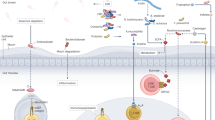
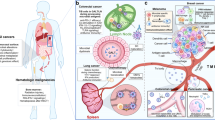
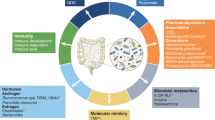
Abstract
The gut microbiome has a crucial role in cancer development and therapy through its interactions with the immune system and tumour microenvironment. Although evidence links gut microbiota composition to cancer progression, its precise role in modulating treatment responses remains unclear. In this Review, we summarize current knowledge on the gut microbiome’s involvement in cancer, covering its role in tumour initiation and progression, interactions with chemotherapy, radiotherapy and targeted therapies, and its influence on cancer immunotherapy. We discuss the impact of microbial metabolites on immune responses, the relationship between specific bacterial species and treatment outcomes, and potential microbiota-based therapeutic strategies, including dietary interventions, probiotics and faecal microbiota transplantation. Understanding these complex microbiota–immune interactions is critical for optimizing cancer therapies. Future research should focus on defining microbial signatures associated with treatment success and developing targeted microbiome modulation strategies to enhance patient outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sender, R., Fuchs, S. & Milo, R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164, 337–340 (2016).
Cani, P. D. & Van Hul, M. Gut microbiota in overweight and obesity: crosstalk with adipose tissue. Nat. Rev. Gastroenterol. Hepatol. 21, 164–183 (2023).
de Vos, W. M., Tilg, H., Van Hul, M. & Cani, P. D. Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032 (2022).
Wiertsema, S. P., van Bergenhenegouwen, J., Garssen, J. & Knippels, L. M. J. The interplay between the gut microbiome and the immune system in the context of infectious diseases throughout life and the role of nutrition in optimizing treatment strategies. Nutrients 13, 886 (2021).
Koren, O., Konnikova, L., Brodin, P., Mysorekar, I. U. & Collado, M. C. The maternal gut microbiome in pregnancy: implications for the developing immune system. Nat. Rev. Gastroenterol. Hepatol. 21, 35–45 (2024).
Rinninella, E. et al. The role of diet in shaping human gut microbiota. Best. Pract. Res. Clin. Gastroenterol. 62–63, 101828 (2023).
Van Hul, M. et al. What defines a healthy gut microbiome? Gut 73, 1893–1908 (2024).
Fidelle, M. et al. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science 380, eabo2296 (2023).
Yang, Q. et al. A review of gut microbiota-derived metabolites in tumor progression and cancer therapy. Adv. Sci. 10, e2207366 (2023).
Belkaid, Y. & Harrison, O. J. Homeostatic immunity and the microbiota. Immunity 46, 562–576 (2017).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Ugai, T. et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 19, 656–673 (2022).
Steck, S. E. & Murphy, E. A. Dietary patterns and cancer risk. Nat. Rev. Cancer 20, 125–138 (2020).
Tsvetikova, S. A. & Koshel, E. I. Microbiota and cancer: host cellular mechanisms activated by gut microbial metabolites. Int. J. Med. Microbiol. 310, 151425 (2020).
Mirzaei, R. et al. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 139, 111619 (2021).
Wu, Y. et al. A systematic review of the gut microbiome, metabolites, and multi-omics biomarkers across the colorectal cancer care continuum. Benef. Microbes 15, 539–563 (2024).
Herlo, L. F. et al. Gut microbiota signatures in colorectal cancer as a potential diagnostic biomarker in the future: a systematic review. Int. J. Mol. Sci. 25, 7937 (2024).
Wong, S. H. et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology 153, 1621–1633 (2017).
Baas, F. S., Brusselaers, N., Nagtegaal, I. D., Engstrand, L. & Boleij, A. Navigating beyond associations: opportunities to establish causal relationships between the gut microbiome and colorectal carcinogenesis. Cell Host Microbe 32, 1235–1247 (2024).
Zepeda-Rivera, M. et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 628, 424–432 (2024).
Kostic, A. D. et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 22, 292–298 (2012).
Sears, C. L. & Pardoll, D. M. Perspective: alpha-bugs, their microbial partners, and the link to colon cancer. J. Infect. Dis. 203, 306–311 (2011).
Tjalsma, H., Boleij, A., Marchesi, J. R. & Dutilh, B. E. A bacterial driver–passenger model for colorectal cancer: beyond the usual suspects. Nat. Rev. Microbiol. 10, 575–582 (2012).
Yu, T. et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548–563 (2017).
Rubinstein, M. R. et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206 (2013).
Bullman, S. et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448 (2017).
Mima, K. et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65, 1973–1980 (2016).
Chen, B. et al. Contribution of pks+ E. coli mutations to colorectal carcinogenesis. Nat. Commun. 14, 7827 (2023).
Rosendahl Huber, A. et al. Improved detection of colibactin-induced mutations by genotoxic E. coli in organoids and colorectal cancer. Cancer Cell 42, 487–496 (2024).
Cornish, A. J. et al. The genomic landscape of 2,023 colorectal cancers. Nature 633, 127–136 (2024).
Pleguezuelos-Manzano, C. et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature 580, 269–273 (2020).
Liu, Y. et al. Peptostreptococcus anaerobius mediates anti-PD1 therapy resistance and exacerbates colorectal cancer via myeloid-derived suppressor cells in mice. Nat. Microbiol 9, 1467–1482 (2024).
Zhang, R., Kang, R. & Tang, D. Gut microbiome mediates ferroptosis resistance for colorectal cancer development. Cancer Res. 84, 796–797 (2024).
Huang, P. et al. Peptostreptococcus stomatis promotes colonic tumorigenesis and receptor tyrosine kinase inhibitor resistance by activating ERBB2-MAPK. Cell Host Microbe 32, 1365–1379 (2024).
Kang, X. et al. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8+ T cells. Gut 72, 2112–2122 (2023).
Chen, D. et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 469, 456–467 (2020).
Pu, W. et al. Inhibitory effects of Clostridium butyricum culture and supernatant on inflammatory colorectal cancer in mice. Front. Immunol. 14, 1004756 (2023).
Fan, S., Zhou, L., Zhang, W., Wang, D. & Tang, D. Role of imbalanced gut microbiota in promoting CRC metastasis: from theory to clinical application. Cell Commun. Signal 22, 232 (2024).
Ayabe, R. I. & White, M. G. Metastasis and the microbiome: the impact of bacteria in disseminated colorectal cancer. Front. Biosci. 29, 152 (2024).
Li, R. et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 26, 2447–2463 (2019).
Coker, O. O. et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 67, 1024–1032 (2018).
Gantuya, B. et al. Gastric mucosal microbiota in a Mongolian population with gastric cancer and precursor conditions. Aliment Pharm. Ther. 51, 770–780 (2020).
Castano-Rodriguez, N., Goh, K. L., Fock, K. M., Mitchell, H. M. & Kaakoush, N. O. Dysbiosis of the microbiome in gastric carcinogenesis. Sci. Rep. 7, 15957 (2017).
Chen, Y., Segers, S. & Blaser, M. J. Association between Helicobacter pylori and mortality in the NHANES III study. Gut 62, 1262–1269 (2013).
Zeng, R., Gou, H., Lau, H. C. H. & Yu, J. Stomach microbiota in gastric cancer development and clinical implications. Gut 73, 2062–2073 (2024).
Kumar, S., Metz, D. C., Ellenberg, S., Kaplan, D. E. & Goldberg, D. S. Risk factors and incidence of gastric cancer after detection of Helicobacter pylori infection: a large cohort study. Gastroenterology 158, 527–536 (2020).
Hayashi, Y. et al. CagA mediates epigenetic regulation to attenuate let-7 expression in Helicobacter pylori-related carcinogenesis. Gut 62, 1536–1546 (2013).
Sun, X. et al. TLR2 mediates Helicobacter pylori-induced tolerogenic immune response in mice. PLoS ONE 8, e74595 (2013).
Cheng, A. S. et al. Helicobacter pylori causes epigenetic dysregulation of FOXD3 to promote gastric carcinogenesis. Gastroenterology 144, 122–133 (2013).
Chauhan, N., Tay, A. C. Y., Marshall, B. J. & Jain, U. Helicobacter pylori VacA, a distinct toxin exerts diverse functionalities in numerous cells: an overview. Helicobacter 24, e12544 (2019).
Yang, I. et al. Different gastric microbiota compositions in two human populations with high and low gastric cancer risk in Colombia. Sci. Rep. 6, 18594 (2016).
Wu, J. et al. Fecal microbiome alteration may be a potential marker for gastric cancer. Dis. Markers 2020, 3461315 (2020).
Yu, C. et al. Dysbiosis of gut microbiota is associated with gastric carcinogenesis in rats. Biomed. Pharmacother. 126, 110036 (2020).
Li, Q. et al. Propionibacterium acnes overabundance in gastric cancer promote M2 polarization of macrophages via a TLR4/PI3K/Akt signaling. Gastric Cancer 24, 1242–1253 (2021).
Xin, Y. et al. Fusobacterium nucleatum-induced exosomal HOTTIP promotes gastric cancer progression through the microRNA-885-3p/EphB2 axis. Cancer Sci. 114, 2360–2374 (2023).
Fu, K. et al. Streptococcus anginosus promotes gastric inflammation, atrophy, and tumorigenesis in mice. Cell 187, 882–896 (2024).
Sung, J. J. Y. et al. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut 69, 1572–1580 (2020).
Yang, Y. et al. Prospective study of oral microbiome and gastric cancer risk among Asian, African American and European American populations. Int. J. Cancer 150, 916–927 (2022).
Hu, J., Han, S., Chen, Y. & Ji, Z. Variations of tongue coating microbiota in patients with gastric cancer. BioMed. Res. Int. 2015, 173729 (2015).
Cani, P. D. & Jordan, B. F. Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 15, 671–682 (2018).
Ponziani, F. R. et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in non-alcoholic fatty liver disease. Hepatology 69, 107–120 (2018).
Ren, Z. et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 68, 1014–1023 (2019).
Grat, M. et al. Profile of gut microbiota associated with the presence of hepatocellular cancer in patients with liver cirrhosis. Transplant. Proc. 48, 1687–1691 (2016).
Xie, G. et al. Distinctly altered gut microbiota in the progression of liver disease. Oncotarget 7, 19355–19366 (2016).
Jinato, T., Anuntakarun, S., Satthawiwat, N., Chuaypen, N. & Tangkijvanich, P. Distinct alterations of gut microbiota between viral- and non-viral-related hepatocellular carcinoma. Appl. Microbiol. Biotechnol. 108, 34 (2024).
Schwabe, R. F. & Greten, T. F. Gut microbiome in HCC - mechanisms, diagnosis and therapy. J. Hepatol. 72, 230–238 (2020).
Ma, H. et al. B. thetaiotaomicron-derived acetic acid modulate immune microenvironment and tumor growth in hepatocellular carcinoma. Gut Microbes 16, 2297846 (2024).
Liu, Q. et al. Alteration in gut microbiota associated with hepatitis B and non-hepatitis virus related hepatocellular carcinoma. Gut Pathog. 11, 1 (2019).
Wu, L. et al. The gut microbiome-bile acid axis in hepatocarcinogenesis. Biomed. Pharmacother. 133, 111036 (2021).
Van Hul, M. et al. Role of the intestinal microbiota in contributing to weight disorders and associated comorbidities. Clin. Microbiol. Rev. 37, e0004523 (2024).
Mishra, Y. et al. The role of the gut microbiome in gastrointestinal cancers. Cell. Signal. 115, 111013 (2024).
Ren, Z. et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 8, 95176–95191 (2017).
Mendez, R. et al. Microbial dysbiosis and polyamine metabolism as predictive markers for early detection of pancreatic cancer. Carcinogenesis 41, 561–570 (2020).
Half, E. et al. Fecal microbiome signatures of pancreatic cancer patients. Sci. Rep. 9, 16801 (2019).
Del Castillo, E. et al. The microbiomes of pancreatic and duodenum tissue overlap and are highly subject specific but differ between pancreatic cancer and noncancer subjects. Cancer Epidemiol. Biomark. Prev. 28, 370–383 (2019).
Pushalkar, S. et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 8, 403–416 (2018).
Paternoster, S. & Falasca, M. The intricate relationship between diabetes, obesity and pancreatic cancer. Biochim. Biophys. Acta Rev. Cancer 1873, 188326 (2020).
Mirji, G. et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci. Immunol. 7, eabn0704 (2022).
Genovese, G. et al. Synthetic vulnerabilities of mesenchymal subpopulations in pancreatic cancer. Nature 542, 362–366 (2017).
Plottel, C. S. & Blaser, M. J. Microbiome and malignancy. Cell Host Microbe 10, 324–335 (2011).
Fuhrman, B. J. et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J. Clin. Endocrinol. Metab. 99, 4632–4640 (2014).
Ervin, S. M. et al. Gut microbial beta-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 294, 18586–18599 (2019).
Martin, F., Peltonen, J., Laatikainen, T., Tikkanen, M. & Pulkkinen, M. Excretion of unconjugated and conjugated progesterone metabolites in pregnancy urine during ampicillin administration. Clin. Chim. Acta. 55, 71–80 (1974).
Doisneau-Sixou, S. F. et al. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr. Relat. Cancer 10, 179–186 (2003).
Luu, T. H. et al. Intestinal proportion of Blautia sp. is associated with clinical stage and histoprognostic grade in patients with early-stage breast cancer. Nutr. Cancer 69, 267–275 (2017).
Crispo, A. et al. Central obesity, body mass index, metabolic syndrome and mortality in Mediterranean breast cancer patients. Sci. Rep. 13, 21208 (2023).
Siiteri, P. K. Adipose tissue as a source of hormones. Am. J. Clin. Nutr. 45, 277–282 (1987).
Zhu, J. et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome 6, 136 (2018).
Goedert, J. J. et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J. Natl Cancer Inst. 107, djv147 (2015).
Bobin-Dubigeon, C. et al. Faecal microbiota composition varies between patients with breast cancer and healthy women: a comparative case-control study. Nutrients 13, 2705 (2021).
Aarnoutse, R. et al. Intestinal microbiota in postmenopausal breast cancer patients and controls. Cancers 13, 6200 (2021).
Zeber-Lubecka, N. et al. Breast cancer but not the menopausal status is associated with small changes of the gut microbiota. Front. Oncol. 14, 1279132 (2024).
Su, J. et al. Prevotella copri exhausts intrinsic indole-3-pyruvic acid in the host to promote breast cancer progression: inactivation of AMPK via UHRF1-mediated negative regulation. Gut Microbes 16, 2347757 (2024).
Ma, J. et al. Alter between gut bacteria and blood metabolites and the anti-tumor effects of Faecalibacterium prausnitzii in breast cancer. BMC Microbiol. 20, 82 (2020).
Wenhui, Y. et al. Variations in the gut microbiota in breast cancer occurrence and bone metastasis. Front. Microbiol. 13, 894283 (2022).
Parida, S. et al. A procarcinogenic colon microbe promotes breast tumorigenesis and metastatic progression and concomitantly activates notch and beta-catenin axes. Cancer Discov. 11, 1138–1157 (2021).
Parida, S. et al. Gut colonization with an obesity-associated enteropathogenic microbe modulates the premetastatic niches to promote breast cancer lung and liver metastasis. Front. Immunol. 14, 1194931 (2023).
Buchta Rosean, C. et al. Preexisting commensal dysbiosis is a host-intrinsic regulator of tissue inflammation and tumor cell dissemination in hormone receptor-positive breast cancer. Cancer Res. 79, 3662–3675 (2019).
Shin, J. H. et al. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res. Microbiol. 170, 192–201 (2019).
Bui, N. N. et al. Clostridium scindens metabolites trigger prostate cancer progression through androgen receptor signaling. J. Microbiol. Immunol. Infect. 56, 246–256 (2023).
Pernigoni, N. et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science 374, 216–224 (2021).
Geller, L. T. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
Zhang, S. et al. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 38, 14 (2019).
Li, B. et al. Fusobacterium nucleatum induces oxaliplatin resistance by inhibiting ferroptosis through E-cadherin/beta-catenin/GPX4 axis in colorectal cancer. Free Radic. Biol. Med. 220, 125–138 (2024).
Tintelnot, J. et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 615, 168–174 (2023).
He, Y. et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 33, 988–1000 (2021).
Daillere, R. et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 45, 931–943 (2016).
Li, Y. et al. Metagenomic analyses reveal distinct gut microbiota signature for predicting the neoadjuvant chemotherapy responsiveness in breast cancer patients. Front. Oncol. 12, 865121 (2022).
Bhatt, A. P. et al. Targeted inhibition of gut bacterial beta-glucuronidase activity enhances anticancer drug efficacy. Proc. Natl Acad. Sci. USA 117, 7374–7381 (2020).
Terrisse, S. et al. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 28, 2778–2796 (2021).
Nguyen, S. M. et al. Gut microbiome in association with chemotherapy-induced toxicities among patients with breast cancer. Cancer 130, 2014–2030 (2024).
Zhang, X. et al. Antibiotics modulate neoadjuvant therapy efficiency in patients with breast cancer: a pilot analysis. Sci. Rep. 11, 14024 (2021).
Chambers, L. M. et al. Disruption of the gut microbiota confers cisplatin resistance in epithelial ovarian cancer. Cancer Res. 82, 4654–4669 (2022).
Maier, L. et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628 (2018).
Benej, M. et al. The tumor microbiome reacts to hypoxia and can influence response to radiation treatment in colorectal cancer. Cancer Res. Commun. 4, 1690–1701 (2024).
Kim, Y. S., Kim, J. & Park, S. J. High-throughput 16S rRNA gene sequencing reveals alterations of mouse intestinal microbiota after radiotherapy. Anaerobe 33, 1–7 (2015).
Jameus, A. et al. Acute impacts of ionizing radiation exposure on the gastrointestinal tract and gut microbiome in mice. Int. J. Mol. Sci. 25, 3339 (2024).
Cui, M. et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol. Med. 9, 448–461 (2017).
Nam, Y. D., Kim, H. J., Seo, J. G., Kang, S. W. & Bae, J. W. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS ONE 8, e82659 (2013).
Reis Ferreira, M. et al. Microbiota- and Radiotherapy-induced Gastrointestinal Side-effects (MARS) study: a large pilot study of the microbiome in acute and late-radiation enteropathy. Clin. Cancer Res. 25, 6487–6500 (2019).
Iacovacci, J. et al. Intestinal microbiota composition is predictive of radiotherapy-induced acute gastrointestinal toxicity in prostate cancer patients. eBioMedicine 106, 105246 (2024).
Meivelu, M. Letter to the editor, “Gut microbiome predicts gastrointestinal toxicity outcomes from chemoradiation therapy in patients with head and neck squamous cell carcinoma”. Oral. Oncol. 155, 106903 (2024).
Acharya, M., Venkidesh, B. S. & Mumbrekar, K. D. Bacterial supplementation in mitigation of radiation-induced gastrointestinal damage. Life Sci. 353, 122921 (2024).
Lu, L. et al. Microbiome in radiotherapy: an emerging approach to enhance treatment efficacy and reduce tissue injury. Mol. Med 30, 105 (2024).
Wang, L., Li, Y., Zhang, Y. J. & Peng, L. H. Intestinal microecological transplantation for a patient with chronic radiation enteritis: a case report. World J. Gastroenterol. 30, 2603–2611 (2024).
Ma, C. Y., Zhao, J. & Zhou, J. Y. Microbiome profiling and co-metabolism pathway analysis in cervical cancer patients with acute radiation enteritis. Heliyon 10, e29598 (2024).
Liu, Y. et al. The effects of preoperative intestinal dysbacteriosis on postoperative recovery in colorectal cancer surgery: a prospective cohort study. BMC Gastroenterol. 21, 446 (2021).
van Praagh, J. B. et al. Mucus microbiome of anastomotic tissue during surgery has predictive value for colorectal anastomotic leakage. Ann. Surg. 269, 911–916 (2019).
Hajjar, R. et al. Gut microbiota influence anastomotic healing in colorectal cancer surgery through modulation of mucosal proinflammatory cytokines. Gut 72, 1143–1154 (2023).
Hajjar, R. et al. Basal levels of microbiota-driven subclinical inflammation are associated with anastomotic leak in patients with colorectal cancer. Gut 73, 1031–1033 (2024).
Tong, J. et al. Changes of intestinal microbiota in ovarian cancer patients treated with surgery and chemotherapy. Cancer Manag. Res. 12, 8125–8135 (2020).
Fan, P., Ding, L., Du, G. & Wei, C. Effect of mastectomy on gut microbiota and its metabolites in patients with breast cancer. Front. Microbiol. 15, 1269558 (2024).
Morris, M. S., Graham, L. A., Chu, D. I., Cannon, J. A. & Hawn, M. T. Oral antibiotic bowel preparation significantly reduces surgical site infection rates and readmission rates in elective colorectal surgery. Ann. Surg. 261, 1034–1040 (2015).
Bachmann, R., Leonard, D., Delzenne, N., Kartheuser, A. & Cani, P. D. Novel insight into the role of microbiota in colorectal surgery. Gut 66, 738–749 (2017).
Alam, A. et al. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat. Microbiol 1, 15021 (2016).
Bachmann, R. et al. Akkermansia muciniphila reduces peritonitis and improves intestinal tissue wound healing after a colonic transmural defect by a MyD88-dependent mechanism. Cells 11, 2666 (2022).
Masaud, K. et al. The gut microbiota in persistent post-operative pain following breast cancer surgery. Sci. Rep. 14, 12401 (2024).
Chen, Y. C. et al. Gut microbiota composition in chemotherapy and targeted therapy of patients with metastatic colorectal cancer. Front. Oncol. 12, 955313 (2022).
Martini, G. et al. Gut microbiota correlates with antitumor activity in patients with mCRC and NSCLC treated with cetuximab plus avelumab. Int. J. Cancer 151, 473–480 (2022).
Saifon, W. et al. Gastrointestinal microbiota profile and clinical correlations in advanced EGFR-WT and EGFR-mutant non-small cell lung cancer. BMC Cancer 22, 963 (2022).
Schettini, F. et al. Faecal microbiota composition is related to response to CDK4/6-inhibitors in metastatic breast cancer: a prospective cross-sectional exploratory study. Eur. J. Cancer 191, 112948 (2023).
Di Modica, M. et al. Gut microbiota condition the therapeutic efficacy of trastuzumab in HER2-positive breast cancer. Cancer Res. 81, 2195–2206 (2021).
Wong, C. W. et al. Analysis of gut microbiome using explainable machine learning predicts risk of diarrhea associated with tyrosine kinase inhibitor neratinib: a pilot study. Front. Oncol. 11, 604584 (2021).
Guardamagna, M. et al. Gut microbiota and therapy in metastatic melanoma: focus on MAPK pathway inhibition. Int. J. Mol. Sci. 23, 11990 (2022).
Sepich-Poore, G. D. et al. The microbiome and human cancer. Science 371, eabc4552 (2021).
Shiravand, Y. et al. Immune checkpoint inhibitors in cancer therapy. Curr. Oncol. 29, 3044–3060 (2022).
Regnier, M., Van Hul, M., Knauf, C. & Cani, P. D. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J. Endocrinol. 248, R67–R82 (2021).
Muschaweck, M. et al. Cognate recognition of microbial antigens defines constricted CD4+ T cell receptor repertoires in the inflamed colon. Immunity 54, 2565–2577 (2021).
Sari, Z. et al. Indolepropionic acid, a metabolite of the microbiome, has cytostatic properties in breast cancer by activating AHR and PXR receptors and inducing oxidative stress. Cancers 12, 2411 (2020).
Luu, T. H. et al. Lithocholic bile acid inhibits lipogenesis and induces apoptosis in breast cancer cells. Cell Oncol. 41, 13–24 (2018).
Miller, K. D. et al. Acetate acts as a metabolic immunomodulator by bolstering T-cell effector function and potentiating antitumor immunity in breast cancer. Nat. Cancer 4, 1491–1507 (2023).
Blaak, E. E. et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 11, 411–455 (2020).
Son, M. Y. & Cho, H. S. Anticancer effects of gut microbiota-derived short-chain fatty acids in cancers. J. Microbiol. Biotechnol. 33, 849–856 (2023).
Yusuf, F., Adewiah, S., Syam, A. F. & Fatchiyah, F. Altered profile of gut microbiota and the level short chain fatty acids in colorectal cancer patients. J. Phys. Conf. Ser. 1146, 012037 (2019).
He, C., Liu, Y., Ye, S., Yin, S. & Gu, J. Changes of intestinal microflora of breast cancer in premenopausal women. Eur. J. Clin. Microbiol Infect. Dis. 40, 503–513 (2021).
He, C. et al. Gut microbial composition changes in bladder cancer patients: a case-control study in Harbin, China. Asia Pac. J. Clin. Nutr. 29, 395–403 (2020).
Hersi, F. et al. Cancer immunotherapy resistance: the impact of microbiome-derived short-chain fatty acids and other emerging metabolites. Life Sci. 300, 120573 (2022).
Carretta, M. D., Quiroga, J., Lopez, R., Hidalgo, M. A. & Burgos, R. A. Participation of short-chain fatty acids and their receptors in gut inflammation and colon cancer. Front. Physiol. 12, 662739 (2021).
Tang, Y., Chen, Y., Jiang, H., Robbins, G. T. & Nie, D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int. J. Cancer 128, 847–856 (2011).
Thangaraju, M. et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 69, 2826–2832 (2009).
Li, Y. & Seto, E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb. Perspect. Med. 6, 1026831 (2016).
Gately, S. Human microbiota and personalized cancer treatments: role of commensal microbes in treatment outcomes for cancer patients. Cancer Treat. Res 178, 253–264 (2019).
Kobayashi, M. et al. A short-chain fatty acid, propionate, enhances the cytotoxic effect of cisplatin by modulating GPR41 signaling pathways in HepG2 cells. Oncotarget 9, 31342–31354 (2018).
Nomura, M. et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw. Open 3, e202895 (2020).
Mann, E. R., Lam, Y. K. & Uhlig, H. H. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat. Rev. Immunol. 24, 577–595 (2024).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Dudakov, J. A., Hanash, A. M. & van den Brink, M. R. Interleukin-22: immunobiology and pathology. Annu. Rev. Immunol. 33, 747–785 (2015).
Qing, C. & Ghorani, E. Two faces: IL-22 effects prevail over defense against metastasis. Immunity 56, 6–8 (2023).
Donohoe, D. R. et al. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 48, 612–626 (2012).
Hu, S. et al. The microbe-derived short chain fatty acid butyrate targets miRNA-dependent p21 gene expression in human colon cancer. PLoS ONE 6, e16221 (2011).
Comerford, S. A. et al. Acetate dependence of tumors. Cell 159, 1591–1602 (2014).
Song, Q. et al. Bifidobacterium pseudolongum-generated acetate suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma. J. Hepatol. 79, 1352–1365 (2023).
Hu, C. et al. Gut microbiota-derived short-chain fatty acids regulate group 3 innate lymphoid cells in HCC. Hepatology 77, 48–64 (2023).
Wang, J. et al. Acetate reprogrammes tumour metabolism and promotes PD-L1 expression and immune evasion by upregulating c-Myc. Nat. Metab. 6, 914–932 (2024).
Le Floc’h, N., Otten, W. & Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 41, 1195–1205 (2011).
Agus, A., Planchais, J. & Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724 (2018).
Gupta, S. K. et al. Microbiota-derived tryptophan metabolism: impacts on health, aging, and disease. Exp. Gerontol. 183, 112319 (2023).
Roager, H. M. & Licht, T. R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 9, 3294 (2018).
Bender, M. J. et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 186, 1846–1862 (2023).
Jia, D. et al. Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer. Cell 187, 1651–1665 (2024).
Fong, W. et al. Lactobacillus gallinarum-derived metabolites boost anti-PD1 efficacy in colorectal cancer by inhibiting regulatory T cells through modulating IDO1/Kyn/AHR axis. Gut 72, 2272–2285 (2023).
Ohta, A. A metabolic immune checkpoint: adenosine in tumor microenvironment. Front. Immunol. 7, 109 (2016).
Xia, C., Yin, S., To, K. K. W. & Fu, L. CD39/CD73/A2AR pathway and cancer immunotherapy. Mol. Cancer 22, 44 (2023).
Chen, S. et al. The expression of adenosine A2B receptor on antigen-presenting cells suppresses CD8+ T-cell responses and promotes tumor growth. Cancer Immunol. Res. 8, 1064–1074 (2020).
Leone, R. D. et al. Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models. Cancer Immunol. Immunother. 67, 1271–1284 (2018).
Kroemer, G. & Zitvogel, L. Inosine: novel microbiota-derived immunostimulatory metabolite. Cell Res 30, 942–943 (2020).
Brown, E. M. et al. Gut microbiome ADP-ribosyltransferases are widespread phage-encoded fitness factors. Cell Host Microbe 29, 1351–1365 (2021).
Kim, I. S. & Jo, E. K. Inosine: a bioactive metabolite with multimodal actions in human diseases. Front. Pharm. 13, 1043970 (2022).
Welihinda, A. A., Kaur, M., Raveendran, K. S. & Amento, E. P. Enhancement of inosine-mediated A(2A)R signaling through positive allosteric modulation. Cell. Signal. 42, 227–235 (2018).
Welihinda, A. A., Kaur, M., Greene, K., Zhai, Y. & Amento, E. P. The adenosine metabolite inosine is a functional agonist of the adenosine A2A receptor with a unique signaling bias. Cell. Signal. 28, 552–560 (2016).
Wang, T. et al. Inosine is an alternative carbon source for CD8+-T-cell function under glucose restriction. Nat. Metab. 2, 635–647 (2020).
Mager, L. F. et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369, 1481–1489 (2020).
Lefort, C. & Cani, P. D. The liver under the spotlight: bile acids and oxysterols as pivotal actors controlling metabolism. Cells 10, 400 (2021).
Kriaa, A. et al. Bile acids: key players in inflammatory bowel diseases? Cells 11, 901 (2022).
Malhi, H. & Camilleri, M. Modulating bile acid pathways and TGR5 receptors for treating liver and GI diseases. Curr. Opin. Pharmacol. 37, 80–86 (2017).
Guo, C., Chen, W. D. & Wang, Y. D. TGR5, not only a metabolic regulator. Front Physiol. 7, 646 (2016).
Okajima, F. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell. Signal. 25, 2263–2271 (2013).
Yoneno, K. et al. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn’s disease. Immunology 139, 19–29 (2013).
Ye, D., He, J. & He, X. The role of bile acid receptor TGR5 in regulating inflammatory signalling. Scand. J. Immunol. 99, e13361 (2024).
Fiorucci, S., Biagioli, M., Zampella, A. & Distrutti, E. Bile acids activated receptors regulate innate immunity. Front. Immunol. 9, 1853 (2018).
Goldberg, A. A., Titorenko, V. I., Beach, A. & Sanderson, J. T. Bile acids induce apoptosis selectively in androgen-dependent and -independent prostate cancer cells. PeerJ 1, e122 (2013).
Miko, E. et al. Lithocholic acid, a bacterial metabolite reduces breast cancer cell proliferation and aggressiveness. Biochim. Biophys. Acta, Bioenerg. 1859, 958–974 (2018).
Serfaty, L., Bissonnette, M. & Poupon, R. Ursodeoxycholic acid and chemoprevention of colorectal cancer. Gastroenterol. Clin. Biol. 34, 516–522 (2010).
Hess, L. M. et al. Results of a phase I multiple-dose clinical study of ursodeoxycholic acid. Cancer Epidemiol. Biomark. Prev. 13, 861–867 (2004).
Bezzio, C., Festa, S., Saibeni, S. & Papi, C. Chemoprevention of colorectal cancer in ulcerative colitis: digging deep in current evidence. Expert Rev. Gastroenterol. Hepatol. 11, 339–347 (2017).
Lee, J. et al. Ursodeoxycholic acid shows antineoplastic effects in bile duct cancer cells via apoptosis induction; p53 activation; and EGFR-ERK, COX-2, and PI3K-AKT pathway inhibition. Mol. Biol. Rep. 48, 6231–6240 (2021).
Song, P., Peng, Z. & Guo, X. Gut microbial metabolites in cancer therapy. Trends Endocrinol. Metab. 36, 55–69 (2024).
Cleusix, V., Lacroix, C., Vollenweider, S., Duboux, M. & Le Blay, G. Inhibitory activity spectrum of reuterin produced by Lactobacillus reuteri against intestinal bacteria. BMC Microbiol. 7, 101 (2007).
Cervantes-Barragan, L. et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science 357, 806–810 (2017).
Wang, T. et al. Probiotics Lactobacillus reuteri abrogates immune checkpoint blockade-associated colitis by inhibiting group 3 innate lymphoid cells. Front. Immunol. 10, 1235 (2019).
He, J., Yin, W., Galperin, M. Y. & Chou, S. H. Cyclic di-AMP, a second messenger of primary importance: tertiary structures and binding mechanisms. Nucleic Acids Res. 48, 2807–2829 (2020).
Danilchanka, O. & Mekalanos, J. J. Cyclic dinucleotides and the innate immune response. Cell 154, 962–970 (2013).
Waters, C. M. Au naturale: use of biologically derived cyclic di-nucleotides for cancer immunotherapy. Open Biol. 11, 210277 (2021).
Brown, J. M. & Hazen, S. L. Microbial modulation of cardiovascular disease. Nat. Rev. Microbiol. 16, 171–181 (2018).
Wang, H. et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 34, 581–594 (2022).
Zarour, H. M. Microbiome-derived metabolites counteract tumor-induced immunosuppression and boost immune checkpoint blockade. Cell Metab. 34, 1903–1905 (2022).
Holbert, C. E., Cullen, M. T., Casero, R. A. Jr. & Stewart, T. M. Polyamines in cancer: integrating organismal metabolism and antitumour immunity. Nat. Rev. Cancer 22, 467–480 (2022).
Casero, R. A. Jr., Murray Stewart, T. & Pegg, A. E. Polyamine metabolism and cancer: treatments, challenges and opportunities. Nat. Rev. Cancer 18, 681–695 (2018).
Nejman, D. et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980 (2020).
Naseemuddin, M. et al. Cell mediated immune responses through TLR4 prevents DMBA-induced mammary carcinogenesis in mice. Int. J. Cancer 130, 765–774 (2012).
Zhang, J. J. et al. Expression and significance of TLR4 and HIF-1 alpha in pancreatic ductal adenocarcinoma. World J. Gastroenterol. 16, 2881–2888 (2010).
Yin, H. et al. Gut-derived lipopolysaccharide remodels tumoral microenvironment and synergizes with PD-L1 checkpoint blockade via TLR4/MyD88/AKT/NF-κB pathway in pancreatic cancer. Cell Death Dis. 12, 1033 (2021).
Melssen, M. M. et al. A multipeptide vaccine plus toll-like receptor agonists LPS or polyICLC in combination with incomplete Freund’s adjuvant in melanoma patients. J. Immunother. Cancer 7, 163 (2019).
Jiang, S. S. et al. Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe 31, 781–797 (2023).
Wang, X. et al. Fusobacterium nucleatum facilitates anti-PD-1 therapy in microsatellite stable colorectal cancer. Cancer Cell 42, 1729–1746 (2024).
Li, X. et al. Ketogenic diet-induced bile acids protect against obesity through reduced calorie absorption. Nat. Metab. 6, 1397–1414 (2024).
Arnone, A. A., Wilson, A. S., Soto-Pantoja, D. R. & Cook, K. L. Diet modulates the gut microbiome, metabolism, and mammary gland inflammation to influence breast cancer risk. Cancer. Prev. Res. 17, 415–428 (2024).
Turati, F. et al. Fiber-type prebiotics and gynecological and breast cancers risk: the PrebiotiCa study. Am. J. Epidemiol. 193, 1693–1700 (2024).
Xu, K. et al. A dose-response meta-analysis of dietary fiber intake and breast cancer risk. Asia Pac. J. Public Health 34, 331–337 (2022).
Jenkins, D. J. A. et al. Association of glycaemic index and glycaemic load with type 2 diabetes, cardiovascular disease, cancer, and all-cause mortality: a meta-analysis of mega cohorts of more than 100,000 participants. Lancet Diabetes Endocrinol. 12, 107–118 (2024).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Andrews, M. C. et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 27, 1432–1441 (2021).
Spencer, C. N. et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 374, 1632–1640 (2021).
Simpson, R. C. et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat. Med. 28, 2344–2352 (2022).
Holmes, Z. C. et al. Microbiota responses to different prebiotics are conserved within individuals and associated with habitual fiber intake. Microbiome 10, 114 (2022).
Gibson, G. R. et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502 (2017).
Zhang, S. L. et al. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics 11, 4155–4170 (2021).
Messaoudene, M. et al. A natural polyphenol exerts antitumor activity and circumvents Anti-PD-1 resistance through effects on the gut microbiota. Cancer Discov. 12, 1070–1087 (2022).
Xiao, X. et al. Synergistic effects of omega-3 polyunsaturated fatty acid supplementation and programmed cell death protein 1 blockade on tumor growth and immune modulation in a xenograft model of esophageal cancer. Clin. Nutr. ESPEN 61, 308–315 (2024).
Xie, X. et al. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutrition 61, 132–142 (2019).
Polakowski, C. B., Kato, M., Preti, V. B., Schieferdecker, M. E. M. & Ligocki Campos, A. C. Impact of the preoperative use of synbiotics in colorectal cancer patients: a prospective, randomized, double-blind, placebo-controlled study. Nutrition 58, 40–46 (2019).
Flesch, A. T., Tonial, S. T., Contu, P. C. & Damin, D. C. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: a randomized, double-blind clinical trial. Rev. Col. Bras. Cir. 44, 567–573 (2017).
Hill, C. et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Yang, D. et al. Effects of probiotics on gastric cancer-related inflammation: a systematic review and meta-analysis. J. Food Biochem. 46, e14034 (2022).
Zhao, R. et al. Effects of fiber and probiotics on diarrhea associated with enteral nutrition in gastric cancer patients: a prospective randomized and controlled trial. Medicine 96, e8418 (2017).
Pitsillides, L., Pellino, G., Tekkis, P. & Kontovounisios, C. The effect of perioperative administration of probiotics on colorectal cancer surgery outcomes. Nutrients 13, 1451 (2021).
Derosa, L. et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 28, 315–324 (2022).
Routy, B. et al. The gut microbiota influences anticancer immunosurveillance and general health.Nat. Rev. Clin. Oncol. 15, 382–396 (2018).
Lee, K. A. et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat. Med. 28, 535–544 (2022).
Cani, P. D., Depommier, C., Derrien, M., Everard, A. & de Vos, W. M. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 19, 625–637 (2022).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013).
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013).
Peled, J. U. et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. J. Clin. Oncol. 35, 1650–1659 (2017).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Vetizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Gunjur, A. et al. A gut microbial signature for combination immune checkpoint blockade across cancer types. Nat. Med. 30, 797–809 (2024).
Muller, E., Shiryan, I. & Borenstein, E. Multi-omic integration of microbiome data for identifying disease-associated modules. Nat. Commun. 15, 2621 (2024).
Qin, W. et al. Multiomics-based molecular subtyping based on the commensal microbiome predicts molecular characteristics and the therapeutic response in breast cancer. Mol. Cancer 23, 99 (2024).
McCulloch, J. A. et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 28, 545–556 (2022).
Frankel, A. E. et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia 19, 848–855 (2017).
Hou, X. et al. Akkermansia muciniphila potentiates the antitumor efficacy of FOLFOX in colon cancer. Front. Pharm. 12, 725583 (2021).
Wang, L. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut 69, 1988–1997 (2020).
Plovier, H. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113 (2017).
Kang, C. S. et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE 8, e76520 (2013).
Zhang, L. et al. Akkermansia muciniphila inhibits tryptophan metabolism via the AhR/beta-catenin signaling pathway to counter the progression of colorectal cancer. Int. J. Biol. Sci. 19, 4393–4410 (2023).
Paz Del Socorro, T. et al. The biotherapeutic Clostridium butyricum MIYAIRI 588 strain potentiates enterotropism of Rorgammat+ Treg and PD-1 blockade efficacy. Gut Microbes 16, 2315631 (2024).
Tomita, Y. et al. Association of Clostridium butyricum therapy using the live bacterial product CBM588 with the survival of patients with lung cancer receiving chemoimmunotherapy combinations. Cancers 16, 47 (2023).
Tomita, Y. et al. Clostridium butyricum therapy restores the decreased efficacy of immune checkpoint blockade in lung cancer patients receiving proton pump inhibitors. Oncoimmunology 11, 2081010 (2022).
Dizman, N. et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat. Med. 28, 704–712 (2022).
Whitfill, T. & Oh, J. Recoding the metagenome: microbiome engineering in situ. Curr. Opin. Microbiol. 50, 28–34 (2019).
Pujo, J. et al. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut 70, 1088–1097 (2021).
Gurbatri, C. R. et al. Engineering tumor-colonizing E. coli Nissle 1917 for detection and treatment of colorectal neoplasia. Nat. Commun. 15, 646 (2024).
Ho, C. L. et al. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat. Biomed. Eng. 2, 27–37 (2018).
Selvanesan, B. C. et al. Listeria delivers tetanus toxoid protein to pancreatic tumors and induces cancer cell death in mice. Sci. Transl. Med. 14, eabc1600 (2022).
Mullish, B. H. et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridioides difficile infection and other potential indications: second edition of joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut 73, 1052–1075 (2024).
Routy, B. et al. 614 Microbiome modification with fecal microbiota transplant from healthy donors before anti-PD1 therapy reduces primary resistance to immunotherapy in advanced and metastatic melanoma patients. J. Immunother. Cancer 10, A646 (2022).
Davar, D. et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371, 595–602 (2021).
Baruch, E. N. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371, 602–609 (2021).
Derosa, L. et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur. Urol. 78, 195–206 (2020).
Rasmussen, T. S. et al. Overcoming donor variability and risks associated with fecal microbiota transplants through bacteriophage-mediated treatments. Microbiome 12, 119 (2024).
Wang, Z. et al. Protective role of fecal microbiota transplantation on colitis and colitis-associated colon cancer in mice is associated with Treg cells. Front. Microbiol. 10, 2498 (2019).
Riquelme, E. et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178, 795–806 (2019).
Salminen, S. et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18, 649–667 (2021).
Gurunathan, S., Thangaraj, P. & Kim, J. H. Postbiotics: functional food materials and therapeutic agents for cancer, diabetes, and inflammatory diseases. Foods 13, 89 (2023).
Xie, W. et al. Postbiotics in colorectal cancer: intervention mechanisms and perspectives. Front. Microbiol. 15, 1360225 (2024).
Teng, Y. et al. Pasteurized Akkermansia muciniphila mitigates 5-FU-induced intestinal mucositis in tumor-bearing mice through suppression of the cGAS-STING pathway and epithelial cell apoptosis. Food Biosci. 61, 104605 (2024).
Porcari, S. et al. International consensus statement on microbiome testing in clinical practice. Lancet Gastroenterol. Hepatol. 10, 154–167 (2024).
Gand, M., Bloemen, B., Vanneste, K., Roosens, N. H. C. & De Keersmaecker, S. C. J. Comparison of 6 DNA extraction methods for isolation of high yield of high molecular weight DNA suitable for shotgun metagenomics Nanopore sequencing to detect bacteria. BMC Genomics 24, 438 (2023).
Fernandez-Pato, A. et al. Choice of DNA extraction method affects stool microbiome recovery and subsequent phenotypic association analyses. Sci. Rep. 14, 3911 (2024).
Thomas, A. M. et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 25, 667–678 (2019).
Aykut, B. et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574, 264–267 (2019).
Nakatsu, G. et al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology 155, 529–541 (2018).
Acknowledgements
B.F.J. is research director at FRS-FNRS (Fonds de la Recherche Scientifique) and P.D.C. is honorary research director at FRS-FNRS. B.F.J. and P.D.C. are the recipients of Actions de Recherches concertees-Communaute Française de Belgique no. ARC19/24–096. P.D.C. is the recipient of grants from FNRS (Projet de Recherche PDR-convention: FNRS T.0032.25, FRFS-WELBIO: WELBIO-CR-2022A-02P, EOS: programme no. 40007505). C.v.M. is supported by a postdoctoral research mandate from the Fonds de Recherche Clinique. A.N. is supported by a doctoral research mandate from the Fondation Saint-Luc.
Author information
Authors and Affiliations
Contributions
A.N., C.v.M., B.F.J., M.V.H. and P.D.C. conceived and designed the Review. P.D.C. coordinated and supervised the Review. A.N., C.v.M., B.F.J., M.V.H. and P.D.C. performed the literature review. A.N., C.v.M., B.F.J., M.V.H. and P.D.C. conducted the writing. M.V.H. prepared the figures. A.N., C.v.M., B.F.J., M.V.H. and P.D.C reviewed the final draft. All authors discussed the Review, reviewed the revised versions, commented on the manuscript and figures before submission, and agreed with the final submitted manuscript.
Corresponding authors
Ethics declarations
Competing interests
P.D.C. and B.F.J. are inventors on patent applications dealing with the use of gut bacteria and their components in the treatment of diseases. P.D.C. was a co-founder of The Akkermansia Company and Enterosys. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nobels, A., van Marcke, C., Jordan, B.F. et al. The gut microbiome and cancer: from tumorigenesis to therapy. Nat Metab 7, 895–917 (2025). https://doi.org/10.1038/s42255-025-01287-w
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01287-w