Abstract
Nutrient limitation regulates phytoplankton growth throughout much of the global ocean and its assessment is important for our understanding of future changes in ocean productivity. The South Pacific Ocean represents a vast region where limiting nutrients have so far been investigated with only a handful of experiments. Here we report the results of eleven nutrient addition bioassay experiments conducted across the South Pacific Ocean at ca. 30°S as part of the GEOTRACES GP21 expedition. Nitrogen addition alone stimulated chlorophyll-a accumulation at all sites, whilst increases were larger following supplementary addition of iron in the eastern and central basin, and phosphate in the western basin. Enhanced iron stress following nitrogen addition in the eastern and central basin further pointed towards ambient iron concentrations approaching co-limiting levels. The overall east-to-west shift in serial limiting nutrients from iron to phosphate was attributed to a broadscale east-west increase in iron availability relative to nitrogen.
Similar content being viewed by others
Introduction
The growth of marine phytoplankton is strongly regulated by the supply of macronutrients (nitrogen (N), phosphorus (P), silica (Si)) and micronutrients (including iron (Fe), manganese (Mn), cobalt (Co), and zinc (Zn))1,2. Furthermore, phytoplankton community structure varies in response to the absolute supply rate of limiting nutrients, the ratios of supply rates between different nutrients, and the chemical forms of nutrients3. In turn, both overall phytoplankton productivity and community structure impact marine food webs and the marine carbon cycle4. Great importance is therefore attached to determining current patterns of nutrient limitation to phytoplankton2. Ultimately, an improved mechanistic understanding will facilitate more reliable predictions of future marine ecosystem responses impacted by climate change5.
Surface waters of the low latitude South Pacific Ocean encompass a range of biogeochemical settings, including the highly productive Chilean upwelling system in the east, the oligotrophic South Pacific subtropical gyre, and regions with enhanced diazotroph (N2-fixing bacteria) growth and nitrogen (N2) fixation in the western basin6,7,8. An earlier study in the Southeast Pacific used the results from three nutrient addition bioassay experiments to show that primary productivity was limited by Fe and N availability at the boundary between upwelling waters off the coast of Chile and the oligotrophic subtropical gyre, whilst N limitation alone was found in the central subtropical gyre9. This matched observations of transitions in limitation by Fe and N in a similar upwelling-to-subtropical gyre system in the Southeast Atlantic10. On the western side of the South Pacific basin, observations of N limitation and one experiment with more complex serial limitations by Fe and P have been reported11, with serial limitations defining a condition where the supply of one nutrient, the primary limiting nutrient, enhances phytoplankton growth, but this is further stimulated by the addition of further nutrient(s). Finally, broad regions of phytoplankton Fe stress throughout the South Pacific have more recently been reported in the form of retained Fe stress genes in Prochlorococcus populations, including the N-depleted waters of the subtropical gyre12.
A coherent, basin-scale assessment of experimentally-determined nutrient limitation is still lacking for the South Pacific Ocean, alongside an assessment of the potential spatial patterns and importance of nutrient co-limitation1. Here we report the results of eleven nutrient addition bioassay experiments conducted using trace-metal-clean protocols along a >10,000 km cruise track of GEOTRACES GP21 at ca. 30°S across this ocean basin (Fig.1a, purple line).
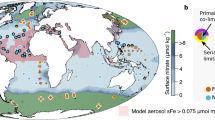
a Experimental locations with color scale showing satellite-derived chlorophyll-a for during the cruise, March 2022. The purple line indicates the cruise GEOTRACES GP21 track and the gray arrows represent the simplified main surface ocean currents. ACC Antarctic Circumpolar Current, EAC East Australian Current, SEC South Equatorial Current. Tran and UP indicate samples at the transition and coastal upwelling regions, respectively. Symbols summarize the nutrient limitation of chlorophyll-a accumulation found at each site as shown in (d). South Pacific upper ocean (b) salinity and (c) chlorophyll-a. The black lines in panel ‘b’ indicate 0.1, 5, and 25 μM nitrate contours, and the white line indicates the 0.1 μM phosphate contour. The black and white lines in (c) are the mixed and euphotic layers, respectively. d chlorophyll-a response to nutrient supply. Bar heights show the mean of triplicate biological replicates, with individual values shown as points. Arrows indicate mean initial values. Bars labeled with the same letter indicate statistically indistinguishable means between treatments with the applied statistical test (one-way ANOVA, α = 0.05, followed by Tukey posthoc test). Central circle color = primary limiting nutrient; outer circle/star color = serial limiting nutrient; star = Zn, green = N, red = Fe, black = P.
Result and discussion
Cross-Pacific nutrient and phytoplankton trends
Field observations were conducted along a transect that spanned the South Pacific Ocean from the end of February to the beginning of April, 2022, which generally corresponds to the time period with strongest stratification/shallowest mixed layer depths13 (Fig. 1a; Table 1). Nutrient addition bioassay experiments were conducted in three oceanographic regimes: (i) Experiment 1: site nearer to the Chilean coast and associated eastern boundary upwelling zone, characterized by cooler (19.5 °C) waters with relatively elevated macronutrient concentrations (nitrate: 0.27 μM, phosphate: 0.47 μM) but depleted DFe (0.17 nM), relatively elevated chlorophyll-a (0.33 mg m−3) and a dominance of nanophytoplankton (56% estimated contribution to chlorophyll-a) (Fig. 2a, b). (ii) Experiment 2: located in a transition zone between the upwelling zone and oligotrophic subtropical gyre, hosting depleted nitrate concentrations (0.044 μM), relatively elevated phosphate (0.275 μM), elevated DFe (0.35 nM), relatively elevated chlorophyll-a (0.25 mg m−3) and dominated by picophytoplankton (62% contribution to chlorophyll-a). (iii) Experiments 3-11: oligotrophic subtropical gyre sites (surface chlorophyll-a < 0.07 mg m−3) with surface nitrate concentrations <0.1 μM at all sites apart from experiment 3 (nitrate=0.172 μM). The vertical distribution of chlorophyll-a at the subtropical gyre sites showed pronounced and deeper subsurface maxima (70–170 m) that generally followed the beginning of nitracline depths (0.1 μM) (27–180 m) (Fig. 1b, c). The euphotic depth (here defined as the 1% of surface PAR depth) was generally located just below the deep chlorophyll maximum (Fig. 1c). The nitracline depth in the eastern basin generally followed the salinity gradient, suggesting a control of the large-scale macronutrient distribution by water mass movements at these depths in this part of the basin (Fig. 1b). Within the oligotrophic subtropical gyre, sea surface temperature (SST), phosphate concentrations, and surface phytoplankton community structure showed spatial gradients from east to west, with higher SST, lower surface phosphate concentrations, a deeper phosphacline (0.1 μM), and an elevated contribution of picophytoplankton to total chlorophyll-a in the western South Pacific (Figs. 1b and 2a, b). This increased picophytoplankton contribution consisted largely of Prochlorococcus, supported by both the cell abundances determined by flow cytometry analysis (Fig. 2c) and the diagnostic pigment analyses (Supplementary Fig. S1).
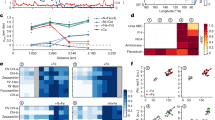
a Physical and chemical parameters including sea surface temperature (SST), dust deposition, dissolved Fe (DFe), dissolved manganese (DMn), dissolved aluminum (DAl), turbulent DFe supply, nitrate and phosphate concentrations. The light lines are the original data, while the bold lines are smoothed with a 5-point moving average. Sampling locations are highlighted with red inverted triangles and filled triangles indicate concentrations measured at experiment start points. b Phytoplankton chlorophyll-a concentrations and size classes variations. The white line is the oligotrophic gyre threshold (here defined as chlorophyll-a ≤ 0.07 mg m−3). c Total picophytoplankton fluorescence (i.e., summed fluorescence of the three groups), and the relative contribution of Prochlorococcus, Synechococcus, and photosynthetic picoeukaryotes (PPE) fluorescence to total picophytoplankton fluorescence. d Photophysiological (Fv/Fm) response of phytoplankton to N, Fe, and N+Fe supply at each bioassay experiments site. Bar heights represent mean responses of the tropical biological replicates for each treatment. Asterisks indicate significant Fv/Fm changes over controls (ANOVA followed by Tukey’s post hoc test, α < 0.05). The black and blue lines respectively represent the magnitude of Fv/Fm drawdown and phytoplankton net growth rates following N supply.
Phytoplankton responses to nutrient amendment
Primary N limitation was found at all experimental sites, with chlorophyll-a significantly increasing after ca. 48 h incubation following a supply of N alone relative to untreated controls or single additions of other non-N nutrients (Fe, Co and/or P; Fig. 1d). Further increases in chlorophyll-a, beyond that to supply of N alone, were observed following combined supply of N + Fe at experiments 1 and 3–8. We define this type of response as serially N-Fe limited. In experiments 9–11, located geographically at the western edge of the subtropical gyre, the serial limiting nutrient switched from Fe to P. Moreover, at the switching point between serial responses to Fe and serial responses to P, experiment 9 showed an additional serial (that is, primary-secondary-tertiary) response to N-P-Fe supply.
In addition to the observed serial chlorophyll-a responses to N + Fe, restricted Fe availability across large portions of the transect were indicated by values of the apparent photosystem II photochemical efficiency parameter, Fv/Fm14 (Fig. 2d). Initial values of Fv/Fm at the bioassay experiment sites showed a significant negative correlation with the residual nitrate concentrations (log10(nitrate) vs. Fv/Fm; R2 = 0.64, p < 0.01, n = 11; Supplementary Fig. S2a), and exhibited higher values (0.45–0.51) when moving west into the subtropical gyre (experiments 3–11). In experiment 1, where DFe concentrations were depleted (0.17 nM) despite relatively elevated chlorophyll-a and nitrate (0.33 mg m−3 and 0.27 μM, respectively), Fv/Fm values significantly increased following Fe-only supply without any concomitant increase in chlorophyll-a, potentially suggesting that Fe stress was present at this site but not limiting phytoplankton growth rates. Furthermore, at experiments 1–8, Fv/Fm showed declines following N supply, whereas any Fv/Fm declines with N+Fe addition were small and not significant. These Fv/Fm reductions were consistent with prior observations, likely a result of tipping N(-Fe serial) limited communities towards stronger Fe stress following the artificial supply of N10,15,16.
Variability between experiments was also observed in terms of the magnitudes of chlorophyll-a responses to N-only supply (Fig. 2d). This variability aligned with the magnitude of Fv/Fm reductions. Specifically, where Fv/Fm reductions following N supply were greatest in the eastern portion of the subtropical gyre (experiments 3–8), temperature-adjusted chlorophyll-a net growth rates were generally low following N supply (0.05–0.08 d−1), suggesting phytoplankton growth rates stimulated by N-only supply were rapidly constrained by insufficient Fe. This was further supported by the significant positive correlation between surface DFe concentration and an index of single N limitation (log10(DFe) vs. Nsingle; Eq. 4; R2 = 0.44, p < 0.05, n = 11; Supplementary Fig. S2b), suggesting phytoplankton growth rates following N supply were larger at sites where initial DFe concentrations were higher. The magnitude of serial Fe limitation responses was less clearly related to DFe concentrations (log10(DFe) vs. NFeserial; Eq. 5; p = 0.13; Fig. S2c). Instead, increases in the dissolved Fe concentration in excess of assumed-average phytoplankton N requirements (FeN*) was diagnostic of N-Fe serial limitation17 (FeN* vs. NFeserial, R2 = 0.50, p < 0.01; Supplementary Fig. S2d and Table 1). Collectively, this analysis suggested that seawater dissolved N and Fe availabilities were important drivers and predictors of the variations in phytoplankton responses to N or N + Fe supply that were observed across this system.
In addition to N and Fe, a modest 1.1-fold additional chlorophyll-a enhancement was observed upon supply of N + Fe + Zn beyond N + Fe in experiment 6 (Fig. 1d). This indicated that serial Zn limitation restricted phytoplankton growth following N and Fe supply at this site, where measured DZn concentrations were amongst the lowest observed at any of the experimental sites (0.09 nM; Table 1). Zinc has a range of functions in phytoplankton including in alkaline phosphatase, an enzyme used to acquire P from DOP under conditions of low phosphate availability18, and in carbonic anhydrase, an enzyme required for carbon concentrating mechanisms in the dark reactions of photosynthesis19. As phosphate concentrations were relatively elevated at this experimental site (0.069 μM), this implied a more likely role for either carbonic anhydrase or an alternative function. However, no response was observed in the parallel N + Fe + Co treatment, which might be expected if the Zn was being directed towards carbonic anhydrase as Co can be potentially substituted by Zn in this function20, albeit with a potential reduction in growth rate21. The lack of response to Co in addition to N + Fe in any of the experiments was also in contrast to experiments offshore of the Benguela upwelling in the Southeast Atlantic10, suggesting that despite the very low dissolved concentrations (Table 1), Co was not sufficiently deficient in ambient seawaters, nor the incubated seawater following the relatively restricted biomass accumulation in N + Fe treatments, to restrict phytoplankton accumulation over experimental timescales22,23.
The switch from Fe to P as the serial limiting nutrient was observed in far western side of the transect starting from experiment 9, and was consistent with the strong decline in both surface phosphate concentrations and derived PN*, the relative excess of P in comparison to N taking into account typical N: P phytoplankton requirements (16:1; both P and PN* <10 nM; Fig. 2a; Table 1). The western (sub)tropical South Pacific is a hot spot of N2 fixation as a result of residual unutilized P and elevated Fe inputs6, the latter of which increases the overall Fe:N supply ratio to the system and strengthens the niche for diazotrophs16,24,25. Any supply of N through diazotrophic N2 fixation without a concomitant supply of P would result in P drawdown26,27, and lead to the serial limitation of phytoplankton by P following N16,27,28. Relatively enhanced concentrations of DFe (0.24 and 0.32 nM at experiment sites 10 and 11, respectively) in this region, alongside enhanced concentrations of both DAl and DMn, were observed (Fig. 2a and Supplementary Fig. S3), consistent with this explanation. Specific supply sources for these relatively enhanced DFe concentrations are more difficult to elucidate. Derived vertical turbulent diffusive DFe supply into the euphotic zone showed unclear cross-basin trends and were not elevated in the far western basin (Fig. 2a). In general, satellite-derived estimates of dust deposition (4-day averages prior to site occupation) matched the surface DFe trend, showing no clear trend between the central subtropical gyre and far western basin (Fig. 2a). In addition, inputs of Fe, either from shallow hydrothermal systems7,29,30 or the major Hunga Tonga-Hunga Haʻapai volcanic eruption that occurred ca. 1.5 months prior to our occupation31, and/or from terrigenous/sediment runoff associated with nearby islands and their subsequent horizontal advection8,32,33 (Supplementary Fig. S4) were potential DFe sources contributing to the regional P drawdown as a result of enhanced N2 fixation.
Phytoplankton community structure responses to nutrient amendment
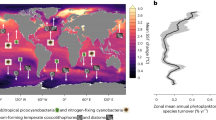
Shifts in phytoplankton community structure were observed following supply of limiting nutrients (Fig. 3). For several experiments where flow cytometry samples were analyzed, chlorophyll-a for individual picophytoplankton groups (Prochlorococcus, Synechococcus, picoeukaryotic phytoplankton) was determined from their total red fluorescence (calculated by multiplying cell concentrations with intracellular fluorescence; Fig. 3a). The photosynthetic picoeukaryote group showed relatively restricted changes apart from experiment 3, where large increases were observed following N + Fe amendment. Synechococcus generally showed increases following N supply that were further enhanced by N + Fe (experiments 1, 6 and 7) or N + P (experiment 10). Prochlorococcus intracellular fluorescence generally showed more restricted changes (apart from in experiment 1), leading to reductions in their estimated contributions to total picophytoplankton chlorophyll-a following N addition (in combination with any other nutrients). For all picophytoplankton groups, changes in cellular fluorescence (reflecting intracellular chlorophyll-a) were overall much stronger than changes in cellular abundances, suggesting more rapid physiological responses (i.e., pigment synthesis) in comparison to bulk carbon biomass increases over the experimental timescales and likely strongly contributing to the overall chlorophyll-a concentration changes observed in the experiments34. However, in experiment 10 the N + P supply (with or without added Fe) clearly increased picophytoplankton abundances, which for Prochlorococcus and Synechococcus was larger than for supply of N or N + Fe (e.g., 1.29-fold increase in Synechococcus abundances in N + Fe + P relative to N + Fe).
a Picophytoplankton derived from flow cytometry analysis from selected experiments. Heat maps indicate fold changes in flow cytometry-derived cell properties following nutrient amendment relative to unamended controls (PPE, photosynthetic picoeukaryotes; Pro, Prochlorococcus; Syn, Synechococcus; F, cellular fluorescence). Treatments in the heatmaps that are labeled with “*” have means that are statistically different from controls with the applied statistical test (one-way ANOVA, α = 0.05, followed by Tukey posthoc test). The superimposed scatter plots are the percentage contributions of each picophytoplankton fluorescence (cell abundances × cellular fluorescence) to total picophytoplankton fluorescence. b Contribution of phytoplankton size classes to total chlorophyll-a for initial experimental conditions and treatments containing at least +N.
An analysis of diagnostic phytoplankton pigments suggested that the fractional contribution of larger microphytoplankton to total chlorophyll-a was initially low (4.7–15.1%) at all experimental sites but exhibited 4.9–11.9% increases following N supply in the oligotrophic subtropical gyre (experiments 3–11) with an additional increase (4.4 ± 1.7%) following the supply of N + Fe for experiments 3–8 where serial Fe limitation was observed (Figs. 2b and 3b).
Pico- and nanophytoplankton groups instead dominated initial contributions to total chlorophyll-a at all experiment sites, but showed an east-to-west shift from a more nanophytoplankton-dominated to a more picophytoplankton-dominated phytoplankton community (Figs. 2b and 3b), consistent with enhanced contributions of cyanobacteria at warmer temperatures under nutrient-depleted conditions35,36,37. An interesting observation was that changes in relative contributions of picophytoplankton and nanophytoplankton to total chlorophyll-a following N supply, regardless of whether additional Fe was also supplied in combination with N, were not consistent throughout the transect. Generally, increased picophytoplankton contributions to chlorophyll-a following N addition were observed in the east (experiments 1–3) and decreased contributions following N addition were observed in the west (experiments 6–11), whereas nanophytoplankton contributions showed the opposite trend (Fig. 3b and Supplementary Fig. S5). Strong, co-varying cross-basin gradients in temperature (Fig. 2a and Supplementary Fig. S6), shifts between serial Fe to P limitation (Fig. 1b), shifts in overall trophic status with likely different responses of the (probably variable) extant grazer communities (Fig. 2b), together with probable changes in phytoplankton species and/or ecotypes along the transect38 could all be hypothesized to play roles in driving this trend. Regardless of the cause, it implies external N supply (e.g., from upwelling, aerosols, N2 fixation) exerts basin-scale variability on its impact on phytoplankton community structure, with potential consequences for ongoing changes in N inputs to the ocean39.
In summary, our basin-scale experimental assessment of phytoplankton nutrient limitation showed N was the primary limiting nutrient throughout the South Pacific in austral summer; however, evidence was found for other nutrients to be approaching co-limiting levels, with serial Fe limitation found in the eastern and central basin and serial phosphate limitation in the west. We propose that Fe:N supply ratios to surface waters are key for establishing these gradients24 and would alter limitation patterns seasonally and with climate changes. Specifically, stronger coastal upwelling offshore of Chile and/or a wintertime northward migration of the south subtropical convergence zone would be expected to lead to surface waters hosting lower Fe:N supply rates than during our expedition, strengthening Fe (co-)limitation in the eastern basin9,40. Conversely, enhanced aerosol, hydrothermal, or sedimentary Fe supply in the west would enhance the Fe:N supply ratio and strengthen the niche for diazotrophs and further draw down phosphate, potentially to levels co-limiting to phytoplankton. Developing the methodology to observe the dynamic nature of these nutrient limitation transitions is an important next step12,41,42.
Methods
Sample collection for water column observations and experiments
Experiments and sample collection were conducted onboard RV Sonne (cruise SO289 representing section GP21 of the GEOTRACES program) from 18th February to 8th April 2022. Near-surface seawater was collected from a trace-metal-clean towed sampling device at approximately 1–2 m depth powered by a Teflon bellows pump (Dellmeco DM15). Water was pumped directly into a purpose-built clean air bubble in a ship laboratory, with positive air pressure maintained via inward airflow passed through a HEPA filter. All experimental sample collection and nutrient spiking were carried out in this trace-metal-clean bubble. Seawater samples for depth profiles of nutrient and trace element concentration were collected throughout the upper 250 m using a titanium frame CTD equipped with trace-metal-clean Niskin-X bottles (Ocean Test Equipment). An in vivo fluorescence sensor and photosynthetically active radiation (PAR) sensor were attached to a separate stainless steel CTD and were used to determine desired sampling depths. The euphotic zone (Zeu) was defined as the depth corresponding to 1% surface PAR. The mixed layer depth (MLD) was determined as the depth at which density changed by 0.03 kg m−3 relative to the near-surface value at 10 m depth. The CTD fluorescence values were converted to chlorophyll-a concentrations using matching observations of high-performance liquid chromatography (HPLC)-derived total chlorophyll-a (chlorophyll-a plus divinyl chlorophyll-a, hereafter simply denoted as chlorophyll-a) with the relationship chlorophyll-a = 0.25 × fluorescence + 0.03 (r2 = 0.72, p < 0.001; n = 118).
Nutrient and trace elements analyses
Macronutrient (nitrite plus nitrate; hereafter referred to as simply as nitrate, and phosphate) and trace element samples were filtered (AcroPak 500 0.8/0.2 μm filter capsule, Pall) before collection. Macronutrient samples were collected into acid-washed 15 mL polypropylene vials. Samples were analyzed on board using a nutrient autoanalyzer system (QuAAtro, SEAL Analytical). Detection limits for the nutrient analyses were: nitrate = 0.005 μM; and phosphate = 0.001 μM. Dissolved trace element samples were collected into acid-washed 125 mL low-density polyethylene bottles (Nalgene) and acidified to pH ~1.9 under a laminar flow hood using concentrated hydrochloric acid (UpA, Romil). After >6 months, samples were preconcentrated on a SeaFAST device and analyzed for dissolved Fe (DFe), Zn (DZn), Co (DCo) and Mn (DMn) concentrations using a high resolution ICP-MS (Element XR, Thermofisher) with quantification by standard addition for DFe, DZn, DCo and DMn43. Certified reference materials (SAFe D2; CASS-6 and NASS-7, National Research Council Canada) were analyzed alongside the samples and produced good agreement with their consensus values (weighted mean absolute percentage error (WMAPE): <5.5% for DFe, <11% for DMn, <17% for DCo and <13% for DZn). Concentrations of dissolved aluminum (DAl) were measured using a modified Al-lumogallion complex method44. The detection limits were 0.05 nM for DFe, 0.001 nM for DCo, 0.06 nM for DZn, 0.005 nM for DMn, and 1.05 nM for DAl.
Turbulent DFe supply rates
The subsurface supply rate of DFe into the euphotic zone was estimated using vertical concentration profiles and a turbulent diffusivity constant. DFe profiles were first interpolated at 1 m vertical resolution, and then the gradient between the Fe concentration at 10 m above and 10 m below the euphotic zone was calculated. Dissolved Fe gradients were multiplied by a typical open ocean turbulent diffusivity constant (1 × 10−5 m2 s−1)45. These values are comparable to other observations in the subtropical Pacific46,47,48. In the case of a negative gradient, we assumed an upward turbulent diffusion of zero.
Phytoplankton biomass and community structure
Seawater samples (200 mL) were filtered onto Fisher MF300 glass fiber filters (nominal pore size of 0.7 μm) and extracted for 12–24 h in 10 mL 90% acetone in a −20 °C freezer in the dark before measurement on a calibrated fluorometer (Turner Designs Trilogy)49. Fluorometrically determined concentrations of chlorophyll-a (Chla) were corrected systematically to HPLC chlorophyll-a concentrations using a linear correlation between duplicate samples taken on the SO289 cruise that were measured by both methods (log10(chlorophyll-a) = 0.92 × log10(Chla)−0.45, R2 = 0.84, p < 0.001; n = 265).
Flow cytometry samples (2 mL) were fixed with neutralized paraformaldehyde at a final concentration of 1%, vortex-mixed, and left in the dark for 10 min before transfer to a −80 °C freezer. Upon return to the land-based laboratory, samples were thawed at room temperature and analyzed using a FACSCalibur flow cytometer (Becton Dickenson, Oxford, UK). Phytoplankton groups were identified and enumerated with CellQuest software.
Samples for diagnostic pigment analyses (initial t = 0 samples 2–4 L; pooled seawater from replicates for some treatments ~2.5 L) were filtered onto Fisher MF300 glass fiber filters, frozen at −80 °C, then later analyzed using a HPLC system50 (Dionex UltiMate 3000 LC, Pigment concentrations were subsequently converted to phytoplankton size classes followed published protocols51.
Phytoplankton photo-physiology
Phytoplankton photo-physiological measurements were performed using a Fast Repetition Rate fluorometer (FASTOcean in bench top mode with an integrated FASTact laboratory system, Chelsea Technologies Ltd). All analyses were conducted at local night-time to remove the influence of non-photochemical quenching. Single acquisitions were performed using 100 × 1 μs blue excitation flashes, and data were fit within the manufacturer’s software (FASTPro8)52. Blanks (0.2 μm syringe-filtered samples) were typically run for one replicate per treatment, and blank fluorescence was subtracted from Fo and Fm before calculation of Fv/Fm = (Fm - Fo)/Fm.
Nutrient addition bioassay experiments
Bioassay experiments directly followed published protocols10,16. Acid-washed polycarbonate 1 L bottles (Nalgene) were filled with seawater using a trace-metal-clean towed sampling system. Initial bottles were used to characterize initial conditions, control bottles were incubated alongside treated bottles, and treatment bottles were spiked with one of the following combinations of nutrients/trace metals: N, Fe, Co, N + Fe, N + Co, Fe + Co, N + Fe + Co, N + Fe + Zn (Experiments 1–8); N, Fe, P, N + Fe, N + P, Fe + P, N + Fe + P (Experiments 9–11). The switch from Co to P as the third nutrient added in factorial combination (alongside N and Fe) was made from experiment 9 as a result of the start of declining P concentrations in surface waters. The bottle filling process distributed total seawater collected throughout the filling period across all bottles, with initial (that is, t = 0) bottles being filled at the beginning, middle, and end of the filling time period. All controls and treatments were conducted in triplicate. The N spike was a combined treatment of nitrate + ammonium (final amended concentration of 1 μM NaNO3 + 1 μM NH4Cl). Solutions of Fe (FeCl3), Co (CoCl2), and Zn (ZnCl2) were prepared using high purity solid standards and 0.01 M hydrochloric acid (Fisher Optima grade diluted in deionized water (Milli-Q, Millipore) and were each added to a final added concentration of 2 nM. Phosphorus (NaH2PO4) was added to a final amended concentration of 0.2 μM. Macronutrient spikes were previously passed through a prepared Chelex 100 column to remove trace metal contamination. Incubation bottles were placed in on-deck incubators connected to the ship’s underway flow-through system to continuously maintain temperatures to those of sea surface waters. Incubators were screened with Blue Lagoon screening (Lee Filters), which maintained irradiance at ~35% of that of the ocean surface. After about 48 h incubation, experiments were taken down and subsampled for chlorophyll-a concentrations, photo-physiology (Fast Repetition Rate fluorometry), and flow cytometry. The remaining water from each triplicate treatment was pooled for collection of samples for HPLC derived pigments analysis. Incubation bottles were rinsed with dilute hydrochloric acid and seawater between experiments.
The calculation of net chlorophyll-a based growth rates (\({\mu }_{{chl}}^{{Net}}\)) were conducted using the equation:
Where \({{Chl}}_{T}\) is the chlorophyll-a concentration in the nutrient amended seawater or controls following the incubation of length t (2 days), and \({{Chl}}_{i}\) is the chlorophyll-a concentration at the experimental start point (t = 0 days).
The relationship between maximum potential growth rates (\({\mu }_{{chl}}^{\max }\)) and temperature was estimated using the equation53:
Where T is the seawater temperature of each bioassay experiment.
Subsequently, \({\mu }_{{chl}}^{{Net}}\) was normalized to the respective value of \({\mu }_{{chl}}^{\max }\) to calculate a temperature-independent relative chlorophyll-a based net growth rate (\({\mu }^{{Max}}\))1:
A phytoplankton N and N-Fe (serial) limitation index was calculated as follows:
Where \({\mu }_{{Control}}^{{Max}}\), \({\mu }_{N}^{{Max}}\) and \({\mu }_{N+{Fe}}^{{Max}}\) are the chlorophyll-a based net growth rates normalized by the maximum potential growth rates for controls, N and N + Fe treatments, respectively. Higher values of Nsingle, and NFeserial indicated stronger N limitation, and serial N-Fe limitation, respectively.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Experimental data are deposited on Zenodo (https://doi.org/10.5281/zenodo.13364985)54. Satellite chlorophyll-a (4 km resolution) was extracted from the MODIS-Aqua Level-3 product from the NASA ocean color website (https://oceancolor.gsfc.nasa.gov). The Modern-Era Retrospective analysis for Research and Applications Version 2 (MERRA-2) satellite re-analysis model for dust deposition (https://doi.org/10.5067/KLICLTZ8EM9D) was extracted along the cruise track. Dust deposition (0.5° × 0.625° grid) for hourly wet and dry deposition for all aerosol size bins was used in this study. The average daily dust deposition flux over a 4-day period before each sample station was determined following published protocols55.
References
Browning, T. J. & Moore, C. M. Global analysis of ocean phytoplankton nutrient limitation reveals high prevalence of co-limitation. Nat. Commun. 14, 5014 (2023).
Moore, C. M. et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 6, 701–710 (2013).
Interlandi, S. J. & Kilham, S. S. Limiting resources and the regulation of diversity in phytoplankton communities. Ecology 82, 1270–1282 (2001).
Falkowski, P. G., Barber, R. T. & Smetacek, V. V. Biogeochemical controls and feedbacks on ocean primary production. Science 281, 200–207 (1998).
Bindoff, N. L. et al. Changing ocean, marine ecosystems, and dependent communities. IPCC special report on the ocean and cryosphere in a changing climate, 477–587 (2019).
Bonnet, S., Caffin, M., Berthelot, H. & Moutin, T. Hot spot of N2 fixation in the western tropical South Pacific pleads for a spatial decoupling between N2 fixation and denitrification. Proc. Natl. Acad. Sci. USA 114, E2800–E2801 (2017).
Bonnet, S. et al. Natural iron fertilization by shallow hydrothermal sources fuels diazotroph blooms in the ocean. Science 380, 812–817 (2023).
Shiozaki, T., Kodama, T. & Furuya, K. Large‐scale impact of the island mass effect through nitrogen fixation in the western South Pacific Ocean. Geophys. Res. Lett. 41, 2907–2913 (2014).
Bonnet, S. et al. Nutrient limitation of primary productivity in the Southeast Pacific (BIOSOPE cruise). Biogeosciences 5, 215–225 (2008).
Browning, T. J. et al. Nutrient co-limitation at the boundary of an oceanic gyre. Nature 551, 242–246 (2017).
Moisander, P. H. et al. Analogous nutrient limitations in unicellular diazotrophs and Prochlorococcus in the South Pacific Ocean. ISME J. 6, 733–744 (2012).
Ustick, L. J. et al. Metagenomic analysis reveals global-scale patterns of ocean nutrient limitation. Science 372, 287–291 (2021).
Sallée, J. B. et al. Summertime increases in upper-ocean stratification and mixed-layer depth. Nature 591, 592–598 (2021).
Behrenfeld, M. J. & Milligan, A. J. Photophysiological expressions of iron stress in phytoplankton. Annu. Rev. Mar. Sci. 5, 217–246 (2013).
Behrenfeld, M. J. et al. Controls on tropical Pacific Ocean productivity revealed through nutrient stress diagnostics. Nature 442, 1025–1028 (2006).
Browning, T. J. et al. Nutrient co‐limitation in the subtropical Northwest Pacific. Limnol. Oceanogr. Lett. 7, 52–61 (2022).
Browning, T. J. et al. Nutrient regulation of late spring phytoplankton blooms in the midlatitude North Atlantic. Limnol. Oceanogr. 65, 1136–1148 (2020).
Mahaffey, C., Reynolds, S., Davis, C. E. & Lohan, M. C. Alkaline phosphatase activity in the subtropical ocean: insights from nutrient, dust and trace metal addition experiments. Front. Mar. Sci. 1, 73 (2014).
Twining, B. S. & Baines, S. B. The trace metal composition of marine phytoplankton. Annu. Rev. Mar. Sci. 5, 191–215 (2013).
Price, N. M. & Morel, F. M. M. Cadmium and cobalt substitution for zinc in a marine diatom. Nature 344, 658–660 (1990).
Kellogg, R. M. et al. Efficient zinc/cobalt inter‐replacement in northeast Pacific diatoms and relationship to high surface dissolved Co: Zn ratios. Limnol. Oceanogr. 65, 2557–2582 (2020).
Hawco, N. J. et al. Minimal cobalt metabolism in the marine cyanobacterium Prochlorococcus. Proc. Natl. Acad. Sci. USA 117, 15740–15747 (2020).
Browning, T. J. et al. Influence of iron, cobalt, and vitamin B12 supply on phytoplankton growth in the tropical East Pacific during the 2015 El Niño. Geophys. Res. Lett. 45, 6150–6159 (2018).
Ward, B. A., Dutkiewicz, S., Moore, C. M. & Follows, M. J. Iron, phosphorus, and nitrogen supply ratios define the biogeography of nitrogen fixation. Limnol. Oceanogr. 58, 2059–2075 (2013).
Wen, Z. et al. Nutrient regulation of biological nitrogen fixation across the tropical western North Pacific. Sci. Adv. 8, eabl7564 (2022).
Wu, J., Sunda, W., Boyle, E. A. & Karl, D. M. Phosphate depletion in the Western North Atlantic Ocean. Science 289, 759–762 (2000).
Moore, C. M. et al. Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nat. Geosci. 2, 867–871 (2009).
Ellwood, M. J. et al. Insights into the biogeochemical cycling of iron, nitrate, and phosphate across a 5,300 km South Pacific Zonal Section (153°E–150°W). Glob. Biogeochem. Cy. 32, 187–207 (2018).
Guieu, C. et al. Iron from a submarine source impacts the productive layer of the Western Tropical South Pacific (WTSP). Sci. Rep. 8, 9075 (2018).
Tilliette, C. et al. Dissolved iron patterns impacted by shallow hydrothermal sources along a transect through the Tonga‐Kermadec Arc. Glob. Biogeochem. Cy. 36, e2022GB007363 (2022).
Yoon, J.-E., King, D., Longman, J. & Cronin, S. J. Differential response of chlorophyll-a concentrations to explosive volcanism in the western South Pacific. Front. Mar. Sci. 10, 1072610 (2023).
Dutheil, C. et al. Modelling N2 fixation related to Trichodesmium sp.: driving processes and impacts on primary production in the tropical Pacific Ocean. Biogeosciences 15, 4333–4352 (2018).
Messié, M. et al. The delayed island mass effect: how islands can remotely trigger blooms in the oligotrophic ocean. Geophys. Res. Lett. 47, e2019GL085282 (2020).
Moore, C. M. et al. Relative influence of nitrogen and phosphorous availability on phytoplankton physiology and productivity in the oligotrophic sub‐tropical North Atlantic Ocean. Limnol. Oceanogr. 53, 291–305 (2008).
Chen, B. Patterns of thermal limits of phytoplankton. J. Plankton Res. 37, 285–292 (2015).
Flombaum, P. et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 110, 9824–9829 (2013).
López‐Urrutia, Á. & Morán, X. A. G. Temperature affects the size‐structure of phytoplankton communities in the ocean. Limnol. Oceanogr. 60, 733–738 (2015).
Raes, E. J. et al. Oceanographic boundaries constrain microbial diversity gradients in the South Pacific Ocean. Proc. Natl. Acad. Sci. USA 115, E8266–E8275 (2018).
Jickells, T. D. et al. A reevaluation of the magnitude and impacts of anthropogenic atmospheric nitrogen inputs on the ocean. Glob. Biogeochem. Cy. 31, 289–305 (2017).
Browning, T. J. et al. Nutrient regimes control phytoplankton ecophysiology in the South Atlantic. Biogeosciences 11, 463–479 (2014).
Browning, T. J. et al. Persistent equatorial Pacific iron limitation under ENSO forcing. Nature 621, 330–335 (2023).
Breier, J. A. et al. Revealing ocean-scale biochemical structure with a deep-diving vertical profiling autonomous vehicle. Sci. Robot. 5, eabc7104 (2020).
Rapp, I., Schlosser, C., Rusiecka, D., Gledhill, M. & Achterberg, E. P. Automated preconcentration of Fe, Zn, Cu, Ni, Cd, Pb, Co, and Mn in seawater with analysis using high-resolution sector field inductively-coupled plasma mass spectrometry. Anal. Chim. Acta 976, 1–13 (2017).
Ren, J. L., Zhang, J., Luo, J. Q., Pei, X. K. & Jiang, Z. X. Improved fluorimetric determination of dissolved aluminium by micelle-enhanced lumogallion complex in natural waters. Analyst 126, 698–702 (2001).
Ledwell, J. R., Watson, A. J. & Law, C. S. Evidence for slow mixing across the pycnocline from an open-ocean tracer-release experiment. Nature 364, 701–703 (1993).
Christian, J. R., Lewis, M. R. & Karl, D. M. Vertical fluxes of carbon, nitrogen, and phosphorus in the North Pacific Subtropical Gyre near Hawaii. J. Geophys. Res. Oceans 102, 15667–15677 (1997).
Du, C., Liu, Z., Kao, S.-J. & Dai, M. Diapycnal fluxes of nutrients in an oligotrophic oceanic regime: the South China Sea. Geophys. Res. Lett. 44, 11,510–511,518 (2017).
Bouruet-Aubertot, P. et al. Longitudinal contrast in turbulence along a ∼ 19°S section in the Pacific and its consequences for biogeochemical fluxes. Biogeosciences 15, 7485–7504 (2018).
Welschmeyer, N. A. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol. Oceanogr. 39, 1985–1992 (1994).
Van Heukelem, L. & Thomas, C. S. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A 910, 31–49 (2001).
Uitz, J., Claustre, H., Morel, A. & Hooker, S. B. Vertical distribution of phytoplankton communities in open ocean: An assessment based on surface chlorophyll. J. Geophys. Res. Oceans 111 https://doi.org/10.1029/2005JC003207 (2006).
Kolber, Z. S., Prasil, O. & Falkowski, P. G. Measurements of variable chlorophyll fluorescence using fast repetition rate techniques: defining methodology and experimental protocols. Bba-Bioenergetics 1367, 88–106 (1998).
Eppley, R. W. Temperature and phytoplankton growth in the sea. Fish. Bull. 70, 1063–1085 (1972).
Liu, H. & Browning, T. J. Experimental dataset for Liu et al. “Patterns of (micro)nutrient limitation across the South Pacific Ocean”. Zenodo https://doi.org/10.5281/zenodo.13364985 (2024).
Westberry, T. K. et al. Atmospheric nourishment of global ocean ecosystems. Science 380, 515–519 (2023).
Acknowledgements
We thank the captain, crew, and scientific team of the SO289 research expedition. A. Mutzberg, D. Jasinski, F. Evers, T. Schott, K. Nachtigall, and T. Klüver are thanked for technical assistance. Z. Steiner is thanked for support on the expedition. The research cruise was funded by the Bundesministerium für Bildung und Forschung (BMBF) grant ‘S Pacific GEOTRACES’. This work was also supported by National Natural Science Foundation of China (No. 92058204 92258302, and 42406110), and the Innovational Fund for Scientific and Technological Personnel of Hainan Province (No. KJRC2023B04). T.J.B. acknowledges funding by the European Union (ERC, Ocean Glow, 101041453). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council. Neither the European Union nor the granting authority can be held responsible for them. H.L. was funded by the Chinese Scholarship Council (No. 202106310098), the China Postdoctoral Science Foundation (No. 2024M750699), and the Postdoctoral Fellowship Program of CPSF (No. GZC20240358).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
H.L., T.J.B., and E.P.A. co-designed the study. H.L., T.J.B., E.P.A., Z.Y., K.J.G., T.L., J.K.T., Z.W., A.E., X.L., B.H., and S.K. contributed to data acquisition and analysis. H.L. and T.J.B. analyzed and interpreted the data and drafted the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Li Qian, Thierry Moutin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Vasco Vieira and Joseph Aslin. A peer review file is available
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, H., Yuan, Z., Gosnell, K.J. et al. Patterns of (micro)nutrient limitation across the South Pacific Ocean. Commun Earth Environ 5, 596 (2024). https://doi.org/10.1038/s43247-024-01749-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-024-01749-1