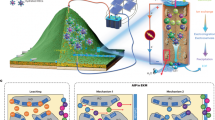
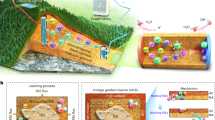
Abstract
The rare earth elements are critically important for a wide range of modern technologies. However, obtaining them selectively and efficiently from natural sources and recycled materials is challenging and often requires harsh or wasteful conditions. Here we show that a macrocyclic chelator appended to a solid resin can overcome this challenge by acting as a robust platform for both the extraction and separation of these elements. This resin preferably captures the large rare earth elements in mixtures of these ions, giving rise to higher extraction efficiencies for them over the smaller ions. We further demonstrate that this resin can be used to separate rare earth elements. As a proof-of-principle validation, this resin was demonstrated to selectively extract rare earth elements in the presence of many different types of competing metal ions in a bioleachate solution obtained from autoslag waste, leading to their enrichment.

Similar content being viewed by others
Introduction
The unique properties of the rare earth elements (REEs) make them critically important for many applications including magnets, electrical devices, and optical displays1,2,3,4,5. To meet the demand for these elements, global REE mining has reached approximately 350,000 tons of rare earth oxides as of 2023, according to the latest Mineral Commodity Summaries released by United States Geological Survey (USGS)6, and this number is predicted to rise in the coming years. In this context, global minable REE reserves are projected to last 60–100 years at the current production rates6,7,8. The increasing demand for these metals coupled with the limited minable reserves of REEs has led to significant challenges in securing a stable and sustainable supply chain. One potential solution is to substitute REEs with other materials, such as ferrite-based magnets for neodymium (Nd)-based magnets, but in many cases this approach leads to less effective materials9,10,11. An alternative strategy to meet the urgent need for REEs is to recover them from end-of-life waste materials, arising from consumer electronics, electric vehicles, and various catalysts12,13,14,15,16. This recycling strategy alleviates the need for mining, which also solves the problem of generating radioactive waste comprised of thorium and uranium that naturally reside in REE mineral deposits17,18,19. Therefore, the development of efficient REE extraction and separation strategies from these end-of-life materials is of significant importance.
The major challenge associated with the separation of REEs originates from their chemical similarity20,21. The most stable oxidation state for all REEs is +3, and these ions all attain high coordination numbers (8 to 10) and preferably bind to chemically hard donor atoms like oxygen22,23. Consequently, much research has been devoted towards the development of extractants based on organophosphonates and carboxylates that can be applied for the liquid-liquid extraction (LLE) separation of the REEs, an approach that leverages the decreasing ionic radii of lanthanides (Ln) across the series24,25,26,27,28. However, the ionic radii change modestly, by about 0.01 Å between adjacent Ln3+ and maximally 0.17 Å between La3+ and Lu3+, thereby giving rise to low separation efficiencies via conventional LLE. For example, the use of Cyanex 572 (Scheme 1) can only separate Lu3+ and Yb3+ with a separation factor (SF) of 1.42 in a single stage. The enrichment of Lu3+ from 7.1% to 78.8% requires 7 sequential extraction and striping processes29. Such a multi-stage process not only increases the separation cost and operational complexity, but also leads to environmental concerns and safety risks because of the consumption of large quantities of flammable solvents, like kerosene, and toxic organic extractants30,31.
As an alternative separation strategy, selective precipitation of REEs has emerged as a promising approach because of its technical simplicity and low-energy requirement. This method leverages the solubility differences of metal ions usually augmented via the addition of additives like ligands or counterions, to differentially convert them into the solid-state. To date, several promising precipitants for REE separations have been reported. For example, an amide-functionalized hexa-substituted arene (Scheme 1) can trigger the selective precipitation of light REEs at a toluene-HNO3 interface, leading to SFs of 248 for La/Eu and 63 for La/Sm32. Using this strategy, near quantitative recovery of Nd/Pr directly from acidic magnet scrap leachate without pH adjustment and pretreatment was accomplished33. A Cu2+ complex of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetra(methylene phosphonic acid) (DOTP, Scheme 1) was shown to selectively separate Lu3+ from a La3+/Lu3+ mixture solution via forming a dark blue heterometallic Lu–(Cu–DOTP) solid34. Some polymerizable metal–organic cages can also selectively bind smaller rare-earth ions over larger ones to achieve their separation35. Several of these systems, however, still suffer from some problems, such as the need for complex ligands, inability to operate in more complex mixtures containing other metal ions, and the need for toxic organic solvents.
Another strategy is solid-phase extraction, which has proven to be an effective method for providing highly efficient, environmentally benign, and reusable REE separation. In a conventional solid-phase extraction, the feedstock containing different metal ions is passed through a column, which is filled with solid adsorbents36,37,38. As the solution is passed through the column, the metal ions are captured by the functional metal-binding groups on these adsorbents and can be sequentially stripped with dilute acid or chelator solutions. After desorption of the metal ions, the column could be used for subsequent extractions and separations. For the REE series, however, commercially available solid materials, like resins, do not afford good separations, unless supplemented with additional reagents in the mobile phase, due to the poor selectivity of the binding groups on their surfaces. For example, α-hydroxyisobutyric acid (α-HIBA, Scheme 1) as an eluent is often required to facilitate their separation with T-42MP cation exchange resin39. Functionalizing these solid matrices with more sophisticated metal-binding groups can also lead to better adsorbents with enhanced REE extraction and separation capabilities. For example, the immobilization of the Ln3+-binding protein lanmodulin on agarose afforded a solid support that could achieve a separation of binary Nd/Y and Nd/Dy pairs40. In addition, cryptand-2,2,2 (Scheme 1) appended on NovaPEG resins can selectively capture electrochemically reduced divalent Eu2+ from a mixture of Eu2+ and Gd3+ 41. Amberlite XAD-4 modified with monoaza dibenzo18-crown-6 ether was also used for the preconcentration and separation of La3+, Nd3+, and Sm3+ in a synthetic solution42. These prior studies show the promise of this approach, which can potentially be optimized via investigations of alternative metal-binding groups with better properties for REE discrimination and extraction.
Macrocyclic ligands based on diaza 18-crown-6 have been studied extensively within f-block coordination chemistry and would be good candidates for adsorption onto solid-state supports for this application. These ligands are promising because of their ability to rapidly form stable complexes with the Ln3+ ions, a property that has led to their use in medicine43,44,45,46,47,48,49,50,51,52,53,54. A unique feature of this chelator class is its reverse-size selectivity, a binding preference for early large REEs over late small REEs. This property is exemplified by macropa (Scheme 1), for which the thermodynamic stability constants of the Ln3+ complexes differ by 7-log units across the series55. This large difference should be considered in the context of conventional chelators, like DOTA and cryptates56,57, which preferably bind smaller REEs and have stability constants only spanning 2–3 log units across the series. Accordingly, chelators from the macropa family have been used for the separation of REEs and actinides. Using macropa as an aqueous complexant, solvent extraction studies have led to effective separations of these f-elements58,59,60. In another recent study, the macrocyclic chelator G-macropa could selectively complex Nd3+ in aqueous solution, allowing Dy3+ to be precipitated cleanly as a carbonate salt from a mixture of these two ions61. Another feature of these chelators that makes them suitable for REE extraction is their relatively low affinity for smaller ions, like Mg2+, Al3+, and Ca2+, which are commonly found in mining solutions and electronic waste62.
Leveraging the promising REE-binding abilities of the macropa family, this work presents a solid-state resin that is functionalized with the bifunctional chelator NH2-BZmacropa through a covalent linkage. The resulting resin, BZmacropa-XAD, retains the reverse-size selectivity of the ligand, and was investigated for its ability to extract and separate REEs using batch experiments and fixed-column experiments. We show that this material is highly effective and selective for adsorbing light REEs selectively in the presence of heavy REEs, even from a complex matrix like bioleachate (a solution of mixed metal ions derived from bioleaching a solid REE-containing feedstock), and can be regenerated for subsequent use. These features establish BZmacropa-XAD to be a valuable material for the recycling and recovery of REEs.
Results and discussion
Chelator synthesis
To make macropa-functionalized resin for REE extraction and separation, a strategy was required to covalently link the chelator onto a solid resin. The solid resin we chose was XAD-4, which contains aromatic phenyl groups that could be modified to contain electrophilic isothiocyanate moieties. To form a stable thiourea linkage on this resin, the chelator needs a terminal amine functional group. For this purpose, we chose the BZmacropa chelator, which we previously showed to be amenable for functionalization with an NH2 group to form NH2-BZmacropa (Scheme 1). Both chelators were synthesized using the reported procedures established by our group (Supplementary Note 1, Schemes S1 and S2)45. BZmacropa, NH2-BZmacropa, and all synthetic intermediates were fully characterized by NMR spectroscopy, electrospray ionization mass spectrometry (ESI-MS) or direct analysis in real-time mass spectrometry (DART-MS), high-performance liquid chromatography (HPLC), and elemental analysis (EA) (Figs. S1–S16).
Solution thermodynamics
Previously, the quantitative binding affinity of BZmacropa was only studied with La3+ 45. Because BZmacropa forms the basis for our functionalized resin, we sought to evaluate its relative efficacy for binding a larger subset of the REEs. For this purpose, pH potentiometric titrations over a pH range of 2.5–11.3 in 0.1 M KCl were carried out to determine its protonation constants (Ka) and stability constants for its La3+, Ce3+, Pr3+, Nd3+, Dy3+, and Lu3+ complexes (KLnL, Supplementary Note 2). These constants, Ka and KLnL, which are defined in Eqs. S1 and S2, are collected in Fig. 1 and Table 1, along with those from the related chelators CHX-macropa and macropa. The four protonation constants of BZmacropa measured in this study are consistent with our previous report45, rather than an earlier investigation of this ligand that used 0.1 M KNO3 as a supporting electrolyte for potentiometric titrations63. The first two protonation constants (log Ka1 = 7.10 and log Ka2 = 6.51) correspond to the sequential protonation of tertiary amines within the macrocycle, and their sum can be used to quantify the basicity of the macrocycle. This value for BZmacropa is 0.65 and 1.1 log units lower than those of macropa and CHX-macropa, respectively. The lower basicity of BZmacropa can be attributed to the electron-withdrawing effect of the phenyl groups, which is embedded in the macrocycle. The third and fourth protonation constants (log Ka3 and log Ka4) are associated with the protonation of the pendent picolinate arms. These values are similar across the three compounds, suggesting that the rigid phenyl and cyclohexyl groups in BZmacropa and CHX-macropa primarily affect the basicity of the macrocycle, without significantly influencing the basicity of the pendent donor arms.
With the protonation constants of BZmacropa in hand, the Ln3+ stability constants (log KLnL) could be measured via pH potentiometric titration using metal-to-ligand molar ratios of 1.0 to 0.95 (Figs. S17–S23). For these studies, La3+, Ce3+, Pr3+, Nd3+, Dy3+, and Lu3+ were investigated as representative light, middle, and heavy REEs. As shown in Fig. 1 and Table 1, BZmacropa preferably binds to the light REEs (log KLnL = 13.45–12.62 for La3+ to Nd3+), over the middle Dy3+ (log KDyL = 9.89) and heavy Lu3+ (log KLuL = 6.69), indicating that this macrocyclic chelator retains the reverse-size selectivity properties of other chelators based on the diaza 18-crown-6 core. Compared to macropa and CHX-macropa, BZmacropa binds to REEs more weakly, as characterized by log KLnL values that are approximately 1–2 log units smaller. For the heaviest REE Lu3+, BZmacropa forms a complex with identical stability to that of CHX-macropa (log KLuL = 6.69 and 6.70, respectively). Although BZmacropa possesses a lower binding affinity for light La3+ and middle Dy3+ than CHX-macropa, the relative differences in these values between La3+ complexes and that of Dy3+ or Lu3+ (Δlog K = 3.56 and 3.20) indicate that BZmacropa still has a strong potential for separations.
Bifunctional NH2-BZmacropa and its coordination chemistry with La3+
With the parent chelator BZmacropa and its REE complexes characterized, its bifunctional derivative NH2-BZmacropa (Scheme 1), which can be conjugated to a solid support, was synthesized via previous procedures45. This compound was characterized by standard methods, including NMR spectroscopy, analytical HPLC, ESI-MS, and EA to confirm that it is isolated as a 3H2O·5HCl adduct with a purity of 96.5% (Figs. S14–S16).
To investigate the effects of the additional amine group on NH2-BZmacropa, its coordination chemistry with La3+ was investigated using single-crystal X-ray diffraction. Suitable crystals of the La3+ complex of NH2-BZmacropa were obtained after anion metathesis of a 1.3:1 mixture of LaCl3 and NH2-BZmacropa with KPF6 at neutral pH (Supplementary Note 3 and Supplementary Data 1). The X-ray crystal structure of one of the two [La(NH₂-BZmacropa)(H2O)]+ cations present in the asymmetric unit is shown in Fig. 2. This structure is analogous to that of [La(BZmacropa)(H2O)]PF6 (Tables S1 and S2). In both complexes, the La3+ ion sits above the plane of O donors in the macrocycle (O13–O16), forming an 11-coordinate geometry, where 10 donor atoms are provided by the macrocyclic chelator, and the 11th donor is contributed by an inner-sphere water molecule. Notably, the interatomic distances between the La3+ and the macrocycle donor atoms within [La(NH2-BZmacropa)(H2O)]+ and [La(BZmacropa)(H2O)]+ are similar, and form near linear N–La–N angles (174.63° and 172.41°, Table S2), demonstrating that the introduced NH2 does not alter the coordination properties of this ligand.
Thermal ellipsoids are depicted at the 50% probability level. For clarity, outer-sphere solvent molecules, counter-anions, and hydrogen atoms attached to carbon centers have been omitted. The color scheme used is as follows: La (navy blue), O (red), N (blue), and C (gray). Only one of the two molecules present in the asymmetric unit is shown.
NH2-BZmacropa immobilization onto NCS-XAD resin
With the coordination chemistry of NH2-BZmacropa studied, an approach to covalently attach it to resin was needed. To address this challenge, an amine-reactive isothiocyanate-containing resin, called NCS-XAD resin, was synthesized. The synthesis of this resin commenced from commercially available XAD-4 resin (Supplementary Note 3 and Scheme S3), which contains aromatic phenyl group side chains. The phenyl groups were nitrated, and the nitro groups were then reduced to amines and converted to isothiocyanates with thiophosgene (Figure S24). The successful introduction of –NO2, –NH2, and –NCS groups on XAD-4 resin in each step was confirmed by Fourier transform infrared spectroscopy (FT-IR, Fig. S25). Treating NCS-XAD resin with NH2-BZmacropa in 100 mM MOPS buffer of pH 7.4 at room temperature resulted in the formation of a thiourea linkage that immobilized the BZmacropa ligand on the solid resin (Scheme 2). This conjugation approach has been frequently used to modify resins and form chelator-antibody conjugates41,45,49. The successful loading of NH2-BZmacropa on the resin was confirmed and determined by UV–vis spectroscopy to be 182 ± 13 μmol ligand g−1 resin (Fig. S26). This loading density is substantially greater than those of other recently reported REE-extracting resins that are functionalized with metal-binding proteins via click chemistry and peptides via amide coupling40,64, indicating that this conjugation strategy is highly effective.
REE extraction and separation
With the BZmacropa-XAD resin formed, its ability to capture individual REEs was investigated using batch adsorption experiments at different pH levels. La3+, Dy3+, and Lu3+ were chosen to represent light, middle, and heavy REEs, respectively. The extraction procedure is described in the Supporting Information, and the metal ion concentrations were determined via inductively coupled plasma mass spectrometry (ICP-MS). The extraction capacities (Qe), the molar quantity of a metal ion per mass of resin after reaching adsorption equilibrium, were calculated with the equation in Methods and are shown in Fig. 3. The efficacy of adsorption of these Ln3+ depends on the pH. At pH 3, their Qe values are 19.5, 13.5, and 11.4 µmol g−1 for La3+, Dy3+, and Lu3+, respectively (Fig. 3a). These values are relatively low with only small differences between the individual REEs. This poor Ln3+ adsorption and ability to discriminate these REEs at this pH is expected because BZmacropa will be protonated under this condition, diminishing metal complexation. As the pH is increased to 4, the Qe increases to 67.0, 43.5, and 27.1 µmol g−1 for La3+, Dy3+, and Lu3+, respectively. These values also reflect the preference of the BZmacropa chelator for binding light over heavy REEs. At pH 5, the adsorption capacity of BZmacropa-XAD is 121.1 µmol g−1 (16.8 mg g−1) for La3+, nearly double that of Dy3+ (67.0 µmol g−1 or 10.9 mg g−1) and triple that of Lu3+ (41.2 μmol g−1 or 7.2 mg g−1). These Qe values measured under these higher pH conditions strongly reflect the Ln3+ binding affinity trend of the free chelator BZmacropa in solution, indicating that its immobilization on solid resins does not substantially alter its reverse-size selectivity. As a control, the adsorption of these three REEs on unmodified NCS-XAD resin was measured. As expected, the lack of an appended chelator or metal-binding group affords very small and similar adsorption capacities (5.0–6.1 µmol g−1) for all three Ln3+ even at pH 5 (Fig. 3b). Thus, the immobilized NH2-BZmacropa group on BZmacropa-XAD resin drives its selectivity and Ln3+ adsorption performance. Under the optimal pH 5 conditions, La3+, Dy3+, and Lu3+ only occupy 66%, 47%, and 23%, respectively, of the chelators on BZmacropa-XAD resin. Thus, quantitative adsorption capacity was not achieved. This phenomenon has also been observed by others when attaching chelators to solid supports in the development of metal-extracting materials64,65,66. This limitation may result from the changes of the resin’s surface area, as confirmed by N2 adsorption experiments, which showed a surface area of 816 m2 g−1 for the XAD-4 resin and a smaller surface area of 192 m2 g−1 for BZmacropa-XAD resin (Fig. S27).
The adsorption of La3+ and Dy3+ on the resin was confirmed by X-ray photoelectron spectroscopy (XPS). After treatment of the resin with solutions of these two ions, the XPS showed characteristic peaks at 839 and 853 eV for the La 3d transition and 1297 and 1333 eV for the Dy 3d transition (Fig. S28). By contrast, resin treated with Lu3+ did not display any of the characteristic transitions of this element, because of its comparatively low adsorption capacity on the BZmacropa-XAD resin. In addition, scanning electron microscopy (SEM) analysis of the BZmacropa-XAD resin showed morphological changes upon adsorption of the Ln3+ ions. BZmacropa-XAD resin has a lamellar structure (Fig. S30a), which becomes more compact upon adsorption of La3+ (Fig. S30b). This effect is less pronounced for Dy3+ and Lu3+ compared to La3+ (Fig. S30c, d), which is consistent with the lower adsorption capacities of the resin for these two Ln3+ ions. Adsorption kinetics studies indicated that equilibrium was reached after 18 h (Fig. 4a). The pseudo-second-order rate law was used to model these adsorption kinetics. This analysis shows that the smaller Ln3+ adsorb slightly more rapidly than La3+, but have substantially poorer overall adsorption capacities (Table S3). Further studies at higher pH values were prevented by the hydrolysis of Ln3+ at pH > 567.
a Plot of Ln3+ adsorption on the resin (Qt in µmol g-1) versus incubation time, fit using the integrated pseudo-second-order adsorption rate law for La3+, Dy3+, and Lu3+ at pH 5. Adsorption conditions: 30 mg resin, [Ln3+] = 600 µM, pH 5 (100 mM ammonium acetate buffer), V = 10 mL, T = 22 ± 1 °C. b La3+ over 6 adsorption/desorption cycles with 0.1 M HCl as eluent. Adsorption condition: 30 mg resin, [Ln3+] = 600 µM, pH 5 (100 mM ammonium acetate buffer), V = 10 mL, T = 22 ± 1 °C, and t = 24 h; Desorption condition: V = 10 mL, T = 22 ± 1 °C, and t = 4 h.
Table 2 displays Ln3+ adsorption conditions and data for a series of resins in addition to BZmacropa-XAD resin. As is apparent from these data, BZmacropa-XAD resin has a higher Qe for Ln3+ compared to the two other related macrocycle-containing resins with a cryptand and a monoaza dibenzo18-crown-6 chelator. Moreover, a more significant difference in the Qe values for light and middle Ln3+ (5.9 mg g−1 between La3+ and Dy3+) was also observed for the BZmacropa-XAD resin compared to that of the dibenzo18-crown-6 (0.9 mg g−1 between La3+ and Sm3+) resin, reflecting the importance of the pendent picolinate groups of the former. Although commercial resins modified with small functional groups, like the SP, SA, Amberlite IRC86, Tulsion CH-93, and TK221 resins, showed larger Qe, these resins also exhibit high adsorption for other metal ions like Ni2+ 68, Cd3+ 69, Ca2+, and Mg2+70, which are common entities in REE waste streams. The REE extraction properties of phosphonate-containing materials, such as BPG, Tulsion CH-93, and poly(DETA)-ν-ECH-MP resins, are quite different. BPG and Tulsion CH-93 resin both show poorer REE extraction compared to BZmacropa-XAD resin. The significantly large REE extraction of netPoly(DETA)-ν-ECH-MP might be a result of its high phosphonate density. However, the required equilibration time of 72 h for this resin could limit its applications71. In comparison to all these other resins, BZmacropa-XAD resin is advantageous with respect to its balance of adsorption capacity, selectivity, and adsorption kinetics.
The pH-dependent adsorption of the REEs suggested that acids could effectively desorb the attached REEs from the BZmacropa-XAD resin. This hypothesis was explored by first saturating the resin with La3+, and then mixing it with 0.1 M HCl (10 mL) for 4 h at room temperature. Under these conditions, >97% of the La3+ was desorbed from the resin (Fig. 4b and Table S4). Consistent with the desorption of this ion, analysis of the remaining resin with XPS showed the disappearance of the La 3d doublet peaks (Fig. S29), and SEM revealed the resin to return to its lamellar structure (Fig. S30e). After desorption, the resin was treated with aqueous NaOH (1 M) to neutralize and regenerate it for subsequent use. The regenerated BZmacropa-XAD resin was then employed for multiple La3+ adsorption/desorption cycles under the same conditions. BZmacropa-XAD resin maintains its integrity for at least 6 consecutive adsorption/desorption cycles, as reflected by a negligible reduction in adsorption performance throughout this process (Fig. 4).
Having validated BZmacropa-XAD resin with individual REE adsorption studies, we next investigated its properties when operating in a solution containing a mixture of 16 REE ions at equal concentration (50 µM). The unmodified resin, NCS-XAD, was also tested as a control. As expected, NCS-XAD resin fails to appreciably extract any of the REEs under these conditions (Fig. 5 and Table S5). For BZmacropa-XAD resin, the light REEs are extracted the most effectively with extraction efficiencies for La3+–Nd3+ ranging between 69.3–54.7% (Fig. S31). The extraction efficiency gradually decreases across the REE series, with less efficient extraction of the middle REEs Sm3+–Dy3+ (45.1–28.3%, Fig. S31), and very poor extraction of the heavy REEs (Ho3+–Lu3+ and Sc3+, 24.5–6.7%). Thus, the reverse-size selectivity of BZmacropa enables the use of BZmacropa-XAD resin as an efficient and selective solid-phase adsorbent for light REEs (La3+–Nd3+). For comparison, lanmodulin immobilized on agarose was shown to equally extract the REEs as Nd3+, Y3+, Dy3+, Lu3+, and La3+40,72. Although diglycolamide-based materials typically exhibit a greater selectivity for heavy over light REEs73, the diglycolamide- and carbomoyl methyl phosphine oxide-functionalized TK221 resin also exhibited higher adsorption capacity for light REEs (43.1 and 45.2 mg g−1 resin for Ce3+ and Nd3+ respectively) over the heavy REEs (35.1 and 15.6 mg g−1 resin for Er3+ and Y3+ respectively) in individual REE extractions, but these studies were not carried out in metal ion mixtures74.
A solution of the REE ions at equal concentration (50 µM) was treated with the resins, and the concentrations of Ln3+ ions remaining in solution after contact with the resin are reported. Adsorption condition: 30 mg resin, [Ln3+]total = 800 µM, pH 5 (100 mM ammonium acetate), V = 7 mL, T = 22 ± 1 °C, and t = 24 h.
On-column separation of binary REE mixtures
We next investigated the application of BZmacropa-XAD resin for the column chromatographic separation of binary mixtures of La3+/Dy3+ and La3+/Lu3+. For these studies, a fixed-bed column was loaded with 1.5 g BZmacropa-XAD resin, and 1000 µM equimolar solutions of La3+ with either Dy3+ or Lu3+ were passed through the column at a flow rate of 0.2 mL/min, which was controlled by a syringe pump (Fig. S32). The fractions eluted from the column were collected in 1 mL portions, and the REE concentrations within them were determined by ICP-optical emission spectroscopy (OES) or ICP-MS (Tables S5–S11). For the first 5 mL of eluate of the La3+/Dy3+ mixture, BZmacropa-XAD resin extracted >98% La3+ and 90% Dy3+ from their mixture (Fig. 6a). Remarkably, highly selective adsorption of La3+ over Dy3+ was observed beyond the first 5 mL fractions. Between the 6 mL fraction and the 14 mL fraction, the concentration of Dy3+ in the eluate increased from 110 µM to 960 µM, corresponding to a drop in Dy3+ adsorption efficiency from 88.7% to 3.8%. By contrast, La3+ was stably captured by BZmacropa-XAD resin effectively, maintaining 88% adsorption efficiency up until 20 mL of eluate flowed through. Between 15 and 20 mL, the Dy3+ adsorption reached equilibrium, leading to the overall composition of the eluate to be 84% Dy3+ and 16% La3+ (Fig. S33). For the La3+/Lu3+ mixture, only 82% Lu3+ is adsorbed within the first fraction. This value continuously decreases to 8.7% upon the elution of 17 mL and reaches equilibrium (Fig. 6c). On the contrary, the resin adsorbed >99% of the La3+ ions over the entire process, resulting in only Lu3+ being detected in all 20 eluate fractions (Fig. S36). Consequently, an overall Lu3+ purity in the eluate was more than 99%. This separation efficiency can be explained by the significantly poorer affinity of BZmacropa for Lu3+ compared to La3+.
a La3+/Dy3+ and c La3+/Lu3+ mixture adsorption profiles. Adsorption condition: 1.5 g resin loaded in column, [La3+] = [Dy3+] = [Lu3+] = 1000 µM, V = 20 mL, pH 5 (100 mM ammonium acetate buffer), flow rate = 0.2 mL/min, T = 22 ± 1 °C. b La3+/Dy3+ and d La3+/Lu3+ mixture two-step pH-based desorption profiles. Desorption condition: pH 1.8 HCl was used for fractions 1–20 and 0.1 M HCl was used for fractions 21–40, flow rate = 0.2 mL/min, T = 22 ± 1 °C.
To recover the adsorbed La3+ and to further separate it from the small quantities of Dy3+ or Lu3+, we performed a stepwise desorption scheme by applying two eluents at different pH values. The use of a HCl solution of pH 1.8 as an eluant stripped 77.8% of the Dy3+ with a purity of 78.5% (Figs. 6b and S34), and 94.3% Lu3+ with a purity of 85% from the Ln-loaded column in the first desorption (Figs. 6d and S37), respectively. The second-step treatment of the columns with a pH 1 solution (0.1 M HCl) led to an elution of 81.2% and 66.6% La3+ from these two columns, corresponding to an overall purity of 97.3% and 91.9% La3+, respectively (Figs. S35 and S38). The greater retention of La3+ on the BZmacropa-XAD resin, which requires higher HCl concentrations for desorption, is a consequence of the reverse-size selectivity of the BZmacropa chelator. Although the La3+ adsorption and desorption profiles in Fig. 6 are similar, there are slight differences in these profiles depending on whether a binary La3+/Lu3+ (Fig. 6c, d) or La3+/Dy3+ (Fig. 6a, b) mixture was used. We hypothesize that these discrepancies are due to slight variations in the resin as prepared from different batches. To probe the mechanical strength of the BZmacropa resin over extended time periods of use, it was subjected to continuous flow through with water at a flow rate of 1 mL min−1 for 5 days. The resin morphology was assessed before and after this treatment via SEM. These data show only minimal morphological changes in the resin after this continuous water flow (Fig. S30f), indicating that this resin is sufficiently robust for column-based separations.
REE extraction from slag bioleachate
Based on the efficacy of BZmacropa-XAD resin for ideal solutions of REEs, we next investigated the ability of this resin to extract REEs from a more complex mixture. A mixture of ions, resulting from bioleaching REEs from smelting slags produced by the recycling of precious metals from catalytic converters, was used for this study. This bioleachate was provided by REEgen, a start-up company that recovers REEs from a variety of recycled and waste materials. REEgen produced the bioleachate by mixing the slag with their biologically derived leaching solution called a biolixiviant. This biolixiviant consists primarily of organic acids generated by Gluconobacter oxydans. G. oxydans converts glucose to organic acids and secretes other factors into solution that contribute to the solubilization of REEs. Biolixiviant from G. oxydans leaches REE more efficiently than a solution with an equivalent amount of organic acids75. The biolixiviant for this work was produced from wild-type G. oxydans in a bioreactor with a proprietary set of optimized parameters. Bioleaching of REEs with G. oxydans biolixiviant presents a nonhazardous and low-impact approach for initial processing of REE feedstocks that does not require strong oxidizing acids like HNO3. The bioleachate was diluted by a factor of 10 with water for these extraction experiments, and the metal ion concentrations in this 10-fold-diluted solution were determined via ICP-OES (Table S12). As these data show, this slag bioleachate contains a wide range of different metal ions, including alkaline earth metals, transition metals, and REEs. The most abundant REEs within this mixture are the light REEs La, Ce, Pr, and Nd. Among these, Ce is the most abundant with a concentration of 1109 µM, followed by La (164 µM), Nd (78.4 µM), and Pr (43.5 µM). In addition to these REEs, the concentrations of the other metal ions within this diluted bioleachate matrix spans a range of over 4 orders of magnitude (from 9.2 µM of Sr to 15.7 mM of Al). Among these, Mg, Al, Ca, and Fe are present in the highest concentrations of 4.2, 15.7, 2.2, and 3.2 mM, respectively.
To isolate REEs from the bioleachate, we used BZmacropa-XAD resin via both batch extraction and on-column adsorption experiments. For the batch experiment, 30 mg BZmacropa-XAD resin was added to 1 mL of the bioleachate and allowed to incubate at room temperature for 24 h, prior to measuring the metal concentration in the supernatant via ICP-OES. As shown in Figs. 7a and S39, 91% La, 89% Ce, 86% Pr, and 45% Nd were adsorbed onto the BZmacropa-XAD resin. The non-REEs, except Fe, are extracted much less efficiently with only 9.9–24.4% of them adsorbed to the resin. Fe, however, is adsorbed with 65.8% efficiency. The apparent adsorption of Fe3+ might be a consequence of its poor solubility, which would result in the formation of insoluble or colloidal Fe2O3 that attached to the resin via physical interactions. The tendency for Fe to adsorb strongly to resins from these complex mixtures has also been noted by others in an experiment involving the use of a lanmodulin-coated agarose40, as well as other resin types76,77. To quantify the selectivity of the resin, the ratio of the initial concentration of a metal ion in the leachate (Cinitial) divided by the final concentration of metal ion in the solution after extraction (Cresidual) was calculated. As shown in Fig. 7b, the Cinitial/Cresidual values show excellent selectivity for the light REEs, compared to the heavier REEs and other metal ions in solution. Furthermore, REE selectivity scales with their ionic radii, consistent with the solution thermodynamics of the BZmacropa chelator. Under the conditions of these extraction experiments, a quantity of 30 mg of resin was verified to be optimal (Fig. S40 and Table S12). Smaller amounts of resin, like 20 mg, were not sufficient to fully extract the REEs from the bioleachate, whereas the use of 60 mg of resin did not lead to substantial improvements in extraction efficiency that would not justify the use of larger quantities.
Following these batch adsorption studies, BZmacropa-XAD resin was loaded into a column, and 10 mL of the slag bioleachate was passed through at a flow rate of 0.2 mL min−1 with the setup shown in Fig. S29. The eluted fractions were collected in 1 mL intervals, and the concentrations of metal ions were measured by ICP-OES. Fig. 8 and Tables S13 indicate that this column extraction approach is highly selective for the REEs. The REEs are absorbed to a much larger extent than the non-REEs, with the exception of Fe. For example, the extraction efficiency of the lightest La3+ ranges from 82.6% in the first fraction to 65.7% in the last fraction (Fig. 8a), resulting in an overall extraction efficiency (percentage of the total metals adsorbed in all fractions relative to initial feed) of 70.2% (Fig. 8b). By contrast, the overall extraction percentages for all other metal ions, except Fe, are limited to a mere 29.2 to 39.1%, even though their initial concentrations are 13 to 96 times greater than that of La3+ in this bioleachate. For the other three REEs, Ce, Pr, and Nd, BZmacropa-XAD resin was also effective, giving rise to extraction efficiencies approaching 80% in the first fraction. Subsequently, 0.01 M HCl (15 mL) was pumped into the column at the same flow rate to strip the small quantity of non-REEs adsorbed on the column. This elution is highly selective for non-REEs, because BZmacropa has a much stronger affinity for REEs over the non-REEs. As shown in Fig. 8c and Table S14, the desorption efficiency of non-REEs (13.4–32.5%) is 1 order magnitude higher than that of REEs (2.6–6.6%). Once the non-REEs were desorbed from the BZmacropa-XAD resin column, the REEs could be efficiently stripped with a solution of 0.1 M HCl, allowing their recovery. Notably, the molar ratios between total quantity of REEs (ƩREEs) and total quantity of non-REEs (Ʃnon-REEs) increased from 0.05 in the feedstock to 0.32 in the eluant (Fig. 8d), with the highest molar ratios of 0.36 being observed in the fourth eluted fraction. As a result, the overall molar percentage of REEs upgrades from 5.2% to 23.3% (Fig. 9 and Table S15). A near 5-fold enrichment was achieved for all REEs, while the molar percentages of Mg, Al, and Ca reduce to 2.16%, 24.9%, and 2.72% from initial 14.8%, 56.0%, and 7.62%, respectively, demonstrating that BZmacopa-XAD resin can be used to enrich REEs from complex mixtures.
a Extraction efficiency of each adsorption fraction (A1–A10); b Overall adsorption efficiency of all metal ions; c The desorption efficiency using 0.01 M HCl of each metal relative to the metal amount adsorbed on the BZmacropa-XAD column. d The molar ratios between the total quantity REEs and total quantity of non-REEs in each desorption fraction (Ʃ is the total REE or non-REE amount in each fraction of eluent and initial feed). Condition: mresin = 1.5 g, 10 mL leachate, pH 5.2, 1 fraction = 1 mL, Flow rate: 0.2 mL min−1. 0.1 M HCl was used as eluent, T = 22 ± 1 °C.
As a final purification, the REEs can be separated from the dominant non-REE Fe via precipitation with oxalate (C2O42−). Fe2(C2O4)3 (Ksp = 1.5 × 10−9) is much more soluble than RE2(C2O4)3 (Ksp = 10−29–10−32)78. Thus, oxalate was added to the final desorbed REE solution and was filtered to remove insoluble oxalate salts after 12 h. The metal ion concentrations in the filtrate were determined via ICP-OES (Table S17). The molar percentage of the REEs was enriched to 57.1% in the solid-state product (Figs. 9 and S41), which corresponds to an 81.2 wt% of REEs.
Conclusions
We have developed a resin modified by the macrocyclic chelator BZmacropa. This resin leverages the solution-phase high affinity of BZmacropa for the light REEs to achieve efficient extractions and separations of these metal ions in batch adsorption and on-column extraction experiments. This resin is advantageous over conventional cation exchange resins, like sulfonic acid-based Amberlite IR-120 and Dowex 50W-8X, which distinguish REEs based on ionic interactions that are often not sufficient for their selective extraction and efficient separation79,80. In addition, its properties compare well to recently reported lanmodulin-modified agarose for binary REE pair separation, and the commercially available cation exchange resin, LEWATIT MDS 200H, which can selectively extract La3+, Ce3+, Pr3+, and Nd3+ in the presence of Al3+, Mg2+, and Ca2+40,81. The relatively slow adsorption kinetics and complicated synthesis of BZmacropa-XAD resin, however, limit our system for the utilization of large scale REE extraction from end-of-life materials. In addition, the low adsorption capacity of this resin within strongly acidic solutions (pH < 4) limits its use to higher pH leachates, like the bioleachate studied here. Further work will focus on developing solid-phase separation with more advanced scaffold materials and new chelators that can be covalently attached to these solid supports to overcome these challenges.
Methods
Preparation of NCS-XAD resin
NH2-XAD-4 resin was synthesized according to the procedure reported in the literature82,83. A mixture of NH2-XAD-4 resin (2 g) and Na2CO3 (1.5 g) in acetone (50 mL) was heated at 60 °C under an argon atmosphere for 30 min. To this suspension, CSCl2 (5 mL) was slowly added, and the mixture was allowed to reflux for 24 h under argon. The reaction progress was monitored by the Kaiser test. After cooling to room temperature, volatiles were removed under vacuum, and the resin was washed with water to remove the Na2CO3 and dried under vacuum to yield 2.35 g of the resin as a black solid.
Kaiser test
To 10–20 beads of resin in a test tube were added 4 drops of 0.66 mg/mL KCN aqueous solution, 4 drops of 2% aqueous ninhydrin (Millipore sigma), and 4 drops of 2 g/mL phenol in n-butanol. The tube was heated at 105 °C for 5 min. When the solution is colorless or pale yellow, no amines remain, and the reaction is complete84,85.
Immobilization of NH2-BZmacropa onto NCS-XAD resin
Solutions of NH2-BZmacropa (≈ 20 mM) were prepared by dissolving the ligand in pure water and determining exact concentrations via endpoint analysis of pH potentiometric titrations. In MOPS buffer (100 mM, 9.5 mL, pH 7.4), a solution of NH2-BZmacropa (20 mM, 10 mL) and 2 g of the NCS-XAD-4 resin were mixed, and the pH of the solution was adjusted to 9 with NaOH (1 M, 0.5 mL). The mixture was shaken for 24 h at room temperature. The modified resin was isolated by filtration, washed with water, and then dried under vacuum. To quantify the amount BZmacropa immobilized on the resin, UV–vis spectroscopy was used. Specifically, the supernatant before and after the conjugation reaction was filtered using 220 nm nylon syringe filter and was diluted from 50 μL to 3000 μL with MOPS buffer (10 mM pH 7.4) in a 3.5 mL quartz cuvette. The absorbance at 265 nm, corresponding to NH2-BZmacropa, was measured (Fig. S25). The amount NH2-BZmacropa remaining unbound was determined via Beer’s law.
Solution preparation
The stock solutions of La3+, Dy3+, and Lu3+ were made by dissolving LnCl3 hydrate salts in pure water to a concentration of 100 mM. Stock solutions of glycine (500 mM, VWR) and ammonium acetate (500 mM, Fisher Scientific) were prepared from their ACS-grade reagents, respectively. These stock solutions were used to make buffered solutions of La3+, Dy3+, and Lu3+ (600 μM final concentration after pH adjustment) containing 100 mM of glycine (pH 3) or ammonium acetate (pH 4 and 5). The pH of buffered Ln3+ solutions was measured using a pH meter (Mettler Toledo FE20). A stock solution containing 16 REEs (0.05 mM final concentration for each metal) was prepared by combining aliquots of ICP standards of individual elements and diluting to a final concentration of 50 μM with 100 mM ammonium acetate (pH 5). The bioleachate of the auto-catalyst slag material was provided by REEgen (a start-up company based in Ithaca, NY). This leachate was generated by mixing a biological leaching solution (biolixiviant) produced by wild-type Gluconobacter oxydans B58. The leaching was conducted at room temperature over the course of 24 h. After leaching, remaining solids were centrifuged and the liquid bioleachate was collected for use in the BZmacropa experiments.
Batch adsorption experiments
All batch adsorption experiments were performed at 22 ± 1 °C. BZmacropa-XAD resin (30 mg, containing 5.5 µmol of NH2-BZmacropa) or NCS-XAD resin was contacted with La3+, Dy3+, and Lu3+ aqueous solutions (10 mL, 600 μM, pH was retained at 3–5 using 100 mM glycine or ammonium acetate buffer) or a mixed REE solution (7 mL, 0.05 mM for each Ln3+, pH was maintained at 5 using 100 mM ammonium acetate buffer) in a 20 mL glass vial. The bioleachate (1 mL, pH 5.2) was contacted with 30 mg BZmacropa-XAD resin in a 3.5 mL glass vial. The resulting mixture was shaken for 24 h at 180 rpm, and the resin beads were removed by filtration. The filtrate was analyzed by ICP-MS after diluting with 2% HNO3 (50 µL → 5000 µL), and the results are summarized in Table S4. All batch adsorption experiments were repeated in triplicate.
Adsorption capacity (Qe, μmol or mg Ln3+ g−1 resin) is calculated using equation below
Where V and m are the Ln3+ solution volume and resin weight.
Adsorption kinetics
All batch adsorption experiments were performed at 22 ± 1 °C. BZmacropa-XAD resin (30 mg, containing about 5.5 µmol of NH2-BZmacropa) was contacted with aqueous solutions of La3+, Dy3+, and Lu3+ (10 mL, 600 μM, pH 5 ammonium acetate buffer) in a 20 mL glass vial. The resulting mixture was shaken at 180 rpm, aliquots of the supernatant were collected at different times (1–24 h) and filtered. The filtrate was analyzed by ICP-MS after diluting with 2% HNO3 (50 µL → 5000 µL). All batch adsorption experiments were repeated in triplicate.
These data were fit with the pseudo-second-order integrate rate law using the OriginPro® 2024b software package:
Where Qt (µmol g−1 resin) is the amount adsorbed at time t (h), Qe (µmol g−1 resin) is the amount adsorbed at equilibrium, k2 is the pseudo-second-order rate constant, and t is adsorption time.
Batch La3+ desorption experiments
All desorption experiments were performed at 22 ± 1 °C. After La3+ adsorption, BZmacropa-XAD (30 mg) resin was isolated by removing the solution with a pipettor and was washed with water and dried at 50 °C. The dry resin was contacted with 10 mL HCl (0.1 M), and the mixture was shaken for 4 h at 180 rpm. The supernatant was isolated and filtered through a 220 nm nylon syringe filter. The filtrate was analyzed by ICP-MS after diluting with 2% HNO3 (50 µL → 5000 µL), and the resin was neutralized with 1 M NaOH, washed with water, and then dried at 50 °C overnight before it was used for the next adsorption/desorption cycle. All adsorption/desorption cycle experiments were repeated in triplicate.
La3+/Dy3+ or Lu3+ on-column separation experiments
The on-column separation experiments were performed at 22 ± 1 °C. A quantity of ~1.5 g BZmacropa-XAD resin was loaded in a chromatography column (Kontes, analytical, 2 mL). The feed solution (20 mL in two equal volume batches) containing La3+ and Dy3+ or Lu3+ was loaded into a syringe, which was dispensed at a controlled flow rate of 0.2 mL min−1 with a syringe pump. The eluate of the column was collected in 1 mL per fraction, which were analyzed by ICP-MS for La3+/Dy3+ and ICP-OES for La3+/Lu3+ after diluting with 2% HNO3 (50 µL → 5000 µL). Prior to carrying out the desorption experiments with 0.1 M HCl solution, the column was washed with 10 mL of pure water to remove physically adsorbed metal ions. The 0.01 M and 0.1 M HCl eluents were prepared from diluting 1 M standard HCl solution (Thermo Scientific).
REE extraction from slag bioleachate
The on-column extraction experiment was performed at 22 ± 1 °C. A quantity of ~1.5 g BZmacropa-XAD resin was loaded in a chromatography column (Kontes, analytical, 2 mL). The leachate feed solution (10 mL) was loaded into a syringe, which was dispensed at a controlled flow rate of 0.2 mL min−1 with a syringe pump. The eluate of the column was collected in 1 mL fractions, which were analyzed by ICP-MS after diluting with 2% HNO3 (50 µL → 5000 µL). Prior to carrying out the desorption experiments with 0.01 M HCl and 0.1 M HCl, the column was washed with 10 mL of pure water to remove the physically adsorbed metal ions. The 0.01 M and 0.1 M HCl eluents were prepared from diluting a 1 M standard HCl solution (Thermo Scientific), and was pumped into the column at a flow rate of 0.2 mL min−1. On-column separation experiments were repeated in duplicate.
REE precipitation from 0.1 M HCl eluents
The REE precipitation experiment was performed at 22 ± 1 °C. To the 0.1 M HCl eluents (~13.5 mL) after its pH was adjusted to 4 using 1 M NaOH (1 mL), 100 mM (NH4)2C2O4 (15 mL) solution was added. The mixture was incubated for 12 h, and filtered through a 220 nm nylon syringe filter. The filtrate was diluted with 2% HNO3 (50 → 5000 µL) and analyzed by ICP-OES.
Data availability
Data to support the conclusions in this paper are available in the main text or the supplementary information for syntheses, potentiometric titrations, UV−Vis studies, NMR spectroscopy studies, X-ray crystallography studies, FTIR studies, REEs extraction and separation studies. The X-ray crystallographic data for [La(NH2-BZmacropa)]+ (CIF) has been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 2420469. This data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. The CIF file for this compound is also provided as Supplementary Data 1.
References
Rare Earth Coordination Chemistry: Fundamentals and Applications (ed. Huang, C.) (Wiley-VCH Verlag GmbH & Co. KGaA, 2008).
Jha, A. R. Chapter 1: History and Discovery of Rare Earth Elements. In Rare Earth Materials Properties and Application (ed. Jha, A. R.) 14–38 (Talyor & Francis Groups, 2014).
Balaram, V. Rare earth elements: a review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 10, 1285–1303 (2019).
Cuadros-Muñoz, J. R., Jimber-del-Río, J. A., Sorhegui-Ortega, R., Zea-De la Torre, M. & Vergara-Romero, A. Contribution of rare earth elements is key to the economy of the future. Land (Basel) 13, 1220 (2024).
Koltun, P. & Tharumarajah, A. Life cycle impact of rare earth elements. ISRN Metall. 2014, 907536 (2014).
Cordier, D. J. Mineral Commodity Summaries 2024, Rare Earth (U.S. Geological Survey, 2024).
Foley, N. K. & Ayuso, R. A. Conventional rare earth element mineral deposits—The global landscape. In Rare Earth Metals and Minerals Industries (eds. Murty, Y. V., Alvin, M. A. & Lifton, J.) 17–56 (Springer, 2023).
Schulz, K. J. & DeYoung, J. H., Seal, R. R. & Bradley, D. Critical Mineral Resources of the United States: Economic and Environmental Geology and Prospects for Future Supply (U.S. Geological Survey, 2017).
Prakht, V., Dmitrievskii, V., Kazakbaev, V. & Ibrahim, M. N. Comparison between rare-earth and ferrite permanent magnet flux-switching generators for gearless wind turbines. Energy Rep. 6, 1365–1369 (2020).
Podmiljšak, B. et al. The future of permanent-magnet-based electric motors: how will rare earths affect electrification? Materials 17, 848 (2024).
Kharbanda, P. et al. Ferrites: magnetic materials as an alternate source of green electrical energy. Heliyon 5, e01151 (2019).
Jyothi, R. K. et al. Review of rare earth elements recovery from secondary resources for clean energy technologies: grand opportunities to create wealth from waste. J. Clean. Prod. 267, 122048 (2020).
El Ouardi, Y. et al. The recent progress of ion exchange for the separation of rare earths from secondary resources – A review. Hydrometallurgy 218, 106047 (2023).
Gaustad, G., Williams, E. & Leader, A. Rare earth metals from secondary sources: review of potential supply from waste and byproducts. Resour. Conserv. Recycl. 167, 105213 (2021).
Tunsu, C., Petranikova, M., Gergorić, M., Ekberg, C. & Retegan, T. Reclaiming rare earth elements from end-of-life products: a review of the perspectives for urban mining using hydrometallurgical unit operations. Hydrometallurgy 156, 239–258 (2015).
Ambaye, T. G., Vaccari, M., Castro, F. D., Prasad, S. & Rtimi, S. Emerging technologies for the recovery of rare earth elements (REEs) from the end-of-life electronic wastes: a review on progress, challenges, and perspectives. Environ. Sci. Pollut. Res. 27, 36052–36074 (2020).
Zhu, Z., Pranolo, Y. & Cheng, C. Y. Separation of uranium and thorium from rare earths for rare earth production - A review. Miner. Eng. 77, 185–196 (2015).
Patel, K. S. et al. Occurrence of uranium, thorium and rare earth elements in the environment: a review. Front. Environ. Sci. 10, 1058053 (2023).
Jun, B. M. et al. Recovery of rare-earth and radioactive elements from contaminated water through precipitation: a review. Chem. Engin. J. 475, 146222 (2023).
Cotton, S. Lanthanide and Actinide Chemistry (John Wiley & Sons Ltd, 2006).
Kagan, H. B. Introduction: frontiers in lanthanide chemistry. Chem. Rev. 102, 1805–1806 (2002).
Mao, W., Xiang, L. & Chen, Y. Rare-earth metal complexes of β-diketiminato ligands bearing pendant nitrogen or oxygen donors. Coord. Chem. Rev. 346, 77–90 (2017).
Mishra, S. Anhydrous scandium, yttrium, lanthanide and actinide halide complexes with neutral oxygen and nitrogen donor ligands. Coord. Chem. Rev. 252, 1996–2025 (2008).
Vargas-Zúñiga, G. I., He, Q. & Sessler, J. L. Liquid-liquid separation by supramolecular systems. In Ion Exchange and Solvent Extraction (ed. Moyer, B. A.) 45–82 (Taylor & Francis Groups, 2019).
Neves, H. P. et al. Liquid-liquid extraction of rare earth elements using systems that are more environmentally friendly: advances, challenges and perspectives. Sep. Purif. Technol. 282, 120064 (2022).
Liu, T. & Chen, J. Extraction and separation of heavy rare earth elements: a review. Sep. Purif. Technol. 276, 119263 (2021).
Ismail, N. A., Abd Aziz, M. A., Mohd Yunus, M. Y. & Hisyam, A. Selection of extractant in rare earth solvent extraction system: a review. IJRTE 8, 728–743 (2019).
Xie, F., Zhang, T. A., Dreisinger, D. & Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 56, 10–28 (2014).
Wang, Y., Li, F., Zhao, Z., Dong, Y. & Sun, X. The novel extraction process based on CYANEX® 572 for separating heavy rare earths from ion-adsorbed deposit. Sep. Purif. Technol. 151, 303–308 (2015).
Vahidi, E. & Zhao, F. Environmental life cycle assessment on the separation of rare earth oxides through solvent extraction. J. Environ. Manag. 203, 255–263 (2017).
Merroune, A. et al. A comprehensive review on solvent extraction technologies of rare earth elements from different acidic media: current challenges and future perspectives. J. Ind. Eng. Chem. 139, 1–17 (2024).
O’Connell-Danes, J. G., Ngwenya, B. T., Morrison, C. A. & Love, J. B. Selective separation of light rare-earth elements by supramolecular encapsulation and precipitation. Nat. Commun. 13, 4497 (2022).
O’Connell-Danes, J. G. et al. A Simple supramolecular approach to recycling rare earth elements. ACS Sustain. Chem. Eng. 12, 9301–9305 (2024).
Otaki, M., Suominen, T., Hietala, S. & Koivula, R. T. Intra-lanthanide separation performance of DOTP: solid-phase extraction and selective precipitation studies. Sep. Purif. Technol. 354, 129082 (2025).
Ou, J. T. & Taylor, M. K. Selective binding of rare-earth ions in polymerizable cages. Chem. Commun. 61, 4784–4787 (2025).
Mikhaylenko, M. Development and screening of resins to recover REE and scandium from different sources. In The Minerals, Metals & Materials Series (ed. Davis, B.) 2113–2122 (Springer, 2018).
Florek, J. et al. Selective recovery of rare earth elements using chelating ligands grafted on mesoporous surfaces. RSC Adv. 5, 103782–103789 (2015).
Chu, S., Feng, X., Liu, C., Wu, H. & Liu, X. Advances in chelating resins for adsorption of heavy metal ions. Ind. Eng. Chem. Res. 61, 11309–11328 (2022).
Trikha, R., Sharma, B. K., Sabharwal, K. N. & Prabhu, K. Elution profiles of lanthanides with α-hydroxyisobutyric acid by ion exchange chromatography using fine resin. J. Sep. Sci. 38, 3810–3814 (2015).
Dong, Z. et al. Bridging hydrometallurgy and biochemistry: a protein-based process for recovery and separation of rare earth elements. ACS Cent. Sci. 7, 1798–1808 (2021).
Kulasekara, D. N., Kajjam, A. B., Praneeth, S., Dittrich, T. M. & Allen, M. J. Cryptands on a solid support for the separation of europium from gadolinium. ACS Appl. Mater. Interfaces 15, 42037–42045 (2023).
Dave, S. R., Kaur, H. & Menon, S. K. Selective solid-phase extraction of rare earth elements by the chemically modified Amberlite XAD-4 resin with azacrown ether. React. Funct. Polym. 70, 692–698 (2010).
Hu, A. & Wilson, J. J. Advancing chelation strategies for large metal ions for nuclear medicine applications. Acc. Chem. Res. 55, 904–915 (2022).
Hu, A., Simms, M. E., Kertesz, V., Wilson, J. J. & Thiele, N. A. Chelating rare-earth metals (Ln3+) and 225Ac3+ with the dual-size-selective macrocyclic ligand Py2-Macrodipa. Inorg. Chem. 61, 12847–12855 (2022).
Kadassery, K. J. et al. H2BZmacropa-NCS: a bifunctional chelator for actinium-225 targeted alpha therapy. Bioconjugate Chem. 33, 1222–1231 (2022).
Fiszbein, D. J. et al. Tuning the kinetic inertness of Bi3+ complexes: the impact of donor atoms on diaza-18-crown-6 ligands as chelators for 213Bi targeted alpha therapy. Inorg. Chem. 60, 9199–9211 (2021).
Abou, D. S. et al. Towards the stable chelation of radium for biomedical applications with an 18-membered macrocyclic ligand. Chem. Sci. 12, 3733–3742 (2021).
Hu, A. et al. Py-Macrodipa: a Janus chelator capable of binding medicinally relevant rare-earth radiometals of disparate sizes. J. Am. Chem. Soc. 143, 10429–10440 (2021).
Thiele, N. A. et al. An eighteen-membered macrocyclic ligand for actinium-225 targeted alpha therapy. Angew. Chem. Int. Ed. 56, 14712 (2017).
King, A. P. et al. 225Ac-MACROPATATE: a novel a-particle peptide receptor radionuclide therapy for neuroendocrine tumors. J. Nucl. Med. 64, 549–554 (2023).
Lee, K. K. et al. Chelation of [111In]In3+ with the dual-size-selective macrocycles py-macrodipa and py2-macrodipa. Dalton Trans. 53, 14634 (2024).
Kanagasundaram, T. et al. Fluorine-18 incorporation and radiometal coordination in macropa ligands for PET imaging and targeted alpha therapy. Chem. Commun. 60, 11940 (2024).
Thiele, N. A., Woods, J. J. & Wilson, J. J. Implementing f-block metal ions in medicine: tuning the size selectivity of expanded macrocycles. Inorg. Chem. 58, 10483–10500 (2019).
McNeil, B. L. et al. Evaluation of the effect of macrocyclic ring size on [203Pb]Pb(II) complex stability in pyridyl-containing chelators. Inorg. Cem. 61, 9638–9649 (2022).
Roca-Sabio, A. et al. Macrocyclic receptor exhibiting unprecedented selectivity for light lanthanides. J. Am. Chem. Soc. 131, 3331–3341 (2009).
Burns, J. H. & Baes, C. F. Stability quotients of some lanthanide cryptates in aqueous solutions. Inorg. Chem. 20, 616–619 (1981).
Tóth, Ė & Brücher, E. Stability constants of the lanthanide(III)-1,4,7,10-tetraazacyclododecane-N,N’,N”,N”’- tetraacetate complexes. Inorg. Chim. Acta 221, 165–167 (1994).
Mark, P. et al. Solvent extraction separation of trivalent americium from curium and the lanthanides. Solvent Extr. Ion. Exch. 33, 329–345 (2015).
Thiele, N. A., Fiszbein, D., Woods, J. J. & Wilson, J. J. Tuning the separation of light lanthanides using a reverse-size-selective aqueous complexant. Inorg. Chem. 59, 16522–16530 (2020).
Jensen, M. P. et al. Aqueous complexes for efficient size-based separation of americium from curium. Inorg. Chem. 53, 6003–6012 (2014).
Gao, Y., Licup, G. L., Bigham, N. P., Cantu, D. C. & Wilson, J. J. Chelator-assisted precipitation-based separation of the rare earth elements neodymium and dysprosium from aqueous solutions. Angew. Chem. Int. Ed. 63, e202410233 (2024).
Evans, M., Brooks, C. & Johnson, M. K. United States Energy Association: Critical Material Recovery from e-Waste (Battelle, 2023).
Panchenko, P. A. et al. Synthesis, structure and metal ion coordination of novel benzodiazamacrocyclic ligands bearing pyridyl and picolinate pendant side-arms. New. J. Chem. 43, 15072–15086 (2019).
Boelens, P. et al. Peptide functionalized dynabeads for the magnetic carrier separation of rare-earth fluorescent lamp phosphors. J. Magn. Magn. Mater. 563, 169956 (2022).
Schrader, M., Bobeth, C. & Lederer, F. L. Quantification of peptide-bound particles: a phage mimicking approach via site-selective immobilization on glass. ACS Omega 7, 187–197 (2022).
Ye, Q., Jin, X., Zhu, B., Gao, H. & Wei, N. Lanmodulin-functionalized magnetic nanoparticles as a highly selective biosorbent for recovery of rare earth elements. Environ. Sci. Technol. 57, 4276–4285 (2023).
Rizkalla, E. N. & Choppin, G. R. Chapter 127 Lanthanides and actinides hydration and hydrolysis. Handb. Phys. Chem. Rare Earths 18, 529–558 (1994).
Karthika, C. & Sekar, M. Comparison studies of adsorption properties on Ni(II) removal by strong and weak acid cation-exchange resins. Res. J. Chem. Sci. 3, 65–69 (2013).
Mendil, D., Kiris, T., Tuzen, M. & Soylak, M. Separation-preconcentration of Cu, Cd, Pb and Ni in various water and food samples on Sepabeads SP-207. Int. J. Food Sci. Technol. 48, 1201–1207 (2013).
Thermax. Ion Exchange Resins. https://www.thermaxglobal.com/chemicals/ion-exchange-resins/specialty-resins/ (2025).
Archer, W. R., Iftekhar, N., Fiorito, A., Winn, S. A. & Schulz, M. D. Synthesis of phosphonated polymer resins for the extraction of rare-earth elements. ACS Appl. Polym. Mater. 4, 2506–2512 (2022).
Cotruvo, J. A., Featherston, E. R., Mattocks, J. A., Ho, J. V. & Laremore, T. N. Lanmodulin: a highly selective lanthanide-binding protein from a lanthanide-utilizing bacterium. J. Am. Chem. Soc. 140, 15056–15061 (2018).
Florek, J., Chalifour, F., Bilodeau, F., Larivière, D. & Kleitz, F. Nanostructured hybrid materials for the selective recovery and enrichment of rare earth elements. Adv. Funct. Mater. 24, 2668–2676 (2014).
Romero, J. L. et al. Modified diglycolamide resin: characterization and potential application for rare earth element recovery. Minerals 13, 1330 (2023).
Reed, D. W., Fujita, Y., Daubaras, D. L., Jiao, Y. & Thompson, V. S. Bioleaching of rare earth elements from waste phosphors and cracking catalysts. Hydrometallurgy 166, 34–40 (2016).
Chikanda, F., Otake, T., Koide, A., Ito, A. & Sato, T. The formation of Fe colloids and layered double hydroxides as sequestration agents in the natural remediation of mine drainage. Sci. Total. Environ. 774, 145183 (2021).
Hirata, S., Yoshihara, H. & Aihara, M. Determination of iron(II) and total iron in environmental water samples by flow injection analysis with column preconcentration of chelating resin functionalized with N-hydroxyethylethylenediamine ligands and chemiluminescence detection. Talanta 49, 1059–1067 (1999).
Nawab, A., Yang, X. & Honaker, R. Parametric study and speciation analysis of rare earth precipitation using oxalic acid in a chloride solution system. Miner. Eng. 176, 107352 (2022).
Monazam, E., Siriwardane, R., Miller, D. & Mcintyre, D. Rate analysis of sorption of Ce3+, Sm3+, and Yb3+ ions from aqueous solution using Dowex 50W-X8 as a sorbent in a continuous flow reactor. J. Rare Earths 36, 648–655 (2018).
Patel, M. & Karamalidis, A. K. Chapter 9: Adsorption-Based Separation and Recovery of Rare Earth Elements. In Rare Earth Elements: Sustainable Recovery, Processing, and Purification (eds. Karamalidis, A. K. & Eggert, R.). 299–376 (ESS open Archive, 2023).
José, L. B., Silva, C. G. & Ladeira, A. C. Q. Pre-concentration and partial fractionation of rare earth elements by ion exchange. Miner. Eng. 205, 108477 (2024).
Hoque, M. I. U., Chowdhury, D. A., Holze, R., Chowdhury, A. N. & Azam, M. S. Modification of Amberlite XAD-4 resin with 1,8-diaminonaphthalene for solid phase extraction of copper, cadmium and lead, and its application to determination of these metals in dairy cow’s milk. J. Environ. Chem. Eng. 3, 831–842 (2015).
Islam, A., Laskar, M. A. & Ahmad, A. Characterization and application of 1-(2-Pyridylazo)-2-naphthol functionalized amberlite XAD-4 for preconcentration of trace metal ions in real matrices. J. Chem. Eng. Data. 55, 5553–5561 (2010).
Wellings, D. A. & Atherton, E. Chapter 4: Standard Fmoc protocols. In Methods in Enzymology: Solid-Phase Peptide Synthesis Vol. 289 (ed. Frederick, M. D.) (1997).
Aapptec. Monitoring of Peptide Coupling and Capping. https://www.peptide.com/resources/solid-phase-peptide-synthesis/monitoring-of-peptide-coupling-and-capping/ (2019).
Kaur, H. & Agrawal, Y. K. Functionalization of XAD-4 resin for the separation of lanthanides using chelation ion exchange liquid chromatography. React. Funct. Polym. 65, 277–283 (2005).
Callura, J. C., Shi, Q., Dzombak, D. A. & Karamalidis, A. K. Selective recovery of rare earth elements with ligand-functionalized polymers in fixed-bed adsorption columns. Sep. Purif. Technol. 265, 118472 (2021).
Page, M. J., Soldenhoff, K. & Ogden, M. D. Comparative study of the application of chelating resins for rare earth recovery. Hydrometallurgy 169, 275–281 (2017).
Radhika, S., Nagaraju, V., Nagaphani, K. B., Kantam, M. L. & Reddy, B. R. Solid-liquid extraction of Gd(III) and separation possibilities of rare earths from phosphoric acid solutions using Tulsion CH-93 and Tulsion CH-90 resins. J. Rare Earths 30, 1270–1275 (2012).
Bezzina, J. P., Ogden, M. D., Moon, E. M. & Soldenhoff, K. L. REE behavior and sorption on weak acid resins from buffered media. Ind. Eng. Chem. 59, 440–455 (2018).
Cui, H. et al. A facile process for enhanced rare earth elements separation from dilute solutions using N, N-di(2-ethylhexyl)-diglycolamide grafted polymer resin. Sep. Purif. Technol. 234, 116096 (2020).
Acknowledgements
This research is based upon work supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under award numbers DE-SC0021662 and DE-SC0025792. Extraction studies involving bioleachate were supported by the U.S. National Science Foundation Small Business Innovation Research award number 2304412 to REEgen Inc. in Ithaca, NY. This research made use of the NMR Facility at Cornell University, which is supported, in part, by the NSF under Award CHE-1531632.
Author information
Authors and Affiliations
Contributions
Y. Gao designed and conducted experimental data and wrote the paper. J.J. Wilson conceived and supervised the project and wrote the paper. K. Ivanovich, B. Pian, and S. Medin prepared the bioleachate. S.N. MacMillan ran the X-ray crystallography and refined the crystal structure. S. Medin ran ICP-MS. S. Medin and A. M Schmitz assisted in the supervision of this project.
Corresponding author
Ethics declarations
Competing interests
A.M.S. and S.M. are co-founders of and hold equity in REEgen Inc., a company that recovers and manufactures REE and other critical metals. B.P. and K.I. hold options for equity in REEgen. All other authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks Freddy Kleitz and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, Y., Ivanovich, K., Medin, S. et al. 18-membered macrocycle appended on resin for selective rare earth element extraction and separation. Commun Chem 8, 176 (2025). https://doi.org/10.1038/s42004-025-01565-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42004-025-01565-4