Abstract
Multiplexed protein imaging offers valuable insights into interactions between tumours and their surrounding tumour microenvironment, but its widespread use is limited by cost, time and tissue availability. Here we present HistoPlexer, a deep learning framework that generates spatially resolved protein multiplexes directly from standard haematoxylin and eosin (H&E) histopathology images. HistoPlexer jointly predicts multiple tumour and immune markers using a conditional generative adversarial architecture with custom loss functions designed to ensure pixel- and embedding-level similarity while mitigating slice-to-slice variations. A comprehensive evaluation of metastatic melanoma samples demonstrates that HistoPlexer-generated protein maps closely resemble real maps, as validated by expert assessment. They preserve crucial biological relationships by capturing spatial co-localization patterns among proteins. The spatial distribution of immune infiltration from HistoPlexer-generated protein multiplex enables stratification of tumours into immune subtypes. In an independent cohort, integration of HistoPlexer-derived features into predictive models enhances performance in survival prediction and immune subtype classification compared to models using H&E features alone. To assess broader applicability, we benchmarked HistoPlexer on publicly available pixel-aligned datasets from different cancer types. In all settings, HistoPlexer consistently outperformed baseline methods, demonstrating robustness across diverse tissue types and imaging conditions. By enabling whole-slide protein multiplex generation from routine H&E images, HistoPlexer offers a cost- and time-efficient approach to tumour microenvironment characterization with strong potential to advance precision oncology.
Similar content being viewed by others
Main
Tumours are complex systems that acquire hallmark traits by establishing a supportive tumour microenvironment (TME) that facilitates tumorigenesis and metastasis1,2. Understanding cancer cell interactions with surrounding tissue provides critical insights into disease progression and therapeutic response3,4,5. Multiplexed immunohistochemistry and immunofluorescence (mIHC/IF) technologies, such as imaging mass cytometry (IMC), enable spatially resolved quantification of up to 40 protein markers, offering comprehensive visualization of tumour–TME interactions4,6,7. These technologies facilitate analysis of spatial cell distribution, phenotype co-localization and cellular interactions, which are valuable for clinical decision-making4,5,8,9. However, IMC is limited by low throughput, high cost and coverage restricted to small regions of interest (ROIs), hindering its broader clinical adoption.
In contrast, haematoxylin and eosin (H&E) staining remains the gold standard for cancer diagnosis in clinical practice due to its low cost, high throughput and entire tissue section coverage. H&E images reveal morphological features crucial for cancer grading, proliferation assessment and staging10. Recent deep learning advances have enabled the prediction of protein markers from H&E images, such as pan-cytokeratin for pancreatic cancer11, HER2 for breast cancer12 and Ki-67 for neuroendocrine and breast cancers13. One study predicted two markers, specifically one tumour and one immune marker14. Only a few studies have attempted to predict a set of protein markers, with a focus solely on either tumour15,16 or immune markers17, limiting their utility for investigation of tumour–TME interactions. These studies either use separate models per marker15,17 or lack quantitative validation of the advantages of multiplexed prediction with a single model14,16,17.
To address these limitations, we introduce HistoPlexer, a deep learning model that generates comprehensive protein multiplexes from H&E images. HistoPlexer simultaneously predicts 11 markers consisting of both tumour and immune markers, enabling an integrative visualization of tumour–host interactions. We train HistoPlexer on metastatic samples from the Tumor Profiler Study (TuPro)18 using paired H&E and IMC images from serial sections. Through rigorous quantitative evaluation, we demonstrate the importance of simultaneous marker prediction through improved model performance and enhanced spatial co-localization of markers. We validate the biological relevance of generated IMC images through cell typing and immune phenotyping, particularly in characterizing immune-hot (inflamed) and immune-cold (excluded/desert) tumours based on CD8+ T cell distributions. We demonstrate the generalizability of the trained HistoPlexer model on an independent patient cohort from the human skin cutaneous melanoma (SKCM) study of The Cancer Genome Atlas (TCGA) project19. Experiments on two public multiplexed datasets14,20 further validate its adaptability across various cancer types.
Our results demonstrate that HistoPlexer generates high-quality IMC images that closely align with real data distributions. These multiplexes enable precise immune phenotyping through spatial analysis of tumour–immune cell interactions, particularly in distinguishing immune-hot and -cold subtypes. Simultaneous marker prediction preserves biologically meaningful relationships. Furthermore, by augmenting H&E whole-slide images (WSIs) with generated IMC multiplex, HistoPlexer improves both survival and immune subtype prediction on the TCGA–SKCM dataset, indicating its potential for enhancing clinical decision-making.
Results
Histopathology-based protein multiplex generation with HistoPlexer
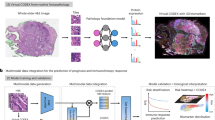
HistoPlexer is a generative model based on a conditional generative adversarial network (cGAN) that predicts spatially resolved profiles of multiple proteins from input H&E images. The model is trained on paired H&E and multiplexed IMC images (Fig. 1a) extracted from aligned H&E and IMC ROIs. During training, the H&E images are input to the translator G, which learns to generate protein multiplexes (that is, IMC images) based on the tissue morphology from high-resolution H&E images. The discriminator D receives the generated IMC and input H&E images and scores their similarity to ground-truth (GT) IMC images (Fig. 1b (i)). The translator and discriminator are trained adversarially using a least-squares GAN loss, such that the generated IMC images can deceive the discriminator into classifying them as real. We incorporate two additional losses to ensure both pixel-level and patch-level consistency between the generated and GT IMC images. The pixel-level consistency loss calculates the L1 distance between the generated and GT IMC images. However, because the H&E and GT IMC images are obtained from serial sections of the tissue block, a degree of spatial displacement in tissue organization exists between consecutive slices (termed slice-to-slice variation). Although aligned at the structural level via template matching, consecutive slides from real-world diagnostic material are not pixel-level aligned. To account for these differences, we adopt the Gaussian pyramid loss12, which relaxes the alignment constraint by evaluating the similarity between the generated and GT IMC images at multiple scales (Fig. 1b (ii)). For patch-level consistency, we utilize a patch-wise contrastive loss to ensure that corresponding patches in the generated and GT IMC images are closer in the embedding space than distant ones (Fig. 1b (iii)). We also incorporate adaptive weights for different patches based on their proximity to GT, as described in ref. 21.
a, The HistoPlexer consists of a translator G that takes H&E and IMC images as input and predicts protein multiplexes from morphology information encoded in the H&E images, ultimately generating protein multiplex on the WSI level from H&E input. b, The objective functions of HistoPlexer contain the GAN adversarial loss (i), Gaussian pyramid loss with average L1 distance across scales (ii), and patch-wise contrastive loss with anchor from generated IMC and positive and negative from GT IMC (iii).
We built the HistoPlexer framework using a multimodal metastatic melanoma dataset generated by TuPro18. Each patient was characterized by multiple modalities, including H&E and IMC images. ROIs of 1 mm2 were selected on each H&E WSI based on visual inspection by a pathology expert, and IMC data were generated for those ROIs on a consecutive section of the same tumour block. Using template matching22, we created a paired dataset of 336 H&E and IMC ROIs from 78 patients. We focused on predicting 11 protein markers that are essential for characterizing the tumour and its surrounding TME. These included tumour markers (MelanA, S100, gp100, SOX10), immune markers (CD3, CD8a, CD20, CD16, CD31) and antigen-presentation markers (HLA-ABC, HLA-DR).
HistoPlexer generates accurate and realistic protein multiplexes
We compared HistoPlexer with Pix2pix23 and PyramidP2P12 in two settings: multiplex (MP), where a single model predicts all markers simultaneously, and singleplex (SP), where separate models predict each marker individually, with predictions stacked to form a pseudomultiplexed output. All models were trained on 231 ROIs and tested on 105 ROIs.
We assessed generated IMC image quality using the multiscale structural similarity index (MS-SSIM)24 for perceptual similarity across multiple scales, root mean squared error using sliding window (RMSE-SW)25 for pixel-level differences and peak signal-to-noise ratio (PSNR)26 for signal fidelity. HistoPlexer in the MP setting achieves the highest MS-SSIM and PSNR and lowest RMSE-SW (Fig. 2a), indicating greater similarity to GT IMC images generated from consecutive tissue sections. The MP setting consistently outperforms SP across all methods, suggesting simultaneous marker prediction captures intermarker correlations. For completeness, we included a comparison with CycleGAN27 (Extended Data Table 1; statistical test results shown in Extended Data Fig. 1) and reported the performance of individual markers for the HistoPlexer-MP model (Extended Data Table 2). Moreover, HistoPlexer surpasses baseline methods on pixel-aligned DeepLIIF datasets14,20, as shown in Extended Data Tables 3 and 4, with details in Supplementary Information.
a, Quantitative assessment and comparison against benchmarks using MS-SSIM, PSNR and RMSE-SW for MP and SP settings. The up arrow indicates higher values are better. The down arrow indicates lower values are better. The best results are highlighted in bold. b, Qualitative assessment of HistoPlexer for three ROIs ((i)–(iii)) with H&E as input to HistoPlexer, and expression profiles of individual markers: MelanA, CD3, CD8a, CD20, SOX10 and CD16. Top rows: GT expression profiles from IMC modality; bottom rows: predicted (Pred) expression profiles from HistoPlexer. Scale bars, 100 μm.
We qualitatively evaluated the generated IMC images by comparing them with the GT (Fig. 2b and Extended Data Fig. 2) and observed strong alignment in global patterns. However, pixel-level correspondence was not expected due to the inherent slice-to-slice variations. In some instances, we observed confusion between CD20 and the CD3/CD8a markers. For instance, in Fig. 2b (ii), CD20 is overexpressed, whereas CD3 and CD8a are underexpressed. This may result from the visually similar morphology of B and T cells in H&E images, leading to confusion between their markers (CD20 for B cells and CD3/CD8a for T cells)28.
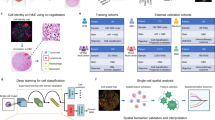
To evaluate the perceived realism of generated IMC images, we used the Human Eye Perceptual Evaluation (HYPE) framework29, in which experts assess real versus generated IMC images for specific markers shown alongside corresponding H&E images. We construct two evaluation sets: tumour-associated markers (MelanA, S100, gp100, SOX10) and lymphocyte markers (CD20, CD3, CD8a). For each set, two experts independently reviewed 250 image pairs, evenly split between real and generated, sampled from test ROIs and augmented through small translations and rotations. The evaluation yielded mean HYPE scores of 41.8% (±0.3%) for lymphocyte markers and 42.8% (±0.6%) for tumour markers. The generated images achieved HYPE scores of 61.6% (±1.3%) and 72.8% (±1.1%), indicating that the majority (>50%) were perceived as real by domain experts.
Next, we went beyond pixel-level evaluation by identifying relevant cell types. We used GT cell-type annotations from the GT IMC training set, following ref. 8, and trained a random forest classifier30 based on average marker expression per cell to classify them into five classes: tumour cells, B cells, CD8+ T cells, CD4+ T cells and others. This classifier was then applied to both GT and generated IMC images in the test set to obtain cell-type maps (Fig. 3a). We visualized ROIs from the tumour centre and the tumour front at the tumour–TME interface and examined spatial patterns based on immune subtype labels. We observed that immune ‘hot’ tumours, characterized by high immune cell infiltration, showed strong interactions between tumour and CD8+ T cells (Fig. 3a (i)), whereas immune ‘cold’ tumours, with low immune presence, displayed minimal immune cell interaction, especially in the tumour centre (Fig. 3a (ii)). Immune ‘cold’ ROIs at the tumour front likewise exhibited sparse or clustered immune cells with minimal interaction with tumour cells (Fig. 3a (iii)–(v)). The strong alignment between predicted and GT cell-type maps suggests that HistoPlexer effectively captures morphological features in H&E images that are relevant for predicting cell types.
a, Cell-typing results: ROIs from the tumour centre ((i) and (ii)) and tumour front ((iii)–(v)), showing H&E, GT and predicted cell types in ROIs grouped by their location within the tissue: tumour centre and tumour front. Scale bars, 100 μm. b, Spearman’s correlation coefficients between protein pairs, comparing the GT with both SP and MP predictions of HistoPlexer (i). The pairs on the x axis are ordered by increasing Spearman’s correlation in the GT. MSE between the GT and predicted Spearman’s correlation coefficients, comparing the SP and MP predictions of HistoPlexer (ii). Bars represent mean values, and error bars indicate standard deviation (s.d.). c, Joint t-SNE visualization of protein co-localization patterns for selected markers: CD3, CD8a, CD31, gp100 and MelanA. The colour represents normalized protein expression.
HistoPlexer preserves spatial co-localization patterns
Given the importance of spatial patterns, as highlighted by refs. 31,32, we assess spatial co-localization by quantifying the correlations between protein markers that are simultaneously expressed within a given region. For each protein pair, we compute the Spearman’s correlation coefficient (SCC) between the two proteins and average it across ROIs, considering only those pairs with strong positive (>0.15) or strong negative (<−0.15) correlation in GT IMC images. We then compare the SCC between GT and generated IMC multiplexes.
The MP model’s predictions match GT SCC better than the SP model, especially for sparse CD-based immune marker pairs like CD16:HLA-DR, CD3:HLA-ABC and CD16:CD8a, which are underrepresented in the training data (Fig. 3b (i)). We hypothesize that sparse markers lack sufficient tissue context for the SP model to generate accurate predictions. In contrast, the MP model benefits from learning intermarker correlations by predicting all markers simultaneously and leveraging auxiliary morphological information from abundant markers. However, for some protein pairs (for example, CD3:CD8a and CD20:CD3), the SCC in MP exceeds that of the GT. This likely stems from similar morphologies of CD8+ T cells, CD3 T cells and B cells (CD20) in H&E images28, potentially leading to overprediction of these sparse markers and overestimated co-localization patterns. Additionally, we compute the mean square error (MSE) between SCC values of GT and generated IMC across test ROIs (Fig. 3b (ii)). The MP model achieves an MSE approximately an order of magnitude lower than that of the SP model, supporting our hypothesis. Comparisons with Pix2Pix23 and PyramidP2P12 are in Extended Data Fig. 3a. See Supplementary Information for biological implications of observed patterns.
To explore spatial patterns beyond protein pairs, we visualized expression profiles using t-distributed stochastic neighbour embedding (t-SNE) of cells from both GT and generated IMC multiplexes, following ref. 33. We observed a strong correspondence between t-SNE embeddings from both GT and generated IMC multiplexes (Fig. 3c). For instance, cells that are positive for CD3 and CD8a are concurrently negative for CD31, gp100 and MelanA. This aligns with biological expectations, as CD3 and CD8a are expressed on T cells but not on endothelial (CD31) or tumour (gp100, MelanA) cells. Full t-SNE plots for all markers are provided in Extended Data Fig. 3b.
In conclusion, our quantitative and qualitative results show that the spatial co-localization patterns in GT can be effectively replicated using the generated IMC images. These patterns remain consistent across tissue sections, providing a robust evaluation metric that mitigates slice-to-slice variations.
HistoPlexer enables multiplexed proteomics profiling on the WSI level
HistoPlexer enables the generation of IMC images from H&E WSIs of up to 100,000 × 100,000 pixels, allowing for the simultaneous visualization of multiple protein markers across entire tissue sections. This capability offers a comprehensive view of tumour and TME interactions at the WSI level. Because GT IMC data are available only for ROIs, we use Ultivue’s InSituPlex technology to obtain multiplexed WSIs using the Immuno8 and MDSC FixVue panels. These panels include key markers, such as SOX10 for tumours, HLA-DR for antigen presentation and CD3/CD8a for T cell profiling, which are shared with the generated protein multiplex. Figure 4 provides a qualitative comparison between the generated IMC and Ultivue multiplex at the WSI level. In both cases, a high concordance in global structures and hotspot regions is observed across all markers. In Fig. 4b, whereas there is good alignment for CD3 and SOX10 markers, differences are observed for CD8A and HLA-DR, particularly at the tissue edges (for example, the bottom-left border). These differences are likely due to slice-to-slice variations between H&E and Ultivue images, which lead to slight shifts in tissue boundaries.
a,b, H&E staining (first column; a and b) and expression profiles of individual markers (CD3, SOX10, CD8a and HLA-DR, from second to last column; a and b). Top rows: GT expression profiles from Ultivue images; bottom rows: Pred expression profiles at the WSI level for samples in a and b. Scale bars, 1 mm.
HistoPlexer facilitates immune phenotyping
We demonstrated the utility of HistoPlexer for immune phenotyping by stratifying immune subtypes based on the spatial distribution of CD8+ T cells, using only H&E images from TuPro metastatic melanoma samples. As illustrated in Fig. 5a, we visualized predicted tumour cells and CD8+ T cells across H&E WSIs. In immune-hot cases, characterized by substantial CD8+ T cell infiltration and typically associated with better response to immunotherapy34,35, we observed both tumour (attacker) cells and infiltrating CD8+ (defender) T cells within the tumour region, suggesting an active immune response. In contrast, immune-cold cases exhibit minimal or no CD8+ T cell infiltration in the tumour area, correlating with poor immunotherapy outcomes. Building on the immune subtype classification framework proposed in ref. 5, we further quantified intratumoral (iCD8) and stromal (sCD8) CD8+ T cell densities within the tumour centre compartment. To do this, we first localized CD8+ T cells using HistoPlexer and then annotated and segmented the tumour centre into intratumoral and stromal regions using the HALOAI platform across 34 TuPro melanoma samples. Figure 5b (i) shows immune subtype stratification using iCD8 and sCD8 densities (measured per μm2). We observe that immune desert cases exhibit very low iCD8 and sCD8 density, indicating the presence of only rare or isolated CD8+ T cells. Immune excluded cases also show very low iCD8 density but slightly higher sCD8 density compared to immune desert cases, suggesting some CD8+ T cells have reached the stroma but not the intratumoral regions. Inflamed cases display high densities of both iCD8 and sCD8, indicating the presence of CD8+ T cells in the stromal compartment and, most importantly, their infiltration into intratumoral regions. These trends are consistent with prior findings5, validating the utility of our approach. To assess the clinical relevance, we analysed the distribution of iCD8 and sCD8 densities across immune-hot (inflamed) versus immune-cold (excluded and desert) cases (Fig. 5b (ii)). As expected, immune-hot cases exhibit substantially higher densities of both iCD8 and sCD8 compared to immune-cold cases. Furthermore, a random forest classifier trained to distinguish between immune-hot and immune-cold cases achieves an F1-score of 0.873 (s.d. 0.006) and a macro-average area under the receiver operating characteristic curve of 0.845 (s.d. 0.047) under 5-fold cross-validation. In conclusion, we demonstrate that HistoPlexer can accurately support immune phenotyping directly from H&E images. This capability offers potential utility in treatment decision-making and patient stratification. For clinical implications of immune phenotyping, refer to Supplementary Information.
a, Four samples from TuPro metastatic melanoma cohort with two immune-hot cases in the top row and two immune-cold samples in the bottom row. For each sample, H&E image on the left along with overlay of predicted tumour and CD8+ T cells within tumour centre region using HistoPlexer model on the right. Heterogeneity average (HTA) index for quantifying the spatial heterogeneity or interaction between tumour and CD8⁺ T cells is indicated for each sample. Scale bars, 1 mm. b, Box plot of iCD8 and sCD8 CD8+ T cell densities in tumour centre compartment, stratified by immune desert, excluded and inflamed classes (i). Box plot of iCD8 and sCD8 CD8+ T cell densities in tumour centre compartment, stratified by immune-hot and -cold classes (ii). Box plots show the median (centre line), the 25th and 75th percentiles (box limits), and whiskers extending to the most extreme data points within 1.5× the interquartile range from the box limits. Points beyond the whiskers represent outliers.
HistoPlexer generalizes to an independent patient cohort
We evaluated the generalizability of the HistoPlexer on independent test data from the TCGA–SKCM cohort19. Figure 6a shows the generated protein multiplex at the WSI level, along with expression profiles for three markers: tumour-associated MelanA, T cell marker CD3 and B cell marker CD20. In the immune-high sample, we observe higher expression and tumour infiltration of CD3 and CD20 markers, contrasting with the minimal or absent expression in the immune-low case, where immune labels are based on bulk RNA sequencing data36.
a, Two examples (immune-high and -low) from the TCGA–SKCM cohort, showing H&E images, predicted protein multiplexes and expression profiles of MelanA, CD3 and CD20 markers. Scale bars, 2 mm (top) and 2.5 mm (bottom). b, Model architecture for multimodal survival and immune subtype prediction. c, Survival prediction results (i), displaying time-dependent C-index scores (left) and Kaplan–Meier survival curves for all test patients aggregated across five cross-validation folds for the multimodal setting, with separation of low- and high-risk groups (right). Immune subtype prediction results (ii), showing the weighted F1-score (left) and confusion matrix (right) for classification into low, intermediate and high immune subtypes. The confusion matrix corresponds to the fold with the highest weighted F1-score. For bar plots, bars represent mean values and error bars indicate s.d. Cls, classifier head; F, residual neural network feature extractor ResNet18; HX, H&E IMC features; HŶ, predicted IMC features.
To explore the clinical utility of the generated IMC images, we assessed their value in augmenting outcome prediction tasks using the MelanA, CD3 and CD20 expression profiles, which are known to have prognostic relevance37,38. We extracted features from both the H&E and generated IMC WSIs using pretrained encoders and input them to an attention-based multiple instance learning (MIL) predictor39. We trained the MIL predictor under two settings: (1) the unimodal setting, where only H&E features were input to the predictor and (2) the multimodal setting, where features extracted from the corresponding H&E and predicted IMC patches were first aggregated via a co-attention layer40, and the bag-level representations of H&E and predicted IMC WSIs after the MIL pooling layer were concatenated before being fed into the classification head (Fig. 6).
We benchmark the model on two clinically relevant tasks: immune subtype classification and survival prediction. For the survival task, we use disease-specific survival from patient metadata, which offers a more accurate representation of the patient’s disease status41. For immune subtyping, we classify patients into low, intermediate and high immune categories based on bulk RNA sequencing–derived labels36. Overall, the multimodal setting consistently outperforms the unimodal configuration for both tasks. For survival prediction, incorporating features from generated IMC images yields a 3.18% improvement in average time-dependent C-index42 across 5-fold cross-validation. Kaplan–Meier curves generated from the multimodal model show clear stratification of patients into low- and high-risk groups based on predicted risk scores (definition provided in the section ‘MIL-based clinical outcome prediction’), with statistical significance confirmed by a log-rank test (P = 5.05 × 10−7). For immune subtype classification, combining features from H&E and generated IMC boosted the average weighted F1-score by 17.02% over 5-fold cross-validation. In summary, these findings highlight both the generalizability of HistoPlexer to external cohorts and the clinical utility of the generated protein expression maps in supporting downstream predictive modelling and decision support.
Discussion
In this study, we introduced HistoPlexer, a generative model for predicting high-order (11-channel) multiplexed protein expression profiles, including both tumour and immune markers, directly from H&E images. By simultaneously predicting markers, HistoPlexer effectively captured sparse markers and preserved biologically meaningful relationships, as validated through spatial correlation analysis of protein co-localization patterns. Our comprehensive evaluation showed consistent outperformance of the MP approach over SP alternatives, with higher MS-SSIM and PSNR and lower RMSE-SW and MSE values. Domain experts found the generated IMC images realistic, with HYPE error rates of 61.6% and 72.8% for lymphocyte and tumour markers. To assess the broader applicability of HistoPlexer across different cancer types, we benchmarked it on the DeepLIIF datasets14,20, which provide pixel-aligned H&E and multiplexed images for lung and head and neck cancers. In both settings, HistoPlexer consistently outperformed baseline methods, indicating robustness across diverse tissue types and imaging conditions (Extended Data Tables 3 and 4).
We further explored integrating features from the UNI computational pathology foundation model43 to enhance the performance of HistoPlexer, marking one of the first attempts to apply pathology foundation models to generative tasks. Surprisingly, end-to-end training of HistoPlexer without UNI-derived features resulted in lower MSE and better preserved biological relationships (Supplementary Information and Extended Data Table 1). This aligns with recent findings44, highlighting the importance of task-specific training for complex biological tasks. Foundation models, typically designed as encoder-only architectures, excel in discriminative tasks like classification and regression by focusing on global high-level features. However, they are less suited for generative tasks that require detailed spatial relationships and fine-grained image details essential for accurate image generation.
We demonstrated clinical utility through two applications. First, HistoPlexer enabled immune phenotyping at the WSI level by quantifying CD8+ T cell infiltration patterns (iCD8, sCD8) in tumour centres. These patterns aligned with a state-of-the-art approach5 and effectively stratified patients into immune-hot and -cold subtypes, relevant for immunotherapy decisions. Second, we showed generalizability to an independent cohort (TCGA–SKCM) where HistoPlexer-generated features, when used alongside H&E features, improved predictive performance in survival analysis (3.18% increase in time-dependent C-index) and immune subtype classification (17.02% improvement in weighted F1-score). These results highlight the potential of HistoPlexer to augment clinical decision-making.
Our study has some limitations. First, in some cases, the model confused CD3/CD8a T cell and CD20 B cell markers, which share morphological similarities. Although this did not substantially impact downstream tasks, it is a priority for future work to refine the model’s ability to accurately distinguish between finer cell types. Second, our focus was on major immune and tumour cell types, such as CD8+ T cells, CD4+ T cells, B cells and tumour cells. This set could be further extended to include more sparse cell types, such as endothelial cells, by obtaining a larger training cohort. Third, for the multimodal learning experiments on TCGA–SKCM, we limited marker selection to MelanA, CD3 and CD20 based on clinical relevance. Extending the marker panel could further improve downstream applications. Finally, due to inherent slice-to-slice variations in the data, we prioritized downstream task performance over strict pixel-level accuracy.
Our work opens several promising research directions. First, expanding HistoPlexer to additional protein markers and cancer types presents an opportunity to uncover deeper insights into disease mechanisms without requiring additional tissue or increasing the cost. Achieving broader generalization, including the development of a universal model across cancer types, remains a key challenge that will require access to larger, more diverse and harmonized datasets. Our current results serve as a proof of concept and provide a foundation for such future efforts. Second, by releasing the Ultivue InSituPlex dataset generated in this study, we encourage researchers to explore new diffusion models for multiplexed protein marker generation, particularly those that account for slice-to-slice variations. Finally, integrating predicted protein expression maps with other modalities could deepen our understanding of tumour biology and improve patient stratification.
In summary, HistoPlexer advances computational pathology by enabling efficient and cost-effective generation of multiplexed protein profiles from standard histology slides. Our findings demonstrate its potential for clinical integration and its utility in enhancing cancer diagnostics and personalized treatment strategies.
Methods
Datasets and preprocessing
Tumour Profiler dataset
We built our HistoPlexer framework using a subset of highly multimodal metastatic melanoma dataset generated by TuPro18. Each patient was characterized using multiple technologies, including Digital Pathology and IMC. A total of six ROIs of 1 mm2 were selected on each H&E WSI, three within the tumour centre and three at the tumour front (intersection of tumour and TME). IMC data were generated for those six ROIs on a consecutive section of the same tumour block. The IMC data were generated at a resolution of 1 μm per pixel, and H&E images were scanned at a resolution of 0.25 μm per pixel. Therefore, ROIs of 1 mm2 were represented by 1,000 pixels for IMC data and 4,000 pixels for H&E images. Because the paired data were generated by visually choosing ROIs, in many cases a considerable positional shift and rotation between the specified H&E regions and the resulting IMC regions can be observed. This was overcome by using template matching45. Specifically, for the IMC modality, we utilized the tumour channel marker SOX10, which provided valuable structural information of the tissue. For the H&E modality, we focused on the H channel, which captures the haematoxylin staining essential for visualizing cellular structures. Given that H&E and IMC represent different modalities, we employed mutual information as a metric for template matching. This approach allowed us to quantify the alignment between the two datasets, ensuring a robust pairing process. This resulted in a paired dataset of 336 H&E and IMC ROIs from 78 patients for training and testing model performance.
IMC profiling was performed using a panel of 40 antibodies, of which 11 were selected for this study based on the biological function of the corresponding proteins as well as high SNR. The proteins targeted by the 11 antibodies include cell-type markers, such as tumour markers (MelanA, gp100, S100, SOX10), lymphocyte markers (CD20, CD16, CD3, CD8a) and an endothelial marker (CD31). Moreover, two functional markers corresponding to proteins involved in antigen presentation (HLA-ABC, HLA-DR) were included in the protein set.
The raw IMC images were processed with CellProfiler software for cell segmentation46. The protein counts extracted from the images were first clipped to 99.9% per protein to exclude outliers and then transformed using the arcsinh-function with cofactor one47. To exclude background noise, we applied Otsu thresholding48 with kernel size three and sigma three and the threshold, separating signal from background, determined per sample using all available ROIs. The resulting data per protein was first centred and standardized and then subjected to min–max transformation, all using data statistics based on the train set only.
The data were split at the patient level into train and test sets, stratified by immune phenotype (inflamed, immune excluded and immune desert). The stratification ensured the representation of both tumour and immune cells in each set. The patient-level splitting guaranteed that all ROIs from a given patient belonged to only one set, preventing undesired information flow. The resulting train and test sets consisted of 231 and 105 ROIs, respectively. During model training, ROIs were chosen at random, and a tile of size 1,024 × 1,024 from the H&E image and a corresponding IMC region of 256 × 256 was extracted.
For WSI inference, we developed a modular pipeline that processes WSIs in a tile-based approach to create spatially resolved protein multiplexes. The process begins with tissue segmentation, which identifies stained tissue areas and removes the background using Otsu thresholding48. Each segmented tissue region is then divided into 1,024 × 1,024 pixel tiles. To optimize performance, we employ a dataloader that batches and parallelizes the processing of these tiles instead of processing them sequentially. We also utilize the Shapely library49 for geometric operations, such as creating polygons for tissue masks, scaling them to match the WSI resolution and efficiently determining overlaps between tiles and tissue regions. This approach reduces computational load by concentrating only on relevant areas of the WSI and avoiding background processing. Each tile may undergo optional stain normalization using the Macenko method50 to reduce staining variability and ensure colour consistency across images. The tiles are then processed through the trained HistoPlexer model to predict protein multiplexes. After prediction, the generated tiles are stitched together to reconstruct the full WSI at multiple resolutions. We also generate quality-control images to visualize the tiling and segmentation results, and we save the coordinates of the tiles for reproducibility. For visualization, we apply min–max normalization using the min–max values from the training set for all markers.
Ultivue dataset
For qualitative evaluation of HistoPlexer on WSIs, we employed Ultivue InSituPlex technology to obtain multiplexed images using the Immuno8 and MDSC FixVue panels. The Immuno8 panel focuses on immune landscape characterization with markers such as CD3, CD4, CD8, CD68, PD-1, PD-L1, FoxP3 and PanCK/SOX10. The MDSC panel identifies myeloid-derived suppressor cells using markers CD11b, CD14, CD15 and HLA-DR. Ultivue images were acquired at a resolution of 0.325 μm per pixel. For evaluation, we used CD3, SOX10, CD8a and HLA-DR markers to assess visual similarity between the generated protein multiplex and Ultivue images.
Paired H&E and Ultivue WSIs were generated by first staining H&E on one tissue section, followed by acquiring Immuno8 and MDSC data on consecutive sections for ten samples. A tonsil tissue was included with each sample as a positive control. Image registration between H&E and Ultivue WSIs was performed using an unsupervised multimodal method51, leveraging the DAPI nuclear stain in Ultivue for alignment with H&E images. Both Ultivue and generated IMC images underwent min–max normalization and histogram equalization. Additionally, adaptive thresholding was applied to Ultivue images to reduce noise and extract true signals. Regions with false signals, particularly those corresponding to haemorrhage, bleeding or erythrocytes in H&E, were manually annotated and excluded from the analysis. The dataset, including H&E and Ultivue images, alignment matrices, and annotated excluded regions, is publicly available and can be accessed on Hugging Face (https://huggingface.co/datasets/CTPLab-DBE-UniBas/HistoPlexer-Ultivue). The dataset could serve as a valuable baseline for the field.
TCGA–SKCM
Diagnostic WSIs of SKCM were downloaded from the TCGA database (https://portal.gdc.cancer.gov/) for a total of 472 cases. Clinical data of SKCM samples including age, gender, sample type (primary tumour/metastatic) and disease-specific survival were also downloaded. For the survival prediction, we discarded cases where the diagnostic WSIs were of low resolution or the disease-specific survival data were missing, leaving 360 cases in total. For the immune subtype prediction, we kept a total of 257 cases where immune subtype labels were available. For each task, we randomly split the cases stratified by age, gender and sample type to create 5-fold cross-validation with a 4:1 ratio of training-validation sets.
HistoPlexer architecture
The HistoPlexer is based on cGAN, which takes an H&E image as input condition and generates multiplexed IMC images where each corresponds to a spatially resolved protein expression profile. The translator of the HistoPlexer is a fully convolutional U-Net52 consisting of an encoder and a decoder. The encoder comprises six downsampling blocks, each with a convolution layer of stride 2 and kernel size 3. The decoder comprises five upsampling blocks, each with nearest-neighbour interpolation, followed by a convolution layer of stride 1 and kernel size 3. Each layer is followed by a batch-norm layer and ReLU activation. The discriminator consists of six blocks, each with a convolution layer followed by a spectral normalization layer and ReLU activation. We used patches extracted from template-matched pairs of H&E and IMC ROIs to train the HistoPlexer and optimize the model with three objectives: an adversarial loss to enforce image-level consistency, a Gaussian pyramid loss to enforce pixel-level consistency and a patch-wise contrastive loss to enforce patch-level consistency.
Adversarial loss
We used the least-squares loss proposed in LSGAN53 as our adversarial loss and the binary coding scheme, where 0 and 1 are the labels for generated (that is, fake) and real IMC images, respectively. We also adopt the multiscale gradient approach54, which allows simultaneous gradient propagation at multiple scales (that is, resolutions). Considering a set of scales {s ∈ S}, the multiscale adversarial losses for the translator G and discriminator D are formulated as
where Xp = {xp ∈ XROI} and Yp = {yp ∈ YROI} denote paired training patches sampled from template-matched H&E and IMC ROIs, respectively; G(s)(⋅) and D(⋅) denote the mapping functions parameterized by the translator (at the output scale s) and discriminator, respectively; and ∣⋅∣ denotes the cardinality of a set.
Gaussian pyramid loss
We also implement a pixel-level L1 loss as in ref. 23. Because our H&E and GT IMC images were not pixel-aligned, we relaxed the constraint on pixel-to-pixel correspondence by calculating the L1 loss at multiresolution representations of the generated and GT IMC images12, termed Gaussian pyramid loss12. More specifically, a Gaussian pyramid was constructed through iterative Gaussian smoothing and downsampling. Each level of resolution, termed an octave, comprised a series of images with increasing degrees of smoothness. Transition between resolutions was achieved by downsampling the image at the highest smoothness level of the current octave to initiate the next:
where #gs denotes the number of Gaussian smoothings at one resolution. Note that for the generated IMC images, we only computed the Gaussian pyramid on the final output scale. Considering a set of resolutions {r ∈ R}, the Gaussian pyramid loss is a weighted sum of L1 loss computed on the primary layer of each octave, formulated as
where \({\hat{{y}}}_{{\rm{p}}}\) denotes the generated IMC image patches, wr denotes the resolution level and wr is the weight of the L1 loss at that level.
Patch-wise contrastive loss
We further incorporated a patch-wise contrastive loss, inspired by ref. 21. More specifically, we first extracted multilayer features using a pretrained feature encoder and applied a transformation via a small projection head (for example, a multilayer perceptron) on the extracted features to enrich their expressiveness55. Then, we randomly selected a set of pixel locations for each feature layer. By aggregating selected patch features from each layer, we could obtain two feature sets for the generated and GT IMC images, respectively.
Let \({\hat{\bf{z}}}_{l}^{\;i}\) denote the anchor feature of the ith patch of the generated IMC image, extracted from the lth layer of the feature encoder; \({\bf{z}}_{l}^{i}\) and \({\bar{\bf{z}}}_{l}^{i}\) denote the positive and negative features of the corresponding patch (that is, at the same pixel location) and the collection of non-corresponding patches (that is, at different pixel locations), extracted from the same layer, respectively. Our patch-wise contrastive loss is defined as
where
is the InfoNCE objective56 and
is the adaptive patch weight21. Here, #layer and #patch denote the number of layers and patches from which we extract features, t and T denote the current and total training steps, h( ⋅ ) denotes some weighting function and sim( ⋅ ) is some similarity measurement.
Although the HistoPlexer translator outputs the prediction of all selected IMC markers, we encountered a practical limitation when employing a pretrained feature encoder, which often requires an RGB image as input. To circumvent this, we first extracted each channel (that is, marker) of the output IMC image and replicated it along the channel dimension to create a pseudo-RGB image. We then passed each of them to the feature encoder. The final patch-wise contrastive loss is the sum of that of each channel.
The total losses for G and D are formulated as
where
is the gradient penalty57 and λgp, contrast and \({\lambda }_{{R}_{1}}\) are the weights for the Gaussian pyramid loss, patch-wise contrastive loss and gradient penalty, respectively.
Implementation and training details
The model was trained for 100 epochs using the ADAM optimizer58 with momentum parameters β1 = 0.5 and β2 = 0.999 with learning rates 0.004 and 0.0008 for translator and discriminator networks, respectively. The weights were initialized using Xavier initialization. The batch size was set to 16 and the patch size to 256 for IMC and 1,024 for H&E images, to accommodate for the higher resolution of the latter. We increased the generalization capabilities of the model by adopting data augmentation, including colour augmentation, random flipping, small translations and rotations. We employed the least-squares GAN objective. The weights for loss terms were as follows: λgp = 5.0, λcontrast = 1.0 and \({\lambda }_{{R}_{1}}\) = 1.0. For model inference and evaluation, we used the latest checkpoint at the end of model training.
Evaluation metrics
To evaluate the quality of generated images, we used three widely adopted metrics: PSNR, MS-SSIM and RMSE-SW.
PSNR was used to measure the reconstruction quality by quantifying the ratio between the maximum possible signal power and the power of corrupting noise. It is expressed in decibels, with higher values indicating better image quality. The PSNR is calculated as
where L is the dynamic range of the pixel values (for example, 255 for 8-bit images) and MSE represents the MSE between the original image I and the generated image I′
MS-SSIM extends the traditional SSIM metric by incorporating multiple scales to capture structural similarity at various resolutions. The SSIM between two images I and I′ is defined as
where μI and \({\mu }_{{I}^{{\prime} }}\) are the means, \({\sigma }_{I}^{2}\) and \({\sigma }_{{I}^{{\prime} }}^{2}\) are the variances, and \({\sigma }_{I{I}^{{\prime} }}\) is the covariance between the two images. C1 and C2 are small constants to stabilize the division. In MS-SSIM, structural similarity is computed at multiple scales, and the final score is a weighted product of SSIM values across these scales:
where M is the number of scales and αj is weighting factor at scale j. Higher MS-SSIM values indicate better perceptual similarity.
RMSE-SW is an image quality metric used to assess the similarity between the original image I and a generated image I′. Unlike traditional RMSE, which computes the error over the entire image globally, RMSE-SW calculates the error within localized regions by moving a fixed-size window across the image. The RMSE-SW between two images I and I′ is defined as
where w × h represents the size of the moving window across the image with a defined stride, resulting in N windows. For each window, we computed the MSE between the corresponding pixel values of I and I′.
These metrics provide a comprehensive assessment of both pixel-level accuracy and perceptual similarity of the generated images. Frechet inception distance and kernel inception distance are widely used metrics for evaluating the quality of generated images; however, they are less effective on small datasets as they rely on the mean and covariance of a cohort. Hence they were not used when evaluating HistoPlexer.
To quantify the evaluation by domain experts, we used HYPE score, which measures the error rate at which humans mistake generated images for real ones or vice versa. It is defined as
where TP is the number of true positives, TN is the number of true negatives, FP is the number of false positives, and FN is the number of false negatives. HYPEfake and HYPEreal are the error rates for generated and real images, respectively.
HistoPlexer for cell-level analysis
Pseudo-cells
Because spatial analyses of IMC data typically rely on cell-level readouts, we created pseudo-single-cell data by extracting circular regions of 10 μm diameter around nuclei coordinates for both input H&E and GT IMC images. Protein expression was averaged across pixels within each pseudo-cell for individual markers. Nuclei coordinates for H&E images were obtained using the HoVer-Net model28, and nuclei coordinates and cell-type labels for GT IMC multiplexes were are derived using Ilastik59 and CellProfiler46, as described in ref. 8. For simplicity, we refer to pseudo-cells as ‛cells’ in the following text.
Cell typing
We used a random forest classifier30 to categorize cells based on the average expression of 11 markers from the HistoPlexer. The classifier distinguished between tumour cells, B cells, CD8+ T cells, CD4+ T cells and other cells. Training was performed using the scikit-learn library60, with hyperparameters (100 base estimators, maximum tree depth of 30) selected based on the lowest out-of-bag error. The model achieved a macro-averaged F1-score of 0.81 on an internal test set. We then applied the trained RF classifier to both GT and generated protein expression data to produce cell-type maps for cells in the test set.
t-SNE on cell-level marker expression
To explore spatial patterns beyond pairwise protein interactions, we conducted a low-dimensional embedding analysis of cell-level marker expression. Following the approach commonly used for mass cytometry data61, we subsampled 1,000 cells per ROI from both GT and generated IMCs, resulting in total 2,000 cells per ROI. A joint t-SNE dimensionality reduction (two dimensions, perplexity of 50 and 1,000 iterations) was then applied. For visualization, protein abundance was scaled and clipped at the 99th percentile, and the t-SNE plots were coloured according to the scaled protein expression61.
Annotations for immune phenotyping
To stratify samples into immune subtypes based on the spatial distribution of CD8+ T cells, we used annotated regions as established in ref. 5. Our dataset included 109 metastatic melanoma H&E WSIs from the TuPro cohort, with metastatic sites in lymph nodes, soft tissue, brain and other distant locations. The primary region for immune-subtyping, termed the ‛tumour centre’, comprised entirely tumour tissue, which was manually defined as a continuous tumour mass excluding a 500 μm margin from the tumour–non-tumour boundary. This ‛tumour centre’ was further segmented into two regions: the ‛intratumoral tumour’ region, consisting of dense clusters of malignant melanocytes without stromal presence, and the ‛intratumoral stromal’ region, which includes extracellular matrix (typically desmoplastic) interwoven within the tumour cell mass but free from malignant melanocytes. These regions were automatically classified using a deep learning model implemented on the HALOAI platform, trained with selected H&E WSI regions. Tissue classification was conducted at 0.30-μm-per-pixel resolution with a minimum object size threshold of 50 μm2. Excluded regions—such as preexisting lymphatic tissue, large adipose and muscle regions, artifacts, necrosis, haemorrhage and background—were omitted from the analysis. Ultimately, we analysed 34 samples with the highest-quality tissue classifications from the HALOAI model predictions. Extended Data Fig. 4 shows an example H&E WSI with region annotation and classification.
MIL-based clinical outcome prediction
Attention-based MIL for survival and immune subtype prediction
MIL is a weakly supervised learning method for set-based data structures. In MIL, an input X is a bag (that is, permutation-invariant set) of instances X = {x1, …, xN}, where N denotes the number of instances in the bag. Given a classification task with K classes, the goal is to learn a function \({\mathcal{F}}\) from M training pairs \({\{({X}^{(m)},{{y}}^{(m)})\}}_{m = 1}^{M}\) that maps X to a bag-level label y ∈ K without knowing label yi ∈ K for each instance in the bag. In our context, the input is a WSI, and the instances denote the extracted patches. More specifically, we follow the embedding-based MIL approach39 and extract a feature vector \({{\bf{h}}}_{i}=h({{x}}_{i})\in {{\mathbb{R}}}^{d}\) from each patch. Then, an attention-pooling operator aggregates the patch features hi=1:N to a single WSI-level representation39
where
is the gated attention39. Here, \({\bf{w}}\in {{\mathbb{R}}}^{L},\,{V}\in {{\mathbb{R}}}^{L\times D},\,{U}\in {{\mathbb{R}}}^{L\times D}\) are learnable parameters with hidden dimension L, ⊙ is element-wise multiplication, and η( ⋅ ) denotes the sigmoid function. Finally, a classifier f( ⋅ ) maps the WSI-level representation to a WSI-level label \(\hat{{y}}\in K\).
The end-to-end prediction takes the following general form:
For survival prediction, we modelled the time-to-event distributions as an ordinal regression task with right censored data (that is, patient death is unobserved until last known follow-up). Following ref. 40, we defined discrete time intervals and modelled each interval using an independent neuron in the output layer. More specifically, we partitioned the continuous time scale into non-overlapping time intervals [tj−1, tj), j ∈ [1, … , J] based on the quartiles of survival time values, denoted as yj. The continuous time-to-event t(m) for each patient was then replaced by a discrete time label \({{y}}_{j}^{(m)}\), where
The problem then simplified to classification, where each patient defined by a triplet \(({{\bf{g}}}^{(m)},{{y}}_{j}^{(m)},{c}^{(m)})\). Here, g is the aggregated bag features; c is the censorship status, where c = 0 if the death of the patient is observed and c = 1 otherwise; and yj is the discrete time GT label. We adopt the negative log-likelihood survival loss62 for modal optimization, formulated as
where \({f}_{{\rm{hazard}}}({{y}}_{j}| {\bf{g}})=\,{\rm{Sigmoid}}({\hat{{y}}}_{j})\) is the discrete hazard function and \({f}_{{\rm{surv}}}({{y}}_{j}| {\bf{g}})=\mathop{\prod }\nolimits_{k = 1}^{j}\left(1-{f}_{{\rm{hazard}}}({{y}}_{k}| {\bf{g}})\right)\) is the discrete survival function. Finally, the patient-level risk was defined as the negative sum of all logits41, which enables the identification of distinct risk groups and the stratification of patients.
For immune subtype prediction, we adopted the cross-entropy loss defined as
Multimodal fusion via co-attention mechanism
To fuse the patch features from different modalities, we adopted the co-attention mechanism proposed in ref. 40. More specifically, given the H&E feature bag \({H}\in {{\mathbb{R}}}^{N\times d}\) and IMC feature bag \({P}\in {{\mathbb{R}}}^{N\times d}\), we guided the feature aggregation of H using P by calculating the cross-attention
where \({{{W}}}_{q},{{{W}}}_{k},{{{W}}}_{v}\in {{\mathbb{R}}}^{d\times d}\) are learnable weights and \({{{A}}}_{P\to H}\in {{\mathbb{R}}}^{N\times N}\) is the co-attention matrix. Intuitively, the co-attention measures the pairwise similarity for how much an H&E instance hi attends to the IMC instance pi for i ∈ N. Similarly, we can guide the feature aggregation of P using H via AH→P. Each co-attention guided feature bag was input to an attention-based MIL module, which outputted an aggregated WSI-level representation. We concatenated the WSI-level representations from multiple modalities and projected them back to the original feature dimension d via a linear layer, resulting in a multimodal WSI-level representation. Then, a classifier f(⋅) used this representation to predict the output label \(\hat{{y}}\).
Implementation and training details
We adopted the original implementation of attention-based MIL39 on GitHub at https://github.com/AMLab-Amsterdam/AttentionDeepMIL. We implemented the co-attention mechanism based on the original implementation of MCAT40 (https://github.com/mahmoodlab/MCAT). Each WSI was cropped to 256 × 256 non-overlapping patches at ×20 magnification to create bags, where patches with more than 10% non-tissue area were discarded. We used ResNet18 (ref. 63) pretrained on pathology-specific datasets using self-supervised learning64 to extract features from H&E patches and ResNet50 pretrained on ImageNet65 to extract features from IMC patches. Because ResNet18 requires three-channel input, we concatenated IMC images of three different protein markers along the channel dimension: one tumour marker (MelanA) and two immune markers (CD8 and CD20). The dimension of extracted features was 512 for both H&E and IMC patches. We ran the survival and immune subtype prediction for 5-fold cross-validation. The model hyperparameters were set as follows: Adam optimizer with initial learning rate of 1e−4 (survival) and 5e−5 (immune subtype), a ReduceLROnPlateau scheme based on validation loss for scheduling and a mini-batch size of 1. The model was trained for 100 epochs with early stopping based on validation loss (survival) and weighted F1-score (immune subtype). The data processing and model training were done on an NVIDIA A100 40GB GPU. The deep learning models were trained using PyTorch (v.1.13.1), and the pipeline was implemented in Python (v.3.8.12).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The multiplexed WSI images for the Immuno8 and MDSC FixVue panels, generated using Ultivue InSituPlex technology, along with paired H&E images, are publicly available on Hugging Face at https://huggingface.co/datasets/CTPLab-DBE-UniBas/HistoPlexer-Ultivue. The H&E WSIs for TCGA–SKCM were obtained from the GDC Data Portal: https://portal.gdc.cancer.gov/. The Tumor Profiler Study data used for model training contain sensitive clinical information and are subject to ethical and privacy restrictions, preventing unrestricted public release. The data are accessible upon request through the Tumor Profiler Consortium’s portal at https://tumorprofilercenter.ch/contacts. Requests must include a brief scientific proposal outlining the intended use. The Consortium reviews requests typically within four to six weeks and determines the scope, duration and conditions of data access. Approved users are required to comply with data use agreements that restrict data use to specified research purposes and prohibit further sharing without authorization. Source data are provided with this paper.
Code availability
The source code and trained model weights for HistoPlexer are available at https://github.com/ratschlab/HistoPlexer and on Zenodo at https://doi.org/10.5281/zenodo.15110117 (ref. 66).
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Egeblad, M., Nakasone, E. S. & Werb, Z. Tumors as organs: complex tissues that interface with the entire organism. Dev. Cell 18, 884–901 (2010).
Jackson, H. W. et al. The single-cell pathology landscape of breast cancer. Nature 578, 615–620 (2020).
Sobottka, B. et al. Establishing standardized immune phenotyping of metastatic melanoma by digital pathology. Lab. Investig. 101, 1561–1570 (2021).
Ptacek, J. et al. Multiplexed ion beam imaging (mibi) for characterization of the tumor microenvironment across tumor types. Lab. Investig. 100, 1111–1123 (2020).
Tan, W. C. C. et al. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. 40, 135–153 (2020).
Windhager, J. et al. An end-to-end workflow for multiplexed image processing and analysis. Nat. Protoc. 18, 3565–3613 (2023).
Jin, M.-Z. & Jin, W.-L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 5, 166 (2020).
Fischer, A. H., Jacobson, K. A., Rose, J. & Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 4986 (2008).
Burlingame, E. A. et al. Shift: speedy histological-to-immunofluorescent translation of a tumor signature enabled by deep learning. Sci. Rep. 10, 17507 (2020).
Liu, S. et al. BCI: breast cancer immunohistochemical image generation through pyramid pix2pix. In Proc. IEEE/CVF Conference on Computer Vision and Pattern Recognition 1815–1824 (IEEE, 2022).
Liu, S. et al. Unpaired stain transfer using pathology-consistent constrained generative adversarial networks. IEEE Trans. Med. imaging 40, 1977–1989 (2021).
Ghahremani, P. et al. An AI-ready multiplex staining dataset for reproducible and accurate characterization of tumor immune microenvironment. In Proc. International Conference on Medical Image Computing and Computer-Assisted Intervention (eds Greenspan, H. et al.) 704–713 (Springer, 2023).
Pati, P. et al. Accelerating histopathology workflows with generative AI-based virtually multiplexed tumour profiling. Nat. Mach. Intell. 6, 1–17 (2024).
Zhang, R. et al. Mvfstain: multiple virtual functional stain histopathology images generation based on specific domain mapping. Med. Image Anal. 80, 102520 (2022).
Zhou, Z. et al. Virtual multiplexed immunofluorescence staining from non-antibody-stained fluorescence imaging for gastric cancer prognosis. eBioMedicine 107, 105287 (2024).
Irmisch, A. et al. The tumor profiler study: integrated, multi-omic, functional tumor profiling for clinical decision support. Cancer Cell 39, 288–293 (2021).
Guan, J., Gupta, R. & Filipp, F. V. Cancer systems biology of tcga skcm: efficient detection of genomic drivers in melanoma. Sci. Rep. 5, 7857 (2015).
Ghahremani, P. et al. Deep learning-inferred multiplex immunofluorescence for immunohistochemical image quantification. Nat. Mach. Intell. 4, 401–412 (2022).
Li, F., Hu, Z., Chen, W. & Kak, A. Adaptive supervised PatchNCE loss for learning H&E-to-IHC stain translation with inconsistent groundtruth image pairs. In Proc. Medical Image Computing and Computer Assisted Intervention – MICCAI 2023 (eds Greenspan, H. et al.) 632–641 (Springer, 2023).
Culjak, I., Abram, D., Pribanic, T., Dzapo, H. & Cifrek, M. A brief introduction to OpenCV. In Proc. 35th International Convention MIPRO 1725–1730 (IEEE, 2012).
Isola, P., Zhu, J.-Y., Zhou, T. & Efros, A. A. Image-to-image translation with conditional adversarial networks. In Proc. IEEE Conference on Computer Vision and Pattern Recognition 1125–1134 (IEEE, 2017).
Wang, Z., Simoncelli, E. P. & Bovik, A. C. Multiscale structural similarity for image quality assessment. In Proc. 37th Asilomar Conference on Signals, Systems & Computers (ed. Matthews, M. B.) 1398–1402 (IEEE, 2003).
Detlefsen, N. S. et al. Torchmetrics-measuring reproducibility in pytorch. J. Open Source Softw. 7, 4101 (2022).
Jain, A. K. Fundamentals of Digital Image Processing (Prentice-Hall, 1989).
Zhu, J.-Y., Park, T., Isola, P. & Efros, A. A. Unpaired image-to-image translation using cycle-consistent adversarial networks. In Proc. IEEE International Conference on Computer Vision 2223–2232 (IEEE, 2017).
Graham, S. et al. Hover-net: simultaneous segmentation and classification of nuclei in multi-tissue histology images. Med. Image Anal. 58, 101563 (2019).
Zhou, S. et al. Hype: a benchmark for human eye perceptual evaluation of generative models. In Proc. 33rd Conference on Neural Information Processing Systems (eds Wallach, H. et al.) 3449–3461 (IEEE, 2019).
Breiman, L. Random forests. Mach. Learn. 45, 5–32 (2001).
Mondello, P. et al. Lack of intrafollicular memory CD4+ T cells is predictive of early clinical failure in newly diagnosed follicular lymphoma. Blood Cancer J. 11, 130 (2021).
Saltz, J. et al. Spatial organization and molecular correlation of tumor-infiltrating lymphocytes using deep learning on pathology images. Cell Rep. 23, 181–193 (2018).
Chevrier, S. et al. An immune atlas of clear cell renal cell carcinoma. Cell 169, 736–74918 (2017).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Ji, R.-R. et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol. Immunother. 61, 1019–1031 (2012).
Godson, L. et al. Immune subtyping of melanoma whole slide images using multiple instance learning. Med. Image Anal. 93, 103097 (2024).
Pfannstiel, C. et al. The tumor immune microenvironment drives a prognostic relevance that correlates with bladder cancer subtypes. Cancer Immunol. Res. 7, 923–938 (2019).
Wouters, M. C. & Nelson, B. H. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin. Cancer Res. 24, 6125–6135 (2018).
Ilse, M., Tomczak, J. & Welling, M. Attention-based deep multiple instance learning. In Proc. International Conference on Machine Learning (eds Dy, J. & Krause, A.) 2127–2136 (PMLR, 2018).
Chen, R. J. et al. Multimodal co-attention transformer for survival prediction in gigapixel whole slide images. In Proc. IEEE/CVF International Conference on Computer Vision 4015–4025 (IEEE, 2021).
Jaume, G. et al. Modeling dense multimodal interactions between biological pathways and histology for survival prediction. In Proc. IEEE/CVF Conference on Computer Vision and Pattern Recognition 11579–11590 (IEEE, 2024).
Antolini, L., Boracchi, P. & Biganzoli, E. A time-dependent discrimination index for survival data. Stat. Med. 24, 3927–3944 (2005).
Chen, R. J. et al. Towards a general-purpose foundation model for computational pathology. Nat. Med. 30, 850–862 (2024).
Mulliqi, N. et al. Foundation models—a panacea for artificial intelligence in pathology? Preprint at https://arxiv.org/abs/2502.21264 (2025).
Bradski, G. The OpenCV Library. Dr. Dobb’s J. 120, 122–125 (2000).
McQuin, C. et al. Cellprofiler 3.0: next-generation image processing for biology. PLoS Biol. 16, 2005970 (2018).
Crowell, H. L. et al. An R-based reproducible and user-friendly preprocessing pipeline for cytof data. F1000Res. 9, 1263 (2020).
Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 9, 62–66 (1979).
Gillies, S. et al. Shapely (2.0.6). Zenodo https://doi.org/10.5281/zenodo.13345370 (2024).
Macenko, M. et al. A method for normalizing histology slides for quantitative analysis. In Proc. IEEE International Symposium on Biomedical Imaging: From Nano to Macro 1107–1110 (IEEE, 2009).
Nan, A., Tennant, M., Rubin, U. & Ray, N. Drmime: differentiable mutual information and matrix exponential for multi-resolution image registration. In Proc. 3rd Conference on Medical Imaging with Deep Learning (eds Arbel, T. et al.) 527–543 (PMLR, 2020).
Ronneberger, O., Fischer, P. & Brox, T. U-net: convolutional networks for biomedical image segmentation. In Proc. International Conference on Medical Image Computing and Computer-Assisted Intervention (eds Navab, N. et al.) 234–241 (Springer, 2015).
Mao, X. et al. Least squares generative adversarial networks. In Proc. IEEE International Conference on Computer Vision 2794–2802 (IEEE, 2017).
Karnewar, A. & Wang, O. Msg-gan: multi-scale gradients for generative adversarial networks. In Proc. IEEE/CVF Conference on Computer Vision and Pattern Recognition 7799–7808 (IEEE, 2020).
Chen, T., Kornblith, S., Norouzi, M. & Hinton, G. A simple framework for contrastive learning of visual representations. In Proc. International Conference on Machine Learning (eds Daumé, H. & Singh, A.) 1597–1607 (PMLR, 2020).
Oord, A. v. d., Li, Y. & Vinyals, O. Representation learning with contrastive predictive coding. Preprint at https://arxiv.org/abs/1807.03748 (2018).
Mescheder, L., Geiger, A. & Nowozin, S. Which training methods for GANS do actually converge? In Proc. International Conference on Machine Learning (eds Dy, J. & Krause, A.) 3481–3490 (PMLR, 2018).
Kingma, D. P. & Ba, J. Adam: a method for stochastic optimization. In Proc. International Conference on Learning Representations (2015).
Berg, S. et al. Ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Wagner, J. et al. A single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell 177, 1330–1345 (2019).
Zadeh, S. G. & Schmid, M. Bias in cross-entropy-based training of deep survival networks. IEEE Trans. Pattern Anal. Mach. Intell. 43, 3126–3137 (2020).
He, K., Zhang, X., Ren, S. & Sun, J. Deep residual learning for image recognition. In Proc. IEEE Conference on Computer Vision and Pattern Recognition 770–778 (IEEE, 2016).
Ciga, O., Xu, T. & Martel, A. L. Self supervised contrastive learning for digital histopathology. Mach. Learn. Appl. 7, 100198 (2022).
Deng, J. et al. Imagenet: a large-scale hierarchical image database. In Proc. IEEE Conference on Computer Vision and Pattern Recognition 248–255 (IEEE, 2009).
Andani, S. et al. Source data for ‘Histopathology-based protein multiplex generation using deep learning’. Zenodo https://doi.org/10.5281/zenodo.15110117 (2025).
Acknowledgements
We gratefully acknowledge funding from the Tumor Profiler Initiative and the Tumor Profiler Center (to V.H.K., G.R. and B.B.). J.F.-P. received partial funding from the Tumor Profiler Consortium. The Tumor Profiler Study is jointly funded by a public–private partnership involving F. Hoffmann-La Roche Ltd., ETH Zurich, University of Zurich, University Hospital Zurich and University Hospital Basel. We also acknowledge funding from the Swiss Federal Institutes of Technology strategic focus area of personalized health and related technologies grant no. 2021-367 (to G.R., V.H.K. and S.A.), the ETH AI Center (to G.R. and B.C.), ETH core funding (to G.R.), UZH, USB and UniBas core funding (to V.H.K), and funding by the Promedica Foundation grant no. F-87701-41-01 (to V.H.K). B.B. was funded by two SNSF project grants (grant no. 310030_205007: Analysis of breast tumour ecosystem properties for precision medicine approaches and grant no. 316030_213512: Cellular-resolution high-performance mass spectrometric imaging of biological samples), an NIH grant (grant no. UC4 DK108132), the CRUK IMAXT Grand Challenge and the European Research Council (ERC) under the European Union’s Horizon 2020 Programme under the ERC grant no. 866074 (‘Precision Motifs’). We thank M. Nowak and A. Frei for their contributions to the generation and review of digital pathology data for immune profiling. We are also grateful to S. Dettwiler, F. Prutek, N. Wey and S. Krackow from the Department of Pathology and Molecular Pathology, University Hospital Zurich, for their excellent collaboration and technical assistance. We acknowledge I. Scapozza (University of Zurich, Switzerland) for his support in the visual assessment evaluation and F. Pedrocchi for her work on pathology image registration. We express our gratitude towards L. Godson (University of Leeds, UK) and J. Nsengimana (Newcastle University, UK) for sharing the immune subtype data for TCGA–SKCM.
Funding
Open access funding provided by Swiss Federal Institute of Technology Zurich
Author information
Authors and Affiliations
Consortia
Contributions
G.R., V.H.K., S.A. and J.F.-P. conceived of the study. G.R., V.H.K., S.A., B.C. and J.F.-P. participated in designing the study and reviewing the literature. V.H.K. and G.R. supervised the study. V.H.K., S.A., J.F.-P., B.S. and R.C. collected the data. S.A., J.F.-P., B.C. and S.H. contributed to code writing, model training and data analysis. V.H.K. and G.R. contributed to expert review and data interpretation. B.C., S.A., V.H.K. and G.R. designed the figures. S.A. and B.C. drafted the paper. T.P.C., V.H.K., R.C., B.B. and B.S. contributed to providing data and tumour material used in this study. B.F.H. contributed towards annotation tasks and qualitative assessment of data. All authors had access to the result data presented in the final paper, and all authors read and approved the final paper. The decision to submit was made by all authors. V.H.K. and G.R. contributed equally to this work and share senior authorship.
Corresponding authors
Ethics declarations
Competing interests
V.H.K. reports being an invited speaker for Sharing Progress in Cancer Care (SPCC) and Indica Labs; advisory board of Takeda; and sponsored research agreements with Roche and IAG, all unrelated to the current study. V.H.K. is a participant of a patent application on the assessment of cancer immunotherapy biomarkers by digital pathology, a patent application on multimodal deep learning for the prediction of recurrence risk in cancer patients and a patent application on predicting the efficacy of cancer treatment using deep learning, all of which are not directly related to the current work. G.R. and J.F.-P. are participants of a patent application on matching cells from different measurement modalities, which is not directly related to the current work. Moreover, G.R. is cofounder of Computomics GmbH, Germany, and one of its shareholders. B.B. has co-founded Navignostics, a spin-off company of the University of Zurich developing precision oncology diagnostics, and is one of its shareholders and a board member. The other authors declare no competing interests.
Peer review
Peer review information
Nature Machine Intelligence thanks Kianoush Falahkheirkhah, Chen Li, Peiliang Lou, Hairong Lv and Saad Nadeem for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Statistical test for HistoPlexer and baselines.
Paired two-sided t-test: Comparison of significance levels between methods in multiplex (left) and singleplex (right) settings for MS-SSIM (row 1), PSNR (row 2) and RMSE-SW (row 3) metrics. No adjustments were made for multiple comparisons. Significance levels are colour-coded: Not Significant (NS) (p≥0.05), Significant (p<0.05), and Strong Significant (p<0.01).
Extended Data Fig. 2 Qualitative RoI-level assessment of HistoPlexer.
H&E (first column) and expression profiles of individual markers: S100, HLA-DR, HLA-ABC, gp100 and CD31 (from second to last column). Top row: ground-truth (GT) expression profiles; bottom row: predicted (pred) expression profiles.
Extended Data Fig. 3 Co-localization patterns.
a(i) Spearman’s correlation coefficients between protein pairs, comparing the ground truth (GT) with both singleplexed (SP) and multiplexed (MP) predictions of HistoPlexer. a(ii) Mean squared error between the GT and predicted Spearman’s correlation coefficients, comparing the SP and MP predictions of HistoPlexer. Bars represent mean values and error bars indicate standard deviation (SD). b Joint t-SNE visualization of protein co-localization patterns.
Extended Data Fig. 4 Region annotation for Immune phenotyping.
H&E WSI (left) and H&E WSI with an outline of the Tumour Center compartment and annotated regions (right).
Supplementary information
Supplementary Information
Supplementary Information on benchmarking on pixel-aligned datasets, TuPro dataset and extended interpretation of results.
Source data
Source Data Figs. 2, 3b and 6c, Extended Data Fig. 2a, and Extended Data Tables 3 and 4
A single file containing all source data with clearly named tabs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Andani, S., Chen, B., Ficek-Pascual, J. et al. Histopathology-based protein multiplex generation using deep learning. Nat Mach Intell 7, 1292–1307 (2025). https://doi.org/10.1038/s42256-025-01074-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42256-025-01074-y