Abstract
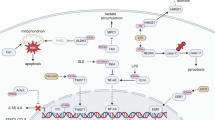
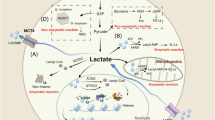
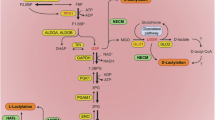
l-Lactate has emerged as a crucial metabolic intermediate, moving beyond its traditional view as a mere waste product. The recent discovery of l-lactate-driven protein lactylation as a post-translational modification has unveiled a pathway that highlights the role of lactate in cellular signalling. In this Perspective, we explore the enzymatic and metabolic mechanisms underlying protein lactylation and its impacts on both histone and non-histone proteins in the contexts of physiology and diseases. We discuss growing evidence suggesting that this modification regulates a wide range of cellular functions and is involved in various physiological and pathological processes, such as cell-fate determination, development, cardiovascular diseases, cancer and autoimmune disorders. We propose that protein lactylation acts as a pivotal mechanism, integrating metabolic and signalling pathways to enable cellular adaptation, and highlight its potential as a therapeutic target in various diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Warburg, O., Wind, F. & Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 8, 519–530 (1927).
Adeva-Andany, M. et al. Comprehensive review on lactate metabolism in human health. Mitochondrion 17, 76–100 (2014).
Rabinowitz, J. D. & Enerback, S. Lactate: the ugly duckling of energy metabolism. Nat. Metab. 2, 566–571 (2020).
Brooks, G. A. et al. Lactate in contemporary biology: a phoenix risen. J. Physiol. 600, 1229–1251 (2022).
Certo, M. et al. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 21, 151–161 (2021).
Li, X. et al. Lactate metabolism in human health and disease. Signal. Transduct. Target Ther. 7, 305 (2022).
Houtkooper, R. H., Canto, C., Wanders, R. J. & Auwerx, J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 31, 194–223 (2010).
Ahmed, K. et al. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 11, 311–319 (2010).
Daw, C. C. et al. Lactate elicits ER-mitochondrial Mg2+ dynamics to integrate cellular metabolism. Cell 183, 474–489 (2020).
Liu, W. et al. Lactate regulates cell cycle by remodelling the anaphase promoting complex. Nature 616, 790–797 (2023).
Cai, X. et al. Lactate activates the mitochondrial electron transport chain independently of its metabolism. Mol. Cell 83, 3904–3920 (2023).
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–57 (2019).
Ramazi, S. & Zahiri, J. Post-translational modifications in proteins: resources, tools and prediction methods. Database 2021, baab012 (2021).
Walsh, C. T., Garneau-Tsodikova, S. & Gatto, G. J. Jr. Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem. Int. Ed. Engl. 44, 7342–7372 (2005).
Su, X. Y., Wellen, K. E. & Rabinowitz, J. D. Metabolic control of methylation and acetylation. Curr. Opin. Chem. Biol. 30, 52–60 (2016).
Choudhary, C. et al. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 15, 536–550 (2014).
Sabari, B. R., Zhang, D., Allis, C. D. & Zhao, Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 18, 90–101 (2017).
Dai, Z., Ramesh, V. & Locasale, J. W. The evolving metabolic landscape of chromatin biology and epigenetics. Nat. Rev. Genet. 21, 737–753 (2020).
Gaffney, D. O. et al. Non-enzymatic lysine lactoylation of glycolytic enzymes. Cell Chem. Biol. 27, 206–213 (2020).
Ahmed, M. U. et al. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J 324, 565–570 (1997).
Zhang, D. et al. Lysine l-lactylation is the dominant lactylation isomer induced by glycolysis. Nat. Chem. Biol. 21, 91–99 (2024).
Gao, J. et al. Identification of 113 new histone marks by CHiMA, a tailored database search strategy. Sci. Adv. 9, eadf1416 (2023).
Yang, D. et al. Identification of lysine-lactylated substrates in gastric cancer cells. iScience 25, 104630 (2022).
Wang, X. et al. YY1 lactylation in microglia promotes angiogenesis through transcription activation-mediated upregulation of FGF2. Genome Biol. 24, 87 (2023).
Yang, Y. H. et al. Global profiling of lysine lactylation in human lungs. Proteomics 23, e2200437 (2023).
Lin, Y. et al. Multi-proteomic analysis reveals the effect of protein lactylation on matrix and cholesterol metabolism in tendinopathy. J. Proteom. Res. 22, 1712–1722 (2023).
Yang, Z. et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat. Metab. 5, 61–79 (2023).
Yao, Y. et al. Global-scale profiling of differential expressed lysine-lactylated proteins in the cerebral endothelium of cerebral ischemia-reperfusion injury rats. Cell Mol. Neurobiol. 43, 1989–2004 (2023).
Zhang, N. et al. Protein lactylation critically regulates energy metabolism in the protozoan parasite Trypanosoma brucei. Front. Cell Dev. Biol. 9, 719720 (2021).
Yin, D. et al. Protein lactylation and metabolic regulation of the zoonotic parasite Toxoplasma gondii. Genomics Proteomics Bioinformatics 21, 1163–1181 (2023).
An, D. et al. Comprehensive analysis of lysine lactylation in Frankliniella occidentalis. Front. Genet. 13, 1014225 (2022).
Zhao, W. et al. Systematic identification of the lysine lactylation in the protozoan parasite Toxoplasma gondii. Parasit. Vectors 15, 180 (2022).
Song, Y. G. et al. Post-translational changes in lysine lactylation during prolonged presence in a patient with a related immune disorder. Front. Immunol. 13, 966457 (2022).
Meng, X., Baine, J. M., Yan, T. & Wang, S. Comprehensive analysis of lysine lactylation in rice (Oryza sativa) grains. J. Agric. Food Chem. 69, 8287–8297 (2021).
Wu, Q. et al. Deciphering the atlas of post-translational modification in sugarcane. J. Agric. Food Chem. 71, 10004–10017 (2023).
Hansen, B. K. et al. Analysis of human acetylation stoichiometry defines mechanistic constraints on protein regulation. Nat. Commun. 10, 1055 (2019).
Olsen, J. V. et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci. Signal 3, ra3 (2010).
Prus, G. et al. Global, site-resolved analysis of ubiquitylation occupancy and turnover rate reveals systems properties. Cell 187, 2875–2892 (2024).
Allis, C. D. et al. New nomenclature for chromatin-modifying enzymes. Cell 131, 633–636 (2007).
Wang, N. et al. Histone lactylation boosts reparative gene activation post-myocardial infarction. Circ. Res. 131, 893–908 (2022).
Zhu, R. et al. ACSS2 acts as a lactyl-CoA synthetase and couples KAT2A to function as a lactyltransferase for histone lactylation and tumor immune evasion. Cell Metab. 37, 361–376 (2024).
Chen, Y. et al. Metabolic regulation of homologous recombination repair by MRE11 lactylation. Cell 187, 294–311 (2024).
Chen, H. X. et al. NBS1 lactylation is required for efficient DNA repair and chemotherapy resistance. Nature 631, 663–669 (2024).
Niu, Z. et al. HBO1 catalyzes lysine lactylation and mediates histone H3K9la to regulate gene transcription. Nat. Commun. 15, 3561 (2024).
Xie, B. et al. KAT8-catalyzed lactylation promotes eEF1A2-mediated protein synthesis and colorectal carcinogenesis. Proc. Natl Acad. Sci. USA 121, e2314128121 (2024).
Wang, Y. G. et al. KAT2A coupled with the α-KGDH complex acts as a histone H3 succinyltransferase. Nature 552, 273–27 (2017).
Liu, R. et al. Nuclear GTPSCS functions as a lactyl-CoA synthetase to promote histone lactylation and gliomagenesis. Cell Metab. 37, 377–394 (2024).
Ibba, M. & Soll, D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 69, 617–650 (2000).
Mao, Y. et al. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res. 34, 13–30 (2024).
Ju, J. Y. et al. The alanyl-tRNA synthetase AARS1 moonlights as a lactyltransferase to promote YAP signaling in gastric cancer. J. Clin. Invest. 134, e174587 (2024).
Zong, Z. et al. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell 187, 2375–2392 (2024).
Sun, L. H. et al. Lactylation of METTL16 promotes cuproptosis via m6A-modification on FDX1 mRNA in gastric cancer. Nat. Commun. 14, 6523 (2023).
Li, H. et al. AARS1 and AARS2 sense l-lactate to regulate cGAS as global lysine lactyltransferases. Nature. 634 1229–1237 (2024).
Seto, E. & Yoshida, M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 6, a018713 (2014).
Lahm, A. et al. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc. Natl Acad. Sci. USA 104, 17335–17340 (2007).
Du, J. T. et al. Sirt5 Is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334, 806–809 (2011).
Peng, C. et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol. Cell Proteomics 10, M111.012658 (2011).
Tan, M. J. et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 19, 605–617 (2014).
Kutil, Z. et al. Histone deacetylase 11 is a fatty-acid deacylase. ACS Chem. Biol. 13, 685–693 (2018).
Moreno-Yruela, C., Galleano, I., Madsen, A. S. & Olsen, C. A. Histone deacetylase 11 is an ε-N-myristoyllysine hydrolase. Cell Chem. Biol. 25, 849–84 (2018).
Madsen, A. S. & Olsen, C. A. Profiling of substrates for zinc-dependent lysine deacylase enzymes: HDAC3 exhibits decrotonylase activity in vitro. Angew. Chem. Int. Ed. Engl. 51, 9083–9087 (2012).
Wei, W. et al. Class I histone deacetylases are major histone decrotonylases: evidence for critical and broad function of histone crotonylation in transcription. Cell Res. 27, 898–915 (2017).
Huang, H. et al. The regulatory enzymes and protein substrates for the lysine beta-hydroxybutyrylation pathway. Sci. Adv. 7, eabe2771 (2021).
Moreno-Yruela, C. et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci. Adv. 8, eabi6696 (2022).
Zessin, M. et al. Uncovering robust delactoylase and depyruvoylase activities of HDAC isoforms. ACS Chem. Biol. 17, 1364–1375 (2022).
Wang, Z. A. et al. Histone H2B deacylation selectivity: exploring chromatin’s dark matter with an engineered Sortase. J. Am. Chem. Soc. 144, 3360–3364 (2022).
Du, R. et al. Sirtuin 1/sirtuin 3 are robust lysine delactylases and sirtuin 1-mediated delactylation regulates glycolysis. iScience 27, 110911 (2024).
Jin, J. et al. SIRT3-dependent delactylation of cyclin E2 prevents hepatocellular carcinoma growth. EMBO Rep. 24, e56052 (2023).
Fan, Z. M. et al. Identification of SIRT3 as an eraser of H4K16la. iScience 26, 107757 (2023).
Yun, M., Wu, J., Workman, J. L. & Li, B. Readers of histone modifications. Cell Res. 21, 564–578 (2011).
Andrews, F. H., Strahl, B. D. & Kutateladze, T. G. Insights into newly discovered marks and readers of epigenetic information. Nat. Chem. Biol. 12, 662–668 (2016).
Dhalluin, C. et al. Structure and ligand of a histone acetyltransferase bromodomain. Nature 399, 491–496 (1999).
Nunez, R. et al. The TRIM33 bromodomain recognizes histone lysine lactylation. ACS Chem. Biol. 19, 2418–2428 (2024).
Ferri, F. et al. TRIM33 switches off Ifnb1 gene transcription during the late phase of macrophage activation. Nat. Commun. 6, 8900 (2015).
Palsson-McDermott, E. M. & O’Neill, L. A. J. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays 35, 965–973 (2013).
Phillips, D. M. The presence of acetyl groups of histones. Biochem. J 87, 258–263 (1963).
Allfrey, V. G., Faulkner, R. & Mirsky, A. E. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc. Natl Acad. Sci. USA 51, 786–794 (1964).
Guarente, L. The logic linking protein acetylation and metabolism. Cell Metab. 14, 151–153 (2011).
Bose, S., Ramesh, V. & Locasale, J. W. Acetate metabolism in physiology, cancer, and beyond. Trends Cell Biol. 29, 695–703 (2019).
Guertin, D. A. & Wellen, K. E. Acetyl-CoA metabolism in cancer. Nat. Rev. Cancer 23, 156–172 (2023).
Kelly, B. & O’Neill, L. A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 25, 771–784 (2015).
Sung, E. et al. Global profiling of lysine acetylation and lactylation in Kupffer cells. J. Proteome Res. 22, 3683–3691 (2023).
Sun, S. et al. Metabolic regulation of cytoskeleton functions by HDAC6-catalyzed alpha-tubulin lactylation. Nat. Commun. 15, 8377 (2024).
Campbell, S. L. & Wellen, K. E. Metabolic signaling to the nucleus in cancer. Mol. Cell 71, 398–408 (2018).
Wellen, K. E. & Thompson, C. B. A two-way street: reciprocal regulation of metabolism and signalling. Nat. Rev. Mol. Cell Biol. 13, 270–276 (2012).
Hagihara, H. et al. Protein lactylation induced by neural excitation. Cell Rep. 37, 109820 (2021).
Wright, W. D., Shah, S. S. & Heyer, W. D. Homologous recombination and the repair of DNA double-strand breaks. J. Biol. Chem. 293, 10524–10535 (2018).
Li, G. et al. Glycometabolic reprogramming-induced XRCC1 lactylation confers therapeutic resistance in ALDH1A3-overexpressing glioblastoma. Cell Metab. 36, 1696–1710 (2024).
Wan, N. et al. Cyclic immonium ion of lactyllysine reveals widespread lactylation in the human proteome. Nat. Methods 19, 854–864 (2022).
Meng, Q. et al. Human papillomavirus-16 E6 activates the pentose phosphate pathway to promote cervical cancer cell proliferation by inhibiting G6PD lactylation. Redox. Biol. 71, 103108 (2024).
Jia, M. et al. ULK1-mediated metabolic reprogramming regulates Vps34 lipid kinase activity by its lactylation. Sci. Adv. 9, eadg4993 (2023).
Folmes, C. D. & Terzic, A. Metabolic determinants of embryonic development and stem cell fate. Reprod. Fertil. Dev. 27, 82–88 (2014).
Ryall, J. G., Cliff, T., Dalton, S. & Sartorelli, V. Metabolic reprogramming of stem cell epigenetics. Cell Stem Cell 17, 651–662 (2015).
Ito, K. & Suda, T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat. Rev. Mol. Cell Biol. 15, 243–256 (2014).
Gatie, M. I. et al. Lactate enhances mouse ES cell differentiation toward XEN cells in vitro. Stem Cells 40, 239–259 (2022).
Dong, Q. et al. Glycolysis-stimulated Esrrb lactylation promotes the self-renewal and extraembryonic endoderm stem cell differentiation of embryonic stem cells. Int. J. Mol. Sci. 25, 2692 (2024).
Panopoulos, A. D. et al. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 22, 168–177 (2012).
Folmes, C. D. L. et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 14, 264–271 (2011).
Li, L. et al. Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat. Metab. 2, 882–892 (2020).
Hu, X. L. et al. Dux activates metabolism-lactylation-MET network during early iPSC reprogramming with Brg1 as the histone lactylation reader. Nucleic Acids Res. 52, 5529–5548 (2024).
Schulz, K. N. & Harrison, M. M. Mechanisms regulating zygotic genome activation. Nat. Rev. Genet. 20, 221–234 (2019).
Li, J. et al. Lactate regulates major zygotic genome activation by H3K18 lactylation in mammals. Natl Sci. Rev. 11, nwad295 (2024).
Oginuma, M. et al. A gradient of glycolytic activity coordinates FGF and Wnt signaling during elongation of the body axis in amniote embryos. Dev. Cell 40, 342–353 (2017).
Bhattacharya, D., Azambuja, A. P. & Simoes-Costa, M. Metabolic reprogramming promotes neural crest migration via Yap/Tead signaling. Dev. Cell 53, 199–211 (2020).
Merkuri, F., Rothstein, M. & Simoes-Costa, M. Histone lactylation couples cellular metabolism with developmental gene regulatory networks. Nat. Commun. 15, 90 (2024).
Govindarajan, G. et al. The cardiometabolic syndrome as a cardiovascular risk factor. Am. J. Med. Sci. 330, 311–318 (2005).
Zhu, W. et al. Lactate and lactylation in cardiovascular diseases: current progress and future perspectives. Metabolism 158, 155957 (2024).
Murashige, D. et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 370, 364–368 (2020).
Zhang, N. et al. Alpha-myosin heavy chain lactylation maintains sarcomeric structure and function and alleviates the development of heart failure. Cell Res. 33, 679–698 (2023).
De Bock, K. et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 (2013).
Li, Y., Lui, K. O. & Zhou, B. Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat. Rev. Cardiol. 15, 445–456 (2018).
Fan, M. et al. Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction. Sci. Adv. 9, eadc9465 (2023).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Liberti, M. V. & Locasale, J. W. The Warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 41, 211–218 (2016).
Noman, M. Z. et al. Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 309, C569–C579 (2015).
Chu, Y. D. et al. Aldolase B-driven lactagenesis and CEACAM6 activation promote cell renewal and chemoresistance in colorectal cancer through the Warburg effect. Cell Death Dis. 14, 660 (2023).
Zhou, J. et al. GPR37 promotes colorectal cancer liver metastases by enhancing the glycolysis and histone lactylation via Hippo pathway. Oncogene 42, 3319–3330 (2023).
Li, W. H. et al. Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy 20, 114–130 (2024).
Sun, X. et al. The diapause-like colorectal cancer cells induced by SMC4 attenuation are characterized by low proliferation and chemotherapy insensitivity. Cell Metab. 35, 1563–1579 (2023).
Yue, Q. et al. Histone H3K9 lactylation confers temozolomide resistance in glioblastoma via LUC7L2-mediated MLH1 intron retention. Adv. Sci. 11, e2309290 (2024).
Yang, L. et al. Nucleolin lactylation contributes to intrahepatic cholangiocarcinoma pathogenesis via RNA splicing regulation of MADD. J. Hepatol. 81, 651–666 (2024).
Qiao, Z. et al. Hypoxia-induced SHMT2 protein lactylation facilitates glycolysis and stemness of esophageal cancer cells. Mol. Cell. Biochem. 479, 3063–3076 (2024).
Yu, J. et al. Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 22, 85 (2021).
Gu, X. et al. Histone lactylation-boosted ALKBH3 potentiates tumor progression and diminished promyelocytic leukemia protein nuclear condensates by m1A demethylation of SP100A. Nucleic Acids Res. 52, 2273–2289 (2024).
Pandkar, M. R., Sinha, S., Samaiya, A. & Shukla, S. Oncometabolite lactate enhances breast cancer progression by orchestrating histone lactylation-dependent c-Myc expression. Transl. Oncol. 37, 101758 (2023).
Xie, B. et al. CircXRN2 suppresses tumor progression driven by histone lactylation through activating the Hippo pathway in human bladder cancer. Mol. Cancer 22, 151 (2023).
Brand, A. et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK Cells. Cell Metab. 24, 657–671 (2016).
Watson, M. J. et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651 (2021).
Angelin, A. et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25, 1282–1293 (2017).
Gottfried, E. et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 107, 2013–2021 (2006).
Puig-Kroger, A. et al. Peritoneal dialysis solutions inhibit the differentiation and maturation of human monocyte-derived dendritic cells: effect of lactate and glucose-degradation products. J. Leukoc. Biol. 73, 482–492 (2003).
Colegio, O. R. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Zhou, C. et al. Mutant KRAS-activated circATXN7 fosters tumor immunoescape by sensitizing tumor-specific T cells to activation-induced cell death. Nat. Commun. 15, 499 (2024).
Gu, J. et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-beta signaling in regulatory T cells. Cell Rep. 40, 111122 (2022).
De Leo, A. et al. Glucose-driven histone lactylation promotes the immunosuppressive activity of monocyte-derived macrophages in glioblastoma. Immunity 57, 1105–1123 (2024).
Xiong, J. et al. Lactylation-driven METTL3-mediated RNA m(6)A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol. Cell 82, 1660–1677 (2022).
Weil, M. H. & Afifi, A. A. Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock). Circulation 41, 989–1001 (1970).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810 (2016).
Zhang, T. et al. Lactate’s impact on immune cells in sepsis: unraveling the complex interplay. Front. Immunol. 15, 1483400 (2024).
Wang, H. C. et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 (1999).
Yang, K. et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 29, 133–146 (2022).
Lelubre, C. & Vincent, J. L. Mechanisms and treatment of organ failure in sepsis. Nat. Rev. Nephrol. 14, 417–427 (2018).
An, S. et al. PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation. Cell Death Dis. 14, 457 (2023).
Wu, D. et al. Histone lactylation-regulated METTL3 promotes ferroptosis via m6A-modification on ACSL4 in sepsis-associated lung injury. Redox. Biol. 74, 103194 (2024).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Caielli, S. et al. Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE. Cell 184, 4464–4479 (2021).
Zhang, J. et al. Mitochondrial DNA programs lactylation of cGAS to induce IFN responses in patients with systemic lupus erythematosus. J. immunol. 213, 795–807 (2024).
Knochelmann, H. M. et al. When worlds collide: TH17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol. 15, 458–469 (2018).
Fan, W. et al. Global lactylome reveals lactylation-dependent mechanisms underlying TH17 differentiation in experimental autoimmune uveitis. Sci. Adv. 9, eadh4655 (2023).
Pan, R. Y. et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab. 34, 634–63 (2022).
Lin, X. et al. Augmentation of scleral glycolysis promotes myopia through histone lactylation. Cell Metab. 36, 511–525 (2024).
Wang, P. et al. H3K18 lactylation promotes the progression of arsenite-related idiopathic pulmonary fibrosis via YTHDF1/m6A/NREP. J. Hazard. Mater. 461, 132582 (2024).
Wang, Y. et al. The glycolytic enzyme PFKFB3 drives kidney fibrosis through promoting histone lactylation-mediated NF-κB family activation. Kidney Int. 106, 226–240 (2024).
Rho, H., Terry, A. R., Chronis, C. & Hay, N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab. 35, 1406–1423 (2023).
Varner, E. L. et al. Quantification of lactoyl-CoA (lactyl-CoA) by liquid chromatography mass spectrometry in mammalian cells and tissues. Open Biol. 10, 200187 (2020).
Li, H., Sun, L., Gao, P. & Hu, H. Lactylation in cancer: current understanding and challenges. Cancer Cell 42, 1803–1807 (2024).
Patel, S. S. & Walt, D. R. Substrate specificity of acetyl coenzyme A synthetase. J. Biol. Chem. 262, 7132–7134 (1987).
Watkins, P. A., Maiguel, D., Jia, Z. & Pevsner, J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J. Lipid Res. 48, 2736–2750 (2007).
McElroy, W. D., DeLuca, M. & Travis, J. Molecular uniformity in biological catalyses. The enzymes concerned with firefly luciferin, amino acid, and fatty acid utilization are compared. Science 157, 150–160 (1967).
Gulick, A. M. Conformational dynamics in the Acyl-CoA synthetases, adenylation domains of non-ribosomal peptide synthetases, and firefly luciferase. ACS Chem. Biol. 4, 811–827 (2009).
Schmelz, S. & Naismith, J. H. Adenylate-forming enzymes. Curr. Opin. Struct. Biol. 19, 666–671 (2009).
Jakubowski, H. Aminoacylation of coenzyme A and pantetheine by aminoacyl-tRNA synthetases: possible link between noncoded and coded peptide synthesis. Biochemistry 37, 5147–5153 (1998).
Dong, H. et al. YiaC and CobB regulate lysine lactylation in Escherichia coli. Nat. Commun. 13, 6628 (2022).
Zhang, X. et al. Screening, expression, purification and characterization of CoA-transferases for lactoyl-CoA generation. J. Ind. Microbiol. Biotechnol. 46, 899–909 (2019).
Li, X. et al. TRAP1 drives smooth muscle cell senescence and promotes atherosclerosis via HDAC3-primed histone H4 lysine 12 lactylation. Eur. Heart J. 45, 4219–4235 (2024).
Xu, X. et al. Sox10 escalates vascular inflammation by mediating vascular smooth muscle cell transdifferentiation and pyroptosis in neointimal hyperplasia. Cell Rep. 42, 112869 (2023).
Dong, M. et al. ASF1A-dependent P300-mediated histone H3 lysine 18 lactylation promotes atherosclerosis by regulating EndMT. Acta Pharm. Sin. B 14, 3027–3048 (2024).
Wang, Y. et al. Exercise-induced endothelial Mecp2 lactylation suppresses atherosclerosis via the Ereg/MAPK signalling pathway. Atherosclerosis 375, 45–58 (2023).
Chen, X. et al. High-intensity interval training induces lactylation of fatty acid synthase to inhibit lipid synthesis. BMC Biol. 21, 196 (2023).
Ma, W. et al. Orphan nuclear receptor NR4A3 promotes vascular calcification via histone lactylation. Circ. Res. 134, 1427–1447 (2024).
Wang, C. et al. Andrographolide regulates H3 histone lactylation by interfering with p300 to alleviate aortic valve calcification. Br. J. Pharmacol. 181, 1843–1856 (2024).
Chen, B. et al. Metabolic recoding of NSUN2-mediated m5C modification promotes the progression of colorectal cancer via the NSUN2/YBX1/m5C-ENO1 positive feedback loop. Adv. Sci.11, e2309840 (2024).
Wang, J. W. et al. Enterobacterial LPS-inducible LINC00152 is regulated by histone lactylation and promotes cancer cells invasion and migration. Front. Cell. Infect. Microbiol. 12, 913815 (2022).
Miao, Z., Zhao, X. & Liu, X. Hypoxia induced beta-catenin lactylation promotes the cell proliferation and stemness of colorectal cancer through the wnt signaling pathway. Exp. Cell. Res. 422, 113439 (2023).
Liao, J. Y. et al. CENPA functions as a transcriptional regulator to promote hepatocellular carcinoma progression via cooperating with YY1. Int. J. Biol. Sci. 19, 5218–5232 (2023).
Li, F. et al. Single-cell transcriptome analysis reveals the association between histone lactylation and cisplatin resistance in bladder cancer. Drug Resist. Updat. 73, 101059 (2024).
Meng, Q. F. et al. Lactylation stabilizes DCBLD1 activating the pentose phosphate pathway to promote cervical cancer progression. J. Exp. Clin. Cancer Res. 43, 36 (2024).
Qiao, J. et al. Histone H3K18 and Ezrin lactylation promote renal dysfunction in sepsis-associated acute kidney injury. Adv. Sci. 11, e2307216 (2024).
Huang, J. et al. YY1 lactylation aggravates autoimmune uveitis by enhancing microglial functions via inflammatory genes. Adv. Sci. 11, e2308031 (2024).
Wei, L. et al. H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer’s disease through the NFκB signaling pathway. J. Neuroinflammation 20, 208 (2023).
Hay, N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat. Rev. Cancer 16, 635–649 (2016).
Martinez-Outschoorn, U. E. et al. Cancer metabolism: a therapeutic perspective. Nat. Rev. Clin. Oncol. 14, 113 (2017).
Stine, Z. E., Schug, Z. T., Salvino, J. M. & Dang, C. V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 21, 141–162 (2022).
Lasko, L. M. et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage specific tumours. Nature 550, 128–132 (2017).
Topper, M. J. et al. The emerging role of epigenetic therapeutics in immuno-oncology. Nat. Rev. Clin. Oncol. 17, 75–90 (2020).
Caruso, J. et al. Ergogenic effects of β-alanine and carnosine: proposed future research to quantify their efficacy. Nutrients 4, 585–601 (2012).
Zhu, F. Y. et al. Inhibiting bridge integrator 2 phosphorylation leads to improved oocyte quality, ovarian health and fertility in aging and after chemotherapy in mice. Nat. Aging 1, 1010–1023 (2021).
Yao, H. et al. Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nat. Biomed. Eng. 3, 306–317 (2019).
Perez-Salvia, M. & Esteller, M. Bromodomain inhibitors and cancer therapy: from structures to applications. Epigenetics 12, 323–339 (2017).
Li, X., Liu, S., Li, X. & Li, X. D. YEATS domains as novel epigenetic readers: structures, functions, and inhibitor development. ACS Chem. Biol. 18, 994–1013 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32270822), the Peking-Tsinghua Center for Life Science, the State Key Laboratory of Gene Function and Modulation Research, the School of Life Sciences at Peking University, the Qidong-SLS Innovation Fund and the Clinical Medicine Plus X-Young Scholars Project at Peking University (PKU2024LCXQ025), the Fundamental Research Funds for the Central Universities to D.Z. We were grateful to the members of the Zhang lab for their assistance in proofreading the manuscript. We sincerely apologize to researchers whose important contributions could not be cited owing to space limitations. All figures were created using BioRender.com.
Author information
Authors and Affiliations
Contributions
H.R. constructed the figures. D.Z. conceived the manuscript and D.Z., H.R. and Y.T. jointly wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Long Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, H., Tang, Y. & Zhang, D. The emerging role of protein l-lactylation in metabolic regulation and cell signalling. Nat Metab 7, 647–664 (2025). https://doi.org/10.1038/s42255-025-01259-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01259-0
This article is cited by
-
Lactylation in cancer: molecular mechanisms and advances in clinical study
Molecular Cancer (2026)
-
Beyond metabolism: exploring the regulatory and therapeutic implications of lactate and lactylation in cancer-regulated cell death
Cell Death & Disease (2026)
-
HSPA8 lactylation attenuates neuronal pyroptosis via E3 ligase-mediated NLRP3 degradation after ischemic stroke
Apoptosis (2026)
-
Nutrients and Metabolites as Signalling Molecules in Osteoclasts
Current Osteoporosis Reports (2026)
-
Glucose metabolism and its direct action in cancer and immune regulation: opportunities and challenges for metabolic targeting
Journal of Biomedical Science (2025)