Abstract
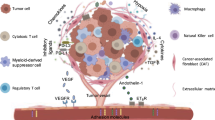
The efficacy of chimeric antigen receptor (CAR) T cells against solid tumors is limited. The molecular mechanisms underlying CAR T cell resistance are yet to be elucidated and new strategies need to be developed to improve treatment outcomes. Here we report that solid tumors respond to CAR T cells by upregulating the secretion of small extracellular vesicles carrying tumor antigens, which are horizontally transferred to CAR T cells, leading to antigen recognition and CAR T cell fratricide. Engineered CAR T cells armored with Serpin B9, a major granzyme B inhibitor, show decreased fratricide and increased vitality, tumor infiltration, and antitumor activity in female mice. Moreover, Serpin B9-armored CAR T cells show higher efficacy than parental CAR T cells in treating solid tumors when combined with the anti-programmed death 1 antibody. Our study demonstrates a mechanism that limits CAR T cell function and suggests an improved strategy in tumor treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data generated from bulk RNA-seq of this study are publicly accessible from the Gene Expression Omnibus under accession number GSE266446. The remaining data are available within the article and Supplementary Information or from the corresponding author upon request. Source data are provided with this paper.
References
Albelda, S. M. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat. Rev. Clin. Oncol. 21, 47–66 (2024).
Hou, A. J., Chen, L. C. & Chen, Y. Y. Navigating CAR-T cells through the solid-tumour microenvironment. Nat. Rev. Drug Discov. 20, 531–550 (2021).
Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023).
Haas, A. R. et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol. Ther. 27, 1919–1929 (2019).
Kershaw, M. H. et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 12, 6106–6115 (2006).
Louis, C. U. et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 (2011).
Wagner, J., Wickman, E., DeRenzo, C. & Gottschalk, S. CAR T cell therapy for solid tumors: bright future or dark reality? Mol. Ther. 28, 2320–2339 (2020).
Pan, J. et al. Donor-derived CD7 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia: first-in-human, phase I trial. J. Clin. Oncol. 39, 3340–3351 (2021).
Mamonkin, M., Rouce, R. H., Tashiro, H. & Brenner, M. K. A T-cell-directed chimeric antigen receptor for the selective treatment of T-cell malignancies. Blood 126, 983–992 (2015).
Hamieh, M. et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 568, 112–116 (2019).
Li, Y. et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat. Med. 28, 2133–2144 (2022).
Lu, Z. et al. ATF3 and CH25H regulate effector trogocytosis and anti-tumor activities of endogenous and immunotherapeutic cytotoxic T lymphocytes. Cell Metab. 34, 1342–1358 (2022).
Dixson, A. C., Dawson, T. R., Di Vizio, D. & Weaver, A. M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 24, 454–476 (2023).
Jeppesen, D. K., Zhang, Q., Franklin, J. L. & Coffey, R. J. Extracellular vesicles and nanoparticles: emerging complexities. Trends Cell Biol. 33, 667–681 (2023).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367, eaau6977 (2020).
Kalluri, R. & McAndrews, K. M. The role of extracellular vesicles in cancer. Cell 186, 1610–1626 (2023).
Mathieu, M., Martin-Jaular, L., Lavieu, G. & Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
Whiteside, T. L. Exosomes and tumor-mediated immune suppression. J. Clin. Invest. 126, 1216–1223 (2016).
Wortzel, I., Dror, S., Kenific, C. M. & Lyden, D. Exosome-mediated metastasis: communication from a distance. Dev. Cell 49, 347–360 (2019).
Guan, L. et al. HRS phosphorylation drives immunosuppressive exosome secretion and restricts CD8+ T-cell infiltration into tumors. Nat. Commun. 13, 4078 (2022).
Zhong, W. et al. Upregulation of exosome secretion from tumor-associated macrophages plays a key role in the suppression of anti-tumor immunity. Cell Rep. 42, 113224 (2023).
Good, C. R. et al. An NK-like CAR T cell transition in CAR T cell dysfunction. Cell 184, 6081–6100 (2021).
Nakao, Y. et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 122, 306–324 (2021).
Kou, X. et al. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci. Transl. Med. 10, eaai8524 (2018).
Ostrowski, M. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 12, 19–30 (2010).
Zhong, W. et al. Tumor-derived small extracellular vesicles inhibit the efficacy of CAR T cells against solid tumors. Cancer Res. 83, 2790–2806 (2023).
Moon, E. K. et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin. Cancer Res. 20, 4262–4273 (2014).
Johnson, L. R. et al. The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell 184, 4981–4995 (2021).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Bird, C. H. et al. The granzyme B–Serpinb9 axis controls the fate of lymphocytes after lysosomal stress. Cell Death Differ. 21, 876–887 (2014).
Kaiserman, D. & Bird, P. I. Control of granzymes by serpins. Cell Death Differ. 17, 586–595 (2010).
Kimman, T. et al. Serpin B9 controls tumor cell killing by CAR T cells. J. Immunother. Cancer 11, e006364 (2023).
Sun, J. et al. A cytosolic granzyme B inhibitor related to the viral apoptotic regulator cytokine response modifier A is present in cytotoxic lymphocytes. J. Biol. Chem. 271, 27802–27809 (1996).
Trapani, J. A. Granzymes: a family of lymphocyte granule serine proteases. Genome Biol. 2, REVIEWS3014 (2001).
Watanabe, K. et al. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight 3, e99573 (2018).
Cherkassky, L. et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 126, 3130–3144 (2016).
Heczey, A. et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol. Ther. 25, 2214–2224 (2017).
Adusumilli, P. S. et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 11, 2748–2763 (2021).
Grosser, R., Cherkassky, L., Chintala, N. & Adusumilli, P. S. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell 36, 471–482 (2019).
Srivastava, S. et al. Immunogenic chemotherapy enhances recruitment of CAR-T cells to lung tumors and improves antitumor efficacy when combined with checkpoint blockade. Cancer Cell 39, 193–208 (2021).
Xiao, Z. et al. Desmoplastic stroma restricts T cell extravasation and mediates immune exclusion and immunosuppression in solid tumors. Nat. Commun. 14, 5110 (2023).
Lu, L. et al. Gambogic acid inhibits TNF-α-induced invasion of human prostate cancer PC3 cells in vitro through PI3K/Akt and NF-κB signaling pathways. Acta Pharmacol. Sin. 33, 531–541 (2012).
Wong, B. R. et al. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol. Cell 4, 1041–1049 (1999).
Rivas, M. A. et al. TNFα acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-κB-dependent pathways. Exp. Cell. Res. 314, 509–529 (2008).
Hirst, C. E. et al. The intracellular granzyme B inhibitor, proteinase inhibitor 9, is up-regulated during accessory cell maturation and effector cell degranulation, and its overexpression enhances CTL potency. J. Immunol. 170, 805–815 (2003).
Bladergroen, B. A. et al. The granzyme B inhibitor, protease inhibitor 9, is mainly expressed by dendritic cells and at immune-privileged sites. J. Immunol. 166, 3218–3225 (2001).
Jiang, P. et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 24, 1550–1558 (2018).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Huang, A. C. & Zappasodi, R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat. Immunol. 23, 660–670 (2022).
Agarwal, S. et al. Deletion of the inhibitory co-receptor CTLA-4 enhances and invigorates chimeric antigen receptor T cells. Immunity 56, 2388–2407 (2023).
Chen, G. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382–386 (2018).
Ma, L. et al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 365, 162–168 (2019).
Ma, L. et al. Vaccine-boosted CAR T crosstalk with host immunity to reject tumors with antigen heterogeneity. Cell 186, 3148–3165 (2023).
Narayan, V. et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 28, 724–734 (2022).
Zhang, W. et al. ICAM-1-mediated adhesion is a prerequisite for exosome-induced T cell suppression. Dev. Cell 57, 329–343 (2022).
Evans, R. A. et al. Lack of immunoediting in murine pancreatic cancer reversed with neoantigen. JCI Insight 1, e88328 (2016).
Liu, D. A. et al. A phosphoinositide switch mediates exocyst recruitment to multivesicular endosomes for exosome secretion. Nat. Commun. 14, 6883 (2023).
Da Rocha, M. N. et al. Coated recombinant target protein helps explore IL-1RAP CAR T-cell functionality in vitro. Immunol. Res. 71, 276–282 (2023).
Yamaguchi, Y. et al. PD-L1 blockade restores CAR T cell activity through IFN-γ-regulation of CD163+ M2 macrophages. J. Immunother. Cancer 10, e004400 (2022).
Acknowledgements
We thank S. M. Albelda and E. Moon (Perelman School of Medicine, University of Pennsylvania) for providing us with the plasmids for the generation of anti-MSLN-CAR retrovirus and the human EM and EM-MSLN cell lines. We also thank S. M. Albelda for his helpful comments on the paper. We thank Y. Lu (Department of Biology, University of Pennsylvania) for his contribution to the RNA-seq analysis. This work was supported by National Institutes of Health grant R35 GM141832 to W.G. The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
W.Z. and W.G. conceptualized and designed this study. W.Z. performed most of the experiments, analyzed and interpreted the data, and wrote the paper. Z.Q., Z.Y. and J.Y. helped with the in vivo experiments. D.Y. contributed to the generation of human MSLN-CAR T cells. N.W.E. helped with the generation of human CD19-CAR T cells. L.M. helped with the production of murine TRP1-CAR T cells. N.C.S., Y.F., R.R., X.X., L.M., S.Y.F. and C.H.J. edited the paper. W.G. supervised the project and edited the paper, with all authors contributing to writing and providing feedback.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Rienk Offringa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Increased secretion of tumor antigens via sEVs in response to CAR T cell treatment.
a, Flow cytometry showing the purity of T cells isolated from mouse spleen used in the study. Experiments consisted of three biologically independent samples with similar results. b, Transfection efficiency of mouse MSLN-CAR T cells. Experiments consisted of three biologically independent samples with similar results. c, Representative electron microscopic images of sEVs isolated from tumor tissues and plasma from mice bearing 4662 tumors. Scale bar, 100 nm. Experiments consisted of three biologically independent samples with similar results. d, NTA of sEVs isolated from tumor tissues from mice bearing 4662 tumors. The X-axis indicates the diameters of the isolated vesicles; the Y-axis indicates the concentration of isolated vesicles (particles/ml). Experiments consisted of six biologically independent samples with similar results. e, NTA of sEVs isolated from plasma of mice bearing 4662 tumors. Experiments consisted of six biologically independent samples with similar results. f, Western blot showing the baseline levels of MSLN in plasma sEVs in tumor-free mice and untreated 4662 tumor-bearing mice. The blot for MSLN was overexposed so that the MSLN in plasma sEVs from tumor free mice could be detected. Relative levels of MSLN on plasma sEVs were quantified and shown to the right. Six mice were used in each group. g, Transfection efficiency of mouse TRP1-CAR T cells. Experiments consisted of three biologically independent samples with similar results. h, TRP1 co-fractionated with exosome marker proteins (CD63, TSG101 and CD9) on iodixanol density gradients from B16-F10 tumor tissue-derived sEVs. Experiments consisted of three biologically independent samples with similar results. i, TRP1 co-fractionated with exosome marker proteins on iodixanol density gradients from the plasma of B16-F10 tumor bearing mice. Experiments consisted of three biologically independent samples with similar results. j, Western blot showing the baseline levels of TRP1 in plasma sEVs in tumor-free mice and untreated B16-F10 tumor-bearing mice. The blot for TRP1 was overexposed so that the TRP1 in plasma sEVs from tumor free mice could be detected. Relative levels of MSLN on plasma sEVs were quantified and shown to the right. Six mice were used in each group. k and l, Western blots showing TRP1 and exosome marker proteins in the sEVs isolated from B16-F10 tumor tissues (k) and plasma (l) of B16-F10 tumor bearing mice. All lanes were loaded with an equal amount of proteins. Six mice were used in each group. Quantification of TRP1 expression in sEVs is shown to the right. m and n, ZetaView analysis showing the percentage of TRP1+ sEVs derived from tumor tissues (m) or the plasma (n) of B16-F10 tumor-bearing mice with indicated treatments. In the left panel, each green dot represents a sEV, while each blue ‘×’ represents TRP1 a positive sEV, as generated from manufacturer’s software. Quantification is shown to the right. Six mice were used in each group. o, Western blots showing MSLN and exosome marker proteins (CD63, TSG101 and CD9) in the sEVs isolated from MOC1 tumor tissues and the plasma of tumor-bearing mice. All lanes were loaded with an equal amount of proteins. Six mice were used in each group. p and q, Quantification of MSLN levels in sEVs from tumor tissues (p) and the plasma of tumor-bearing mice (q). Six mice were used in each group. Data represent mean ± SD (n = 3 or indicated). Statistical analysis was performed using two-sided unpaired t-test (f, j-n, p and q).
Extended Data Fig. 2 The effect of CM from CAR T cells on tumor cell apoptosis and the effect of TNF-α treatment on tumor cell sEV secretion.
a, Flow cytometry showing the effect of MSLN-CAR T cell CM (fresh medium: CM from MSLN-CAR T cells = 1: 1) on the apoptosis of 4662 cells. The quantification is shown at the right. b, flow cytometry showing the effect of TRP1-CAR T cell CM (fresh medium: CM from TRP1-CAR T cells = 1: 1) on the apoptosis of B16-F10 cells. The quantification is shown at the right. c, Quantification from nanoparticle tracking analysis showing the effect of TNF-α on sEV secretion from 4662 cells. d, Quantification from NTA showing the effect of TNF-α on sEV secretion of B16-F10 cells. Experiments consisted of three biologically independent samples for (a-d). Data represent mean ± SD (n = 3). Statistical analysis was performed using two-sided unpaired t-test (a-d).
Extended Data Fig. 3 Knockout of Rab27a inhibits sEV tumor antigen secretion.
a, effect of Rab27a KO on sEV secretion from 4662 cells as examined by NTA. Experiments consisted of three biologically independent samples. b, Western blot showing the effect of Rab27a KO on sEV MSLN secretion from 4662 cells. Quantification of MSLN is shown to the right. Experiments consisted of three biologically independent samples. c, effect of Rab27a KO on sEV secretion from B16-F10 cells. Experiments consisted of three biologically independent samples. d, Western blot showing the effect of Rab27a KO on sEV TRP1 secretion from B16-F10 cells. Quantification of TRP1 is shown to the right. Experiments consisted of three biologically independent samples. e, Western blots showing MSLN and CD63 in the circulating sEVs purified from the plasma of Rab27a KO 4662 tumor-bearing mice with MSLN-CAR T cell or Tnf-a KO MSLN-CAR T cell treatment. Quantification of MSLN levels in circulating sEVs from Rab27a KO 4662 tumor-bearing mice is shown to the right. Six mice were used in each group. f, Western blots showing TRP1 and exosome marker proteins in the circulating sEVs purified from the plasma of Rab27a KO B16-F10 tumor-bearing mice with TRP1-CAR T cell or Tnf-a KO TRP1-CAR T cell treatment. Quantification of MSLN levels in circulating sEVs from Rab27a KO 4662 tumor-bearing mice is shown to the right. Six mice were used in each group. Data represent mean ± SD (n = 3 or 6). Statistical analysis was performed using two-sided unpaired t-test (a-f).
Extended Data Fig. 4 Tumor-derived sEVs transfer tumor antigens to CAR T cells.
a, Western blot showing the MSLN expression in human MSLN-CAR T cells treated with sEVs derived from EM cells and MSLN-expressing EM cells (EM-MSLN cells). Experiments consisted of three biologically independent samples with similar results. b, EM-MSLN cell-derived sEVs increased the percentage of MSLN-positive human MSLN-CAR T cells in a dose-dependent manner as analyzed by flow cytometry. Experiments consisted of three biologically independent samples. c, Western blot showing the PSMA expression in human PSMA-CAR T cells treated with sEVs derived from PC3 cells and PSMA-expressing PC3 cells (PC3-PSMA cells). Experiments consisted of three biologically independent samples with similar results. d, PC3-PSMA cell-derived sEVs increased the percentage of PSMA-positive human PSMA-CAR T cells in a dose-dependent manner as analyzed by flow cytometry. Experiments consisted of three biologically independent samples. e, Flow cytometry showing the PSMA expression on the surface of human PSMA-CAR T cells with or without sEVs from PC3-PSMA cells or PC3 cells. The percentages of PSMA-positive CAR T cells under indicated treatments are shown at the right. Experiments consisted of three biologically independent samples. f, Western blot showing the HER2 expression in human HER2-CAR T cells treated with sEVs derived from SKOV3 cells with or without HER2 knockout. Experiments consisted of three biologically independent samples with similar results. g, SKOV3 cell-derived sEVs increased the percentage of HER2-positive human HER2-CAR T cells in a dose-dependent manner as analyzed by flow cytometry. Experiments consisted of three biologically independent samples. h, Flow cytometry showing the HER2 expression on the surface of human HER2-CAR T cells with sEVs from SKOV3 cells or HER2 KO SKOV3 cells. The percentages of HER2-positive CAR T cells under indicated treatments are shown at the right. Experiments consisted of three biologically independent samples. i, Western blot showing treatment with 4662 cell-derived sEVs increased the level of MSLN in murine MSLN-CAR T cells in a dose-dependent manner. GAPDH was used as loading control. Experiments consisted of three biologically independent samples with similar results. j, 4662 cell-derived sEVs increased the percentage of MSLN-positive murine MSLN-CAR T cells as analyzed by flow cytometry. Experiments consisted of three biologically independent samples. k, Western blot showing MOC1 cell-derived sEVs increased the level of MSLN in murine MSLN-CAR T cells. Experiments consisted of three biologically independent samples with similar results. l, MOC1 cell-derived sEVs increased the percentage of MSLN-positive in murine MSLN-CAR T cells as analyzed by flow cytometry. Experiments consisted of three biologically independent samples. m, Western blot showing the MSLN expression in murine MSLN-CAR T cells with or without sEVs derived from MOC1 or Msln KO MOC1 cells. Experiments consisted of three biologically independent samples with similar results. n, The percentage of MSLN-positive murine MSLN-CAR T cells with or without sEVs from MOC1 or Msln KO MOC1 cells as analyzed by flow cytometry. Experiments consisted of three biologically independent samples. o, Flow cytometry showing the percentage of MSLN+ MSLN-CAR T cell or TRP1-CAR T cells after 4662 sEV treatment. Experiments consisted of three biologically independent samples. p, Flow cytometry showing the percentage of TRP1+ TRP1-CAR T cell or MSLN-CAR T cells after B16-F10 sEV treatment. Experiments consisted of three biologically independent samples. q, Western blot showing B16-F10 cell-derived sEVs increased the level of TRP1 in TRP1-CAR T cells in a dose-dependent manner. Experiments consisted of three biologically independent samples with similar results. r, B16-F10 cell-derived sEV treatment increased the percentage of TRP1-positive TRP1-CAR T cells as analyzed by flow cytometry. Experiments consisted of three biologically independent samples. s, Western blot showing the TRP1 expression in TRP1-CAR T cells after treatment with sEVs from B16-F10 cells or Trp1 KO B16-F10 cells. Experiments consisted of three replicates with similar results. t, Flow cytometry showing the TRP1 expression on the surface of TRP1-CAR T cells with or without treatment of sEVs from B16-F10 cells or Trp1 KO B16-F10 cells. Quantification of TRP1+ CAR T cells with indicated treatment is shown to the right. Experiments consisted of three biologically independent samples. u, Schema of C57BL/6 mice bearing wide type or Rab27a KO B16-F10 tumors with indicated treatments after tumors reach ~150 mm3. Five mice were used in each group. v, Flow cytometry showing TRP1 expression on the surface of TRP1-CAR T cells in B16-F10 or Rab27a KO B16-F10 tumors from C57BL/6 mice with indicated treatments. Quantification of TRP1+ CAR T cells with indicated treatments is shown to the right. Five mice were used in each group. (n = 5). Data represent mean ± SD (n = 3 or indicated). Statistical analysis is performed using one-way ANOVA with Sidak’s multiple comparison tests (b, d, g, j, l, r and v), one-way ANOVA with Dunnett’s multiple comparison tests (e, h, n and t), or using two-sided unpaired t-test (o, p).
Extended Data Fig. 5 sEV-mediated tumor antigen transfer led to CAR T cell fratricide.
a, Representative images of flow cytometric analysis showing the expression of exhaustion markers (LAG3, TIM3 and PD-1) in MSLN-CAR T cells with or without 4662 cell-derived sEV treatment after 24 hrs (upper panel). Quantification of the flow cytometric analysis showing that 4662 cell-derived sEVs had no significant influence on the expression of MSLN-CAR T cell exhaustion markers (lower panel). b, Representative images of flow cytometric analysis showing the expression of exhaustion markers (LAG3, TIM3, and PD-1) in TRP1-CAR T cells with or without treatment with B16-F10 cell-derived sEVs (upper panel). The quantification of the flow cytometric analysis showing that B16-F10 cell-derived sEVs had no significant influence on TRP1-CAR T cell exhaustion markers (lower panel). c, The effect of MOC1 cell-derived sEVs on the viability of MSLN-CAR T cells over time. d, The effect of MOC1 cell-derived sEVs with indicated treatments on the viability of MSLN-CAR T cells. e, Flow cytometry showing the killing of MOC1 cell- or Msln KO MOC1 cell-derived sEV treated MSLN-CAR T cells by fresh MSLN-CAR T cells. Apoptosis of MSLN-CAR T cells detected by flow cytometry analysis of Annexin V (left panel), and the percentage of apoptotic MSLN-CAR T cells is calculated (right panel). f, The effect of EM-MSLN cell-derived sEVs on the viability of human MSLN-CAR T cells over time. g, The effect of EM-MSLN cell-derived sEVs with indicated treatments on the viability of human MSLN-CAR T cells. h, Flow cytometry showing the killing of tumor cell-derived sEV treated human MSLN-CAR T cells by fresh human MSLN-CAR T cells. Apoptosis of MSLN-CAR T cells detected by flow cytometry analysis of Annexin V (left panel), and the percentage of apoptotic MSLN-CAR T cells is calculated (right panel). i, The effect of PC3-PSMA cell-derived sEVs on the viability of PSMA-CAR T cells over time. j, The effect of PC3-PSMA cell-derived sEVs with indicated treatments on the viability of PSMA-CAR T cells. k, Flow cytometry showing the killing of PC3-PSMA cell- or PC3 cell-derived sEV treated PSMA-CAR T cells by fresh PSMA-CAR T cells. Apoptosis of PSMA-CAR T cells detected by flow cytometry analysis of Annexin V (left panel), and the percentage of apoptotic PSMA-CAR T cells is calculated (right panel). l, The effect of SKOV3 cell-derived EVs on the viability of HER2-CAR T cells over time. m, The effect of SKOV3 cell-derived sEVs with indicated treatments on the viability of HER2-CAR T cells. n, Flow cytometry showing the killing of SKOV3 cell- or HER2 KO SKOV3 cell-derived sEV treated HER2-CAR T cells by fresh HER2-CAR T cells. Apoptosis of HER2-CAR T cells detected by flow cytometry analysis of Annexin V (left panel), and the percentage of apoptotic HER2-CAR T cells is calculated (right panel). o, The effect of B16-F10-CD19 cell-derived sEVs on the viability of human CD19-CAR T cells over time. p, The effect of B16-F10 or B16-F10-CD19 cell-derived sEVs with indicated treatments on the viability of human CD19-CAR T cells. q, Flow cytometry showing the killing of B16-F10 cell- or B16-F10-CD19 cell-derived sEV treated CD19-CAR T cells by fresh human CD19-CAR T cells. Apoptosis of human CD19-CAR T cells detected by flow cytometry analysis of Annexin V (left panel), and the percentage of apoptotic CD19-CAR T cells is calculated (right panel). Experiments consisted of three biologically independent samples for a-q. r, Representative IHC images showing the levels of cleaved-caspase 3 in 4662 tumors or 4662 tumors with Rab27a KO, with or without indicated sEV treatments. Cell nuclei were stained with hematoxylin (blue). Cleaved-caspase 3 was stained with primary and secondary antibodies followed by DAB (brown). Scale bar: 100 µm. The quantification is shown at the right. Five mice were used in each group. s, The number of CD45+ immune cells in 4662 tumors for each group of mice quantified based on flow cytometry analysis. Five mice were used in each group. t, Representative IHC images showing the levels of cleaved-caspase 3 in B16-F10 tumors or B16-F10 tumors with Rab27a KO, with or without indicated sEV treatments. Scale bar: 100 µm. The quantification is shown at the right. Five mice were used in each group. u, The number of CD45+ immune cells in B16-F10 tumors for each group of mice quantified based on flow cytometry analysis. Five mice were used in each group. Data represent mean ± SD (n = 3 or indicated). Statistical analysis is performed using two-sided unpaired t-test (a-c, f, i, l and o), one-way ANOVA with Sidak’s multiple comparison tests (d, g, j, m, r-u) or one-way ANOVA with Dunnett’s multiple comparison tests (e, h, k, n, p and q).
Extended Data Fig. 6 The effect of leukemia cell sEVs on CD19-CAR T cell fratricide.
a and b, Flow cytometry analysis showing no significant effect of NALM6 cell (a) or Raji cell (b) derived sEVs on CD19-CAR T cell fratricide. c and d. Western blots showing the presence of CD19 and Serpin B9 in the whole cell lysates (Wcl) and the sEVs from NALM6 (c) and Raji cells (d). e, Western blots showing that sEVs from NALM6 cells or Raji cells increased the expression of CD19 and Serpin B9 in CD19-CAR T cells. f and g, Western blots showing Serpin B9 was barely detectable in the Wcl and the sEVs from 4662 cells (f) and B16-F10 cells (g). h, Western blots showing that sEVs from 4662 cells or B16-F10 cells had no significant effect on the levels of Serpin B9 in MSLN-CAR T cells or TRP1-CAR T cells. i and j, Western blots showing the knockdown of Serpin B9 in NALM6 cells (i) and Raji cells (j) and their sEVs. k and l, Western blots showing Serpin B9 knockdown attenuated the NALM6 cell (k) and Raji cell (l) sEV-mediated Serpin B9 expression in CD19-CAR T cells. Experiments consisted of three biologically independent samples with similar results for c-l. m and n, Flow cytometry analysis showing that sEVs from Serpin B9 knockdown NALM6 cells (m) or Raji cells (n) enhanced CAR T cell fratricide. Experiments consisted of three biologically independent samples for m and n. Data represent mean ± SD (n = 3). Statistical analysis was performed using two-sided unpaired t test (a and b), or using one-way ANOVA with Dunnett’s multiple comparison tests (m and n).
Extended Data Fig. 7 Serpin B9-armored MSLN-CAR T cells show increased tumor cell killing.
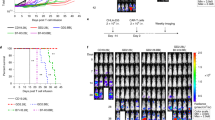
a, Western blot showing the expression of endogenous Serpin B9 and EGFP-Serpin B9 in MSLN-CAR T and Se9-MSLN-CAR T cells using the anti-Serpin B9 antibody. The second-generation anti-MSLN CAR retroviral construct, which included EGFP reporter was used in this study (see ‘Methods’). Experiments consisted of three biologically independent samples with similar results. b, Flow cytometric analysis showing the transfection efficiency of mouse MSLN-CAR T cells and Se9-MSLN-CAR T cells. Experiments consisted of three biologically independent samples. c, Se9-MSLN-CAR T cells showed stronger killing of 4662 cells compared to MSLN-CAR T cells. Experiments consisted of three biologically independent samples. d, Growth curves of tumors in mice with UTD T, MSLN-CAR T and Se9-MSLN-CAR T treatments, respectively (n = 5). e, MSLN-CAR T or Se9-MSLN-CAR T cell treatment did not show a significant difference in the body weights of 4662 tumor-bearing mice (n = 5). Data represent mean ± SD (n = 3 or indicated). Statistical analysis was performed using two-sided unpaired t-tests (b, c) or one-way ANOVA with Dunnett’s multiple comparison tests (e).
Extended Data Fig. 8 Serpin B9 armoring inhibits CAR T cell fratricide and increases the function of CAR T cells.
a, Design of TRP1-CAR and Se9-TRP1-CAR. b, Western blots showing the expression of Serpin B9 in TRP1-CAR T cells and Se9-TRP1-CAR T cells. Experiments consisted of three biologically independent samples with similar results. c, Effect of B16-F10 cell-derived sEVs on the viability of TRP1-CAR T cells and Se9-TRP1-CAR T cells over time. Experiments consisted of three biologically independent samples. d, Flow cytometry showing the killing of TRP1-CAR T cells and Se9-TRP1-CAR T cells by fresh TRP1-CAR T cells. Apoptosis of TRP1-CAR T cells or Se9-TRP1-CAR T cells is detected by flow cytometry analysis of Annexin V (left panel), and the percentage of apoptotic CAR T cells is calculated (right panel). Experiments consisted of three biologically independent samples. e, Schema of C57BL/6 mice bearing B16-F10 tumors treated with indicated T cells. f, Growth curves of tumors in mice with indicated treatments (n = 5). g, Survival curves of mice with indicated treatments (n = 5). h, Percentage of live TRP1-CAR T cells or Se9-TRP1-CAR T cells in total live cells from B16-F10 tumors with indicated treatments as quantified from flow cytometry analysis (n = 5). i, The number of TRP1-CAR T cells or Se9-TRP1-CAR T in tumors for each group of mice quantified based on flow cytometry analysis (n = 5). j, TRP1-CAR T or Se9-TRP1-CAR T cells did not result in a significant difference in the body weights of B16-F10 tumor-bearing mice (n = 5). Data represent mean ± SD (n = 3 or indicated). Statistical analysis was performed using two-sided unpaired t-tests (c, d, i, j), one-way ANOVA with Dunnett’s multiple comparison tests (f, j) or log-rank tests (g).
Extended Data Fig. 9 Treatment of 4662 tumors by the Se9-MSLN-CAR T cells and anti-PD-1 combination.
a, Growth curves of 4662 tumors in mice with indicated treatments. b, Growth curves of 4662 tumors in mice with indicated treatments. c, Body weight of 4662 tumor bearing mice with indicated treatments. Data represent mean ± SD (n = 7). Statistical analysis is performed using Welch ANOVA with Dunnett T3 multiple comparison tests (a, c).
Extended Data Fig. 10 Treatment of B16-F10 tumors by the Se9-TRP1-CAR T cell and anti-PD-1 combination.
a, Treatment with anti-PD-1 antibody or TRP1-CAR T cells alone had no inhibitory effect on the growth of B16-F10 tumors. b, Growth curves of B16-F10 tumors in mice with indicated treatments. c, B16-F10 tumor weights with indicated treatments. d, Survival curves of mice with indicated treatments. e, The number of TRP1-CAR T cells or Se9-TRP1-CAR T cells in tumors for each group of mice quantified based on flow cytometry analysis. f, The percentage of live TRP1-CAR T cells or Se9-TRP1-CAR T cells in total live cells from B16-F10 tumors with indicated treatment as quantified from flow cytometry analysis. g, The percentage of GzmB+ TRP1-CAR T cells as quantified by flow cytometry. h, The percentage of Ki-67+ TRP1-CAR T cells as quantified by flow cytometry. i, The combination of Se9-TRP1-CAR T cell with anti-PD-1 antibody showed no significant effect on the body weight of B16-F10 tumor-bearing mice. Data represent mean ± SD (n = 7). Statistical analysis was performed using Welch ANOVA with Dunnett T3 multiple comparison tests (a-c, e-i), or log-rank tests (d).
Supplementary information
Supplementary Information
Supplementary Figs. 1–16 and Tables 1 and 2.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, W., Qin, Z., Yu, Z. et al. Overcoming extracellular vesicle-mediated fratricide improves CAR T cell treatment against solid tumors. Nat Cancer 6, 1157–1172 (2025). https://doi.org/10.1038/s43018-025-00949-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s43018-025-00949-8