Abstract
Amylin is a glucoregulatory peptide hormone discovered in 1986. Almost 20 years later, pramlintide, a human amylin analogue, emerged as the first amylin-based drug, approved as an adjunct treatment to insulin for type 1 diabetes (T1D) and type 2 diabetes (T2D). Despite its effects on multiple organ systems, the therapeutic potential of amylin has remained relatively underexplored until recently, when growing interest in amylin has prompted advancement of several amylin-based therapies towards clinical use. This Review contextualizes the evolving therapeutic potential of amylin, focusing on recent preclinical and clinical data, amylin receptor pharmacology and its broader biological effects. We discuss the potential and challenges of developing amylin-based treatments for cardiometabolic disease, including milestones in drug development of amylin, and its combination with additional molecules as part of the future landscape of therapies for patients with diabetes or obesity.
Similar content being viewed by others
Enjoying our latest content?
Log in or create an account to continue
- Access the most recent journalism from Nature's award-winning team
- Explore the latest features & opinion covering groundbreaking research
or
References
Young, A. Tissue expression and secretion of amylin. Adv. Pharmacol. 52, 19–45 (2005).
Cooper, G. J. et al. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc. Natl Acad. Sci. USA 84, 8628–8632 (1987).
Cooper, G. J. et al. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proc. Natl Acad. Sci. USA 85, 7763–7766 (1988).
Westermark, P. et al. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc. Natl Acad. Sci. USA 84, 3881–3885 (1987).
Mosselman, S. et al. Islet amyloid polypeptide: identification and chromosomal localization of the human gene. FEBS Lett. 239, 227–232 (1988).
Johnson, K. H. et al. Immunolocalization of islet amyloid polypeptide (IAPP) in pancreatic beta cells by means of peroxidase-antiperoxidase (PAP) and protein A-gold techniques. Am. J. Pathol. 130, 1–8 (1988).
Kanatsuka, A. et al. Secretion of islet amyloid polypeptide in response to glucose. FEBS Lett. 259, 199–201 (1989).
Cluck, M. W., Chan, C. Y. & Adrian, T. E. The regulation of amylin and insulin gene expression and secretion. Pancreas 30, 1–14 (2005).
Butler, P. C. et al. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes 39, 752–756 (1990).
Gasa, R., Gomis, R., Casamitjana, R. & Novials, A. High glucose concentration favors the selective secretion of islet amyloid polypeptide through a constitutive secretory pathway in human pancreatic islets. Pancreas 22, 307–310 (2001).
Bretherton-Watt, D., Gore, N. & Boam, D. S. Insulin upstream factor 1 and a novel ubiquitous factor bind to the human islet amyloid polypeptide/amylin gene promoter. Biochem. J. 313, 495–502 (1996).
Shepherd, L. M. A., Campbell, S. C. & Macfarlane, W. M. Transcriptional regulation of the IAPP gene in pancreatic beta-cells. Biochim. Biophys. Acta 1681, 28–37 (2004).
Martin, C. The physiology of amylin and insulin: maintaining the balance between glucose secretion and glucose uptake. Diabetes Educ. 32, 101S–104S (2006).
Gedulin, B. R., Rink, T. J. & Young, A. A. Dose-response for glucagonostatic effect of amylin in rats. Metabolism 46, 67–70 (1997).
Preskou, M. K. et al. Acute i.v. infusion of the amylin analogue pramlintide does not affect glucagon levels in individuals with type 1 diabetes or in healthy control. Diabetologia 68, S693 (2025).
Cooperberg, B. A. & Cryer, P. E. Insulin reciprocally regulates glucagon secretion in humans. Diabetes 59, 2936–2940 (2010).
Young, A. A., Gedulin, B., Vine, W., Percy, A. & Rink, T. J. Gastric emptying is accelerated in diabetic BB rats and is slowed by subcutaneous injections of amylin. Diabetologia 38, 642–648 (1995).
Lutz, T. A., Geary, N., Szabady, M. M., Del Prete, E. & Scharrer, E. Amylin decreases meal size in rats. Physiol. Behav. 58, 1197–1202 (1995).
Reidelberger, R. D., Arnelo, U., Granqvist, L. & Permert, J. Comparative effects of amylin and cholecystokinin on food intake and gastric emptying in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R605–R611 (2001).
Kolterman, O. G., Gottlieb, A., Moyses, C. & Colburn, W. Reduction of postprandial hyperglycemia in subjects with IDDM by intravenous infusion of AC 137, a human amylin analogue. Diabetes Care 18, 1197–1182 (1995).
Lukinius, A., Wilander, E., Westermark, G. T., Engström, U. & Westermark, P. Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia 32, 240–244 (1989).
Kahn, S. E. et al. Evidence of cosecretion of islet amyloid polypeptide and insulin by β-cells. Diabetes 39, 634–638 (1990).
Inoue, K., Hisatomi, A., Umeda, F. & Nawata, H. Release of amylin from perfused rat pancreas in response to glucose, arginine, β-hydroxybutyrate, and gliclazide. Diabetes 40, 1005–1009 (1991).
Inoue, K., Hisatomi, A., Umeda, F. & Nawata, H. Effects of exogenous somatostatin and insulin on islet amyloid polypeptide (amylin) release from perfused rat pancreas. Horm. Metab. Res. 24, 251–253 (1992).
Ludvik, B., Lell, B., Hartter, E., Schnack, C. & Prager, R. Decrease of stimulated amylin release precedes impairment of insulin secretion in type II diabetes. Diabetes 40, 1615–1619 (1991).
Dechenes, C. J., Verchere, C. B., Andrikopoulos, S. & Kahn, S. E. Human aging is associated with parallel reductions in insulin and amylin release. Am. J. Physiol. 275, E785–E791 (1998).
Paulsson, J. F. et al. High plasma levels of islet amyloid polypeptide in young with new-onset of type 1 diabetes mellitus. PLoS ONE 9, e93053 (2014).
O’Brien, T. D., Westermark, P. & Johnson, K. H. Islet amyloid polypeptide and insulin secretion from isolated perfused pancreas of fed, fasted, glucose-treated, and dexamethasone-treated rats. Diabetes 40, 1701–1706 (1991).
Fehmann, H. C., Weber, V., Göke, R., Göke, B. & Arnold, R. Cosecretion of amylin and insulin from isolated rat pancreas. FEBS Lett. 262, 279–281 (1990).
Opie, E. L. On the relation of chronic interstitial pancreatitis to the islands of langerhans and to diabetes melutus. J. Exp. Med. 5, 397–428 (1901).
Ehrlich, J. C. & Ratner, I. M. Amyloidosis of the islets of Langerhans. A restudy of islet hyalin in diabetic and non-diabetic individuals. Am. J. Pathol. 38, 49–59 (1961).
Westermark, G. T. & Westermark, P. Islet amyloid polypeptide and diabetes. Curr. Protein Pept. Sci. 14, 330–337 (2013).
Akter, R. et al. Islet amyloid polypeptide: structure, function, and pathophysiology. J. Diabetes. Res. 2016, 2798269 (2016).
Wirth, F. et al. A human antibody against pathologic IAPP aggregates protects beta cells in type 2 diabetes models. Nat. Commun. 14, 6294 (2023).
Westermark, G. T., Krogvold, L., Dahl-Jorgensen, K. & Ludvigsson, J. Islet amyloid in recent-onset type 1 diabetes-the DiViD study. Ups. J. Med. Sci. 122, 201–203 (2017).
Oskarsson, M. E. et al. In vivo seeding and cross-seeding of localized amyloidosis: a molecular link between type 2 diabetes and Alzheimer disease. Am. J. Pathol. 185, 834–846 (2015).
Ly, H. et al. The association of circulating amylin with beta-amyloid in familial Alzheimer’s disease. Alzheimers Dement. 7, e12130 (2021).
Jackson, K. et al. Amylin deposition in the brain: a second amyloid in Alzheimer disease? Ann. Neurol. 74, 517–526 (2013).
Albariqi, M. M. et al. Human IAPP is a contributor to painful diabetic peripheral neuropathy. J. Clin. Invest. 133, e156993 (2023).
Gong, W. et al. Amylin deposition in the kidney of patients with diabetic nephropathy. Kidney Int. 72, 213–218 (2007).
Raimundo, A. F., Ferreira, S., Martins, I. C. & Menezes, R. Islet amyloid polypeptide: a partner in crime with Aβ in the pathology of Alzheimer’s disease. Front. Mol. Neurosci. 13, 35 (2020).
Despa, S. et al. Hyperamylinemia contributes to cardiac dysfunction in obesity and diabetes: a study in humans and rats. Circ. Res. 110, 598–608 (2012).
Kruse, T. et al. Development of cagrilintide, a long-acting amylin analogue. J. Med. Chem. 64, 11183–11194 (2021).
Nonoyama, A. et al. A biophysical characterization of the peptide drug pramlintide (AC137) using empirical phase diagrams. J. Pharm. Sci. 97, 2552–2567 (2008).
Esser, N. et al. The islet tissue plasminogen activator/plasmin system is upregulated with human islet amyloid polypeptide aggregation and protects beta cells from aggregation-induced toxicity. Diabetologia 67, 1897–1911 (2024).
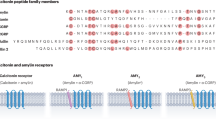
Cao, J. et al. A structural basis for amylin receptor phenotype. Science 375, eabm9609 (2022).
Fletcher, M. M. et al. AM833 is a novel agonist of calcitonin family G protein-coupled receptors: pharmacological comparison with six selective and nonselective agonists. J. Pharmacol. Exp. Ther. 377, 417–440 (2021).
Christopoulos, G. et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol. 56, 235–242 (1999).
Hay, D. L., Chen, S., Lutz, T. A., Parkes, D. G. & Roth, J. D. Amylin: pharmacology, physiology, and clinical potential. Pharmacol. Rev. 67, 564–600 (2015).
Coester, B. et al. RAMP1 and RAMP3 differentially control amylin’s effects on food intake, glucose and energy balance in male and female mice. Neuroscience 447, 74–93 (2020).
Hay, D. L., Garelja, M. L., Poyner, D. R. & Walker, C. S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 175, 3–17 (2018).
Young, A. A. et al. Preclinical pharmacology of pramlintide in the rat: comparisons with human and rat amylin. Drug Dev. Res. 37, 231–248 (1996).
Turner, A. G. et al. The role of the calcitonin receptor in protecting against induced hypercalcemia is mediated via its actions in osteoclasts to inhibit bone resorption. Bone 48, 354–361 (2011).
Zhang, Z. et al. Neuronal receptor activity-modifying protein 1 promotes energy expenditure in mice. Diabetes 60, 1063–1071 (2011).
Fernandes-Santos, C. et al. Amylin acts in the central nervous system to increase sympathetic nerve activity. Endocrinology 154, 2481–2488 (2013).
Cao, J. et al. Structural insight into selectivity of amylin and calcitonin receptor agonists. Nat. Chem. Biol. 20, 162–169 (2024).
Arrigoni, S. et al. A selective role for receptor activity-modifying proteins in subchronic action of the amylin selective receptor agonist NN1213 compared with salmon calcitonin on body weight and food intake in male mice. Eur. J. Neurosci. 54, 4863–4876 (2021).
Carvas, A. O. et al. Cagrilintide lowers bodyweight through brain amylin receptors 1 and 3. EBioMedicine 118, 105836 (2025).
Niall, H. D., Keutmann, H. T., Copp, D. H. & Potts, J. T. Amino acid sequence of salmon ultimobranchial calcitonin. Proc. Natl Acad. Sci. USA 64, 771–778 (1969).
Mack, C. M. et al. Davalintide (AC2307), a novel amylin-mimetic peptide: enhanced pharmacological properties over native amylin to reduce food intake and body weight. Int. J. Obes. 34, 385–395 (2010).
Lee, S. M., Hay, D. L. & Pioszak, A. A. Calcitonin and amylin receptor peptide interaction mechanisms: insights into peptide-binding modes and allosteric modulation of the calcitonin receptor by receptor activity-modifying proteins. J. Biol. Chem. 291, 8686–8700 (2016).
Andreassen, K. V. et al. A novel oral dual amylin and calcitonin receptor agonist (KBP-042) exerts antiobesity and antidiabetic effects in rats. Am. J. Physiol. Endocrinol. Metab. 307, E24–E33 (2014).
Gydesen, S. et al. Optimization of tolerability and efficacy of the novel dual amylin and calcitonin receptor agonist KBP-089 through dose escalation and combination with a GLP-1 analog. Am. J. Physiol. Endocrinol. Metab. 313, E598–E607 (2017).
Andreassen, K. V. et al. KBP-066A, a long-acting dual amylin and calcitonin receptor agonist, induces weight loss and improves glycemic control in obese and diabetic rats. Mol. Metab. 53, 101282 (2021).
Keov, P. et al. Development of a novel assay for direct assessment of selective amylin receptor activation reveals novel differences in behavior of selective and nonselective peptide agonists. Mol. Pharmacol. 105, 359–373 (2024).
Sexton, P. M., Paxinos, G., Kenney, M. A., Wookey, P. J. & Beaumont, K. In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience 62, 553–567 (1994).
Beaumont, K., Kenney, M. A., Young, A. A. & Rink, T. J. High affinity amylin binding sites in rat brain. Mol. Pharmacol. 44, 493–497 (1993).
Ludwig, M. Q. et al. A cross-species atlas of the dorsal vagal complex reveals neural mediators of cagrilintide’s effects on energy balance. Preprint at bioRxiv https://doi.org/10.1101/2025.01.13.632726 (2025).
Liberini, C. G. et al. Amylin receptor components and the leptin receptor are co-expressed in single rat area postrema neurons. Eur. J. Neurosci. 43, 653–661 (2016).
Coester, B., Koester-Hegmann, C., Lutz, T. A. & Le Foll, C. Amylin/calcitonin receptor-mediated signaling in POMC neurons influences energy balance and locomotor activity in chow-fed male mice. Diabetes 69, 1110–1125 (2020).
Zakariassen, H. L., John, L. M. & Lutz, T. A. Central control of energy balance by amylin and calcitonin receptor agonists and their potential for treatment of metabolic diseases. Basic Clin. Pharmacol. Toxicol. 127, 163–177 (2020).
Hay, D. L. & Pioszak, A. A. Receptor activity-modifying proteins (RAMPs): new insights and roles. Annu. Rev. Pharmacol. Toxicol. 56, 469–487 (2016).
Siletti, K. et al. Transcriptomic diversity of cell types across the adult human brain. Science 382, eadd7046 (2023).
Tadross, J. A. et al. A comprehensive spatio-cellular map of the human hypothalamus. Nature 639, 708–716 (2025).
Lutz, T. A., Mollet, A., Rushing, P. A., Riediger, T. & Scharrer, E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int. J. Obes. Relat. Metab. Disord. 25, 1005–1011 (2001).
Lutz, T. A. et al. Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene-related peptide (CGRP) in rats. Peptides 19, 309–317 (1998).
Braegger, F. E., Asarian, L., Dahl, K., Lutz, T. A. & Boyle, C. N. The role of the area postrema in the anorectic effects of amylin and salmon calcitonin: behavioral and neuronal phenotyping. Eur. J. Neurosci. 40, 3055–3066 (2014).
Potes, C. S. et al. Noradrenergic neurons of the area postrema mediate amylin’s hypophagic action. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R623–R631 (2010).
Lundh, S. et al. Spatiocellular mapping of neuronal populations targeted by amylin and GLP-1 in human CNS. Obesity 32, 48–49 (2024).
McLatchie, L. M. et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393, 333–339 (1998).
CZ CELLxGENE. Discover the mechanisms of human health. https://cellxgene.cziscience.com/ (2025).
Maleitzke, T. et al. The calcitonin receptor protects against bone loss and excessive inflammation in collagen antibody-induced arthritis. iScience 25, 103689 (2022).
Nakamura, M. et al. Osteoclast-like cells express receptor activity modifying protein 2: application of laser capture microdissection. J. Mol. Endocrinol. 34, 257–261 (2005).
Fukada, S. et al. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 25, 2448–2459 (2007).
Yamaguchi, M. et al. Calcitonin receptor signaling inhibits muscle stem cells from escaping the quiescent state and the niche. Cell Rep. 13, 302–314 (2015).
Zhang, L. et al. The CalcR-PKA-Yap1 axis is critical for maintaining quiescence in muscle stem cells. Cell Rep. 29, 2154–2163 (2019).
Ikemoto-Uezumi, M. et al. Reduced expression of calcitonin receptor is closely associated with age-related loss of the muscle stem cell pool. JCSM Rapid Commun. 2, 1–13 (2019).
Cao, J. et al. Structural and dynamic features of cagrilintide binding to calcitonin and amylin receptors. Nat. Commun. 16, 3389 (2025).
Hay, D. L., Christopoulos, G., Christopoulos, A., Poyner, D. R. & Sexton, P. M. Pharmacological discrimination of calcitonin receptor: receptor activity-modifying protein complexes. Mol. Pharmacol. 67, 1655–1665 (2005).
Dal Maso, E. et al. Characterization of signalling and regulation of common calcitonin receptor splice variants and polymorphisms. Biochem. Pharmacol. 148, 111–129 (2018).
Larsen, A. T. et al. Does receptor balance matter? - Comparing the efficacies of the dual amylin and calcitonin receptor agonists cagrilintide and KBP-336 on metabolic parameters in preclinical models. Biomed. Pharmacother. 156, 113842 (2022).
Akbari, P. et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science 373, eabf8683 (2021).
Roth, J. D. et al. Interactions of amylinergic and melanocortinergic systems in the control of food intake and body weight in rodents. Diabetes Obes. Metab. 14, 608–615 (2012).
Jacobsen, J. M. et al. CagriSema drives weight loss in rats by reducing energy intake and preserving energy expenditure. Nat. Metab. 7, 1322–1329 (2025).
Gabery, S. et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 5, e133429 (2020).
Larsen, L., Le Foll, C., Dunn-Meynell, A. A. & Levin, B. E. IL-6 ameliorates defective leptin sensitivity in DIO ventromedial hypothalamic nucleus neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R764–R770 (2016).
Trevaskis, J. L., Parkes, D. G. & Roth, J. D. Insights into amylin-leptin synergy. Trends Endocrinol. Metab. 21, 473–479 (2010).
Mack, C. et al. Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference, and body weight. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1855–R1863 (2007).
Chance, W. T., Balasubramaniam, A., Zhang, F. S., Wimalawansa, S. J. & Fischer, J. E. Anorexia following the intrahypothalamic administration of amylin. Brain Res. 539, 352–354 (1991).
Morley, J. E. & Flood, J. F. Amylin decreases food intake in mice. Peptides 12, 865–869 (1991).
Lutz, T. A., Del Prete, E. & Scharrer, E. Reduction of food intake in rats by intraperitoneal injection of low doses of amylin. Physiol. Behav. 55, 891–895 (1994).
Foll, C. L. & Lutz, T. A. Systemic and central amylin, amylin receptor signaling, and their physiological and pathophysiological roles in metabolism. Compr. Physiol. 10, 811–837 (2020).
Roth, J. D. Amylin and the regulation of appetite and adiposity: recent advances in receptor signaling, neurobiology and pharmacology. Curr. Opin. Endocrinol. Diabetes Obes. 20, 8–13 (2013).
Boccia, L. et al. Amylin brain circuitry. Peptides 132, 170366 (2020).
Potes, C. S., Boyle, C. N., Wookey, P. J., Riediger, T. & Lutz, T. A. Involvement of the extracellular signal-regulated kinase 1/2 signaling pathway in amylin’s eating inhibitory effect. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R340–R351 (2012).
Riediger, T., Zuend, D., Becskei, C. & Lutz, T. A. The anorectic hormone amylin contributes to feeding-related changes of neuronal activity in key structures of the gut-brain axis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R114–R122 (2004).
Riediger, T., Schmid, H. A., Lutz, T. & Simon, E. Amylin potently activates AP neurons possibly via formation of the excitatory second messenger cGMP. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1833–R1843 (2001).
Edwards, G. L. et al. Area postrema (AP)-lesions block the regulation of gastric emptying by amylin. Gastroenterology 114, A748 (1998).
Skovbjerg, G. et al. Whole-brain mapping of amylin-induced neuronal activity in receptor activity-modifying protein 1/3 knockout mice. Eur. J. Neurosci. 54, 4154–4166 (2021).
Cheng, W. et al. Calcitonin receptor neurons in the mouse nucleus tractus solitarius control energy balance via the non-aversive suppression of feeding. Cell Metab. 31, 301–312 (2020).
Campos, C. A., Bowen, A. J., Schwartz, M. W. & Palmiter, R. D. Parabrachial CGRP neurons control meal termination. Cell Metab. 23, 811–820 (2016).
Campos, C. A. et al. Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus. Nat. Neurosci. 20, 934–942 (2017).
Carter, M. E., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Genetic identification of a neural circuit that suppresses appetite. Nature 503, 111–114 (2013).
Palmiter, R. D. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 41, 280–293 (2018).
Boccia, L., Le Foll, C. & Lutz, T. A. Noradrenaline signaling in the LPBN mediates amylin’s and salmon calcitonin’s hypophagic effect in male rats. FASEB J. 34, 15448–15461 (2020).
Boccia, L. et al. Hypophagia induced by salmon calcitonin, but not by amylin, is partially driven by malaise and is mediated by CGRP neurons. Mol. Metab. 58, 101444 (2022).
Ludwig, M. Q. et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat. Metab. 3, 530–545 (2021).
Garvey, W. T. et al. Coadministered cagrilintide and semaglutide in adults with overweight or obesity. N. Engl. J. Med. 393, 635–647 (2025).
Billings, L. K. et al. Eloralintide, a selective amylin receptor agonist for the treatment of obesity: a 48-week phase 2, multicentre, double-blind, randomised, placebo-controlled trial. Lancet 406, 2631–2643 (2025).
Secher, A. et al. Cagrilintide and semaglutide target both the hypothalamic and brainstem neurons in the rodent brain. Obesity 30, 55–293 (2022).
Mietlicki-Baase, E. G., Olivos, D. R., Jeffrey, B. A. & Hayes, M. R. Cooperative interaction between leptin and amylin signaling in the ventral tegmental area for the control of food intake. Am. J. Physiol. Endocrinol. Metab. 308, R1116–R1122 (2015).
Mietlicki-Baase, E. G. et al. Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology 38, 1685–1697 (2013).
Mietlicki-Baase, E. G. et al. Amylin modulates the mesolimbic dopamine system to control energy balance. Neuropsychopharmacology 40, 372–385 (2015).
Reiner, D. J. et al. Amylin acts in the lateral dorsal tegmental nucleus to regulate energy balance through gamma-aminobutyric acid signaling. Biol. Psychiatry 82, 828–838 (2017).
Geisler, C. E. et al. Amylin modulates a ventral tegmental area-to-medial prefrontal cortex circuit to suppress food intake and impulsive food-directed behavior. Biol. Psychiatry 95, 938–950 (2024).
Roth, J. D., Hughes, H., Kendall, E., Baron, A. D. & Anderson, C. M. Antiobesity effects of the beta-cell hormone amylin in diet-induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology 147, 5855–5864 (2006).
Blom, I. et al. 1693-P: CagriSema-induced weight loss in diet-induced obese rats relies on preserved mitochondrial leak respiration in skeletal muscle. Diabetes 74 (Suppl. 1), 1693–P (2025).
Boyle, C. N. & Lutz, T. A. Amylinergic control of food intake in lean and obese rodents. Physiol. Behav. 105, 129–137 (2011).
Chapman, I. et al. Effect of pramlintide on satiety and food intake in obese subjects and subjects with type 2 diabetes. Diabetologia 48, 838–848 (2005).
Trevaskis, J. L. et al. Amylin/leptin synergy is absent in extreme obesity and not restored by calorie restriction-induced weight loss in rats. Obes. Sci. Pract. 2, 385–391 (2016).
Nie, T. et al. Altered metabolic gene expression in the brain of a triprolyl-human amylin transgenic mouse model of type 2 diabetes. Sci. Rep. 9, 14588 (2019).
Dahl, K. et al. Preclinical weight loss efficacy of AM833 in animal models of obesity. Obesity 28, 140 (2020).
Halaas, J. L. et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995).
Chrysafi, P. et al. Leptin alters energy intake and fat mass but not energy expenditure in lean subjects. Nat. Commun. 11, 5145 (2020).
Obradovic, M. et al. Leptin and obesity: role and clinical implication. Front. Endocrinol. 12, 585887 (2021).
Duffy, S., Lutz, T. A. & Boyle, C. N. Rodent models of leptin receptor deficiency are less sensitive to amylin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R856–R865 (2018).
Turek, V. F. et al. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology 151, 143–152 (2010).
Trevaskis, J. L. et al. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology 149, 5679–5687 (2008).
Trevaskis, J. L. et al. Interaction of leptin and amylin in the long-term maintenance of weight loss in diet-induced obese rats. Obesity 18, 21–26 (2010).
Osto, M., Wielinga, P. Y., Alder, B., Walser, N. & Lutz, T. A. Modulation of the satiating effect of amylin by central ghrelin, leptin and insulin. Physiol. Behav. 91, 566–572 (2007).
Le Foll, C. et al. Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes 64, 1621–1631 (2015).
Wang, D. et al. Hepatokine Fetuin B expression is regulated by leptin-STAT3 signalling and associated with leptin in obesity. Sci. Rep. 12, 12869 (2022).
Roizen, J. et al. High dose dietary vitamin D allocates surplus calories to muscle and growth instead of fat via modulation of myostatin and leptin signaling. Preprint at Res. Sq. https://doi.org/10.21203/rs.3.rs-4202165/v1 (2024).
Chellappa, K., Perron, I. J., Naidoo, N. & Baur, J. A. The leptin sensitizer celastrol reduces age-associated obesity and modulates behavioral rhythms. Aging Cell 18, e12874 (2019).
Zhang, L., Reed, F. & Herzog, H. Leptin signalling on arcuate NPY neurones controls adiposity independent of energy balance or diet composition. J. Neuroendocrinol. 32, e12898 (2020).
Ravussin, E. et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity 17, 1736–1743 (2009).
Roth, J. D. et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc. Natl Acad. Sci. USA 105, 7257–7262 (2008).
Ogier, V., Ziegler, O., Méjean, L., Nicolas, J. P. & Stricker-Krongrad, A. Obesity is associated with decreasing levels of the circulating soluble leptin receptor in humans. Int. J. Obes. Relat. Metab. Disord. 26, 496–503 (2002).
Herrick, J. E., Panza, G. S. & Gollie, J. M. Leptin, leptin soluble receptor, and the free leptin index following a diet and physical activity lifestyle intervention in obese males and females. J. Obes. 2016, 8375828 (2016).
Kratzsch, J. et al. Circulating soluble leptin receptor and free leptin index during childhood, puberty, and adolescence. J. Clin. Endocrinol. Metab. 87, 4587–4594 (2002).
Frias, J. P. et al. Efficacy and safety of co-administered once-weekly cagrilintide 2.4 mg with once-weekly semaglutide 2.4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet 402, 720–730 (2023).
Mietlicki-Baase, E. G. et al. Amylin receptor activation in the ventral tegmental area reduces motivated ingestive behavior. Neuropharmacology 123, 67–79 (2017).
Kern, K. A. & Mietlicki-Baase, E. G. Distributed amylin receptor signaling and its influence on motivated behavior. Physiol. Behav. 222, 112958 (2020).
Bhimani, R. V., Rzepecki, L., Park, J. & Mietlicki-Baase, E. G. Ventral tegmental area amylin receptor activation differentially modulates mesolimbic dopamine signaling in response to fat versus sugar. eNeuro 11, ENEURO.0133-24.2024 (2024).
Vestergaard, B., Baader-Pagler, T. & Griffin, J. 1661-P: petrelintide (ZP8396) selectively reduces intake of high-fat diet in DIO rats. Diabetes 73 (Suppl. 1), 1661–P (2024).
Gydesen, S. et al. A novel dual amylin and calcitonin receptor agonist, KBP-089, induces weight loss through a reduction in fat, but not lean mass, while improving food preference. Br. J. Pharmacol. 174, 591–602 (2017).
Smith, S. R. et al. Pramlintide treatment reduces 24-h caloric intake and meal sizes and improves control of eating in obese subjects: a 6-wk translational research study. Am. J. Physiol. Endocrinol. Metab. 293, E620–E627 (2007).
Lau, D. C. W. et al. Once-weekly cagrilintide for weight management in people with overweight and obesity: a multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet 398, 2160–2172 (2021).
Whiting, L., McCutcheon, J. E., Boyle, C. N., Roitman, M. F. & Lutz, T. A. The area postrema (AP) and the parabrachial nucleus (PBN) are important sites for salmon calcitonin (sCT) to decrease evoked phasic dopamine release in the nucleus accumbens (NAc). Physiol. Behav. 176, 9–16 (2017).
Kalafateli, A. L. et al. An amylin analogue attenuates alcohol-related behaviours in various animal models of alcohol use disorder. Neuropsychopharmacology 44, 1093–1102 (2019).
Aranäs, C. et al. Salmon calcitonin attenuates some behavioural responses to nicotine in male mice. Front. Pharmacol. 12, 685631 (2021).
Kalafateli, A. L., Vallöf, D. & Jerlhag, E. Activation of amylin receptors attenuates alcohol-mediated behaviours in rodents. Addict Biol. 24, 388–402 (2019).
Leighton, B. & Foot, E. The effects of amylin on carbohydrate metabolism in skeletal muscle in vitro and in vivo. Biochem. J. 269, 19–23 (1990).
Stephens, T. W., Heath, W. F. & Hermeling, R. N. Presence of liver CGRP/amylin receptors in only nonparenchymal cells and absence of direct regulation of rat liver glucose metabolism by CGRP/amylin. Diabetes 40, 395–400 (1991).
Pittner, R. A. Lack of effect of calcitonin gene-related peptide and amylin on major markers of glucose metabolism in hepatocytes. Eur. J. Pharmacol. 325, 189–197 (1997).
Leighton, B. & Cooper, G. J. Pancreatic amylin and calcitonin generelated peptide cause resistance to insulin in skeletal muscle in vitro. Nature 335, 632–635 (1988).
Molina, J. M., Cooper, G. J. S., Leighton, B. & Olefsky, J. M. Induction of insulin resistance in vivo by amylin and calcitonin gene-related peptide. Diabetes 39, 260–265 (1990).
Zierath, J. R. et al. Human islet amyloid polypeptide at pharmacological levels inhibits insulin and phorbol ester-stimulated glucose transport in in vitro incubated human muscle strips. Diabetologia 35, 26–31 (1992).
Castle, A. L., Kuo, C.-H., Han, D.-H. & Ivy, J. L. Amylin-mediated inhibition of insulin-stimulated glucose transport in skeletal muscle. Am. J. Physiol. 275, E531–E536 (1998).
Larsen, A. T., Mohamed, K. E., Melander, S. A., Karsdal, M. A. & Henriksen, K. The enduring metabolic improvement of combining dual amylin and calcitonin receptor agonist and semaglutide treatments in a rat model of obesity and diabetes. Am. J. Physiol. Endocrinol. Metab. 327, E145–E154 (2024).
Melander, S. A., Larsen, A. T., Karsdal, M. A. & Henriksen, K. Are insulin sensitizers the new strategy to treat type 1 diabetes? A long-acting dual amylin and calcitonin receptor agonist improves insulin-mediated glycaemic control and controls body weight. Br. J. Pharmacol. 181, 1829–1842 (2024).
Secher, A., Brand, C. L. & Raun, K. 763-P: CagriSema improves insulin sensitivity in diet-induced obese rats. Diabetes 73 (Suppl. 1), 763–P (2024).
Pullman, J., Darsow, T. & Frias, J. P. Pramlintide in the management of insulin-using patients with type 2 and type 1 diabetes. Vasc. Health. Risk Manag. 2, 203–212 (2006).
Paz-Filho, G., Mastronardi, C., Wong, M. L. & Licinio, J. Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian J. Endocrinol. Metab. 16, S549–S555 (2012).
Zimmet, P. Z. et al. Is there a relationship between leptin and insulin sensitivity independent of obesity? A population-based study in the Indian Ocean nation of Mauritius. Mauritius NCD Study Group. Int. J. Obes. Relat. Metab. Disord. 22, 171–177 (1998).
Bungau, S. et al. Interactions between leptin and insulin resistance in patients with prediabetes, with and without NAFLD. Exp. Ther. Med. 20, 197 (2020).
Morley, J. E., Flood, J. F., Horowitz, M., Morley, P. M. & Walter, M. J. Modulation of food intake by peripherally administered amylin. Am. J. Physiol. 267, R178–R184 (1994).
Wickbom, J., Herrington, M. K., Permert, J., Jansson, A. & Arnelo, U. Gastric emptying in response to IAPP and CCK in rats with subdiaphragmatic afferent vagotomy. Regul. Pept. 148, 21–25 (2008).
Lutz, T. A., Del Prete, E. & Scharrer, E. Subdiaphragmatic vagotomy does not influence the anorectic effect of amylin. Peptides 16, 457–462 (1995).
Coester, B., Foll, C. L. & Lutz, T. A. Viral depletion of calcitonin receptors in the area postrema: a proof-of-concept study. Physiol. Behav. 223, 112992 (2020).
Dacquin, R. et al. Amylin inhibits bone resorption while the calcitonin receptor controls bone formation in vivo. J. Cell Biol. 164, 509–514 (2004).
Young, A. Effects on bone. Adv. Pharmacol. 52, 269–280 (2005).
Cosman, F. et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 25, 2359–2381 (2014).
Stefanakis, K., Kokkorakis, M. & Mantzoros, C. S. The impact of weight loss on fat-free mass, muscle, bone and hematopoiesis health: implications for emerging pharmacotherapies aiming at fat reduction and lean mass preservation. Metabolism 161, 156057 (2024).
Cava, E., Yeat, N. C. & Mittendorfer, B. Preserving healthy muscle during weight loss. Adv. Nutr. 8, 511–519 (2017).
Chaston, T. B., Dixon, J. B. & O’Brien, P. E. Changes in fat-free mass during significant weight loss: a systematic review. Int. J. Obes. 31, 743–750 (2007).
Heymsfield, S. B., Gonzalez, M. C., Shen, W., Redman, L. & Thomas, D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes. Rev. 15, 310–321 (2014).
Dahl, K. et al. NN1213 - a potent, long-acting, and selective analog of human amylin. J. Med. Chem. 67, 11688–11700 (2024).
Boyle, C. N., Lutz, T. A. & Le Foll, C. Amylin - its role in the homeostatic and hedonic control of eating and recent developments of amylin analogs to treat obesity. Mol. Metab. 8, 203–210 (2018).
Briere, D. A. et al. 849-P: eloralintide (LY3841136). A selective amylin mometic, lowered body weight with improved quality of weight loss and GI tolerability in rats compared with cagrilintide. Diabetes 74 (Suppl. 1), 849–P (2025).
Roth, J. D. et al. Effects of prior or concurrent food restriction on amylin-induced changes in body weight and body composition in high-fat-fed female rats. Am. J. Physiol. Endocrinol. Metab. 293, R1112–R1117 (2007).
Cooper, M. E., McNally, P. G., Phillips, P. A. & Johnston, C. I. Amylin stimulates plasma renin concentration in humans. Hypertension 26, 460–464 (1995).
Herrmann, K., Zhou, M., Wang, A. & de Bruin, T. W. A. Cardiovascular safety assessment of pramlintide in type 2 diabetes: results from a pooled analysis of five clinical trials. Clin. Diabetes Endocrinol. 2, 12 (2016).
Vine, W., Smith, P., LaChappell, R., Blase, E. & Young, A. Effects of rat amylin on renal function in the rat. Horm. Metab. Res. 30, 518–522 (1998).
Liu, M. et al. Hyperamylinemia increases IL-1β synthesis in the heart via peroxidative sarcolemmal injury. Diabetes 65, 2772–2783 (2016).
Brie, A. D. et al. Atherosclerosis and insulin resistance: is there a link between them?. Biomedicines 13, 1291 (2025).
Jha, S. et al. pH dependence of amylin fibrillization. Biochemistry 53, 300–310 (2014).
Bower, R. L. & Hay, D. L. Amylin structure-function relationships and receptor pharmacology: implications for amylin mimetic drug development. Br. J. Pharmacol. 173, 1883–1898 (2016).
Colburn, W. A., Gottlieb, A. B., Koda, J. & Kolterman, O. G. Pharmacokinetics and pharmacodynamics of AC137 (25,28,29 tripro-amylin, human) after intravenous bolus and infusion doses in patients with insulin-dependent diabetes. J. Clin. Pharmacol. 36, 13–24 (1996).
Davies, M. J. et al. Cagrilintide-semaglutide in adults with overweight or obesity and type 2 diabetes. N. Engl. J. Med. 393, 648–659 (2025).
Bhattachar, S. N. et al. Eloralintide, a selective, long-acting amylin receptor agonist for obesity—phase 1 proof of concept. Diabetes Obes. Metab. https://doi.org/10.1111/dom.70439 (2026).
Griffin, J. et al. 1668-P: novel once-weekly amylin analog petrelintide (ZP8396) is well tolerated with improved GI tolerability after multiple dosing. Diabetes 73 (Suppl. 1), 1668–P (2024).
Edelman, S. et al. A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care 29, 2189–2195 (2006).
Briere, D. A. et al. Eloralintide (LY3841136), a novel amylin receptor agonist for the treatment of obesity: from discovery to clinical proof of concept. Mol. Metab. 102, 102271 (2025).
Olsen, M. B. et al. Safety, tolerability, and clinical effects of petrelintide: a long-acting amylin analog (P04.047). Obes. Facts 18, 391–392 (2025).
Russell, F. A., King, R., Smillie, S. J., Kodji, X. & Brain, S. D. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol. Rev. 94, 1099–1142 (2014).
Nishimatsu, H. et al. Role of endogenous adrenomedullin in the regulation of vascular tone and ischemic renal injury: studies on transgenic/knockout mice of adrenomedullin gene. Circ. Res. 90, 657–663 (2002).
Ghanizada, H. et al. Amylin analog pramlintide induces migraine-like attacks in patients. Ann. Neurol. 89, 1157–1171 (2021).
Dahl, K. et al. Amycretin, a novel, unimolecular GLP-1 and amylin receptor agonist administered subcutaneously: results from a phase 1b/2a randomised controlled study. Lancet 406, 149–162 (2025).
Tozzi, M. et al. GUBamy: a novel long-acting amylin analogue with significant therapeutic potential for mono- and combination therapy in obesity. Diabetologia 67, 1–593 (2024).
Hornigold, D. C. et al. AZD6234, a novel selective amylin receptor agonist (SARA), demonstrates a preferential profile over DACRA or GLP-1R agonist in rat models of aversion and fat-mass loss. Diabetologia 68, 1–754 (2025).
Ghosh, S., Valdecantos, P., Rada, P., Valverde, A. M. & Rondinone, C. M. 299-OR: discovery of long-acting unimolecular peptide tetra-agonists targeting GLP-1, GIP, amylin, and calcitonin receptors for enhanced metabolic benefits in animal models of obesity. Diabetes 73, 299-OR (2024).
Fang, X. et al. 2184-LB: novel oral small molecule ACCG-2671—a dual amylin and calcitonin receptor agonist development candidate for obesity therapy. Diabetes 74, 2184-LB (2025).
Volčanšek, S., Koceva, A., Jensterle, M., Janež, A. & Muzurović, E. Amylin: from mode of action to future clinical potential in diabetes and obesity. Diabetes Ther. 16, 1207–1227 (2025).
Mullard, A. Amylin takes another shot at the obesity prize. Nat. Rev. Drug Discov. 24, 403–406 (2025).
Zong, L. et al. Discovery of BGM1812, a novel dual amylin and calcitonin receptor agonist for obesity treatment. J. Med. Chem. 68, 14907–14918 (2025).
Larsen, A. T., Gydesen, S., Sonne, N., Karsdal, M. A. & Henriksen, K. The dual amylin and calcitonin receptor agonist KBP-089 and the GLP-1 receptor agonist liraglutide act complimentarily on body weight reduction and metabolic profile. BMC Endocr. Disord. 21, 10 (2021).
Larsen, A. T. et al. Dual amylin and calcitonin receptor agonist treatment improves insulin sensitivity and increases muscle-specific glucose uptake independent of weight loss. Biomed. Pharmacother. 164, 114969 (2023).
Sonne, N., Karsdal, M. A. & Henriksen, K. Mono and dual agonists of the amylin, calcitonin, and CGRP receptors and their potential in metabolic diseases. Mol. Metab. 46, 10110 (2021).
Acknowledgements
This paper was funded by Novo Nordisk A/S. T.A.L.’s research on amylin has been funded to a large extent by the Swiss National Science Foundation (current grant #320030_207763).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception, design, writing and critical revisions of the manuscript at all stages of development and approved the final paper to be submitted.
Corresponding author
Ethics declarations
Competing interests
A.S. and K.R. are employees of Novo Nordisk A/S, which is developing amylin-based drugs for the treatment of metabolic diseases. K.R. is listed as inventor on the patent covering CagriSema, a fixed-dose combination of an amylin analogue and a GLP1R analogue. T.A.L. has research collaborations with Novo Nordisk A/S, had a collaboration with Structure Therapeutics and has consulted for AbbVie, AstraZeneca, Eli Lilly, Roche and Zealand Pharma.
Peer review
Peer review information
Nature Metabolism thanks Kim Henriksen, Florin Despa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Secher, A., Lutz, T.A. & Raun, K. The story of amylin: from physiology to therapy. Nat Metab (2026). https://doi.org/10.1038/s42255-026-01465-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42255-026-01465-4