12Ni/SiC SCALMS (Table 1). In order to quantify the amount of Ni available at the surface of both Ga-Ni SCALMS, ab initio molecular dynamics (AIMD) simulations were carried out using slab models (Fig. 6a) at 900 °C with Ga/Ni ratios of 45 and 12, respectively, equalling those in the experiments. The systems were proven to be in a liquid state at this temperature by computation of the mean square displacement of the atoms from their initial positions, which showed a linear behaviour. Furthermore, density profiles for Ga12Ni and Ga45Ni were calculated (Fig. 6b). Expectedly, a surface depletion of Ni can be observed for both cases. This is in line with previous calculations for Pd, Pt, and Rh SCALMS20,26,33. However, owing to the high mobility of the liquid system at elevated temperatures, the Ni atoms can easily access the surface from time to time to act as single atoms catalytic centres. To quantify the amount of Ni available at the surface, the number of Ni atoms that are present in the first layer of the density profile was evaluated (see Fig. 6a for definition of the first layer). The calculations suggest that 2.5% and 0.7% of the surface atoms are Ni atoms in the case of Ga12Ni and Ga45Ni, respectively. Hence, the availability of Ni atoms at the surface of Ga12Ni is 3.6 times higher than for Ga45Ni, which also approximates the increase in the conversion of CO2 (Fig. 5). The relative depletion of Ni atoms at the surface is nearly equal in both cases. The higher Ni surface concentration in Ga12Ni simply results from the higher total amount of Ni in Ga12Ni. This higher surface concentration may explain the higher catalytic activity going along with better long-term stability due to a higher rate of H2 activation, which ultimately leads to a less oxidised state of the Ga-Ni interface.
a Snapshot of the slab system with a unit cell of Ga165Ni15 corresponding to the experimental composition Ga12Ni. Similarly, a Ga176Ni4 unit cell was used in the simulations of a Ga45Ni composition; Ga is shown in blue, Ni in red. b Density profiles for the compositions Ga12Ni (left) and Ga45Ni (right). The dashed lines mark the end of the first layer. The density profiles of Ni were scaled by a factor of 3 and 9 in the case of Ga12Ni and Ga45Ni, respectively, for the sake of clarity.
In line with the conversion of CH4, a stable conversion of CO2 over the Ga12Ni/SiC SCALMS was achieved after 15 h TOS (Fig. 5). Once again, the level of CO2 conversion is 50% higher than the conversion of CH4 (Fig. S8) suggesting additional conversion of CO2 via the RWGS reaction and oxidation of liquid Ga0 with subsequent reduction of GaxO species by H2 (Fig. 3, reaction 4 & 5). However, the ratio in the conversion of both reactants is constant over 100 h TOS (Fig. S15), while the ratio slowly decreases for the Ga45Ni/SiC SCALMS indicating less conversion of CO2 via oxidation of Ga0 due to a continuous shift of the kinetically controlled Ga0-GaxO redox process to the oxidic side. Contrary, the increased Ni content in the Ga12Ni/SiC SCALMS allows for a kinetically controlled redox process on the metallic side due to sufficient H2 activation. The enhanced consumption of CO2 and H2 in said oxidation-reduction-cycles also results in a lower H2/CO ratio in the produced synthesis gas, which stabilises at 0.45 (Fig. 5c). In contrast, the ratio obtained over the Ga45Ni/SiC SCALMS was continuously increasing from 0.3 to >0.8 over 20 h TOS. This observation, once again, may be linked to the detrimental effect of low Ni concentrations on the stability of SCALMS during DRM causing steady deactivation. The kinetically controlled redox process is far on the oxidic side and not reached over Ga45Ni/SiC SCALMS within 24 h TOS.
Post-run catalyst characterisation
Characterisation of the post-run Ga-Ni SCALMS by means of XRD and comparison with the as-prepared samples suggests an increased fraction of Ga2O3 after catalysis (Fig. 7). The patterns of the fresh samples only feature diffractions for this metal oxide close to the detection limit. A passivation layer after preparation of SCALMS has been reported26,28,29 and the performance of the SCALMS during DRM suggests in situ reduction within the first hours TOS (Fig. 5). Contrary, the spent samples feature pronounced diffractions of Ga2O3 suggesting oxidation of the Ga-rich alloy during DRM. Potential passivation of the (bi-)metallic phase upon exposure to ambient air at room temperature during unloading of the catalyst from the reactor can be expected to form less oxide than the exposure to 500 °C during calcination. In addition, the Ga2O3 diffractions are even more pronounced for the Ga45Ni/SiC catalyst when compared to Ga12Ni/SiC, which supports the hypothesised link between kinetically controlled redox process and Ni concentration.
X-ray diffractograms (Cu K-alpha radiation with λ = 1.541 Å) with an inset of the magnified intensity of Ga-Ni SCALMS employing a mesoporous SiC support, as well as the bare support material before and after application in dry reforming of methane together with reference patterns for SiC (o), graphite (C), and β-Ga2O3 (*).
The Ga-Ni combination was shown to play an essential role as an oxygen shuttle between CO2 and CH4. In particular, the reducibility of the GaxO species is crucial and triggered by H2 activation over the active metal. Here, the Ga-Ni SCALMS (and to a minor extent the Co-Ga SCALMS) were found to allow for closing the DRM catalytic cycle. A nearly constant conversion ratio of CO2/CH4 of approx. 1.5 was observed (Fig. S8) along with a constant hydrogen yield (Fig. S11). On the contrary, the Ga-Cu and Ga-Fe SCALMS underwent rapid deactivation resulting in a decreased conversion of CO2 when compared to CH4. Further, a steadily increasing H2 yield indicates that these systems (after full oxidation) rather cause CH4 pyrolysis under the applied conditions, which is expected to result in excessive coking. The increase in H2 yield is also due to a lower WGS activity after approx. 10 h TOS, as the conversion of CH4 remains constant (Fig. 2a).
TGA of the spent catalysts was performed in order to quantify the deposition of carbon during DRM at 900 °C for 24 h. The smallest weight loss during TPO in 21% O2/N2 was observed for the Ga-Ni SCALMS catalysts, while the catalysts with zero or low level of activity, as well as the SiC reference sample, displayed a high weight loss in the range of 16-19 wt.% (Fig. 8). In fact, TGA suggests a minor coke content of 0.7 wt.% for the Ga12Ni/SiC, which may indicate a significant suppression of coking during DRM over SCALMS when compared to catalysts in the literature17,54,55. This is also evidenced during TGA of the spent Ga12Ni/SiC SCALMS after DRM at 900 °C for 100 h TOS, which exhibited a remarkably low coke content of 2.9 wt.% (Fig. S16). This superior coke resistance underlines the stable performance of this Ga-Ni SCALMS during DRM at 900 °C. In general, the coke content in the SCALMS systems correlates with the observed activity during DRM suggesting the formation of highly reactive carbon species during DRM over Ni atoms. This process seems to be less efficient for Co and Cu dissolved in the liquid Ga matrix. Such highly active species may react with CO2 via the Boudouard reaction (Fig. 3, reaction 2) or even initiate the reduction of oxidised GaxO species (Fig. 3, reaction 6). Another reason may be the observed RWGS activity resulting in the formation of the oxidant H2O, which may oxidise the liquid metal and carbon deposits alike. A similar suppression of coke formation over SCALMS has already been reported for propane dehydrogenation at lower temperatures of 450–550 °C26,28,29. The increased coke content of 12.4 wt.% in the Ga45Ni/SiC is most likely due to the detrimental transformation of the metallic Ga to oxidic species as the kinetically controlled redox process lies on the oxidic side and seemingly does not equilibrate over the studied TOS. In other words, coking may be reasonably suppressed by a liquid Ga-rich alloy, while its (partially) oxidised counterpart cannot restrict coking. This dependency was also observed by means of XRD (Fig. 7, inset). The typical (002) diffraction of carbon was observed for Ga45Ni/SiC and all less active catalysts (Fig. S17), but absent in the case of Ga12Ni/SiC. The final weight gain of all samples during TGA (see range above 800 °C in Fig. 8) is a result of the enhanced SiO2 passivation layer of the SiC support material at elevated temperatures40,56,57.
The spent catalysts were also characterised by means of Raman spectroscopy (green laser with a wavelength of 532 nm) to obtain additional information on coke deposits (Fig. S17). The bare SiC support material also features the characteristic D band (~1350 cm-1) and the G band (~1600 cm-1) of carbon. However, the intensity ratio of D over G band changes when comparing the spectra before and after catalytic application during DRM. The ratio lies in the range of 1.00–1.10 for the bare SiC, as well as the as-prepared SCALMS, and increases to 1.15–1.45 due to the coking of the catalysts (Table S1). Furthermore, said change was more pronounced for less active catalysts, which is in line with the results from TGA. In fact, the ratio was the smallest for the Ga12Ni/SiC SCALMS, which displayed the highest activity during DRM. In addition, the spectra of all spent SCALMS and the bare SiC support after DRM feature the G’ band at ~2700 cm-1 (Fig. S18). No difference in carbon deposits may be identified by means of XRD (Fig. S17).
Potential loss of gallium from the SCALMS catalyst during DRM at 900 °C was investigated by analysing the metal loadings after catalytic testing using ICP-AES (Table 1). Noteworthy, the identified broad range in the coke content (Fig. 8) with an expected link to the hygroscopic behaviour of the spent samples28 lowers the accuracy of the analysis of the metal content after catalytic application. Nevertheless, a certain loss in gallium was identified for all catalysts, which apparently scales with the activity of the catalyst, i.e. the loss of approx. 18% Ga was found to be larger for the more active Ga12Ni SCALMS than for a molar ratio of Ga/Ni of 45. Furthermore, a loss of approx. 30% was identified for the long-term experiment of Ga12Ni after 100 h TOS. This observation renders physical evaporation of liquid gallium unlikely, which is supported by the low vapour pressure of Ga0 at 900 °C of 6.2 ∙ 10-7 bar58. The dependency on the reaction atmosphere rather suggests a strong link to the hypothesised redox cycles. In particular, the formation of more volatile Ga2O, a likely intermediate formed during oxidation to Ga2O3 with CO2 or subsequent re-reduction, may result in loss of gallium from the catalyst bed at reaction temperatures. H2-containing atmospheres may even increase the volatility of gallium oxide59. The potential formation of volatile gallium hydrides cannot be excluded but has been reported for temperatures exceeding the herein applied conditions60. Our thermodynamic calculations also indicate a low feasibility of the formation of GaHx (Fig. S19). Hence, the redox cycles may continuously result in slow leaching from the catalyst bed. However, this process does not heavily affect the catalytic performance in the studied time range. Contrary, a minor increase in conversion between 60–100 h TOS (Fig. S15) was observed during the long-term testing and may indicate a slow and steady decrease of the Ga/Ni ratio below 12 due to the preferential evaporation of Ga species. Such a change in the SCALMS composition may shift the kinetically controlled redox process further to the metallic side, which results in increased activity of the catalyst. Hence, the optimum Ga/Ni ratio for a balanced kinetically controlled redox process during DRM over SCALMS is expected to be below 8.4, which is the final composition of the catalyst after 100 h TOS (Table 1). The liquidus line of Ga-Ni at 900 °C is expected to have a Ga/Ni ratio of 3.139, which leaves significant space for further improvements of the SCALMS composition while complying with the fundamental idea of fully liquid-supported alloys. Furthermore, we assume that the tendency for Ga2O evaporation reduces with higher Ni content of the alloy. Nevertheless, an additional experiment with a bimetallic catalyst with a molecular ratio of Ga/Ni of 0.74 was performed (Fig. S20). While the catalyst initially outperformed the Ga45Ni/SiC SCALMS, rapid deactivation within 5 h TOS and excessive coke formation (16.2 wt.%; Fig. S21) were observed exhibiting the coking affinity of solid-based catalysts. Furthermore, and in line with our expectations, no loss in Ga during DRM was detected when comparing the loading before and after catalysis by means of ICP-AES (Table S2).
Finally, the Ga-Ni SCALMS materials were analysed by means of SEM-EDX after application in DRM to study morphological changes and evaluate the metal distributions. Note, that artefacts from solidification of the liquid alloy during cool-down and subsequent exposure to ambient air may affect this ex situ analysis. Nevertheless, the dispersion of gallium over the SiC support appears to be generally unchanged. However, the spent Ga12Ni/SiC SCALMS features needle-type structures of several micrometres in length and diameters below 100 nm (Fig. 9; Fig. S22). These structures cannot be identified in the as-prepared samples nor in the Ga45Ni/SiC SCALMS after 24 h TOS (Fig. 1; Figs. S4 & S23). According to elemental mapping via SEM-EDX, the needles consist of gallium and oxygen (Fig. 9). No nickel, carbon, or silicon can be detected suggesting the formation of GaxO needles during DRM, most likely as Ga2O3 phase (Fig. S24). In addition, the Ga45Ni/SiC SCALMS features some needles after DRM for an extended duration of 100 h (Fig. S25 & S26), probably because a longer run time was needed to locate the needles in post-run analysis. Hence, the formation of these structures depends on the extent and number of redox cycles as the Ga12Ni SCALMS heavily oxidises and re-reduces due to the increased concentration of Ni in the Ga-rich supported alloys. As extended Ga leaching has been observed for both samples, a needle growth via the gas phase and volatile GaxO species is highly likely. The volatility of oxidised gallium species has already been linked to the extent of redox cycles. Further, the preparation of β-Ga2O3 via chemical vapour deposition (CVD) using Ga and H2O has been reported in literature61, while the presence of β-Ga2O3 in the spent SCALMS has been identified by means of XRD (Fig. 7). Other studies also describe the formation of needle-type structures of gallium oxide with comparable dimensions62,63. Lastly, the formation of needle- or platelet-type structures during water-induced deactivation of metal-based catalysts has been reported for other applications as well64,65.
a Backscattered scanning electron micrograph and elemental mapping of b gallium, c oxygen, and d nickel via energy-dispersive X-ray spectroscopy of Ga12Ni/SiC with metal loadings of Ga and Ni of 5.44 and 0.38 wt.%, respectively, after catalytic application in dry reforming of methane. Magnified insets show needle-type structures of gallium oxide.
Aside from the needle-type structures, EDX mapping suggests a general co-location of nickel with the Ga-rich areas (Fig. 9), i.e., pronounced segregation during DRM is not observed. In contrast to the as-prepared SCALMS with randomly shaped Ni enrichments after impregnation and calcination (Fig. 4), the distribution of Ni after DRM suggests the formation of a liquid alloy under reaction conditions (Fig. 9). Such supported liquid alloy droplets can be expected to solidify during cool-down forming the herein observed spherical bimetallic phases. However, several Ni-enriched Ga-Ni structures may be observed indicating a certain degree of redistribution of the bimetallic phase, which as well may also be an artefact from cool-down.
Summary and conclusion
Supported Ga-rich liquid Ga-Co, Ga-Cu, Ga-Fe, and Ga-Ni alloys were prepared, characterised, and applied in DRM to assess the suitability of SCALMS for high-temperature applications and to push this supported liquid phase catalysis concept to boundaries. While physical evaporation of the liquid metal phase could be excluded, a certain degree of chemical leaching, most likely via oxidic gallium species, was observed. In general, all SCALMS were shown to oxidise during the applied DRM conditions. Only the supported Ga-Ni and, to a smaller extent, the Ga-Co alloy retained activity after a run-in phase. Our study indicates that this is due to the capability of the supported liquid Ga-Ni alloy to efficiently activate H2 (formed during DRM) for the re-reduction of oxidised gallium species. For Ga-Ni SCALMS, the resulting kinetically controlled redox process could be shifted to the metallic side by increasing the concentration of Ni in the Ga-rich alloy, which promotes the activation of H2 and enables stable conversion levels during DRM. A long-term experiment with Ga12Ni/SiC for 100 h time on stream demonstrated the general suitability of this SCALMS system for DRM, but the leaching of gallium species (in combination with the deposition of β-Ga2O3 needles) will affect the stability of Ga-Ni SCALMS with the here-studied composition in even longer operation. Further, the estimated intrinsic activity of Ni is inferior when compared to the literature.
Several important findings for future applications of SCALMS systems were made in this conceptual work:
-
The SCALMS concept allows for the employment of less noble metals, such as Co and Ni, as the secondary active metal dissolved in Ga-rich liquid alloy phase. Ab initio molecular dynamics simulations showed a surface depletion of Ni accompanied by the dynamic temporary appearance of single Ni atoms at the surface, in analogy to the situation of SCALMS containing noble metals.
-
Ga-based SCALMS are generally suitable for high-temperature operation at 900 °C, but volatile Ga-oxide species can cause Ga losses due to evaporation under an oxidising atmosphere.
-
A reduced amount of coke formation was observed for Ga-Ni SCALMS when compared to the other catalysts with low or zero levels of activity. In fact, the Ga12Ni/SiC SCALMS with a high concentration of Ni in the supported liquid alloy almost completely suppressed coking during DRM due to the high dynamics of its supported liquid alloy interface.
Methodology
Materials
Gallium pellets (≥99.9999% purity) were purchased from Alfa Aesar, cobalt(II) chloride (CoCl2; ≥ 98.0% purity), copper(II) chloride (CuCl2; ≥ 99.995% purity), iron(II) chloride (FeCl2; ≥ 98.0% purity), and nickel(II) chloride ethylene glycol dimethyl ether (C4H10Cl2NiO2; ≥ 98.0% purity) were supplied from Sigma Aldrich, and isopropanol (≥99.3% purity) was purchased from Jäkle Chemie (Germany). All chemicals were used as received. Mesoporous 2 mm pellets of β-SiC (SIC3-E3-M) were purchased from SICAT (France), ground, and sieved to yield a particle size range of 500-630 μm.
Preparation of catalysts
Ga-based SCALMS materials were synthesised in a two-step procedure. The first part comprises the deposition of liquid Ga on the SiC support material. Ga nuggets of ~1.00 g were melted and dispersed in 100 mL isopropanol via ultrasonication with a BRANSON-450D sonifier at 80% intensity (maximum power output: 450 W) for 10 min at 40 °C forming an emulsion. Subsequently, a defined amount of SiC (~13.00 g) was added to yield a targeted Ga loading. The mixture was stirred thoroughly for 1 h. Finally, the Ga-decorated SiC was dried with a rotary evaporator at 50 °C and 150 mbar for 2 h and calcined overnight at 500 °C40. The second part of the preparation procedure comprises the addition of the active metal via wetness impregnation of the Ga/SiC material. The corresponding amounts of metal precursors, namely metal chlorides (Co, Cu, Fe) and nickel(II) chloride ethylene glycol dimethyl ether (Ni), were dissolved in distilled water and isopropanol in a proportion of 1:10, added to the previously calcined Ga/SiC sample targeting a molar Ga/secondary metal ratio of 50 and mixed for 1 h. Subsequently, the solvents were evaporated in a rotary evaporator at 50 °C and 150 mbar for 2 h and the obtained SCALMS materials were calcined overnight at 500 °C.
Characterisation of fresh and spent catalysts
The metal loadings of prepared samples and post-run catalysts were determined by means of inductively coupled plasma atomic emission spectroscopy (ICP-AES) using a Ciros CCD (Spectro Analytical Instruments GmbH). The solid samples were digested with concentrated HCl:HNO:HF in a 3:1:1 volumetric ratio using microwave heating up to 220 °C for 20–40 min. The instrument was calibrated with standard solutions of Ni, Co, Fe, Cu, Ga prior to the measurements.
The BET surface area and BJH pore volume of the SiC support material were analysed by N2 physisorption in a QUADROSORB SI Surface Area and Pore Size analyser (Quantachrome Instruments) with a degassing temperature of 200 °C.
Scanning electron microscopy (SEM) was carried out using a Phenom Desktop SEM (BSD detector, 15 kV voltage). The samples were deposited directly onto a conductive sticky carbon pad. In addition, scanning electron microscopy with elemental mapping via energy-dispersive X-ray spectroscopy (SEM-EDX) was performed on a ZEISS Cross Beam 540 Gemini II scanning electron microscope equipped with an EDX detector from Oxford Instruments Group. Silver paste (Acheson Silver DAG 1415) was used to enhance the electrical conductivity. Additionally, the samples were PVD carbon coated prior to the investigations. SEM images were obtained at 3 kV and 2 nA. For images acquired with a backscatter electron detector (BSD) a voltage of 20 kV and a current of 2 nA was used. EDX analysis was performed at 20 kV and 2 nA.
X-ray diffraction (XRD) was conducted using an X’Pert PRO (Philipps) equipped with a Cu anode (λKα1 = 1.54056 Å). The samples were placed in a sample holder and analysed in a continuous scan mode in the 2θ range of 1.992° to 80.000° with a step size of 0.0167113° and a scan time of 1.11 s per step. XRD patterns were compared to references from the Crystallography Open Database (COD)66, namely graphite (COD ID 1200017)67, β-gallium(III) oxide (COD ID 2004987)68, and β-silicon carbide (COD ID 1010995)69.
Raman spectroscopy was conducted using an AvaRaman-PRB-532 (Avantes) probe with an AvaRaman-532HERO-EVO (Avantes) system. The Raman solution consists of a 532 nm (green) solid-state laser (Cobolt) and an AvaSpec-HERO (Avantes) spectrometer with a grating set of 1200 lines mm-1 (HSC1200-0.75). The spectrometer is equipped with a 50 µm slit and the detected wavelength range is 534-696 nm. If not otherwise stated, Raman spectra were collected in 10 repetitions at 15 mW laser power with an exposure time of 20 s. Three different spots of the sample were analysed and averaged. Typically, all three spectra of the samples coincided.
Spent samples were analysed via temperature-programmed oxidation (TPO) in 21% O2/N2 in order to quantify carbon deposits formed during DRM. The weight change was monitored by means of thermogravimetric analysis (TGA) using a SETSYS Evolution high-performance modular TGA (Setaram). A total of 30–45 mg of the particular sample were placed in a quartz crucible, heated to 120 °C (10 °C min-1) for 100 min to remove adsorbed H2O. Subsequently, the temperature was increased with a heating rate of 5 °C min-1 to 1000 °C for 10 min. The overall flow rate was 50 mL min-1 throughout TPO.
Catalytic testing
Catalytic experiments were performed in a high-temperature set-up consisting of a tubular split furnace (Carbolite) with three heating zones of 20 cm length each. Quartz tubes (L: 100 cm, OD: 1.2 cm, ID 1.0 cm) were used as reactors, while three pins at a height of 55.5 cm from the bottom end supported the catalyst bed. A total of 1.0 g of catalyst was placed in between two plugs of 0.1 g quartz wool. DRM was performed at 900 °C and 1 bar for 20 hours with an inlet gas composition of 20% CH4 (≥99.9995% purity) and 20% CO2 (≥99.9995% purity) in Ar (≥99.998% purity and further purified by an Agilent CP17974 gas clean filter) and an overall flow rate of 50 mLN min-1 resulting in a gas hourly space velocity (GHSV) of 3000 mLN h-1 gcat-1. The off-gas was quantitatively analysed in a micro gas chromatograph (I-GRAPHX PR; Industrial Graph Xolutions, Germany) using Ar as the internal standard.
Computational methods
Density-functional theory (DFT) based ab initio molecular dynamics (AIMD) simulations were performed employing the Vienna Ab Initio Simulation Package (VASP) using the projector augmented wave (PAW) method to represent the ion cores and a plane wave basis set with a kinetic energy cutoff of 300 eV70,71,72. The exchange-correlation function of Perdew, Burke and Ernzerhof (PBE) was employed73. The GaNi systems were simulated using periodic slab models with a tetragonal unit cell (12.69 × 12.69 × 40 Å) comprising 180 Ga atoms with 15 and 4 Ni atoms in case of Ga12Ni and Ga45Ni, respectively. A vacuum layer of 12 Å was added in the direction perpendicular to the surface to ensure that periodic images do not interact. To integrate the equations of motion a Verlet algorithm was used with a time step of 5 fs. After sufficient equilibration, a Nosé-Hoover thermostat was applied in order to simulate a canonical ensemble at 900 °C74. To sample the first Brillouin zone a Γ-containing 2x2x1 k-point mesh was used. The SCF convergence criterion was set to 10-7 eV. A total of 10 independent trajectories were sampled for each composition, every trajectory running for at least 150 ps of production time and results were averaged over all trajectories.
Data availability
All relevant data are available from the authors upon request. Please contact the corresponding author.
References
Pakhare, D. & Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 43, 7813–7837 (2014).
Bode, A. et al. in 20th World Hydrogen Energy Conference 2014. Held 15–20 June 2014, Gwangju, South Korea (2014).
Aramouni, N. A. K., Touma, J. G., Tarboush, B. A., Zeaiter, J. & Ahmad, M. N. Catalyst design for dry reforming of methane: analysis review. Renew. Sustain. Energy Rev. 82, 2570–2585 (2018).
Wittich, K., Krämer, M., Bottke, N. & Schunk, S. A. Catalytic dry reforming of methane: insights from model systems. ChemCatChem 12, 2130–2147 (2020).
Song, Y. et al. Dry reforming of methane by stable Ni-Mo nanocatalysts on single-crystalline MgO. Science 367, 777–781 (2020).
Schwab, E., Milanov, A., Schunk, S. A., Behrens, A. & Schödel, N. Dry reforming and reverse water gas shift: alternatives for syngas production. Chem. Ing. Tech. 87, 347–353 (2015).
Moradi, G. R., Rahmanzadeh, M. & Khosravian, F. The effects of partial substitution of Ni by Zn in LaNiO3 perovskite catalyst for methane dry reforming. J. CO2 Util. 6, 7–11 (2014).
Royer, S. et al. Perovskites as substitutes of noble metals for heterogeneous catalysis: dream or reality. Chem. Rev. 114, 10292–10368 (2014).
Mao, X., Foucher, A. C., Stach, E. A. & Gorte, R. J. Changes in Ni-NiO equilibrium due to LaFeO3 and the effect on dry reforming of CH4. J. Catal. 381, 561–569 (2020).
Bartholomew, C. H. Mechanisms of catalyst deactivation. Appl. Catal. A 212, 17–60 (2001).
Zuo, Z.-J., Shen, C.-F., Tan, P.-J. & Huang, W. Ni based on dual-support Mg-Al mixed oxides and SBA-15 catalysts for dry reforming of methane. Catal. Commun. 41, 132–135 (2013).
Han, J. W., Kim, C., Park, J. S. & Lee, H. Highly coke-resistant ni nanoparticle catalysts with minimal sintering in dry reforming of methane. ChemSusChem. 7, 451–456 (2014).
Huang, F. et al. Catalytic performances of Ni/mesoporous SiO2 catalysts for dry reforming of methane to hydrogen. J. Energy Chem. 25, 709–719 (2016).
Zhang, Q. et al. A sintering and carbon-resistant Ni-SBA-15 catalyst prepared by solid-state grinding method for dry reforming of methane. J. CO2 Util. 17, 10–19 (2017).
Yang, E. et al. Al2O3-coated Ni/CeO2 nanoparticles as coke-resistant catalyst for dry reforming of methane. Catal. Sci. Technol. 10, 8283–8294 (2020).
Wolf, M. Thermodynamic assessment of the stability of bulk and nanoparticulate cobalt and nickel during dry and steam reforming of methane. RSC Adv. 11, 18187–18197 (2021).
Ha, Q. L. M. et al. Development of highly stable low Ni content catalyst for dry reforming of CH4‐rich feedstocks. ChemCatChem. 12, 1562–1568 (2020).
Palmer, C. et al. Dry reforming of methane catalysed by molten metal alloys. Nat. Catal. 3, 83–89 (2020).
Yang, Q., Zhou, C., Ni, J. & Guan, X. Methane dry reforming in a coking- and sintering-free liquid alloy-salt catalytic system. Sustain. Energy Fuels 4, 2768–2774 (2020).
Taccardi, N. et al. Gallium-rich Pd-Ga phases as supported liquid metal catalysts. Nat. Chem. 9, 862–867 (2017).
Herman, J. M., Rocourt, A. P. A. F., Van den Berg, P. J., Van Krugten, P. J. & Scholten, J. J. F. The industrial hydroformylation of olefins with rhodium-based supported liquid phase catalyst SLPC. Chem. Eng. J. 35, 83–103 (1987)..
Riisager, A., Fehrmann, R., Haumann, M. & Wasserscheid, P. Supported ionic liquid phase (SILP) catalysis: an innovative concept for homogeneous catalysis in continuous fixed-bed reactors. Eur. J. Inorg. Chem. 2006, 695–706 (2006).
Lijewski, M., Hogg, J. M., Swadźba-Kwaśny, M., Wasserscheid, P. & Haumann, M. Coating of Pd/C catalysts with Lewis-acidic ionic liquids and liquid coordination complexes – SCILL induced activity enhancement in arene hydrogenation. RSC Adv. 7, 27558–27563 (2017).
Marinkovic, J. M., Riisager, A., Franke, R., Wasserscheid, P. & Haumann, M. Fifteen years of supported ionic liquid phase-catalyzed hydroformylation: material and process developments. Ind. Eng. Chem. Res. 58, 2409–2420 (2018).
Rupprechter, G. Popping up to the surface. Nat. Chem. 9, 833–834 (2017).
Raman, N. et al. Highly effective propane dehydrogenation using Ga-Rh supported catalytically active liquid metal solutions. ACS Catal. 9, 9499–9507 (2019).
Sebastian, O. et al. Stable and selective dehydrogenation of methylcyclohexane using supported catalytically active liquid metal solutions–Ga52Pt/SiO2 SCALMS. ChemCatChem. 12, 4533–4537 (2020).
Wolf, M., Raman, N., Taccardi, N., Haumann, M. & Wasserscheid, P. Coke formation during propane dehydrogenation over Ga−Rh supported catalytically active liquid metal solutions. ChemCatChem. 12, 1085–1094 (2020).
Wolf, M. et al. Capturing spatially resolved kinetic data and coking of Ga-Pt supported catalytically active liquid metal solutions during propane dehydrogenation in situ. Faraday Discuss. 229, 359–377 (2021).
Anand, M. et al. Advanced approaches: general discussion. Faraday Discuss. 229, 378–421 (2021).
Grabau, M. et al. Surface enrichment of Pt in Ga2O3 films grown on liquid Pt/Ga alloys. Surf. Sci. 651, 16–21 (2016).
Grabau, M. et al. Spectroscopic observation and molecular dynamics simulation of Ga surface segregation in liquid Pd-Ga alloys. Chem. A Eur. J. 23, 17701–17706 (2017).
Bauer, T. et al. Operando DRIFTS and DFT study of propane dehydrogenation over solid- and liquid-supported GaxPty catalysts. ACS Catal. 9, 2842–2853 (2019).
Hohner, C. et al. Pt–Ga model SCALMS on modified HOPG: growth and adsorption properties. Top. Catal. 62, 849–858 (2019).
Kettner, M. et al. Pd-Ga model SCALMS: characterization and stability of Pd single atom sites. J. Catal. 369, 33–46 (2019).
Nishizawa, T. & Ishida, K. in Binary Alloy Phase Diagrams (eds. Massalski, T. B., Okamoto, H., Subramanian, P. R. & Kacprzak, L.) 1186 (ASM International, 1990).
Subramanian, P. R. & Laughlin, D. E. in Binary Alloy Phase Diagrams (eds. Massalski, T. B., Okamoto, H., Subramanian, P. R. & Kacprzak, L.) 1410–1411 (ASM International, 1990).
Okamoto, H. in Binary Alloy Phase Diagrams (eds. Massalski, T. B., Okamoto, H., Subramanian, P. R. & Kacprzak, L.) 1702–1703 (ASM International, 1990).
Lee, S. Y. & Nash, P. in Binary Alloy Phase Diagrams (eds. Massalski, T. B., Okamoto, H., Subramanian, P. R. & Kacprzak, L.) 1829–1833 (ASM International, Russell Township, USA, 1990).
Raman, N. et al. GaPt supported catalytically active liquid metal solution catalysis for propane dehydrogenation–support influence and coking studies. ACS Catal. 11, 13423–13433 (2021).
Papadopoulou, C., Matralis, H. & Verykios, X. in Catalysis for Alternative Energy Generation (eds L. Guczi & A. Erdôhelyi) Ch. 3, (Springer, 2012).
Shah, Y. T. Water for Energy and Fuel Production. (CRC Press, 2014).
Wang, Y., Yao, L., Wang, S., Mao, D. & Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: a review. Fuel Process. Technol. 169, 199–206 (2018).
Søgaard, A., de Oliveira, A. L., Taccardi, N., Haumann, M. & Wasserscheid, P. Ga–Ni supported catalytically active liquid metal solutions (SCALMS) for selective ethylene oligomerization. Catal. Sci. Technol. 11, 7535–7539 (2021).
Nabaho, D., Niemantsverdriet, J. W., Claeys, M. & van Steen, E. Hydrogen spillover in the Fischer-Tropsch synthesis: an analysis of platinum as a promoter for cobalt-alumina catalysts. Catal. Today 261, 17–27 (2015).
Wolf, M., Mutuma, B. K., Coville, N. J., Fischer, N. & Claeys, M. Role of CO in the water-induced formation of cobalt oxide in a high conversion Fischer-Tropsch environment. ACS Catal. 8, 3985–3989 (2018).
Kocić, S., Corral Valero, M., Schweitzer, J.-M. & Raybaud, P. Surface speciation of Co based Fischer-Tropsch catalyst under reaction conditions: Deactivation by coke or by oxidation? Appl. Catal. A 590, 117332 (2020).
Wolf, M., Fischer, N. & Claeys, M. Water-induced deactivation of cobalt-based Fischer–Tropsch catalysts. Nat. Catal. 3, 962–965 (2020).
Wolf, M., Fischer, N. & Claeys, M. Formation of metal-support compounds in cobalt-based Fischer-Tropsch synthesis: a review. Chem. Catal. 1, 1014–1041 (2021).
Mars, P. & van Krevelen, D. W. Oxidations carried out by means of vanadium oxide catalysts. Chem. Eng. Sci. 3, 41–59 (1954).
Ruckenstein, E. & Wang, H. Y. Carbon dioxide reforming of methane to synthesis gas over supported cobalt catalysts. Appl. Catal. A: Gen. 204, 257–263 (2000).
Otsuka, K., Yasui, T. & Morikawa, A. Production of CO from CO2 by reduced indium oxide. J. Chem. Soc. Faraday Trans. 1, 78, 3281–3286 (1982).
Shang, Z., Li, S., Li, L., Liu, G. & Liang, X. Highly active and stable alumina supported nickel nanoparticle catalysts for dry reforming of methane. Appl. Catal. B: Environ. 201, 302–309 (2017).
Horváth, É. et al. Dry reforming of CH4 on Co/Al2O3 catalysts reduced at different temperatures. Catal. Today 281, 233–240 (2017).
Siew, K. W., Lee, H. C., Gimbun, J. & Cheng, C. K. Production of CO-rich hydrogen gas from glycerol dry reforming over La-promoted Ni/Al2O3 catalyst. Int J. Hydrog. Energ. 39, 6927–6936 (2014).
Roy, J., Chandra, S., Das, S. & Maitra, S. Oxidation behaviour of silicon carbide-a review. Rev. Adv. Mater. Sci. 38, 29–39 (2014).
Berton, B., Bacos, M. P., Demange, D. & Lahaye, J. High-temperature oxidation of silicon carbide in simulated atmospheric re-entry conditions. J. Mater. Sci. 27, 3206–3210 (1992).
Alcock, C. B. Vapor pressure of the metallic elments, in CRC Handbook of Chemistry and Physics (ed. D. R. Lide) Ch. Section 4, (CRC Press, 2003).
Butt, D. P., Park, Y. & Taylor, T. N. Thermal vaporization and deposition of gallium oxide in hydrogen. J. Nucl. Mater. 264, 71–77 (1999).
Breisacher, P. & Siegel, B. Comparative stabilities of gaseous alane, gallane, and indane. J. Am. Chem. Soc. 87, 4255–4258 (1965).
Terasako, T., Kawasaki, Y. & Yagi, M. Growth and morphology control of β-Ga2O3 nanostructures by atmospheric-pressure CVD. Thin Solid Films 620, 23–29 (2016).
Kakoria, A. et al. Gallium oxide nanofibers for hydrogen evolution and oxygen reduction. ACS Appl. Nano Mater. 2, 64–74 (2018).
López, I., Nogales, E., Hidalgo, P., Méndez, B. & Piqueras, J. Field emission properties of gallium oxide micro- and nanostructures in the scanning electron microscope. Phys. Status Solidi A 209, 113–117 (2012).
Kiss, G., Kliewer, C. E., DeMartin, G. J., Culross, C. C. & Baumgartner, J. E. Hydrothermal deactivation of silica-supported cobalt catalysts in Fischer-Tropsch synthesis. J. Catal. 217, 127–140 (2003).
Wolf, M. et al. In-depth characterisation of metal-support compounds in spent Co/SiO2 Fischer-Tropsch model catalysts. Catal. Today 342, 71–78 (2020).
Downs, R. T. & Hall-Wallace, M. The American mineralogist crystal structure database. Am. Mineralogist 88, 247–250 (2003).
Hassel, O. Ueber die Kristallstruktur des Graphits. Z. fuer Phys. 25, 317–337 (1924).
Åhman, J., Svensson, G. & Albertsson, J. A reinvestigation of β-gallium oxide. Acta Crystallogr. Sect. C. 52, 1336–1338 (1996).
Braekken, H. Zur Kristallstruktur des kubischen Karborunds. Z. Kristallogr. Kristallgeomet. Kristallphys. Kristallchem. 75, 572–573 (1930).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81, 511–519 (1984).
Acknowledgements
Financial support by the European Research Council (Project 786475: Engineering of Supported Catalytically Active Liquid Metal Solutions) and the German Research Foundation (DFG) in the frame of the Collaborative Research Centre 1452 Catalysis at Liquid Interfaces (CLINT) is gratefully acknowledged. The authors thank Narayanan Raman and Susanne Pachaly for conducting the N2 physisorption and XRD measurements, respectively.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.W.: conceptualisation; investigation; data curation; formal analysis; validation; visualisation; writing—original draft. A.L.d.O.: Investigation; data curation; formal analysis; writing—review & editing. N.T.: investigation; formal analysis; validation; writing—review & editing. S.M.: investigation; data curation; formal analysis; visualisation. M. Heller: Investigation; data curation; formal analysis; visualisation. SKA: investigation; data curation; formal analysis. AS: resources. PF: resources; supervision. AG: resources; supervision; writing—review & editing. M. Haumann: conceptualisation; project administration; resources; validation; writing—review & editing. PW: conceptualisation; funding acquisition; project administration; resources; validation; writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks Yu Tang, Yasin Orooji and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wolf, M., de Oliveira, A.L., Taccardi, N. et al. Dry reforming of methane over gallium-based supported catalytically active liquid metal solutions. Commun Chem 6, 224 (2023). https://doi.org/10.1038/s42004-023-01018-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-023-01018-w