Abstract
Aluminium–sulfur (Al–S) batteries have emerged as a promising post-lithium alternative owing to aluminium’s abundance, safety, and high theoretical capacity. However, their practical implementation is impeded by key challenges such as sluggish Al3+ redox kinetics, polysulfide shuttle effects, and volumetric changes of the electrodes during cycling. This review critically analysis recent advancements in host structural design engineering, new electrocatalysts, and electrolyte aimed at overcoming these limitations. Advanced host frameworks include 2D/3D porous structures, MXene-based multilayers, and single-atom doped materials that facilitate efficient sulfur confinement, enhance conductivity, and catalyse redox reactions. Embedded catalysts like Mo6S8 and CoS2 within nitrogen-doped carbons lower the decomposition barrier of Al2S3, promote stable Al-polysulfide conversion, and extend cycle life. Electrolyte optimization through ionic liquids, molten salts, and halide-modified systems further enhances ion mobility, suppresses passivation, and supports stable sulfur utilization. Emerging hybrid electrolytes combining high-donicity solvents with ionic or molten salt phases offer synergistic gains in redox kinetics and thermal stability. Density functional theory (DFT) guided designs elucidate key host–electrolyte–polysulfide interactions, revealing pathways for tailored material selection and performance enhancement. These integrated strategies pave the way for high-energy, long-lasting Al–S batteries that perform reliably at both room and elevated temperatures.

Similar content being viewed by others
Introduction
The accelerating global shift towards decarbonization and sustainable energy infrastructure has underscored the need for safe, cost-effective, and high-performance electrochemical energy storage technologies. Meeting net-zero targets by 2030 will require an over eightfold increase in global energy storage capacity, exceeding 1500 GWh, as projected by the IEA1. While lithium-ion batteries remain the industry benchmark, they are increasingly constrained by resource scarcity (Li, Co, Ni), escalating costs (lithium carbonate > $70,000/ton in 2022)2, and safety risks associated with flammable electrolytes.
Sulfur-based batteries are becoming essential owing to sulfur’s combination of high theoretical specific capacity (1672 mAh g−1)3 (S + 2Al → Al2S3), natural abundance (~0.05 wt% of Earth’s crust)3, low-cost, multi-electron redox processes (typically S⁰ → S2−), which enable high energy densities when paired with suitable anodes. Its low atomic weight and high electron affinity also contribute to favorable gravimetric energy densities, making sulfur ideal for lightweight, high-capacity energy storage4. Sulfur undergoes multi-step redox reactions involving intermediate metal polysulfide species (e.g, Al2S6n−, AlS3n−), which offer tunable kinetics when tailored with suitable electrolytes.
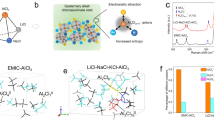
Among them, aluminum–sulfur (Al–S) batteries stand out as the most promising, combining the merits of both sulfur and aluminum, two of the most earth-abundant (Al - ~8.3 wt% of the Earth’s crust) and cost-effective elements. The evolution of performance metrics from 2000 to 2024 for Al–S systems is shown in Fig. 1a. Recently, in 2022, MIT-led researchers mentored by Prof Donald Sadoway developed a safer, potentially cheaper Al–S battery utilizing readily available sulfur and rock salts, demonstrating comparable performance to lithium-ion with a potential cost reduction of approximately 83%, with a faster charging capability. Its non-flammable nature eliminates the safety hazard and the need for complex cooling systems in large-scale making it useful for stationary storage and, longer term. Avanti Battery Company, the startup founded to commercialize this technology, is currently focused on scaling production and ensuring long-term durability5.
a Evolution of specific capacity and cycle life of metal–sulfur batteries from 2000 to 2024. b Schematic of the discharge process in metal–sulfur batteries. c Energy band diagrams showing interfacial energy alignment and electrochemical stability at different interfaces in metal–sulfur batteries. d Comparison of electrolytes used in Al–S batteries, and the overall redox reactions during discharge, showing charge carriers and sulfur reduction pathways.
For Al, the work function (Φ) ranges from 4.0 to 4.3 eV, and sulfur, being a non-metal, does not have a defined work function, but its reduction behavior at the cathode in the Al–S system is influenced by its electronic structure. Sulfur has an atomic number of 16, with the electron configuration [Ne]3S23P4, (outermost electrons are in the 3p orbital)6. In its elemental form (S8), sulfur exists as a molecule with a puckered ring structure consisting of eight sulfur atoms. The bonding involves the overlap of p-orbitals, with some π-bonding interactions between sulfur atoms. In this state, sulfur is insulating (~5 × 10–30 S/cm)6, which makes it difficult for sulfur to conduct electrons without external assistance (through conductive additives). In the molecular form, sulfur has weak interactions between S atoms in the S8 ring, and the electrons in the 3p orbitals are localized within each sulfur atom. As sulfur undergoes reduction, the electron density becomes more delocalized, allowing for better electron flow. However, polysulfides are soluble in organic electrolytes, leading to capacity fading in sulfur-based batteries.
The Al–S system offers a theoretical energy density of up to 1310 Wh kg−1, with high specific capacities of 2980 mAh g−1 (Al) (Al → Al3+ + 3e−) and a practical energy density of 250–400 Wh kg−17,8,9,10. The working mechanism and energy level alignment for the metal–sulfur and Al–S system are shown in Fig. 1b–d. The theoretical electromotive force (EMF) of an Al–S battery is typically between 1.6 and 2.0 V (theoretical - 2–2.3 V). Notably, aluminum’s volumetric capacity (~8046 mAh cm−3)11 far exceeds that of lithium. The Al–S battery operates typically in molten salt or ionic liquid-based electrolytes, where complex aluminum–sulfur species are stabilized. During discharge, Al metal is oxidized at the anode to Al3+, while sulfur at the cathode is reduced through sequential polysulfide intermediates.
The reaction pathway often involves the formation and dissolution of aluminum polysulfide intermediates, which can be engineered to enhance reaction reversibility and reduce shuttle effects.
In Al–S systems, the key challenges are (i) sulfur’s insulating nature, (ii) volume expansion of the host material, (iii) intermediate polysulfide dissolution, and (iv) Al dendrite and corrosion formation. Ideal hosts require a (i) high surface area and moderate porosity to encapsulate sulfur and accommodate volumetric fluctuations, (ii) high electrical conductivity to enhance charge transport, (iii) strong polysulfide anchoring capability to suppress shuttle effects and improve reversibility, and (iv) chemical stability in Lewis-acidic aluminum-based electrolytes. Carbon-based hosts such as mesoporous carbon, graphene, CNTs, and nanofibers offer high conductivity and confinement through physisorption, but require heteroatom doping (N, O, F) to introduce polar sites that improve polysulfide chemisorption and electrolyte interaction. Polar inorganic materials like TiO2, MnO2, and Al2O3 bind polysulfides through strong polar interactions, while metal sulfides (MoS₂, CoP)5,12 provide electrocatalytic activity and ordered porous networks for sulfur confinement. Conductive polymers (polypyrrole and polyaniline) add elasticity and structural adaptability.
This mini review covers the recent advancements, including hybrid carbon–metal oxide/sulfide hosts for dual functionality, core–shell and yolk–shell architectures that buffer expansion and contain sulfur, and 3D hierarchies that enhance sulfur loading and ion/electron pathways. Incorporation of electrocatalytic sites (Co–Ni alloys) accelerates redox reactions, while covalent encapsulation strategies (sulfurized MOFs) prevent polysulfide shuttle and reinforce host integrity. This mini review also covers the mechanistic insights from operando characterizations that have illuminated how these materials influence nucleation, ion diffusion, and redox potential stabilization. Advances in electrolytes and catalysts are covered in detail. Overall, recent Al–S cells have demonstrated sulfur utilization >70%, stable cycling over 300+ cycles with high-capacity retention, and areal capacities of 4–6 mAh cm−2 at sulfur loadings >3 mg cm−2. The article discusses the future research directions: (i) Self-healing hosts that autonomously restore structural and electrochemical integrity, minimizing polysulfide loss, (ii) Embedded redox mediators that accelerate polysulfide conversion by lowering reaction barriers, (iii) New structural design to optimize host properties based on sulfur chemistry and electrolyte interactions.
Structural advancements in host materials
Aluminum’s resistance to dendrite formation and the use of non-flammable electrolytes like ionic liquids or deep eutectic solvents (DESs) enhance safety. However, the sulfur redox mechanism in Al–S batteries differs from Li–S systems. Sulfur is reduced to electrically insulating Al2S3 through complex, multi-step reactions highly dependent on electrolyte composition. Al3+ forms strong chloro-complexes (AlCl4⁻, Al2Cl7⁻) in ionic or eutectic media, affecting both plating/stripping and sulfur conversion12. The system suffers from sluggish ion transport, interfacial instability, and limited reversibility due to Al2S3 formation and volume changes. Three primary mechanisms dominate Al–S batteries, based on cathode and electrolyte combinations: (i) Sulfur in conductive carbon with chloroaluminate ionic liquids (AlCl3–EMImCl): During discharge, Al oxidizes and reacts with sulfur to form Al2S3 via solid-state conversion. While this suppresses polysulfide shuttling, the process is kinetically slow and suffers from poor reversibility due to the insulating nature of Al2S3. (ii) Sulfur embedded in metal/metal oxide hosts with DESs, CILs, or sulfone-based electrolytes: These systems involve stepwise sulfur reduction to soluble Al-polysulfides (AlSx), similar to Li–S batteries. Hosts help trap intermediates, but issues like shuttling, self-discharge, and passivation persist. Interfacial reactions and unstable species often hinder long-term cycling. (iii) Composite cathodes (S/MoS2, S/TiS2) with DESs or sulfonates: Here, Al3+ intercalates into layered hosts while sulfur undergoes conversion, enabling dual mechanisms. Although this offers improved kinetics and potential reversibility, it introduces mixed-phase behavior and structural instability over cycles. Chloroaluminate ionic liquids (AlCl3:EMImCl >1) are favored for their Al3+ transport and strong interactions with polar hosts, supporting sulfur redox and suppressing intermediates. DESs like AlCl3–acetamide provide better wettability for oxides and reduced side reactions. Sulfone-based organic electrolytes (Al(OTf)3 in DMSO), though less explored, stabilize Al–S–O intermediates and facilitate SEI formation5,10,11,12,13. In contrast, aqueous Al3+ electrolytes are unsuitable due to sulfur and host degradation, high polysulfide solubility, and poor cycling stability.
The performance of Al–S system is linked to the design of their host structures, which govern ion transport, mechanical stability, and interfacial integrity. Recent advancements have shifted focus toward engineered architectures that go beyond conventional bulk materials to enable enhanced electrochemical kinetics. This section explores key innovations in host frameworks, including multi-layered architectures, tailored 2D/3D structures, embedded and fibrous designs, and hybrid structures that offer pathways to accommodate volumetric changes and extend cycle life. Each subsection delves into structural characteristics and functional benefits.
Multi-layered hosts frameworks
Multi-layered architectures provide sequential barriers, both physical and chemical, that effectively trap soluble polysulfides, minimizing their diffusion toward the aluminum anode and suppressing the shuttle effect. Further, layered frameworks with hollow, flexible, or porous interlayers accommodate volume expansions without structural degradation. Zheng et al. used a binder-free S@Ti3C2Tx sandwich-structured cathode (Fig. 2a–c) for mitigating the shuttle effect, delivering 489 mAh g−1 initially and retaining 415 mAh g−1 after 280 cycles with ~95% Coulombic efficiency13. Ti3C2Tx acts as both a binder and a conductive agent, while also serving as a promising host for sulfur anchoring. The functional groups on the surface of Ti3C2Tx have a good affinity for polysulfides, and its conductive network accelerates charge transfer. Unlike Ab initio molecular dynamics simulations, which capture atomic dynamics at finite temperatures, density functional theory (DFT) focuses on static, ground-state properties, optimizing the energetics, charge distribution, and bonding. This makes it ideal for material screening, ion diffusion mapping, and active-site optimization in Al–S batteries13,14,15. For material screening, DFT calculates formation and adsorption energies, electronic structures, and phase stability, helping identify cathode hosts that bind sulfur species effectively while maintaining reversibility. It also predicts the electrochemical stability of electrolyte components and electrode–electrolyte interfaces. In ion diffusion studies, DFT combined with the Nudged Elastic Band (NEB) method reveals migration energy barriers for Al3+ in solids or across interfaces, guiding the design of structures with lower transport resistance. For active-site optimization, DFT provides analysis of how sulfur species interact with doped surfaces or functional groups, using tools like charge density mapping and Bader analysis. In one study, DFT calculations suggest that Ti3C2Tx exhibits stronger binding energy toward Al polysulfides compared to graphene. Further, the sandwich-structured S@Ti3C2Tx film has outer Ti3C2Tx layers that block polysulfides and a uniformly distributed S@Ti3C2Tx composite core formed via in situ sulfur deposition13. It offers high conductivity (2954 S cm⁻¹) and strong polysulfide anchoring, confirmed by DFT (Fig. 2b). Wang et al. used single-atom-loaded MXenes as Al–S battery cathodes to suppress Al2Sn dissolution. DFT analysis identified Y, Nb, Mo, and Tc as top atoms, with a low sulfur reduction barrier (0.23 eV), Fig. 2d14. A kinetic activity volcano plot revealed that optimal intermediate binding is key to performance. Single-atom-loaded MXene anchors promote efficient Al2Sn conversion, enhancing stability and rate capability. DFT simulations reveal that optimal binding strength is essential; too weak limits contact, too strong hinders sulfur utilization. High-energy barriers in Al–S batteries hinder sulfur redox kinetics and reversibility, leading to capacity decay due to insoluble Al2Sn formation. Single-atom-doped MXenes (Y@Ti3C2O2, Ea = 0.23 eV) effectively lower these barriers, enhancing sulfur reduction and compensating for low voltage. Optimizing transitions like Al2S12* → Al2S6* → Al2S3* boosts reaction kinetics14. MXene-based cathodes in Al–S batteries show high initial capacity but often suffer from rapid cycling decay and unclear sulfur involvement in reactions. Issues include low Coulombic efficiency due to solid electrolyte interphase (SEI) formation and side reactions, potential oxidation of Ti3C2Tx, and limited capacity primarily driven by [AlCl4]⁻ intercalation rather than sulfur redox activity.
a Schematic representation of the fabrication process for binder-free S@Ti3C2Tx film, pure Ti3C2Tx film, and traditional sulfur–Ti3C2Tx composite cathodes. b Gibbs free energy profiles for the transformation of S8 and Al2Sx intermediates on monolayer graphene (blue) and Ti3C2O2 (red), highlighting catalytic activity and stability differences. c Schematic illustration of the distinct electrochemical reaction pathways in working Al–S cells for both S@Ti3C2Tx and conventional S + Ti3C2Tx cathodes13. d Adsorption energy (E_b) values of S8 and various Al2Sn species (n = 3, 6, 12, 18) on SA@Ti3C2O2 nanosheets, visualized as colored lines (short red: S8*, orange: Al2S18*, green: Al2S12*, blue: Al2S6*, purple: Al2S3*), with corresponding solid lines denoting their binding energies to Ti3C2O214. e Strategy for membrane modification via in situ polymerization incorporating AlCl3/AcA electrolyte, enhancing ion transport and suppressing polysulfide crossover. f Theoretical voltage profiles of different sulfur species referenced against the Al3+/Al redox couple, shedding light on redox behavior during cycling16. g Voltage-capacity profiles of BN/S/C composite cathode at various charge/discharge stages17. h Optical image of a fully assembled Al//[EMIM]Cl–AlCl3//BN/S/C pouch cell with an open-circuit voltage of 1.2 V vs. AlCl4⁻/Al; inset shows the cell structure18. i Galvanostatic charge–discharge curves of GCNT/S composite at different current densities, with accompanying rate capability and long-term cycling stability (600 cycles at 100 mA g−1. j Gibbs free energy diagram of the discharge process on β-germanene, revealing its thermodynamic feasibility as a sulfur host19. k Ab initio molecular dynamics (AIMD) simulations at 400 K showing stable adsorption of Al2S6 species on β-germanene; optimized structures from top and side views show multilayer Al₂S₃ clustering. l Schematic of the synthesis process of Se2.9S5.1@MCNF (metal–carbon nanofiber host) and subsequent energy barrier analysis for Al atom diffusion across SeS2, SeS, Se2.9S5.1, and Se2.9S5.1@MCNF surfaces20. m Synthetic scheme for ZIF-8@ZIF-67 precursor and conversion to Co–NCNHP nanocomposites via thermal treatment21. n Schematic depiction of the formation pathways of NMC–Al2O3 and dual-modified NMC–Al2O3–S cathode materials for improved cycling and interface compatibility in Al–S systems22.
Hollow design increases surface area for better sulfur storage, mitigates volume expansion during cycling, anchors polysulfides to reduce the shuttle effect, and enhances ion and electron transport for improved charge–discharge efficiency. Sun et al. introduced a hollow tubular NiCo2Se4@CS2 cathode, reducing the shuttle effect of Al polysulfides15. The bimetallic selenide lowers the Gibbs free energy barrier, providing active sites that facilitate electron transfer and lower the activation energy required for Al2S3 decomposition, improving cycling stability. The cathode achieves 485 mAh g−1 initially and retains 135 mAh g−1 after 450 cycles at 200 mA g−1. DFT calculations show strong Al polysulfide adsorption, and the morphology helps with sulfur storage and volume expansion mitigation. Here, both diffusion and pseudocapacitive behaviors were observed, with 48% at 0.4 mV s−1 pseudo capacitance contribution increased to 78% at 1 mV s−1. This higher pseudo capacitance was attributed to the hollow tubular structure. In another study, a high-voltage Al–S battery (∼1.8 V, 861 mAh g−1) using reversible sulfur oxidation, forming S2Cl2 via multi-electron transfers, was demonstrated. A polymer membrane with AlCl3/acetamide electrolyte enhances stability, achieving 92.1% capacity retention over 50 cycles and durability over 490 cycles, Fig. 2de, f16. The polymer membrane acts as a barrier that prevents direct contact between reactive species (sulfur and dissolved polysulfides) and the aluminum anode, thereby suppressing parasitic reactions. Despite acting as a barrier, the membrane allows efficient Al3+ ion transport.
Advances in 2D/3D architectures
2D materials (graphene, MoS₂, BN) and 3D frameworks (like porous carbon scaffolds or hollow structures) provide abundant active sites to physically trap sulfur and chemically anchor aluminum polysulfides (AlPSs), mitigating the shuttle effect. Zhang et al. use 2D boron nitride (BN) as a sulfur fixer in aluminum–sulfur batteries, achieving 532 mAh g−1 capacity and 94.3% Coulombic efficiency over 300 cycles17. BN, with MoS2/WS2, stabilizes sulfur by preventing polysulfide dissolution, enabling a discharge plateau at ~1.15 V. In the system, energy storage occurs through a reversible redox mechanism. During discharge, aluminum at the anode oxidizes, releasing electrons and forming Al2Cl7− ions. These electrons travel through the external circuit to the cathode, where sulfur is reduced in the presence of Al2Cl7− to form aluminum sulfide (Al2S3). Upon charging, the process reverses. Al2S3 at the cathode decomposes back into elemental sulfur, releasing Al2Cl7− ions and electrons, while aluminum is re-deposited at the anode. The BN in the cathode anchors sulfur and sulfide compounds, preventing their dissolution and stabilizing the electrode structure, Fig. 2g17.
Interlayer spacing in 2D material hosts is essential because it directly influences ion accessibility, transport kinetics, and storage capacity. Expanded layers reduce the energy barrier for ion migration and buffer volume changes during cycling. Enhancing the interlayer spacing of MWCNTs is essential because their native spacing (~0.34 nm) limits the insertion and diffusion of larger ions (Al3+)18. Ajay et al. presented a strategy to enhance the interlayer spacing of MWCNTs by incorporating sulfur nanoparticles into a graphene–MWCNT framework. The resulting composite boosts performance to 507 mAh g−1 and 600 cycle stability, Fig. 2h. High charge density (Al3+) creates strong electrostatic interactions with the host lattice and sulfide products. This leads to sluggish ion diffusion in dense, non-porous hosts and a higher energy barrier for intercalation/de-intercalation. Expanded interlayer spacing and porous channels in Graphene-CNT ease the transport of Al3+. Sulfur reacts to form Al2S3 via a conversion mechanism rather than intercalation, which accommodates Al3+ readily when supported by a flexible matrix18. Graphene-CNT prevents electrolyte decomposition and dendrite formation by distributing charge uniformly.
Xu et al. explored the potential of 10 different 2D Xenes as cathode using molecular dynamics (MD) simulations and binding energy analysis, which revealed that Xenes exhibit strong adsorption of Al-polysulfides (−1.66 to −2.74 eV). Xenes, particularly β-Germanene, demonstrated high conductivity, low energy barriers for sulfur redox reactions, Al2S3 dissociation, and Al-ion diffusion. β-Germanene also promotes uniform Al2S3 deposition via strong Al–X and S–X interactions Fig. 2i, j, enabling high sulfur loading (39.93 wt%). MD simulations further show the formation of Al2Cl7− and Al3Cl10− species, facilitating Al deposition/stripping19.
Embedded and fibrous designs
Embedded architectures physically trap sulfur species and their soluble intermediates, minimizing the shuttle effect. In fibrous networks, sulfur is immobilized within the porous structure, while surface functional groups or heteroatoms offer strong chemical binding sites for polar polysulfides, Fig. 2k. Li et al. studied a Se-S based cathode, delivering 606 mAh g−1 at 50 mA g−1 and 187 mAh g−1 after 3000 cycles at 0.5 A g−1. The optimized Al3+ bonding enhances capacity (>600%) and lowers diffusion barriers (by 200%) compared to other SexSy compounds. Further, the Se-S structure improves electrical conductivity and suppresses polysulfide dissolution20. The strong Coulombic interactions and complex bonding nature of Al3+ ions often lead to sluggish kinetics due to orbital mismatches with conventional electrodes. To overcome this, Se-S-based cathodes encapsulated in multichannel carbon nanofibers have been developed, offering high capacity and cycling stability. This is attributed to effective bonding orbital overlap with Al3+ (−1.8 eV) and enhanced Al–S interactions, as confirmed by DFT calculations, Fig. 2l. The Se-S structure promotes stronger Al–S bonds and facilitates Al3+ incorporation. Electrode disintegration is another critical concern, often resulting from side reactions involving binders and metallic current collectors in conversion-type electrodes20.
Lin et al. developed CoS2 nanoparticles embedded in N‑doped carbon nanotubes, Fig. 2m21, achieving efficient HER with overpotentials of 158 mV in 0.5 M H2SO4 and 192 mV in 1 M KOH (stable >24 h) and serve as an Al–S battery host delivering 515 mAh g−1 initially and 110 mAh g−1 after 100 cycles. DFT confirms optimal H* binding and suggests that more electrons with a quick round-trip transfer rate undergo the hydrogen evolution reaction (HER) process. The calculations also indicate that the embedded structure’s surface accelerates the dissociation of H2O molecules. DFT analysis further shows that wrapping CoS2 in N‑doped carbon (CoS₂‑NC) creates plentiful electronic states at the Fermi level and shifts its p‑band center furthest from the Fermi level (CoS₂‑NC > Co3O4‑NC > Co‑NC), enabling rapid electron transfer and higher electron density at N sites21. In another study by Sun et al., a nitrogen‑rich mesoporous carbon host decorated with amorphous Al2O3 (Fig. 2n) enables 73.5 wt% sulfur loading, delivers 1212 mAh g−1 at 0.2 C and 755 mAh g⁻¹ at 2 C, and sustains 100% efficiency over 1000 cycles (0.023% decay per cycle). DFT shows Al2O3’s strong polysulfide adsorption and catalytic conversion suppresses the shuttle effect22. Nanostructured carbons (mesoporous carbons, graphene, CNTs) offer tunable pores and heteroatom sites for sulfur hosting but bind polar polysulfides weakly, leading to a shuttle. Adding inorganic materials (oxides, sulfides, nitrides) boosts chemisorption and catalytic conversion. The carbon with Al2O3 host uses an amorphous structure to anchor Li2Sx and catalyze its breakdown, achieving high capacity and nano‑Al2O3 in a carbon framework, synergistically optimizing morphology, porosity, and active-site distribution to suppress shuttle and enhance reversibility22.
While Al2O3 nanoparticles in the cathode serve as Lewis‑acidic anchors and polysulfide catalysts, an uncontrolled Al2O3 layer on the aluminum anode acts as an insulating passivation film that impedes Al3+ transport. In other words, Al2O3 on the cathode chemically binds Li2Sx and accelerates its conversion, but the same oxide on the anode blocks charge transfer and raises overpotentials. This mismatch arises because the beneficial, nano‑dispersed Al2O3 in the host is catalytically active and porous, whereas the thick, native Al2O3 on the metal surface is dense and poorly conductive. Balancing these roles, using just enough Al2O3 at the cathode while preventing excessive passivation at the anode is critical for maintaining both high sulfur utilization and efficient, low‑impedance Al plating/stripping.
Zhou et al. developed a Co/Mo₂C@NCNHP@S composite, with bimetallic Co/Mo₂C nanoparticles embedded in a CNT‑interwoven porous carbon scaffold, which delivers 1016 mAh g−1 at 1 A g−1 and retains 370 mAh g−1 after 200 cycles at 3 mg cm−2 sulfur loading. Here, Co-anchors Al‑polysulfides while Mo₂C catalyzes their reduction, enhancing redox kinetics and reversibility23. Cobalt possesses unfilled d-orbitals and high electronegativity, enabling strong chemical adsorption of polar polysulfides via Lewis acid–base interactions. This helps immobilize aluminum polysulfides, preventing their shuttling and loss into the electrolyte, which is a major issue in Al–S systems. Molybdenum carbide (Mo2C) has a high density of active sites and metallic conductivity, which promotes fast electron transfer. It lowers the activation energy barrier for polysulfide conversion (Al2S6 to Al2S3), thus accelerating redox kinetics. High resistance at electrode–electrolyte interfaces limits overall cell performance, especially under high current densities. Nanoscale integration and porous carbon require multi-step synthesis, affecting scalability and cost.
Three major types of catalytic mechanisms are identified based on their functionality and interaction with sulfur species: (i) redox mediation, (ii) surface adsorption and intermediate anchoring, and (iii) Lewis acid–base catalysis24,25,26,27. Redox mediation involves catalytic materials, typically redox-active metal compounds or heteroatom-doped carbons that participate in electron transfer, lowering the activation energy for the S8 → Al2S3 conversion. Transition metal sulfides (CoS2, MoS2) act as electron shuttles, mediating charge transfer during the multi-step reduction of elemental sulfur and stabilization of Al-polysulfide intermediates24,25. This mechanism improves redox reversibility and suppresses the insulating behavior of sulfur at room temperature. Surface adsorption and intermediate anchoring mechanisms involve polar metal oxides (MnO2, TiO2, Fe2O3) or heterostructured materials that provide abundant active sites and chemical affinity for soluble intermediates25. These materials form coordinate bonds or dipole–dipole interactions with sulfur species, enabling the capture and spatial confinement of Al–Sn intermediates. This confinement not only prevents shuttle-induced loss of active material but also accelerates conversion pathways by facilitating nucleation and growth of discharge products (Al2S3) on catalytic surfaces. Such spatially resolved catalysis also stabilizes solid-liquid-solid conversion reactions during cycling. In Lewis acid–base catalytic systems, surface Lewis basic sites on oxides coordinate with Lewis-acidic AlCl3 species in the electrolyte, tuning the local electron density to lower the energy barrier for Al3+ intercalation. This interfacial catalysis promotes selective pathways and reduces parasitic side reactions. In all these cases, the fundamental principles involve tailoring the electronic structure of catalytic hosts, engineering active sites with optimized binding energies (not too strong to trap, not too weak to release), and maintaining phase stability during redox cycling. In situ characterization is vital for understanding the interaction between catalysts, sulfur intermediates, and electrolytes at the atomic level.
Emerging and hybrid host structures
These structures integrate multiple functional components within a single matrix. (MOFs, heterostructured composites, and hybrid porous carbon doped with oxides/sulfides/nitrides). The structures represent a materials-by-design strategy to holistically address the bottlenecks in sulfur cathodes. Preeti et al. designed a 2D Cu-benzenehexathial (Cu-BHT) MOF as a host, outperforming graphene. DFT calculations show strong Al polysulfide binding (1.11–3.56 eV) and high sulfur loading (45.81 wt%). Cu-BHT’s high conductivity enhances redox kinetics and suppresses polysulfide dissolution28. The dual interactions in Cu-BHT, Al–S (host-sulfur) and S–Cu (polysulfide-copper) bonds synergistically enhance its role as a cathode host (their interactions enable strong binding with Al polysulfides due to charge transfer and the formation of Al–S–Cu and Cu–S–M bonds). This facilitates uniform deposition of Al2S3 across the Cu-BHT surface, prevents agglomeration, ensures better electrical contact, and improves active material utilization.
While the strong binding of Al2S3 on Cu-BHT enhances polysulfide confinement and promotes uniform deposition, it may also introduce certain limitations. Excessively strong interactions may hinder the reversibility of Al2S3 during charging, leading to incomplete sulfur utilization. The accumulation of Al2S3 may passivate the cathode surface, impeding electron and ion transport. Over time, this can saturate active sites on the Cu-BHT host, diminishing its ability to accommodate further reactions. Moreover, repeated Al2S3 deposition and removal can induce mechanical stress within the Cu-BHT framework, causing structural degradation. Strong chemisorption may also increase energy barriers for reoxidation, leading to higher overpotentials and reduced energy efficiency. Therefore, while Cu-BHT offers intact anchoring, an optimal balance must be achieved between binding strength and reversibility.
In another study, Al2Cl7− was replaced with Al2Cl6Br−, which exhibits a 15-fold faster dissociation rate (confirmed via DFT and Arrhenius analysis), significantly improving reaction kinetics. This enhancement was validated experimentally through increased exchange current density during Al electrodeposition. As a result, the Al–S battery using Al2Cl6Br− achieves over 80% sulfur utilization, with four times higher sulfur content and five times the current density compared to previous studies29. The introduction of Br⁻ might impact the long-term stability of the electrolyte or lead to undesired side reactions, as bromine compounds are more corrosive than chloride compounds. Also, while the dissociation rate is improved, the ionic conductivity of Al2Cl6Br− compared to other electrolytes, could still be a limiting factor.
Researchers report the development of sulfur cathodes with a high sulfur loading of 60–70 wt%, and an S loading of 3–5 mg/cm2, the highest yet for Al–S systems, aimed at enhancing energy density. Further, increasing cathode thickness improves energy storage but introduces mass transport limitations and accelerates detrimental side reactions, particularly electrolyte degradation. Electrochemical analyses in general confirm that the capacities were primarily due to sulfur redox activity, with minimal contributions from the carbon matrix. X-ray diffraction (XRD) further revealed complete sulfur utilization without crystalline degradation by-products. Sulfur content, electrode architecture, electrode kinetics, and electrolyte stability must be balanced to improve the reversibility30. Thus, the next section focuses on electrolytes for increasing the ion mobility in Al–S system.
Electrolytes for enhanced ion transport
Improvisation in electrolytes reduces the formation of passivating layers on the aluminum anode and suppresses polysulfide dissolution (shuttling) from the sulfur cathode. This directly benefits the sulfur host by enhancing redox efficiency. Moreover, electrolytes with tailored solvation structures and optimal viscosity facilitate better wetting and ion diffusion, ultimately increasing sulfur utilization. Three primary types of electrolytes have been explored in this section: (i) ionic liquid electrolytes (AlCl3/[EMIm]Cl), (ii) molten salt electrolytes (chloride-based - AlCl3–NaCl–KCl) and halide-modified complex electrolytes (in solvents like THF or DME), (iii) solid electrolytes. While ionic liquids offer thermal stability and reversible Al deposition, they suffer from high viscosity and slow kinetics. Molten salts improve ionic conductivity and sulfur redox activity at elevated temperatures but are impractical for room-temperature applications. Among these, halide-modified complex electrolytes and solid electrolytes are promising, offering enhanced Al3+ dissociation, improved sulfur utilization, and suppressed Al2S3 passivation.
In Al–S cells, electrolytes rich in Lewis‑base donors are key to dissolving aluminum polysulfides and accelerating their multi‑electron redox reactions, which in turn boost sulfur utilization. Ether‑based solvents, proven workhorses in Li–S systems, also suit the relatively low voltage range of Al–S systems and offer better chemical compatibility with both sulfur and its reduced species. Their ether oxygen atoms coordinate strongly with Al3+ and Al‑polysulfide ions, enhancing solubility; for instance, long‑chain glymes like TEGDME, which contain multiple donor sites, can increase capacity by roughly 60% compared to shorter ethers such as DME. Moreover, these high‑donicity ethers bring high boiling points, non‑flammability, and resistance to oxidative breakdown. However, their greater molecular weight raises viscosity and lowers the Al3+ transference number, potentially throttling ionic conductivity. To reconcile these trade‑offs, one can blend a high‑donicity ether (for polysulfide solvation and shuttle suppression) with a lower‑viscosity AlCl3‑based ionic liquid (for efficient Al plating/stripping and improved ion transport)31.
Further, in Al–S system, the nature of the cation affects the stability and reactivity of polysulfide intermediates. Cations with low charge density and weak solvation, like larger alkali ions, can better stabilize mid-chain polysulfides (S3−, S42−). In contrast, highly charged and strongly solvated cations (Al3+) tend to destabilize these soft polysulfide species, shifting speciation toward shorter-chain or less reactive forms. This impacts both the thermodynamics and kinetics of sulfur conversion, especially in thick electrodes where mid-chain polysulfides mediate the full utilization of solid sulfur. To optimize Al–S battery performance, electrolyte design must balance cation–polysulfide interactions, solvent donicity, and intermediate stability to enable efficient redox kinetics31.
Molten salt electrolyte
Molten salts exhibit high ionic conductivity and wide electrochemical windows, enabling fast redox kinetics and deep sulfur utilization. Their high donicity and chloride-rich nature favor the solvation and desolvation of Al3+, supporting fast charge transfer. However, their strong Lewis acidity and moisture sensitivity make them corrosive and unstable in air, requiring inert atmosphere processing and storage. The sulfur redox chemistry in molten salt electrolytes is heavily influenced by the formation and shuttle of polysulfide intermediates, which not only consumes active materials but also passivates the aluminum surface. In molten systems, the higher temperature enhances polysulfide solubility and diffusivity, exacerbating this shuttle effect compared to room-temperature liquid electrolytes. Traditionally, molten salt Al–S systems required high operating temperatures (>150 °C), limiting their practicality. However, recent advancements have enabled sub-boiling operation (~85 °C) using a tailored quaternary alkali chloroaluminate melt. This electrolyte, rich in high-order Al–Cl clusters (Fig. 3a, b), promotes rapid Al3+ desolvation12. The lower melting point of this system not only reduces thermal constraints but also broadens the applicability of molten salt Al–S system. The incorporation of nitrogen-doped porous carbon cathodes further mediates sulfur redox reactions, particularly by stabilizing polysulfide intermediates. Notably, the asymmetric sulfur conversion mechanism, supported by operando X-ray absorption spectroscopy, reveals enhanced redox kinetics even at moderate temperatures. To lower the operating temperature, a quaternary AlCl3–NaCl–LiCl–KCl electrolyte was developed (Fig. 3c, d), reducing the melting point to ~80 °C and enabling water-based thermal management. This system maintained long-chain Al–Cl clusters (Raman peak at 175.3 cm−1 and 2476 cm−1), Fig. 3d enhanced entropy-driven melting, and reduced viscosity. At 85 °C, the cell delivered 931 mAh g−1 initially, 542 mAh g−1 at 1 C, Fig. 3c, d and retained 85.4% capacity over 1400 cycles12.
a Tetrahedral phase diagram representing the quaternary alkali-metal-based inorganic molten salt electrolyte system, elucidating the compositional design space and electrolyte formulation strategies. The presence of high-order chloroaluminate clusters (AlnCl3n+1−) with extended charge delocalization highlights their pivotal role in ionic conductivity and electrochemical stability. b Structural representations of key chloroaluminate anionic clusters—AlCl₄⁻ (black), Al₂Cl₇⁻ (cyan), Al₂Cl₆ (yellow), and Al₃Cl₁₀⁻ (red)—demonstrating the structural diversity of Al-based speciation in molten salt environments. Cationic species (Li⁺, Na⁺, K⁺) are omitted for visual clarity. c Rate performance of Al|Al symmetric cells employing quaternary alkali melt and ionic liquid-based electrolytes under varying current densities from 0.5 to 5 mA cm⁻2, maintaining a fixed plating/stripping areal capacity of 1 mAh cm⁻², demonstrating electrolyte robustness and kinetic compatibility. d Sulfur K-edge X-ray absorption near-edge structure (XANES) spectra collected at various states of discharge and charge, showing the evolution among major sulfur species: neutral sulfur (S⁰, black), Al₂(Sn)3 intermediates (red and blue), and terminal Al₂S₃ (orange). These spectral fingerprints provide mechanistic insights into the redox pathways and reversibility12. e Voltage-capacity profiles of Al–Se batteries using both molten salt and ionic liquid chloroaluminate electrolytes, with performance compared under various charging rates at a constant discharge rate (D/10) at 180 °C, revealing the impact of electrolyte composition on rate-dependent behavior. f Operando Se K-edge XANES spectra (μ(E) vs. incident photon energy E) of the selenium cathode during discharge. Left: stacked spectra indicating evolution of electronic structure; right: contour map visualizing spectral transitions. The magnified region highlights isosbestic points, evidencing a clean two-phase reaction with preserved stoichiometry across initial discharge states5. g Radial distribution functions (RDFs) derived from ab initio molecular dynamics (AIMD) simulations, illustrating coordination environments and atomic correlations in the molten electrolyte: Al–S, S–Li, and S–S pair interactions42. h UV–vis absorption spectra of discharged sulfur cathodes at a capacity of 350 mAh g−1 in different electrolyte configurations: (A) Li | LiCF₃SO₃ (DME/DOL) | S cell, (B) Al | [EMIM]Cl–AlCl₃ | S cell, and (C) hybrid Al | Li⁺–[EMIM]Cl–AlCl₃ | S system. The spectra reveal solvent- and ion-specific electronic transitions correlating with polysulfide speciation42. i Proposed redox reaction pathway schematic for the sulfur cathode and aluminum anode, encompassing the sulfur oxidation and reduction steps in molten-salt-facilitated Al–S batteries. The mechanistic flow delineates intermediate phase transitions, ultimately yielding reversible conversion between S⁰ and Al₂S₃35.
Pang et al. developed a high-performance, cost-effective Al–S battery using a molten NaCl–KCl–AlCl3 electrolyte. This eutectic system melts at ~93 °C due to the formation of polychlorinated anions ([AlnCl3n+1]−), enabling moderate-temperature operation5, Fig. 3e. Compared to [AlCl3]/[EMICl], the molten salt system exhibited ~10× higher exchange current density and lower activation energy (0.19 eV vs. 0.26 eV), Fig. 3f, indicating faster Al³⁺ desolvation and superior interfacial kinetics. Ionic conductivity was also significantly higher, facilitating enhanced ion mobility32.
Raman spectroscopy and AIMD simulations revealed both short ([AlCl4]⁻, [Al2Cl7]⁻) and long-chain ([Al3Cl10]⁻, [Al4Cl13]⁻) anions. Long-chain clusters exhibited lower dissociation energies (63–109 kJ/mol) for Al3+ release compared to short-chain species (138–519 kJ/mol), making them more efficient for sustained redox activity5. Ab initio molecular dynamics (AIMD), unlike classical MD, calculates interatomic forces directly from electronic structure calculations without relying on empirical force fields, making it well-suited for simulating complex electrochemical processes such as bond breaking, formation, and charge transfer. In Al–S batteries, AIMD can simulate the thermal and electrochemical decomposition of electrolytes (ionic liquids, deep eutectic solvents, chloroaluminate salts), helping identify stable formulations. It evaluates the structural stability and sulfur-binding ability of cathode hosts, helping identify compatible materials. In studying ion diffusion pathways, AIMD captures the migration of Al3+ and polysulfides through electrolytes and interfaces, accounting for solvent reorganization and ion pairing31. This allows calculation of diffusion coefficients and informs electrolyte formulations that improve Al3+ mobility. For active-site optimization, AIMD reveals how sulfur species interact with electrode surfaces, identifies transient active sites, and helps monitor SEI formation, guiding the choice of dopants, surface terminations, or coatings that enhance redox kinetics and interfacial stability. Widely used AIMD programs include VASP, Quantum ESPRESSO, CP2K, CASTEP, and ABINIT, each offering features like femtosecond-scale time resolution, temperature control (Nose–Hoover thermostats), and real-time tracking of charge density and atomic structure31.
Al–S cells using molten salts showed two times lesser lower polarization (~50 mV), double the capacity (1350 mAh g−1), better rate capability (500 mAh g−1 at 10 C), and long cycle life (350 mAh g−1 after 500 cycles), outperforming conventional ionic liquids5.
Other systems include AlCl3–EMIMCl (25–60 °C), which offers room-temperature operation with moderate conductivity, and AlCl3–NaCl–CaCl2/MgCl2 (~100 °C), which improves salt stability and sulfur compatibility. Deep eutectic solvents like AlCl3–urea (~30–70 °C) present alternatives but with slower kinetics. Across all systems, the core redox involves Al → Al3+ at the anode, complexed as AlCl4⁻/Al2Cl7⁻, and cathodic S8 → S2− conversion, forming Al2S3 via multi-step redox governed by dynamic Al–Cl cluster dissociation and reformation.
Ionic liquids
Al–S systems employing imidazolium‑based ionic liquids have long suffered from opaque discharge pathways and polysulfide chemistry. Williams et al. introduce a phenomenological model (Al anode, S@CNT cathode, EMIMCl–AlCl3 electrolyte) that integrates the complex redox reactions, Al2Sx speciation, and solid Al2S3 precipitation kinetics, and validates its predictions against Swagelok‑cell experiments33. The model revealed two primary Al2S3 formation routes, direct solid precipitation and via dissolved Al‑polysulfide intermediates, and quantitatively captures how increasing current density reduces capacity by shifting the reaction zone and exacerbating contact resistance at the cathode. Contact resistance is the key rate‑limiting step at high discharge rates; the work underscores the necessity of optimized electrode architectures (enhanced porosity or conductive scaffolds) to improve both energy and power density. While offering essential insights, the model relies on several simplifying assumptions that may limit its real‑world applicability. It treats the complex cathode chemistry as two decoupled steps with identical forward/backward energy barriers, confines ion transport to a single (axial) direction, and assumes polysulfides are short‑lived, fully dissolved species. It further presumes a constant dissolution rate and uniform porosity increase regardless of current density, no accumulation or cross‑interface transfer of sulfide/polysulfide species, and that Al2S3 always precipitates onto either bare CNT or S8 based solely on rate. Built around a specific Swagelok cell design and reliant on fitted parameters, these idealizations may not hold under varied materials, geometries, or operating conditions. In another study, Yu et al. introduced a Li+‑ion mediation strategy by dissolving Li⁺ in an imidazolium‑based ionic liquid, which improved room‑temperature performance. The Li+‑ion–mediated cells deliver an initial capacity near 1000 mAh g−1 and retain about 600 mAh g−1 after 50 cycles, far surpassing conventional Al–S systems. Mechanistic studies show that Li⁺ ions solvate and reactivate aluminum polysulfides, enhancing their solubility and preventing the formation of insulating Al═S surface species that otherwise block redox reactions. By stabilizing soluble intermediates and maintaining active polysulfide turnover, Li⁺ mediation accelerates kinetics and boosts reversibility33. Li+ mediation enhances reaction kinetics and reversibility by stabilizing intermediate polysulfide species, such as S42−, preventing their decomposition into inactive forms. Additionally, Li+ improves ionic conductivity and promotes charge transfer at the electrode–electrolyte interface, leading to better redox processes and cycling stability.
The physical properties of ionic liquids, such as density, viscosity, and conductivity, influence the electrochemical performance. Density is influenced by the concentration of AlCl3−, a higher mole fraction of AlCl3 increases the density due to the presence of heavier Al2Cl7− ions. Viscosity decreases with an increasing mole fraction of AlCl3 because of stronger cation-anion interactions and hydrogen bonding. Higher conductivity was observed with an increasing molar concentration of AlCl4−, as its smaller volume and higher symmetry allow for faster movement in the electrolyte solution34. Xia et al. developed AlCl3/triethylammonium chloride ionic liquid doped with small volumes (20 vol%) of organic solvents toluene, benzene, dichloromethane (DCM), and 1,2‑dichloroethane (DCE) to overcome sluggish kinetics and high viscosity of ionic liquids (IL)34. DCM and DCE blends proved optimal, boosting the discharge capacity from 85 mAh g−1 (pure IL) to 114 mAh g−1 in DCM, while reducing viscosity. The lower the viscosity, the higher the conductivity. For an IL-DCM system, a volume ratio of about 0.9 results in the highest conductivity, measuring 20.4 mS cm−1. The conductivity of a system with a volume ratio of 0.2 was similar to that of an IL-DCE system with a volume ratio of 0.4. For the IL-DCE system, conductivity starts to decline after a volume ratio of 0.8, due to the decreasing concentration of ions. The impact on viscosity and conductivity is most notable before a volume ratio of 0.4 for all four organic solvent-diluted systems, with a weaker trend thereafter. Considering these results and the volatility of organic solvents, a volume ratio of 0.2 is considered the better proportion34. Yu et al. developed a room-temperature Al–S battery using a Li⁺-mediated ionic liquid electrolyte, where Li⁺ reactivates Al polysulfides/sulfides during cycling. DFT confirms Li3AlS3-like discharge products, enabling an initial capacity of ~1000 mAh g−1 and retention of ~600 mAh g−1 after 50 cycles. Figure 3g illustrates the structure of amorphous Li3AlS3 formed via melt-quenching, showing ~50% volume expansion and Al–S polyhedra stabilized by Li⁺ without S–S bonding. Radial distribution function analysis confirms predominant 4-/5-fold Al coordination and a terminal-to-bridging S ratio of ~5:4, with Li⁺ preferentially surrounding terminal S atoms. UV–vis spectra reveal that Li⁺-ion mediation enables the solubility of both S62− and S42− species (Fig. 3h), unlike the Al‖Al[EMI]Cl4‖S cell, which only shows soluble S62−. This enhanced solubility of S42− supports improved electrochemical performance in Li⁺-mediated systems.
Unlike conventional Al–S cells that rely on sulfur reduction (S8 → Al2S3) and are limited to ~0.5 V, this work exploits the reverse reaction electrochemical oxidation of sulfur, using an AlCl3/carbamide ionic liquid. Here, the AlCl4− anion serves as a potent oxidant, extracting electrons from elemental sulfur to form a covalent S–Cl complex, AlSCl7, at the cathode during charge35. On discharge, AlSCl7 accepts electrons to regenerate S8 with ∼94% Coulombic efficiency, demonstrating minimal side reactions. This redox couple operates at ∼1.8 V, a threefold voltage increase over the reduction pathway, directly translating to higher energy density.
DFT calculations corroborate this high voltage, predicting a 1.76 V equilibrium potential for the AlCl4−/AlSCl7 couple and revealing a low 0.52 eV activation barrier for the AlSCl7 → S8 conversion. By contrast, the traditional Al2S3 → S8 reverse reaction faces a steep 3.98 eV barrier Fig. 3i, explaining its poor reversibility. In situ synchrotron near-edge X-ray absorption (NEXAFS) shows a positive shift in the Cl L‑edge consistent with S–Cl bond formation, while X-ray photoelectron spectroscopy (XPS) and operando Raman spectroscopy track the cyclic appearance and disappearance of AlSCl7‑specific signatures35.
Solid-state electrolytes
Solid-state systems offer better safety and thermal stability but face challenges like brittleness (inorganics) or low conductivity (polymers). Quasi-solid-state systems aim to balance these trade-offs. Future work should improve conductivity, interface stability, and cost-effective fabrication of hybrid systems. Jiao et al. designed a quasi‑solid‑state design; sulfur embedded in cobalt‑ and nitrogen‑doped graphene (CoNG) paired with an ionic‑liquid–impregnated MOF (IL@MOF) electrolyte36. This architecture confines polysulfides, maintains Al3+ conduction, and leverages CoNG’s low Al3+ dissociation barrier (1.22 eV) and accelerated Al2S6→Al2S3 conversion (2.25 eV), delivering 820 mAh g−1 at 50 mA g−1 and 78% capacity retention after 300 cycles, better than IL. Replacing CoNG with Pt/N‑doped graphene (PtNG) and IL@MOF with a MOF‑filled gel polymer (MOF@GPE) further boosted kinetics, achieving 1009 mAh g−1 and 65% retention over 300 cycles. Scaling up, a collector‑free pouch cell using an MOF@GPE electrolyte and S@CoNG cathode yielded 288 mAh g−1 (685 on sulfur), ~1.0 V, and 81.6% retention over 400 cycles, achieving > 90 Wh kg−1—surpassing lead‑acid. Despite these advances, challenges remain in improving solid‑electrolyte conductivity, reducing catalyst costs, increasing sulfur loading, and closing the gap to Li‑ion energy densities. Zhang et al. harnessed reversible sulfur oxidation to S2Cl2 rather than conventional sulfur reduction to deliver 1.8 V discharge and 2213 mAh g−1 capacity16. Mass spectrometry confirms S2Cl2 as the main charge product, with other sulfur chlorides present. A polymer membrane swollen with AlCl3/acetamide electrolyte plays three key roles: it blocks parasitic side reactions, stabilizes the Al anode’s interface, and enables selective Al3+ transport. As a result, the cell retains 92.1% of its capacity over 50 cycles and 490 cycles in total. By coupling a high‑voltage redox pathway with membrane‑enabled interfacial control, this design achieves both high energy density and long‑term stability. Polymer membrane here confines dissolved sulfur chlorides, slowing their diffusion and controlling the shuttle effect while also suppressing parasitic oxidation of the electrolyte. Simultaneously, it shields the Al anode from corrosion and uneven deposition.
Moving to a solid‑state electrolyte in a pouch cell combines packaging advantages with enhanced safety, enhanced energy density, and stability. Eliminating flammable liquid electrolytes removes leakage and thermal‑runaway risks, while the thin, ionically conductive electrolyte allows leaner cell designs (thinner separators, more active material). This also helps in achieving high gravimetric energy density (Wh kg−1) due to the reduction of heavy liquid electrolyte weight. Huang et al. demonstrated a solid‑state Al–S pouch cell, essential for scaling lab‑scale concepts to using a composite gel polymer electrolyte and collector‑free sulfur cathode, Fig. 4a9. In a three‑layer S | four‑layer Al architecture, it delivers 288 mAh (cell‑level), >90 Wh kg−1, and 80% capacity retention over 400 cycles by effectively suppressing polysulfide shuttling (Fig. 4b–d). The pouch format enables practical device demonstrations (powering a smartphone). Gel polymer electrolytes (GPEs) can’t fully block polysulfide leakage due to their high liquid fraction and are hard to produce at scale, while common metal current collectors corrode in acidic ionic liquids, forcing the use of expensive refractory metals. Solid‑state pouch cells also suffer from poor ionic conduction in thick cathodes and require external pressure to maintain intimate electrode/electrolyte contact. With the solid electrolyte making up 48 wt% of the cell (versus only 31 wt% for electrodes) and inherently slow sulfur redox kinetics, these issues collectively constrain rate performance, manufacturability, and energy efficiency in Al–S batteries. Sulfide-based solid electrolytes such as Li10GeP2S12 and Li3PS4 are efficient with ionic conductivities up to 2.5 × 10−2 S cm−1 and intrinsic safety over LIBs. These systems achieve energy densities up to 750 Wh L−1 but face hurdles in voltage stability, and electrode–electrolyte interfacial compatibility10.
a Schematic representation of the solid-state Al–S pouch cell configuration, detailing the multilayer structure comprising Al anode, solid electrolyte, sulfur-based composite cathode, and encapsulating flexible packaging. b Comparative charge–discharge voltage profiles of liquid-state and solid-state Al–S pouch cells operated at 0.05 C, highlighting improved voltage polarization and stability in the solid-state configuration. c Discharge curves of the solid-state Al–S pouch cell under varying current rates, demonstrating rate capability and kinetics of sulfur redox processes within the solid-state matrix. d Galvanostatic Al plating/stripping profiles of symmetric Al|electrolyte|Al cells using either ionic liquid electrolyte (ILE) or a composite modified solid electrolyte (MSE@GPE), measured at a constant current density of 0.5 mA cm⁻2, revealing lower overpotentials and improved reversibility in the MSE@GPE-based system9. e Long-term cycling behavior of Al|AE | S full cells using either untreated (u-Al) or previously cycled aluminum (pc-Al) anodes in AE-2Na electrolyte, illustrating the influence of surface conditioning and electrolyte compatibility on capacity retention and stability. f Corresponding voltage profiles of the 50th cycle for cells with u-Al and pc-Al, showing electrochemical maturity, reaction consistency, and improved voltage plateaus with the preconditioned Al anode. g Cycling performance of the Al–S battery using the AE-1K electrolyte at 100 mA g⁻¹, displaying gradual capacity evolution and stabilization indicative of interfacial adaptation and long-term integrity37. h Cyclic voltammetry (CV) profiles of symmetric Al|electrolyte|Al cells under various electrolytic environments, providing insights into redox kinetics, ionic transport, and reversibility of Al electrodeposition/stripping. i Galvanostatic discharge–charge curves at the first cycle (1 A g⁻¹) for Al–S batteries comprising different sulfur composite cathodes, emphasizing the cathode-specific electrochemical behavior, including activation overpotential and specific capacity delivery39. j Schematic of a quasi-solid-state Al–S battery and its working mechanism, delineating ionic transport, phase evolution, and electrochemical redox reactions in the hybrid electrolyte system combining solid framework and liquid infiltration for improved safety and performance. k Comparative cycling stability of liquid-state versus quasi-solid-state Al–S cells tested at 50 mA g⁻¹, underscoring the enhanced lifespan, Coulombic efficiency, and capacity retention of the quasi-solid-state design8. l Schematic illustration of the synthesis and integration of AlMo4S8/CNTs@S composite as an advanced cathode material, engineered to improve conductivity, structural confinement of polysulfides, and electrochemical activity. m Charge–discharge profiles of Al-ion batteries using AlMo4S8 cathodes at 100 mA g-1 and 25 °C40.
Xu et al. added alkaline chlorides (LiCl, NaCl, KCl) to Al–S battery electrolytes to improve the cycle life by forming a thick, NaxAlyO₂‑rich SEI on the Al anode that suppresses polysulfide shuttling37. With NaCl, capacity rises to 473 mAh g−1 after 50 cycles at 100 mA g−1 (vs. 313 mAh g−1 without additive), and with KCl, to 496 mAh g−1 after 100 cycles at 50 mA g−1 Fig. 4e-g. SEM and EDX of aluminum anodes (AMAs) cycled in Al–S cells with (AE‑2Na) and without NaCl (AE) show similar stripping/plating morphologies, but only AE‑2Na exhibits Na signals in its SEI. XPS reveals that AE‑2Na forms a thicker NaxAlyO₂ containing interphase, evidenced by a Na 1s peak at 1073.9 eV and suppressed Al⁰ intensity in Al 2p, while Cl 2p and C 1s spectra confirm additional NaCl and organic SEI components. Crucially, S2p spectra show fewer deposited polysulfides on AE‑2Na. Scaling up of these NaCl additives demands control of NaCl concentration in large volume chloroaluminate electrolytes, and mixing management is required to prevent salt precipitation. Industrial cells must reliably form a uniform, ion-conductive SEI on every aluminum foil, as minor variations in surface roughness, current density, or cell geometry can compromise interphase integrity and re-expose the anode to polysulfides over extended cycling. While a thicker SEI enhances stability, it also increases cell impedance; balancing interphase thickness with power capability, uniform SEI formation in large-format pouch or prismatic cells is essential for commercial viability.
Amaresh et al. presented the synthesis of high-performance lithium ionic conductors based on the Li2S–Al2S3–GeS–P2S5 system. Using short-duration mechanical milling and a single-step heat treatment at 550 °C, crystalline powders with high ionic conductivity comparable to organic liquid electrolytes were obtained. The optimal Al:Ge ratio (30:70) yielded an impressive ionic conductivity of 1.7 × 10−3 S cm−1 at 25 °C and a low activation energy of 17 kJ mol−1 among the best for solid electrolytes38.
Electrocatalysts for boosting reaction kinetics and efficiency
Electrocatalysts accelerate electrochemical reactions by lowering the activation energy (by facilitating the adsorption of reactants, promoting intermediate species formation, and enabling efficient electron transfer at the electrode–electrolyte interface) without being consumed. Their activity depends on factors like surface area, electronic structure, and binding energy to intermediates. Lin et al. introduced a Mo6S8-based electroactive-catalytic conductive framework for tackling sulfur’s low conductivity and sluggish kinetics7. The Mo6S8/S cathode delivers 371 Wh kg−1 with 569 mV reduced voltage hysteresis, enhanced conductivity (138.4 S cm−1), Al-ion storage (70 mAh g−1), and strong polysulfide adsorption. Featuring an Al3+ intercalation–sulfur conversion mechanism, it outperforms conventional C/S cathodes.
Al–S system faces poor reversibility due to the high Gibbs free energy of Al2S3 decomposition (–713.2 kJ/mol), leading to polarization. To overcome this, Mo6S8 lowers the Al2S3 decomposition barrier (1.06 eV vs. 2.32 eV on carbon), enhances catalytic activity, and strongly adsorbs sulfur intermediates (adsorption energy: –4.51 eV).
Single-atom catalysts utilize every metal atom as an active site, offering nearly 100% atomic utilization, and the isolated metal atoms are typically anchored on supports like nitrogen-doped carbon or metal oxides, allowing modulation of the local electronic structure (modulate the d-band center or the charge redistribution). These strong metal-support interactions not only stabilize the active site but also enhance charge transfer. Cobalt (Co2+/Co3+) acts as an effective electrocatalyst, and its incorporation into the sulfur cathode reduces voltage hysteresis from 1.3 to 0.8 V and enables stable capacity retention (~500 mAh g−1 after 200 cycles at 1 A g−1), Fig. 4h-i39. The catalytic activity arises from the reversible formation of cobalt sulfides and Co valence transitions. The presence of Co-(II), Co-(III) increases the reaction activity of polysulfides. Further, the CoN4 sites enhance sulfur conversion, reducing the voltage gap to 0.43 V and achieving a discharge voltage plateau of 0.9 V36. Zheng et al. utilized a single-atom electrocatalyst, platinum/nitrogen co-doped graphene (PtNG), and a MOF-filled gel polymer electrolyte to enhance energy density and cycle life8. The Al–S battery achieved a specific capacity of 1009 mAh g−1 with a discharge plateau of ~0.95 V and maintained 65% capacity retention after 300 cycles (Fig. 4j, k). The PtNG accelerated sulfur redox kinetics, while the electrolyte effectively mitigated the polysulfide shuttle effect. An iron single-atom catalyst supported on nitrogen-doped carbon nanofibers (NCF) as a free-standing interlayer acts as both a catalyst and a chemical barrier, effectively mitigating the shuttle effect and improving the kinetics of polysulfide conversion (specific capacity of 780 mAh g−1)4.
Defects in materials enhance the electrochemical performance by increasing the number of active sites (facilitating better adsorption and activation of intermediates such as AIPSs). These defects improve ion and electron transport by creating additional pathways for charge carriers, reducing resistance, and enabling faster reactions. Moreover, defects alter the material’s electronic structure and lower the activation energy for polysulfide conversion. Zhou et al. introduced defect spinel Aluminum molybdenum sulfide (AlMo4S8) embedded in CNTs (Fig. 4l) as a dual-function catalyst, which addresses polysulfide shuttling by facilitating ion transport and catalyzing the conversion of AlPSs and Al2S340. The material achieves a specific capacity of 304.3 mAh g−1 at 500 mA g−1 with stable cycling over 50 cycles (Fig. 4m). CNTs improve conductivity, DFT confirms strong interactions between AlMo4S8 and AlPSs.
MOFs are strong electrocatalysts, and Zhou et al. studied MOF-derived copper-molybdenum oxide (Cu/MoO2) catalyst, which effectively anchors AIPSs, reducing shuttle effects and promoting their conversion during cycling41. The catalyst’s nanostructure, which combines copper clusters and MoO2 nanoparticles, improves sulfur utilization and conductivity. DFT calculations show strong interactions between AlPSs and Cu/MoO2, facilitating polysulfide conversion and inhibiting Al2S3 deposition. The Al–S battery with Cu/MoO2@C shows an initial capacity of 875 mAh g−1 at 500 mA g−1, maintaining 967 mAh g−1 at 50 °C.
Future research prospects
Future research in Al–S system will strategically converge on mitigating persistent challenges (i) sluggish redox kinetics, (ii) polysulfide shuttle effects, and interfacial degradation. Key directions will include enhancing sulfur utilization, improving long-term cyclability, and increasing areal capacity, critical for practical deployment in flexible and high-energy-density applications. To overcome sulfur-related issues, advances in atomically dispersed electrocatalysts and hierarchical host architectures are anticipated to catalyze rapid and reversible Al-polysulfide conversions while confining intermediate species. The incorporation of functional interlayers and selectively permeable separators will further inhibit the shuttle of soluble polysulfides and stabilize the electrode–electrolyte interface. Concurrently, engineered electrolytes, including multi-ionic liquids, polymer gel matrices, and deep eutectic solvents, are expected to provide wider electrochemical windows, enhanced ionic conductivity, and chemical compatibility with both aluminum and sulfur species. The integration of artificial intelligence (AI) will play an essential role in accelerating new materials discovery and optimization. Quantum-inspired models and AI-guided predictive algorithms will be employed to navigate vast compositional spaces and simulate reaction pathways with high fidelity. Digital twin frameworks will enable real-time monitoring and predictive diagnostics, allowing for adaptive control over battery operation and lifetime. Moreover, high-throughput robotic experimentation and automated combinatorial screening will expedite the evaluation of material libraries under diverse conditions, drastically reducing time-to-discovery and validation cycles. On the structural front, multifunctional electrodes, binder-free architectures, and 3D-printed cathodes with tailored porosity and conductivity will support higher areal loading while reducing inactive mass. These innovations will be necessary for transitioning Al–S systems to commercial domains, particularly in the realm of electronics. Currently, Avanti Battery Company, an MIT-incubated venture, is active in Al–S development, but the field is poised for substantial growth with numerous other companies likely to emerge in the near term.
Conclusions
The performance of Al–S systems is deeply intertwined with the architectural design of their host structures. Traditional bulk materials fall short in addressing the fundamental electrochemical challenges of Al–S systems, especially the shuttle effect of aluminum polysulfides, sluggish redox kinetics, and volumetric changes. Recent innovations focus on advanced host architectures that synergistically combine structural engineering, materials chemistry, and interface science. Multi-layered host frameworks offer an effective approach to immobilize soluble AlPSs through physical and chemical confinement, thereby mitigating shuttle effects and enabling prolonged cycling. Sandwich-structured Ti₃C₂Tx MXene-based hosts not only anchor polysulfides but also provide high electrical conductivity and strong binding energy with sulfur species. The integration of single-atom catalysts further optimizes the redox kinetics by lowering energy barriers, facilitating the conversion of intermediates, and enhancing overall charge transfer efficiency. These structures reveal the balance between adsorption strength and catalytic activity, governed by DFT-calculated binding energies and volcano plot kinetics. 2D and 3D host architectures have high surface areas and tailored porosities to stabilize sulfur and AlPSs. Materials like BN, MoS2, and graphene-based composites serve as dual-function matrices that immobilize polysulfides and accommodate volume expansion. Expanded interlayer spacings and 3D porous networks alleviate ion diffusion limitations associated with trivalent Al3+ ions, ensuring high capacities and structural resilience. Notably, 2D Xenes like β-Germanene show superior sulfur anchoring and ion transport behavior, attributed to their favorable adsorption energies and charge redistribution characteristics. Embedded and fibrous host designs utilize confined nanoarchitectures to trap sulfur species and improve the kinetics of electrochemical reactions. Selenium-sulfur hybrid systems within carbon nanofiber networks exemplify a strategy for enhancing conductivity and suppressing dissolution. Fibrous matrices provide interconnected pathways that improve electron/ion transport while chemically active surfaces stabilize intermediate species. Embedding catalytically active nanoparticles, such as CoS2, within nitrogen-doped carbon, enhances both battery and HER performance by boosting surface electronic states and facilitating fast redox kinetics.
By mitigating Al2S3 passivation, suppressing polysulfide shuttling, and enhancing sulfur redox kinetics, tailored electrolytes are helpful in achieving high sulfur utilization and prolonged cycling stability. Three primary classes of ionic liquids, molten salts, and halide-modified complex/solid-state electrolytes demonstrate varying degrees of success in balancing ion transport, redox activity, and operational feasibility. Ionic liquids, particularly AlCl3/[EMIm]Cl systems, offer intrinsic thermal stability and reversible Al plating/stripping but are hindered by high viscosity and sluggish ion transport. Innovations like solvent co-doping (e.g., DCM, DCE) and Li⁺-ion mediation strategies have markedly improved capacity retention, ionic conductivity, and redox kinetics at room temperature by reactivating Al polysulfides and minimizing Al═S surface blocking. Molten salts, including ternary and quaternary systems like AlCl₃–NaCl–KCl and AlCl3–NaCl–LiCl–KCl, display superior ionic conductivities and facilitate rapid Al3+ desolvation. These electrolytes support enhanced sulfur conversion, deep cycling, and faster kinetics, particularly at moderate temperatures (~85–93 °C). However, challenges remain regarding air stability, corrosion, and system integration due to their Lewis acidity and thermal requirements.
Halide-modified complex electrolytes and high-dicinity ether solvents (TEGDME) emerge as promising candidates, offering improved polysulfide solubility, shuttle suppression, and optimized cation–polysulfide interactions. Nonetheless, their higher molecular weight contributes to increased viscosity and lower transference numbers, necessitating co-solvent blending strategies for performance tuning.
References
Net Zero by 2050 – Analysis - IEA, (n.d.). https://www.iea.org/reports/net-zero-by-2050 (accessed May 1, 2025).
Fleming, M., Kannan, S. G. & Eggert, R. Long-run availability of mineral resources: the dynamic case of lithium. Resour. Policy 97, 105226 (2024).
Baji, D. S. et al. Overarching advancements in building practical Li-S batteries: a holistic review. J. Energy Storage 100, 113412 (2024).
Wang, F. et al. Atomically dispersed iron active sites promoting reversible redox kinetics and suppressing shuttle effect in aluminum–sulfur batteries. Nanomicro Lett. 14, 1–12 (2022).
Pang, Q. et al. Fast-charging aluminium–chalcogen batteries resistant to dendritic shorting. Nature 608, 704–711 (2022).
Shao, S. et al. The exotically stoichiometric compounds in Al–S system under high pressure. NPJ Comput Mater. 6, 1–6 (2020).
Lin, Z. et al. Electroactive-catalytic conductive framework for aluminum-sulfur batteries. Energy Storage Mater. 51, 266–272 (2022).
Huang, Z. et al. Single-atom electrocatalyst and gel polymer electrolyte boost the energy density and life of aluminum-sulfur batteries. J. Mater. Sci. Technol. 152, 86–93 (2023).
Huang, Z. et al. A hundreds-milliampere-hour-scale solid-state aluminum–sulfur pouch cell. Adv. Energy Mater. 13, 2302464 (2023).
Zhang, Q. et al. Sulfide-based solid-state electrolytes: synthesis, stability, and potential for all-solid-state batteries. Adv. Mater. 31, 1901131 (2019).
Liu, S. et al. An advanced high energy-efficiency rechargeable aluminum-selenium battery. Nano Energy 66, 104159 (2019).
Meng, J. et al. Rapid-charging aluminium-sulfur batteries operated at 85 °C with a quaternary molten salt electrolyte. Nat. Commun.15, 1–10 (2024).
Zheng, X., Wang, Z., Li, J. & Wei, L. Binder-free S@Ti3C2Tx sandwich structure film as a high-capacity cathode for a stable aluminum-sulfur battery. Sci. China Mater. 65, 1463–1475 (2022).
Wang, Z. et al. Unraveling the anchoring effect of MXene-supported single atoms as cathodes for aluminum-sulfur batteries. ACS Mater. Lett. 4, 1436–1445 (2022).
Sun, Q., Chai, L., Yang, X., Zhang, W. & Li, Z. Hollow tubular sea-urchin structure with high catalytic activity of NiCo2Se4@CS2 cathodes for high-performance Al/S batteries. J. Colloid Interface Sci. 677, 284–292 (2025).
Zhang, D. et al. High-voltage aluminium-sulfur batteries with functional polymer membrane. Adv. Funct. Mater. 32, 2205562 (2022).
Zhang, K. et al. Two-dimensional boron nitride as a sulfur fixer for high performance rechargeable aluminum-sulfur batteries. Sci. Rep. 9, 1–10 (2019).
Vijaya Kumar Saroja, A. P., Kamaraj, M. & Ramaprabhu, S. Strongly coupled sulfur nanoparticles on graphene-carbon nanotube hybrid electrode for multifunctional sodium and aluminium ion storage. J. Alloy. Compd. 818, 152864 (2020).
Xu, W., Kuang, Y., Feng, T., Xia, J. & Wu, Q. Emerging two-dimensional Xenes for catalyzing aluminum-sulfur batteries: a DFT study. Appl Surf. Sci. 686, 162201 (2025).
Li, L. et al. Novel insight into rechargeable aluminum batteries with promising selenium sulfide@carbon nanofibers cathode. Adv. Mater. 35, 2209628 (2023).
Lin, Y. et al. CoS2 nanoparticles embedded N-doped carbon nanotubes hollow polyhedron for hydrogen evolution and Al−S batteries. ChemCatChem 16, e202401155 (2024).
Sun, F. et al. Vapor deposition of aluminium oxide into N-rich mesoporous carbon framework as a reversible sulfur host for lithium-sulfur battery cathode. Nano Res.14, 131–138 (2021).
Zhou, Q. et al. Polyoxometalates@metal-organic frameworks derived bimetallic Co/Mo2C nanoparticles embedded in carbon nanotube-interwoven hierarchically porous carbon polyhedron composite as a high-efficiency electrocatalyst for Al–S batteries. Small 19, 2304515 (2023).
Mei, Q. et al. TiO2/Fe2O3 heterostructures with enhanced photocatalytic reduction of Cr(VI) under visible light irradiation. RSC Adv. 9, 22764–22771 (2019).
Danewalia, S. S. et al. Effect of transition metals (MO-TiO2, MnO2, Fe2O3, and ZnO) on crystallization and electrical conductivity of SiO2–CaO–Na2O–P2O5-based glass-ceramics. Ionics 26, 2959–2967 (2020).
Deng, G. et al. 3D-printed Co-doped MoS2/Ti3C2Tx/S cathode with accelerated adsorption and conversion of lithium polysulfides for advanced lithium-sulfur batteries. Mater. Today Energy 51, 101877 (2025).
Li, Z., Yang, Z. J., Moloney, J., Yu, C. P. & Chhowalla, M. Quasi-solid-state electrolyte induced by metallic MoS2 for lithium-sulfur batteries. ACS Nano 18, 16041–16050 (2024).
Bhauriyal, P. & Pathak, B. Superior anchoring effect of a Cu-benzenehexathial MOF as an aluminium–sulfur battery cathode host. Mater. Adv. 1, 3572–3581 (2020).
Yang, H. et al. An aluminum–sulfur battery with a fast kinetic response. Angew. Chem. 130, 1916–1920 (2018).
Lampkin, J., Li, H., Furness, L., Raccichini, R. & Garcia-Araez, N. A critical evaluation of the effect of electrode thickness and side reactions on electrolytes for aluminum–sulfur batteries. ChemSusChem 13, 3514–3523 (2020).
Zou, Q. & Lu, Y. C. Liquid electrolyte design for metal-sulfur batteries: Mechanistic understanding and perspective. EcoMat 3, e12115 (2021).
Kang, Z., He, M. & Lu, G. Experimental measurements and thermodynamic optimization of the NaCl+RbCl phase diagram. Materials 15, 6411 (2022).
Appiah, W. A., Li, H., Lampkin, J. & García-Lastra, J. M. Towards understanding aluminum sulfur batteries with imidazolium-based electrolytes: A phenomenological model. J. Power Sources 529, 231254 (2022).
Xia, S., Zhang, X. M., Huang, K., Le Chen, Y. & Wu, Y. T. Ionic liquid electrolytes for aluminium secondary battery: Influence of organic solvents. J. Electroanal. Chem. 757, 167–175 (2015).
Li, H. et al. Reversible electrochemical oxidation of sulfur in ionic liquid for high-voltage Al−S batteries. Nat. Commun. 12, 1–8 (2021).
Huang, Z. et al. Electrocatalysis for continuous multi-step reactions in quasi-solid-state electrolytes towards high-energy and long-life aluminum–sulfur batteries. Angew. Chem. Int. Ed. 61, e202202696 (2022).
Xu, C., Diemant, T., Liu, X. & Passerini, S. Modified solid electrolyte interphases with alkali chloride additives for aluminum–sulfur batteries with enhanced cyclability. Adv. Funct. Mater. 33, 2214405 (2023).
Amaresh, S., Karthikeyan, K., Kim, K. J., Lee, Y. G. & Lee, Y. S. Aluminum based sulfide solid lithium ionic conductors for all solid state batteries. Nanoscale 6, 6661–6667 (2014).
Guo, Y. et al. Rechargeable aluminium–sulfur battery with improved electrochemical performance by cobalt-containing electrocatalyst. Angew. Chem. Int. Ed. 59, 22963–22967 (2020).
Zhou, Q. et al. Defect spinel aluminum molybdenum sulfide: a dual-function catalyst for polysulfide conversion and aluminum intercalation in aluminum–sulfur batteries. Adv. Sci. 12, 2417061 (2025).
Li, J. S. en et al. Polyoxometalate-based metal–organic framework-derived hybrid electrocatalysts for highly efficient hydrogen evolution reaction. J. Mater. Chem. A Mater. 4, 1202–1207 (2016).
Yu, X., Boyer, M. J., Hwang, G. S. & Manthiram, A. Room-temperature aluminum-sulfur batteries with a lithium-ion-mediated ionic liquid electrolyte. Chem 4, 586–598 (2018).
Author information
Authors and Affiliations
Contributions
B.R. conceptualized the study, conducted the literature review, wrote the initial draft, performed data visualization, and contributed to the review and editing of the manuscript. D.G. and R.P.R. reviewed and edited the manuscript. S.R. conceptualized and supervised the overall work
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ramasubramanian, B., Ganguly, D., Rao, R.P. et al. Progress, pitfalls, and prospects in emerging materials for aluminum-sulfur batteries. Commun Chem 8, 301 (2025). https://doi.org/10.1038/s42004-025-01690-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-025-01690-0