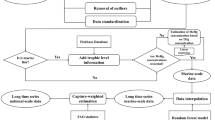
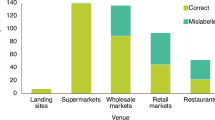
Abstract
Seafood consumption is a major pathway for exposure to methylmercury (MeHg), a globally pervasive neurotoxin. Yet, how upstream processes in the seafood value chain influence MeHg exposure remains poorly understood. Here we quantified MeHg in seafood production, trade and consumption in 2019 around the world. We found that countries with seafood-MeHg exposures beyond the recommended threshold by the World Health Organization were predominately high-income countries. These countries experienced a tenfold increase in exposure levels compared with low-income countries, due to greater consumption and long-overlooked higher MeHg concentrations in seafood inherited from production. Notably, 43% of seafood MeHg in production was redistributed through seafood trade, marked by inequality, as exports from high-income to lower-income countries contained higher seafood-MeHg concentrations. These exposures may have resulted in 61,800 global premature deaths and economic losses of around US$2.87 trillion, underscoring the need to change seafood production practices and trade patterns.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All Hg-related data generated from this study are available in the Supplementary Information. The seafood quantity data are publicly available via the FAO FishStatJ online database at www.fao.org/fishery/en/topic/166235?lang=en. Source data are provided with this paper.
Code availability
The R codes for data analyses and visualization are available upon request to S.W. at shxw@tsinghua.edu.cn or qrwu@tsinghua.edu.cn.
References
Zhang, Y. X. et al. Global health effects of future atmospheric mercury emissions. Nat. Commun. 12, 10 (2021).
Streets, D. G. et al. Global and regional trends in mercury emissions and concentrations, 2010–2015. Atmos. Environ. 201, 417–427 (2019).
Global Mercury Assessment 2018 (UNEP, 2019).
Streets, D. G. et al. Total mercury released to the environment by human activities. Environ. Sci. Technol. 51, 5969–5977 (2017).
Seco, J. et al. Mercury biomagnification in a Southern Ocean food web. Environ. Pollut. 275, 116620 (2021).
Lavoie, R. A., Jardine, T. D., Chumchal, M. M., Kidd, K. A. & Campbell, L. M. Biomagnification of mercury in aquatic food webs: a worldwide meta-analysis. Environ. Sci. Technol. 47, 13385–13394 (2013).
Bose-O’Reilly, S., McCarty, K. M., Steckling, N. & Lettmeier, B. Mercury exposure and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 40, 186–215 (2010).
Zhang, H., Feng, X. B., Larssen, T., Qiu, G. L. & Vogt, R. D. In Inland China, rice, rather than fish, is the major pathway for methylmercury exposure. Environ. Health Perspect. 118, 1183–1188 (2010).
Liu, M. et al. Significant elevation of human methylmercury exposure induced by the food trade in Beijing, a developing megacity. Environ. Int. 135, 105392 (2020).
Liu, M. D. et al. Rice life cycle-based global mercury biotransport and human methylmercury exposure. Nat. Commun. 10, 5164 (2019).
Polak-Juszczak, L. Total mercury and methylmercury in garfish (Belone belone) of different body weights, sizes, ages, and sexes. J. Trace Elem. Med. Biol. 79, 127220 (2023).
Zampetti, C. J. & Brandt, J. E. Co-considering selenium concentrations alters mercury-based fish and seafood consumption advice: a data compilation and critical assessment. Environ. Sci. Technol. Lett. 10, 179–185 (2023).
Bjørklund, G., Dadar, M., Mutter, J. & Aaseth, J. The toxicology of mercury: current research and emerging trends. Environ. Res. 159, 545–554 (2017).
The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation (FAO,2022).
Xing, Z. et al. International trade shapes global mercury–related health impacts. PNAS Nexus 2, pgad128 (2023).
Lavoie, R. A., Bouffard, A., Maranger, R. & Amyot, M. Mercury transport and human exposure from global marine fisheries. Sci. Rep. 8, 6705 (2018).
Evaluation of Certain Food Additives and Contaminants: Sixty-First Report of the Joint FAO/WHO Expert Committee on Food Additives (WHO, 2003).
Xie, J. et al. Mercury and selenium in squids from the Pacific Ocean and Indian Ocean: the distribution and human health implications. Mar. Pollut. Bull. 173, 112926 (2021).
Médieu, A., Lorrain, A. & Point, D. Are tunas relevant bioindicators of mercury concentrations in the global ocean? Ecotoxicology 32, 994–1009 (2023).
Schartup, A. T. et al. Climate change and overfishing increase neurotoxicant in marine predators. Nature 572, 648–650 (2019).
Jiskra, M. et al. Mercury stable isotopes constrain atmospheric sources to the ocean. Nature 597, 678–682 (2021).
Chen, L. et al. Trans-provincial health impacts of atmospheric mercury emissions in China. Nat. Commun. 10, 1484 (2019).
Giang, A. & Selin, N. E. Benefits of mercury controls for the United States. Proc. Natl Acad. Sci. USA 113, 286–291 (2016).
Sunderland, E. M., Krabbenhoft, D. P., Moreau, J. W., Strode, S. A. & Landing, W. M. Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models. Glob. Biogeochem. Cycles 23, GB2010 (2009).
Bratkic, A. et al. Mercury presence and speciation in the South Atlantic Ocean along the 40°S transect. Glob. Biogeochem. Cycles 30, 105–119 (2016).
Kroodsma, D. A. et al. Tracking the global footprint of fisheries. Science 359, 904–907 (2018).
Pauly, D. et al. China’s distant-water fisheries in the 21st century. Fish Fish. 15, 474–488 (2014).
Liu, M. et al. Impacts of farmed fish consumption and food trade on methylmercury exposure in China. Environ. Int. 120, 333–344 (2018).
Springmann, M., Kennard, H., Dalin, C. & Freund, F. International food trade contributes to dietary risks and mortality at global, regional and national levels. Nat. Food 4, 886–893 (2023).
Trade map. ITC https://www.trademap.org/Index.aspx (2023).
Muir, D. C. G. Toxic chemical exposure from global fish trade. Nat. Food 1, 259–259 (2020).
Xu, Y. J., Li, J., Bo, Y. & Wang, R. P. Comparison of feeding behaviour characteristics between wild-caught and captive-reared Hippocampus kuda Bleeker. Appl. Anim. Behav. Sci. 259, 105850 (2023).
Govzman, S. et al. A systematic review of the determinants of seafood consumption. Br. J. Nutr. 126, 66–80 (2021).
Sharma, B. M., Sáňka, O., Kalina, J. & Scheringer, M. An overview of worldwide and regional time trends in total mercury levels in human blood and breast milk from 1966 to 2015 and their associations with health effects. Environ. Int. 125, 300–319 (2019).
Calder, R. S. D., Bromage, S. & Sunderland, E. M. Risk tradeoffs associated with traditional food advisories for Labrador Inuit. Environ. Res. 168, 496–506 (2019).
Technical Background Report for the Global Mercury Assessment 2018 (AMAP/UNEP, 2019).
Murray, G. D. et al. Seafood consumption and the management of shellfish aquaculture. Mar. Policy 150, 105534 (2023).
Racine, P. et al. A case for seaweed aquaculture inclusion in US nutrient pollution management. Mar. Policy 129, 104506 (2021).
Zhang, J., Wu, W., Li, Y., Liu, Y. & Wang, X. Environmental effects of mariculture in China: an overall study of nitrogen and phosphorus loads. Acta Oceanolog. Sin. 41, 4–11 (2022).
Sumaila, U. R. et al. Illicit trade in marine fish catch and its effects on ecosystems and people worldwide. Sci. Adv. 6, eaaz3801 (2020).
Peterson, S. A., Van Sickle, J., Herlihy, A. T. & Hughes, R. M. Mercury concentration in fish from streams and rivers throughout the western United States. Environ. Sci. Technol. 41, 58–65 (2007).
Nowosad, J. et al. Dynamics of mercury content in adult sichel (Pelecus cultratus L.) tissues from the Baltic Sea before and during spawning. Mar. Environ. Res. 148, 75–80 (2019).
Erasmus, V. N., Hamutenya, S., Iitembu, J. A. & Gamatham, J. C. Mercury concentrations in muscles and liver tissues of Cape monkfish (Lophius vomerinus) from the Northern Benguela, Namibia. Mar. Pollut. Bull. 135, 1101–1106 (2018).
Jinadasa, K. K., Herbello-Hermelo, P., Peña-Vázquez, E., Bermejo-Barrera, P. & Moreda-Piñeiro, A. Mercury speciation in edible seaweed by liquid chromatography—inductively coupled plasma mass spectrometry after ionic imprinted polymer-solid phase extraction. Talanta 224, 121841 (2021).
Global production by production source 1950-2021 (FishStatJ). FAO https://www.fao.org/fishery/en/statistics/software/fishstatj (2023).
Global aquatic trade statistics – Global aquatic trade – by partner country. FAO https://www.fao.org/fishery/en/collection/global_commodity_prod (2023).
Rodrigues, E. T., Coelho, J. P., Pereira, E. & Pardal, M. A. Are mercury levels in fishery products appropriate to ensure low risk to high fish-consumption populations? Mar. Pollut. Bull. 186, 114464 (2023).
Perugini, M. et al. Effect of cooking on total mercury content in Norway lobster and European hake and public health impact. Mar. Pollut. Bull. 109, 521–525 (2016).
Food balance sheets of aquatic products 1961–2019 (FishStatJ) v2023.1.0. FAO https://www.fao.org/fishery/en/collection/global_fish_consump (2023).
Anderson, G. Error propagation by the Monte Carlo method in geochemical calculations. Geochim. Cosmochim. Acta 40, 1533–1538 (1976).
Taylor, J. Introduction to Error Analysis, the Study of Uncertainties in Physical Measurements (University Science Books, 1997).
Soerensen, A. L., Faxneld, S., Pettersson, M. & Sköld, M. Fish tissue conversion factors for mercury, cadmium, lead and nine per- and polyfluoroalkyl substances for use within contaminant monitoring. Sci. Total Environ. 858, 159740 (2023).
Boalt, E., Miller, A. & Dahlgren, H. Distribution of cadmium, mercury, and lead in different body parts of Baltic herring (Clupea harengus) and perch (Perca fluviatilis): implications for environmental status assessments. Mar. Pollut. Bull. 78, 130–136 (2014).
Rice, G. E., Hammitt, J. K. & Evans, J. S. A probabilistic characterization of the health benefits of reducing methyl mercury intake in the United States. Environ. Sci. Technol. 44, 5216–5224 (2010).
Acknowledgements
This study is funded by National Natural Science Foundation of China (grant no. 42407138, Q.C.; grant no. 22222604, Q.W.; and grant no. 42394094, Q.W.), International Postdoctoral Exchange Fellowship Program (Talent Introduction Program) (grant no. YJ20210103, Q.C.), Shuimu Scholar Fellowship from Tsinghua University (grant no. 2020SM075, Q.C.), Special fund of State Key Joint Laboratory of Environment Simulation and Pollution Control (grant no. 22L02ESPC, S.W.) and New Cornerstone Science Foundation through the XPLORER PRIZE (S.W.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Q.C., Q.W. and S.W. conceived the idea. Q.C. compiled the seafood Hg concentration database, conducted the statistical analysis and drafted and revised the manuscript. Y.C. performed the health and economic damage modelling. Q.W. and S.W. provided expertise in interpreting results and provided important critiques, supervised the project and were in charge of the overall study. All authors contributed to the discussion, revision and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Food thanks Jessica Brandt, Ping Li, Zhencheng Xing and Jun Xu for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6 and Supplementary Tables 1–5.
Source data
Source Data
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Q., Wu, Q., Cui, Y. et al. Global seafood production practices and trade patterns contribute to disparities in exposure to methylmercury. Nat Food 6, 491–502 (2025). https://doi.org/10.1038/s43016-025-01136-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s43016-025-01136-9