Abstract
The emergence of molecular oxygen on early Earth is conventionally attributed to the evolution of oxygenic photosynthesis. A persistent challenge for early life, however, was the management of reactive oxygen species such as hydrogen peroxide (H2O2), which could arise through a variety of abiotic processes. Here we report that some RNA molecules, when coordinated with ferrous iron (Fe2+), catalyze the oxidation of H2O2 into O2 and H2O under anoxic conditions that mimic the early Earth environment. This previously unrecognized RNA-based redox activity suggests that ancient RNA-metal complexes may have contributed to the detoxification of H2O2 and the management of oxidative stress prior to the evolution of protein enzymes. Such RNA–Fe complexes provide a plausible molecular mechanism linking early geochemical oxidants to primitive biological redox chemistry.
Similar content being viewed by others
Data availability
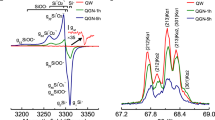
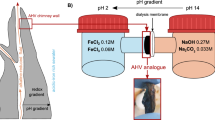
All data generated and analyzed in this study are included in this published article and its Supplementary Information. Primary datasets supporting the main findings are provided as Supplementary Data files: Supplementary Data 1 (Fig. 3), Supplementary Data 2 and 3 (Fig. 4), and Supplementary Data 4 (Fig. 5).
References
Moody, E. R. R. et al. The nature of the last universal common ancestor and its impact on the early Earth system. Nat. Ecol. Evol. 8, 1654–1666 (2024).
Schopf, J. W., Kudryavtsev, A. B., Agresti, D. G., Wdowiak, T. J. & Czaja, A. D. Laser-Raman imagery of Earth’s earliest fossils. Nature 416, 73–76 (2002).
Holland, H. D. The oxygenation of the atmosphere and oceans. Philos. Trans. R. Soc. B: Biol. Sci. 361, 903–915 (2006).
Anbar, A. D. Elements and evolution. Science 322, 1481–1483 (2008).
Olson, J. M. & Blankenship, R. E. Thinking about the evolution of photosynthesis. Photosynth Res. 80, 373–386 (2004).
Sessions, A. L., Doughty, D. M., Welander, P. V., Summons, R. E. & Newman, D. K. The continuing puzzle of the great oxidation event. Curr. Biol. 19, R567–R574 (2009).
Awramik, S. M. The oldest records of photosynthesis. Photosynth Res. 33, 75–89 (1992).
Ueno, Y., Ono, S., Rumble, D. & Maruyama, S. Quadruple sulfur isotope analysis of ca. 3.5Ga Dresser Formation: new evidence for microbial sulfate reduction in the early Archean. Geochim. Cosmochim. Acta 72, 5675–5691 (2008).
Olson, J. M. Evolution of photosynthesis. Science 168, 438 (1970).
Blankenship, R. E. & Hartman, H. The origin and evolution of oxygenic photosynthesis. Trends Biochem. Sci. 23, 94–97 (1998).
He, H. et al. A mineral-based origin of Earth’s initial hydrogen peroxide and molecular oxygen. Proc. Natl. Acad. Sci. USA 120, e2221984120 (2023).
Wu, X. et al. Geodynamic oxidation of Archean terrestrial surfaces. Commun. Earth Environ. 4, 132 (2023).
Slesak, I., Slesak, H. & Kruk, J. Oxygen and hydrogen peroxide in the early evolution of life on earth: in silico comparative analysis of biochemical pathways. Astrobiology. 12, 775–784 (2012).
Stone, J., Edgar, J. O., Gould, J. A. & Telling, J. Tectonically-driven oxidant production in the hot biosphere. Nat. Commun. 13, 4529 (2022).
Schwartz, R. M. & Dayhoff, M. O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science 199, 395–403 (1978).
Weiss, M. C. et al. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 1, 16116 (2016).
Bray, M. S. et al. Multiple prebiotic metals mediate translation. Proc. Natl. Acad. Sci. USA 115, 12164 (2018).
Hsiao, C. et al. RNA with iron(II) as a cofactor catalyses electron transfer. Nat. Chem. 5, 525–528 (2013).
Lin, S.-Y., Wang, Y.-C. & Hsiao, C. Prebiotic iron originates the peptidyl transfer origin. Mol. Biol. Evol. 36, 999–1007 (2019).
Cotruvo, J. A. & Stubbe, J. Class I ribonucleotide reductases: metallocofactor assembly and repair in vitro and in vivo. Annu. Rev. Biochem. 80, 733–767 (2011).
Poole, A. M., Logan, D. T. & Sjöberg, B.-M. The evolution of the ribonucleotide reductases: much ado about oxygen. J. Mol. Evol. 55, 180–196 (2002).
López, M. B., Oterino, M. B. & González, J. M. In Macromolecular Protein Complexes V: Structure and Function (eds Robin Harris, J. & Jon Marles-Wright) 33–47 (Springer International Publishing, 2024).
Zámocký, M., Gasselhuber, B., Furtmüller, P. G. & Obinger, C. Turning points in the evolution of peroxidase-catalase superfamily: molecular phylogeny of hybrid heme peroxidases. Cell Mol. Life Sci. 71, 4681–4696 (2014).
Hsiao, C. et al. In Nucleic Acid Metal Ion Interactions (ed. Hud, N.) 1–35 (The Royal Society of Chemistry, 2008).
Limpanuparb, T., Areekul, C., Montriwat, P. & Rajchakit, U. Blue bottle experiment: learning chemistry without knowing the chemicals. J. Chem. Educ. 94, 730–737 (2017).
Hsiao, C. & Williams, L. D. A recurrent magnesium-binding motif provides a framework for the ribosomal peptidyl transferase center. Nucleic Acids Res. 37, 3134–3142 (2009).
Petrov, A. S. et al. History of the ribosome and the origin of translation. Proc. Natl. Acad. Sci. USA 112, 15396–15401 (2015).
Fox, G. E. Origin and evolution of the ribosome. Cold Spring Harb. Perspect. Biol. 2, a003483 (2010).
Cantor, C. & Schimmel, P. Biophysical Chemistry (I-III) (Academic Press, 1984).
Job, P. Studies on the formation of complex minerals in solution and on their stability. Annal. De. Chim. Fr. 9, 113–203 (1928).
Bandarian, V. & Reed, G. H. Hydrazine cation radical in the active site of ethanolamine ammonia-lyase: mechanism-based inactivation by hydroxyethylhydrazine. Biochemistry 38, 12394–12402 (1999).
Equbal, A. et al. Role of electron spin dynamics and coupling network in designing dynamic nuclear polarization. Prog. Nucl. Magn. Reson. Spectrosc. 126-127, 1–16 (2021).
Cloud, P. Working model of primitive Earth. Am. J. Sci. 272, 537–548 (1972).
He, H. et al. An abiotic source of Archean hydrogen peroxide and oxygen that pre-dates oxygenic photosynthesis. Nat. Commun. 12, 6611 (2021).
Balk, M. et al. Oxidation of water to hydrogen peroxide at the rock–water interface due to stress-activated electric currents in rocks. Earth Planet. Sci. Lett. 283, 87–92 (2009).
Xia, Y. et al. Contact between water vapor and silicate surface causes abiotic formation of reactive oxygen species in an anoxic atmosphere. Proc. Natl. Acad. Sci. USA 120, e2302014120 (2023).
Schmeing, T. M. et al. A pre-translocational intermediate in protein synthesis observed in crystals of enzymatically active 50S subunits. Nat. Struct. Biol. 9, 225–230 (2002).
Murray, L. J., Arendall, W. B. 3rd, Richardson, D. C. & Richardson, J. S. RNA backbone is rotameric. Proc. Natl. Acad. Sci. USA 100, 13904–13909 (2003).
Petrov, A. S., Bowman, J. C., Harvey, S. C. & Williams, L. D. Bidentate RNA-magnesium clamps: on the origin of the special role of magnesium in RNA folding. Rna 17, 291–297 (2011).
Fried, S. D., Fujishima, K., Makarov, M., Cherepashuk, I. & Hlouchova, K. Peptides before and during the nucleotide world: an origins story emphasizing cooperation between proteins and nucleic acids. J. R. Soc. Interface 19, 20210641 (2022).
Sánchez, M., Sabio, L., Gálvez, N., Capdevila, M. & Dominguez-Vera, J. M. Iron chemistry at the service of life. IUBMB Life 69, 382–388 (2017).
Athavale, S. S. et al. Domain III of the T. thermophilus 23S rRNA folds independently to a near-native state. Rna 18, 752–758 (2012).
Baranovskii, S. F., Bolotin, P. A. & Evstigneev, M. P. Aggregation of 1,3,7-trimethylxanthine with methylene blue in aqueous solution. J. Appl. Spectrosc. 73, 171–177 (2006).
Acknowledgements
The authors thank Drs. Steve Sheng-Fa Yu and Ivan Tsai (Institute of Chemistry, Academia Sinica, Taiwan) for training and access to the EPR facility. We also acknowledge Drs. S. Yu, I. Tsai, J. Lin, and C. Chang for helpful discussions. This work is supported by the National Science and Technology Council (NSCT-112-2311-B-002-010).
Author information
Authors and Affiliations
Contributions
Y.C.W., J.H.T., L.C.Y., and C.H. conceived and designed the study. Y.C.W. and J.H.T. performed the experiments and analyzed the data. Y.C.W. and L.C.Y. provided key materials. C.H. supervised the project. Y.C.W., J.H.T., L.C.Y., and C.H. co-wrote the manuscript. All authors discussed the results and contributed to the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, YC., Tu, JH., Yu, LC. et al. RNA−Iron complexes catalyse prebiotic oxygen generation. Commun Chem (2026). https://doi.org/10.1038/s42004-026-01935-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-026-01935-6