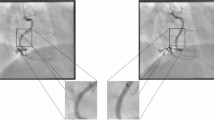
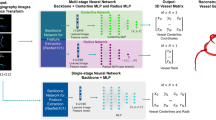
Abstract
Three-dimensional (3D) structural information of cardiac vessels is crucial for the diagnosis and treatment of cardiovascular disease. In clinical practice, interventionalists have to empirically infer 3D cardiovascular topology from multi-view X-ray angiography images, which is time-consuming and requires extensive experience. Owing to the dynamic nature of heartbeats and sparse-view observations in clinical practice, accurate and efficient reconstruction of 3D cardiovascular structures from X-ray angiography images remains challenging. Here we introduce AutoCAR, a fully automated transfer learning-based algorithm for dynamic 3D cardiovascular reconstruction. AutoCAR comprises three main components: pose domain adaptation, sparse backwards projection and vascular graph optimization. By merging the X-ray angiography imaging parameter statistics of over 1,000 clinical cases into synthetic data generation, and exploiting the intrinsic spatial sparsity of cardiac vessels for computational design, AutoCAR outperforms state-of-the-art methods in both qualitative and quantitative evaluations, enabling dynamic cardiovascular reconstruction in real-world clinical settings. We envision that AutoCAR will facilitate current diagnostic and intervention procedures and pave the way for real-time visual guidance and autonomous catheter navigation in cardiac intervention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated and analysed in this study, including the training dataset, synthetic and real-world evaluation dataset, pretrained weights and model predictions of existing method and proposed method results, are publicly available at under a CC BY-NC-ND 4.0 license at GitHub (https://github.com/zhuyinheng/autocar_release) and Zenodo50 (https://doi.org/10.5281/zenodo.15004536). Please follow the instructions in either public repository for data preparation, reproduction (including training or inference with the provided weights), and for inspecting and comparing the predictions of the proposed method, existing methods and ground truth.
Code availability
The source code is publicly available under a CC BY-NC-ND 4.0 license at GitHub (https://github.com/zhuyinheng/autocar_release) and Zenodo50 (https://doi.org/10.5281/zenodo.15004536). Please follow the instructions in either public repository for data preparation, reproduction (including training or inference with the provided weights), and for inspecting and comparing the predictions of the proposed method, existing methods and ground truth.
References
Mézquita, A. J. V. et al. Clinical quantitative coronary artery stenosis and coronary atherosclerosis imaging: a consensus statement from the quantitative cardiovascular imaging study group. Nat. Rev. Cardiol. 20, 696–714 (2023).
Mahmoud, K. D. & Zijlstra, F. Thrombus aspiration in acute myocardial infarction. Nat. Rev. Cardiol. 13, 418–428 (2016).
Çimen, S., Gooya, A., Grass, M. & Frangi, A. F. Reconstruction of coronary arteries from X-ray angiography: a review. Med. Image Anal. 32, 46–68 (2016).
Zhao, H. et al. Self-supervised learning enables 3D digital subtraction angiography reconstruction from ultra-sparse 2D projection views: a multicenter study. Cell Rep. Med. 3, 100775 (2022).
Feldkamp, L. A., Davis, L. C. & Kress, J. W. Practical cone-beam algorithm. J. Opt. Soc. Am. A 1, 612 (1984).
Neubauer, A. M. et al. Clinical feasibility of a fully automated 3D reconstruction of rotational coronary X-ray angiograms. Circ. Cardiovasc. Interv. 3, 71–79 (2010).
Liu, J. et al. 5D respiratory motion model based image reconstruction algorithm for 4D cone-beam computed tomography. Inverse Probl. 31, 115007 (2015).
Blondel, C., Malandain, G., Vaillant, R. & Ayache, N. Reconstruction of coronary arteries from a single rotational X-ray projection sequence. IEEE Trans. Med. Imaging 25, 653–663 (2006).
Unberath, M., Taubmann, O., Hell, M., Achenbach, S. & Maier, A. Symmetry, outliers, and geodesics in coronary artery centerline reconstruction from rotational angiography. Med. Phys. 44, 5672–5685 (2017).
Banerjee, A. et al. Point-cloud method for automated 3D coronary tree reconstruction from multiple non-simultaneous angiographic projections. IEEE Trans. Med. Imaging 39, 1278–1290 (2020).
Cong, W. et al. Quantitative analysis of deformable model-based 3-D reconstruction of coronary artery from multiple angiograms. IEEE Trans. Biomed. Eng. 62, 2079–2090 (2015).
Yang, J. et al. External force back-projective composition and globally deformable optimization for 3-D coronary artery reconstruction. Phys. Med. Biol. 59, 975–1003 (2014).
Liao, R., Luc, D., Sun, Y. & Kirchberg, K. 3-D reconstruction of the coronary artery tree from multiple views of a rotational X-ray angiography. Int. J. Cardiovasc. Imaging 26, 733–749 (2010).
Zheng, S., Meiying, T. & Jian, S. Sequential reconstruction of vessel skeletons from X-ray coronary angiographic sequences. Comput. Med. Imaging Graph. 34, 333–345 (2010).
Yang, J., Wang, Y., Liu, Y., Tang, S. & Chen, W. Novel approach for 3-D reconstruction of coronary arteries from two uncalibrated angiographic images. IEEE Trans. Image Process. 18, 1563–1572 (2009).
Cong, W., Yang, J., Liu, Y. & Wang, Y. Energy back-projective composition for 3-D coronary artery reconstruction. In Annual International Conference of the IEEE Engineering in Medicine and Biology Society 5151–5154 (IEEE, 2013).
Galassi, F. et al. 3D reconstruction of coronary arteries from 2D angiographic projections using non-uniform rational basis splines (NURBS) for accurate modelling of coronary stenoses. PLoS ONE 13, e0190650 (2018).
Hwang, M. et al. A simple method for automatic 3D reconstruction of coronary arteries from X-ray angiography. Front. Physiol. 12, 724216 (2021).
Bransby, K. M. et al. 3D coronary vessel reconstruction from bi-plane angiography using graph convolutional networks. In IEEE International Symposium on Biomedical Imaging 1–5 (IEEE, 2023).
Wang, G., Ye, J. C. & De Man, B. Deep learning for tomographic image reconstruction. Nat. Mach. Intell. 2, 737–748 (2020).
Iyer, K., Nallamothu, B. K., Figueroa, C. A. & Nadakuditi, R. R. A multi-stage neural network approach for coronary 3D reconstruction from uncalibrated X-ray angiography images. Sci. Rep. 13, 17603 (2023).
Liang, D. & Chen, S. Weakly-supervised 3D coronary artery reconstruction from two-view angiographic images. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-3703340/v1 (2023).
Gao, C. et al. Synthetic data accelerates the development of generalizable learning-based algorithms for X-ray image analysis. Nat. Mach. Intell. 5, 294–308 (2023).
Ying, X. et al. X2CT-GAN: reconstructing CT from biplanar X-rays with generative adversarial networks. In Proc. IEEE/CVF Conference on Computer Vision and Pattern Recognition 10619–10628 (IEEE, 2019).
Kasten, Y., Doktofsky, D. & Kovler, I. End-to-end convolutional neural network for 3D reconstruction of knee bones from bi-planar X-ray images. In International Workshop on Machine Learning for Medical Image Reconstruction 123–133 (Springer, 2020).
Kini, A. & Sharma, S. K. (eds) Practical Manual of Interventional Cardiology (Springer, 2021).
Graham, B., Engelcke, M. & Van Der Maaten, L. 3D semantic segmentation with submanifold sparse convolutional networks. In Proc. IEEE/CVF Conference on Computer Vision and Pattern Recognition 9224–9232 (IEEE, 2018).
Shen, L., Zhao, W. & Xing, L. Patient-specific reconstruction of volumetric computed tomography images from a single projection view via deep learning. Nat. Biomed. Eng. 3, 880–888 (2019).
Li, M., Yang, H. & Kudo, H. An accurate iterative reconstruction algorithm for sparse objects: application to 3D blood vessel reconstruction from a limited number of projections. Phys. Med. Biol. 47, 2599 (2002).
Hansis, E., Schafer, D., Dossel, O. & Grass, M. Evaluation of iterative sparse object reconstruction from few projections for 3-D rotational coronary angiography. IEEE Trans. Med. Imaging 27, 1548–1555 (2008).
Jandt, U., Schäfer, D., Grass, M. & Rasche, V. Automatic generation of 3D coronary artery centerlines using rotational X-ray angiography. Med. Image Anal. 13, 846–858 (2009).
Unberath, M. et al. Respiratory motion compensation in rotational angiography: graphical model-based optimization of auto-focus measures. In IEEE International Symposium on Biomedical Imaging 227–230 (IEEE, 2017).
Antiga, L., Ene-Iordache, B. & Remuzzi, A. Computational geometry for patient-specific reconstruction and meshing of blood vessels from MR and CT angiography. IEEE Trans. Med. Imaging 22, 674–684 (2003).
Zheng, J.-Q., Zhou, X.-Y., Riga, C. & Yang, G.-Z. Towards 3D path planning from a single 2D fluoroscopic image for robot assisted fenestrated endovascular aortic repair. In International Conference on Robotics and Automation 8747–8753 (IEEE, 2019).
Sumner, R. W., Schmid, J. & Pauly, M. Embedded deformation for shape manipulation. ACM Trans. Graph. 26, 80 (2007).
Habert, S., Habert, S., Dahdah, N. & Cheriet, F. A novel method for an automatic 3D reconstruction of coronary arteries from angiographic images. In International Conference on Information Science, Signal Processing and their Applications 484–489 (IEEE, 2012).
Shit, S. et al. clDICE—a novel topology-preserving loss function for tubular structure segmentation. In Proc. IEEE/CVF Conference on Computer Vision and Pattern Recognition 16560–16569 (IEEE, 2021).
Royer-Rivard, R., Girard, F., Dahdah, N. & Cheriet, F. End-to-end deep learning model for cardiac cycle synchronization from multi-view angiographic sequences. In Annual International Conference of the IEEE Engineering in Medicine and Biology Society 1190–1193 (IEEE, 2020).
Vlontzos, A. & Mikolajczyk, K. Deep segmentation and registration in X-ray angiography video. In British Machine Vision Conference 267–278 (BMVA, 2018).
Schonberger, J. L. & Frahm, J.-M. Structure-from-motion revisited. In Proc. IEEE/CVF Conference on Computer Vision and Pattern Recognition 4104–4113 (IEEE, 2016).
Yu, T. et al. DoubleFusion: real-time capture of human performances with inner body shapes from a single depth sensor. IEEE Trans. Pattern Anal. Mach. Intell. 42, 2523–2539 (2019).
Rohkohl, C., Lauritsch, G., Keil, A. & Hornegger, J. CAVAREV—an open platform for evaluating 3D and 4D cardiac vasculature reconstruction. Phys. Med. Biol. 55, 2905 (2010).
Zeng, A. et al. ImageCAS: a large-scale dataset and benchmark for coronary artery segmentation based on computed tomography angiography images. Comput. Med. Imaging Graph. 109, 102287 (2023).
Antiga, L., Ene-Iordache, B., Remuzzi, G. & Remuzzi, A. Automatic generation of glomerular capillary topological organization. Microvasc. Res. 62, 346–354 (2001).
Hartley, R. & Zisserman, A. Multiple View Geometry in Computer Vision (Cambridge Univ. Press, 2003).
Ravi, N. et al. Accelerating 3D deep learning with PyTorch3D. Preprint at https://arxiv.org/abs/2007.08501 (2020).
Riba, E., Mishkin, D., Ponsa, D., Rublee, E. & Bradski, G. Kornia: an open source differentiable computer vision library for PyTorch. In Proc. IEEE/CVF Winter Conference on Applications of Computer Vision 3674–3683 (IEEE, 2020).
Newell, A., Yang, K. & Deng, J. Stacked hourglass networks for human pose estimation. In European Conference on Computer Vision 483–499 (2016).
Gwak, J., Choy, C. & Savarese, S. Generative sparse detection networks for 3D single-shot object detection. In European Conference on Computer Vision 297–313 (2020).
Zhu, Y. et al. Sparse and transferable 3D dynamic vascular reconstruction for instantaneous diagnosis. Zenodo https://doi.org/10.5281/zenodo.15004536 (2025).
Acknowledgements
We thank Y. Yang and Z. Guo for XA pre-processing advice; K. Hu for advices in visualization; Y. Zheng for clinical advice; and H. Xu, Z. Zhang and L. Yang for discussion. This work was supported by National Natural Science Foundation of China (grant number 32371470,82341019, S.M.), National Key Research and Development Program of China (2024YFA0919800, S.M.), Department of Science and Technology of Guangdong Province (grant number 2023B0909020003, S.M.), and Cross-disciplinary Research and Innovation Fund of Tsinghua SIGS (grant number JC2022007, S.M.).
Author information
Authors and Affiliations
Contributions
S.M. and Y.Z. conceived of the project. Y.Z. designed the methodology of the reconstruction algorithms with suggestions from F.L. Y.Z. conducted the main experiments and developed the software. Y.Z. and C.D. performed data curation. Y.Z., H.L. and Y.W. conducted the validation experiments. Y.Z., S.M. and Y.W. prepared the figures and visualization, and wrote the paper with input from all authors. The whole project was supervised by S.M.
Corresponding authors
Ethics declarations
Competing interests
Y.Z., S.M. and F.L. have applied for a patent related to the work reported in this paper. The other authors declare no competing interests.
Peer review
Peer review information
Nature Machine Intelligence thanks Haoran Dou, Jan Egger, Alejandro Frangi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The training pipeline of AutoCAR.
Synthetic XA images are generated by forward-projecting the 3D coronary surface mesh from the CCTA dataset with given imaging parameters. The projected results are naturally vascular mask images and processed by the SBP module. Specifically, two mask images are backward-projected into 3D space, fused into one 3D volume, and processed by the sparse backbone network to output per-voxel vascular occupancy and centerness. During the inference process, only the part in the dashed box (that is, the SBP module) needs to be performed.
Extended Data Fig. 2 The inference pipeline of AutoCAR.
AutoCAR directly receives two image sequences captured from different views as input, and gives the 3D reconstruction result in a fully-automated manner.
Extended Data Fig. 3 Weakly synchronization property and infeasibility of 3D ground truths.
a, Scanning protocol and exemplar input. The rows correspond to different viewing poses, while the first column indicates the C-Arm rotation state and the exemplar frames in X-ray angiography are shown starting from the second column. Due to the deformation of cardiovasculature structures, instead of using a continuous circular scanning approach common in head and neck angiography, the protocol recommends capturing a video from a fixed viewpoint before transitioning to the next, yielding multiple multi-view image sequences. b, Weak synchronization issues. Even with ECG for synchronization, and with patients holding their breath, which is often challenging for emergencies, with elderly/young patients or heart rate variability, temporal misalignment persist. c, Challenges in paired multi-view 2D X-ray angiography and 3D ground truth. Each dot in view time space represents an image at a unique pose and time, and a sequence forms a scanning pattern (Pattern I, red segments). For 3D reconstruction of blue-dot image, simultaneous multi-view images (Pattern II) are essential. However, capturing images within 1/6 of a cardiac cycle would require the C-arm to rotate 1080 deg/s and record at 900 fps (Pattern III), far exceeding current systems like Siemens Axiom Artis at 60 fps.
Extended Data Fig. 4 AutoCAR reconstruction of 3D Models and 2D Correspondences Across 21 Cases.
For each case, the top section displays the 3D visualization, while the left and right images provide views from two distinct perspectives. The areas highlighted by the red circle denotes suspicious regions, where clinicians may wish to investigate the 3D shapes or their corresponding 2D regions from a different view angle. The colour coding is used to indicate correspondence among 3D and multi-views for each individual cases.
Extended Data Fig. 5 Illustration of imaging parameters in DICOM.
a, Primary angle α is defined as the rotation angle with respect to craniocaudal axis. Positive direction is defined at the patient left hand side. b, Secondary angle β is defined as the rotation angle with respect to secondary axis, which is in the patient plane (horizontal) and is perpendicular to the craniocaudal axis at the isocentre. Cranial direction is regarded as positive direction. c, Pixel spacing μ is defined as the physical length of each pixel. d, Source intensifier distance (SID) is defined as the distance between intensifier (detector) and X-ray source. Source object distance (SOD) is defined as the distance between isocentre and X-ray source. The above definitions are consistent with DICOM standard (C.8.7.5.1.2, Section 10.7.1.1).
Extended Data Fig. 6 Pairwise scatterplot of imaging parameter distributions.
α, β, μ denote primary angle, secondary angle and pixel spacing, respectively. All statistics are summarized from the imaging parameters of 1215 real-world XA cases.
Supplementary information
Supplementary Information
Supplementary Notes 1–8, Figs. 1–12 and Tables 1–9.
Source data
42256_2025_1025_MOESM3_ESM.csv
Source Data Fig. 1. Statistical source data. Fig. 1a, left, and Extended Data Fig. 6 share the same source data because Extended Data Fig. 6 is the detailed pair-wise scatter plot while Fig. 1a, left, is the simplified (due to the space limit) marginal plot (2-joint plot). Source Data Extended Data Fig. 6. Statistical source data, Fig. 1a, left, and Extended Data Fig. 6 share the same source data because Extended Data Fig. 6 is the detailed pair-wise scatter plot while the Fig. 1a, left, is the simplified (due to the space limit) marginal plot (2-joint plot).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Y., Wang, Y., Di, C. et al. Sparse and transferable three-dimensional dynamic vascular reconstruction for instantaneous diagnosis. Nat Mach Intell 7, 730–742 (2025). https://doi.org/10.1038/s42256-025-01025-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s42256-025-01025-7