Abstract
Resistance to human epidermal growth factor receptor 2 (HER2)-targeted therapies and immuno-oncology agents poses a major challenge in treating HER2-positive breast cancer. Here we demonstrate that p95HER2, a truncated form of HER2, drives immune evasion in HER2-positive female breast cancer, enhancing tumor growth and conferring therapy resistance. This stems from the unique ability of p95HER2 to promote cancer cell-intrinsic programmed death ligand 1 expression and secretion of immunosuppressive mediators including interleukin 6. In preclinical models, this impairs the efficacy of trastuzumab deruxtecan, a HER2-directed antibody–drug conjugate (ADC) that relies on immunogenic responses to cell death for full efficacy. Importantly, we find that neratinib potently directs proteasomal degradation of p95HER2, relieving its immunosuppressive effects, and provide proof of concept that neratinib and/or agents targeting p95HER2 downstream mediators can restore antitumor immunity and trastuzumab deruxtecan efficacy. This study reveals a p95HER2-specific therapy resistance mechanism in HER2-positive female breast cancer and highlights the potential value of targeting p95HER2 to improve outcomes with ADCs or immuno-oncology agents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All raw RNA-seq data from mouse tumors are available from the Gene Expression Omnibus through accession numbers GSE220203 and GSE256132. FinHer gene expression data are available from the Gene Expression Omnibus through accession number GSE65095. The MSigDB used for GSEA can be accessed online (https://www.gsea-msigdb.org/gsea/msigdb). Raw p95HER2 and total HER values for FinHer samples are available in Supplementary Table 5. All other data supporting the findings of this study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
Code availability
No custom code or algorithms were developed for this study.
References
Goutsouliak, K. et al. Towards personalized treatment for early stage HER2-positive breast cancer. Nat. Rev. Clin. Oncol. 17, 233–250 (2020).
Rimawi, M. F., Schiff, R. & Osborne, C. K. Targeting HER2 for the treatment of breast cancer. Annu. Rev. Med. 66, 111–128 (2015).
Swain, S. M., Shastry, M. & Hamilton, E. Targeting HER2-positive breast cancer: advances and future directions. Nat. Rev. Drug Discov. 22, 101–126 (2023).
Nahta, R. et al. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat. Clin. Pract. Oncol. 3, 269–280 (2006).
Arribas, J., Baselga, J., Pedersen, K. & Parra-Palau, J. L. p95HER2 and breast cancer. Cancer Res. 71, 1515–1519 (2011).
Pedersen, K. et al. A naturally occurring HER2 carboxy-terminal fragment promotes mammary tumor growth and metastasis. Mol. Cell. Biol. 29, 3319–3331 (2009).
Ward, T. M. et al. Truncated p110 ERBB2 induces mammary epithelial cell migration, invasion and orthotopic xenograft formation, and is associated with loss of phosphorylated STAT5. Oncogene 32, 2463–2474 (2013).
Chumsri, S. et al. High p95HER2/HER2 ratio associated with poor outcome in trastuzumab-treated HER2-positive metastatic breast cancer NCCTG N0337 and NCCTG 98-32-52 (Alliance). Clin. Cancer Res. 24, 3053–3058 (2018).
Lipton, A. et al. HER3, p95HER2, and HER2 protein expression levels define multiple subtypes of HER2-positive metastatic breast cancer. Breast Cancer Res. Treat. 141, 43–53 (2013).
Nishimura, R. et al. Role of HER2-related biomarkers (HER2, p95HER2, HER3, PTEN, and PIK3CA) in the efficacy of lapatinib plus capecitabine in HER2-positive advanced breast cancer refractory to trastuzumab. Oncology 93, 51–61 (2017).
Saez, R. et al. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin. Cancer Res. 12, 424–431 (2006).
Carvajal-Hausdorf, D. E. et al. Measurement of domain-specific HER2 (ERBB2) expression may classify benefit from trastuzumab in breast cancer. J. Natl Cancer Inst. 107, djv136 (2015).
Sperinde, J. et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin. Cancer Res. 16, 4226–4235 (2010).
Kruger, J. M. et al. Detection of truncated HER2 forms in formalin-fixed, paraffin-embedded breast cancer tissue captures heterogeneity and is not affected by HER2-targeted therapy. Am. J. Pathol. 183, 336–343 (2013).
Duchnowska, R. et al. Quantitative measurements of tumoral p95HER2 protein expression in metastatic breast cancer patients treated with trastuzumab: independent validation of the p95HER2 clinical cutoff. Clin. Cancer Res. 20, 2805–2813 (2014).
Duchnowska, R. et al. Quantitative HER2 and p95HER2 levels in primary breast cancers and matched brain metastases. Neuro Oncol. 17, 1241–1249 (2015).
Nuciforo, P., Radosevic-Robin, N., Ng, T. & Scaltriti, M. Quantification of HER family receptors in breast cancer. Breast Cancer Res. 17, 53 (2015).
Angelini, P. D. et al. Constitutive HER2 signaling promotes breast cancer metastasis through cellular senescence. Cancer Res. 73, 450–458 (2013).
Iwata, T. N. et al. A HER2-targeting antibody–drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol. Cancer Ther. 17, 1494–1503 (2018).
Dongre, A. et al. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res. 77, 3982–3989 (2017).
Veglia, F., Perego, M. & Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 19, 108–119 (2018).
Penichet, M. L. et al. In vivo properties of three human HER2/neu-expressing murine cell lines in immunocompetent mice. Lab. Anim. Sci. 49, 179–188 (1999).
Yong, C. S. et al. Embryonic lethality in homozygous human Her-2 transgenic mice due to disruption of the Pds5b gene. PLoS ONE 10, e0136817 (2015).
Cho, H. M. et al. Delivery of NKG2D ligand using an anti-HER2 antibody–NKG2D ligand fusion protein results in an enhanced innate and adaptive antitumor response. Cancer Res. 70, 10121–10130 (2010).
D’Amico, L. et al. A novel anti-HER2 anthracycline-based antibody–drug conjugate induces adaptive anti-tumor immunity and potentiates PD-1 blockade in breast cancer. J. Immunother. Cancer 7, 16 (2019).
Iwata, T. N., Sugihara, K., Wada, T. & Agatsuma, T. [Fam-] trastuzumab deruxtecan (DS-8201a)-induced antitumor immunity is facilitated by the anti-CTLA-4 antibody in a mouse model. PLoS ONE 14, e0222280 (2019).
Joensuu, H. et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N. Engl. J. Med. 354, 809–820 (2006).
Sperinde, J. et al. p95HER2 methionine 611 carboxy-terminal fragment is predictive of trastuzumab adjuvant treatment benefit in the FinHer trial. Clin. Cancer Res. 24, 3046–3052 (2018).
Sonnenblick, A. et al. Integrative proteomic and gene expression analysis identify potential biomarkers for adjuvant trastuzumab resistance: analysis from the Fin-her phase III randomized trial. Oncotarget 6, 30306–30316 (2015).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Charoentong, P. et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 18, 248–262 (2017).
Yoshihara, K. et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 4, 2612 (2013).
Singer, M. et al. A distinct gene module for dysfunction uncoupled from activation in tumor-infiltrating T cells. Cell 166, 1500–1511 (2016).
Gupta, R. & Brunak, S.Prediction of glycosylation across the human proteome and the correlation to protein function. Pac. Symp. Biocomput. 2002, 310–322 (2002).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Pander, G. et al. Antibody–drug conjugates: what drives their progress? Drug Discov. Today 27, 103311 (2022).
Ahmed, A. & Tait, S. W. G. Targeting immunogenic cell death in cancer. Mol. Oncol. 14, 2994–3006 (2020).
Kroemer, G., Galassi, C., Zitvogel, L. & Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 23, 487–500 (2022).
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22, 5097–5108 (2016).
Citri, A., Kochupurakkal, B. S. & Yarden, Y. The achilles heel of ErbB-2/HER2: regulation by the Hsp90 chaperone machine and potential for pharmacological intervention. Cell Cycle 3, 51–60 (2004).
Klapper, L. N., Waterman, H., Sela, M. & Yarden, Y. Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res. 60, 3384–3388 (2000).
Marx, C., Held, J. M., Gibson, B. W. & Benz, C. C. ErbB2 trafficking and degradation associated with K48 and K63 polyubiquitination. Cancer Res. 70, 3709–3717 (2010).
Raja, S. M. et al. A combination of trastuzumab and 17-AAG induces enhanced ubiquitinylation and lysosomal pathway-dependent ErbB2 degradation and cytotoxicity in ErbB2-overexpressing breast cancer cells. Cancer Biol. Ther. 7, 1630–1640 (2008).
Xu, W. et al. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc. Natl Acad. Sci. USA 99, 12847–12852 (2002).
Zhou, P. et al. ErbB2 degradation mediated by the co-chaperone protein CHIP. J. Biol. Chem. 278, 13829–13837 (2003).
Zhang, Y. et al. Neratinib induces ErbB2 ubiquitylation and endocytic degradation via HSP90 dissociation in breast cancer cells. Cancer Lett. 382, 176–185 (2016).
Chandarlapaty, S. et al. Inhibitors of HSP90 block p95-HER2 signaling in trastuzumab-resistant tumors and suppress their growth. Oncogene 29, 325–334 (2010).
Wolf, Y., Anderson, A. C. & Kuchroo, V. K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 20, 173–185 (2020).
Zhang, M. et al. Galectin-9 in cancer therapy: from immune checkpoint ligand to promising therapeutic target. Front. Cell Dev. Biol. 11, 1332205 (2023).
Zhou, T. et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature 583, 609–614 (2020).
Fabbi, M., Carbotti, G. & Ferrini, S. Context-dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP. J. Leukoc. Biol. 97, 665–675 (2015).
Reschke, R. & Gajewski, T. F. CXCL9 and CXCL10 bring the heat to tumors. Sci. Immunol. 7, eabq6509 (2022).
Pan, M. et al. Targeting CXCL9/10/11-CXCR3 axis: an important component of tumor-promoting and antitumor immunity. Clin. Transl. Oncol. 25, 2306–2320 (2023).
Komar, A. A. & Merrick, W. C. A retrospective on eIF2A—and not the alpha subunit of eIF2. Int. J. Mol. Sci. 21, 2054 (2020).
Kwon, O. S. et al. An mRNA-specific tRNAi carrier eIF2A plays a pivotal role in cell proliferation under stress conditions: stress-resistant translation of c-Src mRNA is mediated by eIF2A. Nucleic Acids Res. 45, 296–310 (2017).
Lu, P. D., Harding, H. P. & Ron, D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167, 27–33 (2004).
Sendoel, A. et al. Translation from unconventional 5′ start sites drives tumour initiation. Nature 541, 494–499 (2017).
Nishikawa, R. et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc. Natl Acad. Sci. USA 91, 7727–7731 (1994).
Erdem-Eraslan, L. et al. Mutation specific functions of EGFR result in a mutation-specific downstream pathway activation. Eur. J. Cancer 51, 893–903 (2015).
Lavictoire, S. J. et al. Interaction of Hsp90 with the nascent form of the mutant epidermal growth factor receptor EGFRvIII. J. Biol. Chem. 278, 5292–5299 (2003).
Li, B. T. et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov. 10, 674–687 (2020).
Dongre, A. et al. Direct and indirect regulators of epithelial–mesenchymal transition-mediated immunosuppression in breast carcinomas. Cancer Discov. 11, 1286–1305 (2021).
Ye, X. et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 525, 256–260 (2015).
Ekambaram, P. et al. The CARMA3–Bcl10–MALT1 signalosome drives NFκB activation and promotes aggressiveness in angiotensin II receptor-positive breast cancer. Cancer Res. 78, 1225–1240 (2018).
Lee, J. L. et al. MALT1 is a targetable driver of epithelial-to-mesenchymal transition in Claudin-low, triple-negative breast cancer. Mol. Cancer Res. 20, 373–386 (2022).
Sachs, N. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172, 373–386 (2018).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis 2nd edn (Springer, 2016).
Hanzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 14, 7 (2013).
Cappellesso, F. et al. Targeting the bicarbonate transporter SLC4A4 overcomes immunosuppression and immunotherapy resistance in pancreatic cancer. Nat. Cancer 3, 1464–1483 (2022).
Acknowledgements
We wish to thank all members of the P.C.L. and L.M.M.-L. lab and the NSABP Foundation team for their insights, suggestions and support. We thank the Translational Pathology Imaging Core and the Flow Cytometry Core of the UPMC Hillman Cancer Center for exceptional guidance and resources. This research was supported by the Mayo Clinic Breast Cancer SPORE grant (National Institutes of Health (NIH), P50-CA116201, to P.C.L. and L.M.M.-L.), Breast Cancer Research Foundation awards (to N.W., A.V.L. and S.O.), the Sigrid Juselius Foundation (to H.J.), the Tsinghua University Educational Foundation North America and Scholarship Fund of the China Scholarship Council awards (to X.L. and Z.C.), a Conover Scholar award (to H.C.), a University of Pittsburgh MSTP T32 training grant (T32-GM008208, to N.M.C., H.C. and J.L.), the NIH (F30-CA2649632, to N.M.C.), the Wheeler Family Charitable Foundation Endowed Chair (to L.M.M.-L.), The University of Pittsburgh UPMC Institute for Precision Medicine (to J.M.A., D.D.B. and A.V.L.), a research gift from the NSABP Foundation (to P.C.L.), the UPMC Hillman Cancer Center Support Grant (NIH, P30-CA047904), including use of the UPMC Hillman Cancer Center Tissue and Research Pathology/Pitt Biospecimen Core, a UPMC Hillman Cancer Center Pilot and Feasibility Award and a Shear Family Foundation Grant (to P.C.L.) and the Mayo Clinic Cancer Center Support Grant (NIH, P30-CA15083). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
D.H., L.M.M.-L. and P.C.L. conceptualized and oversaw the study. D.H., X.L., P.E., Z.C., N.M.C., H.E.C., J.L. 4th, F.K., L.R.K. and M.B. conducted and interpreted the cell-based and in vivo experiments. A.D., J.M.A., D.D.B., H.F., A.V.L. and S.O. generated and evaluated the cell-based and organoid-based models. T.F. and M.J. performed and interpreted the multispectral IF analyses. J.S., W.H. and H.J. developed the assay systems and evaluated the clinical trial specimens and data. J.Z. and G.C.T. conducted the statistical analyses. Z.L. and Y.W. performed the bioinformatic analyses. T.C.B. helped to design and oversee the Cytek Aurora flow cytometry strategy. A.S., K.L.P.-G., L.D.E., A.S.L., A.V.L, S.O., N.W., C.J.A., S.A.J., L.M.M.-L. and P.C.L. acquired funding, designed the methodology and approach and evaluated the data. D.H., L.M.M.-L. and P.C.L. wrote the manuscript. All authors revised the manuscript and approved the submission.
Corresponding authors
Ethics declarations
Competing interests
P.E. declares equity interest (class P unit shares) in Inceptor Bio outside the submitted work. J.S. and W.H. are employees of Monogram Biosciences, Laboratory Corporation of America Holdings. H.J. has a leadership role in Orion Pharma, Neutron Therapeutics and Sartar Therapeutics, has a consulting or advisory role in Orion Pharma and Neutron Therapeutics, has received honoraria for scientific meetings from Deciphera Pharmaceuticals and has equity interest in Orion Pharma and Sartar Therapeutics. L.D.E. is an employee of Puma Biotechnology and has equity interest in Puma Biotechnology. A.S.L. is an employee of Puma Biotechnology and has equity interest in Puma Biotechnology. L.M.M.-L. has equity interest in AMGN and has received speaker compensation from Schrödinger, both outside the submitted work. P.C.L. has equity interest in AMGN outside the submitted work. All other authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Mothaffar Rimawi, Alfred Zippelius and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 p95HER2 promotes tumor growth by suppressing anti-tumor immunity.
(a) Cell proliferation assay evaluating the effect of expressing FL-HER2 or p95HER2 on cell growth in culture. Cell proliferation was monitored in real time using the IncuCyte imaging system. N = 4 replicate wells per cell line; data are expressed as mean ± SEM; results are representative of n = 3 biological repeats. (b) Growth curves of PB2 tumorgrafts in both immunodeficient NSG mice and immunocompetent C57BL/6 mice. PB2 cells expressing either FL-HER2 or p95HER2 under the control of doxycycline were implanted into the mammary fat pads of mice which were then fed doxycycline diet. Empty vector (EV) PB2 tumorgrafts served as a control. Growth for each tumor type is plotted normalized to the initial (baseline) EV tumor size. EV: left n = 10, right n = 14; FL-HER2: left n = 10, right n = 13; p95HER2: left n = 10, right, n = 13. Data represent mean ± SEM. Statistical analyses by ordinary one-way ANOVA with Tukey’s correction for multiple comparisons. P values are indicated within the plots. (c) Individual growth trajectories for PB2 tumors expressing either p95HER2 or FL-HER2 in C57BL/6 mice, analogous to panel b, but without normalization (FL-HER2 n = 10, p95HER2 n = 10). Statistical analysis by unpaired, two-tailed Student’s t test (p = 0.0133). (d) Extended growth trajectories for individual PB2 tumors expressing p95HER2 in C57BL/6 mice, along with associated Kaplan Meier curve depicting Event Free Survival (EFS) which includes mandated sacrifice as an event. (e) qRT-PCR demonstrating the effect of p95HER2 on expression of immune-related genes in PB2 tumors grown 21 days in the mammary glands of immunocompetent C57BL/6 mice (n = 8 for p95HER2+ tumor-bearing mice compared to n = 10 for EV tumor-bearing mice). Data represent mean ± SEM. Statistical analysis by unpaired, two-tailed Student’s t test with Welch’s correction. P values are indicated within the plot. (f) Gene Set Enrichment Analysis (GSEA) of RNA-seq data from PB2 tumors expressing p95HER2 versus control PB2 tumors, performed using GOBP and KEGG databases. (g) Enrichment plots of gene sets that are involved in IFN-γ response and antigen processing and presentation. Both gene sets are found to be downregulated in p95HER2+ PB2 tumors grown in immunocompetent C57BL/6 mice. GSEA was performed against the GOBP database. The significance is reported as a Normalized Enrichment Score (NES) and adjusted for multiple testing using False Discovery Rate (FDR) control. (h) Ingenuity pathway analysis (IPA) of the same RNAseq data. The top 20 pathways that are significantly altered in p95HER2+ PB2 tumors and have absolute z-score = or >2 are included. Pathways downregulated in association with p95HER2 are in blue, while those upregulated in association with p95HER2 are in orange. Statistical analyses by a right-tailed Fisher’s exact test adjusted with false discovery rate control. (i) Proportion of CD45+ and CD8+ immune cells positive for IFN-γ in PB2 tumors that express either p95HER2 or FL-HER2 (n = 4 EV tumors and n = 7 p95HER2+ and HER2+ tumors). Data represent mean ± SEM. Statistical analysis by unpaired, two-tailed Student’s t test. P values are indicated within the plot.
Extended Data Fig. 2 PB2 and EMT6 tumor models for the evaluation of immune cell infiltrates.
(a) Correlations between p95HER2 and both CD3 protein levels and tumor size. Protein levels determined by Western densitometry (arbitrary units). Simple linear regressions with 95% confidence bands are shown. Pearson correlations (R) with associated one-tailed p values are listed in the plot. (b) Example of pancytokeratin staining of a PB2 tumor using the Akoya PhenoImager HT, with staining presented as a Pathview pseudochromogenic IHC image. Corresponding example of tumor/stromal mask, generated based on the pancytokeratin staining. (c) Quantification of Ly6G, CD161, F4/80, and CD19 as determined by mIF analysis of PB2 tumors described in Fig. 2. No significant difference in the infiltration of cells expressing any of these markers was seen between p95HER2-low and p95HER2-high expressing tumors, within either cancer cell dominated ‘tumor’ or stromal regions. Each data point indicates marker quantification from a single ROI. Data represent mean ± SEM. Statistical analyses by two-tailed Mann-Whitney U test. P values are indicated within the plots. (d) Schematic depicting the strategy for constitutive expression of human FL-HER2 in EMT6 cells, along with dox-induced mouse p95HER2. Flow cytometric analysis demonstrating cell-surface expression of FL-HER2 in EMT6 cells. (e) Growth of orthotopic EMT6 tumorgrafts in syngeneic, immunocompetent BALB/c mice. The expression of human FL-HER2 in EMT6 cells slows tumor growth but not as dramatically as when expressed in the PB2 – C57BL/6 model depicted in Fig. 2c. N = 10 per group. Data represent mean ± SEM. Statistical analysis by unpaired, two-tailed Student’s t test with Welch’s correction and two-stage Benjamini-Hochberg correction for multiple comparisons. P values are indicated within the plot.
Extended Data Fig. 3 p95HER2 is associated with signatures of immune suppression in FinHER specimens.
(a,b) GSEA analysis of FinHER dataset (GSE65095). The log-transformed ratio of p95HER2 protein versus total HER2 protein [log(p95HER2/HER2)] was used as a continuous (a) or median-split categorical (b) variable for GSEA analysis against databases including Hallmark, KEGG, Reactome and GOBP. (c) Correlation between the p95HER2/HER2 ratio (as a continuous variable, n = 180) and the log2 transformed mRNA levels of specific immunomodulatory molecules as per the GSE65095 dataset. Simple linear regressions with 95% confidence bands are shown. Pearson correlations (R) with associated two-tailed p values are listed in the plot.
Extended Data Fig. 4 p95HER2 induces the expression of several immune-related cytokines independently of activating senescence.
(a) p95HER2 acutely induces IL-6 protein secretion. ELISAs were performed to measure IL-6 protein level in the media from PB2 cells expressing either p95HER2 or FL-HER2 under control of doxycycline (0.5 µg/mL for 48 h). Similarly, p95HER2 induces IL-6 secretion from MDA-MB-453 cells, which is abolished by neratinib (50 nM for 24 h). N = 3 independent biologic samples per condition. Data represent mean ± SEM. Statistical analyses by ordinary one-way ANOVA with Tukey’s correction for multiple comparisons. P values are indicated within the plots. (b) The same cytokine array as depicted in Fig. 4i, presented with different exposure levels. Cytokines that are induced by p95HER2 are annotated in red. (c) Short-term expression of p95HER2 fails to induce cellular senescence in both MCF-7 and PB2 cells. Cells were treated with doxycycline for 48 h to induce either FL-HER2 or p95HER2, then fixed and evaluated with cellular senescence assay kit (β-galactosidase). As a positive control, cells were treated with etoposide for 24 h and allowed to recover prior to assaying senescence. (d) Expression of p95HER2 fails to induce p21 expression in PB2 cells. Cells were treated with increasing doses of doxycycline for 48 h to induce either FL-HER2 or p95HER2, then harvested for Western analysis to measure expression of proteins as indicated. (e) Cellular senescence signatures are downregulated in FinHER specimens with a high p95HER2/HER2 ratio. GSVA enrichment score calculations for cellular senescence signatures in HER2+ tumors within the FinHER dataset (median-split categorical analysis. p95HER2/HER2 ratio: low n = 90, high n = 90 for each plot). Boxes span the upper to lower quartiles with a median center line. Whiskers extend from the minimum to the maximum values in the dataset. Statistical analyses by unpaired, two-tailed Student’s t test. P values are indicated within the plots.
Extended Data Fig. 5 p95HER2-dependent signal transduction in vivo and in vitro: role of p95HER2 glycosylation and establishment of an autocrine loop for effective PD-L1 induction.
(a) Glycosylation analysis of p95HER2 protein. p95HER2 protein migrates as two closely spaced bands. The upper band was verified as the glycosylated form by its disappearance in response to either the treatment of MCF7-p95HER2 cells with the glycosylation inhibitor, tunicamycin, or the incubation of cell lysates with PNGase F. (b) The NetNGlyc algorithm predicts two N-linked glycosylation sites on the p95HER2 protein, at positions N629 and N758, although the score for the N758 residue is below threshold. (c) Western blotting demonstrates that site directed mutagenesis of N629 results in loss of the upper p95HER2 band, verifying this residue as a glycosylation site. Simultaneous loss of p95HER2 autophosphorylation emphasizes the importance of glycosylation for signal transduction. Results are representative of n = 3 biological repeats. (d) Site directed mutagenesis of N629 and N758 also results in impaired p95HER2-dependent PD-L1 induction. Results are representative of n = 3 biological repeats. (e) MDA-MB-453 cells expressing either empty vector (EV) or p95HER2 were orthotopically implanted into the mammary fat pads of immunodeficient NCG mice. The developed tumors (EV n = 5, p95HER2 n = 5) were harvested and subjected to Western blotting to evaluate signal transduction pathway activation. Tumor growth curves and terminal tumor weights are shown. Data represent mean ± SEM. Statistical analyses by unpaired, two-tailed Student’s t test (p >0.05). (f) Conditioned media from p95HER2-expressing cells can rapidly activate STAT3 in recipient cells in as little as 10 min. (g) IL-6 neutralizing antibody does not abrogate autocrine JAK-STAT activation induced by p95HER2. (h) Working model to depict how p95HER2 leverages the coordinated activation of multiple pathways, including AKT, ERK, and JAK/STAT pathways, to effectively induce PD-L1 expression. Schematic constructed with assistance from BioRender (https://BioRender.com/u81a258).
Extended Data Fig. 6 Role of PD-L1 as a mediator of p95HER2-dependent tumor immune suppression.
(a) Flow cytometry analyzing the impact of PD-L1 knockout (KO) on cell surface PD-1 binding. Control or PD-L1 KO PB2 cells were treated with 10 ng/mL of IFN-γ for 24 h. Cells were then incubated with PD-1-Fc chimeric protein and PE conjugated F(ab’)2-anti-human IgG Fc 2nd antibody and analyzed by flow cytometry. Results demonstrate loss of PD-1 binding to cells harboring knockout of the PD-L1 locus. (b) PD-L1 KO does not impact cell proliferation in culture. Cell proliferation was monitored in real time using the IncuCyte imaging system. N = 6 replicate wells per cell line; data are expressed as mean ± SEM; results are representative of n = 3 biological repeats. (c) Flow cytometry analysis of single cells isolated from the same p95HER2+ PB2 tumors as shown in Fig. 6c. N = 9 control and n = 11 PD-L1 KO tumors, respectively. Data represent mean ± SEM. Statistical analyses by unpaired, two-tailed Student’s t test. No significant alterations were observed for B-cells, dendritic cells, or M1/M2 macrophages. (d) Western blot verification of PD-L1 depletion by shRNA in PB2 cells. (e) shRNA-mediated PD-L1 knockdown slows growth of p95HER2+ PB2 tumorgrafts in syngeneic, immunocompetent C57BL/6 mice. Control shRNA n = 7, PD-L1 shRNA n = 7. Data represent mean ± SEM. Statistical analysis by unpaired, two-tailed Student’s t test (p = 0.0344). (f) Flow cytometric analysis of single cells isolated from the tumors depicted in (e) (Control shRNA n = 7, PD-L1 shRNA n = 7). Boxes span the upper to lower quartiles with a median center line. Whiskers extend from the minimum to the maximum values in the dataset. Statistical analyses by unpaired, two-tailed Student’s t test. P values are indicated within the plots.
Extended Data Fig. 7 p95HER2 abrogates T-DXd-induced immune infiltration but is exquisitely sensitive to neratinib.
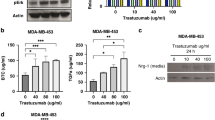
(a,b) T-DXd is effective against FL-HER2+ EMT6 tumorgrafts, regardless of their size at time of treatment initiation. Cells were orthotopically implanted into BALB/c mice. After 13 days, mice were divided into two groups based on tumor size and received two doses of T-DXd. Tumor growth curves in (a), terminal tumor weights in (b) along with percent growth inhibition. Large tumors at the start of treatment: IgG n = 10, T-DXd n = 10; Small tumors at the start of treatment: IgG n = 10, T-DXd n = 10. Data represent mean ± SEM. Statistical analyses by unpaired, two-tailed Student’s t test. P values are indicated within the plots. (c) qRT-PCR quantifying the levels of immune-related genes in bulk RNA harvested from the T-DXd-treated tumors described in Fig. 7b. N = 8 EV tumors and n = 10 p95HER2+ tumors. Data represent mean ± SEM. Statistical analyses by unpaired, two-tailed Student’s t test. P values are indicated within the plots. (d) qRT-PCR analysis of dendritic cell marker Cd11c in tumors treated with IgG only. N = 10 EV tumors and n = 8 p95HER2+ tumors. Data represent mean ± SEM. Statistical analyses by unpaired, two-tailed Student’s t test (p = 0.0031). (e) mIF analysis of dendritic cell subtypes to complement mIF analysis shown in Fig. 7d. N = 5 EV tumors and n = 5 p95HER2+ tumors. Data presented as truncated violin plots. Statistical analyses by ordinary one-way ANOVA with Tukey’s correction for multiple comparisons. P values are indicated within the plots. (f) The glycosylated form of p95HER2 is selectively targeted for proteasomal degradation by irreversible TKIs (neratinib, pyrotinib, and afatinib) but not reversible TKIs (tucatinib, lapatinib). Both classes of TKI inhibit PD-L1 expression. (g,h) Western blotting demonstrates that proteasome inhibitors Bortezomib (g) and MG132 (h) prevent the downregulation of glycosylated p95HER2 induced by irreversible TKIs. For all panels (f-h), western blot data results are representative of n = 3 biological repeats.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3.
Supplementary Table
Supplementary Table 1: Antibodies for western blot analysis, flow cytometry and Akoya multiplexed IF. Supplementary Table 2: TaqMan assays. Supplementary Table 3: Customized NanoString assay panel. Supplementary Table 4: Immune cell categorization by flow cytometry. Supplementary Table 5: FinHER trial sample data.
Source data
Source Data Figs. 1, 2 and 4–8 and Extended Data Figs. 4–7
Combined file of unprocessed western blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, D., Lyu, X., Li, Z. et al. p95HER2, a truncated form of the HER2 oncoprotein, drives an immunosuppressive program in HER2+ breast cancer that limits trastuzumab deruxtecan efficacy. Nat Cancer 6, 1202–1222 (2025). https://doi.org/10.1038/s43018-025-00969-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43018-025-00969-4