Abstract
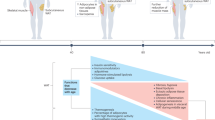
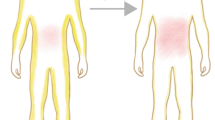
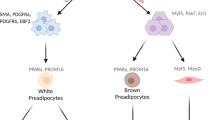
Ageing is a conserved biological process, modulated by intrinsic and extrinsic factors, that leads to changes in life expectancy. In humans, ageing is characterized by greatly increased prevalence of cardiometabolic disease, type 2 diabetes and disorders associated with impaired immune surveillance. Adipose tissue displays species-conserved, temporal changes with ageing, including redistribution from peripheral to central depots, loss of thermogenic capacity and expansion within the bone marrow. Adipose tissue is localized to discrete depots, and also diffusely distributed within multiple organs and tissues in direct proximity to specialized cells. Thus, through their potent endocrine properties, adipocytes are capable of modulating tissue and organ function throughout the body. In addition to adipocytes, multipotent progenitor/stem cells in adipose tissue play a crucial role in maintenance and repair of tissues throughout the lifetime. Adipose tissue may therefore be a central driver for organismal ageing and age-associated diseases. Here we review the features of adipose tissue during ageing, and discuss potential mechanisms by which these changes affect whole-body metabolism, immunity and longevity. We also explore the potential of adipose tissue-targeted therapies to ameliorate age-associated disease burdens.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Reynolds, C. A. et al. A decade of epigenetic change in aging twins: genetic and environmental contributions to longitudinal DNA methylation. Aging Cell 19, e13197 (2020).
Brooks-Wilson, A. R. Genetics of healthy aging and longevity. Hum. Genet. 132, 1323–1338 (2013).
Mirisola, M. G. & Longo, V. D. Yeast Chronological lifespan: longevity regulatory genes and mechanisms. Cells https://doi.org/10.3390/cells11101714 (2022).
Roux, A. E. et al. Individual cell types in C. elegans age differently and activate distinct cell-protective responses. Cell Rep. 42, 112902 (2023).
Riera, C. E., Merkwirth, C., De Magalhaes Filho, C. D. & Dillin, A. Signaling networks determining life span. Annu. Rev. Biochem. 85, 35–64 (2016).
Kamminga, L. M. et al. Impaired hematopoietic stem cell functioning after serial transplantation and during normal aging. Stem Cells 23, 82–92 (2005).
Sethe, S., Scutt, A. & Stolzing, A. Aging of mesenchymal stem cells. Aging Res. Rev. 5, 91–116 (2006).
Korstanje, R., Peters, L. L., Robinson, L. L., Krasinski, S. D. & Churchill, G. A. The Jackson Laboratory Nathan Shock Center: impact of genetic diversity on aging. Geroscience 43, 2129–2137 (2021).
Zhou, W. et al. High-resolution aging niche of human adipose tissues. Signal Transduct. Target. Ther. 8, 105 (2023).
Sparks, L. et al. A single nuclei atlas of aging human abdominal subcutaneous white adipose tissue. Preprint at Res. Sq. https://doi.org/10.21203/rs.3.rs-3097605/v1 (2023).
Tabula Muris, C. A single-cell transcriptomic atlas characterizes aging tissues in the mouse. Nature 583, 590–595 (2020).
Schaum, N. et al. Aging hallmarks exhibit organ-specific temporal signatures. Nature 583, 596–602 (2020).
Palovics, R. et al. Molecular hallmarks of heterochronic parabiosis at single-cell resolution. Nature 603, 309–314 (2022).
Bluher, M. Fat tissue and long life. Obes. Facts 1, 176–182 (2008).
Gavalda-Navarro, A., Villarroya, J., Cereijo, R., Giralt, M. & Villarroya, F. The endocrine role of brown adipose tissue: an update on actors and actions. Rev. Endocr. Metab. Disord. 23, 31–41 (2022).
Cinti, S. The endocrine adipose organ. Rev. Endocr. Metab. Disord. 23, 1–4 (2022).
Massier, L. et al. An integrated single cell and spatial transcriptomic map of human white adipose tissue. Nat. Commun. 14, 1438 (2023).
Maniyadath, B., Zhang, Q., Gupta, R. K. & Mandrup, S. Adipose tissue at single-cell resolution. Cell Metab. 35, 386–413 (2023).
Emont, M. P. et al. A single-cell atlas of human and mouse white adipose tissue. Nature 603, 926–933 (2022).
Merrick, D. et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science https://doi.org/10.1126/science.aav2501 (2019).
Min, S. Y. et al. Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells. Proc. Natl Acad. Sci. USA 116, 17970–17979 (2019).
Zuk, P. A. et al. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13, 4279–4295 (2002).
Traktuev, D. O. et al. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ. Res. 104, 1410–1420 (2009).
Lima, J. G. et al. Causes of death in patients with Berardinelli-Seip congenital generalized lipodystrophy. PLoS ONE 13, e0199052 (2018).
Cypess, A. M. Reassessing human adipose tissue. N. Engl. J. Med. 386, 768–779 (2022).
Slawik, M. & Vidal-Puig, A. J. Lipotoxicity, overnutrition and energy metabolism in aging. Aging Res. Rev. 5, 144–164 (2006).
Porter, J. W. et al. Age, sex, and depot-specific differences in adipose-tissue estrogen receptors in individuals with obesity. Obesity 28, 1698–1707 (2020).
Li, Z. et al. SPATA4 improves aging-induced metabolic dysfunction through promotion of preadipocyte differentiation and adipose tissue expansion. Aging Cell 20, e13282 (2021).
Turner, R. B. S., Tyrrell, D., Hepworth, G., Dunshea, F. R. & Mansfield, C. S. Compartmental fat distribution in the abdomen of dogs relative to overall body fat composition. BMC Vet. Res. 16, 104 (2020).
McKenzie, B. A. Comparative veterinary geroscience: mechanism of molecular, cellular, and tissue aging in humans, laboratory animal models, and companion dogs and cats. Am. J. Vet. Res. https://doi.org/10.2460/ajvr.22.02.0027 (2022).
Mansoor, A. et al. Echocardiographic determination of epicardial adipose tissue in healthy bonnet macaques. Echocardiography 27, 180–185 (2010).
Cefalu, W. T. et al. Caloric restriction and cardiovascular aging in cynomolgus monkeys (Macaca fascicularis): metabolic, physiologic, and atherosclerotic measures from a 4-year intervention trial. J. Gerontol. A Biol. Sci. Med. Sci. 59, 1007–1014 (2004).
Colman, R. J. et al. Body fat distribution with long-term dietary restriction in adult male rhesus macaques. J. Gerontol. A Biol. Sci. Med Sci. 54, B283–B290 (1999).
Yan, Y. et al. HDAC6 suppresses age-dependent ectopic fat accumulation by maintaining the proteostasis of PLIN2 in Drosophila. Dev. Cell 43, 99–111 (2017).
Sasaki, T. et al. Status and physiological significance of circulating adiponectin in the very old and centenarians: an observational study. Elife https://doi.org/10.7554/eLife.86309 (2023).
Li, N. et al. Adiponectin preserves metabolic fitness during aging. Elife https://doi.org/10.7554/eLife.65108 (2021).
Di Nicola, V. Omentum a powerful biological source in regenerative surgery. Regen. Ther. 11, 182–191 (2019).
Meza-Perez, S. & Randall, T. D. Immunological functions of the omentum. Trends Immunol. 38, 526–536 (2017).
Ha, C. W. Y. et al. Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell 183, 666–683 (2020).
Cao, E. et al. Mesenteric lymphatic dysfunction promotes insulin resistance and represents a potential treatment target in obesity. Nat. Metab. 3, 1175–1188 (2021).
Macdougall, C. E. et al. Visceral adipose tissue immune homeostasis is regulated by the crosstalk between adipocytes and dendritic cell subsets. Cell Metab. 27, 588–601 (2018).
Meek, S. E., Nair, K. S. & Jensen, M. D. Insulin regulation of regional free fatty acid metabolism. Diabetes 48, 10–14 (1999).
Madsen, S. et al. Deep proteome profiling of white adipose tissue reveals marked conservation and distinct features between different anatomical depots. Mol. Cell Proteomics 22, 100508 (2023).
Hruska, P. et al. Unraveling adipose tissue proteomic landscapes in severe obesity: insights into metabolic complications and potential biomarkers. Am. J. Physiol. Endocrinol. Metab. 325, E562–E580 (2023).
Khan, S., Chan, Y. T., Revelo, X. S. & Winer, D. A. The immune landscape of visceral adipose tissue during obesity and aging. Front. Endocrinol. 11, 267 (2020).
Lumeng, C. N. et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J. Immunol. 187, 6208–6216 (2011).
Verboven, K. et al. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Sci. Rep. 8, 4677 (2018).
Hoffstedt, J., Arner, P., Hellers, G. & Lonnqvist, F. Variation in adrenergic regulation of lipolysis between omental and subcutaneous adipocytes from obese and non-obese men. J. Lipid Res. 38, 795–804 (1997).
Blondin, D. P. Human thermogenic adipose tissue. Curr. Opin. Genet. Dev. 80, 102054 (2023).
Saito, M. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009).
Pfannenberg, C. et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes 59, 1789–1793 (2010).
Becher, T. et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 27, 58–65 (2021).
Rogers, N. H., Landa, A., Park, S. & Smith, R. G. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging Cell 11, 1074–1083 (2012).
McDonald, R. B. & Horwitz, B. A. Brown adipose tissue thermogenesis during aging and senescence. J. Bioenerg. Biomembr. 31, 507–516 (1999).
Ashwell, M., Stirling, D., Freeman, S. & Holloway, B. R. Immunological, histological and biochemical assessment of brown adipose tissue activity in neonatal, control and beta-stimulant-treated adult dogs. Int. J. Obes. 11, 357–365 (1987).
Du, K. et al. De novo reconstruction of transcriptome identified long non-coding RNA regulator of aging-related brown adipose tissue whitening in rabbits. Biology https://doi.org/10.3390/biology10111176 (2021).
Huang, Z. et al. Brown adipose tissue involution associated with progressive restriction in progenitor competence. Cell Rep. 39, 110575 (2022).
Garside, J. C., Kavanagh, K., Block, M. R., Williams, A. G. & Branca, R. T. Xenon-enhanced computed tomography assessment of brown adipose tissue distribution and perfusion in lean, obese, and diabetic primates. Obes. 30, 1831–1841 (2022).
Burkhardt, R. et al. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone 8, 157–164 (1987).
Rosen, C. J. & Horowitz, M. C. Nutrient regulation of bone marrow adipose tissue: skeletal implications of weight loss. Nat. Rev. Endocrinol. 19, 626–638 (2023).
Pachon-Pena, G. & Bredella, M. A. Bone marrow adipose tissue in metabolic health. Trends Endocrinol. Metab. 33, 401–408 (2022).
Beekman, K. M. et al. Gender- and age-associated differences in bone marrow adipose tissue and bone marrow fat unsaturation throughout the skeleton, quantified using chemical shift encoding-based water-fat MRI. Front. Endocrinol. 13, 815835 (2022).
Al Saedi, A. et al. Age-related increases in marrow fat volumes have regional impacts on bone cell numbers and structure. Calcif. Tissue Int. 107, 126–134 (2020).
Ricci, C. et al. Normal age-related patterns of cellular and fatty bone marrow distribution in the axial skeleton: MR imaging study. Radiology 177, 83–88 (1990).
Bigelow, C. L. & Tavassoli, M. Fatty involution of bone marrow in rabbits. Acta Anat. 118, 60–64 (1984).
Liu, L. F., Shen, W. J., Ueno, M., Patel, S. & Kraemer, F. B. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics 12, 212 (2011).
Duque, G. et al. Differential effects of long-term caloric restriction and dietary protein source on bone and marrow fat of the aging rat. J. Gerontol. A Biol. Sci. Med. Sci. 75, 2031–2036 (2020).
Miggitsch, C. et al. Human bone marrow adipocytes display distinct immune regulatory properties. EBioMedicine 46, 387–398 (2019).
Mattiucci, D. et al. Bone marrow adipocytes support hematopoietic stem cell survival. J. Cell. Physiol. 233, 1500–1511 (2018).
Tratwal, J. et al. Raman microspectroscopy reveals unsaturation heterogeneity at the lipid droplet level and validates an in vitro model of bone marrow adipocyte subtypes. Front. Endocrinol. 13, 1001210 (2022).
Suchacki, K. J. et al. The effects of caloric restriction on adipose tissue and metabolic health are sex- and age-dependent. Elife https://doi.org/10.7554/eLife.88080 (2023).
Li, Z. et al. Constitutive bone marrow adipocytes suppress local bone formation. JCI Insight https://doi.org/10.1172/jci.insight.160915 (2022).
Zhang, X. et al. A bone-specific adipogenesis pathway in fat-free mice defines key origins and adaptations of bone marrow adipocytes with age and disease. Elife https://doi.org/10.7554/eLife.66275 (2021).
Ambrosi, T. H. et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20, 771–784 (2017).
Zhou, B. O. et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat. Cell Biol. 19, 891–903 (2017).
Naveiras, O. et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259–263 (2009).
Schraut, N. B. et al. What protects certain nerves from stretch injury? Anat. Rec. 299, 111–117 (2016).
Ahmed, A., Bibi, A., Valoti, M. & Fusi, F. Perivascular adipose tissue and vascular smooth muscle tone: friends or foes? Cells https://doi.org/10.3390/cells12081196 (2023).
Hillock-Watling, C. & Gotlieb, A. I. The pathobiology of perivascular adipose tissue (PVAT), the fourth layer of the blood vessel wall. Cardiovasc. Pathol. 61, 107459 (2022).
Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 19, 593–606 (2022).
Favaretto, F., Bettini, S., Busetto, L., Milan, G. & Vettor, R. Adipogenic progenitors in different organs: pathophysiological implications. Rev. Endocr. Metab. Disord. 23, 71–85 (2022).
Shulman, G. I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 371, 2237–2238 (2014).
Hausman, G. J., Basu, U., Du, M., Fernyhough-Culver, M. & Dodson, M. V. Intermuscular and intramuscular adipose tissues: bad vs. good adipose tissues. Adipocyte 3, 242–255 (2014).
Biltz, N. K. et al. Infiltration of intramuscular adipose tissue impairs skeletal muscle contraction. J. Physiol. 598, 2669–2683 (2020).
Pouliopoulos, J. et al. Intramyocardial adiposity after myocardial infarction: new implications of a substrate for ventricular tachycardia. Circulation 128, 2296–2308 (2013).
Burton, J. S., Sletten, A. C., Marsh, E., Wood, M. D. & Sacks, J. M. Adipose tissue in lymphedema: a central feature of pathology and target for pharmacologic therapy. Lymphat. Res. Biol. 21, 2–7 (2023).
Landgraf, K. et al. Short-term overfeeding of zebrafish with normal or high-fat diet as a model for the development of metabolically healthy versus unhealthy obesity. BMC Physiol. 17, 4 (2017).
Palikaras, K. et al. Ectopic fat deposition contributes to age-associated pathology in Caenorhabditis elegans. J. Lipid Res. 58, 72–80 (2017).
Corvera, S. Cellular heterogeneity in adipose tissues. Annu. Rev. Physiol. 83, 257–278 (2021).
Yang Loureiro, Z. et al. Wnt signaling preserves progenitor cell multipotency during adipose tissue development. Nat. Metab. 5, 1014–1028 (2023).
Matacchione, G. et al. Senescent macrophages in the human adipose tissue as a source of inflammaging. Geroscience 44, 1941–1960 (2022).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254 (2000).
Arner, E. et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 59, 105–109 (2010).
Craig, B. W., Garthwaite, S. M. & Holloszy, J. O. Adipocyte insulin resistance: effects of aging, obesity, exercise, and food restriction. J. Appl. Physiol. 62, 95–100 (1987).
Cruz-Garcia, L. et al. Changes in adipocyte cell size, gene expression of lipid metabolism markers, and lipolytic responses induced by dietary fish oil replacement in gilthead sea bream (Sparus aurata L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 158, 391–399 (2011).
Kim, S. M. et al. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 20, 1049–1058 (2014).
Varlamov, O. et al. Combined androgen excess and Western-style diet accelerates adipose tissue dysfunction in young adult, female nonhuman primates. Hum. Reprod. 32, 1892–1902 (2017).
Goncalves, L. F. et al. Aging is associated with brown adipose tissue remodelling and loss of white fat browning in female C57BL/6 mice. Int. J. Exp. Pathol. 98, 100–108 (2017).
Kirkland, J. L., Hollenberg, C. H., Kindler, S. & Gillon, W. S. Effects of age and anatomic site on preadipocyte number in rat fat depots. J. Gerontol. 49, B31–B35 (1994).
Chen, H. T. et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J. Cell. Mol. Med. 16, 582–593 (2012).
Zhu, M. et al. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J. Tissue Eng. Regen. Med. 3, 290–301 (2009).
Alt, E. U. et al. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res 8, 215–225 (2012).
Van Harmelen, V., Rohrig, K. & Hauner, H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism 53, 632–637 (2004).
Caso, G. et al. Peripheral fat loss and decline in adipogenesis in older humans. Metabolism 62, 337–340 (2013).
Le Pelletier, L. et al. Metformin alleviates stress-induced cellular senescence of aging human adipose stromal cells and the ensuing adipocyte dysfunction. Elife https://doi.org/10.7554/eLife.62635 (2021).
Karagiannides, I. et al. Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1772–R1780 (2001).
Hotta, K. et al. Age-related adipose tissue mRNA expression of ADD1/SREBP1, PPARgamma, lipoprotein lipase, and GLUT4 glucose transporter in rhesus monkeys. J. Gerontol. A Biol. Sci. Med Sci. 54, B183–B188 (1999).
Liu, Q. et al. Convergent alteration of the mesenchymal stem cell heterogeneity in adipose tissue during aging. FASEB J. 37, e23114 (2023).
Nguyen, H. P. et al. Aging-dependent regulatory cells emerge in subcutaneous fat to inhibit adipogenesis. Dev. Cell 56, 1437–1451 (2021).
Holman, C. D. et al. Aging impairs cold-induced beige adipogenesis and adipocyte metabolic reprogramming. eLife 12, RP87756 (2023).
Palani, N. P. et al. Adipogenic and SWAT cells separate from a common progenitor in human brown and white adipose depots. Nat. Metab. 5, 996–1013 (2023).
Donato, A. J. et al. The impact of aging on adipose structure, function and vasculature in the B6D2F1 mouse: evidence of significant multisystem dysfunction. J. Physiol. 592, 4083–4096 (2014).
Camell, C. D. et al. Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab. 30, 1024–1039 (2019).
Bapat, S. P. et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 528, 137–141 (2015).
Brigger, D. et al. Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat. Metab. 2, 688–702 (2020).
Chavakis, T., Alexaki, V. I. & Ferrante, A. W. Jr. Macrophage function in adipose tissue homeostasis and metabolic inflammation. Nat. Immunol. 24, 757–766 (2023).
Kosteli, A. et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Invest. 120, 3466–3479 (2010).
Subramanian, M., Ozcan, L., Ghorpade, D. S., Ferrante, A. W. Jr. & Tabas, I. Suppression of adaptive immune cell activation does not alter innate immune adipose inflammation or insulin resistance in obesity. PLoS ONE 10, e0135842 (2015).
Song, J. et al. Age-associated adipose tissue inflammation promotes monocyte chemotaxis and enhances atherosclerosis. Aging Cell 22, e13783 (2023).
Camell, C. D. et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during aging. Nature 550, 119–123 (2017).
Feng, X. et al. Senescent immune cells accumulation promotes brown adipose tissue dysfunction during aging. Nat. Commun. 14, 3208 (2023).
Abdullahi, A. et al. Adipose browning response to burn trauma is impaired with aging. JCI Insight https://doi.org/10.1172/jci.insight.143451 (2021).
Fabbiano, S. et al. Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metab. 24, 434–446 (2016).
Fulop, T. et al. Immunology of aging: the birth of inflammaging. Clin. Rev. Allergy Immunol. 64, 109–122 (2023).
Islam, M. T. et al. Aging differentially impacts vasodilation and angiogenesis in arteries from the white and brown adipose tissues. Exp. Gerontol. 142, 111126 (2020).
Corvera, S., Solivan-Rivera, J. & Yang Loureiro, Z. Angiogenesis in adipose tissue and obesity. Angiogenesis 25, 439–453 (2022).
Honek, J. et al. Modulation of age-related insulin sensitivity by VEGF-dependent vascular plasticity in adipose tissues. Proc. Natl Acad. Sci. USA 111, 14906–14911 (2014).
Willows, J. W. et al. Age-related changes to adipose tissue and peripheral neuropathy in genetically diverse HET3 mice differ by sex and are not mitigated by rapamycin longevity treatment. Aging Cell 22, e13784 (2023).
Blaszkiewicz, M. et al. Neuropathy and neural plasticity in the subcutaneous white adipose depot. PLoS ONE 14, e0221766 (2019).
Cristante, E. et al. Late neuroprogenitors contribute to normal retinal vascular development in a Hif2a-dependent manner. Development https://doi.org/10.1242/dev.157511 (2018).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell. Res. 25, 585–621 (1961).
Steinert, S., White, D. M., Zou, Y., Shay, J. W. & Wright, W. E. Telomere biology and cellular aging in nonhuman primate cells. Exp. Cell. Res. 272, 146–152 (2002).
Andriani, G. A. et al. Whole chromosome instability induces senescence and promotes SASP. Sci. Rep. 6, 35218 (2016).
Wright, W. E. & Shay, J. W. Historical claims and current interpretations of replicative aging. Nat. Biotechnol. 20, 682–688 (2002).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays aging-associated disorders. Nature 479, 232–236 (2011).
Chen, Y. W., Harris, R. A., Hatahet, Z. & Chou, K. M. Ablation of XP-V gene causes adipose tissue senescence and metabolic abnormalities. Proc. Natl Acad. Sci. USA 112, E4556–E4564 (2015).
Xu, M. et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl Acad. Sci. USA 112, E6301–E6310 (2015).
Minamino, T. et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 15, 1082–1087 (2009).
Gurkar, A. U. et al. Spatial mapping of cellular senescence: emerging challenges and opportunities. Nat. Aging 3, 776–790 (2023).
Cohn, R. L., Gasek, N. S., Kuchel, G. A. & Xu, M. The heterogeneity of cellular senescence: insights at the single-cell level. Trends Cell Biol. 33, 9–17 (2023).
Li, K. et al. Age-related alteration in characteristics, function, and transcription features of ADSCs. Stem Cell Res. Ther. 12, 473 (2021).
Wang, B. et al. Transplanting cells from old but not young donors causes physical dysfunction in older recipients. Aging Cell 19, e13106 (2020).
Gustafson, B., Nerstedt, A. & Smith, U. Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat. Commun. 10, 2757 (2019).
Kavanagh, K. et al. Biomarkers of senescence in non-human primate adipose depots relate to aging. Geroscience 43, 343–352 (2021).
Smith, C. D. et al. Genetically increasing flux through beta-oxidation in skeletal muscle increases mitochondrial reductive stress and glucose intolerance. Am. J. Physiol. Endocrinol. Metab. 320, E938–E950 (2021).
Koves, T. R. et al. Pyruvate-supported flux through medium-chain ketothiolase promotes mitochondrial lipid tolerance in cardiac and skeletal muscles. Cell Metab. 35, 1038–1056 (2023).
Nowak, C. et al. Glucose challenge metabolomics implicates medium-chain acylcarnitines in insulin resistance. Sci. Rep. 8, 8691 (2018).
Lytrivi, M., Castell, A. L., Poitout, V. & Cnop, M. Recent insights into mechanisms of beta-cell lipo- and glucolipotoxicity in type 2 diabetes. J. Mol. Biol. 432, 1514–1534 (2020).
Cheng, X. et al. Targeting DGAT1 ameliorates glioblastoma by increasing fat catabolism and oxidative stress. Cell Metab. 32, 229–242 (2020).
Wang, X. et al. Peroxisome proliferator-activated receptor gamma is essential for stress adaptation by maintaining lipid homeostasis in female fish. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 1867, 159162 (2022).
Meyer, L. K., Ciaraldi, T. P., Henry, R. R., Wittgrove, A. C. & Phillips, S. A. Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte 2, 217–226 (2013).
Cohen, K. E., Katunaric, B., SenthilKumar, G., McIntosh, J. J. & Freed, J. K. Vascular endothelial adiponectin signaling across the life span. Am. J. Physiol. Heart Circ. Physiol. 322, H57–H65 (2022).
Bik, W. & Baranowska, B. Adiponectin—a predictor of higher mortality in cardiovascular disease or a factor contributing to longer life? Neuro Endocrinol. Lett. 30, 180–184 (2009).
Gabriely, I., Ma, X. H., Yang, X. M., Rossetti, L. & Barzilai, N. Leptin resistance during aging is independent of fat mass. Diabetes 51, 1016–1021 (2002).
Martin, M. G. & Dotti, C. G. Plasma membrane and brain dysfunction of the old: do we age from our membranes? Front. Cell Dev. Biol. 10, 1031007 (2022).
Frank, S. M., Raja, S. N., Bulcao, C. & Goldstein, D. S. Age-related thermoregulatory differences during core cooling in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R349–R354 (2000).
Bornstein, M. R. et al. Comprehensive quantification of metabolic flux during acute cold stress in mice. Cell Metab. 35, 2077–2092 (2023).
Ma, X., Xu, L., Gavrilova, O. & Mueller, E. Role of forkhead box protein A3 in age-associated metabolic decline. Proc. Natl Acad. Sci. USA 111, 14289–14294 (2014).
Vatner, D. E., Oydanich, M., Zhang, J., Campbell, S. C. & Vatner, S. F. Exercise enhancement by RGS14 disruption is mediated by brown adipose tissue. Aging Cell 22, e13791 (2023).
Tavassoli, M. & Crosby, W. H. Bone marrow histogenesis: a comparison of fatty and red marrow. Science 169, 291–293 (1970).
Li, Z. et al. Lipolysis of bone marrow adipocytes is required to fuel bone and the marrow niche during energy deficits. Elife https://doi.org/10.7554/eLife.78496 (2022).
Chandra, R. K. & Au, B. Spleen hemolytic plaque-forming cell response and generation of cytotoxic cells in genetically obese (C57Bl/6J ob/ob) mice. Int. Arch. Allergy Appl. Immunol. 62, 94–98 (1980).
Kara, N. et al. Endothelial and leptin receptor+ cells promote the maintenance of stem cells and hematopoiesis in early postnatal murine bone marrow. Dev. Cell 58, 348–360 e346 (2023).
Trinh, T. & Broxmeyer, H. E. Role for leptin and leptin receptors in stem cells during health and diseases. Stem Cell Rev. Rep. 17, 511–522 (2021).
de Candia, P. et al. The pleiotropic roles of leptin in metabolism, immunity, and cancer. J. Exp. Med. https://doi.org/10.1084/jem.20191593 (2021).
Wang, L. & Shan, T. Factors inducing transdifferentiation of myoblasts into adipocytes. J. Cell. Physiol. 236, 2276–2289 (2021).
Venz, R., Pekec, T., Katic, I., Ciosk, R. & Ewald, C. Y. End-of-life targeted degradation of DAF-2 insulin/IGF-1 receptor promotes longevity free from growth-related pathologies. Elife https://doi.org/10.7554/eLife.71335 (2021).
Green, C. L., Lamming, D. W. & Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 23, 56–73 (2022).
Mattison, J. A. et al. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 8, 14063 (2017).
Liu, S. et al. The health-promoting effects and the mechanism of intermittent fasting. J. Diabetes Res. 2023, 4038546 (2023).
Brandhorst, S. et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 22, 86–99 (2015).
Ravussin, E. et al. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1097–1104 (2015).
Zietara, P., Dziewiecka, M. & Augustyniak, M. Why is longevity still a scientific mystery? Sirtuins—past, present and future. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24010728 (2022).
Boutant, M. et al. SIRT1 gain of function does not mimic or enhance the adaptations to intermittent fasting. Cell Rep. 14, 2068–2075 (2016).
Solivan-Rivera, J. et al. A neurogenic signature involving monoamine oxidase-a controls human thermogenic adipose tissue development. Elife https://doi.org/10.7554/eLife.78945 (2022).
Bannister, C. A. et al. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes. Metab. 16, 1165–1173 (2014).
Kulkarni, A. S. et al. Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults. Aging Cell https://doi.org/10.1111/acel.12723 (2018).
Xu, L. et al. PPARγ agonists delay age-associated metabolic disease and extend longevity. Aging Cell 19, e13267 (2020).
Gealekman, O. et al. Effect of rosiglitazone on capillary density and angiogenesis in adipose tissue of normoglycaemic humans in a randomised controlled trial. Diabetologia 55, 2794–2799 (2012).
Belfort, R. et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 355, 2297–2307 (2006).
Nakamura, T. et al. Thiazolidinedione derivative improves fat distribution and multiple risk factors in subjects with visceral fat accumulation–double-blind placebo-controlled trial. Diabetes Res. Clin. Pract. 54, 181–190 (2001).
Sulston, R. J. et al. Increased circulating adiponectin in response to thiazolidinediones: investigating the role of bone marrow adipose tissue. Front. Endocrinol. 7, 128 (2016).
Hidayat, K., Du, X., Wu, M. J. & Shi, B. M. The use of metformin, insulin, sulphonylureas, and thiazolidinediones and the risk of fracture: systematic review and meta-analysis of observational studies. Obes. Rev. 20, 1494–1503 (2019).
Liu, C. et al. Fibroblast growth factor 6 promotes adipocyte progenitor cell proliferation for adipose tissue homeostasis. Diabetes 72, 467–482 (2023).
Mandl, M. et al. Sprouty1 prevents cellular senescence maintaining proliferation and differentiation capacity of human adipose stem/progenitor cells. J. Gerontol. A Biol. Sci. Med. Sci. 75, 2308–2319 (2020).
Tang, W., Zeve, D., Seo, J., Jo, A. Y. & Graff, J. M. Thiazolidinediones regulate adipose lineage dynamics. Cell Metab. 14, 116–122 (2011).
Okada-Iwabu, M. et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503, 493–499 (2013).
Evans, D. S. et al. Proteomic analysis of the senescence associated secretory phenotype (SASP): GDF-15, IGFBP-2, and cystatin-C are associated with multiple aging traits. J. Gerontol. A Biol. Sci. Med. Sci. 79, glad265 (2023).
Baker, D. J. et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Wang, B. et al. An inducible p21-Cre mouse model to monitor and manipulate p21-highly-expressing senescent cells in vivo. Nat. Aging 1, 962–973 (2021).
Wang, L. et al. Targeting p21Cip1 highly expressing cells in adipose tissue alleviates insulin resistance in obesity. Cell Metab. 34, 75–89 (2022).
Power, H., Valtchev, P., Dehghani, F. & Schindeler, A. Strategies for senolytic drug discovery. Aging Cell 22, e13948 (2023).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Gonzales, M. M. et al. Senolytic therapy in mild Alzheimer’s disease: a phase 1 feasibility trial. Nat. Med. 29, 2481–2488 (2023).
Murakami, T., Inagaki, N. & Kondoh, H. Cellular senescence in diabetes mellitus: distinct senotherapeutic strategies for adipose tissue and pancreatic beta cells. Front. Endocrinol. 13, 869414 (2022).
Salaami, O. et al. Antidiabetic effects of the senolytic agent dasatinib. Mayo Clin. Proc. 96, 3021–3029 (2021).
Hickson, L. J. et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456 (2019).
Islam, M. T. et al. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell 22, e13767 (2023).
Ruggiero, A. D. et al. Long-term dasatinib plus quercetin effects on aging outcomes and inflammation in nonhuman primates: implications for senolytic clinical trial design. Geroscience https://doi.org/10.1007/s11357-023-00830-5 (2023).
Hernandez-Silva, D. et al. Senescence-independent anti-inflammatory activity of the senolytic drugs dasatinib, navitoclax, and venetoclax in zebrafish models of chronic inflammation. Int. J. Mol. Sci. https://doi.org/10.3390/ijms231810468 (2022).
Chen, J., Bardes, E. E., Aronow, B. J. & Jegga, A. G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 37, W305–W311 (2009).
Author information
Authors and Affiliations
Contributions
T.T.N. and S.C. contributed to manuscript concept and writing, and analysis of publicly available datasets.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Ming Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, T.T., Corvera, S. Adipose tissue as a linchpin of organismal ageing. Nat Metab 6, 793–807 (2024). https://doi.org/10.1038/s42255-024-01046-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-024-01046-3
This article is cited by
-
ER remodelling is a feature of ageing and depends on ER-phagy
Nature Cell Biology (2026)
-
Osteoarthritis as a systemic disease
Nature Reviews Rheumatology (2026)
-
Interleukin-10 expressing B lineage cells in visceral adipose tissue protect against aging-related insulin resistance and extend lifespan
Nature Communications (2026)
-
Early-onset Palmijihwang-hwan treatment modulates phospholipid metabolism and gut microbiota for healthy aging: reducing adipose inflammation and oxidative stress
npj Aging (2026)
-
Premature aging in serious mental illness
Neuropsychopharmacology (2026)