Abstract
Photolysis is an attractive method in organic synthesis to produce free radicals through direct bond cleavage. However, in this method, specific irradiation wavelengths of light have been considered indispensable for excitation through S0–Sn or S0–Tn transitions. Here we report the photoinduced homolysis of electronegative interelement bonds using light at wavelengths much longer than theoretically and spectroscopically predicted for the S0–Sn or S0–Tn transitions. This long-wavelength photolysis proceeds in N–Cl, N–F, and O–Cl bonds at room temperature under blue, green, and red LED irradiation, initiating diverse radical reactions. Through experimental, spectroscopic, and computational studies, we propose that this “hidden” absorption is accessible via electronic excitations from naturally occurring vibrationally excited ground states to unbonded excited states and is due to the electron-pair repulsion between electronegative atoms.
Similar content being viewed by others
Introduction
Photolysis, the homolytic cleavage of chemical bonds through the excited state, offers an attractive method to produce free radical species using traceless photons1,2,3,4,5,6,7. The first report of an organic reaction initiated by photoinduced homolysis dates back to the accidental discovery of N–Cl bond cleavage of nitrosyl chloride under sunlight irradiation in 19198. Since then, by using ultraviolet light, light-emitting diodes (LEDs) or sunlight, this strategy has evolved to be elegantly applied to many organic reactions, including radical addition reactions, radical-mediated functionalizations, and radical-initiated polymerizations9,10,11,12,13,14,15.
In photolysis, there are two routes to the excited state from the ground state (S0), and the use of an appropriate wavelength of light is necessary for the electronic excitation of the target molecules. The first route is a transition between the singlet excited states (S0–Sn), which may be followed by internal conversion to another singlet excited state or intersystem crossing (ISC) to the triplet excited state (Tn). Many examples of photolysis of electronegative bonds, such as O–O, O–X, N–X, and X–X (X = halogen) bonds (Fig. 1a-(I))16,17,18,19,20,21,22, as well as carbon-containing C–C or C–X bonds, via the excitation to the Sn state have been reported (Fig. 1a-(II))23,24,25,26. Our group has also developed borylation and silylation reactions based on the photolysis of electropositive interelement B–B and B–Si bonds via S0–Sn transition (Fig. 1a-(III))27,28.
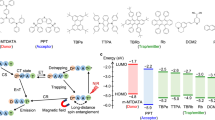
a Photolysis of electronegative, carbon-containing, or electropositive bonds via S0–Sn transition. b Photolysis of heavy atom-containing or electronegative bonds via S0–Tn transition. c Long-wavelength photolysis of electronegative bonds via S0μ0–S0μn–Sn or Tn transition (this work). d Potential energy surface and S0–Sn or Tn transition. e Potential energy surface of electronegative bonds and S0μ0–S0μn–Sn or Tn transition (this work).
The second route to the excited state is a direct excitation to the triplet state (Tn), although S0–Tn transition is generally restricted due to the intrinsic need for a spin inversion29,30. In 2020, Nakajima and Nemoto established that photoinduced reactions of heavy-atom-containing molecules, such as hypervalent iodine or bismuth compounds, proceed via direct S0–Tn transition, with the aid of the relaxation of spin restrictions based on the internal heavy-atom effect (Fig. 1b-(I))31,32. Subsequently, in 2022, Mita and Maeda also featured the homolysis of P–P bonds via light absorption through direct S0–Tn transition (Fig. 1b-(II))33. In these cases, the potential energy of the triplet state (Tn) is usually lower than that of the singlet state (Sn), and thus longer wavelengths of light can be employed in these reactions (Fig. 1d). Thus, when designing photolysis-driven organic reactions, the appropriate irradiation wavelength has conventionally been determined by investigating the absorption spectrum of the target molecule or by predicting S0–Sn as well as S0–Tn transition energies with theoretical calculations.
Here we report the photoinduced homolysis of electronegative interelement bonds using light at wavelengths much longer than theoretically and spectroscopically predicted for the S0–Sn or S0–Tn transitions (Fig. 1c). Specifically, this report includes the photohomolysis of the following interelement bonds: (1) the N–Cl bond in N-chlorosuccinimide (NCS, 1a) and the N–F bond in N-fluorobenzenesulfonimide (NFSI, 1b), (2) the N–Cl bond in N-chlorosaccharin (NCSA, 1c), and (3) the O–Cl bond in t-butyl hypochlorite (1d), under blue, green, and red LEDs irradiation, respectively. Through experimental, spectroscopic, and computational studies, we propose that this “hidden” absorption occurs via an electronic transition from a thermally populated vibrational excited state (S0μ0–S0μn–Sn or Tn, Fig. 1e), referred to as vibration-mediated photodissociation (VMP) in physical chemistry34,35,36. VMPs of small molecules such as water, acetylene, ammonia, nitric acid, hydrogen peroxide, pyrrole, and methanethiol have been detected by laser-based double-resonance techniques34,35,36,37,38,39. VMP has also been used to investigate intramolecular vibrational redistribution dynamics40,41,42, but not for organic synthesis. The proposed mechanism is shown in Fig. 1c. Molecules bearing N–Cl, N–F, and O–Cl bonds are stabilized in the excited state as their bond lengths increase, reducing the energy necessary for S0μn–Sn or Tn transitions and allowing their photolysis and “hidden” absorption in the long-wavelength region. Hence, the electronic excitation of a small fraction of molecules, specifically those in the excited vibrational state (S0μn) at thermal equilibrium, would allow direct access to the excited state (S0μn–Sn or Tn). Although such long-wavelength photolysis generates only trace amounts of the radical species, combining it with a chain process enables reactions on a bulk scale. VMP first meets organic synthesis in this paper to enable the C(sp2)–H chlorination/fluorination of aldehydes and allylic C(sp3)–H chlorination/aminochlorination of alkenes using long wavelengths of light beyond S0–S1 and S0–T1 transitions.
Results and discussion
Computational analysis
We have previously reported the photolysis of electropositive interelement B–B and B–Si bonds by the formation of light-absorbing complexes under irradiation with light of longer wavelengths than the absorption of B–B and B–Si reagents27,28. During the application of this complexation strategy to the electronegative N–Cl bond of 1a, we unexpectedly found the blue-light-induced photolysis of 1a without any complexations (Supplementary Fig. 1a). However, our measurement of the absorption spectrum of 1a showed that the major absorption peaks were observed below 250 nm, and the absorption tail cannot reach even UV-A (<320 nm) under standard measurement conditions (Supplementary Fig. 1b).
Considering that the spin-restricted S0–Tn transition might enable the absorption of such longer wavelength31,32,33, we theoretically predicted the S0–Sn and S0–Tn transition wavelengths and bond dissociation energies (BDEs) for N–Cl bonds by density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations43,44,45,46 at the (U)M06/6-31 + G* or (U)M06/6-311 + G* levels of theory. Typical compounds involving 1a are shown in Table 1 (for the detailed list of calculated N-chloroamines, see: Supplementary Table 2). For such small molecules with only N–Cl bonds, carbonyl groups, and phenyl groups, the S0–S1 transition is limited to wavelengths below 320 nm, as expected, and the spin-restricted S0–T1 transition was assigned to UV-A (320–400 nm) for 1e, 1 f, 1 h and 1i (N–chlorophthalimide, NCP), or visible light (>400 nm) for 1c. These calculations well explained previous reports where photolysis occurs in 1a and 1c–i at the above-predicted irradiation wavelengths19,47,48,49, but the present visible-light-induced photolysis of 1a remained inexplicable. In addition, according to our prediction, two reports of catalyst-free blue-light-induced N–Cl excitation reactions for 1e50 and 1 h51, where the absorption tails can reach visible light, may not be explained by S0–Sn and S0–Tn transition. On the basis of these contradictions between experimental and theoretical results, we hypothesized that the electron-pair repulsions in electronegative bonds could facilitate photolysis at much longer irradiation wavelengths than both the theoretically predicted S0–Sn or S0–Tn transitions and the spectroscopically observed absorption tails to enable diverse radical reactions using “hidden” absorption.
Experimental analysis
To test the validity of our hypothesis, we first conducted C(sp2)–H chlorination of benzaldehyde (2a), which proceeded using 1 h under the irradiation wavelength corresponding to absorption tails reported by Luca51, with the most stable N–Cl reagent 1a under irradiation wavelengths beyond S0–S1 and S0–T1 transitions (Table 2). The efficacy of the halogenation step was evaluated by derivatizing the unstable acyl chloride produced to the corresponding stable amide using dimethylamine. Without light irradiation, the chlorination of 2a did not proceed with 1a (entry 1). In contrast, irradiation with blue LEDs (390–470 nm, λmax = 450 nm) in a wavelength range much longer than the theoretically estimated for the S0–S1 (242 nm, Table 1) or the S0–T1 absorption peaks (275 nm, Table 1) of 1a, could promote the C(sp2)–H chlorination of 2a to give the corresponding acyl chloride, which was subjected to amidation to form N,N–dimethylbenzamide 3a in 83% yield (entry 2). However, green LEDs (490–570 nm, λmax = 525 nm) could not activate the present chlorination (entry 3). Notably, heating could not promote this reaction either, indicating that this reaction proceeded via photoinduced homolysis of the N–Cl bond (entry 4). This reaction also proceeded under air (entry 5), showing that the bond dissociation might occur faster than the oxygen quenching of the excited state. Furthermore, the yields correlate with the irradiance of the used blue LEDs (entries 6–8), and the present “hidden” absorption is accessible even with lower irradiances. Hence, two-photon excitation is unlikely.
Next, we examined the present reaction using 1i and 1c (entries 9–11). Reagent 1i gave a similar result to 1a under blue LEDs irradiation, but it was also activatable with green LEDs, albeit with low product yield. In the case of 1c, the product was obtained in high yield under green LEDs (entry 11) irradiation with wavelengths much longer than the theoretically estimated for the S0–S1 (277 nm, Table 1) or S0–T1 (408 nm, Table 1) absorption peaks. It is worth mentioning that previous reports have used light-driven photocatalytic radical initiators52,53,54, and thus this is the first example of a catalyst-free visible-light-induced C(sp2)–H chlorination of aldehydes by NCS (1a).
Substrate scope
The substrate scope of the photoinduced C(sp2)–H chlorination of aldehydes followed by amidation is shown in Fig. 2a. For blue LEDs irradiation using 1a (Method A, Fig. 2a), aromatic aldehydes bearing various electron-withdrawing groups (p-Ph, p-CO2Me, p-CF3, o-/m-/p-Cl, p-F, p-Br, and m-cyano) as well as electron-donating groups (p-Me and o-/m-/p-OMe) could be used to give the corresponding amide products in good to excellent yields (3b–3o). π-extended arenes- (2p) and heteroarenes-substituted (2q and 2r) aldehydes were also compatible. Importantly, not only aromatic aldehydes but aliphatic aldehydes can participate in the present chlorination to afford the amide products 3s–3 v in moderate yields, indicating that the formation of an NCS (1a)-substrate complex that allows longer wavelength absorption is unlikely11,12. Additionally, sequential one-pot amidation with various amines was possible, giving the amide products 3ab–3ae without isolation of the acyl chlorides. Similarly, under green LEDs irradiation (525 nm), NCSA (1c) could also afford the amide products 3a and 3d (Method B, Fig. 2a). According to solvent screening experiments (Supplementary Table 1), the present C(sp2)–H chlorination proceeds in not only CCl4 but also other solvents, such as MeCN and CH2Cl2, or even under solventless conditions, to afford the desired products 3a, 3d, and 3t (Method A’ for MeCN, Fig. 2a).
a C(sp2)–H chlorination of aldehydes. 2 (0.10 mmol), 1a (0.11 mmol), and CCl4 (0.20 mL) or MeCN (0.10 mL) were used [Method A or A’]. 2 (0.10 mmol), 1c (0.11 mmol), and CCl4 (0.20 mL) were used [Method B]. b Aminochlorination of alkenes. 4 (0.50 mmol), 1a (0.55 mmol), and CH2Cl2 (1.0 mL) were used [Method A], and 4 (0.10 mmol), 1c (0.11 mmol), and CH2Cl2 (0.2 mL) were used [Method B]. c, C(sp2)–H fluorination of aldehydes. 2 (0.20 mmol), 1b (0.20 mmol), and MeCN (0.4 mL) were used. d, Allylic C(sp3)–H chlorination. 4d (0.5 mmol), 1d (1.0 mmol), and CH2Cl2 (1.0 mL) were used. Cited yields are of isolated products. Blue LEDs (λmax = 450 nm, >2000 W m-2), green LEDs (λmax = 525 nm, >2000 W m-2), or red LEDs (λmax = 640 nm, <771 W m-2) were used. a Yields determined by 1H or 19F NMR of the crude mixture using mesitylene as the internal standard. b Product was isolated after amidation or amination.
This visible-light-induced activation mode also enabled the aminochlorination of alkenes 4 (Fig. 2b). In previous reports, a combination of short wavelength UV light irradiation and stable N–Cl reagents such as 1a47,48 and 1i19 or fluorescent lamp irradiation and an unstable N–Cl reagent such as 1c49 enabled this transformation. In contrast, aminochlorination with 1a under blue LEDs irradiation afforded the corresponding alkanes 5aa–5ca bearing both amino and chloride groups in moderate yields, analogous to those previously reported (Method A, Fig. 2b). As in the C(sp2)–H chlorination of aldehydes, these aminochlorination reactions did not proceed without photo-irradiation. Moreover, green light irradiation enabled the aminochlorination of cyclohexene 4d and styrene derivatives 4e and 4 f to afford 5dc, 5ec, and 5 fc, respectively, in good yields, where interestingly, the aminochlorination of 4d with 1c afforded trans-5dc product stereoselectively (Method B, Fig. 2b). This selectivity can be explained as follows. In the reaction of succinimide/chlorine radicals with alkenes, the addition of the nitrogen-centered radical is favorable for unactivated alkenes (4b and 4c)47,48. Vinyl ether derivate 4a may simultaneously react ionically via halogenium intermediates (in addition to the free radical mechanism), giving both products as reported for the reaction with NCP55. In contrast, changing the nitrogen-centered radical to saccharin favors the addition of the chlorine radical, yielding 5ec and 5 fc, as previously reported49.
Next, we examined whether unexpectedly long wavelengths of light would similarly activate electronegative interelement bonds involving the F atom in the second row instead of the Cl atom in the third row. We were pleased to find that blue LEDs irradiation enables the fluorination of aldehydes using 1b through the N–F bond photolysis (Fig. 2c). Thus, we have established that the heavy atom effect is not the key to the present photolysis. Aldehydes 3a, 3d, and 3 s were successfully converted and isolated in good to high yields as the corresponding fluorinated derivates, more stable than their chlorinated counterparts. The fluorination product from the electron-rich aldehyde 1 l was unstable and thus was isolated after amidation, yielding the target product in high yield. As in the other cases, according to the theoretically predicted S0–S1 (246 nm) and S0–T1 (307 nm) absorption peaks for 1b (Supplementary Table 2), absorption of blue LEDs is not expected. It is worth mentioning that the present reaction constitutes the first example of a catalyst-free visible-light-induced C(sp2)–H fluorination of aldehydes by reagents with N–F bonds56.
In addition, the O–Cl bond of 1d, for which S0–S1 (333 nm) and S0–T1 (399 nm) absorption peaks are theoretically predicted (Supplementary Table 2), underwent photoinduced homolysis with cyclohexene (4d) under green LEDs irradiation (525 nm) to give the allylic C(sp3)–H chlorination57 product 7d, as shown in Fig. 2d. Furthermore, red LEDs irradiation (>600 nm) also enabled this chlorination to give 7d in good yield.
Mechanistic studies
We conducted spectroscopic studies to elucidate the mechanism of this activation (Fig. 3). When we measured the absorption spectrum of 1a in MeCN (1 × 10–3 M), the major absorption peaks, corresponding to the spin-allowed S0–Sn transitions, were observed below 250 nm, as predicted in Table 1 (Fig. 3a). Although we observed a considerable broadening of the original absorption peak at the high concentration of 1 M, the absorption of visible light is still almost undetectable. On the other hand, when we used a 10 cm cell (10 times longer than a conventional cell), the peak broadening can finally reach the visible light range, but the molar absorption coefficient (ε) is only 2 × 10–3 at 400 nm. In addition, spectroscopic measurements of 1b and 1d in MeCN gave similar results. We observed very weak absorption of longer wavelengths (visible light) only in highly concentrated solutions, but no discernible peaks could be observed (Figs. 3b and 3c). Therefore, these results support the possibility that weak transitions to Sn or Tn states can occur at much larger wavelengths than theoretically predicted and experimentally observed at standard measuring conditions.
a–c Absorption spectra of 1a, 1b, and 1d in MeCN, respectively. DFT calculated absorption was obtained at the M06/6-31 + G* level of theory. d–e Temperature dependence of the photoinduced C(sp2)–H chlorination of 2a with 1a, the photolysis of 1a, and the photolysis of 1b. Yields were determined by 1H NMR using mesitylene as the internal standard. Calc, calculated. Abs, absorption. f Proposed mechanism.
Next, we conducted experimental mechanistic studies with external stimuli. In the present photoinduced C(sp2)–H chlorination of aldehydes with 1a, the temperature has a critical impact on the product yields (Fig. 3d). The high temperature of 60 °C drastically accelerated the blue-light-induced reaction to afford 3a in a high 87% yield, but the low temperature of 0 °C reduced the yield to 8%. More importantly, green LEDs irradiation promoted the reaction at 60 °C, in contrast to no reaction at room temperature (Fig. 3d). Additionally, the simple photolysis of 1a and 1b in highly diluted solutions (0.01 or 0.001 M), where radical chain propagation processes are minimized, was also accelerated at 60 °C, while heating alone could not promote these reactions (Fig. 3e). These results indicate that heating cannot be used to overcome the energy barrier and promote homolysis directly from the ground state. In contrast, based on VMP theories34,35,36, heating could enhance photoexcitation.
Therefore, we propose the reaction proceeds as shown in Fig. 3f. The unexpectedly red-shifted absorption could be derived from the excitation from a naturally occurring vibrationally excited state (S0μn) that is energetically closer to the excited state. This state could theoretically absorb light since electronic transitions (S0μn–Sn or Tn) are a faster process (10-14–10-15 s) than internal conversion (S0μn– S0μ0) (10-12 s)58. In the excited state, the homolysis of the N–Cl bond of 1a can afford the nitrogen-centered radical I, which reacts with the aldehyde 2 to provide the C(sp2)-centered radical II. II affords the desired chlorinated product III by abstracting the chlorine from 1a, which regenerates the nitrogen-centered radical I. Therefore, a propagation process enables trace amounts of photo-generated radicals to drive the reactions in bulk.
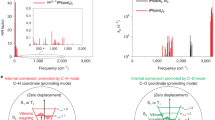
To examine this hypothesis, we predicted both S0–S1 and S0–T1 transition wavelengths from the ground state surfaces by scanning the potential energy along the electronegative-bond lengths using DFT and TD-DFT methods (Fig. 4). When the distances of the electronegative N–Cl, N–F, and O–Cl bonds increase, not only the destabilization of the ground state but also the stabilization of the excited states reduces the energy required for S0–Sn or S0–Tn transitions to allow an increase of absorption wavelengths (Fig. 4a–c and Supplementary Table 14). For instance, in the S0–T1 transitions of 1a (Fig. 4a), the destabilization of the ground state of 7.9 kcal mol-1 (N–Cl = 1.89 Å) leads to an increase of over 100 nm in the absorption wavelength (from 251 nm to 396 nm), reaching the visible range. Vibrational analysis showed that this energy state is accessible for the 4th vibrational state of the N–Cl vibrational mode, for example, which has a Boltzmann factor of 4.0× 10–6 at 27 °C and 1.9× 10–5 at 60 °C (vibration number = μ10-4 in Supplementary Tables 16 and 17). Similarly, in the S0–T1 transitions of 1b (Fig. 4b), the destabilization of the ground state of 7.7 kcal mol-1 (N–F = 1.60 Å) leads to a larger absorption wavelength from 307 nm to 405 nm. Furthermore, in the S0–T1 transitions of 1d (Fig. 4c), the absorption wavelength of the 1.6 kcal mol-1 unstable structure (O–Cl = 1.81 Å) reaches the green light region, and that of the even more unstable one (5.7 kcal mol-1, O–Cl = 1.91 Å) extends into the red light range. In contrast, in 1j where the N–C bond is not an electronegative interelement bond, absorption wavelengths do not increase due to the destabilization of both the ground and excited state (Fig. 4d).
a–d Potential energy surface and transition values for various N–Cl, N–F, O–Cl, and N–C bond lengths of 1a, 1b, 1d, and 1j, respectively, by DFT and TD-DFT calculations at the (U)M06/6-311 + G** levels of theory. The vertical line in each graph illustrates the calculated BDE. The changes in the electronic energies are shown as ⊿E in kcal mol-1.
Thus, the observed red-shifts common to electronegative interelement bonds are thought to be dependent on the repulsive character of the excited state. These unstable molecular configurations would be accessible via naturally occurring vibrationally excited states, which are expected to follow a Boltzmann distribution in equilibrium. Not only the S0–T1 but the S0–S1 transition, which does not require a spin inversion, can also contribute to the red-shift of the absorption. For example, in the S0–S1 transitions of 1a (Fig. 4a), a destabilization of the ground state of 15.0 kcal mol–1 (N–Cl = 1.99 Å) can reach the visible region from 240 nm to 408 nm. Thus, we propose that these visible-light-induced infinitesimal transitions facilitated by the vibration in electronegative interelement bonds could generate trace amounts of radical initiators that allow a variety of radical reactions to proceed. Importantly, these bonds were cleaved with irradiation energies lower than the respective BDEs. For example, 1i (calculated BDE of N–Cl bond = 75.3 kcal mol–1, Table 1) was successfully cleaved with green LEDs, whose irradiation energy is up to only 58.4 kcal mol–1 ( > 490 nm).
Conclusion
We have established that electronegative interelement bonds involving N–Cl, N–F, and O–Cl bonds can be cleaved by long-wavelength light irradiation at room temperature, contradicting conventional prediction methods used to determine appropriated irradiation wavelengths, such as absorption spectroscopy and S0–Sn or S0–Tn transition energy calculations. Experimental, spectroscopic, and computational studies support that this “hidden” absorption is accessible via electronic excitations from vibrationally excited ground states to unbonded excited states without external stimulus. Consistent with vibration-mediated photodissociation theories, this absorption would lead to bond homolysis, giving the corresponding free radicals. Hence, the observed red-shift in absorption common to electronegative interelement bonds would be dependent on the repulsive character of the excited state. We propose that this vibration-mediated photolysis, although infinitesimal when compared with the typical excitation from a vibrationally stable ground state, could initiate diverse visible-light-induced radical reactions, such as the C(sp2)–H chlorination and fluorination of aldehydes and the aminochlorination or allylic C(sp3)–H chlorination of alkenes under mild conditions. Visible light photolysis of specific bonds without external stimuli is valuable for materials recycling, such as polymer degradation, especially when using light in the low-energy deep red or near-infrared region (λ > 600 nm), which is essential for in vivo reactions. Although, we are still unable to exclude alternative mechanisms, such as two photon absorption, complexation, or an impurity-initiated chain reaction, and further studies, such as UV-vis/IR transient absorption spectroscopy, temperature dependence studies, and theoretical calculations are required to further examine this interpretation, we believe that this electronegative bond-selective photolysis, using a wide range of light wavelengths from blue to red can become a fundamental technology for these applications.
Methods
General procedure for the C(sp2)–H chlorination of aldehydes (3, Fig. 2a)
Aldehyde 2 freshly passed through a basic silica pad (0.01 mmol), 1a or 1c (0.11 mmol), and CCl4 (0.20 mL) or MeCN (0.10 mL) were placed in a 3 mL screw cap vial. The vial was capped and wrapped with a Teflon seal. The mixture was stirred at room temperature under argon with blue LEDs irradiation for 18 h. To the resulting mixture, Et3N (0.30 mL) and a THF solution of HNMe2 (2.0 mol/L, 0.5 mL) were added, and the mixture was stirred at room temperature under argon for an additional period of 2 h. The final mixture was concentrated under reduced pressure and purified by silica gel preparative thin-layer chromatography (PTLC) to give the desired product 3.
General procedure for the aminochlorination of olefins (5, Fig. 2b)
Alkene 4 (0.50 mmol), 1a or 1c (0.55 mmol), and CH2Cl2 (1.0 mL) were placed in a 3 mL screw cap vial. The vial was capped and wrapped with a Teflon seal. The mixture was stirred at room temperature under argon with blue LEDs irradiation for 18 h. The final mixture was concentrated under reduced pressure and purified by silica gel PTLC to give the desired product 5.
General procedure for the C(sp2)–H fluorination of aldehydes (6, Fig. 2c)
Aldehyde 3, freshly passed through a basic silica pad, (0.20 mmol), 1b (0.20 mmol), and MeCN (0.40 mL) were placed in a 3 mL screw cap vial. The vial was capped and wrapped with a Teflon seal. The mixture was stirred at room temperature under argon with blue LEDs irradiation for 18 h. The final mixture was concentrated under reduced pressure and purified by silica gel PTLC to give the desired product 6.
Procedure for the allylic chlorination of cyclohexene (7d, Fig. 2d)
Cyclohexene (4d, 0.50 mmol), 1d (1.0 mmol), and CH2Cl2 (1.0 mL) were placed in a 3 mL screw cap vial. The vial was capped and wrapped with a Teflon seal. The mixture was stirred at room temperature with red LEDs irradiation for 8 h to give the desired crude product 7d, the yield of which was determined by 1H NMR using mesitylene as an internal standard.
Data availability
The data that supports the findings of the present study are available in this article and its Supplementary Information, which includes materials and methods, synthetic experiments, computational studies, Supplementary Fig. 1, Supplementary Tables 1–17, Supplementary Data 1 for computational studies, and Supplementary Data 2 (Supplementary Figs. 2–80) for NMR spectra.
References
Oster, G. & Mark, H. The production of organic free radicals by light. J. Opt. Soc. Am. 43, 283–289 (1953).
Albini, A. & Fagnoni, M. Green chemistry and photochemistry were born at the same time. Green Chem 6, 1–6 (2004).
Hoffmann, N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 108, 1052–1103 (2008).
Abderrazak, Y., Bhattacharyya, A. & Reiser, O. Visible-light-induced homolysis of earth-abundant metal-substrate complexes: a complementary activation strategy in photoredox catalysis. Angew. Chem. Int. Ed. 60, 21100–21115 (2021).
Kariofillis, S. K. & Doyle, A. G. Bhatt synthetic and mechanistic implications of chlorine photoelimination in nickel/photoredox C(sp3)–H Cross-Coupling. Acc. Chem. Res. 54, 988–1000 (2021).
Protti, S., Ravelli, D. & Fagnoni, M. Designing radical chemistry by visible light-promoted homolysis. Trends Chem 4, 305–317 (2022).
Cheung, K. P. S., Sarkar, S. & Gevorgyan, V. Visible light-induced transition metal catalysis. Chem. Rev. 112, 1543–1625 (2022).
Lynn, E. V. A new reaction of paraffin hydrocarbons. J. Am. Chem. Soc. 41, 368–370 (1919).
Yi, H. et al. Recent Advances in Radical C–H Activation/Radical Cross-Coupling. Chem. Rev. 117, 9016–9085 (2017).
Cao, H., Tang, X., Tang, H., Yuan, Y. & Wu, J. Photoinduced intermolecular hydrogen atom transfer reactions in organic synthesis. Chem. Catalysis 1, 523–598 (2021).
Sumida, Y. & Ohmiya, H. Direct Excitation Strategy for Radical Generation in Organic Synthesis. Chem. Soc. Rev. 50, 6320–6332 (2021).
Zheng, L. et al. Progress in Photoinduced Radical Reactions using Electron Donor-Acceptor Complexes. Asian J. Org. Chem. 10, 711–748 (2021).
Tay, N. E. S., Lehnherr, D. & Rovis, T. Photons or Electrons? A Critical Comparison of Electrochemistry and Photoredox Catalysis for Organic Synthesis. Chem. Rev. 122, 2487–2649 (2022).
Aydogan, C., Yilmaz, G., Shegiwal, A., Haddleton, D. M. & Yagci, Y. Photoinduced Controlled/Living Polymerizations. Angew. Chem. Int. Ed. 61, e202117377 (2022).
Fouassier, J. P., Allonas, X. & Burget, D. Photopolymerization reactions under visible lights: principle, mechanisms and examples of applications. Prog. Org. Coat. 47, 16–36 (2003).
Kitamura, A., Sakuragi, H., Yoshida, M. & Tokumaru, K. Photolysis of dibenzoyl peroxide under direct and singlet sensitized irradiations. mechanism for the formation of the geminate product. Bull. Chem. Soc. Jpn. 53, 1393–1398 (1980).
Savitsky, A. N., Paul, H. & Shushin, A. I. Electron Spin Polarization after Photolysis of AIBN in Solution: Initial Spatial Radical Separation. J. Phys. Chem. A. 104, 9091–9100 (2000).
Sedlacek, A. J., Mansueto, E. S. & Wight, C. A. Laser-Initiated Chain Reactions of Chlorine with Propane and Cyclopropane in Amorphous Films at 77. K. J. Am. Chem. Soc. 109, 6223–6229 (1987).
Kirsch, A. & Lüning, U. Imidyl radicals. 2. Radical Addition of N-Chlorophthalimide and N-Bromophthalimide to Alkenes. J. prakt. Chem. 340, 129–134 (1998).
Bonfield, H. E. et al. A Detailed Study of Irradiation Requirements Towards an Efficient Photochemical Wohl-Ziegler Procedure in Flow. ChemPhotoChem 2, 938–944 (2018).
Paul, H., Small, R. D. Jr & Scaiano, J. C. Hydrogen abstraction by tert-butoxy radicals. A laser photolysis and electron spin resonance study. J. Am. Chem. Soc. 100, 4520–4527 (1978).
Thelen, M. A., Felder, P., Frey, J. G. & Huber, J. R. Photodissociation of tert-butyl hypochlorite and decomposition of the tert-butoxy radical fragment. J. Phys. Chem. 97, 6220–6225 (1993).
Norrish, R. G. W. & Bamford, C. H. Photodecomposition of Aldehydes and Ketones. Nature 138, 1016–1016 (1936).
Mfuh, A. M., Doyle, J. D., Chhetri, B., Arman, H. D. & Larionov, O. V. Scalable, Metal- and Additive-Free, Photoinduced Borylation of Haloarenes and Quaternary Arylammonium Salts. J. Am. Chem. Soc. 138, 2985–2988 (2016).
Sumiyoshi, T., Schnabel, W., Henne, A. & Lechtken, P. On the photolysis of acylphosphine oxides: 1. Laser flash photolysis studies with 2,4,6-trimethylbenzoyldiphenylphosphine oxide. Polymer 26, 141–149 (1985).
Minisci, F., Vismara, E., Fontana, F. & Barbosa, M. C. N. A new general method of homolytic alkylation of protonated heteroaromatic bases by carboxylic acids and iodosobenzene diacetate. Tetrahedron Lett 30, 4569–4572 (1989).
Yukimori, D., Nagashima, Y., Wang, C., Muranaka, A. & Uchiyama, M. Quadruple borylation of terminal alkynes. J. Am. Chem. Soc. 141, 9819–9822 (2019).
Ishigaki, S., et al. Dearomative triple elementalization of quinolines driven by visible light. Nat. Commun. 14, 652 (2023).
Turro, N. J., Ramamurthy, V., Scaiano, J. C. Principles of Molecular Photochemistry–An Introduction, University Science Books, Sausalito (2009).
Harvey, N. J. Spin-forbidden reactions: computational insight into mechanisms and kinetics. WIREs Comput. Mol. Sci. 4, 1–14 (2014).
Nakajima, M. et al. A Direct S0 → Tn transition in the photoreaction of heavy-atom-containing molecules. Angew. Chem. Int. Ed. 59, 6847–6852 (2020).
Nakajima, M., Nagasawa, S., Matsumoto, K., Matsuda, Y. & Nemoto, T. Synthesis of Visible-Light–Activated Hypervalent Iodine and Photo-Oxidation under Visible Light Irradiation via a Direct S0 → Tn Transition. Chem. Pharm. Bull. 70, 235–239 (2022).
Takano, H. et al. A Theory-Driven Synthesis of Symmetric and Unsymmetric 1,2-Bis(Diphenylphosphino)Ethane Analogues via Radical Difunctionalization of Ethylene. Nat. Commun. 13, 7034 (2022).
Crim, F. F. Vibrationally mediated photodissociation: exploring excited-state surfaces and controlling decomposition pathways. Annu. Rev. Phys. Chem. 44, 397–428 (1993).
Crim, F. F. Bond-Selected Chemistry: Vibrational State Control of Photodissociation and Bimolecular Reaction. J. Phys. Chem. 100, 12725–12734 (1996).
Bar, I. & Rosenwaks, S. Controlling bond cleavage and probing intramolecular dynamics via photodissociation of rovibrationally excited molecules. Int. Rev. Phys. Chem. 20, 711–749 (2001).
Ticich, T. M., Likar, M. D., Dübal, H. ‐R., Butler, L. J. & Crim, F. F. Vibrationally mediated photodissociation of hydrogen peroxide. J. Chem. Phys. 87, 5820–5829 (1987).
Epshtein, M., Portnov, A., Rosenwaks, S. & Bar, I. Mode-specific photodissociation of vibrationally excited pyrrole. J. Chem. Phys. 134, 201104 (2011).
Lee, H. & Kim, S. K. Vibration mediated photodissociation dynamics of CH3SH: manipulation of the dynamic energy disposal into products. Phys. Chem. Chem. Phys. 22, 19713–19717 (2020).
Bingemann, D., Gorman, M. P., King, A. & Fleming Crim, F. Time-Resolved Vibrationally Mediated Photodissociation of HNO3: Watching Vibrational Energy Flow. J. Chem. Phys. 107, 661–664 (1997).
Portnov, A., Rosenwaks, S. & Bar, I. Vibrational spectroscopy and intramolecular dynamics of 1-butyne. J. Chem. Phys. 121, 5860–5867 (2004).
Roberts, G. M. et al. Probing ultrafast dynamics in photoexcited pyrrole: timescales for 1πσ* mediated H-atom elimination. Faraday Discuss 163, 95–116 (2013).
Hohenberg, P. & Kohn, W. In homogeneous electron gas. Phys. Rev. 136, B864–B872 (1964).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965).
Runge, E. & Gross, E. K. U. Density-functional theory for time-dependent systems. Phys. Rev. Lett. 52, 997–1000 (1984).
Neese, F. Prediction of molecular properties and molecular spectroscopy with density functional theory: From fundamental theory to exchange-coupling. Coord. Chem. Rev. 253, 526–563 (2009).
Lessard, J., Mondon, M. & Touchard, D. The Photochemical Addition of N-Haloamides to Olefins: The Influence of Various Factors on the Competition between 1,2-Addition and Hydrogen Abstraction. Can. J. Chem. 59, 431–450 (1981).
Lessard, J., Couture, Y., Mondon, M. & Touchard, D. The Photochemical Addition of N-Haloamides to Olefins: A Comparison of Cyclic and Acyclic N-Halo-N-Alkylamides and N-Halo-N-Acylamides (N-Haloimides). Can. J. Chem. 62, 105–112 (1984).
Song, L., Luo, S. & Cheng, J.-P. Visible-light promoted intermolecular halofunctionalization of alkenes with N-Halogen Saccharins. Org. Chem. Front. 3, 447–452 (2016).
Short, M. A., Blackburn, J. M. & Roizen, J. L. Sulfamate esters guide selective radical‐mediated chlorination of aliphatic C–H bonds. Angew. Chem. Int. Ed. 57, 296–299 (2018).
Gaspa, S. et al. Visible light-induced transformation of aldehydes to esters, carboxylic anhydrides and amides. New J. Chem. 43, 10711–10715 (2019).
Nikitas, N. F., Apostolopoulou, M. K., Skolia, E., Tsoukaki, A. & Kokotos, C. G. Photochemical Activation of Aromatic Aldehydes: Synthesis of Amides, Hydroxamic Acids and Esters. Chem. Eur. J. 27, 7915–7922 (2021).
Xiang, M. et al. Visible Light-Catalyzed Benzylic C–H Bond Chlorination by a Combination of Organic Dye (Acr+‐Mes) and N‐Chlorosuccinimide. J. Org. Chem. 85, 9080–9087 (2020).
Rogers, D. A. et al. U.S. Food and Drug Administration-Certified Food Dyes as Organocatalysts in the Visible Light-Promoted Chlorination of Aromatics and Heteroaromatics. ACS Omega 5, 7693–7704 (2020).
Zhu, N., Lia, Y. & Bao, H. Metal-free intermolecular aminochlorination of unactivated alkenes. Org. Chem. Front. 5, 1303–1307 (2018).
Liang, Y., Zhao, Z., Taya, A. & Shibata, N. Acyl Fluorides from Carboxylic Acids, Aldehydes, or Alcohols under Oxidative Fluorination. Org. Lett. 23, 847–852 (2021).
Walling, C. & Thaler, W. Positive Halogen Compounds. III. Allylic Chlorination with t-Butyl Hypochlorite The Stereochemistry of Allylic Radicals. J. Am. Chem. Soc. 83, 3877–3884 (1961).
Omary, M. A. & Patterson, H. H. Luminescence, Theory. Encyclopedia of Spectroscopy and Spectrometry (Third Edition), 636–653 (2017).
Acknowledgements
The authors are grateful for Grants-in-Aid for Scientific Research (Nos. JP23K26649/JP22H05346/ JP24H01067/JP24H01839 to Y.N., and Nos. JP22H00320/JP22H05125 to M.U.) from JSPS (Japan), as well as grants from JST FOREST (No. JPMJFR221Y) (to Y.N.), JST CREST (No. JPMJCR19R2) (to M.U.), NAGASE Science Technology Foundation, Naito Foundation, Chugai Foundation, (to M.U.), UBE Foundation, The Asahi Glass Foundation, Tokyo Tech Challenging Research Award (to Y.N.), and Uehara Memorial Foundation (to Y.N. and M.U.). A generous allotment of computational resources from TSUBAME (Tokyo Institute of Technology) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
A.J.A.D. and Y.N. conceived and designed the project. A.J.A.D. conducted the experiments. A.J.A.D., Y.N., A.M., and M.U. performed computational studies. A.J.A.D., Y.N., A.M., and M.U. performed the spectroscopic analysis. A.J.A.D. wrote a draft manuscript. Y.N. and K.T. directed the project and wrote the manuscript. All authors participated in data analyses and discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Araujo Dias, A.J., Muranaka, A., Uchiyama, M. et al. Vibration-mediated long-wavelength photolysis of electronegative bonds beyond S0–S1 and S0–T1 transitions. Commun Chem 7, 126 (2024). https://doi.org/10.1038/s42004-024-01208-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-024-01208-0