Abstract
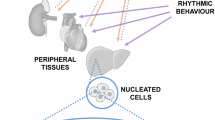
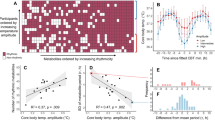
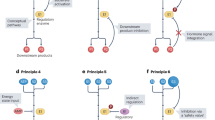
The constant expansion of the field of metabolic research has led to more nuanced and sophisticated understanding of the complex mechanisms that underlie metabolic functions and diseases. Collaborations with scientists of various fields such as neuroscience, immunology and drug discovery have further enhanced the ability to probe the role of metabolism in physiological processes. However, many behaviours, endocrine and biochemical processes, and the expression of genes, proteins and metabolites have daily ~24-h biological rhythms and thus peak only at specific times of the day. This daily variation can lead to incorrect interpretations, lack of reproducibility across laboratories and challenges in translating preclinical studies to humans. In this Review, we discuss the biological, environmental and experimental factors affecting circadian rhythms in rodents, which can in turn alter their metabolic pathways and the outcomes of experiments. We recommend that these variables be duly considered and suggest best practices for designing, analysing and reporting metabolic experiments in a circadian context.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Percie du Sert, N. et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 18, e3000411 (2020).
Nelson, R. J. et al. Time of day as a critical variable in biology. BMC Biol. 20, 142 (2022).
la Fleur, S. E., Kalsbeek, A., Wortel, J., Fekkes, M. L. & Buijs, R. M. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes 50, 1237–1243 (2001).
Zimmet, P. Z., Wall, J. R., Rome, R., Stimmler, L. & Jarrett, R. J. Diurnal variation in glucose tolerance: associated changes in plasma insulin, growth hormone, and non-esterified fatty acids. Br. Med. J. 1, 485–488 (1974).
Klerman, E. B. et al. Keeping an eye on circadian time in clinical research and medicine. Clin. Transl. Med. 12, e1131 (2022).
Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 (2017).
Hatori, M. & Panda, S. The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol. Med. 16, 435–446 (2010).
Ono, D. et al. The suprachiasmatic nucleus at 50: looking back, then looking forward. J. Biol. Rhythms 39, 135–165 (2024).
Todd, W. D. et al. Suprachiasmatic VIP neurons are required for normal circadian rhythmicity and comprised of molecularly distinct subpopulations. Nat. Commun. 11, 4410 (2020).
Saini, C. et al. Real-time recording of circadian liver gene expression in freely moving mice reveals the phase-setting behavior of hepatocyte clocks. Genes Dev. 27, 1526–1536 (2013).
Bass, J. & Takahashi, J. S. Circadian integration of metabolism and energetics. Science 330, 1349–1354 (2010).
Damiola, F. et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961 (2000).
Stokkan, K. A., Yamazaki, S., Tei, H., Sakaki, Y. & Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493 (2001).
Mukherji, A. et al. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc. Natl Acad. Sci. USA 112, E6691–E6698 (2015).
Vollmers, C. et al. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl Acad. Sci. USA 106, 21453–21458 (2009).
Izumo, M. et al. Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. eLife 3, e04617 (2014).
Rao, F. & Xue, T. Circadian-independent light regulation of mammalian metabolism. Nat. Metab. 6, 1000–1007 (2024).
Panda, S. Circadian physiology of metabolism. Science 354, 1008–1015 (2016).
Chouchani, E. T. & Kajimura, S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 1, 189–200 (2019).
Bideyan, L., Nagari, R. & Tontonoz, P. Hepatic transcriptional responses to fasting and feeding. Genes Dev. 35, 635–657 (2021).
Hui, S. et al. Quantitative fluxomics of circulating metabolites. Cell Metab. 32, 676–688 (2020).
Petrenko, V. et al. The core clock transcription factor BMAL1 drives circadian beta-cell proliferation during compensatory regeneration of the endocrine pancreas. Genes Dev. 34, 1650–1665 (2020).
Stenvers, D. J., Scheer, F., Schrauwen, P., la Fleur, S. E. & Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 15, 75–89 (2018).
Challet, E. Keeping circadian time with hormones. Diabetes Obes. Metab. 17, 76–83 (2015).
Martchenko, A., Martchenko, S. E., Biancolin, A. D. & Brubaker, P. L. Circadian rhythms and the gastrointestinal tract: relationship to metabolism and gut hormones. Endocrinology 161, bqaa167 (2020).
Voigt, R. M., Forsyth, C. B. & Keshavarzian, A. Circadian rhythms: a regulator of gastrointestinal health and dysfunction. Expert Rev. Gastroenterol. Hepatol. 13, 411–424 (2019).
Cao, R. mTOR signaling, translational control, and the circadian clock. Front. Genet. 9, 367 (2018).
Liu, S. et al. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature 502, 550–554 (2013).
Guan, D. et al. Diet-induced circadian enhancer remodeling synchronizes opposing hepatic lipid metabolic processes. Cell 174, 831–842 (2018).
Perino, A., Demagny, H., Velazquez-Villegas, L. & Schoonjans, K. Molecular physiology of bile acid signaling in health, disease, and aging. Physiol. Rev. 101, 683–731 (2021).
Yeung, J. & Naef, F. Rhythms of the genome: circadian dynamics from chromatin topology, tissue-specific gene expression, to behavior. Trends Genet. 34, 915–926 (2018).
Mermet, J., Yeung, J. & Naef, F. Systems chronobiology: global analysis of gene regulation in a 24-hour periodic world. Cold Spring Harb. Perspect. Biol. 9, a028720 (2017).
Kim, Y. H. & Lazar, M. A. Transcriptional control of circadian rhythms and metabolism: a matter of time and space. Endocr. Rev. 41, 707–732 (2020).
Lim, C. & Allada, R. Emerging roles for post-transcriptional regulation in circadian clocks. Nat. Neurosci. 16, 1544–1550 (2013).
Brown, S. A. Circadian metabolism: from mechanisms to metabolomics and medicine. Trends Endocrinol. Metab. 27, 415–426 (2016).
Adamovich, Y., Dandavate, V. & Asher, G. Circadian clocks’ interactions with oxygen sensing and signalling. Acta Physiol. 234, e13770 (2022).
Liu, X., Cai, Y. D. & Chiu, J. C. Regulation of protein O-GlcNAcylation by circadian, metabolic, and cellular signals. J. Biol. Chem. 300, 105616 (2024).
Chaix, A., Zarrinpar, A. & Panda, S. The circadian coordination of cell biology. J. Cell Biol. 215, 15–25 (2016).
Aviram, R., Adamovich, Y. & Asher, G. Circadian organelles: rhythms at all scales. Cells 10, 2447 (2021).
Manella, G. & Asher, G. The circadian nature of mitochondrial biology. Front. Endocrinol. 7, 162 (2016).
Neufeld-Cohen, A. et al. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc. Natl Acad. Sci. USA 113, E1673–E1682 (2016).
Masri, S. et al. Circadian acetylome reveals regulation of mitochondrial metabolic pathways. Proc. Natl Acad. Sci. USA 110, 3339–3344 (2013).
Aguilar-Lopez, B. A., Moreno-Altamirano, M. M. B., Dockrell, H. M., Duchen, M. R. & Sanchez-Garcia, F. J. Mitochondria: an integrative hub coordinating circadian rhythms, metabolism, the microbiome, and immunity. Front. Cell Dev. Biol. 8, 51 (2020).
Ma, D., Li, S., Molusky, M. M. & Lin, J. D. Circadian autophagy rhythm: a link between clock and metabolism? Trends Endocrinol. Metab. 23, 319–325 (2012).
Juste, Y. R. et al. Reciprocal regulation of chaperone-mediated autophagy and the circadian clock. Nat. Cell Biol. 23, 1255–1270 (2021).
Welsh, D. K., Yoo, S. H., Liu, A. C., Takahashi, J. S. & Kay, S. A. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 14, 2289–2295 (2004).
Balsalobre, A., Damiola, F. & Schibler, U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93, 929–937 (1998).
Balsalobre, A. et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347 (2000).
Brown, S. A., Zumbrunn, G., Fleury-Olela, F., Preitner, N. & Schibler, U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr. Biol. 12, 1574–1583 (2002).
Yagita, K. & Okamura, H. Forskolin induces circadian gene expression of rPer1, rPer2 and dbp in mammalian rat-1 fibroblasts. FEBS Lett. 465, 79–82 (2000).
Brown, S. A. et al. Molecular insights into human daily behavior. Proc. Natl Acad. Sci. USA 105, 1602–1607 (2008).
Manella, G., Aizik, D., Aviram, R., Golik, M. & Asher, G. Circa-SCOPE: high-throughput live single-cell imaging method for analysis of circadian clock resetting. Nat. Commun. 12, 5903 (2021).
Schwartz, W. J. & Zimmerman, P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J. Neurosci. 10, 3685–3694 (1990).
Kopp, C. et al. Effects of a daylight cycle reversal on locomotor activity in several inbred strains of mice. Physiol. Behav. 63, 577–585 (1998).
Moore, T. M. et al. Conserved multi-tissue transcriptomic adaptations to exercise training in humans and mice. Cell Rep. 42, 112499 (2023).
Reiter, R. J. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 12, 151–180 (1991).
Cipolla-Neto, J. & Amaral, F. G. D. Melatonin as a hormone: new physiological and clinical insights. Endocr. Rev. 39, 990–1028 (2018).
Persaud, S. J. & Jones, P. M. A wake-up call for type 2 diabetes? N. Engl. J. Med. 375, 1090–1092 (2016).
Karamitri, A. & Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 15, 105–125 (2019).
Goto, M., Oshima, I., Tomita, T. & Ebihara, S. Melatonin content of the pineal gland in different mouse strains. J. Pineal Res. 7, 195–204 (1989).
Roseboom, P. H. et al. Natural melatonin ‘knockdown’ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res. Mol. Brain Res. 63, 189–197 (1998).
Enriquez, J. A. Mind your mouse strain. Nat. Metab. 1, 5–7 (2019).
Buckley, T. N. et al. High-fat feeding disrupts daily eating behavior rhythms in obesity-prone but not in obesity-resistant male inbred mouse strains. Am. J. Physiol. Regul. Integr. Comp. Physiol. 320, R619–R629 (2021).
Lempiainen, P. A., Ylitalo, A., Huikuri, H., Kesaniemi, Y. A. & Ukkola, O. H. Non-dipping blood pressure pattern is associated with cardiovascular events in a 21-year follow-up study. J. Hum. Hypertens. 38, 444–451 (2024).
Kudo, T. et al. Night-time restricted feeding normalises clock genes and Pai-1 gene expression in the db/db mouse liver. Diabetologia 47, 1425–1436 (2004).
Hou, T. et al. Time-restricted feeding protects the blood pressure circadian rhythm in diabetic mice. Proc. Natl Acad. Sci. USA 118, e2015873118 (2021).
Zhang, T. et al. High-throughput discovery of genetic determinants of circadian misalignment. PLoS Genet. 16, e1008577 (2020).
Yang, G. et al. Systemic PPARγ deletion impairs circadian rhythms of behavior and metabolism. PLoS ONE 7, e38117 (2012).
Canaple, L. et al. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor a defines a novel positive feedback loop in the rodent liver circadian clock.Mol. Endocrinol. 20, 1715–1727 (2006).
Liu, C., Li, S., Liu, T., Borjigin, J. & Lin, J. D. Transcriptional coactivator PGC-1α integrates the mammalian clock and energy metabolism. Nature 447, 477–481 (2007).
Hong, H. K. et al. Requirement for NF-κB in maintenance of molecular and behavioral circadian rhythms in mice. Genes Dev. 32, 1367–1379 (2018).
Shen, Y. et al. NF-κB modifies the mammalian circadian clock through interaction with the core clock protein BMAL1. PLoS Genet. 17, e1009933 (2021).
Duncan, M. J. et al. Effects of aging and genotype on circadian rhythms, sleep, and clock gene expression in APP × PS1 knock-in mice, a model for Alzheimer’s disease. Exp. Neurol. 236, 249–258 (2012).
Krizo, J. A. & Mintz, E. M. Sex differences in behavioral circadian rhythms in laboratory rodents. Front. Endocrinol. 5, 234 (2014).
Alvord, V. M., Kantra, E. J. & Pendergast, J. S. Estrogens and the circadian system. Semin. Cell Dev. Biol. 126, 56–65 (2022).
Alvord, V. M. & Pendergast, J. S. The estrous cycle coordinates the circadian rhythm of eating behavior in mice. J. Biol. Rhythms 39, 413–422 (2024).
Palmisano, B. T., Stafford, J. M. & Pendergast, J. S. High-fat feeding does not disrupt daily rhythms in female mice because of protection by ovarian hormones. Front. Endocrinol. 8, 44 (2017).
Weger, B. D. et al. The mouse microbiome is required for sex-specific diurnal rhythms of gene expression and metabolism. Cell Metab. 29, 362–382 (2019).
Nakamura, T. J. et al. Influence of the estrous cycle on clock gene expression in reproductive tissues: effects of fluctuating ovarian steroid hormone levels. Steroids 75, 203–212 (2010).
Iwasaki-Sekino, A., Mano-Otagiri, A., Ohata, H., Yamauchi, N. & Shibasaki, T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology 34, 226–237 (2009).
Weinert, D. Age-dependent changes of the circadian system. Chronobiol. Int. 17, 261–283 (2000).
Welsh, D. K., Richardson, G. S. & Dement, W. C. Effect of age on the circadian pattern of sleep and wakefulness in the mouse. J. Gerontol. 41, 579–586 (1986).
Valentinuzzi, V. S., Scarbrough, K., Takahashi, J. S. & Turek, F. W. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am. J. Physiol. 273, R1957–R1964 (1997).
Buresova, M., Benesova, O. & Illnerova, H. Aging alters resynchronization of the circadian system in rats after a shift of the light–dark cycle. Experientia 46, 75–77 (1990).
Davidson, A. J. et al. Chronic jet-lag increases mortality in aged mice. Curr. Biol. 16, R914–R916 (2006).
Acosta-Rodriguez, V. A., Rijo-Ferreira, F., Green, C. B. & Takahashi, J. S. Importance of circadian timing for aging and longevity. Nat. Commun. 12, 2862 (2021).
Speakman, J. R. & Keijer, J. Not so hot: optimal housing temperatures for mice to mimic the thermal environment of humans. Mol. Metab. 2, 5–9 (2012).
Fischer, A. W., Cannon, B. & Nedergaard, J. Optimal housing temperatures for mice to mimic the thermal environment of humans: an experimental study. Mol. Metab. 7, 161–170 (2018).
Ganeshan, K. & Chawla, A. Warming the mouse to model human diseases. Nat. Rev. Endocrinol. 13, 458–465 (2017).
Kajimura, S. & Spiegelman, B. M. Confounding issues in the ‘humanized’ BAT of mice. Nat. Metab. 2, 303–304 (2020).
Rabearivony, A. et al. Housing temperature affects the circadian rhythm of hepatic metabolism and clock genes. J. Endocrinol. 247, 183–195 (2020).
Skop, V. et al. Mouse thermoregulation: introducing the concept of the thermoneutral point. Cell Rep. 31, 107501 (2020).
Keijer, J., Li, M. & Speakman, J. R. What is the best housing temperature to translate mouse experiments to humans? Mol. Metab. 25, 168–176 (2019).
Fischer, A. W., Cannon, B. & Nedergaard, J. The answer to the question ‘what is the best housing temperature to translate mouse experiments to humans?’ is: thermoneutrality. Mol. Metab. 26, 1–3 (2019).
Keijer, J., Li, M. & Speakman, J. R. To best mimic human thermal conditions, mice should be housed slightly below thermoneutrality. Mol. Metab. 26, 4 (2019).
Manzanares, G., Brito-da-Silva, G. & Gandra, P. G. Voluntary wheel running: patterns and physiological effects in mice. Braz. J. Med. Biol. Res. 52, e7830 (2018).
Wolff, G. & Esser, K. A. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med. Sci. Sports Exerc. 44, 1663–1670 (2012).
Yasumoto, Y., Nakao, R. & Oishi, K. Free access to a running-wheel advances the phase of behavioral and physiological circadian rhythms and peripheral molecular clocks in mice. PLoS ONE 10, e0116476 (2015).
Schroeder, A. M. et al. Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J. Physiol. 590, 6213–6226 (2012).
Pendergast, J. S., Branecky, K. L., Huang, R., Niswender, K. D. & Yamazaki, S. Wheel-running activity modulates circadian organization and the daily rhythm of eating behavior. Front. Psychol. 5, 177 (2014).
Karamihalev, S., Flachskamm, C., Eren, N., Kimura, M. & Chen, A. Social context and dominance status contribute to sleep patterns and quality in groups of freely-moving mice. Sci. Rep. 9, 15190 (2019).
Robbers, Y., Tersteeg, M. M. H., Meijer, J. H. & Coomans, C. P. Group housing and social dominance hierarchy affect circadian activity patterns in mice. R. Soc. Open Sci. 8, 201985 (2021).
Ota, S. M., Kong, X., Hut, R., Suchecki, D. & Meerlo, P. The impact of stress and stress hormones on endogenous clocks and circadian rhythms. Front. Neuroendocrinol. 63, 100931 (2021).
Tahara, Y. et al. Entrainment of the mouse circadian clock by sub-acute physical and psychological stress. Sci. Rep. 5, 11417 (2015).
Manno, F. A. M. et al. Environmental enrichment leads to behavioral circadian shifts enhancing brain-wide functional connectivity between sensory cortices and eliciting increased hippocampal spiking. NeuroImage 252, 119016 (2022).
Peirson, S. N., Brown, L. A., Pothecary, C. A., Benson, L. A. & Fisk, A. S. Light and the laboratory mouse. J. Neurosci. Methods 300, 26–36 (2018).
Small, L. et al. Seasonal light hours modulate peripheral clocks and energy metabolism in mice. Cell Metab. 35, 1722–1735 (2023).
Fonken, L. K. et al. Light at night increases body mass by shifting the time of food intake. Proc. Natl Acad. Sci. USA 107, 18664–18669 (2010).
Zhang, S., Zhang, Y., Zhang, W., Chen, S. & Liu, C. Chronic exposure to green light aggravates high-fat diet-induced obesity and metabolic disorders in male mice. Ecotoxicol. Environ. Saf. 178, 94–104 (2019).
Lucas, R. J. et al. Recommendations for measuring and standardizing light for laboratory mammals to improve welfare and reproducibility in animal research. PLoS Biol. 22, e3002535 (2024).
Kohsaka, A. et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 6, 414–421 (2007).
Pendergast, J. S. et al. High-fat diet acutely affects circadian organisation and eating behavior. Eur. J. Neurosci. 37, 1350–1356 (2013).
Mendoza, J., Pevet, P. & Challet, E. High-fat feeding alters the clock synchronization to light. J. Physiol. 586, 5901–5910 (2008).
Chaix, A., Deota, S., Bhardwaj, R., Lin, T. & Panda, S. Sex- and age-dependent outcomes of 9-hour time-restricted feeding of a Western high-fat high-sucrose diet in C57BL/6J mice. Cell Rep. 36, 109543 (2021).
Benegiamo, G. et al. The genetic background shapes the susceptibility to mitochondrial dysfunction and NASH progression. J. Exp. Med. 220, e20221738 (2023).
Montgomery, M. K. et al. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 56, 1129–1139 (2013).
Bachmann, A. M. et al. Genetic background and sex control the outcome of high-fat diet feeding in mice. iScience 25, 104468 (2022).
Chaix, A., Manoogian, E. N. C., Melkani, G. C. & Panda, S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu. Rev. Nutr. 39, 291–315 (2019).
Ikeda, Y. et al. Glucagon and/or IGF-1 production regulates resetting of the liver circadian clock in response to a protein or amino acid-only diet. EBioMedicine 28, 210–224 (2018).
Oishi, K. et al. Ketogenic diet disrupts the circadian clock and increases hypofibrinolytic risk by inducing expression of plasminogen activator inhibitor-1. Arterioscler. Thromb. Vasc. Biol. 29, 1571–1577 (2009).
Tognini, P. et al. Distinct circadian signatures in liver and gut clocks revealed by ketogenic diet. Cell Metab. 26, 523–538 (2017).
Acosta-Rodriguez, V. A., de Groot, M. H. M., Rijo-Ferreira, F., Green, C. B. & Takahashi, J. S. Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab. 26, 267–277 (2017).
Acosta-Rodriguez, V. et al. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science 376, 1192–1202 (2022).
Pendergast, J. S. & Yamazaki, S. The mysterious food-entrainable oscillator: insights from mutant and engineered mouse models. J. Biol. Rhythms 33, 458–474 (2018).
Xin, H. et al. A multi-tissue multi-omics analysis reveals distinct kineztics in entrainment of diurnal transcriptomes by inverted feeding. iScience 24, 102335 (2021).
Froy, O., Chapnik, N. & Miskin, R. Effect of intermittent fasting on circadian rhythms in mice depends on feeding time. Mech. Ageing Dev. 130, 154–160 (2009).
Frazier, K. & Chang, E. B. Intersection of the gut microbiome and circadian rhythms in metabolism. Trends Endocrinol. Metab. 31, 25–36 (2020).
Frazier, K. et al. High-fat diet disrupts REG3γ and gut microbial rhythms promoting metabolic dysfunction. Cell Host Microbe 30, 809–823 (2022).
Murakami, M. et al. Gut microbiota directs PPARγ-driven reprogramming of the liver circadian clock by nutritional challenge. EMBO Rep. 17, 1292–1303 (2016).
Tahara, Y. et al. Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci. Rep. 8, 1395 (2018).
Montagner, A. et al. Hepatic circadian clock oscillators and nuclear receptors integrate microbiome-derived signals. Sci. Rep. 6, 20127 (2016).
Smith, J. G. et al. Antibiotic-induced microbiome depletion remodels daily metabolic cycles in the brain. Life Sci. 303, 120601 (2022).
Allaband, C. et al. Time of sample collection is critical for the replicability of microbiome analyses. Nat. Metab. 6, 1282–1293 (2024).
Zarrinpar, A. et al. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat. Commun. 9, 2872 (2018).
Gabriel, B. M. & Zierath, J. R. Circadian rhythms and exercise — re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 15, 197–206 (2019).
Martin, R. A., Viggars, M. R. & Esser, K. A. Metabolism and exercise: the skeletal muscle clock takes centre stage. Nat. Rev. Endocrinol. 19, 272–284 (2023).
Shirai, H., Oishi, K., Kudo, T., Shibata, S. & Ishida, N. PPARα is a potential therapeutic target of drugs to treat circadian rhythm sleep disorders. Biochem. Biophys. Res. Commun. 357, 679–682 (2007).
Antoch, M. P. & Kondratov, R. V. Pharmacological modulators of the circadian clock as potential therapeutic drugs: focus on genotoxic/anticancer therapy. In Circadian Clocks. Handbook of Experimental Pharmacology Vol. 217 (eds Kramer, A. & Merrow, M.) 289–309 (Springer, 2013).
Tamai, T. K. et al. Identification of circadian clock modulators from existing drugs. EMBO Mol. Med. 10, e8724 (2018).
Crosby, P. et al. Insulin/IGF-1 drives PERIOD synthesis to entrain circadian rhythms with feeding time. Cell 177, 896–909 (2019).
Weger, M., Weger, B. D. & Gachon, F. Understanding circadian dynamics: current progress and future directions for chronobiology in drug discovery. Expert Opin. Drug Discov. 18, 893–901 (2023).
Manella, G. et al. Hypoxia induces a time- and tissue-specific response that elicits intertissue circadian clock misalignment. Proc. Natl Acad. Sci. USA 117, 779–786 (2020).
Koritala, B. S. C. et al. Intermittent hypoxia alters the circadian expression of clock genes in mouse brain and liver. Genes 12, 1627 (2021).
Koritala, B. S. C. et al. Obstructive sleep apnea in a mouse model is associated with tissue-specific transcriptomic changes in circadian rhythmicity and mean 24-hour gene expression. PLoS Biol. 21, e3002139 (2023).
Niu, L. et al. Chronic sleep deprivation altered the expression of circadian clock genes and aggravated Alzheimer’s disease neuropathology. Brain Pathol. 32, e13028 (2022).
Takahashi, K. et al. Chronic mild stress alters circadian expressions of molecular clock genes in the liver. Am. J. Physiol. Endocrinol. Metab. 304, E301–E309 (2013).
Logan, R. W. et al. Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in mice. Biol. Psychiatry 78, 249–258 (2015).
Miyazaki, K., Itoh, N., Ohyama, S., Kadota, K. & Oishi, K. Continuous exposure to a novel stressor based on water aversion induces abnormal circadian locomotor rhythms and sleep–wake cycles in mice. PLoS ONE 8, e55452 (2013).
Gil-Lozano, M., Mingomataj, E. L., Wu, W. K., Ridout, S. A. & Brubaker, P. L. Circadian secretion of the intestinal hormone GLP-1 by the rodent L cell. Diabetes 63, 3674–3685 (2014).
Kalsbeek, A. & Strubbe, J. H. Circadian control of insulin secretion is independent of the temporal distribution of feeding. Physiol. Behav. 63, 553–558 (1998).
Pak, H. H. et al. Non-canonical metabolic and molecular effects of calorie restriction are revealed by varying temporal conditions. Cell Rep. 43, 114663 (2024).
Dantas Machado, A. C. et al. Diet and feeding pattern modulate diurnal dynamics of the ileal microbiome and transcriptome. Cell Rep. 40, 111008 (2022).
Parra-Vargas, M., Ramon-Krauel, M., Lerin, C. & Jimenez-Chillaron, J. C. Size does matter: litter size strongly determines adult metabolism in rodents. Cell Metab. 32, 334–340 (2020).
Yu, H. et al. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin. Chem. 57, 691–700 (2011).
Andersen, B., Beck-Nielsen, H. & Hojlund, K. Plasma FGF21 displays a circadian rhythm during a 72-h fast in healthy female volunteers. Clin. Endocrinol. 75, 514–519 (2011).
Tong, X. et al. Transcriptional repressor E4-binding protein 4 (E4BP4) regulates metabolic hormone fibroblast growth factor 21 (FGF21) during circadian cycles and feeding. J. Biol. Chem. 285, 36401–36409 (2010).
Fenzl, T., Flachskamm, C., Rossbauer, M., Deussing, J. M. & Kimura, M. Circadian rhythms of basal orexin levels in the hypothalamus are not influenced by an impaired corticotropin-releasing hormone receptor type 1 system. Behav. Brain Res. 203, 143–145 (2009).
Surya, S., Symons, K., Rothman, E. & Barkan, A. L. Complex rhythmicity of growth hormone secretion in humans. Pituitary 9, 121–125 (2006).
Bodosi, B. et al. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R1071–R1079 (2004).
Sinha, M. K. et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J. Clin. Invest. 97, 1344–1347 (1996).
Acknowledgements
F.G. receives funding from the Australian National Health and Medical Research Council (Synergy Grant 2019260), the National Institutes of Health (R01AG078241) and the Novo Nordisk Foundation (Hallas-Møller Ascending Investigator grant 0087882). Research in S.P.’s laboratory is supported by NIH grants AG068550 and CA258221, the Wu Tsai Human Performance Alliance and the Joe and Clara Tsai Foundation.
Author information
Authors and Affiliations
Contributions
S.D. and S.P. wrote the initial draft of the manuscript and led the revisions. All authors contributed to the content and organization of this Review; contributed to writing, editing and/or revising the manuscript; and approved the final version.
Corresponding author
Ethics declarations
Competing interests
S.A.B. is a cofounder and scientific advisor of Ona Therapeutics. G.S.H. is a scientific advisory board member of Crescenta Biosciences. J.S.T. is a founder and scientific advisory board member of Synchronicity Pharma. J.D.R. is a paid adviser and/or stockholder in Colorado Research Partners, L.E.A.F. Pharmaceuticals, Faeth Therapeutics and Empress Therapeutics; a paid consultant of Pfizer; a founder and stockholder in Marea Therapeutics; and a founder, director and stockholder of Farber Partners, Raze Therapeutics and Sofro Pharmaceuticals. S.K. serves as a scientific advisory board member of Moonwalk Biosciences. V.D.L. has equity interest in L-Nutra, a company making medical food, and has filed patents related to fasting-mimicking diets and their medical use. M.A.L. is on the advisory board of Pfizer and serves on the advisory board and is a cofounder of Flare Therapeutics. E.V. is a scientific cofounder of Napa Therapeutics and BHB Therapeutics and serves on the scientific advisory board of Seneque. J.A. is a board member of NOV Metapharma, a founder and/or consultant for Vandria and Amprenta Therapeutics and consults for OrsoBio, MetroBiotech and Amazentis (now Timeline); none of these companies develop products regulating circadian activity. D.J.D. has served as a consultant or speaker within the past 12 months for Amgen, AstraZeneca, Boehringer Ingelheim, Kallyope, Novo Nordisk and Pfizer. S.P. is the author of the books the Circadian Code and the Circadian Diabetes Code and is a scientific advisor to Hooke London, Avadel and WndrHlth. Other authors have no conflict of interests to declare.
Peer review
Peer review information
Nature Metabolism thanks Kevin Koronowski, Annie Curtis and Min-Dian Li for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deota, S., Pendergast, J.S., Kolthur-Seetharam, U. et al. The time is now: accounting for time-of-day effects to improve reproducibility and translation of metabolism research. Nat Metab 7, 454–468 (2025). https://doi.org/10.1038/s42255-025-01237-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01237-6
This article is cited by
-
Circadian ontogenetic metabolomics atlas: an interactive resource with insights from rat plasma, tissues, and feces
Cellular and Molecular Life Sciences (2025)