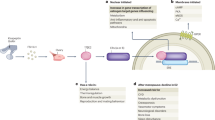
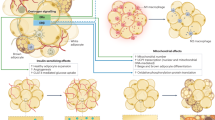
Abstract
Oestradiol (E2), a steroid hormone derived from cholesterol, has long been recognized for its central role in female reproduction and pathobiology of menopause. However, accumulating evidence underscores a critical role for E2 in the regulation of systemic metabolism in both women and men. The metabolic actions of E2 are predominantly mediated by oestrogen receptor α (encoded by ESR1), a nuclear receptor with heritable expression patterns and tissue-specific transcript levels highly correlated with indices of metabolic health in both sexes. Here we provide an overview of the cell-specific actions of E2 and its receptors (α and β) in modulating key metabolic pathways. We contextualize these mechanistic preclinical studies with epidemiological data linking the menopausal transition to a marked rise of metabolic disease risk and provide evidence that E2 replacement mitigates this risk by preserving metabolic health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Finkelstein, J. S. et al. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med. 369, 1011–1022 (2013).
Eriksson, A. L. et al. Genetic variations in sex steroid-related genes as predictors of serum estrogen levels in men. J. Clin. Endocrinol. Metab. 94, 1033–1041 (2009).
Jones, M. E. et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc. Natl Acad. Sci. USA 97, 12735–12740 (2000).
Simpson, E. R. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 86, 225–230 (2003).
Simpson, E. & Santen, R. J. Celebrating 75 years of oestradiol. J. Mol. Endocrinol. 55, T1–T20 (2015).
Tabatadze, N., Sato, S. M. & Woolley, C. S. Quantitative analysis of long-form aromatase mRNA in the male and female rat brain. PLoS ONE 9, e100628 (2014).
Cornil, C. A. On the role of brain aromatase in females: why are estrogens produced locally when they are available systemically? J. Comp. Physiol. A Neuroethol. Sens Neural Behav. Physiol. 204, 31–49 (2018).
Sondern, C. W. & Sealey, J. L. The comparative estrogenic potency of diethyl stilbestrol, estrone, estradiol and estriol. Endocrinology 27, 670–672 (1940).
Punnonen, R. & Lukola, A. High-affinity binding of estrone, estradiol and estriol in human cervical myometrium and cervical and vaginal epithelium. J. Endocrinol. Invest. 5, 203–207 (1982).
Shadyab, A. H. et al. Ages at menarche and menopause and reproductive lifespan as predictors of exceptional longevity in women: the Women’s Health Initiative. Menopause 24, 35–44 (2017).
Cui, R. et al. Relationships of age at menarche and menopause, and reproductive year with mortality from cardiovascular disease in Japanese postmenopausal women: the JACC study. J. Epidemiol. 16, 177–184 (2006).
Carr, M. C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 88, 2404–2411 (2003).
Jeong, H. G. & Park, H. Metabolic disorders in menopause. Metabolites.https://doi.org/10.3390/metabo12100954 (2022).
Gurka, M. J., Vishnu, A., Santen, R. J. & DeBoer, M. D. Progression of metabolic syndrome severity during the menopausal transition. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.116.003609 (2016).
Greendale, G. A. et al. Changes in body composition and weight during the menopause transition. JCI Insight. https://doi.org/10.1172/jci.insight.124865 (2019).
Hulley, S. et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 280, 605–613 (1998).
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002).
Anderson, G. L. et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 291, 1701–1712 (2004).
Lobo, R. A. Hormone-replacement therapy: current thinking. Nat. Rev. Endocrinol. 13, 220–231 (2017).
Bluming, A. Z., Hodis, H. N. & Langer, R. D. Tis but a scratch: a critical review of the Women’s Health Initiative evidence associating menopausal hormone therapy with the risk of breast cancer. Menopause 30, 1241–1245 (2023).
Lacey, J. V. Jr. The WHI ten year’s later: an epidemiologist’s view. J. Steroid Biochem. Mol. Biol. 142, 12–15 (2014).
Hodis, H. N., Collins, P., Mack, W. J. & Schierbeck, L. L. The timing hypothesis for coronary heart disease prevention with hormone therapy: past, present and future in perspective. Climacteric: J. Int. Menopause Soc. 15, 217–228 (2012).
Bhupathiraju, S. N. & Stampfer, M. J. Menopausal Hormone therapy and cardiovascular disease: unraveling the role of age and time since menopause onset. Clin. Chem. 64, 861–862 (2018).
Allen, E. & Doisy, E. A. Landmark article Sept 8, 1923. An ovarian hormone. Preliminary report on its localization, extraction and partial purification, and action in test animals. JAMA 250, 2681–2683 (1983).
Doisy, E. A. The crystals of the follicular ovarian homrone. Exp. Biol. Med. 27, 417–419 (1930).
Corbin, C. J. et al. Isolation of a full-length cDNA insert encoding human aromatase system cytochrome P-450 and its expression in nonsteroidogenic cells. Proc. Natl Acad. Sci. USA 85, 8948–8952 (1988).
Sanghera, M. K. et al. Immunocytochemical distribution of aromatase cytochrome P450 in the rat brain using peptide-generated polyclonal antibodies. Endocrinology 129, 2834–2844 (1991).
Kilgore, M. W., Means, G. D., Mendelson, C. R. & Simpson, E. R. Alternative promotion of aromatase P-450 expression in the human placenta. Mol. Cell. Endocrinol. 83, R9–R16 (1992).
Simpson, E. R. et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 15, 342–355 (1994).
Brodie, A. M. & Santen, R. J. Aromatase in breast cancer and the role of aminoglutethimide and other aromatase inhibitors. Crit. Rev. Oncol. Hematol. 5, 361–396 (1986).
Santen, R. J., Brodie, H., Simpson, E. R., Siiteri, P. K. & Brodie, A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr. Rev. 30, 343–375 (2009).
Yi, M., Negishi, M. & Lee, S. J. Estrogen sulfotransferase (SULT1E1): its molecular regulation, polymorphisms, and clinical perspectives. J. Pers. Med. https://doi.org/10.3390/jpm11030194 (2021).
Qian, Y., Deng, C. & Song, W. C. Expression of estrogen sulfotransferase in MCF-7 cells by cDNA transfection suppresses the estrogen response: potential role of the enzyme in regulating estrogen-dependent growth of breast epithelial cells. J. Pharmacol. Exp. Ther. 286, 555–560 (1998).
Falany, C. N., Wheeler, J., Oh, T. S. & Falany, J. L. Steroid sulfation by expressed human cytosolic sulfotransferases. J. Steroid Biochem. Mol. Biol. 48, 369–375 (1994).
Fashe, M., Yi, M., Sueyoshi, T. & Negishi, M. Sex-specific expression mechanism of hepatic estrogen inactivating enzyme and transporters in diabetic women. Biochem. Pharmacol. 190, 114662 (2021).
Green, S. et al. Cloning of the human oestrogen receptor cDNA. J. Steroid Biochem. 24, 77–83 (1986).
Kuiper, G. G., Enmark, E., Pelto-Huikko, M., Nilsson, S. & Gustafsson, J. A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl Acad. Sci. USA 93, 5925–5930 (1996).
Hewitt, S. C. & Korach, K. S. Estrogen receptors: new directions in the new millennium. Endocr. Rev. 39, 664–675 (2018).
Green, S., Kumar, V., Krust, A., Walter, P. & Chambon, P. Structural and functional domains of the estrogen receptor. Cold Spring Harb. Symp. Quant. Biol. 51, 751–758 (1986).
Helsen, C. et al. Structural basis for nuclear hormone receptor DNA binding. Mol. Cell. Endocrinol. 348, 411–417 (2012).
Ponglikitmongkol, M., Green, S. & Chambon, P. Genomic organization of the human oestrogen receptor gene. EMBO J. 7, 3385–3388 (1988).
Zhou, Z. et al. Estrogen receptor α controls metabolism in white and brown adipocytes by regulating Polg1 and mitochondrial remodeling. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aax8096 (2020).
Nilsson, M. et al. Oestrogen receptor α gene expression levels are reduced in obese compared to normal weight females. Int J. Obes. 31, 900–907 (2007).
Kozniewski, K. et al. Epigenetic regulation of estrogen receptor genes’ expressions in adipose tissue in the course of obesity. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23115989 (2022).
Danielian, P. S., White, R., Lees, J. A. & Parker, M. G. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 11, 1025–1033 (1992).
Shang, Y., Hu, X., DiRenzo, J., Lazar, M. A. & Brown, M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103, 843–852 (2000).
McKenna, N. J., Lanz, R. B. & O’Malley, B. W. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 20, 321–344 (1999).
Arao, Y. & Korach, K. S. The physiological role of estrogen receptor functional domains. Essays Biochem. 65, 867–875 (2021).
Torres, M. J. et al. 17β-estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab. 27, 167–79 e7 (2018).
Scheidt, H. A., Badeau, R. M. & Huster, D. Investigating the membrane orientation and transversal distribution of 17β-estradiol in lipid membranes by solid-state NMR. Chem. Phys. Lipids 163, 356–361 (2010).
Ribas, V. et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERα-deficient mice. Am. J. Physiol. Endocrinol. Metab. 298, E304–E319 (2010).
Ribas, V. et al. Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci. Transl. Med. 8, 334ra54 (2016).
Drew, B. G. et al. Estrogen receptor (ER)α-regulated lipocalin 2 expression in adipose tissue links obesity with breast cancer progression. J. Biol. Chem. 290, 5566–5581 (2015).
Zhou, Z. et al. Estrogen receptor alpha protects pancreatic beta-cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. J. Biol. Chem. 293, 4735–4751 (2018).
Ribas, V. et al. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc. Natl Acad. Sci. USA 108, 16457–16462 (2011).
Couse, J. F., Yates, M. M., Walker, V. R. & Korach, K. S. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol. Endocrinol. 17, 1039–1053 (2003).
Chen, J. Q., Delannoy, M., Cooke, C. & Yager, J. D. Mitochondrial localization of ERα and ERβ in human MCF7 cells. Am. J. Physiol. Endocrinol. Metab. 286, E1011–E1022 (2004).
Chen, J. Q., Yager, J. D. & Russo, J. Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim. Biophys. Acta 1746, 1–17 (2005).
Karakas, B. et al. Mitochondrial estrogen receptors alter mitochondrial priming and response to endocrine therapy in breast cancer cells. Cell Death Discov. 7, 189 (2021).
Monje, P. & Boland, R. Subcellular distribution of native estrogen receptor α and β isoforms in rabbit uterus and ovary. J. Cell. Biochem. 82, 467–479 (2001).
Melanson, E. L. et al. Regulation of energy expenditure by estradiol in premenopausal women. J. Appl Physiol. 119, 975–981 (2015).
Moorman, P. G. et al. A prospective study of weight gain after premenopausal hysterectomy. J. Women’s Health (Larchmt.). 18, 699–708 (2009).
Rogers, N. H. et al. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150, 2161–2168 (2009).
Moran, A. L., Nelson, S. A., Landisch, R. M., Warren, G. L. & Lowe, D. A. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J. Appl Physiol. 102, 1387–1393 (2007).
Hamilton, D. J. et al. Estrogen receptor α activation enhances mitochondrial function and systemic metabolism in high-fat-fed ovariectomized mice. Physiol Rep. https://doi.org/10.14814/phy2.12913 (2016).
Said, S. A. et al. Effects of long-term dietary administration of estrogen receptor-β agonist diarylpropionitrile on ovariectomized female ICR (CD-1) mice. Geroscience 40, 393–403 (2018).
Cooke, P. S., Heine, P. A., Taylor, J. A. & Lubahn, D. B. The role of estrogen and estrogen receptor-α in male adipose tissue. Mol. Cell. Endocrinol. 178, 147–154 (2001).
Heine, P. A., Taylor, J. A., Iwamoto, G. A., Lubahn, D. B. & Cooke, P. S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl Acad. Sci. USA 97, 12729–12734 (2000).
Ohlsson, C. et al. Obesity and disturbed lipoprotein profile in estrogen receptor-α-deficient male mice. Biochem. Biophys. Res. Commun. 278, 640–645 (2000).
Osterlund, M., Kuiper, G. G., Gustafsson, J. A. & Hurd, Y. L. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res. Mol. Brain Res. 54, 175–180 (1998).
Xu, Y. et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 14, 453–465 (2011).
Martinez de Morentin, P. B. et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 20, 41–53 (2014).
Musatov, S. et al. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl Acad. Sci. USA 104, 2501–2506 (2007).
Velickovic, K. et al. Expression and subcellular localization of estrogen receptors α and β in human fetal brown adipose tissue. J. Clin. Endocrinol. Metab. 99, 151–159 (2014).
van Veen, J. E. et al. Hypothalamic estrogen receptor α establishes a sexually dimorphic regulatory node of energy expenditure. Nat. Metab. 2, 351–363 (2020).
Krause, W. C. et al. Oestrogen engages brain MC4R signalling to drive physical activity in female mice. Nature 599, 131–135 (2021).
Mosca, L., Barrett-Connor, E. & Wenger, N. K. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation 124, 2145–2154 (2011).
Allard, M. F., Schonekess, B. O., Henning, S. L., English, D. R. & Lopaschuk, G. D. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am. J. Physiol. 267, H742–H750 (1994).
Lopaschuk, G. D. & Jaswal, J. S. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J. Cardiovasc. Pharmacol. 56, 130–140 (2010).
Herrero, P. et al. Impact of hormone replacement on myocardial fatty acid metabolism: potential role of estrogen. J. Nucl. Cardiol. 12, 574–581 (2005).
Tham, Y. K. et al. Estrogen receptor α deficiency in cardiomyocytes reprograms the heart-derived extracellular vesicle proteome and induces obesity in female mice. Nat. Cardiovasc Res. 2, 268–289 (2023).
Baskin, K. K. et al. MED13-dependent signaling from the heart confers leanness by enhancing metabolism in adipose tissue and liver. EMBO Mol. Med. 6, 1610–1621 (2014).
Dewey C. M., Spitler, K. M., Ponce, J. M., Hall, D. D. & Grueter, C. E. Cardiac-secreted factors as peripheral metabolic regulators and potential disease biomarkers. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.115.003101 (2016).
Yang, W. et al. An estrogen receptor α-derived peptide improves glucose homeostasis during obesity. Nat. Commun. 15, 3410 (2024).
Qiu, S. et al. Hepatic estrogen receptor α is critical for regulation of gluconeogenesis and lipid metabolism in males. Sci. Rep. 7, 1661 (2017).
Gao, H. et al. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol. Endocrinol. 20, 1287–1299 (2006).
Meda, C. et al. Hepatic ERα accounts for sex differences in the ability to cope with an excess of dietary lipids. Mol. Metab. 32, 97–108 (2020).
Zhu, L., Martinez, M. N., Emfinger, C. H., Palmisano, B. T. & Stafford, J. M. Estrogen signaling prevents diet-induced hepatic insulin resistance in male mice with obesity. Am. J. Physiol. Endocrinol. Metab. 306, E1188–E1197 (2014).
Goodman, M. P. Are all estrogens created equal? A review of oral vs. transdermal therapy. J. Women’s Health (Larchmt.). 21, 161–169 (2012).
Larson, A. A., Baumann, C. W., Kyba, M. & Lowe, D. A. Oestradiol affects skeletal muscle mass, strength and satellite cells following repeated injuries. Exp. Physiol. 105, 1700–1707 (2020).
Zhou, Z. et al. Enhanced metabolic resilience and exercise adaptation of skeletal muscle in males by Esr1-induced remodeling of mitochondrial cristae-nucleoid architecture. Cell Rep. Med. 6, 102116 (2025).
Heinonen, S. et al. Mitochondria-related transcriptional signature is downregulated in adipocytes in obesity: a study of young healthy MZ twins. Diabetologia 60, 169–181 (2017).
Gannon, M., Kulkarni, R. N., Tse, H. M. & Mauvais-Jarvis, F. Sex differences underlying pancreatic islet biology and its dysfunction. Mol. Metab. 15, 82–91 (2018).
Marchese, E. et al. Enumerating β-cells in whole human islets: sex differences and associations with clinical outcomes after islet transplantation. Diabetes Care 38, e176–e177 (2015).
Mauvais-Jarvis, F., Manson, J. E., Stevenson, J. C. & Fonseca, V. A. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr. Rev. 38, 173–188 (2017).
Contreras, J. L. et al. 17β-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: molecular mechanisms and islet functionality. Transplantation 74, 1252–1259 (2002).
Herz, C. T. et al. Sex differences in brown adipose tissue activity and cold-induced thermogenesis. Mol. Cell. Endocrinol. 534, 111365 (2021).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Chella Krishnan, K. et al. Sex-specific genetic regulation of adipose mitochondria and metabolic syndrome by Ndufv2. Nat. Metab. 3, 1552–1568 (2021).
Kautzky-Willer, A., Harreiter, J. & Pacini, G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 37, 278–316 (2016).
Ahmed, F. et al. Altered expression of aromatase and estrogen receptors in adipose tissue from men with obesity or type 2 diabetes. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgaf038 (2025).
Yin, X. et al. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J. Clin. Endocrinol. Metab. 99, E209–E216 (2014).
Norheim, F. et al. Gene-by-sex interactions in mitochondrial functions and cardio-metabolic traits. Cell Metab. 29, 932–49 e4 (2019).
Davis, K. E. et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol. Metab. 2, 227–242 (2013).
Saavedra-Pena, R. D. M., Taylor, N. & Rodeheffer, M. S. Insights of the role of estrogen in obesity from two models of ERα deletion. J. Mol. Endocrinol. 68, 179–194 (2022).
Cioffi, M. et al. Cytokine pattern in postmenopause. Maturitas 41, 187–192 (2002).
Ghisletti, S., Meda, C., Maggi, A. & Vegeto, E. 17β-Estradiol inhibits inflammatory gene expression by controlling NF-κB intracellular localization. Mol. Cell. Biol. 25, 2957–2968 (2005).
Hodgin, J. B. et al. Estrogen receptor α is a major mediator of 17β-estradiol’s atheroprotective effects on lesion size in Apoe-/- mice. J. Clin. Invest. 107, 333–340 (2001).
Yu, Y. et al. Systematic analysis of adverse event reports for sex differences in adverse drug events. Sci. Rep. 6, 24955 (2016).
Rushovich, T. et al. Adverse drug events by sex after adjusting for baseline rates of drug use. JAMA Netw. Open. 6, e2329074 (2023).
Lee, K. M. N. et al. A gender hypothesis of sex disparities in adverse drug events. Soc. Sci. Med. 339, 116385 (2023).
Shih, Y. H., Yang, C. Y., Wang, S. J. & Lung, C. C. Menopausal hormone therapy decreases the likelihood of diabetes development in peri‑menopausal individuals with prediabetes. Diabetes Metab. 50, 101546 (2024).
Margolis, K. L. et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 47, 1175–1187 (2004).
Flores, V. A., Pal, L. & Manson, J. E. Hormone therapy in menopause: concepts, controversies, and approach to treatment. Endocr. Rev. 42, 720–752 (2021).
Amar, D. et al. Temporal dynamics of the multi-omic response to endurance exercise training. Nature 629, 174–183 (2024).
Wang, J. et al. Exploring the mechanisms of genome-wide long-range interactions: interpreting chromosome organization. Brief. Funct. Genomics 15, 385–395 (2016).
Acknowledgements
A.L.H. holds the Sidney Roberts and Clara Szego Roberts Chair in Molecular and Cellular Endocrinology and her work is funded by the Iris Cantor-UCLA Women’s Health Center, UCLA Jonsson Comprehensive Cancer Center and the NIH (R01 DK128957, U54 HL170326, R01 DK060484, P30DK063491 and previously NURSA NDSP U24DK097748). S.M.C. is supported by the NIH (R01 AG066821, NIH R01 DK136073 and R21 CA249338), Iris Cantor-UCLA Women’s Health Center and Allen Distinguished Investigator Award in Sex Hormones grant no. 202211-13640.
Author information
Authors and Affiliations
Contributions
A.L.H. and S.M.C. jointly wrote this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Sylvia Hewitt, Manuel Tena-Sempere and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Jean Nakhle and Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hevener, A.L., Correa, S.M. Metabolic Messengers: oestradiol. Nat Metab 7, 1114–1122 (2025). https://doi.org/10.1038/s42255-025-01317-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01317-7