Abstract
The phase 2, multicohort, ongoing ELM-2 study evaluates odronextamab, a CD20×CD3 bispecific antibody, in patients with relapsed/refractory (R/R) B cell non-Hodgkin lymphoma after ≥2 lines of therapy. Here primary analysis of the diffuse large B cell lymphoma (DLBCL) cohort is reported. Patients received intravenous odronextamab in 21-day cycles until progression or unacceptable toxicity, with cycle 1 step-up dosing to mitigate cytokine release syndrome (CRS) risk. The primary endpoint was objective response rate (ORR). Secondary endpoints included complete response (CR) rate, duration of response, progression-free survival (PFS) and overall survival. A total of 127 patients were enrolled. At the 29.9-month efficacy follow-up, the ORR was 52.0% and CR rate was 31.5%. Median durations of response and CR were 10.2 and 17.9 months, respectively. Undetectable minimal residual disease at cycle 4 day 15 was associated with PFS benefit. With a step-up of 0.7 to 4 to 20 mg (n = 60), CRS was the most common treatment-emergent adverse event (53.3% (grade ≥3, 1.7%)). No immune effector cell-associated neurotoxicity syndrome was reported. Infections were reported in 82/127 (64.6%) patients (grade ≥3, 38.6%; coronavirus disease 2019, 18.1% (grade ≥3, 12.6%)). In conclusion, odronextamab showed encouraging efficacy in heavily pretreated R/R DLBCL and generally manageable safety with supportive care. Clinical trial registration: NCT03888105.
Similar content being viewed by others
Main
Diffuse large B cell lymphoma (DLBCL) is an aggressive form of B cell non-Hodgkin lymphoma (B-NHL)1. Approximately 30% of people will relapse after first-line treatment with immunochemotherapy (for example, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone) and poor outcomes are observed in this relapsed/refractory (R/R) setting; the median overall survival (OS) is approximately 6–7 months in people with primary refractory disease2,3,4.
T cell-engaging therapies, including chimeric antigen receptor (CAR) T cell therapies and bispecific antibodies, are important modalities in the management of R/R DLBCL. CAR T cell treatments were initially approved in people with two or more prior therapy lines5,6,7,8,9,10 and have since received approval in DLBCL refractory to or relapsed after first-line immunochemotherapy8,10,11,12. The shift to earlier CAR T cell therapy necessitates the development of effective options in the third-line setting.
Bispecific antibodies, which bind T cells to a tumor antigen on cancer cells, have shown encouraging activity in solid and hematologic malignancies and are manufactured to allow off-the-shelf administration13,14,15. Recently, glofitamab and epcoritamab received accelerated US approval for DLBCL treatment after at least two prior lines of systemic therapy16,17.
Odronextamab is an Fc-silenced, human CD20×CD3 bispecific antibody that simultaneously engages CD20 on malignant B cells and CD3 on cytotoxic T cells to induce T cell-mediated cytotoxicity of the former18. The phase 1 dose-escalation and expansion ELM-1 study demonstrated encouraging activity and generally manageable safety of odronextamab monotherapy in patients with heavily pretreated B-NHL, including those with R/R DLBCL after CAR T cell therapy (at the active dose for aggressive lymphoma (≥80 mg); the objective response rate (ORR) was 33% in those who had previous CAR T cell therapy)19. Following further safety, pharmacokinetic and pharmacodynamic evaluation, the recommended dose for DLBCL was established as 160 mg (ref. 19). Here, we report the long-term efficacy and safety results of odronextamab in patients with R/R DLBCL from the phase 2 ELM-2 study (NCT03888105).
Results
Patient disposition and characteristics
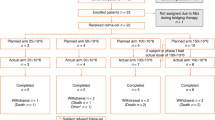
Between March 24, 2020 and May 18, 2022, 127 patients with DLBCL were enrolled, treated with odronextamab and evaluated for efficacy and safety. At data cutoff (August 18, 2023), the median duration of exposure was 18.0 weeks (range: 0.9–168.1) and 19 (15.0%) patients remained in the study. In total, 91.3% of patients completed cycle 1 (C1); of these, 67 (52.8%) received the C1 regimen with a step up of 1 to 20 mg, and 60 (47.2%) received the regimen with a step up of 0.7 to 4 to 20 mg. In total, 63.0% of patients completed four or more cycles of odronextamab treatment. The most common reasons for treatment discontinuation were progressive disease (PD; 47.2%), death (15.7%) and adverse events (AEs; 13.4%), occurring in a similar proportion of patients by step-up regimen (Extended Data Fig. 1).
Anti-infection prophylaxis was recommended as part of a protocol amendment; eight patients (6.3%) received prophylaxis for cytomegalovirus (CMV) infection, 84 patients (66.1%) received prophylaxis for Pneumocystis jirovecii pneumonia and nine patients (7.1%) received intravenous (IV) immunoglobulin prophylaxis.
At baseline, the median age of patients was 67 years (range: 24–88), with 23.6% of patients aged ≥75 years (Table 1). Most patients (81.1%) had advanced disease (Ann Arbor stages III–IV) and 55.9% had high–intermediate or high International Prognostic Index scores. A total of 31 (24.4%) patients had transformed DLBCL (24, non-Richter’s; 7, Richter’s) and 11 (8.7%) had double-hit or triple-hit cytogenetic rearrangements by local assessment. The median number of prior therapy lines was two (range: 2–8), with 20.5% of patients having received four or more prior lines, 55.1% of patients being primary refractory, 64.6% of patients being double refractory to an alkylator and anti-CD20 antibody and 17.3% of patients with prior autologous stem cell transplantation (ASCT). Baseline demographics were generally similar irrespective of step-up dosing regimen used (1 to 20 mg versus 0.7 to 4 to 20 mg) (Supplementary Table 1).
Efficacy
The primary endpoint of ORR per independent central review (ICR) was 52.0% (66/127 (95% confidence interval (CI): 42.9–60.9); Table 2). The complete response (CR) rate was 31.5% (40/127 (95% CI: 23.5–40.3)). The median time to response was 2.6 months (range: 0.8–6.4) and 87.9% (58/66) of patients who responded did so by their first assessment at week 12. Response rates by ICR were similar in patients who received the step-up regimen of 1 to 20 mg and in those who received the step-up regimen of 0.7 to 4 to 20 mg, with overlapping CIs for ORR, CR and partial response (PR; Supplementary Table 2). Response rates as reported by local investigator assessment were similar to those reported by ICR, with an ORR of 49.6% (63/127 (95% CI: 40.6–58.6)) and a CR rate of 38.6% (49/127 (95% CI: 30.1–47.6)).
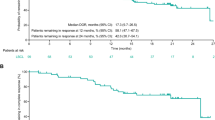
Tumor size was reduced in 78.9% (71/90) of patients with postbaseline imaging (Fig. 1). With a median efficacy follow-up of 29.9 months (95% CI: 20.4–32.6), the median duration of response (DOR) per ICR was 10.2 months (95% CI: 5.0–17.9). Patients with a best response of CR had a median CR duration of 17.9 months (95% CI: 10.2–not evaluable (NE)). Among the 21 patients who sustained a CR for 9 months and were eligible to transition to dosing once every 4 weeks, 18 transitioned and the median DOR from the time of transition was 18.5 months (95% CI: 6.0–NE).
Data for each evaluable patient are shown as a separate bar on the figure (n = 90 patients). SD, stable disease.
Odronextamab demonstrated antitumor activity in patients across a range of key subgroups, including in patients aged 75 years and older (ORR = 50.0%), in those with more than two lines of prior therapy (ORR = 46.7%) and in those who were double refractory to an alkylator and anti-CD20 antibody (ORR = 40.2%) (Fig. 2).
ORR data are presented as the mean values ± 95% CIs. The vertical dashed line indicates the ORR for all patients (N = 127).
The median progression-free survival (PFS) was 4.4 months (95% CI: 3.6–5.9) and median OS was 9.2 months (95% CI: 6.5–12.7). The median PFS in patients with CR (20.4 months) was longer than that in those with PR (5.8 months; hazard ratio (HR) = 0.29 (95% CI: 0.2–0.5)), as was the OS (not reached (NR) versus 17.0 months, respectively; HR = 0.48 (95% CI: 0.2–1.0)) (Fig. 3a,b).
a, PFS. b, OS. Data are presented as Kaplan–Meier curves, with tick marks indicating patients with censored data (N = 127 patients; n = 40 with CR, n = 26 with PR). Median values with 95% CIs are presented alongside the respective curves.
Biomarker assessment
Among 63 patients evaluable for circulating tumor DNA (ctDNA) assessment who had a response assessment at C4 day 15 (C4D15), all were positive for minimal residual disease (MRD+) at baseline. At C4D15, 43 remained MRD+ and 20 were MRD−. PFS was longer in patients who were MRD− by C4D15 versus those who were MRD+ (HR = 0.27; 95% CI: 0.12–0.62) (Fig. 4). MRD negativity also predicted PFS benefit in patients who did not achieve CR by positron emission tomography–computed tomography at C4D15 (MRD− versus MRD+: HR = 0.11; 95% CI: 0.03–0.49) (Extended Data Fig. 2).
Data are presented as Kaplan–Meier curves, with tick marks indicating patients with censored data (n = 63 ctDNA-evaluable patients; n = 20 with cleared MRD at C4D15, n = 43 with detected MRD at C4D15). Median values with 95% CIs are presented alongside the respective curves. The HR for PFS in patients with MRD cleared versus MRD detected was calculated by univariate Cox regression.
Safety
Treatment-emergent AEs (TEAEs) were reported in 126 (99.2%) patients, with 111 (87.4%) patients experiencing at least one treatment-related (per investigator assessment) TEAE (Table 3). Overall, the most common TEAEs were cytokine release syndrome (CRS; 55.1%), pyrexia (43.3%), anemia (38.6%) and neutropenia (30.7%). Grade ≥3 TEAEs occurred in 107 (84.3%) patients, the most common being neutropenia (26.0%), anemia (22.8%), thrombocytopenia (15.0%), and coronavirus disease 2019 (COVID-19; 10.2%). Serious TEAEs occurred in 82 (64.6%) patients and were considered treatment related in 62 (48.8%) patients.
A total of 17 (13.4%) patients had TEAEs that led to treatment discontinuation. Treatment-related TEAEs leading to discontinuation were encephalopathy (n = 2), CRS, COVID-19, CMV reactivation, pulmonary tuberculosis, aphasia, supraventricular tachycardia, and cholangitis sclerosing (n = 1 each); CRS, tachycardia, pancreatitis, septic shock, pneumonia plus cough in one patient; and P. jirovecii plus neutrophil count decrease in one patient. TEAEs leading to death were reported in 20 (15.7%) patients (Supplementary Table 3); these were considered treatment related in five (3.9%) patients (COVID-19, pneumonia, P. jirovecii pneumonia, pseudomonal sepsis (n = 1 each), and CMV pneumonia plus CMV reactivation in one patient).
With the step-up regimen of 0.7 to 4 to 20 mg, CRS was reported in 32/60 (53.3%) patients and mostly occurred during C1 (Extended Data Fig. 3). With this regimen, the majority of events were of low grade (grade 1, 40%; grade 2, 11.7%), with one grade 3 case occurring in the setting of acute pancreatitis (pancreatic lymphoma mass causing obstruction of biliary drainage) at week 6; this was numerically lower than the rate of grade 3 CRS with the original step-up regimen of 1 to 20 mg (n = 5 (7.5%)) (Supplementary Table 4). There were no cases of grade 4 or grade 5 CRS. CRS was managed with tocilizumab in 15 (25.0%) patients and systemic steroids in 13 (21.7%) patients (Supplementary Table 5); no patients required mechanical ventilation or intensive care unit admission for CRS. The median time to onset of CRS was 18.0 h (range: −3.4 to 221.0) and CRS events resolved in a median of 7.7 h (range: 0.1–143.9). Infusion-related reactions occurred in five (8.3%) patients (all grade 1 or 2). Two cases of encephalopathy that led to discontinuation of odronextamab treatment occurred in the setting of CRS during step-up dosing: one grade 3 event with the step-up regimen of 1 to 20 mg in a 79-year-old patient, and one grade 2 event with the step-up regimen of 0.7 to 4 to 20 mg in an 85-year-old patient; both events resolved with steroids.
Neurologic AEs of any grade occurred in 54 (42.5%) patients (grade ≥3, five (3.9%) patients), including in 22 (36.7%) patients with the step-up regimen of 0.7 to 4 to 20 mg (all grade 1 or 2). Neurologic AEs reported in >5% of patients were insomnia (n = 20 (15.7%)), dizziness (n = 12 (9.4%)) and headache (n = 8 (6.3%)). No cases of immune effector cell-associated neurotoxicity syndrome (ICANS; preferred term) were reported with either regimen. Tumor lysis syndrome occurred in one patient (grade 3–4) with the step-up regimen of 1 to 20 mg (Supplementary Table 4). There was one case of low-grade tumor flare.
Infections occurred in 82/127 (64.6%) patients (grade ≥3, 38.6%). The most frequent type of infection was COVID-19, which was reported in 18.1% (grade ≥3, 12.6%) of patients. Febrile neutropenia was also observed in three (2.4%) patients. Overall, six (4.7%) patients discontinued treatment because of treatment-related infections. Grade 5 infection occurred in 15 patients, with five cases because of COVID-19. Other grade 5 infections included pneumonia and sepsis (n = 3 each), P. jirovecii pneumonia, CMV infection, and pseudomonal sepsis (n = 1 each), and CMV infection reactivation plus CMV pneumonia in one patient.
Discussion
In this phase 2 study, odronextamab monotherapy demonstrated substantial efficacy in heavily pretreated patients with R/R DLBCL. These results are consistent with those from the ELM-1 study in patients with R/R DLBCL who had received prior CAR T cell therapy and no new safety signals were observed19,20, indicating that odronextamab may have an important role in maintaining effective disease control in this aggressive lymphoma.
Overall, the baseline characteristics of enrolled patients were representative of a heavily pretreated, highly refractory population. High-risk factors included double-hit and triple-hit cytogenetic rearrangements (9% of patients), transformed disease (24%), age ≥ 75 years (24%), Ann Arbor stage III–IV (81%) and prior ASCT (17%). Despite the difficult-to-treat nature of this population, odronextamab demonstrated consistent ORRs across high-risk subgroups.
T cell-engaging therapies including CAR T cell therapies are now established as an important treatment option for people with R/R DLBCL2. Despite encouraging ORRs (52–82%), eligibility for CAR T cell therapy is low (6–22%) and uptake among eligible people is variable because of access barriers (for example, administration complexities, manufacturing timelines and costs), associated toxicities (including CRS, ICANS and prolonged cytopenias) and potential risk of secondary malignancy5,6,7,21,22,23,24,25. These challenges may underpin the less frequent use of CAR T cell therapies reported in elderly people26. Potential earlier use of CAR T cell therapy also highlights the need for therapies that are active after these agents, where outcomes are typically dismal27. Bispecific antibodies have, thus, assumed greater importance as an alternative treatment option, particularly in the third line following the recent approvals of axicabtagene ciloleucel and lisocabtagene maraleucel for people with DLBCL who are R/R within 12 months of first-line immunochemotherapy8,10. Although cross-study comparisons of different single-arm studies are challenging, in the third-line setting, odronextamab demonstrated ORR and CR rates of 52% (95% CI: 42.9–60.9) and 31% (95% CI: 23.5–40.3), respectively, similar to those across the field of bispecific antibodies in R/R DLBCL (glofitamab, 52% (95% CI: 43–60) and 39% (95% CI: 32–48); epcoritamab, 63% (95% CI: 55.0–70.6) and 39% (95% CI: 31.2–46.9), respectively)28,29. Although this study did not include people treated with prior CAR T cell therapy, the efficacy of odronextamab in this population is supported by data from a prospective cohort of patients with disease progression after CAR T cell therapy in ELM-1 (n = 44), where the ORR was 48% (CR rate, 30%) and median DOR was NR after a median efficacy follow-up of 4.9 months. These data are consistent with those reported in CAR T cell therapy-naive patients in the current study and there were no major differences in safety profile20.
Biomarker assessment revealed MRD clearance in 20/63 patients at C4D15 of odronextamab treatment, with improved PFS in patients with cleared versus detectable MRD. The observed association between MRD clearance and PFS benefit, even in patients without positron emission tomography–computed tomography CR, indicates the prognostic utility of MRD measurement at this early time point and supports further investigation at later stages of treatment and potentially even after treatment.
The odronextamab administration schedule involved step-up dosing during C1, which was optimized during the study to help mitigate the risk of CRS. The step-up regimens used differed by just 1 week before reaching full dose and pharmacokinetic data indicated that exposure levels were similar for both regimens after the first full dose was received30. Following step-up, patients received weekly odronextamab in C2–C4, before dosing once every 2 weeks (once every 4 weeks with durable CR) as maintenance treatment until disease progression or unacceptable toxicity. This treatment regimen provided compelling antitumor control while maintaining or improving patient-reported outcomes over 42 weeks31. Alternative treatment paradigms, including fixed duration, have been explored in this setting28,29 but the optimal treatment approach for R/R DLBCL is yet to be determined. The potential for growth of subclonal cell populations supports treatment to progression32,33,34 in highly refractory people with aggressive lymphoma and appeared feasible. In addition, administration frequency could be reduced to once every 4 weeks in patients with a durable CR, enabling continued antitumor control in the context of reduced treatment burden.
Among the key AEs associated with bispecific antibodies, severe CRS risk was generally mitigated with the optimized C1 step-up regimen, with a numerically lower rate of grade 3 CRS compared with the original step-up regimen of 1 to 20 mg. One case of grade 3 CRS occurred with the revised regimen, although this was confounded by concurrent acute pancreatitis. Grade ≥3 CRS was reported in 4% (6/154) and 2.5% (4/157) of patients treated with glofitamab and epcoritamab, respectively28,29. Tocilizumab and corticosteroids for CRS were given to 25% and 22% of patients, respectively, in the current study according to evolving institutional guidelines35,36,37,38, although no patients required ventilatory or intensive care unit support. Given the low incidence of severe CRS, ongoing studies are evaluating odronextamab dosing in the outpatient setting, an important consideration for promoting equitable access to effective treatment options in underserved communities. No ICANS was reported with odronextamab in contrast to glofitamab (8%) and epcoritamab (6%)28,29. Neurologic AEs occurred in 43% of patients treated with odronextamab, similar to rates reported with glofitamab in R/R B-NHL (40%) and epcoritamab in R/R DLBCL (35%)39,40. Neurologic AEs were mostly grade ≤2 with odronextamab and the events observed were generally consistent with those reported with other bispecific antibodies16,40. Two cases of grade 2–3 encephalopathy that led to treatment discontinuation were reported in elderly patients; however, both events occurred in the setting of CRS during step-up dosing and resolved with steroids.
Infections were observed in 65% of patients, which may be common in a population with impaired B cell functionality because of underlying malignancy, prior exposure to immunosuppressive agents and chemotherapy, and anticipated B cell depletion and hypogammaglobulinemia induced by odronextamab41,42. Anti-infection prophylaxis was added to the protocol during the study, although local practices for infection management and IV immunoglobulin supplementation may have differed between global sites. COVID-19 was the most frequent infection reported and the most frequent grade 5 infection, reflecting the course of ELM-2 enrollment. Enrollment began early in the pandemic when viral severity and mortality were high and no vaccines or anti-COVID-19 treatments were available. Later enrollment occurred when more transmissible variants were prevalent, vaccine availability had improved and social-distancing measures were relaxing. Randomized controlled trials are required to further investigate the risk of infections with odronextamab and better characterize the kinetics of B cell depletion following fixed durations of treatment.
TEAEs resulting in death were reported in 15.7% of patients treated with odronextamab, of which five (3.9%) were because of COVID-19 infection. With glofitamab and epcoritamab, TEAEs leading to death were reported in approximately 5% of patients, which were related to COVID-19 in five (3.2%) and two (1.3%) patients, respectively28,29. Most fatal TEAEs were caused by infections. The differences in fatal infection rates may be attributed to variations in populations, regional infection rates and local supportive care practices. In addition, the timing of enrollment during different phases of the COVID-19 pandemic, including the availability of treatments and vaccines, may have influenced outcomes.
In conclusion, odronextamab demonstrated highly encouraging clinical activity, including durable CRs, in heavily pretreated, highly refractory patients with R/R DLBCL. AEs were experienced by nearly all patients treated with odronextamab. However, these events were generally manageable with supportive care measures. Odronextamab is a potential treatment option for people with highly refractory R/R DLBCL. Phase 3 trials are currently enrolling in earlier lines of therapy and will inform the future management paradigm for aggressive lymphomas.
Methods
Study design and patients
ELM-2 is an ongoing phase 2, open-label, multicohort, multicenter, single-arm study of odronextamab monotherapy in R/R B-NHL (ClinicalTrials.gov identifier: NCT03888105). Here, we report long-term follow-up results of the primary analysis in the cohort of patients with R/R DLBCL. Patients were recruited from various centers across multiple countries, including the USA, Australia, Canada, China, France, Germany, Italy, Japan, the Republic of Korea, Poland, Singapore, Spain, Taiwan and the United Kingdom. Methods for the follicular lymphoma cohort of ELM-2, which used the same endpoints as the DLBCL cohort, have been published43. Eligible patients for the DLBCL cohort were aged ≥18 years with DLBCL (de novo or transformed) refractory to or relapsed after two or more prior lines of systemic therapy, including an anti-CD20 antibody and an alkylator. Other inclusion criteria were measurable disease on cross-sectional imaging, Eastern Cooperative Oncology Group (ECOG) performance status 0–1, and adequate bone marrow and hepatic functions. Patients with high-grade lymphoma (double-hit and triple-hit cytogenetic rearrangements) were accepted. People with primary central nervous system lymphoma or prior ASCT, CAR T cell therapy or CD20×CD3 bispecific antibody treatment were excluded.
Measures to ensure diverse and inclusive enrollment included diverse trial sites, translated consent forms for under-represented populations, extended screening windows for patients with access constraints, broad eligibility criteria to include patients with controlled human immunodeficiency virus, hepatitis B and hepatitis C infection, and lower thresholds for those with compromised organ function because of lymphoma.
Prophylaxis for P. jirovecii pneumonia was recommended for all patients. Other anti-infection prophylaxis measures included IV immunoglobulin supplementation and antivirals, in accordance with the protocol and local institutional standard, as well as the National Comprehensive Cancer Network44, American Society of Clinical Oncology45 or European Society for Medical Oncology guidelines46. In patients with severe hypogammaglobulinemia (<400 mg dl−1) or in patients with recurrent episodes of infection with immunoglobulin levels between 400 and 600 mg dl−1, supplementation with IV immunoglobulin was recommended. For patients with positive hepatitis B surface antigens, hepatitis B core antibodies and/or measurable viral load, an appropriate antiviral agent for hepatitis B virus was recommended. Appropriate antiviral prophylaxis was recommended for patients with prior herpes simplex virus or CMV infection.
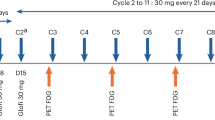
Patients received IV odronextamab in 21-day cycles. The original step-up regimen (1 to 20 mg) during C1 comprised a dose of 1 mg split over day 1 (0.5 mg) and day 2 (0.5 mg) and 20 mg split over day 8 (10 mg) and day 9 (10 mg), followed by the full dose of 160 mg on day 15. The step-up dosing regimen was optimized during the study to further mitigate the risk of CRS by reducing the initial dose and adding an intermediary dose. The revised step-up regimen of 0.7 to 4 to 20 mg regimen consisted of 0.7 mg split over day 1 (0.2 mg) and day 2 (0.5 mg), 4 mg split over day 8 and day 9 and 20 mg split over day 15 and day 16 of C1. Following C1 step-up dosing, patients received odronextamab 160 mg on days 1, 8 and 15 of C2–C4 and then 320 mg once every 2 weeks as maintenance until disease progression or another protocol-defined reason for treatment discontinuation. Patients were admitted for inpatient monitoring for 24 h following each infusion up to and including C2D1.
In patients who had a CR that lasted for 9 months or longer by investigator evaluation, the frequency of dosing was reduced to 320 mg once every 4 weeks.
Premedication with dexamethasone, diphenhydramine and acetaminophen was given during C1 step-up dosing to help mitigate the risk of CRS. All patients received 20 mg of IV dexamethasone 1–3 h before each split or single infusion dose for both regimens. Patients on the step-up regimen of 0.7 to 4 to 20 mg also received 10 mg of oral dexamethasone 12–24 h before the first split infusion and 25 mg of IV diphenhydramine and 650 mg of oral acetaminophen 30–60 min before each split or single infusion. Patients then received 10 mg of oral dexamethasone 24 h after the second split infusion or first single infusion. Premedication was continued until the patient received the full weekly dose without experiencing infusion-related reactions or CRS. Patients who developed symptoms consistent with severe CRS were considered for treatment with tocilizumab, corticosteroid and other interventions according to the clinical judgment of the investigator.
The protocol and amendments were approved by the relevant institutional review boards and ethics committees (Supplementary Table 6). The study protocol is included in the Supplementary Information. The study was conducted according to applicable regulatory requirements, guidelines of Good Clinical Practice as specified by the International Conference on Harmonization and principles originating from the Declaration of Helsinki. All patients provided written informed consent before enrollment. Where possible, the present report was developed in accordance with CONSORT reporting guidelines47. Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Endpoints and assessments
The primary endpoint was ORR, assessed by ICR and in accordance with Lugano criteria48. Secondary endpoints included ORR assessed by local investigator, CR rate, DOR, PFS, OS and patient-reported quality-of-life outcomes.
Disease assessments using computed tomography/magnetic resonance imaging and positron emission tomography were performed during screening, at week 12 and then every 8 weeks in year 1, every 12 weeks in year 2 and during follow-up as described in the protocol.
Exploratory endpoints included changes in select cytokine levels and MRD status using ctDNA, with samples taken at baseline, at week 12 and at every radiologic response assessment in patients with CR. A modified AVENIO ctDNA analysis workflow (Roche; research only) was used for next-generation sequencing according to the cancer personalized profiling obtained by deep sequencing49. Whole-blood-cell pellets were used to filter out germline allele variants and MRD negativity was reported when the P value for allele frequency was greater than 0.005 (ref. 50). The study was not powered for statistical testing of MRD analyses given their exploratory nature.
Safety and tolerability were assessed until 90 days after the last dose of study drug or initiation of another antilymphoma therapy, with AEs graded according to the National Cancer Institute Common Terminology Criteria for AEs (version 5). CRS grading was adapted from American Society for Transplantation and Cellular Therapy guidelines51. TEAE and treatment-related TEAE data are presented. TEAEs were defined as AEs that newly occurred or worsened during the on-treatment period and any treatment-related serious AEs that occurred during the post-treatment period. TEAEs were deemed treatment related by the investigator.
Statistics and reproducibility
In the ELM-2 study of B-NHL, approximately 512 patients were planned for enrollment into five disease-specific cohorts (DLBCL, follicular lymphoma, marginal zone lymphoma, mantle cell lymphoma and ‘other B-NHL’). This report included all patients in the DLBCL global cohort (160 mg once weekly or 320 mg once every 2 weeks), with no data excluded. Data distribution was assumed to be normal, but not formally tested.
An exact binomial design was adopted for the primary endpoint of ORR. The two-sided 95% CIs for the observed ORR were calculated on the basis of a sample size of 112. Assuming a clinically meaningful ORR as being greater than 35%, with 112 patients, an ORR of at least 45% would have a lower CI bound that excludes 35%. In addition, if the observed ORR was at least 50%, 55% or 60%, the lower bound of the 95% CI would exclude an ORR of 40%, 45% and 50%, respectively. With a sample size of 112 patients, if the true treatment effect of odronextamab was 50%, the probability of the observed lower bound of 95% CI excluding 35% was 89%. Enrollment was increased to include at least 60 patients treated with a step-up regimen of 0.7 to 4 to 20 mg, and up to 127 patients with 160 mg of weekly dosing. The step-up dosing regimen allocation for patients was nonrandomized and unblinded.
Patients NE for best overall response were considered nonresponders. The primary analysis for the primary endpoint was performed after all patients had completed 36 weeks of tumor assessment or withdrawn from study. Efficacy and safety analyses were performed in all patients who received odronextamab. DOR, PFS and OS were analyzed using Kaplan–Meier estimation. Data collection and analysis were not performed blind to the conditions in the experiments.
All analyses were performed using SAS (SAS Institute) version 9.4 or above. The statistical analysis plan is included in the Supplementary Information.
Subgroup analysis
Patient demographics were summarized for the DLBCL cohort, including age (<65 years, 65–75 years, and ≥65 years), sex (male or female; self-reported by patients), race (White, Black or African American, American Indian or Alaska native, native Hawaiian or other Pacific Islander, not reported, unknown, or other) and ethnicity (Hispanic or Latino, or not Hispanic or Latino).
ORR per ICR was analyzed in subgroups of patients with DLBCL defined by baseline characteristics, including age, cell of origin (germinal center B cell-like (GCB) DLBCL, activated B cell-like (ABC) DLBCL/non-GCB, or unclassified DLBCL) and cytogenetic status (triple hit or double hit). If a subgroup included fewer than ten patients, the analysis for the given subgroup was not performed or combined with another subgroup.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Patient personal data will be treated in compliance with all applicable laws and regulations. The sponsor shall take all appropriate measures to safeguard and prevent access to these data by any unauthorized third party. Qualified researchers can request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form and statistical analysis plan) that support the methods and findings in this paper. Individual anonymized patient data will be considered for sharing (1) once the product and indication have been approved by major health authorities (the U.S. Food and Drug Administration, European Medicines Agency, Pharmaceuticals and Medical Devices Agency, etc.) or development of the product has been discontinued globally for all indications on or after April 2020 and there are no plans for future development; (2) if there is legal authority to share the data; and (3) if there is not a reasonable likelihood of patient reidentification. Requests should be submitted to https://vivli.org/. Once the criteria for data availability have been fulfilled, the time frame from data request to access of data will be approximately 1–6 months. Source data are provided with this paper.
Change history
16 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s43018-025-00967-6
References
Susanibar-Adaniya, S. & Barta, S. K. 2021 update on diffuse large B cell lymphoma: a review of current data and potential applications on risk stratification and management. Am. J. Hematol. 96, 617–629 (2021).
Duarte, C. & Kamdar, M. Management considerations for patients with primary refractory and early relapsed diffuse large B-cell lymphoma. Am. Soc. Clin. Oncol. Educ. Book 43, e390802 (2023).
Harrysson, S. et al. Outcomes of relapsed/refractory diffuse large B-cell lymphoma and influence of chimaeric antigen receptor T trial eligibility criteria in second line—a population-based study of 736 patients. Br. J. Haematol. 198, 267–277 (2022).
Flowers, C. R. & Odejide, O. O. Sequencing therapy in relapsed DLBCL. Hematology Am. Soc. Hematol. Educ. Program 2022, 146–154 (2022).
Abramson, J. S. et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396, 839–852 (2020).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Highlights of Prescribing Information: BREYANZI® (U.S. Food and Drug Administration, accessed 21 January 2025); https://www.fda.gov/media/145711/download
Highlights of Prescribing Information: KYMRIAH® (U.S. Food and Drug Administration, accessed 21 January 2025); https://www.fda.gov/media/107296/download
Highlights of Prescribing Information: YESCARTA® (U.S. Food and Drug Administration, accessed 21 January 2025); https://www.fda.gov/media/108377/download
Locke, F. L. et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N. Engl. J. Med. 386, 640–654 (2022).
Kamdar, M. et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 399, 2294–2308 (2022).
Duell, J. et al. Bispecific antibodies in the treatment of hematologic malignancies. Clin. Pharmacol. Ther. 106, 781–791 (2019).
Hutchings, M. The evolving therapy of DLBCL: bispecific antibodies. Hematol. Oncol. 41, 107–111 (2023).
van de Donk, N. W. C. J. & Zweegman, S. T-cell-engaging bispecific antibodies in cancer. Lancet 402, 142–158 (2023).
Highlights of Prescribing Information: COLUMVITM (U.S. Food and Drug Administration, accessed 21 January 2025); https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761309s000lbl.pdf
Highlights of Prescribing Information: EPKINLYTM (U.S. Food and Drug Administration, accessed 21 January 2025) https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761324s003lbl.pdf
Smith, E. J. et al. A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci. Rep. 5, 17943 (2015).
Bannerji, R. et al. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 9, e327–e339 (2022).
Crombie, J. L. et al. Odronextamab demonstrates durable complete responses in patients with diffuse large B-cell lymphoma (DLBCL) progressing after CAR-T therapy: outcomes from the ELM-1 study. Blood 142, 4461 (2023).
Smedby, K. E. et al. Evaluation of eligibility for CAR-T cell therapy in a population-based cohort of 3550 patients with incident diffuse large B-cell lymphoma (DLBCL) in Sweden. Blood 136, 38–39 (2020).
Canales Albandea, M. Á. et al. Comparative analysis of CAR T-cell therapy access for DLBCL patients: associated challenges and solutions in the four largest EU countries. Front. Med. (Lausanne) 10, 1128295 (2023).
Di Rocco, A. et al. Relapsed/refractory diffuse large B-cell lymphoma patients. A multicenter retrospective analysis of eligibility criteria for car-T cell therapy. Leuk. Lymphoma 62, 828–836 (2021).
Hoffmann, M. S., Hunter, B. D., Cobb, P. W., Varela, J. C. & Munoz, J. Overcoming barriers to referral for chimeric antigen receptor T cell therapy in patients with relapsed/refractory diffuse large B cell lymphoma. Transplant Cell. Ther. 29, 440–448 (2023).
Mikhael, J., Fowler, J. & Shah, N. Chimeric antigen receptor T-cell therapies: barriers and solutions to access. JCO Oncol. Pract. 18, 800–807 (2022).
Chihara, D. et al. Real-world experience of CAR T-cell therapy in older patients with relapsed/refractory diffuse large B-cell lymphoma. Blood 142, 1047–1055 (2023).
Di Blasi, R. et al. Outcomes of patients with aggressive B-cell lymphoma after failure of anti-CD19 CAR T-cell therapy: a DESCAR-T analysis. Blood 140, 2584–2593 (2022).
Dickinson, M. J. et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 387, 2220–2231 (2022).
Thieblemont, C. et al. Epcoritamab, a novel, subcutaneous CD3×CD20 bispecific T-cell–engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J. Clin. Oncol. 41, 2238–2247 (2023).
Zhu, M. et al. Optimization of intravenous odronextamab step-up regimen for reducing the risk of high-grade cytokine release syndrome. Blood 140, 11560–11561 (2022).
Iskierka-Jażdżewska, E. et al. Health-related quality of life and symptoms in patients with relapsed or refractory diffuse large B-cell lymphoma treated with odronextamab monotherapy in the phase 2 ELM-2 study. Blood 142, 4504 (2023).
Coiffier, B. & Sarkozy, C. Diffuse large B-cell lymphoma: R-CHOP failure—what to do? Hematology Am. Soc. Hematol. Educ. Program 2016, 366–378 (2016).
Melchardt, T. et al. Clonal evolution in relapsed and refractory diffuse large B-cell lymphoma is characterized by high dynamics of subclones. Oncotarget 7, 51494–51502 (2016).
Brouwer-Visser, J. et al. Molecular assessment of intratumoral immune cell subsets and potential mechanisms of resistance to odronextamab, a CD20×CD3 bispecific antibody, in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. J. Immunother. Cancer 12, e008338 (2024).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014).
Brudno, J. N. & Kochenderfer, J. N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016).
Maus, M. V. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J. Immunother. Cancer 8, e001511 (2020).
Neelapu, S. S. et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15, 47–62 (2018).
EU Risk Management Plan for Columvi/Glofitamab Version 1.2 (F. Hoffmann-La Roche, Ltd, accessed 21 January 2025); https://www.ema.europa.eu/en/documents/rmp/columvi-epar-risk-management-plan_en.pdf
EU Risk Management Plan for Epcoritamab Version 1.4 (AbbVie, Inc., accessed 21 January 2025); https://www.ema.europa.eu/en/documents/rmp/tepkinly-epar-risk-management-plan_en.pdf
Lutz, C. et al. Patients with indolent lymphomas are at high risk of infections: experience from a German outpatient clinic. BMC Immunol. 24, 2 (2023).
Schoevaerdts, D., Sibille, F. X. & Gavazzi, G. Infections in the older population: what do we know?. Aging. Clin. Exp. Res. 33, 689–701 (2021).
Kim, T. M. et al. Safety and efficacy of odronextamab in patients with relapsed or refractory follicular lymphoma. Ann. Oncol. 35, 1039–1047 (2024).
Clinical Practice Guidelines in Oncology for Prevention and Treatment of Cancer-Related Infections Version 3 (National Comprehensive Cancer Network, accessed 17 January 2025); https://www.nccn.org/professionals/physician_gls/pdf/infections.pdf
Taplitz, R. A. et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J. Clin. Oncol. 36, 3043–3054 (2018).
Klastersky, J. et al. Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann. Oncol. 27, v111–v118 (2016).
Schulz, K. F. et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 340, c332 (2010).
Cheson, B. D. et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J. Clin. Oncol. 32, 3059–3068 (2014).
Kurtz, D. M. et al. Circulating tumor DNA measurements as early outcome predictors in diffuse large B-cell lymphoma. J. Clin. Oncol. 36, 2845–2853 (2018).
Stokowski, R. P. et al. Detection of early molecular response (EMR) and minimal residual disease (MRD) in patients with diffuse large B-cell lymphoma (DLBCL) using a validated next generation sequencing (NGS) assay for the detection of tumor variants in circulating tumor (ct)DNA. Blood 140, 9257–9258 (2022).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25, 625–638 (2019).
Acknowledgements
We would like to thank the patients, their families, the ELM-2 study team, all other investigators and all investigational site members involved in this study, especially during the challenging times of the COVID-19 pandemic. This study was funded by Regeneron Pharmaceuticals, Inc. J. Li of Regeneron Pharmaceuticals, Inc. assisted with clinical data analysis. Medical writing support was provided by N. Harrison and G. Bartle of Oberon, OPEN Health Communications and was funded by Regeneron Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Consortia
Contributions
W.S.K., T.M.K., S.G.C., I.J., E.I.-J., L.M.P., H.M.P., H.Z., J. Cao, M.Z., B.T., S.Y.O., F.L., C.C., T.-D.T., S. Ayyappan, A.G. and J.W. participated in the acquisition of clinical data from their study sites. J. Cai performed the clinical data analysis. M.U., A.C., H.M. and S. Ambati participated in the design of the study, including defining patient selection criteria, response definitions, follow-up methodology and data collection. S.S. oversaw analysis and interpretation of safety data. J.B.-V. oversaw biomarker strategy implementation, sample collection and analysis. All authors contributed to the development of the first and subsequent drafts and approved the final submission draft of the paper.
Corresponding author
Ethics declarations
Competing interests
W.S.K. reports research funding from BeiGene, Boryung, Donga, Kyowa-Kirin, Roche and Sanofi. T.M.K. reports honoraria from Amgen, AstraZeneca, IMBDx, Janssen, MedImmune and Takeda, trial research funding to institution from AstraZeneca, consultancy for AstraZeneca, Boryung, F.Hoffmann-La Roche, Janssen, MedImmune, Novartis, Regeneron Pharmaceuticals, Inc., Roche, Samsung Bioepis, Takeda and Yuhan, speaker bureau fees for IMBDx, Janssen and Takeda, membership on an entity’s board of directors or advisory committees for BeiGene, Janssen, Novartis, Regeneron Pharmaceuticals, Inc., Roche and Takeda and an uncompensated relationship with AstraZeneca, Boryung, MedImmune, Novartis and Roche. I.J. reports consultancy for Amgen, AstraZeneca, Kyowa-Kirin, Novartis, Pfizer, Sobi and Takeda, research funding from Amgen, AstraZeneca, BeiGene, Incyte, Janssen, Regeneron Pharmaceuticals, Inc., Sobi and Takeda and honoraria fees from AstraZeneca, Incyte, Janssen, Novartis, Pfizer, Sobi and Takeda. E.I.-J. reports consultancy for AbbVie and AstraZeneca and honoraria from AbbVie, AstraZeneca, Janssen and Novartis. L.M.P. reports research funding from Regeneron Pharmaceuticals, Inc. B.T. reports honoraria from AbbVie, Gilead, Incyte and Kite. C.C. reports honoraria from Gilead, Novartis, Regeneron Pharmaceuticals, Inc. and Takeda, consultancy for BMS, Regeneron Pharmaceuticals, Inc. and Takeda and membership on an entity’s board of directors or advisory committees for Regeneron Pharmaceuticals, Inc. and Takeda. Sa.A. reports membership on an entity’s board of directors or advisory committee for AbbVie, AstraZeneca, BeiGene, Fate Therapeutics and Regeneron Pharmaceuticals, Inc., consultancy for ADC Therapeutics and AstraZeneca and speaker bureau fees from BeiGene and Genentech. J. Cai, M.U., S.S., J.B.-V., A.C., H.M. and S. Ambati hold stock or stock options for and are employees of Regeneron Pharmaceuticals, Inc. J.W. reports consultancy for AbbVie, Gilead, Novartis, Roche, Takeda and MSD, research or clinical trial funding from GSK, Novartis and Roche and honoraria from AbbVie, Amgen, Gilead, GSK, Novartis, Roche and Takeda. The other authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Tilly Herve and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Patient disposition for the DLBCL cohort.
N = 127 patients enrolled into the DLBCL cohort and evaluated for efficacy and safety. n numbers represent the number of patients who received the 1/20 mg or 0.7/4/20 mg cycle 1 step-up dosing regimens, those who discontinued treatment (and reasoning), and those with ongoing treatment at the time of data cut-off (August 18, 2023). DLBCL, diffuse large B-cell lymphoma.
Extended Data Fig. 2 PFS by C4D15 ctDNA MRD status in patients with DLBCL and no CR at C4D15.
N = 35 ctDNA-evaluable patients (n = 8 with cleared MRD at C4D15; n = 27 with detected MRD at C4D15). Data are presented as Kaplan–Meier curves, with tick marks indicating patients with censored data. The HR for PFS in patients with MRD cleared versus MRD detected was calculated by univariate Cox regression. C4D15, cycle 4 day 15; CI, confidence interval; CR, complete response; ctDNA, circulating tumor DNA; DLBCL, diffuse large B-cell lymphoma; HR, hazard ratio; MRD, minimal residual disease; PFS, progression-free survival.
Extended Data Fig. 3 Treatment-emergent CRS by dose and severity grade with the 0.7/4/20 mg regimen.
The percentage of patients in the 0.7/4/20 mg regimen cohort who experienced at least one CRS event are shown according to CRS grade (1, 2, or 3). n numbers represent the number of patients treated at each of the dose points shown. CRS, cytokine release syndrome.
Supplementary information
Supplementary Information
Redacted Study Protocol and redacted Statistical Analysis Plan.
CONSORT checklist
Completed CONSORT 2010 checklist.
CONSORT abstract checklist
Completed CONSORT 2010 abstract checklist.
REMARK checklist
Completed REMARK checklist.
Supplementary Tables 1–6
Supplementary Table 1: Patient demographics and baseline characteristics of patients who received the step-up dosing regimens of 1 to 20 mg or 0.7 to 4 to 20 mg (n = number of patients). Supplementary Table 2: Best overall responses of patients who received the step-up dosing regimens of 1 to 20 mg or 0.7 to 4 to 20 mg, according to ICR (n = number of patients). Supplementary Table 3: TEAEs resulting in death among 127 patients treated with odronextamab (n = number of events). *CMV reactivation and CMV pneumonia were reported as the cause of death in one patient. Supplementary Table 4: AEs of special interest of any grade or grade ≥3 in patients treated with the step-up dosing regimens of 1 to 20 mg or 0.7 to 4 to 20 mg (n = number of events). *Events included psychiatric disorders and nervous system disorders; †treatment related. Supplementary Table 5: Rates of CRS and CRS management approaches among 60 patients treated with the C1 step-up regimen of 0.7 to 4 to 20 mg (n = number of patients). Supplementary Table 6: Countries, names and site numbers of institutional review boards and ethics committees who approved the ELM-2 study protocol and amendments. HREC, Health Research Ethics Committee; IRB, Institutional Review Board; MEC, Medical Ethics Committee; WCG, Western Institutional Review Board—Copernicus Group.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, W.S., Kim, T.M., Cho, SG. et al. Odronextamab monotherapy in patients with relapsed/refractory diffuse large B cell lymphoma: primary efficacy and safety analysis in phase 2 ELM-2 trial. Nat Cancer 6, 528–539 (2025). https://doi.org/10.1038/s43018-025-00921-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43018-025-00921-6
This article is cited by
-
Infektionen unter Therapie mit bispezifischen Antikörpern bei malignen Lymphomen, dem multiplen Myelom und der akuten lymphatischen Leukämie
best practice onkologie (2026)
-
Therapeutic landscape of primary refractory and relapsed diffuse large B-cell lymphoma: Recent advances and emerging therapies
Journal of Hematology & Oncology (2025)
-
Entwicklungen in der Rezidivtherapie des diffusen großzelligen B-Zell-Lymphoms
Die Onkologie (2025)
-
SEOM–GOTEL clinical guidelines on diffuse large B-cell lymphoma (update 2025)
Clinical and Translational Oncology (2025)
-
Efficacy and safety of epcoritamab in Japanese patients with relapsed or refractory diffuse large B-cell lymphoma: 3-year follow-up from the EPCORE NHL-3 trial
International Journal of Clinical Oncology (2025)