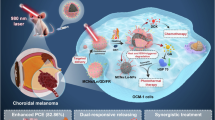
Abstract
Photothermal therapy (PTT) has emerged as a promising strategy for treating solid tumors and topical infections by converting the incident light energy into localized heat using photothermal agents. Among these, gold nanoparticles (GNPs) are particularly attractive due to their strong surface plasmon resonance, tunable surface chemistry, biocompatibility and scalability. However, their limited biodegradability and inefficient clearance remain significant translational challenges. In this study, we have developed gold-coated calcium peroxide nanoparticles (CPAu-NPs) that offer dual advantages, enhanced photothermal conversion and intrinsic reactive oxygen species generation. The self-release of oxygen and hydrogen peroxide from CPAu-NPs addresses tumor hypoxia, a key barrier to effective therapy. To further augment therapeutic efficacy, we incorporated Sorafenib, a multi-kinase inhibitor known to induce ferroptosis and inhibit tumor progression in melanoma, a cancer type marked by dysregulated iron metabolism and vulnerability to ferroptosis. This combinatorial approach disrupts critical survival pathways while promoting lipid peroxidation, potentially overcoming resistance to standard treatments. Additionally, we explored the antifungal potential of this system, recognizing the increased susceptibility of immunocompromised cancer patients to fungal infections. Our results suggest that CPAu-NPs, in combination with Sorafenib, provide a multifunctional theranostic platform capable of targeting melanoma cells, modulating the tumor microenvironment, and addressing opportunistic fungal infections.
Similar content being viewed by others
Introduction
Photothermal therapy (PTT) is an affordable alternative to temperature-augmented therapy for treating solid tumors and topical microbial infections. PTT takes advantage of photothermal agents (PTA), which convert the light energy into localized hyperthermia when excited by near-infrared radiations (NIR)1,2,3. PTT is advantageous over conventional therapies owing to their selectivity, accuracy, and minimally invasive nature. Among the different metallic PTAs, Gold nanoparticles (GNPs) have been widely employed for PTT owing to their unique properties. The surface plasmon resonance (SPR) phenomenon is particularly well-suited for noble metal nanoparticles, which improves their radioactive absorption and dispersal characteristics. Since PTT uses the SPR effect to generate heat through laser absorption, GNPs are promising materials for temperature-augmented PTT. GNPs possess good biocompatibility, surface modification window, and high-yield scale-up possibility. Though efficient as imaging agents, size and biodegradability of these nanosystems play a major role for use in photothermal treatment4,5,6,7,8. A size, less than 5–6 nm, in case of inorganic metal system is required for an effective renal clearance through the glomerular basement membrane. Hence, multifunctional nanosystems capable of effective clearance via both the hepato-biliary route and renal route and also serving as efficient PTA are of increasing demand. The use of SPR of NGPs in PTT dates back decades to the development of silica-gold-based nanostructures. This led to the development of various biodegradable templates (cores) for tuning the NIR absorbance of gold coated over their surfaces9,10,11,12.
A strong interlink between microbial infections and cancer progression has been established in the recent past. Due to the compromised immune system, occasional neutropenia (an adverse effect of prolonged chemotherapy), and disruption of the normal anatomical barriers in superficial tumors (owing to surgical resection or radiation damage), microbial infections are the most complicated consequences in cancer patients. Recently, it has been reported that patients with candidiasis are prone to increased cancer risks13,14,15,16,17. Hence, a theranostic strategy capable of eliminating microbial infections and simultaneously killing tumor cells is warranted18.
In this study, we have reported a gold coated calcium peroxide nanoparticles (CPAu NPs) for photothermal applications. The advantage of the used CaO2 NPs is the stimuli responsive and O2/H2O2 self-sufficient nature, to tackle the hypoxic tumor microenvironment19,20,21,22,23. Melanoma cells often exhibit alterations in iron metabolism and increased susceptibility to ferroptosis, making it a relevant therapeutic avenue24,25,26,27,28. Sorafenib’s dual action as a tyrosine kinase inhibitor and ferroptosis inducer may contribute to its effectiveness against melanoma. By disrupting key signaling pathways and promoting lipid peroxidation-induced cell death, Sorafenib may offer a targeted and multifaceted approach to combating melanoma, especially in cases where traditional treatments face challenges29,30,31,32,33,34 as illustrated in Scheme 1. We further explored the antifungal effects of sorafenib, aiming to enhance anticancer efficacy while potentially mitigating fungal co-infections in immunocompromised states. This strategic combination could offer a comprehensive and synergistic therapeutic approach, leveraging the unique attributes of each component for enhanced efficacy against cancer.
a Illustrating the mechanism of ferroptosis mediated cell death after the treatment with CPSAu. b The scheme depicts how the CaO₂ core generates reactive oxygen species when integrated with the gold coated on surface, enabling photothermal heating and enhances sorafenib-induced lipid peroxidation, together driving ferroptosis-mediated cell death upon CPSAu treatment. This figure was created by the authors using BioRender.com.
Results and discussion
Synthesis and characterization of CPAu NPs
Gold coated calcium peroxide nanoparticles were synthesized using in situ reduction method. Briefly, 200 µL of 20 mM ascorbic acid was added to 1:1 mixture of CaO2 NPs and HAuCL4.3H2O, resulting in an immediate color change of the solution to dark blue. The developed CPAu NPs exhibited a broad absorbance in the 600–800 nm range (Fig. 1a). TEM images revealed uniform nanoparticles of 70-80 nm size, concordant with the particle size distribution analysis (Fig. 1b and c). The elemental mapping using Scanning Transmission Electron Microscopy (STEM) and Energy-Dispersive X-ray Spectroscopy (EDS) confirmed the presence of gold on the surface of the NPs (Fig. 1d and Fig. S1). The elemental mapping conducted using STEM coupled with EDS confirmed the successful deposition of gold on the surface of the nanoparticles. In Fig. 1d and Supplementary Fig. S1, the spatial distribution of elements clearly illustrates the presence of gold localized on the outer region of the CaO₂ nanoparticles, indicating effective surface coating. This confirms that the gold was not incorporated into the core but rather formed a shell-like layer around the CaO₂ core, validating the intended core–shell architecture.
Additionally, to further support our interpretation, we have included a TEM image of GNPs synthesized under identical conditions but without the CaO₂ core. These particles appear as small spherical nanoparticles (10–20 nm) shown in Fig. S2. Furthermore, as previously described in our earlier publications9,10,11, the in situ reduced gold on the nanoparticles can disintegrate into smaller GNPs (2–5 nm) upon NIR irradiation. This photothermal effect facilitates the detachment or breakdown of gold nanostructures from the composite system.
Subsequently, the FTIR spectral analysis was conducted to evaluate the surface chemistry modifications during the stepwise functionalization of calcium peroxide nanoparticles. The pristine CaO₂ nanoparticles (green spectrum) exhibited characteristic peaks around 3400 cm⁻¹ (O–H stretching of adsorbed water), 1650 cm⁻¹ (H–O–H bending), ~1400 cm⁻¹ (O₂²⁻ stretching), and in the 850–950 cm⁻¹ region (Ca–O vibrations), confirming their typical structural profile. Upon sorafenib loading (blue spectrum), additional peaks appeared at ~2920–2850 cm⁻¹ corresponding to C–H stretching, and ~1600–1500 cm⁻¹ attributed to aromatic C = C stretching, along with features between 1250–1000 cm⁻¹ related to C–O or C–N vibrations. These changes indicate successful conjugation of sorafenib onto the CaO₂ nanoparticle surface.
Further modification with gold coating (red spectrum) led to broadening and slight shifts in the vibrational bands, particularly in the O–H and C–H regions, suggesting interactions between the gold layer and surface functional groups. The attenuation or masking of certain peaks can be attributed to the conductive nature of the gold shell, which alters the local vibrational environment. These spectral modifications collectively confirm the sequential surface engineering of CaO₂ nanoparticles with sorafenib and gold, supporting the structural integrity and functional integration of the designed nanocomposite system (Fig. S2b).
The synthesized CPAu NPs and CPSAu NPs were irradiated with the continuous laser (750 nm, 650 mW). It was found that there was a time dependent temperature rise to 65 °C and 63 °C respectively, upon 5 min of irradiation as seen in Fig. 2a and b, indicating that the presence of sorafenib did not affect the photothermal properties of the nanosystem.
Further, The Sorafenib release mechanism is triggered by NIR laser irradiation. The heat generated by the photothermal conversion of the gold layer leads to disruption of the lipid bilayer and acceleration of calcium peroxide decomposition, increasing ROS levels. This synergistic photothermal and oxidative effect causes disintegration of the particle and facilitates Sorafenib release. The release profile was evaluated under physiological conditions (pH 7.4, 37 °C) with and without NIR exposure and observed that 40% of the drug was released within 5 min of NIR exposure (Fig. 2c).
In vitro studies
PTT mediated cytotoxicity in B16 cells
Prior to testing the anti-cancer efficacy of the nanosystem, the cellular internalization of CPSAu NPs were evaluated. Figure S3 shows the internalization of CPAu loaded with Nile red in cells compared to that of bare CP NPs upon 6 h of incubation. As seen, surface coating of gold did not affect the internalization of CP NPs in B16 cells.
Further, the biocompatibility of CPAu and CPSAu NPs was evaluated in L929 fibroblast cells. The results indicated that cells treated with CPAu nanoparticles at various concentrations maintained a viability more than 80%. Similarly, cells treated with CPSAu NPs exhibited around 80% viability up to a concentration of 100 µg/mL which confirms the biocompatibility of the nanosystem (Fig. 3a).
The NIR laser mediated cytotoxicity was evaluated in B16 cells. Briefly, the cells were treated with 100 µg/mL of CPAu and CPSAu NPs and irradiated with 750 nm laser for 5 min. Upon 24 h of laser irradiation, the cell viability was quantified using MTT assay. There was significant reduction in the cell viability in case of CPSAu treated cells upon laser irradiation compared to the respective control groups (Fig. 3b).
The PTT induced apoptosis/necrosis was also qualitatively evaluated using Acridine orange/EtBr staining. As seen in Fig. 3c, without laser irradiation, the cells were viable and stained with acridine orange. EtBr, being impermeable to live cells, did not stain the cells. However, in case CPAu and CPSAu treated cells, upon laser irradiation, the orange color-stained cells reveal the late apoptotic cells.
PTT mediated ROS generation and mitochondrial damage in B16 cells
ROS generated upon various external stimuli, is one of the basic phenomena leading to several cell death pathways. PTT mediated ROS generation was evaluated using DCFDA assay. Briefly, the cells treated with CPAu and CPSAu NPs, upon 6 h post irradiation, was stained with DCFHDA stain. The cells were then imaged under the fluorescence microscope. As seen in Fig. 4a and b, there was increase in the ROS generation upon laser irradiation in both the CPAu and CPSAu treated groups, compared to their respective controls (Fig. S4).
Increased ROS generation primarily leads to potential mitochondrial damage. Hence, the JC1 dye was used to qualitatively assess the PTT mediated change in the mitochondrial membrane potential. As discussed in earlier chapters, JC1, upon encountering a healthy mitochondrial membrane, aggregates to fluoresce in red, whereas in case of dead or dying cells, remains as a monomer and fluoresces in green. As seen in Fig. 4c and Fig. S5, there is a significant damage to the mitochondrial membrane potential, evident from the increase in green fluorescence in case of laser irradiated CPAu and CPSAu treated groups. To confirm this, the cells treated with LCIF and ɸCIF, upon 12 h of irradiation, were stained with lipid peroxidation sensor dye. Upon imaging, it was evident that in case of ɸCIF treated group, there was a strong enhancement in the green fluorescence, indicating the enhancement in ferroptotic cell death as compared with the LCIF treated and non-laser irradiated groups (Fig. 4d and S6).
PTT mediated Anti-migration activity in B16 cells
Impeding cancer cell invasion and migration is pivotal in combating cancer metastasis. The two processes form an integral part of metastasis, from dissemination of cells from primary tumor site to forming a secondary tumor at distant organ site. Hence, PTT mediated anti-migratory effect was evaluated to understand the potential of the treatment to impede metastasis. As seen in the Fig. 5, in the case of CPAu NPs treated cells, even upon laser irradiation, the cells could acquire resistance and still migrate to cover the wound area. However, in presence of the drug Sfb, the cells completely lost their morphology, leading to early cell death compared to absence of Sfb. This also indicates, the synergistic photothermal ferroptosis is crucial in impeding cancer metastasis.
Evaluation of Anti-Migration effect in B16 cells treated with CPSAu NPs and irradiated with 750 nm NIR laser (Scale bar: 100 µm). The images demonstrate that CPSAu treatment combined with 750 nm NIR irradiation significantly suppresses B16 cell migration, as evidenced by reduced wound closure relative to control groups.
Colony formation assay in B16 cells
In addition to cancer metastasis, cancer recurrence at the treated site is a substantial concern. Colony formation assay was performed to evaluate the long-term proliferative abilities of treated cancer cells, and its self-renewal and clonogenic potential. As seen in Fig. 6a, upon laser irradiation of CPAu treated cells, there were few small colonies form. Whereas, in case of CPSAu treated cells, upon laser irradiation, there were no colonies found, showing the potential of synergistic photothermal ferroptosis in overcoming cancer cell recurrence.
Activation of macrophages
A combination of photothermal ferroptosis, is expected to elicit an enhanced immune activation, which could be leveraged in treating secondary tumors. To evaluate this, RAW 246.6 murine macrophages were used. Briefly, pre-seeded RAW cells were treated with the supernatant of 4T1 cells treated with CPSAu and irradiated with NIR laser. LPS was used as positive control. CPAu treated groups were considered as internal controls. The morphology of the activated macrophages were also captured under the microscope. As seen in the Fig. 6b, RAW cells treated with supernatant of CPSAu irradiated cancer cells showed qualitatively higher number of elongated macrophages, compared to that of LPS treated group, showing potential activation of the macrophages, owing to the presence of Sorafenib in the nanoformulation. However, in-depth analysis using in vitro immuno-assays and in vivo models is required to validate the same.
Anti-Cancer efficacy in 3D spheroid model
The anti-cancer efficacy of CPSAu NPs was also tested in 3D spheroids of B16. The growth of the cells within the spheroids was observed and visualized under the microscope (Fig. S7). After 5 days of forming alginate-based spheroids, the beads were treated with CPAu NPs and CPSAu NPs and irradiated with 750 nm laser for 5 min. 24 h post irradiation, the spheroids were stained with FDA/PI to assess the viability of the cells within the spheroids. As seen in Fig. 7, in case of CPAu treated cells, upon laser irradiation, there was a comparable cell death compared to the respective control groups. However, in case of CPSAu group, the cell death is significantly higher, due to the presence of Sfb, proving the efficacy of combining PTT with ferroptosis inducer, Sfb.
CAM assay
CAM assay is a simple and reproducible model to study the angiogenic and anti-angiogenic potential of novel drugs. To this, the anti-angiogenic behavior of Au coated CaO2 loaded hydrogel is evaluated through CAM assay. The chick embryos on the 4th day of incubation are treated with (a) Au coated CaO2 loaded hydrogel+ laser (b) Au coated CaO2 loaded hydrogel (c) only hydrogel (d) hydrogel + laser (e) only laser, were given followed by incubation with laser for 10 min in the respective groups. Results reveal that, in the Au coated CaO2 loaded hydrogel+ laser treatment group there is disruption of blood vessel in the chick embryo indicating the antiangiogenic behavior of the nanoparticles (Fig. 8). However, in the other treatment groups (b) Au coated CaO2 loaded hydrogel (c) only hydrogel (d) hydrogel + laser (e) only laser, there is less inhibition of blood vasculature which further potentiating
the antiangiogenic behavior of Au coated CaO2 loaded hydrogel+ laser treatment group. The percentage fold change in blood vessel diameter is quantified by IMAGEJ software which corroborates with the above observation.
Evaluation of Anti-fungal efficacy against C. albicans
PTT mediated anti-fungal efficacy of CPAu and CPSAu was observed on Candida albicans using spot assay. 5 µL of 8-fold dilution of treated sample in 1 mL of SD broth were spotted on SD agar plate with the appropriate controls. After 24 h of incubation at 37 °C, the difference in the number of colonies and spot size was evaluated. It was observed that the C.albicans treated with CPSAu showed PTT mediated effect and significantly reduced the colony number and spot size after 5 min and 10 min of NIR exposure, compared to control and CPAu (Fig. 9a).
Conclusion
In this study, we successfully synthesized gold-coated calcium peroxide nanoparticles (CPAu NPs) using an in-situ reduction method, which demonstrated promising characteristics for PTT. The CPAu NPs exhibited a broad absorbance in the 600–800 nm range, consistent with their intended use for near-infrared (NIR) light absorption. Transmission electron microscopy (TEM) confirmed the uniform size of the nanoparticles, and elemental mapping verified the presence of gold on their surfaces. The photothermal transduction efficiency (PTE) of CPAu NPs was calculated to be 53.11% with continuous laser irradiation and 45.46% with femtosecond pulsed laser irradiation, indicating effective heat generation upon NIR exposure. In vitro studies demonstrated that CPAu NPs, when combined with Sorafenib (Sfb), significantly enhanced cytotoxicity in B16 melanoma cells under laser irradiation. This combination treatment not only induced cell death but also resulted in increased reactive oxygen species (ROS) generation mitochondrial damage which further lead to lipid peroxidation, highlighting the efficacy of CPSAu NPs in promoting ferroptosis. The anti-migratory effects of the CPAu NPs with Sfb further illustrated their potential in impeding cancer metastasis, as evidenced by reduced cell migration and colony formation. Additionally, the combination therapy was shown to activate macrophages, suggesting a potential for enhanced immune responses against cancer cells. The efficacy of CPAu NPs was confirmed in a 3D spheroid model, where CPSAu NPs demonstrated superior anti-cancer activity compared to CPAu NPs alone. The chorioallantoic membrane (CAM) assay revealed that CPAu NPs, with Sfb, significantly impaired blood vessel formation and exhibited strong anti-angiogenic properties. Moreover, the CPAu and CPSAu NPs displayed effective anti-fungal activity against Candida albicans, demonstrating their potential utility beyond cancer treatment. Overall, this study underscores the potential of CPAu NPs, particularly in combination with Sorafenib, for effective PTT against cancer and microbial infections, providing a multifaceted approach to therapeutic interventions.
Methods
Materials
Tetrachloroauric acid trihydrate (HAuCl4.3H2O), ascorbic acid, and fluorescein diacetate were purchased from Sigma Aldrich Pvt.Ltd., India. Sorafenib (Sfb) was purchased from Sigma Aldrich, USA. Calcium hydroxide, PVP and Hydrogen peroxide were purchased from SRL Chemicals, India. Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum (FBS), Pen-strep solution, phosphate-buffered saline (PBS), Thiazolyl Blue Tetrazolium Bromide (MTT), and Trypsin EDTA solution were purchased from Himedia Laboratories, Mumbai (India). Propidium iodide and (40,6-diamidino-2-phenylindole) DAPI were purchased from Sigma. All other reagents used were of analytical grade.
Synthesis and characterization of CPAu NPs
Calcium peroxide nanoparticles were prepared using previously reported methods. Gold was coated on the surface of CaO2 NPs to tune its NIR absorbance through an in-situ reduction method. Briefly, CaO2 and HauCl4.3H2O were taken 1:1 ratio and 200 µL of 20 mM ascorbic acid was added. A visible color change was observed from white colored solution to dark blue colored solution. The developed nanoparticle, CPAu NPs was characterized for its UV–Vis absorbance, Hydrodynamic diameter, and morphology using TEM analysis. The release of peroxide groups from the core CaO2 NPs was tested using a simple colorimetric test using potassium permanganate (KMNO4).
Loading method of sorafenib
Sorafenib was incorporated into the system via a liposomal encapsulation strategy. Specifically, 1 mg of calcium peroxide nanoparticles (CPNPs) and 1 mg of Sorafenib were dissolved in ethanol and added to 10 mg of a lipid mixture (Hydrogenated Soy Phosphatidylcholine [HSPC] and cholesterol in a 3:1 molar ratio), which was dissolved in a 3:1 chloroform:methanol solution. This mixture was subjected to rotary evaporation to form a thin lipid film, followed by hydration in 10 mL of ultrapure water to produce a 1 mg/mL lipid stock solution encapsulating Sorafenib and CP NPs.
Formation of CPSAu NPs
From the above lipid stock, 200 µL was taken and mixed with 200 µL of 2 mM HAuCL4 solution. In situ reduction was then performed by adding 100 µL of 20 mM ascorbic acid, leading to the deposition of gold on the surface of the lipid-coated CPNPs. A visible color change to blue confirmed the reduction and formation of CPSAu NPs.
Drug loading ratio and quantification
The weight ratio of Sorafenib to calcium peroxide used during loading was 1:1. Sorafenib concentration in the final formulation was quantified using UV–Vis spectroscopy at its characteristic absorbance peak (~265–270 nm). The encapsulation efficiency (EE%) and drug loading content (DL%) were calculated using standard equations.
Mechanism of release
The Sorafenib release mechanism is triggered by NIR laser irradiation. The heat generated by the photothermal conversion of the gold layer leads to disruption of the lipid bilayer and acceleration of calcium peroxide decomposition, increasing ROS levels. This synergistic photothermal and oxidative effect causes disintegration of the particle and facilitates Sorafenib release. The release profile was evaluated under physiological conditions (pH 7.4, 37 °C) with and without NIR exposure.
In vitro studies
Biocompatibility in L929 cells
The biocompatibility of the synthesized CPAu NPs were tested in L929 cells. Briefly, 7 × 103 cells/well was seeded in a 96-well plate and upon 24 h, were treated with varying concentrations of CPAu NPs. The % cell viability was quantified using MTT assay after 24 h of treatment.
Cytotoxicity in B16 cells
The cytotoxicity of Sorafenib (Sfb) loaded CPAu NPs (CPSAu NPs) was tested in B16 cells. Briefly, 7 × 103 cells/well was seeded in a 96-well plate and upon 24 h, were treated with varying concentrations of CPSAu NPs. The % cell viability was quantified using MTT assay after 24 h of treatment.
PTT mediated cytotoxicity in B16 cells
Laser mediated cytotoxicity of CPAu and CPSAu NPs was evaluated in B16 cells. Briefly, 1 × 104 cells/well were seeded in 96-well plate. Upon attachment, the cells were treated with 100 µg/mL of CPAu and CPSAu NPs and irradiated with 750 nm laser for 5 min. The cells treated with the nanoparticles and not irradiated with NIR laser were considered as respective controls. After 24 h of laser treatment, the cell viability was quantified using MTT assay. Also, the cell death was qualitatively assessed using acridine orange/EtBr staining. After 24 h of irradiation, the cells were stained with 10 µg/mL of acridine orange and EtBr each simultaneously and incubated for 20 min. The cells were further washed and imaged under the live cell imager.
PTT mediated ROS generation and mitochondrial damage in B16 cells
DCFHDA assay and JC1 staining was done to evaluate the PTT mediated ROS generation and mitochondrial damage respectively. Briefly, the cells were treated with 100 µg/mL of CPAu and CPSAu NPs. The cells were them added with 1 µL of DCFDA and then irradiated with 750 nm laser for 5 min. The cells were then imaged under a fluorescence microscope. For JC1 staining, 6 h post irradiation with 750 nm laser, the cells were stained with 10 µg/mL of JC-1 dye and incubated for 20 min. The cells were then washed and imaged using a Live cell imager.
PTT mediated Anti-migration activity in B16 cells
Briefly, 5 × 104 cells/well were seeded in a 24-well plate and incubated. Upon attachment, the cells were then treated with 100 µg/mL of CPAu and CPSAu NPs and then irradiated with 750 nm NIR laser for 5 min. 6 h post irradiation, a scratch was introduced using 200 µL tip. The cells were washed and imaged at 0th hour and after 24 h of incubation.
Colony formation assay in B16 cells
For colony formation assay, cells were initially seeded in a 24-well plate at a density of 5 × 104 cells/well. Upon attachment, the cells were then treated with 100 µg/mL of CPAu and CPSAu NPs and then irradiated with 750 nm NIR laser for 5 min. Upon 24 h of laser irradiation, the cells were trypsinised, washed and re-seeded into 35 mm culture dishes at a low seeding density. The cells were incubated for 7 days and then fixed using 4% PFA for 10 min. Upon fixation, the cells were then stained with 0.5% crystal violet solution for 30 min. The cells were then gently washed with tap water and allowed to air-dry overnight. The colonies were then imaged using mobile camera.
Activation of RAW 264.4 macrophages
Briefly. 50 × 104 B16 cells/well were seeded in a 24-well plate and upon attachment, treated with CPAu & CPSAu NPs, and irradiated using 750 nm laser for 5 min. Upon 24 h of irradiation, the supernatant of the treated cells was added to pre-seeded RAW264.6 cells. After 24 h, the morphology of the macrophages were analyzed under the microscope. LPS treated macrophages were used as positive control.
Anti-Cancer efficacy in 3D spheroid model
3D spheroids of B16 cell were prepared using alginate beads. Briefly, pellet containing 0.5 × 106 cells were mixed with 1 mL of 0.5% sodium alginate solution. The solution was added carefully dropwise to chilled calcium chloride solution. Uniformly sized beads of cells were incubated at 37 °C in a humidified atmosphere with 5% CO2.
Chorioallantoic membrane assay (CAM)
The CAM assay is widely employed to study the angiogenesis, anti-angiogenesis effect of drug molecules on the blood vessels of chick embryo. The progression of tumor in the tumor microenvironment depends on the development of new blood vessels that nourish tumor cells and play an important role in tumor growth. Therefore, to demonstrate the effect of Au coated CaO2 loaded hydrogel on the blood vessels of chick embryo CAM assay was performed using the fertilized eggs. Briefly, fertilized eggs were procured from ICAR-Directorate of Poultry Research, Rajendranagar, Hyderabad (India). These eggs were maintained an egg incubator at 37 ◦C with relative humidity of 60–70%. On fourth day, the egg shell was broken to create a small gap on top of the egg and various treatments (a) Au coated CaO2 loaded hydrogel with laser (b) Au coated CaO2 loaded hydrogel (c) only hydrogel (d) hydrogel + laser (e) only laser, were given followed by incubation with laser for 10 min in the respective groups. The egg containing embryos were incubated in the egg incubator for another 6 h and images were captured at both 0 and 6 h using Leica Stereo Microscope at 0 h and 6 h.
Evaluation of anti-fungal efficacy against C. albicans
The NIR mediated PTT mediated anti-fungal effect of CPAu and CPSAu was performed in 96 well plate as per the previous protocol. Briefly, inoculum with 2 × 103 CFU/ml of C. albicans was prepared from the overnight culture, followed by the treatment with CPAu and CPSAu at 100 µg/ml concentration. Afterwards, the wells with the respective treatment group were exposed to NIR for 5 min and 10 min, further incubated at 37 °C for 24 h. After 24 h of incubation the spot assay was performed by diluting the sample to 8-fold (10-2, 10-4, 10-6 and 10-8) and spotted 5 µL of each sample on SD agar plate.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are included in the article and its Supplementary Information files. Any additional information required to evaluate the conclusions of this work is available from the corresponding author upon reasonable request.
References
Yang, K. et al. Communication. Adv. Mater. 24, 5586–5592 (2012).
Doughty, A. C. V. et al. Nanomaterial applications in photothermal therapy for cancer. Materials 12, 779, (2019).
Zhi, D., Yang, T., O’Hagan, J., Zhang, S. & Donnelly, R. F. Photothermal therapy. J. Control. Release 325, 52–71 (2020).
Riley, R. S. & Day, E. S. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. WIREs Nanomed. Nanobiotechnol. 9, e1449 (2017).
Turkmen Koc, S. N., Rezaei Benam, S., Aral, I. P., Shahbazi, R. & Ulubayram, K. Gold nanoparticles-mediated photothermal and photodynamic therapies for cancer. Int. J. Pharm. 655, 124057 (2024).
Kim, H. et al. Gold nanoparticle-Carrying T cells for the combined immuno-photothermal therapy. Small 19, 2301377 (2023).
Kim, D., Paik, J. & Kim, H. Effect of gold nanoparticles distribution radius on photothermal therapy efficacy. Sci. Rep. 13, 12135 (2023).
Gupta, N. & Malviya, R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Biochim. Biophys. Acta Rev. Cancer 1875, 188532 (2021).
Rengan, A. K., Jagtap, M., De, A., Banerjee, R. & Srivastava, R. Multifunctional gold coated thermo-sensitive liposomes for multimodal imaging and photo-thermal therapy of breast cancer cells. Nanoscale 6, 916–923 (2014).
Appidi, T. et al. A plasmon-enhanced fluorescent gold coated novel lipo-polymeric hybrid nanosystem: synthesis, characterization and application for imaging and photothermal therapy of breast cancer. Nanoscale 14, 9112–9123 (2022).
Yadav, D. N., Sankaranarayanan, S. A., Thanekar, A. M. & Rengan, A. K. Bioinspired gold coated phage nanosomes for anti-microbial and anti-cancer theranostics. Mater. Today Nano 23, 100348 (2023).
Rengan, A. K. et al. In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer. Nano Lett 15, 842–848 (2015).
Azzimonti, B. et al. Microbiota, oxidative stress, and skin cancer: an unexpected triangle. Antioxidants 12, 546 (2023).
Talapko, J. et al. A putative role of candida albicans in promoting cancer development: a current state of evidence and proposed mechanisms. Microorganisms 11, 1476 (2023).
Liu, N. et al. Target- and prodrug-based design for fungal diseases and cancer-associated fungal infections. Adv. Drug Deliv. Rev. 197, 114819 (2023).
Wang, X. et al. Is Candida albicans a contributor to cancer? A critical review based on the current evidence. Microbiol. Res. 272, 127370 (2023).
Yang, L., Li, A., Wang, Y. & Zhang, Y. Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy. Signal Transduct. Target. Ther. 8, 35 (2023).
Srivastava, R. et al. Bioinspired photoresponsive algosomes as a nanocarrier for combating cancer and bacterial infections. https://doi.org/10.1021/acsanm.4c05104 (2024).
Yan, Y. et al. pH Switchable Nanozyme Platform for Healing Skin Tumor Wound Infected with Drug-Resistant Bacteria. Adv. Healthc. Mater. 12, 2301375 (2023).
Jiang, C. et al. A Robust ROS generation and ferroptotic lipid modulation nanosystem for mutual reinforcement of ferroptosis and cancer immunotherapy. Adv. Healthc. Mater. 13, 2401502 (2024).
Shen, H. et al. Microneedle patch loaded with calcium peroxide nanoparticles for oxygen healing and biofilm inhibition in diabetic wound healing. ACS Appl. Nano Mater. 7, 15171–15184 (2024).
Sankaranarayanan, S. A. et al. In situ thermosensitive H2O2/NO self-sufficient hydrogel for photothermal ferroptosis of triple-negative breast cancer. Nanoscale 16, 18899–18909 (2024).
Chen, N. et al. H2O2 Self-Supplying CaO2/POM@MOF bimodal nanogeneration materials for photothermal and chemodynamic synergistic antimicrobials. Appl. Organomet. Chem. 39, e7788 (2024).
Feng, T. et al. Exosome camouflaged coordination-assembled Iridium(III) photosensitizers for apoptosis-autophagy-ferroptosis induced combination therapy against melanoma. Biomaterials 301, 122212 (2023).
Ta, N., Jiang, X., Zhang, Y. & Wang, H. Ferroptosis as a promising therapeutic strategy for melanoma. Front. Pharmacol. 14, 1252567 (2023).
Zhang, D., Zhang, M., Pang, Y., Li, M. & Ma, W. Folic acid–modified long-circulating liposomes loaded with sulfasalazine for targeted induction of ferroptosis in melanoma. ACS Biomater. Sci. Eng. 10, 588–598 (2024).
Manzari Tavakoli, G., Mirzapour, M. H., Razi, S. & Rezaei, N. Targeting ferroptosis as a cell death pathway in Melanoma: from molecular mechanisms to skin cancer treatment. Int. Immunopharmacol. 119, 110215 (2023).
Khorsandi, K., Esfahani, H., Ghamsari, S. K. - & lakhshehei, P. Targeting ferroptosis in melanoma: cancer therapeutics. Cell Commun. Signal. 21, 337 (2023).
Allam, R. M., El Kerdawy, A. M., Gouda, A. E., Ahmed, K. A. & Abdel-Mohsen, H. T. Benzimidazole-oxindole hybrids as multi-kinase inhibitors targeting melanoma. Bioorg. Chem. 146, 107243 (2024).
Zhang, J. et al. Targeted therapy, immunotherapy, and small molecules and peptidomimetics as emerging immunoregulatory agents for melanoma. Cancer Lett. 586, 216633 (2024).
Xu, F. et al. SLC27A5 promotes sorafenib-induced ferroptosis in hepatocellular carcinoma by downregulating glutathione reductase. Cell Death Dis. 14, 22 (2023).
Kuche, K., Yadav, V., Patel, M., Ghadi, R. & Jain, S. Exploring Sorafenib and Simvastatin combination for ferroptosis-induced cancer treatment: cytotoxicity screening, in vivo efficacy, and safety assessment. AAPS PharmSciTech 24, 180 (2023).
Li, Y. et al. Targeting fatty acid synthase modulates sensitivity of hepatocellular carcinoma to sorafenib via ferroptosis. J. Exp. Clin. Cancer Res. 42, 6 (2023).
Li, Q. et al. Understanding sorafenib-induced ferroptosis and resistance mechanisms: Implications for cancer therapy. Eur. J. Pharmacol. 955, 175913 (2023).
Acknowledgements
The authors would like to acknowledge ICMR-IIRPIG/2024/01/2351, ICMR-FIW-2024/01/00000208, ICMR-CoE, SUPRA(SPR/2022/000230), ANRF-ARG -2025/008857/LS, MoE/STARS (STARS 2/2023-640),MoE/SPARC/P2082 and IITH/BME/SOCH3 project grants. S.A.S. would like to gratefully acknowledge MoE PMRF (ID 2000832) for funding her fellowship. K.E. would like to thank PMRF for the funding support (2003331). A project funding from the Department of Biotechnology, Ministry of Science & Technology, Government of India (DBT/CE/F064/2020-21/G307) is gratefully acknowledged by R.S. The authors would also like to acknowledge Dr Prabusankar Ganesan for providing his laboratory facilities. Graphical abstracts and schematics were created using the licensed version of BioRender.com.
Author information
Authors and Affiliations
Contributions
Sri Amruthaa Sankaranarayanan: conceptualization, data curation, methodology, software, validation, writing—original draft, review and editing; Rupali Srivastava: data curation and validation, writing—original draft, review and editing; Kalyani Eswar: data curation, validation, writing—original draft, review and editing; Sanchita Tripathy- Data curation; Proma Nagchowdhury- Data curation; Maddila Jagapathi Rao- Data curation; Chitaranjan Patra- supervision, methodology and validation; Aravind Kumar Rengan: conceptualization, supervision, funding acquisition, project administration, validation, writing—original draft, review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sankaranarayanan, S.A., Srivastava, R., Eswar, K. et al. Development of Gold coated calcium peroxide nanoparticles for photothermal ferroptosis against skin cancer and C. albicans. Commun Chem 9, 75 (2026). https://doi.org/10.1038/s42004-025-01878-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42004-025-01878-4