Abstract
Fiddler crabs, as coastal ecosystem engineers, play a crucial role in enhancing biodiversity and accelerating the flow of material and energy. Here we show how widespread crab burrows modify the carbon sequestration capacity of different habitats across a large climatic gradient. The process of crab burrowing results in the reallocation of sediment organic carbon and humus. Crab burrows can increase more greenhouse gases emissions compared to the sediment matrix (CO2: by 17–30%; CH4: by 49–141%). Straightforward calculations indicate that these increased emissions could offset 35–134% of sediment carbon burial in these two ecosystems. This research highlights the complex interactions between crab burrows, habitat type, and climate which reveal a potential lower carbon sink function of blue carbon ecosystems than previously expected without considering crab burrows.
Similar content being viewed by others
Introduction
Coastal wetlands at the land-ocean interface provide habitat for organisms, enhance biodiversity, filter nutrient pollution, and exhibit the highest area-based rates of carbon burial on Earth1,2. Saltmarshes and mangroves, referred to as “blue carbon” ecosystems, are perceived as global champions in carbon sequestration3,4. However, coastal wetlands release carbon and greenhouse gases when sediment porewater flushes out sediments5,6. The net carbon sequestration potential and overall carbon budget of coastal wetlands remain subject to debate due to the intricate interplay of processes driving carbon sequestration and greenhouse gas emissions7.
Coastal wetlands are unique blue carbon ecosystems with low primary sediment permeability but high secondary permeability due to benthic burrows8,9,10,11, which can lead to oxygen penetration periodically and highly variable organic matter accumulation and decomposition rates12,13,14. In most coastal wetlands, fiddler crabs act as ecological engineers15 enhancing soil-water-atmosphere exchange through burrowing, foraging, and courtship behaviors12,16. Burrows promote hydrological connectivity17,18 and sediment transport19,20, biogeochemical cycles of redox-sensitive elements (e.g., Fe, Mn, S21), and greenhouse gas emissions (N2O, CO2, CH49). As a result, burrows may reduce carbon sequestration by promoting organic carbon mineralization and flushing of greenhouse gases9,11,22,23. Indeed, CO2 efflux through crab burrows can be orders of magnitude greater than through aqueous diffusion alone9,24. However, crabs and their burrows can also have a positive impact on carbon burial by increasing organic matter accumulation and improving plant primary productivity11,25,26. For example, crab burrows promote seedling growth that indirectly increases carbon sequestration27. Because of these contrasting perspectives, further clarifying the impact of crab burrows on the carbon budget is crucial for understanding the complexity of blue carbon systems and the relative importance of biological interactions. Therefore, large-scale assessments of the impact of crab burrows on blue carbon sequestration have long been limited by logistical and scientific constraints.
Here, we test whether crab burrows alter soil carbon sequestration by enhancing greenhouse gas emissions from blue carbon sediments. We conducted a continental-scale investigation covering large gradients in climate, sediment, and habitat types (Fig. 1; Supplementary Table 1). Our paired investigations in bare mudflats, saltmarshes, mangroves, and tidal creek banks resulted in 53 control plots and surveys of > 1000 crab burrows (see Methods) to resolve links between organic carbon composition, enzyme activities, and greenhouse gas emissions (CO2, CH4, and N2O). We also assessed whether crab burrows modify the blue carbon sink capacity of large areas of saltmarshes replaced by mangroves due to coastal restoration projects. Overall, our large-scale investigation resolves a major carbon cycle feedback of widespread fiddler crab burrows.
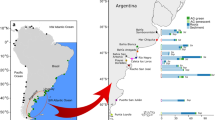
a Each sampling site (red circles) has 1-4 types of coastal habitats, including bare mudflats (orange square), saltmarshes (green square), mangroves (red square) and tidal creek banks (blue square). The white arrows indicate sites with sediment incubation experiments for measuring greenhouse gas emissions and sediment enzyme activity. Interannual mean values of (b) temperature and (c) rainfall reveal a climatic gradient along the 21 sampling sites. The error bars indicate annual temperature ranges. d Photos of saltmarshes, mangroves, bare mudflat, and tidal creek banks as well as a chimney shaped crab burrow showed the field scene and sampling schemes. For easier comparison, sites 1-8 and 9−21 were grouped into southern (mangrove-dominated) and northern (saltmarsh-only) systems.
Results and Discussion
Crab burrowing reallocates soil organic carbon
Our continental-scale observations revealed contrasting soil organic carbon (SOC) compositions in crab burrows and sediment matrix in all fiddler crab habitats (Fig. 2 and Supplementary Fig. 1). Crab burrowing and foraging activities increased permeability and oxygen exposure thereby altering SOC compositions8,17,25. Heavy-fraction organic carbon (HFOC) was consistently enriched relative to light-fraction organic carbon (LFOC) (p < 0.01; Supplementary Figs. 1 and 2). The SOC composition varied as a function of latitude and habitat type (Supplementary Fig. 2). For example, light SOC was stable from south to north, while heavy SOC showed pronounced peaks in some southern mangroves. The average LFOC was 2.9 times higher in the southern mangroves (1.22 ± 1.53 (0.76) g kg−1; average ± SE (median)) than in the northern saltmarshes (0.42 ± 0.38 (0.3) g kg−1). The average LFOC content in mangroves was at least three times greater than in the other three habitats (p < 0.01; Supplementary Fig. 1). Tidal creek banks had the highest proportion of HFOC at 94% and 95% in burrow and matrix, respectively, reflecting lower storage capability of LFOC. Finally, the average contents of both heavy and light SOC were 3% and 8% higher in the burrows than in the matrix (Supplementary Fig. 1).
GPP (Gross primary production), NPP (Net primary production), R (Respiration), LFOC (Light-fraction organic carbon), HFOC (Heavy-fraction organic carbon), HU (humin), HA (Humic acid), FA (Fulvic acid). The data in the parenthesis indicate the median fluxes (g m−2 yr−1) of different carbon transport pathways based on published literature (Supplementary Table 3). The red arrow shows the net increase of GHGs emission fluxes per unit area under the influence of crab burrows. The different colored boxes in the subplots indicate significant differences between crab burrows and sediment matrix (p < 0.05). The brown, blue, and red boxes indicate that the values in crab burrows were significantly larger (>), lower (<), and approximately equal (≈) than those in sediment matrix, respectively.
The effects of crab burrowing on humus compositions were complex. Fulvic acid (FA) and humin (HU) were higher in the southern warmer climates than northern colder sites in both burrow and matrix samples. Total humus in mangroves was 5 to 140% greater than in the other three habitats. The HU fraction in humus exceeded 80% in both burrow and matrix across different habitats (Supplementary Figs. 1 and 3). In saltmarshes and tidal creek banks, humus contents in burrows were consistently larger than those in the matrix, but an opposite trend occurred in mudflats and mangroves (p < 0.01; Supplementary Fig. 1).
Crab burrows have great influence on soil pH, moisture level, nitrogen, and enzyme activity which can indirectly control organic carbon compositions (Supplementary Text 1; Supplementary Figs. 4 and 5). The organic carbon proxies such as dissolved organic carbon (DOC), SOC, LFOC and humus were correlated with nitrogen, clay content, and climatic factors (Supplementary Fig. 6). Furthermore, crab burrowing influenced LFOC and HFOC through the indirect controls of β-glucosidase and cellobiosidase on SOC (Supplementary Fig. 7; Supplementary Table 2), indicating that sediment enzymes can catalyze reactions and directly influence carbon retention rates and material transformation28,29. The overall impact of crab burrows on organic carbon content and compositions was not as strong as geographical location, climate, and sediment structure (Supplementary Fig. 7; Supplementary Table 2).
In salt marshes and mangroves, organic carbon indicators such as SOC, DOC, and humus compositions in crab burrows are significantly higher than those in the sediment matrix (p < 0.05; Supplementary Fig. 1). The presence of feeding and metabolic residues of crabs inside the burrows creates effective traps of organic matter11,26. The δ13C-SOC values (Supplementary Fig. 1), and high fluorescence index confirms the abundance of biogenic organic matter within crab burrows30 (Supplementary Fig. 1). Sediment properties have a significant impact on carbon indicators (p < 0.05; Fig. 3a, b; Supplementary Fig. 8), with a noticeable increase in mineral and carbon indicators within crab burrows compared to the surrounding matrix. Clay within crab burrows exert a stronger influence on carbon distribution than those found in the matrix (Fig. 3a, b; Supplementary Fig. 8).
Relative effect and parameter estimate of environmental predictors on carbon distribution in (a) crab burrow and (b) sediment matrix. The “*” indicate the significance levels at p < 0.01. The error bars indicate parameter estimates ranges. Structural equation model (SEM) showing the strong relationships between greenhouse gases and chemical indices in crab burrow (c, CO2; e, CH4) and sediment matrix (d, CO2; f, CH4). The solid lines indicate the significance level at p < 0.05 while the dashed lines indicate non-significant differences. The climate factor includes the daily temperature difference (DT) and the annual rainfall (AnR). The sediment characteristics include the mineral (clay) and moisture level. The sediment carbon components include light-fraction organic carbon (LFOC) and heavy-fraction organic carbon (HFOC).
Crab burrows enhance greenhouse gas emissions
Crab burrows enhanced sediment CO2, CH4 and N2O emission rates from blue carbon ecosystems by 23, 120, and 30% relative to fluxes from the sediment matrix (Fig. 4; Supplementary Figs. 9–12), respectively. The average emissions of CO2 (2.0 ± 3.2 (3.1) mg kg−1 d−1), CH4 (2.7 ± 2.7 (1.5) μg kg−1 d−1) and N2O (4.6 ± 4.1 (4.4) μg kg−1 d−1) in crab burrows were 17, 49 and 46 % higher than those from the sediment matrix in saltmarshes during a one-month incubation. In mangroves the average emissions of CO2, CH4 and N2O in crab burrows were 30, 141% and 9% higher than those samples in the sediment matrix. CO2 and CH4 were significantly different (p < 0.05, Fig. 4) between crab burrows and matrix but N2O was not (p > 0.05; Fig. 4).
The violin plots compare emission rates of (a) CO2, (b) CH4 and (c) N2O in burrows and matrix. The cumulative bar charts integrate greenhouse gas fluxes (d CO2; e CH4; f N2O) in burrows versus matrix during the 28-day incubation period. The box plots indicate the range in cumulative fluxes for different sampling sites. The red font indicates the multiple of mean value of emission rates or fluxes in burrow versus matrix. The “**” and “*” indicate the significance levels at p < 0.01 and p < 0.05, respectively.
The substantial fluxes observed can be attributed to the enhanced penetration of oxygen induced by burrows, leading to increased oxidation of soil organic carbon (SOC) and subsequent greenhouse gas production12,23. A comparative analysis demonstrated that the emission rates of greenhouse gases on the 1st, 14th, and 28th day of the incubations exhibited a stronger correlation with chemical proxies in crab burrows than in the sediment matrix (Supplementary Fig. 13). The emission rates of CO2 were found to be correlated with indicators for total nitrogen and organic carbon (TNs, TNw, SOC, HFOC, HU, FA and HA) both within burrows and matrix. However, CO2 emissions were primarily influenced by organic carbon content (e.g., DOC) and enzyme activity (e.g., cellobiosidase) within burrows, while in the matrix they were predominantly controlled by moisture levels regulating oxygen penetration into sediments31.
In contrast to CO2, the CH4 fluxes were driven by the cm-scale micro-environment conditions, which were in turn controlled by enzyme activities and humus in burrows (Supplementary Fig. 13). CH4 emission rates both in burrows and matrix were positively correlated with total nitrogen, SOC, LFOC, and C/N ratio. CH4 emissions correlated more significantly with humus and cellobiosidase in burrows than in matrix (p < 0.05; Supplementary Fig. 13). N2O emissions in crab burrows exhibited contrasting trends (Supplementary Fig. 14). The overall correlations between N2O emissions and all environmental proxies was barely significant (p > 0.05, Supplementary Figs. 12 and 14), indicating more complex processes driving N2O dynamics than CO2 and CH4. SOC, especially HFOC, is an important factor affecting greenhouse gas emissions and is significantly correlated with CO2 and CH4 emission fluxes (p < 0.05; Fig. 3c–f). Compared to the indirect limitation through HFOC and LFOC inside the burrows, the annual rainfall and other climatic conditions in the substrate are directly related to greenhouse gas emissions (p < 0.05; Fig. 3c–f).
Implications and prospects
Ecosystem-engineer crabs are common in nearly all coastal wetlands except for the polar regions32 and may impact blue carbon ecosystem budgets. Crab burrows enable hydrological connectivity and efficient solute transport across the sediment-water interface18. Although our incubation experiments cannot fully mimic in-situ conditions regulating microbial process and carbon turnover33, the enhancement of greenhouse gas emissions due to crab burrowing still has the potential to attenuate carbon sequestration in coastal wetlands. Greenhouse gas emissions from coastal blue carbon ecosystems account for only 11–37% of net ecosystem production and vertical carbon burial (Fig. 2; Supplementary Table 3). Despite their small mass contribution, CH4 and N2O can play a crucial role in local carbon budgets34.
The impacts of crab burrows on carbon cycling are highly heterogeneous preventing global upscaling from our lab-based incubations. However, our continental-scale observations along a climate gradient allows for large-scale insight. There were only weak correlations between burrow density and greenhouse gas fluxes (Supplementary Fig. 15) because the underground burrow shape, size, and connectivity cannot be evaluated at scale. The median value of greenhouse gas fluxes (Supplementary Table 3) from the sediment matrix were multiplied by the increase in emissions due to crab burrows. The increased CO2 emissions due to crab burrows were 95.4 ± 71.8 (111.5) and 256.1 ± 392.3 (156.7) g C m−2 yr−1 in saltmarshes and mangroves, respectively. If these emissions remained as soil organic carbon, carbon burial would increase by 29 ± 22 (34) % from 330.0 g C m−2 yr−1 (median; Supplementary Table 3) to 425.4 ± 401.8 (441.5) g C m−2 yr−1 in saltmarshes, and 97 ± 148 (59) % from 265.0 g C m−2 yr−1 (median; Supplementary Table 3) to 521.1 ± 392.3 (421.7) g C m−2 yr−1 in mangroves.
The increased CH4 emissions (27 times CO2 equivalents35) due to crab burrows were 3.5 ± 3.4 (2.8) and 382.6 ± 383.5 (198) g C-CO2eq m−2 yr−1 in saltmarshes and mangroves, respectively. If these CH4 emissions remained as soil organic carbon, carbon burial would increase by 1 ± 1 (0.9) % and 144 ± 145 (75) % in saltmarshes and mangroves, respectively. There are differences in CH4 emissions between saltmarshes and mangroves likely because greater SO42− in saltmarshes inhibit CH4 emission36,37. Overall, burrow-related greenhouse gas emissions can minimize the potential for soil carbon sequestration by 35% and 134% (median) in saltmarshes and mangroves, respectively. There are large uncertainties in the net increase of N2O emissions due to the large scatter in the data, preventing similar estimates for N2O (Supplementary Table 3).
Compared to saltmarshes, crab burrows in mangroves produced more CO2 and CH4. In recent years, the Chinese government has strengthened the restoration of the mangrove ecological environment (Special Action Plan for Mangrove Conservation and Restoration 2020–2025, Ministry of Natural Resources, PRC38) as a response to the ongoing expansion of saltmarshes. Some coastal saltmarshes have now been fully replaced by mangroves. In China, mangroves were distributed in the southern subtropical provinces39 with slight seasonal cycles in solar radiation, temperature, crab species, and precipitation (Supplementary Table 1). We estimate that if all saltmarshes in these provinces were replaced by mangroves, crab burrows could minimize blue carbon burial by −6 to 45% (median 41%; Supplementary Fig. 16).
Multiple feedback loops exist among crab burrows, vegetation, sediment properties, and carbon components (Fig. 5). These feedbacks create a highly complex intertidal sediment mosaic that modify carbon sequestration and habitat structure. Although crab burrows can increase SOC accumulation through the traps of organic matter, the high oxygen content in sediments caused by burrows can also promote aerobic degradation of organic matter40. Wetland vegetation is a source of nutrition and influences burrow construction41,42. Moderate-density vegetation facilitates burrow maintenance, while high-density vegetation reduces crab burrow abundance43. The enhanced greenhouse gas emissions create a feedback loop. For example, warm conditions aggravate water scarcity, disrupt normal burrowing activities, and can lead to widespread crab mortality44. As a result of climate change, individual fiddler crab species may migrate to higher latitudes, resulting in shifts in community composition45 and carbon sequestration. From the perspective of carbon emissions and storage, the importance of crab burrows was greatest in mangroves > tidal creek banks > saltmarshes > mudflats.
However, the interaction between crab burrows and other bioturbators (mesofauna, vegetation) can lead to changes in sediment organic matter characteristics46. For example, any interaction between vegetation and burrows can dramatically change sediment properties due to radial oxygen loss and exudates released by roots47. The increase in soil organic matter and humus will affect carbon storage and promote greenhouse gas emissions (Fig. 3; Supplementary Fig. 8). Simultaneously, crab burrows and greenhouse gas emissions can stimulate vegetation metabolism, improve productivity, and buffer stresses (i.e. salinity). Other crab species and burrowing animals may build deeper and more complex burrows, which in turn affect carbon accumulation and sediment characteristics25,48. Further estimates of large-scale blue carbon fluxes should thus consider the complex impacts of crab burrows on blue carbon biogeochemistry.
Overall, our continental-scale experiments reveal a major impact of fiddler crab burrowing on methane emissions and blue carbon accounting. Sediment properties and biogeochemical fluxes in crab burrows were less variable than in the sediment matrix. Considering the combined effects of CO2 and CH4, crab burrows reduced the soil carbon burial potential by 35% in saltmarshes and 134% in mangroves, potentially making mangroves a net carbon source. The influence of crab burrows was lower in colder, drier northern regions than tropical regions dominated by mangroves. Human activities (i.e., eradication of Spartina alterniflora-dominated saltmarshes) will further minimize net carbon sequestration by enhancing greenhouse gas emissions. Considering crab burrows weakens the carbon sink function of coastal blue carbon habitats by enhancing greenhouse gas emissions.
Methods
Field campaign
This study covered 18,000 km of shoreline along a latitudinal gradient from 20°N to 45°N in China encompassing three climate zones (tropical, subtropical, and temperate) and four marginal seas (the South China Sea, the East China Sea, the Yellow Sea, and the Bohai Sea) (Fig. 1). A total of 21 research sites were established from Guangxi Province in the south to Liaoning Province in the north (Fig. 1). The annual temperature variation among the 21 research sites increased from south to north (Supplementary Table 1). For instance, annual temperature variation in southern sites was around 20 °C while it reached 44 °C in the north. Annual precipitation showed a decreasing trend from south to north.
Sediment samples were collected from all crab habitats including bare mudflats, saltmarshes, mangroves, and tidal creek banks (Fig. 1). Mudflats are the most widespread habitat type, present at all research sites along the coastline. Saltmarshes, known for their resilience and ability to invade mangroves in China, were almost ubiquitous from south to north49. Mangroves, consisting mainly of evergreen shrubs or trees, are limited to tropical or subtropical intertidal zones. Tidal creek banks, characterized by strong hydrodynamic exchange, abundant food availability, and higher crab burrow densities, serve as unique habitats for fiddler crabs compared to wetland interior50.
This study focused on comparing sediment samples from crab burrow walls with the surrounding sediment matrix in the four crab habitat zones (Fig. 1). Different crab species can lead to completely different sediment characteristics25,46,48. Here, we investigated three types of fiddler crabs: Tubuca arcuata, Gelasimus borealis, and Austruca lactea (Supplementary Table 1). The burrow sediments were sampled at least 30 cm from the roots because roots change the sediment characteristic due to radial oxygen exchange47. Sampling took place during low tides when crabs leave their burrows. Burrows were randomly selected, prioritizing fresh ones with chimney-shaped structures. Before sampling, the exposed chimney-shaped sediments around the burrow openings were removed due to prolonged exposure to oxygen. Sediment samples, approximately 0.5 cm thick, were collected using a small knife by rotating around the burrow walls at a depth of 2–5 cm below the opening. Around 10–30 burrows were collected from each habitat and pooled until reaching a weight of 150 g. In total, 1137 crab burrow samples were collected across 53 habitats. The density of burrows was assessed by counting burrow openings within a 0.5 m × 0.5 m square area. Samples were collected both from the crab burrow walls (i.e., burrow samples) and the surrounding sediment matrix (i.e., matrix samples) located 5 cm away from the burrow center, allowing for a direct comparison of physical and chemical characteristics. A total of 106 paired burrow-matrix samples were collected from 53 habitats. Additionally, we collected more sediment samples (300 g) from 17 saltmarsh and mangrove habitats (white arrows in Fig. 1) for measuring greenhouse gas production and sediment enzyme activity. All field sampling were completed from March 2022 to May 2022. At that time, the direct impact of human activities was negligible due to the COVID-19 pandemic.
Sediment and porewater properties
We measured 24 environmental variables including water saturation, sediment particle size, organic carbon compositions, humus, total nitrogen content and sediment enzyme activities in sediment and/or porewater. After weighing the wet sediment samples, they were heated at 105 °C ± 2 °C for 12 h and then reweighed to determine sediment dry weight. The difference between the wet and dry sediment represents the water content in the sediment. The sediments for particle size analysis were first dispersed with sodium hexametaphosphate solution and then measured on a MasterSizer-2000 Laser Particle Size Analyzer (measurement range: 0.02 ~ 2000 μm). To measure EC and pH through a HACH Multi-Parameter Meter, we fully mixed 3 g wet sediment with 60 mL Milli-Q water (Milli-Q Integral 5, Thermo Scientific) for 24 h. After being centrifuged at high speed, the supernatant passed through a 0.45 μm PES membrane (Millipore Express®PLUS). Total sediment organic carbon (SOC) and total nitrogen in sediment (TNs) were tested using a carbon and nitrogen analyzer (CN802, Velp, Italy). Dissolved organic carbon (DOC) and total dissolved nitrogen (TNw) were determined using the potassium dichromate - concentrated sulfuric acid oxidation method with a total organic carbon analyzer (TOC-V series SSM-5000A, Shimadzu, Japan; detection limit: ~4 μg L−1). δ13C-SOC isotope analysis was conducted using a GasBench II coupled to a 253 Plus mass spectrometer (Thermo Fischer Scientific).
Determination of humic substances and organic carbon fractions
HA is the major composition of humic substances. FA is a type of organic acid that occurs as a composition of humus. HU is a carbon-based macromolecular substance that occurs in sediment or as a byproduct of saccharide-dependent biorefinery processes, which usually constitutes about half of the humic substances in sediment. The key differences among HA, FA and HU are that: 1) HA is insoluble under acidic conditions (pH < 2) but soluble at higher pH; 2) FA is soluble at all pH conditions; 3) HU is the fraction not soluble in water at any pH value51. The HA and FA contents were obtained through the standard potassium dichromate oxidation volumetric method52, including the extraction solution after acidification, precipitation, and abstraction53,54. The HU was calculated by subtracting the HA and FA contents from SOC54.
Determination of light and heavy fractions of organic carbon
The ratio of LFOC to HFOC in organic carbon particles reflects carbon storage characteristics55. The content of LFOC ( < 1.7 g cm−3) and HFOC ( >1.7 g cm−3) was determined using the Titrette titration device from Prandtl, Germany. The method involved the use of potassium dichromate-concentrated sulfuric acid with additional heating (Supplementary Method 1). LFOC serves as a transitional reservoir between fresh residues and stable organic matter, which can be easily decomposed into CO2 or CH456. As the main composition of SOC in most wetlands, HFOC is the organic carbon wrapped by particles with more stable physical and chemical properties than LFOC57.
Sediment enzyme activities
Sediment enzymes, a major active organic component of sediment, play important roles in the material cycle of coastal wetland ecosystems58. Sediment enzyme activity is a useful indicator of microbial activity linked to substrate dynamics because enzymes catalyze rate-limiting steps in sediment organic matter decomposition28. An inverse relationship often exists between nutrient availability and enzyme activity, i.e., high enzyme activity indicates nutrient limitation59. Generally, enzyme activities in the vegetated sediments are ordinarily higher than in bare mudflat sediments due to larger biomass and more developed aerenchyma in both aboveground and belowground parts in vegetated sediments60. The typical enzyme activities (polyphenol oxidase, peroxidase, β-glucosidase, and cellulase) were measured through the fluorescence method (Supplementary Method 2).
Anaerobic incubation experiment to resolve greenhouse gas production
Sediment incubation experiments were performed to measure sediment greenhouse gas production rates. The incubations were conducted under anaerobic conditions which dominate in coastal sediments owing to the high-water content, low-permeability, and periodic inundation21. Each incubation was carried out in the laboratory for 28 days. Briefly, sediment samples (20.0 g) taken from burrows and sediment matrixs were placed in 500 mL conical flasks and 40 mL of ultrapure water was added into each flask to simulate the intertidal moisture content61. All samples were sealed with a rubber stopper, and then vacuum-filled with Ar three times to remove O2 and mimic flooding. 40 mL of ultra-pure water was added in each conical flask before being re-evacuated and filled with Ar three times, and incubated for 28 days.
During the incubation period, 20 mL of gas was extracted from the top of the conical flask on days 1, 3, 7, 14, 21, and 28 to measure concentrations of CO2, CH4, and N2O using gas chromatography (Model 7890 N, Agilent, USA). After sampling, an equal amount of Ar was added to ensure that air pressure was maintained in the culture flasks. The concentrations of CO2, CH4, and N2O were converted to the concentrations of CO2-C, CH4-C, and N2O-N. The production rate of CO2, CH4, and N2O was estimated by comparing gas concentrations over a fixed incubation period as described by refs. 62, 54.
Statistical analyses
The analysis employed Pearson correlation and Spearman’s R to assess the relationships between variables. Principal composition analysis (PCA) was used to reduce the dimensionality of the dataset, considering sediment characteristics and greenhouse gas production rates63. Prior to PCA, the suitability of the data was confirmed through the Kaiser-Meyer-Olkin and Bartlett’s tests64. Only compositions with eigenvalues greater than 1 were considered65. Statistical analysis included one-way analysis of variance (ANOVA) and non-parametric tests (Wilcoxon Signed Rank Test; Kendall). Set 95% and 99% confidence intervals to identify significant differences between variables. Significance levels were set at p < 0.05 (*) and highly significant at p < 0.01 (**). “NA” indicates no significant difference. We performed structural equation model (SEM) analyses to determine the relative importance of influencing factors such as temperature and rainfall, and sediment physicochemical properties in the emissions of greenhouse gases (i.e., CO2, CH4, and N2O), both inside and outside the crab burrows. A detailed description of SEM building and validation processes are provided in supplementary methods 3 and 4. SPSS 27.0 and Origin 2023 were used for statistical calculations. In southern China, the extensive coastal restoration projects have been rapidly increasing to eradicate Spartina alterniflora-dominated saltmarshes66. Thus we further assessed the dynamic influence of crab burrows on blue carbon sink as large areas of saltmarshes were replaced by mangroves (Supplementary Method 5).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The source data underlying all the figures in the main article and supplementary information that support the findings of this study are available in “figshare” with the link (https://doi.org/10.6084/m9.figshare.25347760.v3). Specifically, it includes field sampling data (SOC, HFOC, LFOC, HU, FA, HA, environmental variables) and incubation experiment data (enzyme activity, greenhouse gas emission rate, greenhouse gas cumulative flux).
References
Donato, D. C. et al. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 4, 293–297 (2011).
Macreadie, P. I. et al. Blue carbon as a natural climate solution. Nat. Rev. Earth Environ. 2, 826–839 (2021).
Atwood, T. B. et al. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Change 7, 523–528 (2017).
Bertram, C. et al. The blue carbon wealth of nations. Nat. Clim. Change 11, 886–886 (2021).
Crooks, S. et al. Coastal wetland management as a contribution to the US National Greenhouse Gas Inventory. Nat. Clim. Change 8, 1109–1112 (2018).
Noyce, G. L. et al. Oxygen priming induced by elevated CO2 reduces carbon accumulation and methane emissions in coastal wetlands. Nat. Geosci. 16, 63–68 (2023).
Reithmaier, G. M. S. et al. Carbonate chemistry and carbon sequestration driven by inorganic carbon outwelling from mangroves and saltmarshes. Nat. Commun. 14, 8196 (2023).
Guimond, J. A. et al. A physical-biogeochemical mechanism for negative feedback between marsh crabs and carbon storage. Environ. Res. Lett. 15, 034024 (2020).
Agusto, L. E. et al. Fiddling with the blue carbon: Fiddler crab burrows enhance CO2 and CH4 efflux in saltmarsh. Ecol. Indic. 144, 109538 (2022).
Kristensen, E. et al. Pneumatophores and crab burrows increase CO2 and CH4 emission from sediments in two Brazilian fringe mangrove forests. Mar. Ecol.: Prog. Ser. 698, 29–39 (2022).
Martinetto, P. et al. The blue carbon of southern southwest Atlantic salt marshes and their biotic and abiotic drivers. Nat. Commun. 14, 8500 (2023).
Kristensen, E. et al. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 89, 201–219 (2008).
Alongi, D. M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 6, 195–219 (2014).
Glud, R. N. Oxygen dynamics of marine sediments. Mar. Biol. Res. 4, 243–289 (2008).
Kristensen, E. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. J. Sea Res. 59, 30–43 (2008).
Backwell, P. R. Y. & Jennions, M. D. Coalition among male fiddler crabs. Nature 430, 417–417 (2004).
Xin, P. et al. Effects of crab burrows on pore water flows in salt marshes. Adv. Water Resour. 32, 439–449 (2009).
Yin, M. S. et al. Randomly distributed crab burrows enhance groundwater flow and salt transport in creek-marsh systems. Water Resour. Res. 59, e2023WR035612 (2023).
Wasson, K. et al. Pattern and scale: evaluating generalities in crab distributions and marsh dynamics from small plots to a national scale. Ecology 100, e02813 (2019).
Liu, Y., Reible, D. & Hussain, F. Roles of tidal cycling, hyporheic exchange and bioirrigation on metal release from estuary sediments. Water Resour. Res. 58, e2021WR030790 (2022).
Pan, F. et al. Integrated effects of bioturbation, warming and sea-level rise on mobility of sulfide and metalloids in sediment porewater of mangrove wetlands. Water Res. 233, 119788 (2023).
Xiao, K. et al. Large CO2 release and tidal flushing in salt marsh crab burrows reduce the potential for blue carbon sequestration. Limnol. Oceanogr. 66, 14–29 (2021).
Grow, A. K., Schutte, C. A. & Roberts, B. J. Fiddler crab burrowing increases salt marsh greenhouse gas emissions. Biogeochemistry 158, 73–90 (2022).
Howes, B. L., Dacey, J. W. H. & Teal, J. M. Annual carbon mineralization and belowground production of spartina-alterniflora in a new-england salt-marsh. Ecology 66, 595–605 (1985).
Andreetta, A. et al. Mangrove carbon sink. Do burrowing crabs contribute to sediment carbon storage? Evidence from a Kenyan mangrove system. J. Sea Res. 85, 524–533 (2014).
Toniolo, M. A., Seitz, C. & Perillo, G. M. E. Origin and evolution of tidal depressions in a tidal flat and their role in carbon sequestration in the Bahia Blanca Estuary (Argentina). Mar. Geol. 436, 106467 (2021).
Macusi, E. & Tipudan, C. D. Effects of bioturbation of fiddler crabs in relation to the growth of mangrove saplings (Rhizophora apiculata) in a mangrove reforested area. Journal of Marine and Island Cultures 9, 1–10 (2020).
Sinsabaugh, R. L. et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 11, 1252–1264 (2008).
Moeskops, B. et al. The impact of exogenous organic matter on SOM contents and microbial soil quality. Pedobiologia 55, 175–184 (2012).
McKnight, D. M. et al. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 46, 38–48 (2001).
Pepper, I. L. & Brusseau, M. L. Chapter 2 - Physical-Chemical Characteristics of Soils and the Subsurface. In: Brusseau M. L., Pepper I. L., Gerba C. P. (eds). Environmental and Pollution Science (Third Edition). Academic Press, 9−22 (2019).
Rosenberg, M. S. Contextual cross-Referencing of species names for fiddler crabs (Genus Uca): an experiment in cyber-taxonomy. Plos One 9, e101704 (2014).
Zhang, L. et al. Changes in soil carbon and enzyme activity as a result of different long-term fertilization regimes in a greenhouse field. Plos One 10, e0118371 (2015).
Cotovicz, L. C. et al. Methane oxidation minimizes emissions and offsets to carbon burial in mangroves. Nat. Clim. Change 14, 275–281 (2024).
Forster, P. et al. The Earth’s Energy Budget, Climate Feedbacks, and Climate Sensitivity. in Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (eds. Masson-Delmotte, V. et al.) (Cambridge University Press, 2021).
Capone, D. G. & Kiene, R. P. Comparison of microbial dynamics in marine and fresh-water sediments - contrasts in anaerobic carbon catabolism. Limnol. Oceanogr. 33, 725–749 (1988).
Capooci, M. et al. Experimental influence of storm-surge salinity on soil greenhouse gas emissions from a tidal salt marsh. Sci. Total Environ. 686, 1164–1172 (2019).
Ministry of Natural Resources (PRC), Special Action Plan for Mangrove Conservation and Restoration 2020−2025. https://www.forestry.gov.cn/main/216/20201001/113924624667920.html (2020).
Liao, B. & Zhang, Q. Area, distribution and species composition of mangroves in China. Wetland Science 12, 435–440 (2014).
Martinetto, P. et al. Crab Bioturbation and Herbivory May Account for Variability in Carbon Sequestration and Stocks in South West Atlantic Salt Marshes. Front. Mar. Sci. 3, https://doi.org/10.3389/fmars.2016.00122 (2016).
Nobbs, M. Effects of vegetation differ among three species of fiddler crabs. J. Exp. Mar. Biol. Ecol. 284, 41–50 (2003).
He, Q. & Cui, B. S. Multiple mechanisms sustain a plant-animal facilitation on a coastal ecotone. Sci. Rep. 5, 8612 (2015).
Bertness, M. D. & Miller, T. The distribution and dynamics of uca-pugnax (smith) burrows in a new-england salt-marsh. J. Exp. Mar. Biol. Ecol. 83, 211–237 (1984).
Ryer, C., H., vanMontfrans, J. & Moody, K. E. Cannibalism, refugia and the molting blue crab. Mar. Ecol.: Prog. Ser. 147, 77–85 (1997).
Lee, S. Ecology of Brachyura. In: Castro D., Guinot, S. & von Klein. V. (ed). The Crustacea 9:C-I Decapoda:Brachyura. Brill, Leiden, 469-541(2015).
Booth, J. M. et al. The microbial landscape in bioturbated mangrove sediment: A resource for promoting nature-based solutions for mangroves. Microb. Biotechnol. 16, 1584–1602 (2023).
Booth, J. M. et al. Fiddler crab bioturbation determines consistent changes in bacterial communities across contrasting environmental conditions. Sci. Rep. 9, 3749 (2019).
Booth, J. M. et al. The role of fungi in heterogeneous sediment microbial networks. Sci. Rep. 9, 7537 (2019).
Zhang, Y. H. et al. Interactions between mangroves and exotic Spartina in an anthropogenically disturbed estuary in southern China. Ecology 93, 588–597 (2012).
McCraith, B. J. et al. The effect of fiddler crab burrowing on sediment mixing and radionuclide profiles along a topographic gradient in a southeastern salt marsh. J. Mar. Res. 61, 359–390 (2003).
Stevenson, F. J. Humus chemistry: genesis, composition, reactions, second edition (Stevenson, F. J.). J. Chem. Educ. 72, A93 (1995).
Ministry of Agriculture and Rural Affairs (PRC), Method NY/T 1867−2010: Determination of humus content in soil: potassium dichromate oxidation volumetric. 1–3 (2010).
Weber, J. et al. Optimized isolation method of humin fraction from mineral soil material. Environ. Geochem. Health 44, 1289–1298 (2022).
Ye, H. et al. Extreme rainstorms change organic matter compositions and regulate greenhouse gas production in mangrove sediments. Agric., Ecosyst. Environ. 357, 108694 (2023).
Qin, X., Wu, H. M. & Jian, L. Methane emissions from wetlands in China: effects of wetland type and climate zone. Carbon Manage. 5, 535–541 (2015).
Janzen, H. H. et al. Light-fraction organic-matter in soils from long-term crop rotations. Soil Sci. Soc. Am. J. 56, 1799–1806 (1992).
Ji, H. et al. Soil organic carbon pool and chemical composition under different types of land use in wetland: Implication for carbon sequestration in wetlands. Sci. Total Environ. 716, 136996 (2020).
Liu, L. et al. Pattern of soil extracellular enzyme activities along a tidal wetland with mosaic vegetation distributions in Chongming Island, China. J. Cleaner Prod. 315, 127991 (2021).
Sinsabaugh, R. L. et al. Wood decomposition - nitrogen and phosphorus dynamics in relation to extracellular enzyme-activity. Ecology 74, 1586–1593 (1993).
Shao, X., Yang, W. & Wu, M. Seasonal dynamics of soil labile organic carbon and enzyme activities in relation to vegetation types in Hangzhou Bay tidal flat wetland. Plos One 10, e0142677 (2015).
Zhang, Y. H. et al. Exogenous nitrogen addition inhibits sulfate-mediated anaerobic oxidation of methane in estuarine coastal sediments. Ecol. Eng. 158, 106021 (2020).
Das, S. & Adhya, T. K. Dynamics of methanogenesis and methanotrophy in tropical paddy soils as influenced by elevated CO2 and temperature interaction. Soil Biol. Biochem. 47, 36–45 (2012).
Cloutier, V. et al. Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J. Hydrol. 353, 294–313 (2008).
Maurya, P. & Kumari, R. Toxic metals distribution, seasonal variations and environmental risk assessment in surficial sediment and mangrove plants (A. marina), Gulf of Kachchh (India). J. Hazard. Mater. 413, 125345 (2021).
Abdi, H. & Williams, L. J. Principal component analysis. WIREs Computational Statistics 2, 433–459 (2010).
Lyu, C. X. et al. The effect of Spartina alterniflora eradication on waterbirds and benthic organisms. Restor. Ecol. 31, e14023 (2023).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Nos. 42177046, 12372383, U22A20580), GuangDong Basic and Applied Basic Research Foundation (No. 2022A1515010572), Shenzhen Science and Technology Program (No. 20200925174525002), and the Ministry of Education of China (No. D20020).
Author information
Authors and Affiliations
Contributions
K.X. conceptualized the study; F.P. and Y.L. provided resources and material; H.B.P. and his team conducted the field work; K.X., Y.C.W., Y.R.H., and M.Q.L. analyzed the physicochemical properties of sediment and porewater and conducted statistical analysis; H.L.L., Y. Zheng, C.M.Z., N.W.C., Y. Zhang, L.L.X., G.X.H., Y.S.L., P.X., R.L.L, B.C.X., F.M.W., J.J.T., A.M.W., D.M.A and I.R.S. contributed to formal data analysis and data interpretation; K.X. wrote the original draft; all authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Nadine Schubert and Alireza Bahadori. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiao, K., Wu, Y., Pan, F. et al. Widespread crab burrows enhance greenhouse gas emissions from coastal blue carbon ecosystems. Commun Earth Environ 5, 437 (2024). https://doi.org/10.1038/s43247-024-01621-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-024-01621-2
This article is cited by
-
Forest Species Composition, Not Crab Bioturbation, Drives Greenhouse Gas Emissions in Subtropical Mangroves
Ecosystems (2025)
-
Current status of coastal blue carbon assessment: Theory, methods, and carbon sequestration pathways
Science China Earth Sciences (2025)