Abstract
Reef-building coral populations face unprecedented threats from climate warming. Standing variation in heat tolerance is crucial for evolutionary processes necessary for corals to persist. Yet, the spatial distribution of heat-tolerant corals and the underlying factors that determine heat tolerance are poorly understood from individual to ecosystem scales. Here, we show extensive variation in the heat tolerance of a foundational coral species complex across the Great Barrier Reef. Thermal thresholds of 569 individuals differed by up to 7.3 °C across scales from meters to >1250 km. Variation in thresholds among reefs was consistent with local adaptation and acclimatization to historical and recent thermal history. However, variation within reefs was sometimes greater than among reefs and largely unexplained by environmental predictors, putative host species, or symbiont communities. This indicates that within-reef heat tolerance differences may be informed primarily by other factors, such as adaptive genomic variation. We anticipate our findings will inform conservation and restoration actions, including targeting individuals for selective breeding of enhanced heat tolerance.
Similar content being viewed by others
Introduction
Climate warming and unprecedented marine heatwaves have resulted in bleaching and mortality of reef-building corals1. However, not all corals will fare equally well under sea temperature rise as heat tolerance varies widely among2,3 and within species4,5,6,7. Intraspecific variation in coral heat tolerance may hold equal or even greater amounts of variation and ecological value compared to among-species, as has been shown in a wide variety of taxa and traits8. Likewise, heat tolerance variation within closely related species complexes may be uniquely ecologically important, and especially valuable for adaptation if intraspecific variation is boosted through occasional hybridization between closely related taxa9. Thus, understanding the heat tolerance repertoire of populations and closely related species is fundamental to understanding the future of coral reefs under climate change.
Intraspecific variation in the heat tolerance of corals is apparent among reef populations10,11,12,13, but can also occur within and among habitats across smaller spatial scales4,6,7,14. This has been attributed in part to environmental factors such as thermal history, either by adaptation through natural selection and/or through the acclimatization of individuals13,15,16. Consequently, heat tolerance has been found to be positively associated with greater numbers of historical thermal anomalies12,17,18, or temperature variability19. However, further studies are required to understand the distribution and drivers of intraspecific variation in heat tolerance at individual and ecosystem scales.
Quantifying intraspecific variation in key traits that affect fitness is important as it informs the potential for rapid evolution in response to environmental change. For example, intraspecific variation can affect community structure and dynamics, and thus is an important consideration for ecological or evolutionary models20. Intraspecific variation may also be incorporated into coral projection models that calculate survival under future climate scenarios21. Further, heat-tolerant individuals are key targets for restoration and adaptation programs as they and their offspring may be more likely to persist under future warming scenarios compared to less tolerant conspecifics. Thus, a more comprehensive understanding of the drivers of heat tolerance and the distribution and abundance of adaptive variation in reef metapopulations is crucial to inform evolutionary models, projections of future reef populations, selective breeding efforts, and conservation planning.
Coral heat tolerance may differ among closely related or cryptic species12,22, including those within the Acropora hyacinthus complex, which is the focus of this study. A. hyacinthus represents a group of Indo-Pacific tabular corals23,24,25,26 that hold exceptional value in ecosystem function but are threatened by a high vulnerability to climate warming27. Although morphological differences exist among some taxa within this complex, it is often difficult or impossible to identify them without genetic data23. These cryptic lineages may vary in their growth rate, microhabitat distribution, and heat tolerance23,24,26,28. Unresolved taxonomic diversity may lead to an overestimation of true intraspecific heat tolerance and may obscure investigations into drivers of heat tolerance. As species delineations within the A. hyacinthus complex is an active area of research24,25,29, heat tolerance differences among putative species also need to be characterized. Currently, only two studies have assessed heat tolerance differences within species in the A. hyacinthus complex in American Samoa24,28, and information is lacking for other regions including the Great Barrier Reef (GBR).
The community composition of algal endosymbionts (Symbiodiniaceae30) can also exert a strong influence on coral heat tolerance31. Acroporid corals on the GBR have been shown to associate with primarily generalist Cladocopium species32,33, and associations with heat-tolerant Cladocopium and Durusdinium species following bleaching have increased heat tolerance by over 1 °C34,35,36. Yet, heat tolerance conferred by Symbiodiniaceae associations may be species-specific due to co-evolved symbiont-host partnerships and host-dependent effects34,37. Thus, to understand general patterns of heat tolerance conferred by Symbiodiniaceae associations, their effect on heat tolerance needs to be characterized in additional host species, including within the A. hyacinthus species complex.
Here, we quantify the heat tolerance traits of 569 colonies of A. hyacinthus from 17 reefs across 9.42° latitude of the GBR, in one of the largest-scale studies of intraspecific variation in coral heat tolerance to date. We use an acute heat stress assay to identify the spatial distribution of heat-tolerant individuals and reef populations in a multi-metric approach. We then evaluate the relative influence of environmental factors, Symbiodiniaceae community, and host genomic identity on heat tolerance traits in this ecologically important species complex.
Results
Variation in heat tolerance across the Great Barrier Reef
Standardized assays were used to rapidly phenotype numerous colonies by exposing them to acute heat stress (Fig. 1). This approach has been shown to provide rankings of heat tolerance that corresponded with rankings determined by traditional longer-term heat stress experiments38 and recovery from heat stress39. Following heat exposure, tolerance was measured as photochemical efficiency (Fv/Fm) and chlorophyll content (Normalized Difference Vegetation Index; NDVI), which were expressed as absolute thermal thresholds (i.e., the effective temperature inducing a 50% decline in trait performance; ED50), as well as their retained performance (i.e., the proportion of retained trait performance under heat stress at 9 °C above local climatology; see “Methods”).
A Experimental design and heat tolerance trait data collection for Acropora hyacinthus colonies across the Great Barrier Reef. B Colonies were sampled from 17 sites to be fragmented to allow 3 replicate fragments in each of 4 temperature treatments in experimental aquaria. C Aquaria were controlled to follow ramp-hold temperature profiles. Photochemical efficiency and chlorophyll content were measured after the ramp down from temperature (dashed lines). D Trait values at temperature treatments were used to calculate retained performance and fit log-logistic curves to generate ED50 values.
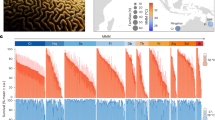
Responses of colonies within the A. hyacinthus complex to acute heat stress varied considerably across the GBR (Fig. 2). Overall mean ED50 thermal thresholds were 36.4 °C and 35.6 °C for Fv/Fm and NDVI respectively, yet these thresholds differed by as much as 3.5 °C (Fv/Fm: 34.6–38.1) and 7.3 °C (NDVI: 32.4–39.7) among individual colonies (Table 1). For both traits, colonies with the highest ED50 thresholds predominantly occupied northern reefs, however, a few individuals ranked within the top 20% were also found at central and southern reefs (Fig. 2A, B). Mean retained performance values were 0.39 and 0.26 for Fv/Fm and NDVI respectively, ranging by 0.93 (Fv/Fm: 0.00–0.93) and 0.91 (NDVI: 0.03–0.95). Colonies with the highest retained performance generally occupied southern reefs with some individuals within the top 20% also occupying central and northern reefs, a pattern that was stronger for retained Fv/Fm (Fig. 2B) than retained NDVI (Fig. 2C).
For absolute metrics (i.e., ED50 thresholds), of the maximum quantum yield of photosystem II (Fv/Fm, (A)) and the normalized difference vegetation index (NDVI, (B)), most heat-tolerant individuals (i.e., within the top 20%) were from the northern GBR. In contrast, for retained performance metrics of Fv/Fm (C) and NDVI (D), most heat-tolerant individuals were from the southern GBR. The distribution of top-performing colonies also varied by metric, whereby top performers according to Fv/Fm were found in a smaller number of sites and top performers according to NDVI can be found across most sites. The dashed line indicates the latitudinal divide between north-central sites (above dashed line) and southern sites (below dashed line).
Within-site rankings of heat tolerance were largely consistent between absolute and retained performance metrics for both photochemical capacity (Fv/Fm; R = 0.71) and chlorophyll content (NDVI; R = 0.65; Supplementary Fig. 2). The relationship of tolerance rankings between Fv/Fm and NDVI traits was weaker (R = 0.36–0.47; Supplementary Fig. 3), potentially as these traits capture different biological aspects of the heat stress response with Fv/Fm capturing a breakdown in the photochemical pathways within Symbiodiniaceae and NDVI capturing the loss of Symbiodiniaceae during bleaching. Consequently, trade-offs existed between heat tolerance responses across the GBR, where few colonies performed highly for both ED50 and retained performance metrics and tended to be restricted to northern-central and southern reefs, respectively (Fig. 2).
For ED50 and retained metrics of Fv/Fm, variance was 1.6–6 times higher among sites than within sites (Table 1). However, for both metrics of NDVI, variance was 1.3–1.5 times higher within sites than among sites, and this trend was most pronounced for ED50 thresholds (Table 1). Variance within and among sites for all heat tolerance metrics deviated by <0.01 from variances stated above when also accounting for host genomic cluster as a random factor (Supplementary Table 3).
Host genomic cluster assignments
Principal components analysis of 1,238,851 loci indicated that coral colonies (n = 625) belonged to four distinct genomic clusters (Fig. 3A) and these assignments were also supported in Bayesian clustering analysis (Fig. 3C, see “Methods”). Most colonies (472 of 625) were assigned to group 1 (Grey, Fig. 3A) which represents our target morphotype, A. hyacinthus “neat”. Groups 2–4 contained 27, 36, and 90 colonies, respectively. Group 1 differentiated from groups 2–4 across PC1 and its divergence was also supported in Bayesian admixture analyses for all values of K (Fig. 3C). Groups 2–4 likely represent other putative species (or ecotypes) within the A. hyacinthus species complex. Colony morphology and branchlet structure varied among genomic clusters (Fig. 3B), yet colonies of the same cluster often presented variable features in different sites and may be very difficult to distinguish based on morphology alone (i.e., cryptic).
A Principal Components Analysis of genome-wide SNPs showing four distinct clusters representing four distinct putative species within the A. hyacinthus complex. Group 1 represents the target morphotype, A. hyacinthus “neat” which comprised the majority of colonies sampled (472 of 625 total colonies), with groups 2–4 containing 27, 36, and 90 colonies, respectively. B Colony morphology and branching structure of genomic clusters 1–4 within the A. hyacinthus species complex on the Great Barrier Reef. Colonies pictured to represent groups 1–4 were all sampled on Sykes Reef. C Admixture plot assuming 2–5 ancestral populations (k) within the A. hyacinthus species complex sampled across the Great Barrier Reef, supporting the grouping of these colonies into four putative species clusters.
Host genomic clusters (Fig. 3A) varied spatially, with group 1 (A. hyacinthus “neat”) sampled primarily in the central and northern GBR and groups 2–4 sampled primarily in the southern GBR and at Fitzroy Island (inshore northern GBR; Fig. 4A). While differences may exist in the distribution and abundance of these genomic clusters (or putative species), the relationship between latitude and cluster prevalence seen here could also reflect collection bias and identification error at different sites. Thus, we restricted our analysis of heat tolerance variation among clusters to the 5 southern sites to reduce the confounding latitudinal effects on heat tolerance (groups 1–4: n = 44, 14, 27, 51). Site-adjusted heat tolerance metrics exhibited higher variation within than among genomic clusters (Fig. 4B–E), although group 2 displayed a non-significant trend for lower NDVI ED50 (Fig. 4D). This relationship was more pronounced during a natural bleaching event, where groups 2 and 3 exhibited lower CoralWatch Color Scores than group 4, indicating a lighter appearance and greater bleaching sensitivity of these putative species (Supplementary Fig. 4).
A Variation in A. hyacinthus host genomic cluster assignments across sites on the Great Barrier Reef. B–E Heat tolerance traits from the southern reefs (sites shown in rectangle in A). Groups 1–4 from southern reefs shown in rectangle contained 44, 14, 27, and 51 individuals, respectively. Host genomic clusters were assigned based on genome-wide SNPs with group 1 representing A. hyacinthus “neat” and groups 2–4 representing other putative species in the A. hyacinthus complex. Heat tolerance traits have been adjusted to remove variation due to site using residuals from site-specific means. P-values are shown for each trait using one-way ANOVAs.
Symbiodiniaceae communities
Symbiodiniaceae ITS2 communities in A. hyacinthus colonies were overwhelmingly composed of Cladocopium variants (≥99% of sequencing reads in all colonies; Fig. 5B), with rare occurrences of Symbiodinium (4% of reads in 22% of colonies). The major Cladocopium variants in our sample set co-occurred within colonies across the GBR, indicating they are of intragenomic origin. Variants differed spatially, most remarkably across the inshore-offshore gradient (Fig. 5A). Two closely related variants, C50a and C50c were disproportionately detected in inshore sites (yellow, Fig. 5B), while C50b was detected primarily in southern sites (purple, Fig. 5B). The C3k variant was detected in every colony (n = 421), although northern mid and outer reef sites contained proportionally more C3k reads compared to southern and inshore sites. Consequently, 70% of colonies were assigned a single dominant ITS2-type profile (C3k/C50a/C50b/C50c-C3ba-C50q-C3-C50f) which occurred at all reefs but was hosted almost exclusively on inshore reefs (blue, Supplementary Fig. 5). A further 16% were assigned a closely related ITS2-type profile (C3k-C50a-C3-C3ba-C50q-C50f-C3dq-C3a), which was most common on mid and offshore reefs (yellow, Supplementary Fig. 5). The remaining 9 ITS2-type profiles were each hosted by less than 4% of colonies, with C3k/C3bo-C3ba-C50a-C50q-C50b occurring primarily on southern reefs (purple, Supplementary Fig. 5).
A Principal components analysis of ITS2 sequences in 404 colonies of A. hyacinthus showing ITS2 sequence differentiation between colonies in inshore habitats versus those in outer and mid-shelf habitats. B Variation in Symbiodiniaceae communities within A. hyacinthus colonies across the Great Barrier Reef. ITS2 sequences are shown for individuals (vertical columns) within sites (arranged by latitude). C Shelf position (bottom row) is shown to visualize ITS2 sequence differences at inshore sites (yellow) versus mid-shelf (green) and offshore sites (blue). The dashed line indicates the latitudinal divide between north-central sites (left) and southern sites (right).
Symbiodiniaceae ITS2-type profiles and sequences were most strongly differentiated across shelf position and latitude; a trend that was 10 times more pronounced for ITS2 sequence variants (PERMANOVA, partial ω2 = 0.41 and 0.23, p = 0.001; Supplementary Table 4) than type profiles (PERMANOVA, partial ω2 = 0.035 and 0.026, p = 0.001; Supplementary Table 5). ITS2 sequences revealed similar trends between offshore and mid-shelf sites, with more distinct sequences found in inshore sites (Fig. 5A) While we did not detect variation in ITS2-type profiles by host genomic cluster, there were minor differences in sequence variants among host genomic clusters (PERMANOVA, partial ω2 = 0.090, p = 0.001), that interacted with the effects of shelf position and latitude (PERMANOVA, partial ω2 = 0.027 and 0.091, p = 0.001; Supplementary Table 4). Despite this spatial variation, we did not observe any relationship between the first PC value summarizing Symbiodiniaceae ITS2 sequence variants in A. hyacinthus and heat tolerance metrics across the GBR, apart from a very weak relationship with Fv/Fm ED50 (R = −0.12, p = 0.026; Supplementary Fig. 6). This was also the case at the site-level, apart from Fv/Fm ED50 at North Direction (R = −0.39, p = 0.019, Supplementary Fig. 7) and both Fv/Fm metrics at Lady Musgrave (R = −0.45 and −0.51; p = 0.029 and 0.027; Supplementary Fig. 7).
Top predictors of heat tolerance traits
Boosted Regression Trees (BRTs), incorporating environmental, Symbiodiniaceae, and host genomic cluster predictors, explained 36–81% of the variation in heat tolerance traits of A. hyacinthus across the GBR (Fig. 6). Temperature variables were the most predictive of trait variation for all metrics. Maximum Monthly Mean (MMM) related variables held strong relative predictive influence for both ED50 metrics whereby a MMM > ~28.5 °C signaled an increase in Fv/Fm and NDVI ED50s (Fig. 6A, C). However, MMM showed a negative relationship with retained performance, indicating that colonies on cooler reefs are living further from their upper thermal limits (Supplementary Fig. 8). Fv/Fm metrics tended to have stronger relationships to MMM than NDVI metrics, likely because Fv/Fm primarily varied across sites while NDVI varied more strongly within sites. Degree Heating Weeks (DHW) at the time of collection was an important predictor for 2 of 4 metrics, Fv/Fm ED50 and retained NDVI, indicating that exposure to recent heat stress (DHW > 0.5) improved performance in our heat stress assays (Fig. 6A, D). Thermal anomalies were also an important predictor for retained metrics, where performance was highest at southern reefs that have experienced fewer thermal anomalies (Fig. 6B, D). Symbiodiniaceae variables appeared in the top 4 predictors for NDVI metrics (Fig. 6C, D), and host genomic cluster was not a top predictor in any of the BRT models.
BRT models of retained performance metrics explained 12–13% more variation than absolute (ED50) metrics, likely because retained traits varied more among sites and ED50s varied more within sites, and most environmental predictors were stratified at the site level. Additionally, predictors explained 31–33% more variation in Fv/Fm than NDVI, likely due to the high within-site variability of NDVI metrics. These results were supported by linear models which showed that the amount of variance explained by predictors and traits was largely consistent with BRTs, though tended to retain additional predictors including genomic cluster and coral health chart score (Supplementary Fig. 9; Supplementary Table 6).
Discussion
Our study provides one of the largest-scale investigations of intraspecific variation in the heat tolerance of reef-building corals. Using acute heat stress assays, we quantified the multi-trait heat tolerance of 569 individual colonies belonging to the Acropora hyacinthus species complex across 9.42° latitude of the Great Barrier Reef (GBR). We demonstrate considerable variation in the thermal thresholds of individual colonies both among (up to 7.3 °C) and within (up to 5.7 °C) reefs. Thermal history was the main driver of this variation, supporting the adaptation of populations to their local climatology (i.e., historical MMM) as well as acclimatization to recent thermal anomalies (i.e., DHW during collection). While Symbiodiniaceae community and the distribution of four host genomic clusters varied spatially, these factors had minimal contribution to variation in heat tolerance. Thus, unexplained variation in heat tolerance within putative species within the A. hyacinthus species complex may be controlled by functional genomic variation that was not measured in this study.
Sea surface temperature climatology was the strongest predictor of variation in the heat tolerance of colonies within the A. hyacinthus species complex across the GBR. This aligns with previous findings indicating that heat tolerance likely reflects local adaptation to temperature10,40. A. hyacinthus on northern and inshore reefs had higher thermal thresholds (Fv/Fm and NDVI ED50s; Fig. 2A, B) but were living on average 0.6–0.8 °C closer to those thresholds than those on southern reefs. These populations living near their upper thermal limits may have higher absolute thermal thresholds but less capacity to adapt to future warming41. While southern populations in this study exhibited lower absolute thermal thresholds, their greater distance from summer maximum temperatures and higher tolerance in retained traits indicate that they may be better positioned to persist through future warming, perhaps enhanced by gene flow of heat-adapted alleles from northern populations42,43. Similar latitudinal patterns in thermal thresholds have also been identified for A. spathulata in a parallel study44 and in other non-acroporid species2,12,45, highlighting the potential for high latitude populations to serve as refugia as sea temperatures continue to rise.
In addition to climatology, recent accumulated thermal stress (i.e., DHW at time of collection) was an important predictor for two heat tolerance metrics (NDVI retention and Fv/Fm ED50; Fig. 6A, D). Colonies that were pre-exposed to mild to moderate recent heat stress (DHW > 0.5 and < 4) at the time of experimentation exhibited increased heat tolerance in our short-term assays. This supports that preconditioned colonies can display higher heat tolerance, likely via acclimatization. However, more extreme heat stress would likely have opposite effects of mild heat stress and instead exacerbate damage to coral during subsequent experimental stress44,46. As heat tolerance varies with recent environmental conditions, acclimatization to these conditions may complicate understanding of heritable phenotypic variation available for natural or assisted evolution. Thus, future work should aim to reduce variation in heat stress preconditioning prior to assessing heat tolerance differences by performing experiments within the same season or including recent heat stress exposure in explanatory models.
Analysis of genome-wide variation revealed four putative species in the A. hyacinthus complex on the GBR, with most samples representing the “neat” morphotype, the primary target for this study (Fig. 3). This aligns with past work detecting four putative species within the A. hyacinthus complex on the GBR and six putative species across the Asia-Pacific23,25. Our findings support the importance of a taxonomic revision to resolve morphological and genomic differences in this species complex.
Putative species displayed differences in their responses to a natural bleaching event, with groups 2 and 3 exhibiting lower heat tolerance than group 4 (Supplementary Fig. 4). This suggests a role of host genomic identity in setting heat tolerance, although these differences may be confounded with low sample sizes of non-target putative species (n = 5, 7, and 31 for groups 2–4). Further, it is possible that putative species within the A. hyacinthus complex hold distinct environmental preferences, as has been found in past work23,26,28. Thus, species effects on heat tolerance can be difficult to disentangle from other drivers (e.g., thermal history). In this study, our analysis of tolerance to experimental heat stress among genomic clusters was restricted to southern GBR colonies, which did not reveal differences among putative species, perhaps due to high trait variation within each genomic cluster. This conflicts with past work showing differences in responses to natural and experimental heat stress between two putative species within the A. hyacinthus complex living in the same backreef pool28. Consequently, further work is needed to evaluate differences in heat tolerance among putative species within the A. hyacinthus complex while explicitly accounting for known genomic identities and their environmental preferences.
For two of four heat tolerance metrics measured, we found variation among colonies within the A. hyacinthus complex was greater within reefs than among reefs (Table 1; Supplementary Table 3), indicating that even small spatial scales may hold genomic variation in heat tolerance to allow adaptation to climate warming, even when accounting for host genomic cluster. This aligns with past work showing high trait variability among colonies within a single reef, including bleaching and mortality responses to experimental and natural heat stress4,6,47. Here, thermal threshold variation in color paling (NDVI) was over 2.5 times more variable within sites compared to photochemistry (Fv/Fm). This may reflect the later measurement of this trait after the accumulation of more thermal damage (i.e., 17 versus 10 h after the start of the experiment), or a more variable holobiont response in NDVI versus symbiont response in Fv/Fm. A later measurement of Fv/Fm may have produced more variable results as time-series Fv/Fm was shown to remain fairly consistent after an acute heat stress assay until 48 h after the end of heat stress in A. tenuis48. High phenotypic variability within small scales may also reflect the high potential larval dispersal of A. hyacinthus as a broadcast spawner and may not reflect species with different life history strategies.
Predictors of heat tolerance were largely uninformative within-reef sites, indicating that the high variability we measured among individual colonies is primarily determined by factor(s) other than depth, host genomic cluster identity, colony pigmentation, or Symbiodiniaceae community composition. Although we accounted for thermal and nutrient variables along depth stratifications, there may be additional unmeasured microhabitat features such as water flow49 that could affect within-reef heat tolerance. It is also likely that within-reef heat tolerance variation is controlled in part by host genomic variation given the high heritability of coral bleaching and survival under heat stress47. Functional genomic variation within the coral host or Symbiodiniaceae may occur even across small scales (i.e., within a reef) and underpin heat tolerance variation that could not be accounted for in this study. Future work should aim to quantify this fine-scale genomic variation as it may be harnessed by natural or assisted evolution to support restoration goals.
Symbiodiniaceae communities in all A. hyacinthus clusters were composed of closely related and co-occurring variants of Cladocopium across the GBR. The predominant differentiation of Symbiodiniaceae communities by shelf position, followed by latitude, aligns with previous findings on the Symbiodiniaceae communities in acroporid corals on the GBR32,50,51. Inshore environments may exert unique selective pressures on Symbiodiniaceae communities due to higher temperatures, greater temperature or water variability, and/or increased turbidity32,33,50,52. We found minor differences in Symbiodiniaceae communities among different host genomic clusters, although this may be partially explained by unequal sampling of groups 2, 3, and 4 in the southern GBR, where certain Symbiodiniaceae ITS2 variants were more abundant. Yet, this finding is consistent with past work showing that Symbiodiniaceae communities can vary between closely related putative host species24,53.
While Symbiodiniaceae communities varied spatially and by host cluster identity, they had minor effects on the heat tolerance of coral colonies in our study. This challenges previous findings showing that symbiont identity is a strong driver of heat tolerance in acroporid corals on the GBR; a pattern especially evident when coral colonies host multiple symbiont genera34,36. When limited to naturally occurring variation in Symbiodiniaceae associations at the intrageneric level (e.g., within Cladocopium), heat tolerance conferred by the Symbiodiniaceae community may be less apparent. Yet, naturally heat-adapted Cladocopium has been shown to enhance the heat tolerance of corals37, suggesting potential for heat tolerance variation among Cladocopium communities of A. hyacinthus across the GBR. Minor effects of Symbiodiniaceae on host heat tolerance detected in this study may be due to limitations of the ITS2 marker to detect species delineations and functional associations54. Genome-wide studies of adaptive variation55 may reveal insight into functional differences among closely related Symbiodiniaceae taxa and their effect on heat tolerance. Further, symbiotic bacteria are another component of the coral holobiont that may influence heat tolerance56 but were not explored in this study.
Our results follow recent applications of standardized acute heat stress experiments to quantify population thermal thresholds2,12,14,57, extend this approach to colony-level applications14,58, and is one of the first to quantify individual-level thresholds across environmental gradients in wild populations (but see ref. 44). This method allows rapid high-throughput phenotyping of corals and has been shown to measure relative differences in heat tolerance between sites and among individuals in a short-term assay (18 h) comparable to a longer-term assay (21 days38,58). Previous work has shown agreeability in experimental acute heat tolerance rankings among nursery-bred colonies during two seasons in the same year14 and with bleaching resistance during a natural bleaching event59, but further work is required to conclude that experimental heat tolerance responses are consistent across time and are indicative of survival during bleaching events.
Since no single trait can fully capture the coral heat tolerance phenotype, we measured both photochemical stress and color paling after acute heat stress. Recent work has shown that interpretations based on a single heat tolerance trait (i.e., bleaching vs photochemistry) after acute heat stress can lead to disparate conclusions, suggesting the importance of measuring multiple traits to yield a more comprehensive picture of heat tolerance58. Future work should continue to assess multiple traits, especially to provide insight into both Symbiodiniaceae and host responses to heat stress. Standardized comparisons of heat tolerance utilizing multiple traits can allow for greater comparative frameworks across coral taxa and ocean regions.
In conclusion, we find that heat tolerance within the A. hyacinthus species complex varies considerably across small and large spatial scales on the GBR. Depending on the heat tolerance trait measured, variation was sometimes greater within-reef sites than among reef sites, suggesting that even small scales hold wide phenotypic variability within each putative species that can be harnessed by natural or artificial selection. Variation in heat tolerance rankings among traits suggests the importance in aligning tolerance metrics with research, restoration, or conservation goals. For example, absolute heat tolerance metrics may be of special interest to restoration initiatives aiming to enhance tolerance traits, while retained performance metrics may be more useful to understand how coral populations under climate warming will fare in their changing environments. Extensive variation in within-reef heat tolerance measured here indicates that heat-tolerant alleles may be found across multiple reefs, supporting the notion that identifying heat-tolerant broodstock for restoration is not as simple as sourcing from warm reefs17,60. Additional unexplained variation in heat tolerance may be due in part to functional genomic differences in the coral host or Symbiodiniaceae not measured here, which may uncover evolutionary potential in climate adaptation and standing variation available for restoration initiatives.
Methods
Field collections and acute heat stress assays
Individual colonies of Acropora hyacinthus (n = 583) were sampled from 17 reefs across the Great Barrier Reef (GBR; Supplementary Table 1). Within reefs, colonies were primarily sampled from the reef crest or upper slope at a single site (i.e., within 5 km), apart from Hicks where a small number of colonies (n = 7) were sampled from an additional site. Since A. hyacinthus represents a species complex23,26,29, the “neat” morphotype was targeted in this study to focus on intraspecific variation in heat tolerance. The “neat” morphotype is characterized by tightly packed and neatly arranged vertical branchlets with crowded labellate radial corallites forming a rosette-like arrangement, tightly reticulate basal branches, and a neater oval-shaped tabular structure. These characters distinguish it from similar species, where branch lengths and radial corallites are more irregular in shape and arrangement. However, a minority of colonies representing non-target morphotypes were also inadvertently sampled, particularly at sites where “neat” morphotypes were rarely encountered. The identity of colony morphotypes was confirmed by assignment to clusters based on genomic data (described below).
Colonies were sampled on SCUBA over the course of six field trips from January 2021 to March 2022. For each colony, 14 replicate fragments were collected; 12 fragments were placed in an acute heat stress assay, one was fixed in 100% ethanol for genomic analysis, and one provided a skeletal taxonomic reference. Colonies were sampled if they met size thresholds (>20 cm) and showed no signs of disease or predation, though disease and predation were rarely encountered. Upon sampling, colony metadata was recorded including depth, GPS coordinates (as per61), visual CoralWatch Health Chart Score62, and photographs of each colony were taken for species identification. All colonies were sampled under permits from the Great Barrier Reef Marine Park Authority (G19/43148.1 and G21/45166.1) and with Free Prior and Informed Consent of Traditional Owners of the Sea Country where the study sites were located.
To quantify the heat tolerance of each colony within the A. hyacinthus complex, replicate fragments were exposed to a standardized acute heat stress assay (Fig. 1). Assays were performed with treatments at each site’s Maximum Monthly Mean (MMM) and the MMM + 3, +6, and +9 °C (n = 3 fragments per colony per treatment). The MMM was calculated according to the protocol established by the National Oceanic and Atmospheric Administration (NOAA) and represents the upper limit of baseline historical temperatures. It is defined as the warmest sea surface temperature (SST) of the 12 monthly mean values for the years 1985–1990 and 1993. The MMM is a widely used metric of coral climatology as it is used to calculate Degree Heating Weeks (DHW), an established metric to predict coral bleaching and compare heat tolerance.
Heat stress assays were undertaken with a portable experimental system on board a research vessel at sea developed by the National Sea Simulator at the Australian Institute of Marine Science. The system had four independent temperature treatments with three tanks per treatment. Tanks were individually housed with exterior insulating jackets of seawater kept at treatment temperatures by independent heating elements (Omega 2 kW titanium) in separate sumps for each temperature treatment. Separate seawater lines passed through titanium coils (Waterco titanium heat exchanger), which proceeded through temperature-controlled sumps to reach their programmed temperature, and were directed into tanks at a flow rate of approximately 0.8 L/min. Tanks contained temperature probes (TC Direct PT-100) and PAR sensors (Skye Quantum PAR – SKL26250) to maintain target temperatures and light levels. Independent temperature loggers (HOBO) were also placed in select tanks to confirm appropriate temperature ramps after each run. Light levels were maintained at approximately 300 PAR (mmol photons/m2s2) and were gradually turned on and off at sunrise and sunset to mimic natural light conditions.
Fragments were mounted onto experimental racks immediately upon collection and held in flow-through aquaria overnight before being placed in their experimental tanks. Assay profiles involved a ramp up to treatment temperatures over 3 h, a temperature hold for 3 h, a ramp down to MMM over 2 h, and an overnight hold at MMM. Profiles typically began at 11:00, with exceptions for Reef 21-550 (run 1 at 11:30 and run 2 at 12:00), Davies (12:00), East Cay (12:00), St. Crispin (run 1 at 12:30), and Martin (12:30). Downstream phenotypic measurements were delayed correspondingly to maintain consistent time spent in aquaria between the end of heat stress and trait measurements. One experimental assay has been removed from this dataset (run 2 at Reef 21-550) due to poor temperature control of aquaria during the experiment, reducing the final sample size to 569 colonies.
Heat tolerance was evaluated in an additional 60 colonies within the A. hyacinthus complex at North Direction during a natural bleaching event in March 2021. Tolerance was measured using a CoralWatch Color Health Chart Score62 to quantify bleaching and a sample was preserved in 100% ethanol for genomic analyses. Since these colonies were found to encompass multiple putative species, they were used only for comparisons of natural bleaching responses among closely related putative species (described below).
Measuring heat tolerance traits
The photochemical performance (Fv/Fm) of the symbionts within fragments was measured with an imaging PAM (Walz) two hours after the ramp-down to MMM was complete (dashed line, Fig. 1). Imaging PAM settings were as follows: measuring light intensity = 3 (in 2021) or 2 (in 2022, following replacement of LEDs), saturating pulse intensity = 7, gain = 1, damping = 1. From each fragment, three areas of interest were haphazardly selected, and the maximum quantum yield of photosystem II (Fv/Fm) was measured. The three Fv/Fm values were averaged to attain a mean measurement per fragment. Inadvertently, imaging PAM chlorophyll fluorescence images were missing for one or two of the three replicate fragments for 6, 5, 1 and 47 of the 569 colonies at MMM, +3 °C, +6 °C, and +9 °C respectively.
Color paling was measured using a hyperspectral camera (Resonon Pica XC2) ten hours after the ramp-down to MMM was complete (dashed line, Fig. 1). Three areas of interest were haphazardly selected on each fragment and a custom MATLAB script was used to extract a median Normalized Difference Vegetation Index (NDVI) per fragment. NDVI is an established metric to monitor land vegetation using satellites but has also been used to measure chlorophyll a reflectance of soft corals in experimental aquaria63. Reflectance was normalized using a grey standard and NDVI was computed as (R720 – R670)/(R720 + R670). This approach was validated in our sample set by comparing hyperspectral-image generated NDVI values to total chlorophyll content in a subset of tissue samples from two reefs (Chicken and Davies). Total chlorophyll was extracted from homogenized powder and measured on a microplate spectrophotometer (Bio-tek Powerwave). Chlorophyll was quantified and standardized to ash-free dry weight. NDVI values were highly correlated to total chlorophyll when compared among fragments (R = 0.74, p = <0.001, n = 293; Supplementary Fig. 1A) and averaged across replicate fragments of each colony (R = 0.86, p < 0.001, n = 98; Supplementary Fig. 1B). This demonstrates that the NDVI trait captures the decline in chlorophyll that occurs with color paling during the bleaching stress response, due to a loss of endosymbiont cells, their pigments and/or tissue necrosis. NDVI metrics were not computed for colonies in the first run at Hicks (n = 20 colonies) or the first run at Reef 21-550 (n = 15 colonies), as hyperspectral images were not taken.
From each of these two phenotypic traits (Fv/Fm and NDVI), two heat tolerance metrics were computed: an absolute metric which represents an absolute thermal threshold independent of local thermal history and a retained heat tolerance metric that represents how traits decline relative to their local site MMM. Absolute heat tolerance was measured as the effective dose (ED) temperature (°C) at which trait performance declines by 50% (i.e., ED5064). ED50 values were estimated by fitting three-parameter log-logistic dose response curves in the drc package in R with the following constraints: slope = [10,120]; ED50 = [28,40], Fv/Fm = [0,0.8], NDVI = [0,1]. ED50 values with confidence intervals >5 °C were excluded, and 83.7% and 91.8% of colony values were retained for downstream analyses for Fv/Fm and NDVI, respectively. Retained heat tolerance was quantified as the proportion of trait performance retained at the highest heat stress treatment (MMM + 9 °C). Metric values were corrected for any deviation between target treatment and actual tank temperatures, such that retained heat tolerance = (TraitMMM + 9 °C/TraitMMM) * ((TempMMM + 9 °C – TempMMM)/9).
Variation within and among sample sites was investigated using linear mixed models in the package lme465. In the first iteration, site was set as a random factor, with residual variance attributed to within-site variation. In a second iteration, site and genomic cluster (described below) were each set as random factors to assess residual variation within both sites and genomic clusters.
Genomic assignment to divergent species clusters
Genomic DNA was extracted using the QIAGEN Blood and Tissue kit and whole genome libraries were prepared using the Lotus DNA Library Prep Kit for NGS. Sequencing was performed by Azenta Life Sciences on the NovaSeq S4 300 cycle using 150 bp paired end reads. Reads were assessed and trimmed for quality, and trimmed reads were mapped to the A. hyacinthus genome66. Individuals with less than 80% of primary mapped reads to the reference genome were excluded from downstream analyses. We also removed technical replicates, retaining a total of 565 samples for which we had viable genomic and phenotype data and 60 colonies collected during a natural bleaching event. Additional details on genomic data preparation can be found in the Supplementary Methods and Results.
Genomic data were visualized using ANGSD67 to identify genetically distinct host clusters. We identified polymorphic sites and estimated genotype likelihoods to account for statistical uncertainty associated with sequencing errors or missing genotypes. Polymorphic sites were filtered as: mapping quality > 30, base quality > 30, coverage ≥ 3 reads in at least 95% of individuals, and sites mapping to 14 assembled chromosomes. We called major and minor alleles directly from the genotype likelihoods assuming biallelic sites and considered only polymorphic sites with a likelihood ratio test p-value < 0.000001. To evaluate population genetic structure, we extracted an individual covariance matrix with PCAngsd68 applying a 0.05 minor allele frequency (MAF) threshold. We computed eigenvectors and eigenvalues in R and performed a Principal Components Analysis. We also performed Bayesian hierarchical clustering admixture analyses in PCAngsd68 using the “admix” option to estimate individual ancestry proportions assuming 2–5 ancestral populations (K genomic clusters) and applying a MAF threshold of 0.05.
For 625 samples, including those placed in the acute heat assay (n = 565) and those assessed for their response natural bleaching (n = 60), the number of quality filtered sequence reads ranged between 15,603,604 and 63,841,053 reads per sample, with a mean of 26,493,269 reads. Sequenced samples had an average of 92.5% primary reads mapped to the reference genome. The mean coverage among individuals was 11.4x genome-wide and 7.02× considering only scaffolds corresponding to 14 assembled chromosomes.
Quantifying Symbiodiniaceae communities
Symbiodiniaceae communities were quantified using the ITS2 rDNA marker69 using established protocols for PCR amplification, library preparation, and ITS2 sequencing (see Supplementary Methods and Results).
The SymPortal framework (https://symportal.org/) was used to assign reads within each sample to Symbiodiniaceae genera, subgeneric ITS2-type profiles, and unique sequence variants. The Symbiodiniaceae ITS2 rDNA region can resolve genera and some species-level variation, however closely related species and sub-species can be challenging to delineate due to high intragenomic sequence diversity54. The SymPortal framework addresses this issue by assigning ITS2-type profiles to sets of co-occurring sequence variants which are more likely to belong to the same genotype when they repeatedly co-occur in biological samples69. Therefore, ITS2-type profiles represent putative Symbiodiniaceae taxa while sequence variants represent intragenomic variation that may or may not confer phenotypic or species differences54. ITS2 reads were normalized by median sequencing depth and sequences with fewer than 10 reads were removed, resulting in a total of 52 unique sequence variants across the sample set. ITS2-type profiles were also removed if they contained fewer than 10 reads, resulting in 12 ITS2-type profiles. To reduce the prevalence of metabarcoding artifacts, samples with low overall read counts were discarded from the analysis. Minimum read count thresholds were set as the number of reads found in negative control samples: 7500 reads for ITS2 sequence variants and 6,100 reads for ITS2-type profiles. This reduced the sample size to 424 colonies with assigned ITS2-type profiles and 421 colonies with ITS2 sequence variant data.
To characterize the Symbiodiniaceae community composition in each A. hyacinthus colony, the dominant ITS2-type profile was examined since over 97% of samples were assigned only one profile. However, as ITS2-type profile assignments in A. hyacinthus on the GBR have not yet been validated with other genetic makers, we also examine the relative abundance of all ITS2 amplicon sequence variants (ASVs) within our samples. These were summarized using Principal Components (PC) values of normalized read counts of all ITS2 sequences. Both ITS2-type profiles and ITS2 sequence variants (expressed as PCs) were examined for their relationships to shelf position, latitude, host genomic cluster, and their interactions using PERMANOVA on Bray-Curtis dissimilarities with 999 permutations. This was performed in R with the adonis2 function within the vegan package70.
Environmental variables
A total of 22 environmental variables were computed to quantify site- and colony-level variation in thermal history, accumulated heat stress, nutrient availability, and light penetration (Supplementary Table 2). This included data obtained from NOAA CoralWatch71, eReefs72, and NASA MODIS73, as well as in situ measurements (i.e., colony depth and pigmentation) (Supplementary Table 2). Variables computed from eReefs are based on hydrodynamic or biogeochemical models that estimate variables at different vertical stratifications. Thus, eReefs-derived variables were computed using values from models at the closest vertical stratification to the true colony depth (from −0.5, −2.35, −5.35, −9, or −13 m for the GBR1_hydro hydrodynamic model and −0.5, −1.5, −3, −5.5, −8.8, −12.75 m for the GBR4_BGC biogeochemical model). Additionally, some temperature variables were calculated using eReefs while others were calculated using CoralWatch, since these datasets vary in their spatial resolution and temporal range. Variables of climatology and thermal anomalies requiring a historical baseline were computed using CoralWatch which ranges from 1985 and has a 5 km resolution, while variables of warming rate and temperature variability requiring finer-scale resolution were computed using eReefs which ranges from 2014 and has a 1 km resolution (Supplementary Table 2).
Gradient boosting and prediction models
Predictors encapsulating variation in environment, endosymbiont community, and host genomic cluster were examined for their influence on heat tolerance traits using boosted regression trees (BRT; Supplementary Table 2). BRTs are a machine learning algorithm that evaluates the relationship between predictor variables and outcomes by building decision trees, that are boosted such that after each tree is built, a subsequent tree is trained using the residuals not explained by the previous tree74. BRT algorithms perform best when they learn slowly, building many trees and then averaging the patterns across all the trees to determine the influence and effect of predictor variables74. BRTs can accommodate continuous and categorical variables and are robust to non-linear interactions and missing data74. Collinear predictor variables do not affect predictive accuracy computation but may compete within the BRT model such that collinear variables may be elevated or repressed in variable importance rankings. Thus, the favored collinear variable in a BRT is more likely to be related to the response than its collinear predictors which were repressed. BRTs were implemented as gradient-boosting machine models in the R package gbm. Model parameters were chosen as follows: [Learning rate = 0.001, Interaction depth = 5, Bag fraction = 0.5, CV folds = 10]. Diagnostic plots of holdout deviance by tree iteration were used to assess overfitting. To assess deviance explained by each model, an identical model was fit using the gbm.step function in the dismo package and was calculated as (total deviance − cross-validated residual deviance)/total deviance75. Linear models and stepwise multiple regression were also used to investigate effects of predictors on heat tolerance traits and are explained in further detail in the Supplementary Methods and Results.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data and scripts that support the findings of this study are available at https://github.com/melissanaugle/Ahya_heat_tol_variation, coral colony metadata is available at https://doi.org/10.25845/J1JH-Q660, and genomics scripts can be found at https://github.com/ivapops/Acropora_cryptic_species. Raw sequence data for Symbiodiniaceae is available under the NCBI Sequence Read Archive: PRJNA1105655.
Code availability
Code used for analyses and figure generation is available at https://github.com/melissanaugle/Ahya_heat_tol_variation and code used for genomics analyses is available at https://github.com/ivapops/Acropora_cryptic_species.
References
Hughes, T. P. et al. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377 (2017).
Evensen, N. R. et al. Empirically derived thermal thresholds of four coral species along the Red Sea using a portable and standardized experimental approach. Coral Reefs https://doi.org/10.1007/s00338-022-02233-y. (2022)
Loya, Y. et al. Coral bleaching: the winners and the losers. Ecol. Lett. 4, 122–131 (2001).
Cornwell, B. et al. Widespread variation in heat tolerance and symbiont load are associated with growth tradeoffs in the coral Acropora hyacinthus in Palau. eLife https://doi.org/10.7554/eLife.64790. (2021)
Fuller, Z. L. et al. Population genetics of the coral Acropora millepora: toward genomic prediction of bleaching. Science 369, eaba4674 (2020).
Humanes, A. et al. Within-population variability in coral heat tolerance indicates climate adaptation potential. Proc. R. Soc. B Biol. Sci. 289, 20220872 (2022).
Marhoefer, S. R. et al. Signatures of adaptation and acclimatization to reef flat and slope habitats in the coral Pocillopora damicornis. Front. Marine Sci. 8, 704709 (2021).
Des Roches, S. et al. The ecological importance of intraspecific variation. Nat. Ecol. Evol. 2, 57–64 (2018).
Riginos, C. et al. Cryptic species and hybridisation in corals: challenges and opportunities for conservation and restoration. EcoEvoRxiv (2024).
Howells, E. J., Berkelmans, R., van Oppen, M. J. H., Willis, B. L. & Bay, L. K. Historical thermal regimes define limits to coral acclimatization. Ecology 94, 1078–1088 (2013).
Kenkel, C. D. et al. Evidence for a host role in thermotolerance divergence between populations of the mustard hill coral (Porites astreoides) from different reef environments. Mol. Ecol. 22, 4335–4348 (2013).
Marzonie, M. R. et al. The effects of marine heatwaves on acute heat tolerance in corals. Glob. Change Biol. 29, 404–416 (2023).
Thomas, L. et al. Mechanisms of thermal tolerance in Reef-building corals across a fine-grained environmental mosaic: lessons from Ofu, American Samoa. Front. Marine Sci. 4, 434 (2018).
Cunning, R. et al. Census of heat tolerance among Florida’s threatened staghorn corals finds resilient individuals throughout existing nursery populations. Proc. R. Soc. B: Biol. Sci. 288, 20211613 (2021).
Foo, S. A. & Byrne, M. Chapter two—acclimatization and adaptive capacity of marine species in a changing ocean. In Proc. Advances in Marine Biology (ed. Curry, B. E.) Vol. 74 69–116 (Academic Press, 2016).
Palumbi, S. R., Barshis, D. J., Traylor-Knowles, N. & Bay, R. A. Mechanisms of reef coral resistance to future climate change. Science 344, 895–898 (2014).
Quigley, K. M. & van Oppen, M. J. H. Predictive models for the selection of thermally tolerant corals based on offspring survival. Nat. Commun. 13, 1543 (2022).
Sully, S., Burkepile, D. E., Donovan, M. K., Hodgson, G., & van Woesik, R. A global analysis of coral bleaching over the past two decades. Nat. Commun. 10, 1264 (2019).
Carilli, J., Donner, S. D. & Hartmann, A. C. Historical temperature variability affects coral response to heat stress. PLOS ONE 7, e34418 (2012).
Bolnick, D. I. et al. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evolut. 26, 183–192 (2011).
Logan, C. A., Dunne, J. P., Eakin, C. M. & Donner, S. D. Incorporating adaptive responses into future projections of coral bleaching. Glob. Change Biol. 20, 125–139 (2014).
Gómez-Corrales, M. & Prada, C. Cryptic lineages respond differently to coral bleaching. Mol. Ecol. 29, 4265–4273 (2020).
Ladner, J. T. & Palumbi, S. R. Extensive sympatry, cryptic diversity and introgression throughout the geographic distribution of two coral species complexes. Mol. Ecol. 21, 2224–2238 (2012).
Rose, N. H. et al. Genomic analysis of distinct bleaching tolerances among cryptic coral species. Proc. R. Soc. B Biol. Sci. 288, 20210678 (2021).
Sheets, E. A., Warner, P. A. & Palumbi, S. R. Accurate population genetic measurements require cryptic species identification in corals. Coral Reefs 37, 549–563 (2018).
Suzuki, G. et al. Genetic evidence of peripheral isolation and low diversity in marginal populations of the Acropora hyacinthus complex. Coral Reefs 35, 1419–1432 (2016).
Ortiz, J. C. et al. Important ecosystem function, low redundancy and high vulnerability: The trifecta argument for protecting the Great Barrier Reef’s tabular Acropora. Conserv. Lett. 14, e12817 (2021).
Rose, N. H., Bay, R. A., Morikawa, M. K. & Palumbi, S. R. Polygenic evolution drives species divergence and climate adaptation in corals. Evolution 72, 82–94 (2018).
Ramírez-Portilla, C. et al. Solving the coral species delimitation conundrum. Syst. Biol. 71, 461–475 (2022).
LaJeunesse, T. C. et al. Systematic revision of sSymbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 28, 2570–2580.e6 (2018).
Quigley, K. M., Baker, A. C., Coffroth, M. A., Willis, B. L. & van Oppen, M. J. H. Bleaching Resistance and the Role of Algal Endosymbionts. In Coral Bleaching: Patterns, Processes, Causes and Consequences (eds. van Oppen, M. J. H. & Lough, J. M.) 111–151 (Springer International Publishing, Cham, 2018). https://doi.org/10.1007/978-3-319-75393-5_6.
Matias, A. M. A. et al. Cryptic diversity and spatial genetic variation in the coral Acropora tenuis and its endosymbionts across the Great Barrier Reef. Evolut. Appl. 16, 293–310 (2022).
Tonk, L., Sampayo, E. M., Weeks, S., Magno-Canto, M. & Hoegh-Guldberg, O. Host-specific interactions with environmental factors shape the distribution of Symbiodinium across the Great Barrier Reef. PLOS ONE 8, e68533 (2013).
Abrego, D., Ulstrup, K. E., Willis, B. L. & van Oppen, M. J. H. Species–specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proc. R. Soc. B Biol. Sci. 275, 2273–2282 (2008).
Berkelmans, R. & van Oppen, M. J. H. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc. R. Soc. B Biol. Sci. 273, 2305–2312 (2006).
Jones, A. M., Berkelmans, R., van Oppen, M. J. H., Mieog, J. C. & Sinclair, W. A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc. R. Soc. B Biol. Sci. 275, 1359–1365 (2008).
Howells, E. J. et al. Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Change 2, 116–120 (2012).
Voolstra, C. R. et al. Standardized short-term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Glob. Change Biol. 26, 4328–4343 (2020).
Walker, N. S. et al. Persistence of phenotypic responses to short-term heat stress in the tabletop coral Acropora hyacinthus. PLOS ONE 17, e0269206 (2022).
van Oppen, M. J. H. et al. Adaptation to reef habitats through selection on the coral animal and its associated microbiome. Mol. Ecol. 27, 2956–2971 (2018).
Somero, G. N. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers. J. Exp. Biol. 213, 912–920 (2010).
Matz, M. V., Treml, E. A., Aglyamova, G. V. & Bay, L. K. Potential and limits for rapid genetic adaptation to warming in a Great Barrier Reef coral. PLOS Genet. 14, e1007220 (2018).
Riginos, C. et al. Asymmetric dispersal is a critical element of concordance between biophysical dispersal models and spatial genetic structure in Great Barrier Reef corals. Divers. Distrib. 25, 1684–1696 (2019).
Denis, H. et al. Quantifying the thermal tolerance traits of individual corals reveals widespread variation across the Great Barrier Reef. bioRxiv, (2024).
Woolsey, E. S., Keith, S. A., Byrne, M., Schmidt-Roach, S. & Baird, A. H. Latitudinal variation in thermal tolerance thresholds of early life stages of corals. Coral Reefs 34, 471–478 (2015).
Ainsworth, T. D. et al. Climate change disables coral bleaching protection on the Great Barrier Reef. Science 352, 338–342 (2016).
Cunning, R., Ritson-Williams, R. & Gates, R. D. Patterns of bleaching and recovery of Montipora capitata in Kāne’ohe Bay, Hawai’i, USA. Mar. Ecol. Prog. Ser. 551, 131–139 (2016).
Nielsen, J. J. V. et al. Experimental considerations of acute heat stress assays to quantify coral thermal tolerance. Sci. Rep. 12, 16831 (2022).
Fifer, J. et al. Going with the flow: How corals in high-flow environments can beat the heat. Mol. Ecol. 30, 2009–2024 (2021).
Cooper, T. F. et al. Environmental factors controlling the distribution of Symbiodinium harboured by the coral Acropora millepora on the Great Barrier Reef. PLOS ONE 6, e25536 (2011).
Hoadley, K. D. et al. Host–symbiont combinations dictate the photo-physiological response of reef-building corals to thermal stress. Sci. Rep. 9, 9985 (2019).
de Souza, M. R. et al. Community composition of coral-associated Symbiodiniaceae differs across fine-scale environmental gradients in Kāne’ohe Bay. R. Soc. Open Sci. 9, 212042 (2022).
Johnston, E. C., Cunning, R. & Burgess, S. C. Cophylogeny and specificity between cryptic coral species (Pocillopora spp.) at Mo′orea and their symbionts (Symbiodiniaceae). Mol. Ecol. 31, 5368–5385 (2022).
Davies, S. W. et al. Building consensus around the assessment and interpretation of Symbiodiniaceae diversity. PeerJ 11, e15023 (2023).
Ishida, H., John, U., Murray, S. A., Bhattacharya, D. & Chan, C. X. Developing model systems for dinoflagellates in the post-genomic era. J. Phycol. 59, 799–808 (2023).
Ziegler, M., Seneca, F. O., Yum, L. K., Palumbi, S. R. & Voolstra, C. R. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat. Commun. 8, 14213 (2017).
Voolstra, C. R. et al. Contrasting heat stress response patterns of coral holobionts across the Red Sea suggest distinct mechanisms of thermal tolerance. Mol. Ecol. 30, 4466–4480 (2021).
Klepac, C. N. et al. Assessing acute thermal assays as a rapid screening tool for coral restoration. Sci. Rep. 14, 1898 (2024).
Morikawa, M. K. & Palumbi, S. R. Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. PNAS 116, 10586–10591 (2019).
Howells, E. J. et al. Enhancing the heat tolerance of reef-building corals to future warming. Sci. Adv. https://doi.org/10.1126/sciadv.abg6070 (2021).
Lukoschek, V., Riginos, C. & van Oppen, M. J. H. Congruent patterns of connectivity can inform management for broadcast spawning corals on the Great Barrier Reef. Mol. Ecol. 25, 3065–3080 (2016).
Siebeck, U. E., Marshall, N. J., Klüter, A. & Hoegh-Guldberg, O. Monitoring coral bleaching using a colour reference card. Coral Reefs 25, 453–460 (2006).
Leal, M. C. et al. Concurrent imaging of chlorophyll fluorescence, Chlorophyll a content and green fluorescent proteins-like proteins of symbiotic cnidarians. Mar. Ecol. 36, 572–584 (2015).
Evensen, N. R., Fine, M., Perna, G., Voolstra, C. R. & Barshis, D. J. Remarkably high and consistent tolerance of a Red Sea coral to acute and chronic thermal stress exposures. Limnol. Oceanogr. 66, 1718–1729 (2021).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
López-Nandam, E. H., Albright, R., Hanson, E. A., Sheets, E. A. & Palumbi, S. R. Mutations in coral soma and sperm imply lifelong stem cell renewal and cell lineage selection. Proc. R. Soc. B Biol. Sci. 290, 20221766 (2023).
Korneliussen, T. S., Albrechtsen, A., Nielsen, R. & ANGSD Analysis of next generation sequencing data. BMC Bioinforma. 15, 356 (2014).
Meisner, J. & Albrechtsen, A. Inferring population structure and admixture proportions in low-depth NGS data. Genetics 210, 719–731 (2018).
Hume, B. C. C. et al. SymPortal: a novel analytical framework and platform for coral algal symbiont next-generation sequencing ITS2 profiling. Mol. Ecol. Resour. 19, 1063–1080 (2019).
Oksanen, J. et al. Vegan: community ecology package. (2022).
Liu, G. et al. Reef-scale thermal stress monitoring of coral ecosystems: new 5-km global products from NOAA coral reef watch. Remote Sens. 6, 11579–11606 (2014).
Steven, A. D. L. et al. eReefs: an operational information system for managing the Great Barrier Reef. J. Op. Oceanogr. 12, S12–S28 (2019).
Savtchenko, A. et al. Terra and Aqua MODIS products available from NASA GES DAAC. Adv. Space Res. 34, 710–714 (2004).
Elith, J., Leathwick, J. R. & Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 77, 802–813 (2008).
Hijmans, R. J., Phillips, S., Leathwick, J., Elith, J. & Hijmans, M. R. J. Package ‘dismo’ (2013).
Acknowledgements
This work was supported by the Reef Restoration and Adaptation Program (RRAP), funded by the partnership between the Australian Government’s Reef Trust and the Great Barrier Reef Foundation. We thank Australian Institute of Marine Science (AIMS) technical staff and volunteers for field assistance, especially to R. Forester, C. Thompson, G. Cameron, S. Goyen, and M. Wooster. We also thank T. Bridge and A. Baird for assistance with species identification, C. Klass for image labeling, E. Hochberg and J. Kok for assistance with hyperspectral NDVI data extraction, M. Logan for statistical input, I. Byrne, C. Coppin, S. Howitt, Z. Meziere, and K. Prata for lab assistance, and C. Riginos for input on sample collection and sequencing. We acknowledge that Figs. 1 and 3 were created with BioRender.com. We thank the Traditional Owners of the Great Barrier Reef for their free prior and informed consent to enter and undertake coral sampling in their Sea Country. We pay our respect to their Elders, past, present, and future.
Author information
Authors and Affiliations
Contributions
E.J.H. and L.K.B. designed and funded the study. E.J.H., L.K.B., V.M.M., M.S.N., and H.D. conducted the field sampling and heat stress experiments. H.D. and M.S.N. extracted the environmental variables and V.M.M. extracted coordinate data. M.S.N. extracted phenotypic data. P.L. and M.S.N. conducted the ITS2 analysis. I.P. conducted the bioinformatic analysis and genomic cluster assignments. M.S.N. led the analysis of heat tolerance drivers. M.S.N. wrote the manuscript with input from E.J.H. All authors contributed to and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Jiangyuan Liang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Clare Davis and Alice Drinkwater. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Naugle, M.S., Denis, H., Mocellin, V.J.L. et al. Heat tolerance varies considerably within a reef-building coral species on the Great Barrier Reef. Commun Earth Environ 5, 525 (2024). https://doi.org/10.1038/s43247-024-01649-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-024-01649-4
This article is cited by
-
Species-specific retention vs. recovery of coral thermal tolerance following nursery propagation
Communications Biology (2025)
-
A rapidly closing window for coral persistence under global warming
Nature Communications (2025)
-
Marine heatwaves select for thermal tolerance in a reef-building coral
Nature Climate Change (2025)
-
Coral reefs span borders, so must solutions: transboundary conservation in complex political environments
npj Ocean Sustainability (2025)