Abstract
The Brazilian Amazon Satellite Monitoring Program (PRODES Amazonia) tracks forest loss in the Brazilian Amazon but excludes about 6.6% ( ~ 280,000 km²) of non-forest vegetation (NF). Here we developed the PRODES NF system to address this gap by adapting the well-established methodology of PRODES Amazonia. Initial findings from PRODES NF show that the Brazilian Amazon lost 10.46% ( ~ 30,000 km²) of NF area, mainly in the last two decades, with the estates of Mato Grosso, Roraima, and Amapá being primary hotspots of losses. Savannas were the most affected (13.3% of their extent). NF loss strongly correlates (r = 0.87; p < 0.0001) with deforestation, suggesting a continuum of vegetation loss in the biome regardless of the predominant vegetation type. Combining data from PRODES Amazonia and PRODES NF reveals an official estimate of ~798,000 km² in total primary vegetation loss ( ~ 19% of the entire biome) in the Brazilian Amazon by 2022.
Similar content being viewed by others
Introduction
Forest loss in Brazilian Amazon have been continuously monitored since 1988 through the Brazilian Amazon Satellite Monitoring Program (PRODES Amazonia1. PRODES Amazonia data is internationally recognized as a crucial tool to assess and control the extent and rate of deforestation processes, being critical for public policy proposals and enforcement, as well as research on varied topics that include biodiversity, climate change, and human well-being2. PRODES Amazonia is designed for primary forest loss, whereas companion projects like TerraClass and DETER3 complement it by providing data on land use and land cover changes in the Brazilian Amazon. This includes information on forest regrowth, primary land use classes, and the detection of smaller areas of forest loss or forest degradation events in near real-time throughout the year. Throughout the PRODES Amazonia data series, however, a considerable challenge persisted in addressing the need to map a consistent historical series of natural nonforest vegetation (NF) loss across an area spanning 279,492.08 km², equivalent to 6.6% of the Amazon biome.
NF stands for natural vegetation other than strictly forest ecosystems and embraces different types of vegetation. In the Brazilian Amazon, NF occurs as open-like formations such as savannas and grasslands; seasonally flooded areas with sandy soils and sparse trees; ecotones; isolated forest patches with deciduous, semi-deciduous, and even broadleaf characteristics; and natural areas of bare lands4 (Supplementary Fig. 1). These landscape features receive names such as pioneer formations, ecological refuges, lavrados, campinas and campinaranas or white-sand ecosystems4. Despite the lack of knowledge5,6,7, NF ecosystems are important sites for biodiversity conservation with endemic species of different taxa5,7,8,9,10, and information about their status along time is crucial to better understand their functioning and ecology, and their response to climate change.
Previous mapping attempts11,12,13 have shown the expansion of human activities within NF formations, leading to relatively high percentages ( ~ 17%) of accumulated losses in NF areas up to 2021 in selected Amazonian municipalities13. The overall extent of NF losses in the Brazilian Amazon biome and their specific locations were still unknown, which precludes the assessment of the impacts on Amazonian ecosystems and understanding on the drivers behind this destruction.
To ensure the continuous mapping of natural vegetation loss throughout the entire Brazilian Amazon biome, we have developed PRODES NF, a fully operational systematic monitoring system for NF in the region14. PRODES NF adapts the PRODES Amazonia forest mapping methodology, and utilizes multi-sensor satellite imagery to identify vegetation loss in predominantly open ecosystems (see the Methods section). PRODES NF generates official data regarding NF loss in the Amazon, expected to be released annually by the Brazilian Federal Government through the National Institute for Space Research (INPE) and it is available at the Terrabrasilis platform14. This type of data is crucial to develop policies to face and control of NF loss and to support governmental and corporate commitments to reduce not only forest, but also non-forest vegetation loss and ecosystem conversion15,16,17.
In this study, we present the PRODES NF system and the spatial and temporal distribution of NF vegetation loss (referred to as NF loss) in the Brazilian Amazon biome. Initial findings from PRODES NF show that the Brazilian Amazon lost 10.46% ( ~ 30,000 km²) of natural non forest vegetation, mainly in the last two decades, with significant losses in the states of Mato Grosso, Roraima, and Amapá. Savannas were the most affected non forest vegetation type, losing 13.3% of their extent. We conclude with an overview of the total extent of vegetation loss monitored by the PRODES systems, encompassing both forests and non-forest ecosystems, showing that the total primary vegetation loss in the Brazilian Amazon by 2022 is estimated at ~798,000 km² ( ~ 19% of the biome).
Results and discussion
NF loss hotspots
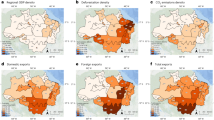
Accumulated NF losses in the Brazilian Amazon biome until 2000 (baseline map) accounted for 12,934.75 km² (4.63% of the total NF area, Fig. 1) and reached 29,247.44 km² (10.46%) up to 2022, meaning that more than half of the accumulated loss happened in the last 20 mapped years. The prevailing pattern of NF loss up to 2022 unfolded from the southern to the northern regions of the Brazilian Amazon (Fig. 1a), evidencing three main hotspots of NF loss located in the states of Mato Grosso, Roraima, and Amapá. Substantial and earlier losses (pre-2000) were primarily evident in the southwest sector of Mato Grosso (Fig. 1a, blue color), with additional minor occurrences in isolated zones within the central-eastern of Rondônia. The loss of NF persisted in Mato Grosso during 2001–2010 (cyan) and starts to be visible in the southeastern sector of Amapá in the same period. Emerging regions of NF loss (Fig. 1a, green color) appeared in the northern and southwestern sectors of Mato Grosso during 2001–2010, alongside sporadic patches within Roraima. More recent instances of NF loss (2011–2022) happened across extensive areas in Roraima (denoted by the red color) and localized patches in Rondônia. In summary, Mato Grosso exhibited a higher degree of established and longstanding NF loss, while Rondônia, Roraima, and Amapá showed an escalating contribution and growing relative values for NF losses.
a RGB color composite of NF loss through time. Additive colors in the RGB system represent older (blue), intermediate (green), and younger loss (red). The subtractive colors (cyan, yellow, and magenta) represent continuous loss among the analyzed period. Black areas indicate regions of low NF loss through all the period, with relatively equal amounts of subtractive colors (cyan, yellow and magenta). b Primary y-axis showing annual increments (km²) of NF loss. The flattening effect in NF data is due to those periods when the NF mapping was performed at two-year intervals. Bars with a transparency level and starlike symbols are those not included in the monitoring process and corresponds to half the value of the subsequent mapped year (see the methodology). Secondary y-axis showing the percentage of NF and forest formations (orange and red dashed lines, respectively relative to the area of the Brazilian Amazon biome (NF formations) and Brazilian Legal Amazon (forest formations). Historical vegetation loss data simplified from PRODES monitoring program14.
The state of Mato Grosso had the highest absolute lost area (14,469.20 km²), the second in relative terms (32.1%) (Supplementary Fig. 2). This state ranks second in area of deforestation in the Brazilian Amazon14 and holds the highest cultivated area (75.91% of total) and largest cattle herd in the country (35.68%)18. The concentration of land in large properties contributes to mechanized vegetation loss of large areas, along with the expansion of mechanized agriculture19,20. In Mato Grosso, the transition region between the Amazon with the Pantanal and Cerrado savannas is highly affected by agricultural expansion in the Amazon21,22,23, appearing among the top three hotspots of NF loss (Fig. 1a and Supplementary Fig. 3). Figure 1a shows that the majority of NF loss in transition areas between biomes is older (up to 2000), with large hotspots appearing in the early 2000s in the central region of Mato Grosso, following the advance of the east-to-west agricultural frontier and the increasing conversion of pastures to soybean fields20,24.
The states of Roraima and Amapá have been considered the last agricultural frontiers in the Amazon25,26. In both States, the influx of capital and technology found in old frontier areas are among the main the causes of NF loss19. The expansion of agricultural production was facilitated by various factors, such as highways, technological innovations in seeds, low land prices, and proximity to the capital7,27,28. State governments have been playing an important role in attracting farmers from other states to the lavrado savannas through economic subsidies and flexibility in state environmental legislation27,28,29.
Roraima ranks third in total NF loss, with 3527.70 km² (Fig. 1b). It hosts the Amazon biome’s largest continuous savanna area ( ~ 43,000 km²)28. NF loss notably intensified from 2001 to 2022, resulting in substantial cleared areas. Soybean cultivation saw exponential growth (191% in four years), driven by locally adapted seeds30. Roraima’s savanna floodplains also favor rice cultivation31. This, along with silviculture expansion, regional road projects, and port infrastructure improvements, heightens the risk of savanna loss5,7,27,29. In 2014, the region around the state capital, Boa Vista, witnessed a considerable fourfold increase in agriculture and other land uses, including forestry and urban development. Urban growth reached 22% in the same year27.
While Amapá initially experienced limited forest and NF loss due to its lack of road connections with other states5, upcoming projects like the asphalt paving of BR-210 between Boa Vista (state capital of Roraima) and Macapá (state capital of Amapá) and the establishment of a port at the Amazon River’s mouth may heighten pressure on its savannas5,9. Compounding this risk is the fact that only 9.2% of the Amapá savannas are protected, with just 0.3% falling under strict conservation units32. In contrast, 72% its primarily forested lands are protected. The protection gap between savannas and forests implies that land clearing leakage33,34 into the savannas might be the reason behind its larger area lost (7.3%) compared to deforestation (2.8%)14, making Amapá the only state with this discrepancy.
Despite not having extensive, continuous areas of NF loss, Rondônia ranks as the third state with the highest relative losses (11.4%). This has resulted in a loss of 2656.70 km², making it the fourth-largest state in terms of area (Supplementary Fig. 2a). While small hotspots of NF losses were observed as early as 2000, new focal points have emerged in the 2010’s (Fig. 1a and Supplementary Fig. 2), with a consistent increase in the proportion of NF loss up to 2022 (Supplementary Fig. 2b). These recent changes may be attributed primarily to the conversion of land to pasture, with a growing portion being allocated for soybean production11,35,36 The central region has older conversion areas, aligning with substantial deforestation rates experienced in the 1990s, especially near the BR-364 highway35,37. It’s important to note that most NF areas in Rondônia are located within protected areas and are sparingly distributed across the landscape38. This fragmented distribution could explain the presence of isolated hotspots of NF loss (Supplementary Fig. 2) and the relatively modest ~10% loss in a state that has already experienced a substantial 46.4% reduction in its forest cover39.
Temporal trend of NF loss
Examining yearly NF loss spanning from 2001 to 2022 (Fig. 1b) unveils three distinctive patterns: (1) between 2001 and 2008, losses exhibited a noteworthy decline, ranging from 1555.60 km².yr−1 to 581.50 km².yr−1; (2) between 2009 and 2013, NF loss was relatively stable, not surpassing 400 km².yr−1; (3) from 2014 to 2022, NF loss increased, oscillating between 515.00 km².yr−1 and 726.50 km².yr−1. Notably, the highest losses were recorded in 2001–2002 (1555.60 km²), whereas the lowest values occurred during 2011–2014 (351.90 km²; Fig. 1b). Proportionally to their extents, deforestation almost always showed higher values than that of NF loss during 2003–2013, with an inverted behavior during 2014–2022. In 2014, NF relative loss was almost twice as high as the deforestation ( ~ 0.23% and 0.13%, respectively; Fig. 1b). Nonetheless, the relative differences between deforestation and NF loss yielded no significant statistical differences (Student t-test: t = 0.77; df = 40.51; p = 0.45), while both processes exhibited a robust positive correlation (Spearman’s rank correlation: r = 0.87; p < 0.0001) (Fig. 1b), suggesting that the magnitude of vegetation loss in both forest and non-forest vegetation types did not exhibit significant relative differences over time.
The strong correlation between deforestation and NF loss suggests a common response to common factors. The observed decline in deforestation in the Brazilian Amazon, particularly after 2004 (Fig. 1b), can be largely attributed to environmental policies implemented by the Brazilian government in response to the high deforestation rates in the Brazilian Amazon that have played a crucial role in reducing forest loss rates in the region24,40,41,42,43,44,45. These policies include the Plan for Prevention and Control of Deforestation in the Legal Amazon (PPCDAM); the Amazon Protected Areas Program (ARPA); the prioritization of Amazonian municipalities for preventing, monitoring, and controlling illegal forest loss; the Cattle Agreement; and the soy moratorium. However, it remains unclear to what extent NF loss during the same period responded to these policies, as they were specifically designed and enforced for forested areas only. On the other hand, it is conceivable that the decline in commodity prices during this period, which has been shown to reduce new land clearings in the Amazon46, could have played a role in the observed decline in NF loss.
In line with the Amazon’s deforestation trend, NF loss increased from 2013 onward (Fig. 1b). From 2013 to 2022, both NF loss and deforestation exhibited a rate of 0.21 km2.km-2.y-1 (relative to their area extents). Several factors could have contributed to this rise, including the growing value of soybeans, which led to the conversion of pasture areas in states like Mato Grosso with better logistical infrastructure for soybean production, and the shift of pastures towards the active deforestation frontier24,47. Additionally, the increase in the global demand for meat resulted in the expansion of the cattle herd in the Amazon18. The discussed environmental policies were identified as drivers of deforestation leakage33,34 over NF formations by different studies5,9,22,28,48,49. Similar events were observed in the biome towards its neighbor Cerrado22,50,51.
Political decisions have played an important role in driving natural vegetation loss increase. Changes in the Forest Code in 2012, including amnesty for pre-2008 conversion of natural vegetation into agricultural and pasture lands, and other bills aimed at easing environmental licensing, likely incentivized new conversions in anticipation of further legislative changes due to political pressure from the ruralist caucus in the National Congress52,53,54. The political influence on vegetation loss intensified in the Amazon during the period of 2018-2021, likely influenced by the incentives and discourse by then-president Jair Bolsonaro, coupled with the weakening of command-and-control measures to halt forest loss in the Brazilian Amazon under his government54,55,56. Consequently, there was a rapid surge in vegetation loss, human-induced fires, and illegal mining activities in various Brazilian biomes, well documented elsewhere53,55,56,57,58. In addition, NF formations, even in the Amazon Biome, have less protection under the Brazilian Forest Code. A key limitation lies in the lack of differentiation among the various Amazonian vegetation types, which instead results in their protection being generalized across the entire Brazilian Legal Amazon region. For instance, whereas private properties are required by law to preserve 80% of forest ecosystems in the biome, open ecosystems are less protected, ranging from 35% in cerrado areas to 20% in grasslands59.
Losses by vegetation types
Cross-referencing spatial data of NF loss with the official map of Brazilian vegetation types of coverage60 allowed us to estimate the losses related to different NF vegetation types (Table 1). The ecotones (see Supplementary Fig. 1 and Supplementary Table 3 for distribution and area of vegetation types) had the largest accumulated suppressed area (12,388.40 km²). They correspond to mixtures of different vegetation types (e.g., contact of the savanna-ombrophilous forest, ombrophilous forest-deciduous forest; see Supplementary Table 2), where their separation is limited through image interpretation4,6. In addition to ecotones, patches of forest formations also occur within the NF mask. During the analyzed period (2000–2022), 5732.92 km² of forests were cleared inside the NF mask, and when considering ecotone areas (Supplementary Table 2) with other NF formations in contact with forests, forest loss may reach 17,619.43 km², an average of 590.44 km² ( ± 619.94 km²) lost annually. By standardizing forest loss within the NF mask by its respective area and applying the same approach to forest loss within the PRODES Amazonia mask14 from 2001 to 2022, both processes displayed a loss rate of 0.04 km².year⁻¹ per km² of forest. This value suggests that forest loss is occurring at the same pace irrespective of the predominant vegetation type in the landscape (i.e., no matter if it is continuous forest or forest occurring within NF vegetation).
Savannas, which represent approximately 30% - the largest proportion - of the NF mask, experienced the largest losses (excluding ecotones; Table 1). In the Amazon, these formations are more susceptible to clearing compared to dense forested areas27. Considering the combined area of savannas and savanna steppe, the loss amounted to 10,253.51 km², accounting for approximately 35% of the NF losses and 13.3% of their total extent within the NF mask. If the contact areas of savannas with other formations (ecotones; Supplementary Table 2) are also included, losses would reach 21,550.58 km². The three main hotspots of NF loss are located precisely within this ecosystem, specifically in the states of Mato Grosso, Roraima, and Amapá (Fig. 1a and Supplementary Fig. 3).
The main factor driving losses in these states has been linked to the expansion of soybean cultivation, as discussed above. Even when not suppressed, the savannas may be affected by fire and grazing27,61. The threats primarily arise from neglect and biases towards these ecosystems, often seen as successional stages of forests with low biodiversity and ecological significance62,63,64. However, the Amazonian savannas are old formations65 and constitute vegetation islands (Supplementary Fig. 1) with distinct characteristics from the neighbor Brazilian Cerrado7,66. In the states of Roraima and Amapá, the savannas show a great heterogeneity and embraces different vegetation types (e.g., the lavrados) with a biodiversity that is still poorly understood and home to endemic species that are threatened to extinction due to limited protection within conservation units5,7,28,67. Some studies have highlighted their conservation importance due to their richness, rarity, and endemic species, as well as species adapted to these ecosystems5,9, low protection ( ~ 12% within strictly protected areas9), and limited research on their ecology and biodiversity5,7,32.
Pioneer formations within the NF mask have experienced a 1.75% reduction in their extent. These formations consist of pioneering vegetation elements such as grasses, bryophytes, therophytes, cryptophytes, among others, which undergo a continuous succession due to the seasonal instability of the inundated terrain, occurring predominantly in lacustrine and alluvial soils4. They constitute a major portion of the vegetation in the floodplains along the major Amazonian rivers (Supplementary Fig. 1). These floodplains are periodically inundated and have historically been inhabited by local riverine populations who have relied on the fertile soils for agriculture, extractive activities, and fishing68,69. Vegetation loss in these areas is primarily driven by agriculture and livestock70. The floodplains of the Solimões/Amazonas River have experienced moderate NF loss, with higher intensity during the first period (Fig. 1a and Supplementary Fig. 3), particularly concentrated around the city of Manaus and in the Lower Amazon region between the states of Amazonas and Pará. The central section of the Amazon River channel (ranging from 56°W to 55°W) has been recognized as one of the regions most affected by vegetation loss, encompassing both recent and historical losses, even though certain areas might still maintain up to 70% of their forest cover70,71.
The campinaranas, also known as white-sand ecosystems, and the savanna-steppes were the least affected by NF loss (0.46% and 0.10% of lost area, respectively; Table 1). While the latter has the smallest total area within the NF mask among the open vegetation types, the campinaranas rank second in area, behind only the savannas. They encompass a gradient of grassland to forest formations, showing a broad geographic distribution (Supplementary Fig. 1), and occur as predominant formations in large patches of hundreds of square kilometers in the Negro River basin (ottobasin, level 2) and in border regions with Colombia, as well as exhibiting an island-like distribution pattern throughout the Amazon embedded in other habitats4,6,10. Their greater geographical distribution in the northwest of the basin, a region with the lowest anthropogenic pressure in the Brazilian Amazon72,73, may explain the low levels of losses in this formation. Furthermore, the nutrient-poor, acidic, and water-limited sandy soils6,10,74 may limit their suitability for agricultural use.
The white-sand ecosystems have lower species richness and diversity of fauna and flora compared to adjacent upland forests, but these are adapted to specific soil conditions and include endemic species75,76,77,78,79, making them of utmost biological importance and strategically for conservation6,10. However, like other NF ecosystems in the Amazon, knowledge of biodiversity and even the functioning of these ecosystems are still limited, especially in those island-like areas with patches smaller than 1 km² and immersed within forested areas, which are difficult to map using satellite imagery6. Due to the nature of their soils, they are fragile systems and highly sensitive to anthropogenic disturbances80, and their low protection ( < 1 km² within conservation units)6 makes them extremely vulnerable. Although of low agricultural interest, sand extraction poses a considerable threat of white-sand ecosystems in the state of Pará81.
Final remarks
The strong correlation we identified between NF loss and deforestation (Fig. 1b) suggests a common response to prevalent factors contributing to the loss of Amazonian vegetation. Conversion to agriculture and pasture has long been recognized as the primary driver of forest loss in the Brazilian Amazon82 and, as showed here, it is also believed to be the main cause of NF loss, although further investigation is needed. The development of road and port infrastructure in states like Roraima and Amapá, as discussed above, has facilitated the expansion of agricultural lands, reminiscent of past frontiers in the Amazon83,84. These states have become emerging agricultural frontiers, driven by the cultivation of crops adapted to the region’s soil and climate conditions. Additionally, public policies such as the beef agreement and soy moratorium, which only restrict forest clearing, may have inadvertently led to the encroachment of cattle and soy production into NF ecosystems that were not previously monitored by the PRODES system5,9,22,28,48,49.
The limited protection of Amazon NF vegetation types is one of the common factors observed in different states where large areas of NF have been lost, accentuated by the low protection granted by the Brazilian Forest Code, as discussed before. Regarding Amazonian savannas, only 12.3% are within conservation units, reaching 58% when Indigenous Lands are considered9. However, a more detailed analysis of the representativeness of different types of NF within protected areas is still lacking, as the protection of each NF vegetation types is uneven29,32,62,63. Additionally, political pressures and approval delays/denial of ecological-economic zoning plans have posed a frequent threat to these ecosystems, particularly in states where agricultural expansion is the main proposal for economic development5,7,11,28.
The join analysis of PRODES Amazonia and PRODES NF has provided the quantification of total primary vegetation loss in the Brazilian Amazon at the biome level. Taking into account the total NF loss (29,247.44 km²) and the total deforestation (768,930.88 km²)14, overall, the Brazilian Amazon biome has experienced a total loss of 798,178.32 km², representing 18.93% of its original vegetation cover (Fig. 2). The lower proportional loss of NF compared to forests reflects their island-like spatial distribution, mostly located outside the deforestation arc (Supplementary Fig. 1) and lower agriculture suitability. However, the escalating trend of NF loss over the past decade, mirroring the rates of forest loss, coupled with the expanding risk from agribusiness expansion into NF formations, inadequate protective measures, and undervaluation, paints a scenario marked by substantial losses, particularly impacting small, island-like NF formations. A more detailed analysis currently underway for each vegetation type may provide further insights into the threats.
Nonforest data are from 2000 to 2022. Vegetation loss data in forest formations are simplified from the PRODES monitoring program based on the cumulative mask until 2007 and from 2008 to 2022 (http://terrabrasilis.dpi.inpe.br/downloads/).
We emphasize the recommendations proposed by researchers who have examined the threats faced by NF formations5,7,9,32,63: establishing protected areas, whether for sustainable use or integral protection, have shown strong evidence of effectively safeguarding the local biodiversity and reducing forest loss85,86,87, and represents a critical measure for strategically planning the conservation of these ecosystems. However, it will require an effort to map the heterogeneity within these ecosystems to effectively protect distinct phytogeographic units of these vegetation types. In this regard, the limited knowledge about the biodiversity and social importance of these vegetation types5,6,7,9,10 needs to be overcome to obtain minimal informed decisions regarding the protection and sustainable use of these ecosystems through the suitable, responsible, and participatory ecological-economic zoning.
As stated by Overbeck et al.63, savanna conservation often requires different strategies than that of forests (e.g., prescribed fire, grazing). Protecting and sustainably managing these ecosystems will require research to harness their contributions to biodiversity, ecosystem services, and climate change. Extending the soybean moratorium and cattle agreement, or implementing any sectoral or national mechanism with a robust monitoring and verification system to promote vegetation loss-free agricommodities supply chains, represents crucial measures to curb accelerated NF loss45.
In this context, the introduction of PRODES NF laid the groundwork for the extension of the Near Real-Time Deforestation Detection System (DETER), now referred to as DETER NF88. This extension is designed to provide daily alerts regarding NF vegetation suppression to government environmental agencies. Furthermore, PRODES NF contributes to the TerraClass program89, which aims to characterize and quantify different land use classes and secondary vegetation within the PRODES NF mapped area. In this way, the conception of PRODES NF provides essential data (i) to allow the monitoring of socioenvironmental compliance of production sites and traceability systems in the search for more sustainable production, and (ii) to plan effective actions to control and regulate the conversion of non-forest vegetation areas in the Brazilian Amazon.
Methods
We used a multi-sensor satellite imagery approach with digital image processing and visual image interpretation techniques to map NF vegetation loss within the Brazilian Amazon biome for the period from 2000 to 2022. Images from the Landsat series (MSS, TM, ETM + , OLI sensors) were used to create the baseline map for 2000 and track changes until 2014. From 2016 onwards, Sentinel-2A and 2B images (MSI sensor with a 20–10 m spatial resolution) were utilized due to their improved temporal resolution, allowing for images with less cloud coverage (see Supplementary Table 2 for details). The number of images per year ranged from 182 to 210 for Landsat and from 546 to 885 for Sentinel (Supplementary Table 2). Images from the sensor MSS (12 images) were used to assist the identification of changes in the floodplains of the Amazon and Solimões rivers for the 2000 base map.
The images were processed using scripts in the Google Earth Engine (GEE) cloud computing platform90. Selection filters were applied to obtain cloud-free or minimally cloud-covered images. PRODES methodology established an optimal mapping period for each Landsat orbit/point during the dry season, considering the region’s extensive longitudinal and latitudinal range1, and the images available were filtered (ranked) by the lowest percentage of cloud. Areas with persistent cloud cover were classified as unobserved. The annual average of nonobserved area was 25,466.66 km², what corresponds to 9.09 % of NF formations. Additional preprocessing steps included resampling the red spectral band of Sentinel-2 from 10 m to 20 m resolution for compatibility with other bands, creating mosaics of Sentinel-2 images based on the Landsat grids, and enhancing image contrast through histogram manipulation.
NF loss mapping
NF loss was mapped using the incremental approach in which NF loss of a given year is mapped using a cumulative or exclusion mask of NF loss from previous years, similar to the PRODES approach1. This procedure ensures that only newly cleared areas are mapped each year, preventing duplicate mapping of the same area. The year of 2000 was defined as the basemap to create the mask of NF loss. The inclusion of older satellite images (1970s, 1980s, and 1990s) from the Landsat MSS and TM sensors was necessary to create this mask. Satellite images of the year 2000 were compared to the older images, due to the greatest existence of natural NF formations in previous decades, which helped to identify changes over time.
The mapping of NF loss is provided for the period from 2000 to 2022. However, mapping was not carried out at continuous time intervals. We initiated biennial mapping from 2001 to 2018 and completed it annually from 2019 onwards. This strategy was employed due to the limited availability of human resources and a continuous supply of satellite images with moderate temporal resolution. For the 2-year time intervals, the total NF loss of the mapped year was divided into two, approximating the NF loss amounts in the unmapped year. The only exception was for the interval of 2011–2013, which was divided into three years. This deviation was due to the failure of the Landsat-5/TM sensor in November 2011, disrupting the continuous monitoring of the Earth’s surface. Monitoring only resumed in February 2013 with the launch of Landsat-8. As a result, deforestation values are flatted in these periods. While recognizing that this approach introduces uncertainties regarding the actual distribution of NF suppression in the historical series, which may be influenced by various factors in specific years, the strategy of dividing the value by the unmapped year enables a more objective analysis of the historical series. This prevents atypical spikes in NF suppression in the mapped year due to the inclusion of NF suppression events occurring over two years.
NF vegetation was mapped by visual image interpretation techniques using specific elements and interpretation keys to better distinguish between preserved and suppressed vegetation patches. The elements color, shade, texture, shape, and context were used for this purpose. Together with PRODES NF team of interpreters, experts with knowledge in Amazonian NF vegetation also helped to define standard visual interpretation keys (Supplementary Table 1). NF mapping was performed in the TerraAmazon software91 at maximum (1:125,000) and minimum (1:75,000) scales based on a same false color composite: shortwave infrared (R), near infrared or near infrared narrow (G) and red (B). For the mapping of NF loss in the floodplains of some large Amazonian rivers, a mask of water bodies (available at TerraBrasilis14) was used to avoid mapping of anthropogenic land use on riverbanks and seasonally dry lakes.
Audit of NF loss data and post-processing
The NF data underwent post-mapping operations to ensure data quality. An independent team of expert auditors was established to check all NF loss polygons mapped by the interpreters and correct any eventual errors (e.g., omission, commission, and topology), using freely available high-resolution satellite images when necessary.
Polygons from the same year that touch each other are joined into one. A spatial filter was applied to remove polygons smaller than one hectare (1 ha), which was the minimum area mapped in this study. The polygons are submitted to topological verification (e.g., overlaps and gaps) and when necessary, corrections are made. The area of each polygon is calculated and inserted, and an identifier (ID) is assigned.
Accuracy assessment
PRODES NF accuracy assessment was implemented using a stratified random sampling by class92,93. We sampled 2,100 points (Supplementary Table 4) and its validation was carried out on the Temporal Visual Inspection (TVI) platform (Supplementary Fig. 4), an open-source tool that simplifies the visualization of points in Landsat images for long time series and allows to perform analyzes quickly, practically and with simultaneous supervision94. A time series from 2000 to 2022 was used, with an image in false color composition (RGB / NIR, SWIR, RED) and the mosaic in the composition (RGB / SWIR, NIR, RED). The validation process was carried out by the Image Processing and Geoprocessing Laboratory (LAPIG)/Federal University of Goiás (UFG), a completely independent team, which did not work in the mapping stage. The overall map accuracy was 0.96 ( ± 0.006 CI), and the natural class (NF vegetation) have 0.99 ( ± 0.002-0.006 CI) of producer’s and user’s accuracy (Supplementary Tables 4, 5, 6).
Spatiotemporal analysis of NF data
We synthetized the spatiotemporal distribution of NF data in the Brazilian Amazon through maps and graphs, depicting annual state-wise increments and percentages of NF loss as well as the remaining natural NF vegetation. NF maps included the percentage of NF loss for two periods, until 2000 and between 2001 and 2022, based on grids of 10 km x 10 km according to previous tests. This strategy was used in order to better represent the spatial distribution of NF loss data in the study area. The grid approach was also derived for three periods (up to 2000, 2002–2010 and 2013–2022) aiming to produce a RGB color composite showing the temporal dynamics of NF loss. The cumulative NF data through time was also compared to forest loss in the Brazilian Amazon. Forest loss data was downloaded from the PRODES digital collections14.
Vegetation types loss was assessed by overlaying NF loss polygons with official Brazilian vegetation mapping data at a 1:250,000 scale60. The calculation presented in Table 1 utilized the Legend_1 attribute from the vegetation data, while the aggregated information of the ecotone features (described in the nm_contat legend) was included in Supplementary Table 3.
Data availability
The annual NF loss data in the Brazilian Amazon biome for the period from 2000 to 2022 can be downloaded at https://terrabrasilis.dpi.inpe.br/app/dashboard/deforestation/biomes/amazon_nf/increments.
References
Almeida, C. A. et al. Methodology for Forest Monitoring used in PRODES and DETER projects − 2nd edition (updated). (INPE, São José dos Campos, 2022).
INPE. PRODES Amazonia citation track, <http://www.obt.inpe.br/OBT/assuntos/programas/amazonia/prodes/citacoes-ao-prodes> (2023).
Diniz, C. G. et al. DETER-B: The New Amazon Near Real-Time Deforestation Detection System. IEEE J. Sel. Top. Appl. Earth Observations Remote Sens. 8, 3619–3628 (2015).
IBGE. Manual técnico da vegetação brasileira. 2 edn, (IBGE, 2012).
Mustin, K. et al. Biodiversity, threats and conservation challenges in the Cerrado of Amapá, an Amazonian savanna. Nat. Conserv. 22, 107–127 (2017).
Adeney, J. M., Christensen, N. L., Vicentini, A. & Cohn‐Haft, M. White‐sand ecosystems in Amazonia. Biotropica 48, 7–23 (2016).
Barbosa, R. I., Campos, C., Pinto, F. & Fearnside, P. M. The “Lavrados” of Roraima: biodiversity and conservation of Brazil’s Amazonian savannas. Funct. Ecosyst. Commun. 1, 29–41 (2007).
Araujo, M. A. M., da Rocha, A. E. S., Miranda, I. S. & Barbosa, R. I. Hydro-edaphic conditions defining richness and species composition in savanna areas of the northern Brazilian Amazonia. Biodiv. Data J, e13829, (2017).
de Carvalho, W. D. & Mustin, K. The highly threatened and little known Amazonian savannahs. Nat. Ecol. Evol. 1, 100 (2017).
Fine, P. V. A. & Bruna, E. M. Neotropical white-sand forests: origins, ecology and conservation of a unique rain forest environment. Biotropica 48, 5–6 (2016).
Santos, L. B. et al. Proposta metodológica para mapeamento das áreas de não-floresta presentes no projeto de monitoramento de áreas desflorestadas da Amazônia Legal Brasileira. Res. Soc. Dev. 11, e20411425794 (2022).
Sano, E. E. et al. Desmatamento em áreas de cobertura não-florestais do bioma Amazônia: resultados preliminares baseados em imagens do satélite Landsat-8 OLI de 2013. in XVIII Simpósio Brasileiro de Sensoriamento Remoto. (eds Gherardi, D. F. M. & Aragao, L. E. O. C.) 3369–3376 (INPE, 2017).
Almeida, C. A. et al. Mapping natural non-forest vegetation removal in the Brazilian Amazon – a pilot project. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. XLIII-B3-2022, 1341–1348 (2022).
INPE. Terrabrasilis - Plataforma de dados geográficos, <http://terrabrasilis.dpi.inpe.br/en/home-page/> (2024).
van der Hoff, R. & Rajão, R. The politics of environmental market instruments: coalition building and knowledge filtering in the regulation of forest certificates trading in Brazil. Land Use Policy 96, 104666 (2020).
Bernasconi, P., Blumentrath, S., Barton, D. N., Rusch, G. M. & Romeiro, A. R. Constraining Forest Certificate’s Market to Improve Cost-Effectiveness of Biodiversity Conservation in Sao Paulo State, Brazil. PLoS One 11, e0164850 (2016).
Soares-Filho, B. et al. Brazil’s market for trading forest certificates. PLoS One 11, e0152311 (2016).
IBGE. Produção Agropecuária, <https://www.ibge.gov.br/explica/producao-agropecuaria/> (2022).
Alves, D. S. The changing rates and patterns of deforestation and land use in Brazilian Amazonia. in Amazonia and Global Change (eds et al.) 11–23 (American Geophysical Union, 2009).
Morton, D. C. et al. Cropland expansion changes deforestation dynamics in the southern Brazilian Amazon. Proc. Natl Acad. Sci. USA 103, 14637–14641 (2006).
Bonini, I. et al. Collapse of ecosystem carbon stocks due to forest conversion to soybean plantations at the Amazon-Cerrado transition. Ecol. Manag. 414, 64–73 (2018).
Magalhães, I. B. et al. Brazilian Cerrado and soy moratorium: effects on biome preservation and consequences on grain production. Land Use Policy 99, 105030 (2020).
Bezerra, F. G. S. et al. New land-use change scenarios for Brazil: Refining global SSPs with a regional spatially-explicit allocation model. PLoS One 17, e0256052 (2022).
Macedo, M. N. et al. Decoupling of deforestation and soy production in the southern Amazon during the late 2000s. PNAS 109, 1341–1346 (2012).
Staevie, P. M. Um balanço das discussões sobre os impactos do agronegócio sobre a Amazônia brasileira. Rev. NERA 21, 98–112 (2018).
Silva, E. A última fronteira da soja. in Revista Globo Rural, Vol. 371 28-33 (Editora Globo, 2016).
da Silva, G. D. F. N. & Oliveira, I. J. Reconfiguration of the landscape in the Amazonian savannas. Mercator 17, e17028 (2018).
Barbosa, R. I. & Campos, C. Detection and geographical distribution of clearing areas in the savannas (‘lavrado’) of Roraima using Google Earth web tool. J. Geogr. Reg. Plann. 4, 122–136 (2011).
Yokomizo, G. K. I. & Costa, L. D. N. O uso do cerrado amapaense e os recursos vegetais. Des. Reg. Deb. 6, 164–177 (2016).
Rodrigues, C. Produção de soja aumenta 191% em RR em quatro anos e deve bater novo recorde em 2023. in g1, (2023). <https://g1.globo.com/rr/roraima/noticia/2023/08/17/producao-de-soja-aumenta-191percent-em-rr-em-quatro-anos-e-deve-bater-novo-recorde-em-2023.ghtml>.
Cordeiro, A. C. C., Suhre, E., Medeiros, R. D. & Vilarinho, A. A. Sistemas de cultivo e manejo de água na produção de diferentes genótipos de arroz em várzea, no estado de Roraima. Pesqui. Agropecu. Trop. 40, 362–369 (2010).
Hilário, R. R. et al. The fate of an Amazonian savanna: government land-use planning endangers sustainable development in Amapá, the most protected Brazilian state. Trop. Conserv. Sci. 10, 1–8 (2017).
Pfaff, A. & Robalino, J. Spillovers from conservation programs. Annu. Rev. Resour. Econ. 9, 299–315 (2017).
Meyfroidt, P. et al. Focus on leakage and spillovers: informing land-use governance in a tele-coupled world. Environ. Res. Lett. 15, 090202 (2020).
Alves, D. S., Escada, M. I. S., Pereira, J. L. G. & de Albuquerque Linhares, C. Land use intensification and abandonment in Rondônia, Brazilian Amazônia. Int. J. Remote Sens 24, 899–903 (2010).
Costa, O. B. D., Matricardi, E. A. T., Pedlowski, M. A., Cochrane, M. A. & Fernandes, L. C. Spatiotemporal mapping of soybean plantations in Rondônia, Western Brazilian Amazon. Acta Amazon. 47, 29–38 (2017).
Ferraz, S. Fd. B., Vettorazzi, C. A., Theobald, D. M. & Ballester, M. V. R. Landscape dynamics of Amazonian deforestation between 1984 and 2002 in central Rondônia, Brazil: assessment and future scenarios. Ecol. Manag. 204, 69–85 (2005).
Rosa, M. C. O que está por trás das áreas de “não-floresta” do projeto PRODES-INPE no estado de Rondônia? Bachelor thesis, Universidade de Brasília, (2017).
INPE. PRODES Amazônia - Monitoramento do Desmatamento da Floresta Amazônica Brasileira por Satélite, <http://www.obt.inpe.br/OBT/assuntos/programas/amazonia/prodes> (2024).
Assunção, J. & Rocha, R. Getting greener by going black: the effect of blacklisting municipalities on Amazon deforestation. Environ. Dev. Econ. 24, 115–137 (2019).
Nepstad, D. et al. Slowing Amazon deforestation through public policy and interventions in beef and soy supply chains. Science 344, 1118–1123 (2014).
West, T. A. P. & Fearnside, P. M. Brazil’s conservation reform and the reduction of deforestation in Amazonia. Land Use Policy 100, 105072 (2021).
Silva, A. L. d. & Bueno, M. A. F. The Amazon Protected Areas Program (ARPA): participation, local development, and governance in the Brazilian Amazon. Biodivers. Bras. 7, 122–137 (2017).
Soares-Filho, B. S. et al. Contribution of the Amazon protected areas program to forest conservation. Biol. Conserv. 279, 109928 (2023).
Heilmayr, R., Rausch, L. L., Munger, J. & Gibbs, H. K. Brazil’s Amazon soy moratorium reduced deforestation. Nat. Food 1, 801–810 (2020).
Assunção, J., Gandour, C. C. & Rocha, R. in Climate Policy Innitiative Working Paper (PUC Rio, Rio de Janeiro, 2012).
Song, X. P. et al. Massive soybean expansion in South America since 2000 and implications for conservation. Nat. Sustain. 4, 784–792 (2021).
Richards, P., Arima, E., VanWey, L., Cohn, A. & Bhattarai, N. Are Brazil’s deforesters avoiding detection? Conserv. Lett. 10, 470–476 (2017).
Soterroni, A. C. et al. Expanding the Soy Moratorium to Brazil’s Cerrado. Sci. Adv. 5, eaav7336 (2019).
Moffette, F. & Gibbs, H. K. Agricultural displacement and deforestation leakage in the Brazilian Legal Amazon. Land Econ. 97, 155–179 (2021).
Castro, P. et al. Redesigning nature and managing risk: Social Representation, change and resistance. in Environment, Health, and Sustainable Development (eds Abdel-Hadi, A. et al.) 227–242 (Hogrefe, 2010).
Ferrante, L. & Fearnside, P. M. Brazil’s new president and ‘ruralists’ threaten Amazonia’s environment, traditional peoples and the global climate. Environ. Conserv. 46, 261–263 (2019).
Pereira, E. J. A. L., Ribeiro, L. Cd. S., Freitas, L. F. S. & Pereira, H. B. B. Brazilian policy and agribusiness damage the Amazon rainforest. Land Use Policy 92, 104491 (2020).
Rajão, R. et al. The rotten apples of Brazil’s agribusiness. Science 369, 246–248 (2020).
Ramos, A. The Amazon under Bolsonaro. Aisthesis 70, 287–310 (2021).
Ferrante, L. & Fearnside, P. M. Brazil’s political upset threatens Amazonia. Science 371, 898 (2021).
Pelicice, F. M. & Castello, L. A political tsunami hits Amazon conservation. Aquat. Conserv.: Mar. Freshwat. Ecosyst. 31, 1221–1229 (2021).
Vale, M. M. et al. The COVID-19 pandemic as an opportunity to weaken environmental protection in Brazil. Biol. Conserv. 255, 108994 (2021).
Brasil. Lei n° 12.651, de 25 de maio de 2012, <https://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/lei/l12651.htm> (2012).
IBGE. Vegetação 1:250.000, <https://www.ibge.gov.br/geociencias/informacoes-ambientais/vegetacao/22453-cartas-1-250-000.html?=&t=acesso-ao-produto> (2022).
Lima, J. M. et al. Influência do regime de queimadas sobre a riqueza e composição florística de uma savana isolada na Amazônia - PELD Oeste do Pará. Oecologia Aust. 24, 301–316 (2020).
Overbeck, G. E. et al. Placing Brazil’s grasslands and savannas on the map of science and conservation. Perspect. Plant Ecol. Evol. Syst. 56, 125687 (2022).
Overbeck, G. E. et al. Conservation in Brazil needs to include non-forest ecosystems. Divers. Distrib. 21, 1455–1460 (2015).
Bond, W. J. & Parr, C. L. Beyond the forest edge: ecology, diversity and conservation of the grassy biomes. Biol. Conserv. 143, 2395–2404 (2010).
Mayle, F. E., Langstroth, R. P., Fisher, R. A. & Meir, P. Long-term forest-savannah dynamics in the Bolivian Amazon: implications for conservation. Philos. Trans. R. Soc. Lond., Ser. B: Biol. Sci. 362, 291–307 (2007).
Prance, G. T. Islands in Amazonia. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351, 823–833 (1996).
Barbosa, R. I. & Miranda, I. S. Fitofisionomias e diversidade vegetal das savanas de Roraima. in Savanas de Roraima - Etnoecologia, Biodiversidade e Potencialidades Agrossilvipastoris (eds Barbosa, R. I. et al.) (FEMACT, 2004).
Pinedo-Vasquez, M., Ruffino, M. L., Padoch, C. & Brondízio, E. S. The Amazon Várzea: The Decade Past and the Decade Ahead. (Springer, 2011).
Junk, W. J. The Central Amazon floodplain: ecology of a pulsing system. (Springer, 1997).
Renó, V. F., Novo, E. M. L. M., Suemitsu, C., Rennó, C. D. & Silva, T. S. F. Assessment of deforestation in the Lower Amazon floodplain using historical Landsat MSS/TM imagery. Remote Sens. Environ. 115, 3446–3456 (2011).
Renó, V. & Novo, E. Forest depletion gradient along the Amazon floodplain. Ecol. Indic. 98, 409–419 (2019).
Barreto, P. et al. Human pressure on the Brazilian Amazon forests. (Imazon, Global Forest Watch, WRI, 2006).
Numata, I. & Cochrane, M. A. Forest Fragmentation and Its Potential Implications in the Brazilian Amazon between 2001 and 2010. Open J. 02, 265–271 (2012).
Luizão, F. J., Luizão, R. C. C. & Proctor, J. Soil acidity and nutrient deficiency in central Amazonian heath forest soils. Plant Ecol. 192, 209–224 (2007).
Fine, P. V. A. & Baraloto, C. Habitat endemism in white‐sand forests: insights into the mechanisms of lineage diversification and community assembly of the Neotropical flora. Biotropica 48, 24–33 (2016).
Matos, M. V. et al. Comparative phylogeography of two bird species,Tachyphonus phoenicius (Thraupidae) and Polytmus theresiae (Trochilidae), specialized in Amazonian white-sand vegetation. Biotropica 48, 110–120 (2016).
Alonso, J. Á., Metz, M. R. & Fine, P. V. A. Habitat specialization by birds in Western Amazonian white-sand forests. Biotropica 45, 365–372 (2013).
Guevara, J. E. et al. Low phylogenetic beta diversity and geographic neo-endemism in Amazonian white-sand forests. Biotropica 48, 34–46 (2016).
Anderson, A. B. White-sand vegetation of Brazilian Amazonia. Biotropica 13, 199–210, (1981).
Ramalho, W. P. et al. Impacto do assoreamento sobre a diversidade de peixes em igarapés de um complexo vegetacional de campinarana no noroeste do Acre, Brasil. Neotrop. Biol. Conserv. 9, 105–114 (2014).
Ferreira, L. V., Chaves, P. P., Cunha, D. D. A., Rosário, A. S. D. & Parolin, P. A extração ilegal de areia como causa dodesaparecimento de campinas e campinaranas no estado do Pará, Brasil. Pesqui., Bot.ânica 64, 157–173 (2013).
Berenguer, E. et al. Drivers and ecological impacts of deforestation and forest degradation. in Amazon Assessment Report 2021 (eds Nobre, C. et al.) Ch. 19, 1-41 (United Nations Sustainable Development Solutions Network, 2021).
Moran, E. F. Developing the Amazon. 292 (Indiana University Press, 1981).
Wood, C. H. & Porro, R. Deforestation and land use in the Amazon. (University Press of Florida, 2002).
Nepstad, D. et al. Inhibition of Amazon deforestation and fire by parks and Indigenous Lands. Conserv. Biol. 220, 65–73 (2006).
Nolte, C., Agrawal, A., Silvius, K. M. & Soares-Filho, B. S. Governance regime and location influence avoided deforestation success of protected areas in the Brazilian Amazon. PNAS 110, 4956–4961 (2013).
Pfaff, A., Robalino, J., Herrera, D. & Sandoval, C. Protected areas’ impacts on Brazilian Amazon deforestation: examining conservation-development interactions to inform planning. PLoS One 10, e0129460 (2015).
Messias, C. G. et al. Extending DETER into non-forest vegetation areas in the Brazilian Amazon. in XXIV Simpósio Brasileiro de Geoinformática. (eds Feitosa, F. F. & Vinhas, L.) 155–166 (MCTIC/INPE, 2023).
INPE/Embrapa. TerraClass: Levantamento de informações de uso e cobertura da terra na Amazônia, <http://www.inpe.br/cra/projetos_pesquisas/dados_terraclass.php> (2023).
Gorelick, N. et al. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 202, 18–27 (2017).
TerraAmazon, v. 7.3.2 (2023).
Stehman, S. V. Impact of sample size allocation when using stratified random sampling to estimate accuracy and area of land-cover change. Remote Sens. Lett. 3, 111–120 (2012).
Olofsson, P. et al. Good practices for estimating area and assessing accuracy of land change. Remote Sens. Environ. 148, 42–57 (2014).
Nogueira, S. H. M., Parente, L. L. & Ferreira, L. G. Temporal visual inspection: uma ferramenta destinada à inspeção visual de pontos em séries históricas de imagens de sensoriamento remoto. in XXVII Congresso Brasileiro de Cartografia e XXVI Exposicarta. 624-628 (2017).
Acknowledgements
We thank the INPE’s Geospatial Big Data Program, funded by the Brazil Ministry of Science, Technology, and Innovation (MCTI) [PO 216 W.0004], the National Council of Technological and Scientific Development (CNPq) for funding project Monitoring and Alerts of Land Cover Changes in Brazilian Biomes—Training and Semi-Automation of the BiomasBR Program [422354/2023-6]. The Brazilian Association of Vegetable Oil Industries (ABIOVE) for financial support of the fieldwork. We also appreciate the partnership and fieldwork insights from Embrapa Roraima, including their assistance with planning and transportation. Thanks to the PRODES Cerrado team for their support in mapping. We are grateful to David Galbraith (University of Leeds) for his comments on the initial manuscript and the three anonymous reviewers whose feedback improved our work. Lastly, we acknowledge the valuable contributions of experts Adriano Venturieri (Embrapa Eastern Amazon), Andréa dos Santos Coelho (SEMAS Pará), Evelyn Moraes Novo (INPE), and Tassio Koiti Igawa (INPE). M.A. thanks CNPq [grant 309045/2023-1].
Author information
Authors and Affiliations
Contributions
C.G.M., C.A.A., D.E.S., L.S., and L.E.M. were involved in the coordination of the project and in the article development; V.L.C., F.C.A., L.J.S., and M.S.R. contributed to article development, data analysis, and figures; T.C.L., V.R., D.L.C.L., A.P.B., D.C.M.B., and Douglas R.V.M. served as auditors; C.B.Q., D.C.M.B., Douglas R.V.M., E.F.M.B., I.P.C., J.J.S., L.S.B., L.H.A.G., R.A., Dayane R.V.M., D.M.S., E.H.S.C., J.F.S.K.P., M.R.R.N., M.H.H.M., N.A.P.M., R.C.S.T., G.M.R.A., A.C.S.A., L.P.P., B.M.P.B., H.C.C.F., I.S.S., L.C.L.A., M.M., M.B.S. comprised the team of interpretation experts; F.C.P. and A.C. were responsible for project database maintenance; H.X., M.X. acted as external consultants and participated in fieldwork; A.P.M., L.B., E.B.S., and L.G.F. formed the external validation team for the results; M.A. analyzed the validation results, conducted a thorough review, and made substantial contributions to the writing of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Carlos Souza and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Rodolfo Nóbrega, Joe Aslin and Aliénor Lavergne. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Messias, C.G., de Almeida, C.A., Silva, D.E. et al. Unaccounted for nonforest vegetation loss in the Brazilian Amazon. Commun Earth Environ 5, 451 (2024). https://doi.org/10.1038/s43247-024-01542-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-024-01542-0