Abstract
Northern Foothills of Victoria Land, Antarctica contains numerous hydrological formations, ranging from small surface streams and ponds fed by glacial or snow meltwater to permafrost lakes containing briny pockets. Here we describe the discovery of a massive body of unfrozen stratified oligotrophic water in Lake Enigma, a permanently ice-covered lake previously thought to be frozen from top to bottom. A remarkable feature of the Lake Enigma microbial ecosystem is the presence, and sometimes even dominance, of ultrasmall bacteria belonging to the superphylum Patescibacteria, a group apparently absent from Antarctic lakes in the well-studied McMurdo Dry Valleys. Cyanobacteria are virtually absent from Lake Enigma ice and water column although they are well represented in its extensive and diverse benthic microbial mats. Collectively, these features reveal a new complexity in Antarctic lake food webs and demonstrate that in addition to phototrophic and simple chemotrophic metabolisms, both symbiotic and predatory lifestyles may exist.
Similar content being viewed by others
Introduction
Limnologically studies of perennially ice-covered freshwater Antarctic lakes including physical, hydrological, geochemical, and microbiological investigations have been conducted primarily in two ice-free desertic regions: the McMurdo Dry Valleys (MDV), located in the southern part of Victoria Land (77.7°S; 162.6°E), and the Untersee Oasis (UO), located in Dronning Maud Land (71.3°S; 13.5°E). The MDV contains over 20 perennially ice-covered lakes and many more meltwater streams and ponds that have been intensively studied since 19571,2,3,4,5,6,7,8,9,10,11, whereas the UO contains two large hydrological formations, Lake Untersee and Lake Obersee, systematically studied since 198812,13,14,15. Studies of MDV lakes have shown that their major properties are a function of several factors including the permanent presence of an ice cover that prevents wind mixing and atmospheric exchange, geochemical and biogeochemical activities in the ice, water column, and sediments that form distinct and highly stable chemical gradients, and variations in water supply and types16,17,18,19,20. Similar relations were found in Lakes Untersee and Obersee21,22. Strong physical and chemical gradients within MDV and UO ice-covered aquatic habitats create a unique microbial-dominated ecosystem that is both complex and diverse14,19,22,23,24,25,26,27,28,29,30,31,32,33.
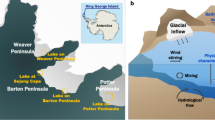
In this study, we focus on Lake Enigma (74°42’55”S 164°01’19”E), a perennially ice-covered lake positioned between Boulder Clay and Amorphous Glaciers in the Northern Foothills (Northern Victoria Land) of Antarctica (Fig. 1). While some Antarctic lakes, like Lake Vanda, maintain relatively warm bottom water temperatures1,2 others like Lake Vida (Victoria Valley, MDV) are frozen from top to bottom34. Well-studied MDV lakes such as Lakes Bonney, Hoare, and Fryxell, and UO lakes Untersee and Obersee have subzero water columns under a permanent ice cover25,27, and Lake Enigma falls into this category. Collectively, these lakes provide an exceptional environment for microbial life in with temperatures hovering near 0 °C and where shifts in density of bulk-phase H2O can affect cellular systems35. Thus, knowledge of the similarities and differences in the microbial communities of these lakes is foundational to understanding biology in Antarctica, a primarily microbial continent.
Lake Enigma36 is irregularly lobate-shaped and elongated between its northwest and southeast edges and fills a depression with an area of about 140,000 m2. The average annual air temperature in this region is –14 °C (maximum +9.6 °C; minimum –40.7 °C) and mean annual precipitation about 250 kg m−2 per year (www.climantartide.it). Ice melt in Lake Enigma occurs only in the summer season and only by a few decimeters in the shallower parts of the lake37,38,39. Prior ground-penetrating radar (GPR) surveys have shown that this region contains numerous frozen saltwater bodies with characteristic lake-ice blisters40,41. Lake Enigma is the largest hydrological formation among them and received its informal name from the presence on the surface near its center of a peculiar thin veneer of debris and ice-cored debris cone formed from the glacial drift that characterizes the Boulder Clay area39,42.
Based on a GPR survey carried out by Lozej et al43., the bottom of Lake Enigma was described as dipping gently from NW to SE and up to 10 m in depth, with a likelihood that the water column was completely frozen to the bottom; this conclusion is consistent with results from drilling adjacent to the ice-cored debris cone, which reported no liquid water down to a depth of 4.3 m depth42. The veneer of debris has an irregular elliptical shape that varies from a few centimeters to one decimeter in depth and is composed of angular and sub-angular granitoid, diorite, and metamorphic clasts up to 1 m in diameter. Additional drillings have shown that this large frozen mound contains an ice core beneath a shallow surficial debris cover38,42,43. This suspended arrangement of the ‘debris cone’ separated from the lake bottom can best be explained by upward accretion of basal ice beneath the lake ice sheet, resulting in the gradual uplift of debris from the lake bottom38. Such a process of ascending lake ice formation almost certainly requires the periodic influx of liquid water from beneath, the origin of which is yet to be discovered.
In addition to low precipitation and intense solar evaporation, ice in Lake Enigma experiences strong sublimation from katabatic winds that blow for more than 100 days a year at speeds up to 135 km h−1 39,44. Due to such harsh climatic conditions, it is estimated that Lake Enigma should lose 70,000–200,000 m3 of ice annually and its ice surface drop by 50–150 cm per year39,44. From over 60 years of aerial photography data, the area of Lake Enigma has fluctuated significantly, with a maximum lake ice cover of 141,000 m2 recorded in 198538,39 (Supplementary Fig. 1). Given the aridity and low snowpack of the Northern Hills region, precipitation alone is insufficient to recharge Lake Enigma at the rate of estimated annual sublimation losses, and hence some form of drainage system must exist. However, none of the drillings undertaken prior to our research has revealed liquid water, leading previous researchers to conclude that the Lake Enigma ice sheet extends to an undetermined depth36,38.
Our objective in this work is to explore the geochemistry and microbiology of Lake Enigma and compare these with the limnology and microbial diversity of MDV lakes10,16,23,27,28,29,30,31,32. During the XXXV Italian Expedition to Antarctica (November 2019–January 2020), we drilled through the ice cover at several points selected from a GPR survey. Using a clean-access and compliance approach to sampling Antarctic subglacial aquatic environments45, a massive body of unfrozen stratified oligotrophic water covered by up to 11 m of ice was discovered and sampled. The height of the present-day water column under the ice cover reaches at least 12 m but may be even deeper. In our study, several geochemical properties were assessed that showed that the lake basin is tightly isolated from the atmosphere and is meromictic, with a pronounced thermocline and chemocline. The composition of microbial communities sampled from surface ice, various layers of the stratified water column, and well-developed benthic microbial mats, was also characterized. Analysis of these communities demonstrate that Lake Enigma supports a phylogenetically diverse and high-biomass microbial ecosystem that stands unique among Antarctic perennially ice-covered lakes.
Results and discussion
Discovery of unfrozen water masses in Lake Enigma
Our GPR survey conducted on Lake Enigma consisted of a grid of 12 profiles, oriented ENE/WSW, i.e., perpendicular to the major axes of the lake, and five profiles, oriented NNW/SSW for a total of about 40 km (Supplementary Fig. 2A). The reflectivity of GPR signals depends on the contrast in the dielectric constant of the materials through which the radar wave passes. The strongest contrast generates a high-energy reflected wave, and this behavior allows assumptions to be made about materials and their physical state. In particular, the ice/rock–ice/water interface varies considerably, suggesting the presence of liquid phases such as a subglacial lake, pond, or thin film over the bedrock. Three GPR profiles from Lake Enigma (Supplementary Fig. 2B) exhibited a powerful specular reflection due to the strong change in reflectivity compared with that observed along the shores of the lake where the contact between ice and rocks occurs. The returned energy was high enough to generate up to three reflections in the first layer of ice. The amplitude’s increment of reflected waves often exceeded 11 dB corresponding to a transition from an ice/bedrock to an ice/water interface46. In addition, the presence of a thick layer of water led to strong attenuation of radar signals preventing significant reflection from the lake bottom.
Based on the above soundings, a total of six lake sites were identified as candidates for drilling operations and were named drilling points DP#1 to 4 and DP#C21 to 22 (Supplementary Table 1). The selection of drilling locations was aimed at intercepting the various interfaces identified from GPR data analysis (presence of potential ice/water and/or ice/rock interfaces, as well as ice blisters/bubbles). Drilling operations at DP#1, DP#3 and DP#C21 sites ceased at ice depths of 6, 12 and 9 m, respectively, when drill penetration was blocked due to significant amounts of clay (DP#1), sand (DP#3) and small stones (DP#C21). The estimated lake depth estimated by the GRP survey at DP#1 and DP#3 matched the achieved depths and confirmed that the lake was frozen to the bottom at these two locations. By contrast, GPR data showed that the lake depth at DP#C21 was > 20 m. Thus, the fine rock deposits encountered during drilling were considered to be an intermediate layer, which prevented drilling from being completed using the thermal head penetration method.
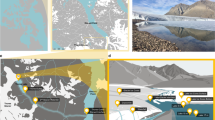
Liquid water was discovered in DP#2, DP#4 and DP#C22. Ice thickness at these sites according to extrapolation of the GPR data varied from a few meters to about 16 m. Drilling operations confirmed these estimates, showing an ice depth of 6.3 m at DP#2, 8.4 m at DP#4 and 11.0 m at DP#C22. The total lake depth (including ice cover) measured at these points was thus 9.3, 23.0, and 22.5 m, respectively. It should be noted that, starting from 480 m on the C-C′ section, GPR data revealed a topographical depression of unknown depth with a steep rise at the end, thus closing the lake basin. Underwater camera video taken at the bottom of DP#C22 confirmed the presence of such a depression southeast of the drilling site, suggesting that the lake is indeed much deeper than previously suspected (Supplementary Video 1). These data place Lake Enigma among the deepest lakes in Victoria Land behind the MDV Lakes Bonnie, Hoare, Joyce, and Vanda47.
Water column stratification of Lake Enigma
Using field multi-sensor measurements, profiling of key chemical and physical parameters was initially performed in situ. A comparative analysis of the data obtained from the deepest drilling points DP#4 and DP#C22 showed that the geochemistry of the water column of Lake Enigma resembles that of a meromictic lake. The main water body of the lake at a depth of more than 12.5 m had a near-zero temperature with subzero temperatures observed near the bottom at DP#C22 (Fig. 2). The upper layers of the Lake Enigma water column had a higher pH than lower layers (pH 10.0–10.2 versus 9.5–9.7 at the 6 m-thick metalimnion). When approaching the hypolimnion, pH values gradually decreased more from 8.8 to 8.2 near the lake bottom at DP#4 and DP#C22, respectively. The metalimnion, located at a depth of 12.5 to 18 m, was additionally characterized by relatively stable and extremely high concentrations of dissolved oxygen (up to 50 mg L−1) and total salinity of 1.05–1.12 g L−1. Marginally higher salinity (up to 1.50 g L−1) with a simultaneous decrease in both dissolved oxygen concentration and pH was observed in the deepest part of the Lake Enigma hypolimnion. According to all analyzed profiles and taking into account different depths at all three drilling points, the water column of DP#2 accurately reproduced the characteristics of the epilimnion of both the water columns of DP#4 and DP#C22 (Fig. 2). Thus, it is likely that the stratified water masses discovered and measured at locations separated by a distance of 245 to 300 m are actually interconnected and thus parts of a single hydrological entity—the body of Lake Enigma (Supplementary Fig. 3).
Geochemistry of the Lake Enigma water column and sediments
Total dissolved solids (TDS) as determined in the laboratory were significantly higher than those measured in situ using the YSI multiprobe sensor (Supplementary Table 2). These corrected values increased from the ice surface to the lake bottom from 91.5 to 1802 mg L−1, indicating that the Lake Enigma hypolimnion can be considered brackish, according to Rusydi48. The overall chemical composition was dominated by Na+ and Cl− (Fig. 3A), which represented on average 68% of total cations and 71% of total anions. This composition is in agreement with the literature data recently reviewed by Porcino et al49. for Terra Nova Bay lakes. On average, the predominance of major cations and anions in Lake Enigma was in the order of Na+ > Ca2+ > Mg2+ > K+ and Cl− > SO42- > HCO3− + CO32-. The measured Na+/Cl− molar ratios in the lake water samples (from 0.77 to 1.3; Fig. 3B), lower than those recorded in ice samples (ca. 1.8), were in agreement with those reported for ground ice from Amorphous Glacier (from 0.79 to 2.2650) but lower than those from Boulder Clay Glacier (from 1.98 to 1150). Accordingly, lake water replenishment in Lake Enigma likely occurs from the Amorphous Glacier. A more detailed description of Lake Enigma hydrochemistry is presented in Supplementary Text.
Piper diagram for the collected ice (circles) and liquid water (squares) samples from DP#2 (black symbols), DP#4 (red symbols) and DP#C22 (blue symbols) sites collected from Lake Enigma (A); Na/Cl molar ratio vs. TDS (mg L−1) diagram for ice (circles) and liquid water (squares) samples from DP#2 (black symbols), DP#4 (red symbols) and DP#C22 (blue symbols) sites collected from Lake Enigma (B); δD-H2O vs. δ18O-H2O (in ‰ vs. V-SMOW) diagram for ice (circles) and liquid water (squares) samples from DP#2 (black symbols), DP#4 (red symbols) and DP#C22 (blue symbols) sites collected from Lake Enigma (C). The literature data from (i) lakes in the Terra Nova Bay (grey triangles), including Lake Enigma (magenta triangles), compiled by Porcino et al.49 and (ii) Boulder Clay and Amorphous Glaciers (green cross and star symbols, respectively) from Gragnani et al.50 are also reported for comparison. The Na/Cl molar ratios for seawater (0.86; blue dashed line), marine aerosol (0.87; dark cyan dashed line) and halite dissolution (1; black solid line) are also shown. The Global Meteoric (GMWL)54, the Local Meteoric (LMWL)55 and the Antarctic Meteoric Water Line (AMWL)56 are also shown.
The lithology of the Northern Foothills region is composed primarily of granite, granodiorite, and metamorphic rocks, best described as muscovite-bearing monzogranites with characteristic microcline- and quartz-plagioclase mineral assemblages37,51,52. Accordingly, the spectrochemical analysis of sediments collected at all drilling points revealed a predominance in the bedrock of the silicate-rich minerals albite, kaolinite, muscovite and quartz (Supplementary Fig. 4). Silicate weathering under closed hydrological conditions, i.e. where no replenishment of CO2 from the atmosphere can occur, likely contributed to the alkaline pH values measured in the lake water body, as previously proposed for Lake Untersee53. Generally speaking, Br (in mg L−1) and As, B, Li and Rb (in μg/L) mimicked those for Na and Cl, with the highest concentrations found below the depth of 13 m. This would suggest a common source for both the main and trace solutes. No further information can be obtained by NO3− and NH4+ since their content is often slightly over or below the instrumental detection limit (0.1 and 0.01 mg L−1, respectively).
The δ18O and δD values obtained from the analysis of samples collected from Lake Enigma are reported in Supplementary Table 3 and Fig. 3C. The lake water samples showed δ18O values ranging between −27.6 to −24.6‰ vs. V-SMOW whilst those of δD varied from −212 to −193‰ vs. V-SMOW. All of them did not have a typical refreezing slope, but are distributed on or near the Global Meteoric Water Line (GMWL: \(\delta D=8\times {\delta }^{18}O+10\)54) and align along the local meteoric water lines defined by Masson-Delmotte et al.55 and Fernandoy et al.56, i.e. AMWL (\(\delta D=7.75\times {\delta }^{18}O-4.93\)) and LMWL (\(\delta D=7.83\times {\delta }^{18}O-0.12\)), respectively. Unlike lake water, the surface layer of the Lake Enigma ice sheet was isotopically much heavier (δ18O = −18.4‰ vs. V-SMOW and δD = −146 ‰ vs. V-SMOW) (Fig. 3C). Notably, epilimnetic lake waters showed a slight enrichment in heavier isotopes with respect to deeper waters tending towards the ice composition, possibly supporting the hypothesis of meltwater inflow from neighboring glacier(s) (Fig. 4). The adjacent Boulder Clay and Amorphous Glaciers display distinct isotopic compositions related to their diverse origin: Amorphous Glacier was interpreted as pure continental ice, whereas Boulder Clay was suggested to include a marine ice component36. As highlighted in Fig. 3C, the measured isotopic compositions determined in water samples were consistent with those reported for the ground ice from surface and deeper layers of Amorphous Glacier (δ18O: from −28.9 to −17.3‰ vs. V-SMOW and δD: from −224 to −139‰ vs. V-SMOW) and lighter than those from Boulder Clay Glacier (δ18O: from −22.3 to −7.82‰ vs. V-SMOW and δD: from −175 to −63.1‰ vs. V-SMOW). Accordingly, and in agreement with evidence from geochemical data, water isotopic data indicate that Lake Enigma is fed by subsurface waters most likely originating from the Amorphous Glacier. This hypothesis, however, assumes that subglacial water of Amorphous Glacier exists, and perhaps only sporadically, in an unfrozen state.
Underwater survey of the bottom of Enigma Lake made at three different drilling points: A, B DP#2 (depth 9.3 m); C, D DP#4 (depth 22.5 m); E, F, DP#C22 (sampling depth 22.0 m). Evidence of supraglacial meltwater inflow from Amorphous Glacier to the surface of Lake Enigma evidenced during the XXXV Italian Antarctic Expedition on January 3, 2020 (G, H).
Among other anomalies concerning the bulk seawater components, we observed more than 100-fold elevated F− anion contents in Lake Enigma waters (Supplementary Table 4). For subglacial Lake Whillans, a trend towards high F− concentrations has been explained as a potential influence of subglacial volcanism in the upstream catchment57. Although this lake is located in close proximity to the active volcanoes Mt. Melbourne (74°21'06''S 164°41'50''E) and Mt. Rittmann (73°26'57.2''S 165°29'46.9''E), the presence of geothermally active zones near Lake Enigma is not documented58. Considering all these prerequisites, as well as the difference in the altitudes of these hydrological formations (175 m asl for Lake Enigma and 360 to 1100 m asl for the Amorphous Glacier), an interconnected cryptic drainage system, sealed from the atmosphere, may experience significant hydraulic pressure on the lake bottom from a tightly closed hidden drainage system of pressurized water flowing from Amorphous Glacier to Lake Enigma. If true, this could explain two of its most puzzling features: the existence of a stable hydrological formation in the cold Northern Foothills desert and the occurrence of a huge boulder (that takes the form of both a hill and an island) in the center of the lake, separated from the lake bottom.
Macroscopic observation of microbial life
The perennially ice-covered lakes of Antarctic cold deserts provide a stable, low-disturbance environment that supports only microbial life19,26,27,30,59. In the photic zone, the bottoms of these lakes are commonly covered with complex three-dimensional microbial mats of varying morphology14,28,29,31,32,33, reminiscent of microbial assemblages from Earth’s early biosphere widespread in thermal regions and shallow marine basins on Earth today60,61. Thus, these lacustrine ecosystems of the Antarctic cold desert have been considered by many to be suitable sites for early life on planet Earth3,24,28,62,63.
Consistent with the above, underwater videos (Supplementary Video 2, 3) taken at drilling points DP#2 and DP#4 showed the bottom of Lake Enigma to be covered with microbial mats of two different sympatric morphologies. At the bottom of DP#2 (depth 9.3 m), prostrate benthic microbial mats had a variable morphology resembling a crumpled thick carpet, sometimes forming large amorphous tree-like structures up to 40 cm high and up to 50 to 60 cm in diameter (Fig. 4A, B). In contrast, the prostrate benthic microbial mats observed at DP#4 (23.0 m) were flat and very thin ( < 3 cm) with a congeneric distribution of vertically raised small elongated cuspate pinnacles ranging from 25 to 45 mm in diameter and up to 50 to 100 mm in height (Fig. 4C, D). Both types of microbial mats collected from drilling points DP#2 and DP#4 consisted of a superficial filamentous green-brown layer covering a soft, creamy (both in color and consistency) amorphous structure without signs of lithification. Visual inspection of the surface laminae of these prostrate microbial mats using epifluorescence and confocal microscopy confirmed the absolute dominance of autofluorescent filamentous cyanobacterial (oscillatorians) morphotypes, densely grouped in agglomerated structures along with bacterial cells identified by both DAPI and CARD-FISH (Fig. 5). Mat samples were initially analyzed by confocal laser scanning microscopy, and their upper layers were found to have an absolute dominance of filamentous cyanobacterial (oscillatorians) morphotypes. The dominance of filamentous cyanobacteria in benthic phototrophic communities in Antarctic perennially ice-covered lakes is well documented29,31,32,33,64. No cells of Archaea were observed in microbial mat samples using CARD-FISH. A similar morphology of microbial mats with cuspate pinnacles, consisting primarily of cyanobacteria, was found in ice-covered Lake Vanda (McMurdo Dry Valleys, Antarctica)65,66. Their photosynthetic activity has been demonstrated in microbial mats at depths of at least 40 m, suggesting that these benthic communities are responsible for the bulk of primary production in this highly oligotrophic lake67. In addition to high biomass yield, intense oxygenic photosynthesis causes oxygen supersaturation of unfrozen water, a phenomenon observed in many perennially ice-covered Antarctic lakes68, including Lake Enigma.
The bottom of the DP#C22 site was observed as a steeply plunging slope in a southeast direction. Located at a shallower depth than DP#4, the bottom of DP#C22 was devoid of microbial mats (Fig. 4E, F). This absence of microbial mats is likely due to a combination of two factors that limit light penetration of solar energy necessary for the development of photosynthetic microbial mats: a thicker ice cover and the aforementioned presence of a huge field of boulders lying on the surface of the ice sheet in the immediate vicinity of DP#22 and thus obscuring the bottom at this drilling point.
Diversity estimation of Lake Enigma microbial communities
General characteristics of DNA obtained from a total of 19 samples are summarized in Supplementary Table 5 and Supplementary Fig. 5. A total of 551,427 16S rRNA gene sequences were obtained, ranging from 16,485 to 76,343 sequences per sample, which belonged to a total of 1531 distinct amplicon sequence variants (ASVs) (Supplementary Table 6). Consistent with the approach used to characterize both prokaryotic and algal communities in lacustrine and hydro-terrestrial environments of East Antarctica69, filtered ASVs derived from eukaryotic organellar DNA (chloroplast 16S rRNA sequences) were also analyzed.
A total of eighteen bacterial phyla and three eukaryotic phyla (all unicellular phototrophs) were identified in the surface ice, layers of stratified water column, and microbial mats. 16S rRNA gene sequences belonging to Archaea were not detected, even in assays using archaea-specific primers. Rarefaction curves with 99.9–100% coverage were obtained for each ASVs library processed, indicating that a sufficient sampling of microbial diversity was obtained from all three Lake Enigma compartments. Despite the limited number of drilling sites sampled, alpha and beta diversity analyses revealed a clear pattern of differentiation of microbial communities in various macro-scale habitats of Lake Enigma. Both DP#2 and DP#4 showed an increase in microbial biodiversity with depth, with the fewest number of identified ASVs in the surface ice and the water layer just below the ice (Supplementary Table 6). At the DP#2 site, approximately 80 to 90 ASVs were detected at a depth of 7.8 m and just above the microbial mat covering the lake bottom. In contrast, despite the average similarity (90.7 ASVs in the corresponding samples), in the more stratified water column of DP#4, large variations in the number of ASVs were found—from 50 to 178 ASVs. The Inverse Simpson, Shannon, and Chao1 indices showed comparable values, and microbial biodiversity increased significantly in the deepest part of Lake Enigma, especially in the benthic microbial mats at DP#2 and DP#4; at these sites, 245 and 278 ASVs were identified with indices of 116.59 and 75.90 (Inverse Simpson) and 4.84 and 4.98 (Shannon Diversity), respectively.
Beta diversity between samples collected from different depths and matrices (surface ice, water column, and microbial mats) was analyzed using UniFrac (weighted and unweighted)70 and Jaccard and Bray−Curtis distances71 as detailed in the Materials and Methods. The contributions of the two selected unweighted UniFrac principal components to differences in microbial community compositions were 16.0% and 29.2 individually (Supplementary Fig. 6). Beta diversity analysis separated Lake Enigma microbial communities into four main categories: microbial mats, surface ice, shallow water (up to 17 m), and deep water as confirmed by an ANOSIM test (Supplementary Table 7). Water sample DP#2-NEAR represents a fifth category, positioning itself closer to the microbial mat cluster than the water column groups. Considering that this sample was collected only a few centimeters above the microbial mat surface, the possible migration/displacement of organisms from the mat cannot be ruled out. Because most samples collected are grouped into distant clusters, regardless of location, habitat type is the most important factor determining the microbial community structure of Lake Enigma. As shown by statistical analysis using envfit and ordisurf functions in vegan (Supplementary Table 8, 9; Supplementary Fig. 7), there are several important hydrological factors in the stratified water column of Lake Enigma, such as fluctuations in both pH and temperature, which likely shape the planktonic microbial populations. The Mantel test using the R microeco package72 was further applied to evaluate the correlation between physicochemical parameters and structure of five target phyla: Cryptophyta, Actinobacteriota, Bacteroidota, Pseudomonadota, and Candidate Phylum Patescibacteria. Along with pH and temperature, chemical factors that statistically influenced variability in the composition of Bacteroidota, Actinobacteriota and Ca. Patescibacteria included sodium and boron (Fig. 6 and Supplemented Table 10). By contrast, ammonium, lithium and fluorine, although showing a significant p value (0.03), have very low r values, indicating that their variation does not significantly influence microbial population structure in Lake Enigma.
The coefficient of correlation (r) represents the correlation between the two factors, the width of the line denotes the level of the correlation, and the color indicates statistical significance based on 9999 permutations (p < 0.01, p < 0.05). Pearson’s correlation coefficient matrix shows the relationships among dependent variables.
Microbiome profiling
Bacteroidota, Actinobacteriota and Pseudomonadota (previously Proteobacteria) were among the most abundant bacterial phyla in all Lake Enigma habitats, accounting for nearly 60% of all taxonomically analyzed 16S rRNA gene sequences (Fig. 7, Supplementary Fig. 8). However, already at the phylum level, strong differences were found in the microbial communities in ice, in the water column, and in microbial mats. With the exception of the bacterial phyla, all remaining 16S rRNA gene sequences (25.3%) recovered from the ice were greater than 99% identical to chloroplast sequences obtained from various samples of Antarctic glaciers and high-altitude Arctic snows and were distantly related to chrysophytic (Heterokontophyta) chloroplasts. Taken together, these data indicate extremely low diversity of the ice-associated microbial community, and this contrasts with what has been found in the ice cover of MDV lakes9,26.
A All retrieved taxa at the phylum level are represented according to Illumina-sequencing read counts and habitat types (upper hemisphere) and proportional to overall Lake Enigma diversity richness (lower hemisphere). B Proportional composition of microbiota at the phylum level reconstructed from each Lake Enigma habitat type. The relative distribution of minor phyla (presented in <0.1% of total Illumina-sequencing reads) is shown in detail separately as bubble chart.
The Lake Enigma water column was characterized by a high relative abundance of 16S rRNA gene sequences from cryptophyte chloroplasts (20.6% of all 404,704 water column sequences analysed). These phototrophic organisms were found predominantly in the water column and represented by 65 distinct ASVs distantly related to Teleaulax amphioxea (highest sequence identity of 98.87%). Their distribution in the water column was fairly uniform, with a slight preference for the warmest layers (Fig. 8). The second phytoplankton-specific phylotype, represented by Nannochloropsis-related species (Eustigmatophyceaea), was present at almost 2% in all water column sequences. Unlike cryptophytes, the last group of phototrophic protists was found only in the water column of DP#4. Given the absence of cyanobacterial 16S rRNA gene sequences in any of the water column layers analyzed—in contrast to MDV Lake Fryxell29—these eukaryotic phototrophs may play a key role in carbon cycling in the Lake Enigma water column, fixing inorganic carbon during austral summer. It is worth noting that cryptophyte populations inhabiting MDV Lakes Vanda and Bonney are able to supplement phototrophy with heterotrophy (bacterial grazing) and in this way maintain metabolic activity during the dark polar winter73. Thus, the DP#4 group of phytoplankton may also play an important role in carbon and nutrient cycling during the dark fall and winter seasons.
Cyanobacteria (Cyanobacteriota) are an important part of the phototrophic components of both planktonic and benthic communities in permanently ice-covered Antarctica lake14,28,29,74,75,76. However, in stark contrast to these ecosystems, cyanobacteria in Lake Enigma are only present in benthic microbial mats (Fig. 8). Molecular analyses revealed 10 distinct cyanobacterial ASVs in Lake Enigma microbial mats. Despite the macromorphological differences between the DP#2 and DP#4 microbial mats, significant structural similarities were present in their cyanobacterial composition (Supplementary Fig. 9). Additionally, there were two specific ASVs that accounted for 44.51 and 2.46% of the relative cyanobacterial abundance in the mat communities of DP#2 and DP#4, respectively. Although we were unable to unambiguously assign them to any recognized cyanobacterial phylotypes, both clustered with uncultured cyanobacteria distantly related to the deeply divergent class Vampirovibrionia (order Vampirovibrionales) (Supplementary Fig. 10), none of which are known to be phototrophic77,78. Overall, the majority of assigned cyanobacterial ASVs were closely related to environmental sequences, enrichments, and isolates from microbial mats from several Antarctic lakes, including MDV Lakes Fryxell, Hoare and Vanda31,32,33,79,80.
In addition to the heterotrophic bacterial phyla of Bacteroidota, Actinobacteriota and Pseudomonadota, there were eight other phyla common to planktonic and benthic mat microbiota (Fig. 9, Supplementary Fig. 11). Four of them, Patescibacteria (5.99%), Chloroflexota (4.58%), Verrucomicrobiota (4.39%) and Planctomycetota (2.72%), collectively account for almost one fifth of taxonomically assigned Lake Enigma 16S rRNA gene sequences. The phyla Acidobacteriota, Bacillota, and Gemmatimonadota had relative abundances greater than 1% in their respective microbial communities and were more abundant in microbial mats compared with the water column, whereas the phylum Dependentiae, although minimally represented, showed the opposite trend. This is where the similarity at the phylum level between these two communities ends, and, in addition to the already discussed phylum Cyanobacteria, the remaining rare ( < 1% presence) phyla Armatimonadota, Bdellovibrionota, Chlorophyta, Myxococcota, Deinococcota, Desulfobacterota, Fibrobacterota, Latescibacterota and Nitrospirota were specific to microbial mats (Supplementary Fig. 11). Phylogenetic evidence for both N2-fixing cyanobacteria and nitrite-oxidizing Nitrospira species was obtained but only from microbial mats. Moreover, the sixfold predominance of nitrite-reducing Gemmatimonadaceae in mats compared to the entire water column suggests that nitrogen cycling occurs primarily if not exclusively in benthic mat communities.
Absolute numbers of Illumina-sequencing reads are shown only for the abundant bacterial groups, namely >2% of the total number of reads detected in the surface ice, water column, and microbial mats, respectively. *According to NCBI taxonomy, members of the phylum Pseudomonadota have been divided into Alpha-, Beta- and Gammaproteobacteria classes.
The Lake Enigma microbiota is enriched by ultrasmall bacteria of the superphylum Patescibacteria
A remarkable feature of the Lake Enigma ecosystem was the presence and sometimes even dominance (for example, constituting 54.3% of the community in the DP#2-NEAR water layer) of organisms belonging to the superphylum Patescibacteria, also called Candidate Phyla Radiation (CPR) (Fig. 10). Phylogenetic affiliation of 133 related ASVs showed a distribution within at least 9 different classes of Patescibacteria, with only three similar sequences found in the ice cover (4.8 m deep)81 and brine of Lake Vida (MVD)82 and in benthic moss pillars of the fresh water Lake Hitoke-Ike (Skarvsnes Forland, Antarctica)83. Although a diverse array of ultrasmall bacteria were present in Lake Enigma, no clear phylogenetic link to source, trophic state of the ecosystem, ambient temperature, salinity or the presence of oxygen was observed (Supplementary Fig. 12).
These tiny bacteria, representing 5.53% and 8.84% of the total planktonic and benthic microbial communities of Lake Enigma, respectively, are extremely small cells (average diameter 200–350 nm) and contain highly reduced genomes (average size 1 Mbp) and minimal cellular activities including limited metabolic potential84,85. As a consequence, these bacteria have adopted an obligate symbiotic or predatory lifestyle, relying entirely on their respective prokaryotic host cells, either of Bacteria85,86,87 or Archaea88. Species of CPR are phylogenetically diverse and ubiquitous but are particularly abundant in microaerobic and/or anaerobic zones of lakes, sediments, and groundwater89,90. This is because all known CPR species lack genes encoding citric acid cycle and oxidative phosphorylation functions, suggesting an adaptation to a strictly fermentative lifestyle84,86. Therefore, the discovery of a relative high abundance of Patescibacteria in the hyperoxic water column of Lake Enigma was surprising and hints at additional metabolic capabilities of this group.
CPRs have been reported in hypoxic habitats in Antarctica such as permafrost, desert soils90,91, and endoglacial hypersaline brines92. However, before our study of Lake Enigma, CPRs had not been found in perennially ice-covered lakes. One explanation for this may be a bias caused by the commonly used ≥0.22 µm pore size filters to collect bacterioplankton; such filters would allow the smaller CPR cells to pass through. To test this hypothesis, additional filtration of 0.22 µm-penetrates of samples DP#4-21.3 m and DP#4-22.5 m samples was carried out using a 0.1 µm filter. On such filters, Patescibacteria were highly enriched (Wilcoxon signed-rank test, P < 0.001) by 4.22 and 15.32 times compared to their recovery on 0.22 µm-filters (Fig. 10). This agrees with previous studies in which free-living ultrasmall Patescibacteria cells were enriched using fine-pore filters86,93,94. The significant abundance of Patescibacteria in the 0.22 µm-filtered sample DP#2-NEAR ( > 50% of community) likely reflects cells of Patescibacteria firmly attached to much larger host cells. That such cells were recovered from Lake Enigma but not from MDV lake waters is further evidence of the novelty of the Lake Enigma ecosystem and the apparent absence of ultrasmall bacteria from MDV lakes.
Based on 16S rRNA gene sequence data (35,103 sequences taxonomically assigned to Patescibacteria and grouped into 133 different ASVs), the hypolimnion and microbial mats of Lake Enigma had the highest percentage of CPR classes; ‘Ca. Paceibacteria’, ‘Ca. Saccharimonadia’, ‘Ca. Gracilibacteria’ and ‘Ca. Microgenomatia’ were the most abundant groups. These findings are consistent with an extensive analysis of bacterioplankton from 17 Eurasian lakes that showed that representatives of these classes are more common in the hypolimnion, regardless of their trophic state95,96. Because most Patescibacteria remain uncultured, it is difficult to make assumptions about their specific hosts. However, some representatives of the class ‘Ca. Saccharimonadia’ were successfully co-cultivated with an actinobacterial host97,98,99, while members of the class ‘Ca. Gracilibacteria’ have been maintained in stable binary cultures with gammaproteobacterial hosts87,100; both of these hosts are abundant in Lake Enigma. Thus, it is likely that Patescibacteria are important components of the Lake Enigma microbiome and may express unique metabolic traits in order to thrive under hyperoxic conditions. This finding highlights the complexity and diversity of food webs in Antarctic permanently ice-covered lakes, with symbiotic and predatory lifestyles a possibility not previously recognized.
Conclusion
Perennially ice-covered freshwater ecosystems are hotspots of biodiversity in Antarctica’s polar deserts, providing a year-round oasis for microbial life. This study describes a unique example of these ecosystems by adding the massive freshwater Lake Enigma, one of the deepest hydrological formations in Victoria Land. The lake has an unusual geochemistry and microbial diversity and is isolated from the external environment by a permanent ice cover 9 to 11 meters thick. The ice overlies a highly stable, pressurized, and chemically stratified water column of at least 12 m, the source of which is likely the nearby Amorphous Glacier. It is clear that Lake Enigma contains distinct ice-associated, planktonic, and benthic microbial communities. The ice-sealed planktonic and benthic microbiota of Lake Enigma likely represent persistent legacy biota that arose from the lake’s ancient microbial ecosystem before the freeze-up. Overall, Lake Enigma microbiota occupy different trophic levels within a simple aquatic food web, ranging from primary production to ectosymbiosis and predation. The ultrasmall Patescibacteria in particular may play unusual roles in the lake’s ecosystem that do not play out in other ice-covered Antarctic lakes.
Materials and methods
Drilling activity using the ‘clean access’ methodology
During the XXXV Italian Expedition to Antarctica (November 2019-January 2020), three different approaches were successively used to drill Lake Enigma: the initial phase of drilling to a depth of up to 3 m using an electric drill (StrikeMaster Combo 40 V 150 mm, Rapala, USA) followed by two different types of ‘thermal drilling’ for deeper penetration. The first employed a custom-made thermal head melt drilling (THMD) system that used thermal resistors integrated in the perforator head, similar to those designed by Li et al. 101. The second employed hot water drilling (HWD) for thermal penetration. To do this, the ice crumbs formed as a result of mechanical drilling at the initial phase were collected, boiled, and hot water was returned back into the drilled well. The THMD consisted of a 40 cm long x 3.5 cm-wide stainless tube (3 mm thick) connected to a 40 cm long solid brass head containing 6 RS PRO cartridge-heating elements (200 W, 220 V [RS Components MI, Italy]). The device can be used either tied to a rope or securely screwed to a rigidly assembled guide system consisted of modular stainless tubes (1.5 m long) extending to the surface. Both THMC and HWD reuse water obtained from crushed ice to create the access port to the ice-sealed lake. As the returned drilling water passes through the drilling system at the surface, it is filtered, UV-treated and pasteurized to 100 °C before being returned into the drilling well. THMD and HWD were used individually or together depending on drilling depth and drilling difficulties, such as deposits of ice-cemented rubble that did not allow mechanical drilling using ice-specific tools. These thermal drilling approaches, are fully compliant with the SCAR Code of Conduct for Subglacial Research and are known as ‘clean hot water drills’102.
Sampling procedures and field measurements
After access to the lake, redox potential, conductivity/total salinity, water temperature, pH and dissolved oxygen were measured using a YSI model 6600 Environmental Monitoring System (multiprobe) sensor at regular 0.5-m depth intervals along each vertical profile in boreholes DP#2, DP#4 and DP#C22. After initial data analysis, the water column was sampled from the ice-water interface to the bottom, according to the identified stratification. Between 2 and 5 L of lake water was collected from each chosen layer using a peristaltic pump (Millipore C419, Merck, Italy) and pre-sterilized tubing and polycarbonate bottles. The collected samples were immediately transported to the Italian base Mario Zucchelli Station (MZS) and processed in the MZS laboratory within 6 h after sampling for chemical and biological analyses. From each sample, two unfiltered aliquots were transferred to 125 mL polyethylene bottles for the analysis of major anions and water stable isotopes. One filtered (0.45 μm) and acidified (0.5 mL ultrapure HCl) aliquot from each sample was transferred to 50 mL polyethylene bottle for the analysis of major cations.
To analyze Lake Enigma microbiota, samples of melted surface ice and lake water were filtered through 0.22 µm Sterivex filters (Durapore; Millipore, Billerica, MA, USA) using a peristaltic pumping system. Filters were then stored in lysis buffer (40 mM EDTA, 50 mM Tris/HCl, 0.75 M sucrose) at −20 °C. Since the identified stratification was identical in the nearby drilling wells DP#4 and DP#C22, a total of 15 samples representing the shallow (DP#2) and deep (DP#4) compartments of Lake Enigma were selected for further biomolecular analyses. To gain insight into the diversity of ultra-small ( < 220 nm in size) bacteria, after filtering the deepest lake water samples, DP#4-21.5 m and DP#4-22.5 m, the 0.22 µm penetrant was further filtered using a 0.1 μm pore-size polycarbonate filters (type GTTP; Millipore, Eschborn, Germany). All filters were stored at −20 °C until processed for total DNA extraction in Italy. For community composition analysis by CARD-FISH, aliquots (15 mL each) were fixed with a formaldehyde solution (Sigma Aldrich; final concentration 1%). Before proceeding with the DNA extraction, the Sterivex filters were first thawed on ice, then 400 μL of TE buffer (pH 8.0), containing lysozyme (5 mg mL−1) and proteinase K (0.2 mg mL−1, final concentrations) was added inside the cartridges; the cartridges were then shaken for 5 s and incubated for 10 min at room temperature. A total of 1.6 mL of QRL1 lysis buffer (containing β-mercaptoethanol) was added inside the Sterivex cartridges and DNA extraction was performed using a Qiagen RNA/DNA Mini Kit (Qiagen, Milan, Italy) according to the manufacturer’s instructions. The DNA samples were further concentrated using a microconcentrator (Centricon 100; Amicon, Millipore, Billerica, MA, USA). The quantity, integrity and purity of DNA was checked and evaluated using both agarose gel electrophoresis, and a NanoDrop® ND-1000 Spectrophotometer (Wilmington, DE, USA).
As video recording showed, the lake floor at the drilling points DP#2 and DP#4 was completely covered with benthic microbial mats. For biological analyses, they were sampled from both boreholes using a modified gravity coring approach. Due to the extremely soft composition of microbial mat spread on the rocky bottom, it was not possible to recover intact benthic samples using traditional gravity coring technology. To collect the microbial mat material, a pre-sterilized stainless-steel cylinder (25 cm long, 10 cm wide and 4 mm thick), sharpened from below, was inserted into the microbial mat and the detached material trapped inside the cylinder was lifted to the surface through a presterilized one-inch diameter sampling tube (Masterflex 96499 or Norprene®) tightly connected to the core using a peristaltic pump (Millipore C419, Merck, Italy). The collected mat material was immediately delivered to the MZS field laboratory, where it was centrifuged at 2000 x g and 4 °C for 2 min and divided into two sub-aliquots (50 − 100 mL each): one was stored directly at −20 °C, while the other was fixed with 50% ethanol (Sigma Aldrich) before storage at −20 °C until further processing.
Epifluorescence and confocal microscopy
In order to visualize the 3D structure of the mat, Catalyzed Reported Deposition - Fluorescence in situ Hybridization (CARD-FISH) was applied as described previously103,104. Specific rRNA-target Horseradish peroxidase labeled oligonucleotidic probes (Biomers, Ulm, Germany) targeted Bacteria (EUB338 I-III), and Archaea (ARCH915). Cells were then stained with DAPI solution, at room temperature in the dark for 15 min, followed by a second staining of 0.15 mM Calcofluor-white (Sigma-Aldrich Chemie GmbH, Steinheim, Germany), at room temperature in the dark for 4 min, for Extracellular Polymeric Substances (EPS) visualization. The stained mats were then observed under a confocal laser scanning microscope (CSLM; Olympus FV1000). Both DAPI stained cells and EPS were excited by 405 nm light and emitted at 430 to 470 nm (blue color). The hybridized Bacterial cells were excited with the 488 nm line of an Ar laser (excitation) and observed in the green channel from 500 to 530 nm (emission). Cyanobacteria were discriminated by their red autofluorescence. Cyanobacteria were excited with the 543 nm line of a He−Ne laser and observed in the red channel from 550 to 660 nm. The three-dimensional reconstruction of CSLM images was elaborated by the software IMARIS 7.6 (Bitplane, Switzerland) with 3D volume rendering mode.
Chemical and isotopic analyses of water samples
HCO3− and CO32− were analyzed by acidimetric titration (HCl 0.01 N). The main dissolved anions (Cl−, SO42−, Br−, F−) and cations (Na+, K+, Ca2+, Mg2+) were analyzed by ion chromatography on Metrohm 761 and Metrohm 861 chromatographs (analytical error <5%). Water samples (100 mL) were filtered (0.45 μm pore-size nylon filters) and acidified with ultrapure HCl for dissolved inorganic nitrogen (DIN = NH4+ + NH3 + NO2− + NO3−) determination. NO2− + NO3− were assessed on a Bran + Luebbe autoanalyzer after the reduction of nitrate on a Cu-Cd column. NH4+ + NH3 and phosphate concentrations were estimated by the salicylate method, as previously described104. To eliminate the interference of silica, the pH of the solution was adjusted to 1. Water isotope ratios 18O/16O and D/1H, expressed as δ18O-H2O and δD-H2O in ‰ vs. V-SMOW, were analyzed using a Finnigan Delta Plus XL mass spectrometer. Analytical errors for δ18O-H2O and δD-H2O were ±1‰ and ±0.1‰, respectively.
High-throughput 16S rRNA amplicon sequencing, bioinformatics and statistics
Next generation sequencing was used to study the biodiversity of the Lake Enigma study sites. The V3 − V4 variable regions of the SSU rRNA gene were amplified using the primer pair S-D-Bact-0341-b-S-17 (5’-CCTACGGGNGGCWGCAG-3’) and S-D-Bact-0785-a-A-21 (5’-GACTACHVGGGTATCTAATCC-3)105. Amplicons were sequenced on an Illumina MiSeq platform by FISABIO, Valencia, Spain (http://fisabio.san.gva.es/en/servicios). Library preparation and Illumina sequencing were performed according to standard protocols.106 Raw reads were cleaned of barcodes and primers, then reads shorter then <150 bp or with ambiguous base calls, and with homopolymer runs exceeding 6 bp, were removed. Reads were imported in RStudio package107 and analyzed using the DADA2 package (v 1.26.0)108 as described in Cordone et al.109. Due to preprocessing by the sequencing facility, reads were trimmed using modified filtering parameters (truncLen=c(280,280), maxN = 0, truncQ = 0, rm.phix = TRUE) according to Crisafi et al.110. A parametric error model, based on the convergence between the estimation of error rate and the inference of the sample composition, was applied to remove PCR biases108. Then paired-end reads were merged and the amplicon sequence variants (ASVs) table was constructed.
After chimeric sequences were removed, taxonomy was assigned to sequence variants using the naive Bayesian classifier method against the Silva Database v138 (v138; https://www.arb-silva.de/documentation/release-138/, and https://zenodo.org/record/4587955#.YgKJlb_MJH4). Diversity analyses, as Alpha diversity for each sample analyzed (“Observed”, “Chao1”, “Shannon”, “Simpson” and “Fisher” diversity index) and beta diversity among the sampled areas (nMDSs using UNIFRAC both weighted and unweighted and Jaccard and Bray−Curtis distance) based on ASVs abundance table obtained from DADA2, were processed using Phyloseq and Microbiome packages111,112. A Chord diagram was performed using the Jockergoo/Circlize package in R113. The R function envfit and ordisurf in vegan were used to investigate the role of environmental factors in the distribution of microbial diversity. The linearity of the correlation between the rate of change in beta diversity and the variables identified as significant by envfit was assessed by plotting the non-metric multidimensional scaling (nMDS) axis against the respective variable. Additionally, the R microeco package was used to perform a Mantel’s test applying the phylum abundance table and the environmental variables72. The ASVs abundance table obtained from DADA2 was used by PAST (PALeontological Statistics V3.25; https://palaeo-electronica.org/) to calculate the ANOSIM test (ANalysis Of SIMilarities)114 and SIMPER (Similarity Percentage) to assess which taxa were primarily responsible for any observed differences between groups of samples100 in order to elucidate abiotic factors that may be contributing to the composition of microbial communities.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The sequencing dataset obtained in this study is freely available through the European Nucleotide Archive (ENA)/NCBI under the accession number PRJNA1109609.
References
Wilson, A. T. & Wellman, H. W. Lake Vanda: an Antarctic Lake. Nature 196, 1171–1173 (1965).
Canfield, D. E. & Green, W. J. The cycling of nutrients in a closed-basin Antarctic lake: Lake Vanda. Biogeochemistry 1, 233–256 (1985).
Wharton, R. A., Simmons, G. M. & McKay, C. P. Perennially ice-covered Lake Hoare, Antarctica: physical environment, biology and sedimentation. Hydrobiologia 172, 305–320 (1989).
Priscu, J. C. Phytoplankton nutrient deficiency in lakes of the McMurdo dry valleys, Antarctica. Freshw. Biol. 34, 215–227 (1995).
Priscu, J. C. Ecosystem dynamics in a polar desert: The McMurdo Dry Valleys, Antarctica Vol. 72 (American Geophysical Union Publications: Washington, DC, USA 1998).
Spigel, R. H. & Priscu, J. C. Physical limnology of the Mcmurdo Dry Valleys lakes. in Ecosystem Dynamics in a Polar Desert: the Mcmurdo Dry Valleys, Antarctica 72, 153–187 (American Geophysical Union Publications: Washington, DC, USA 1998).
Siegert, M. J., Carter, S., Tabacco, I., Popov, S. & Blankenship, D. D. A revised inventory of Antarctic subglacial lakes. Antarct. Sci. 17, 453–460 (2005).
Greenfield, S. R. et al. Life and its traces in Antarctica’s McMurdo Dry Valley paleolakes: a survey of preservation. Micron 131, 102818 (2020).
Gordon, D. A., Priscu, J. & Giovannoni, S. Origin and phylogeny of microbes living in permanent Antarctic lake ice. Microbial Ecol. 39, 197–202 (2000).
Green, W. J. & Lyons, W. B. The saline lakes of the McMurdo Dry Valleys, Antarctica. Aquatic Geochem. 15, 321–348 (2009).
Sohm, J. A. et al. Microbial mats of the McMurdo Dry Valleys, Antarctica: oases of biological activity in a very cold desert. Front. Microbiol. 11, 537960 (2020).
Kaup, E., Loopman, A., Klokov, V., Simonov, I. & Haendel, D. Limnological Investigations in the Untersee Oasis (Queen Maud Land, East Antarctica). In Limnological studies in Queen Maud Land (East Antarctica) (ed. Martin, J.) 28–42 (Academy of Sciences of Estonia, Tallinn 1988).
Haendel, D., Hermichen, W.-D., Höfling, R. & Kowski, P. Hydrology of the lakes in Central Wohlthat Massif, East Antarctica: new results. Isotopes Environ. Health Stud. 47, 402–406 (2011).
Greco, C. et al. Microbial diversity of pinnacle and conical microbial mats in the perennially ice-covered Lake Untersee, East Antarctica. Front. Microbiol. 11, 607251 (2020).
Marsh, N. B. et al. Sources of solutes and carbon cycling in perennially ice-covered Lake Untersee. Antarctica. Sci. Rep. 10, 12290 (2020).
Lyons, W. B. et al. The Mcmurdo Dry Valleys long‐term ecological research program: new understanding of the biogeochemistry of the Dry Valley lakes: A review. Polar Geogr. 25, 202–217 (2001).
Levy, J. S., Fountain, A. G., Gooseff, M. N., Welch, K. A. K. & Lyons, W. B. Water tracks and permafrost in Taylor Valley, Antarctica: extensive and shallow groundwater connectivity in a cold desert ecosystem. Geol. Soc. Am. Bull. 123, 2295–2311 (2011).
Bowman, J. S. et al. Microbial community dynamics in two polar extremes: The lakes of the McMurdo Dry Valleys and the West Antarctic Peninsula marine ecosystem. BioScience 66, 829–847 (2016).
Gooseff, M. N. et al. Decadal ecosystem response to an anomalous melt season in a polar desert in Antarctica. 1, 1334–1338 (2017).
Lawrence, J. P., Doran, P. T., Winslow, L. A. & Priscu, J. C. Subglacial brine flow and wind-induced internal waves in Lake Bonney. Antarctica. Antarct. Sci. 32, 223–237 (2020).
Faucher, B., Lacelle, D., Marsh, N. B., Fisher, D. A. & Andersen, D. T. Ice-covered ponds in the Untersee Oasis (East Antarctica): Distribution, chemical composition, and trajectory under a warming climate. Arctic, Antarct. Alp. Res. 53, 324–339 (2021).
Faucher, B. et al. Glacial lake outburst floods enhance benthic microbial productivity in perennially ice-covered Lake Untersee (East Antarctica). Commun. Earth Environ. 2, 211 (2021).
Lyons, W. B., Laybourn-Parry, J., Welch, K. A., & Priscu, J. C. Antarctic lake systems and climate change. In Trends in Antarctic terrestrial and limnetic ecosystems: Antarctica as a global indicator (eds. Bergstrom, D., Conveyand, M. P. A. & Huiskes, H. L.) 273-295 (Springer, Dordrecht, The Netherlands 2006).
Mikucki, J., Lyons, W. B., Hawes, I., Lanoil, B. D., & Doran, P. T. Saline lakes and ponds in the McMurdo Dry Valleys: ecological analogs to Martian paleolake environments. In Life in Antarctic deserts and other cold dry environments: astrobiological analogs (eds. Doran, P. T., Lyons, W. B. & McKnight, D. M.) 160–194 (Cambridge University Press 2010).
Takacs-Vesbach, C. et al. Factors promoting microbial diversity in the McMurdo Dry Valleys, Antarctica. In Life in Antarctic deserts and other cold dry environments: astrobiological analogs (eds. Doran, P. T., Lyons, W. B. & McKnight, D. M.) 221-257 (Cambridge University Press 2010).
Dieser, M. et al. Viable microbes in ice: application of molecular assays to McMurdo Dry Vally lake ice communities. Anarctic Sci 22, 470–476 (2010).
Laybourn-Parry J. & Wadham J. L. Antarctic Lakes. (2014) Oxford University Press.
Sumner, D. et al. Antarctic microbial mats: A modern analog for Archean lacustrine oxygen oases. Geology 43, 887–890 (2015).
Jungblut, A. D. et al. Microbial mat communities along an oxygen gradient in a perenially ice-covered Antarctic lake. Appl. Environ. Microbiol. 82, 620–630 (2016).
Kwon, M. et al. Niche specialization of bacteria in permanently ice-covered lakes of the McMurdo Dry Valleys, Antarctica. Environ. Microbiol. 19, 2258–2271 (2017).
Dillon, M. L. et al. Energetic and environmental constraints on the community structure of benthic microbial mats in Lake Fryxell, Antarctica. FEMS Microbiol, Ecol. 96, fiz207 (2020).
Dillon, M. L. et al. Environmental control on the distribution of metabolic strategies of benthic microbial mats in Lake Fryxell, Antarctica. PLoS One 15, e0231053 (2020).
Zoumplis, A. et al. Impact of meltwater flow intensity on the spatiotemporal heterogeneity of microbial mats in the McMurdo Dry Valleys, Antarctica. ISME Comms. 3, 3 (2023).
Doran, P. T., Fritsen, C. H., McKay, C. P., Priscu, J. C. & Adams, E. E. Formation and character of an ancient 19-m ice cover and underlying trapped brine in an “ice-sealed” east Antarctic lake. Proc. Natl. Acad. Sci. 100, 26–31 (2003).
Pavankumar, T. L., Mittal, P. & Hallsworth, J. E. Molecular insights into the ecology of a psychrotolerant Pseudomonas syringae. Environ. Microbiol. 23, 3665–3681 (2021).
Chinn, T. J. H. Glacial History and Glaciology of Terra Nova Bay Area. (1985).
French, H. M. & Guglielmin, M. Cryogenic Weathering of Granite, Northern Victoria Land, Antarctica. Permafr. Periglac. Process. 11, 305–314 (2000).
French, H. M. & Guglielmin, M. Frozen ground phenomena in the vicinity of the Terra Nova bay, Northern Victoria land, Antarctica: A preliminary report. Geogr. Ann. Ser. A Phys. Geogr. 82, (2000).
Urbini, S. et al. Multi-Temporal investigation of the Boulder Clay Glacier and Northern Foothills (Victoria Land, Antarctica) by integrated surveying techniques. Remote Sens. 11, 1501 (2019).
Guglielmin, M., Lewkowicz, A. G., French, H. M. & Strini, A. Lake‐ice blisters, terra nova bay area, northern victoria land, antarctica. Geogr. Ann. Ser. A, Phys. Geogr. 91, 99–111 (2009).
Forte, E., Dalle Fratte, M., di Rosamarina, M. & Guglielmin, M. Pressurized brines in continental Antarctica as a possible analogue of Mars. Sci. Rep. 6, 33158 (2016).
Chinn, T. J. H., Whitehouse, I. E. & Höfle, H.-C. Report on a reconnaissance of the glaciers of Terra Nova Bay Area. Geol. Jahrbuch. R. E, Geophys. E 38, 299–319 (1989).
Lozej, A. et al. Radio-echo soundings of Enigma Lake (Northern Foothills, Victoria Land, Antarctica). Mem. della Soc. Geol. Ital. 46, 103–115 (1992).
Baroni, C. et al. Mount Melbourne Quadrangle, Victoria Land, Antarctica 1:250,000 (Antarctic Geomorphological and Glaciological Map Series). In Fluctuations of glaciers 8, 38-40 (ICSU[FAGS]/IUGG [IACS]/UNEP/UNESCO/WMO 2005).
Michaud, A. B. et al. Environmentally clean access to Antarctic subglacial aquatic environments. Antarct. Sci. 32, 329–340 (2020).
Zirizzotti, A., Cafarella, L. & Urbini, S. Ice and bedrock characteristics underneath Dome C (Antarctica) from radioecho sounding data analysis. IEEE Trans. Geosci. Remote Sens. 50, 37–43 (2012).
Faucher, B. Hydrochemistry of ice-covered lakes and ponds in the Untersee Oasis (Queen Maud Land, Antarctica). (Faucher, B. (2021). Hydrochemistry of ice-covered lakes and ponds in the Untersee Oasis (Queen Maud Land, Antarctica) (Doctoral dissertation, Université d’Ottawa/University of Ottawa 2021).
Rusydi, A. F. Correlation between conductivity and total dissolved solid in various type of water: A review. IOP Conf. Ser. Earth Environ. Sci. 118, 12019 (2018).
Porcino, N., Cosenza, A. & Azzaro, M. A review on the geochemistry of lakes in Victoria Land (Antarctica). Chemosphere vol. 251 at https://doi.org/10.1016/j.chemosphere.2020.126229 (2020).
Gragnani, R. et al. Origins of the ground ice in the ice-free lands of the Northern Foothills (Northern Victoria Land, Antarctica). in (Centre d’études nordiques, Université Laval, 1998).
Carmignani, L. et al. Geology of the Wilson Terrane in the area between David and Mariner Glaciers, Victoria Land (Antarctica). in (1987).
Libera, V. Osservazioni fisico-limnologiche su un lago Antartico nell’ambito di una ricognizione dei corpi d’acqua dolce nell’area di Baia Terra Nova. in Proceedings of the seminar ‘“Role of remote areas in the study of global changes.”’) Pp. 133-139. Rome: Italian National Research Council (1993).
Andersen, D. T., Sumner, D. Y., Hawes, I., Webster-Brown, J. & Mckay, C. P. Discovery of large conical stromatolites in Lake Untersee, Antarctica. Geobiology 9, 280–293 (2011).
Craig, H. Isotopic variations in meteoric waters. Science. 133, 1702–1703 (1961).
Masson-Delmotte, V. et al. A Review of Antarctic Surface Snow Isotopic Composition: Observations, Atmospheric Circulation, and Isotopic Modeling. J. Clim. 21, 3359–3387 (2008).
Fernandoy, F. et al. New insights into the use of stable water isotopes at the northern Antarctic Peninsula as a~tool for regional climate studies. Cryosph. 12, 1069–1090 (2018).
Christner, B. C. et al. A microbial ecosystem beneath the West Antarctic ice sheet. Nature 512, 310–313 (2014).
Cremisini, C., Gianelli, G., Mussi, M. & Torcini, S. Geochemistry and isotope chemistry of surface waters and geothermal manifestations at Terra Nova Bay,(Victoria Land, Antarctica). Mem. della Soc. Geol. Ital. 463, 475 (1991).
Parker, B. C., Simmons, G. M. Jr., Seaburg, K. G., Cathey, D. D. & Allnutt, F. C. T. Comparative ecology of plankton communities in seven Antarctic oasis lakes. J. Plankton Res. 4, 271–286 (1982).
Allwood, A. C., Walter, M. R., Kamber, B. S., Marshall, C. P. & Burch, I. W. Stromatolite reef from the Early Archaean era of Australia. Nature 441, 714–718 (2006).
Allwood, A. C., Walter, M. R., Burch, I. W. & Kamber, B. S. 3.43 billion-year-old stromatolite reef from the Pilbara Craton of Western Australia: ecosystem-scale insights to early life on Earth. Precambrian Res. 158, 198–227 (2007).
McKay, C. P., Andersen, D. & Davila, A. Antarctic environments as models of planetary habitats: University Valley as a model for modern Mars and Lake Untersee as a model for Enceladus and ancient Mars. Polar J. 7, 303–318 (2017).
McKay, C. P. Exobiology and future Mars missions: The search for Mars’ earliest biosphere. Adv. Sp. Res. 6, 269–285 (1986).
Wharton, R. A. Jr., Parker, B. C. & Simmons, G. M. Jr Distribution, species composition and morphology of algal mats in Antarctic dry valley lakes. Phycologia 22, 355–365 (1983).
Kaspar, M. et al. Bryum Hedw. Collected from Lake Vanda, Antarctica. Bryologist 85, 424–430 (1982).
Love, F. G., Simmons, G. M., Parker, B. C., Wharton, R. A. & Seaburg, K. G. Modern conophyton‐like microbial mats discovered in Lake Vanda, Antarctica. Geomicrobiol. J. 3, 33–48 (1983).
Hawes, I., Moorhead, D., Sutherland, D., Schmeling, J. & Schwarz, A.-M. Benthic primary production in two perennially ice-covered Antarctic lakes: patterns of biomass accumulation with a model of community metabolism. Antarct. Sci. 13, 18–27 (2001).
Wharton, R. A. Jr., Meyer, M. A., McKay, C. P., Mancinelli, R. L. & Simmons, G. M. Jr. Sediment oxygen profiles in a super‐oxygenated antarctic lake. Limnol. Oceanogr. 39, 839–853 (1994).
Hirose, Y. et al. Investigating algal communities in lacustrine and hydro-terrestrial environments of East Antarctica using deep amplicon sequencing. Microorganisms 8, 497 (2020).
Lozupone, C. & Knight, R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71, 8228–8235 (2005).
Ricotta, C. & Podani, J. On some properties of the Bray-Curtis dissimilarity and their ecological meaning. Ecol. Complex. 31, 201–205 (2017).
Liu, C., Cui, Y., Li, X. & Yao, M. Microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 97, fiaa255 (2021).
Bielewicz, S. et al. Protist diversity in a permanently ice-covered Antarctic lake during the polar night transition. ISME J. 5, 1559–1564 (2011).
Vincent, W. F. Evolutionary origins of Antarctic microbiota: invasion, selection and endemism. Antarct. Sci. 12, 374–385 (2000).
Singh, S. M., & Elster, J. Cyanobacteria in Antarctic lake environments: a mini-review. In Algae and Cyanobacteria in Extreme Environments (ed. Seckbach, J.) 11, 303-320 (Springer: Dordrecht, The Netherlands, 2007).
Lyon, B. R. & Mock, T. Polar microalgae: new approaches towards understanding adaptations to an extreme and changing environment. Biology 3, 56–80 (2014).
Soo, R., Woodcroft, B., Parks, D., Tyson, G. & Philip, H. Back from the dead; the curious tale of the predatory cyanobacterium Vampirovibrio chlorellavorus. PeerJ 3, e968 (2015).
Grettenberger, C. L. et al. A phylogenetically novel cyanobacterium most closely related to Gloeobacter. ISME J. 14, 2142–2152 (2020).
Taton, A., Grubisic, S., Brambilla, E., De Wit, R. & Wilmotte, A. Cyanobacterial diversity in natural and artificial microbial mats of Lake Fryxell (McMurdo Dry Valleys, Antarctica): a morphological and molecular Approach. Appl. Environ. Microbiol. 69, 5157–5169 (2003).
Hawes, I., Jungblut, A. D., Obryk, M. K. & Doran, P. T. Growth dynamics of a laminated microbial mat in response to variable irradiance in an Antarctic lake. Freshw. Biol. 61, 396–410 (2016).
Mosier, A. C., Murray, A. E. & Fritsen, C. H. Microbiota within the perennial ice cover of Lake Vida, Antarctica. FEMS Microbiol. Ecol. 59, 274–288 (2007).
Murray, A. E. et al. Microbial life at −13 °C in the brine of an ice-sealed Antarctic lake. Proc. Natl. Acad. Sci. USA. 109, 20626–20631 (2012).
Nakai, R. et al. Microflorae of aquatic moss pillars in a freshwater lake, East Antarctica, based on fatty acid and 16S rRNA gene analyses. Polar Biol. 35, 425–433 (2012).
Tian, R. et al. Small and mighty: adaptation of superphylum Patescibacteria to groundwater environment drives their genome simplicity. Microbiome 8, 1–15 (2020).
Castelle, C. J. & Banfield, J. F. Major new microbial groups expand diversity and alter our understanding of the tree of life. Cell 172, 1181–1197 (2018).
Brown, C. T. et al. Unusual biology across a group comprising more than 15% of domain Bacteria. Nature 523, 208–211 (2015).
Yakimov, M. M. et al. Cultivation of a vampire: ‘Candidatus Absconditicoccus praedator’. Environ. Microbiol. 24, 30–49 (2022).
Chen, X. et al. Candidatus Nealsonbacteria” are likely biomass recycling ectosymbionts of methanogenic archaea in a stable benzene-degrading enrichment culture. Appl. Environm. Microbiol. 89, e00025–23 (2023).
Hug, L. A. et al. A new view of the tree of life. Nat. Microbiol. 1, 1–6 (2016).
Alekseev, I., Zverev, A. & Abakumov, E. Microbial communities in permafrost soils of Larsemann Hills, Eastern Antarctica: environmental controls and effect of human impact. Microorganisms 8, 1202 (2020).
Ortiz, M. et al. Multiple energy sources and metabolic strategies sustain microbial diversity in Antarctic desert soils. Proc. Natl. Acad. Sci. 118, e2025322118 (2021).
Guglielmin, M. et al. A possible unique ecosystem in the endoglacial hypersaline brines in Antarctica. Sci. Rep. 13, 177 (2023).
Luef, B. et al. Diverse uncultivated ultra-small bacterial cells in groundwater. Nat. Commun. 6, 6372 (2015).
Proctor, C. R. et al. Phylogenetic clustering of small low nucleic acid-content bacteria across diverse freshwater ecosystems. ISME J. 12, 1344–1359 (2018).
Cabello-Yeves, P. et al. Microbiome of the deep Lake Baikal, a unique oxic bathypelagic habitat. Limnol. Oceanogr. 65, 1471–1488 (2019).
Baricz, A. et al. Spatio-temporal insights into microbiology of the freshwater-to-hypersaline, oxic-hypoxic-euxinic waters of Ursu Lake. Environ. Microbiol. 23, 3523–3540 (2021).
Bor, B. et al. Insights obtained by culturing Saccharibacteria with their bacterial hosts. J. Dent. Res. 99, 685–694 (2020).
Cross, K. L. et al. Targeted isolation and cultivation of uncultivated bacteria by reverse genomics. Nat. Biotechnol. 37, 1314–1321 (2019).
He, X. et al. Cultivation of a human-associated TM7 phylotype reveals a reduced genome and epibiotic parasitic lifestyle. Proc. Natl. Acad. Sci. 112, 244–249 (2015).
Moreira, D., Zivanovic, Y., López-Archilla, A. I., Iniesto, M. & López-García, P. Reductive evolution and unique predatory mode in the CPR bacterium Vampirococcus lugosii. Nat. Commun. 12, 2454 (2021).
Li, Y. et al. Thermal heads for melt drilling to subglacial lakes: design and testing. Astrobiology 20, 142–156 (2020).
Makinson, K. et al. Clean subglacial access: Prospects for future deep hot-water drilling. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 374, 20140304 (2016).
Lupini, G., Proia, L., Di Maio, M., Amalfitano, S. & Fazi, S. CARD–FISH and confocal laser scanner microscopy to assess successional changes of the bacterial community in freshwater biofilms. J. Microbiol. Methods 86, 248–251 (2011).
Fazi, S. et al. High concentrations of dissolved biogenic methane associated with cyanobacterial blooms in East African lake surface water. Commun. Biol. 4, 845 (2021).
Herlemann, D. P. R. et al. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 5, 1571–1579 (2011).
Caporaso, J. G. et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624 (2012).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Open J. Stat. 13, (2023).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Cordone, A. et al. Bacterioplankton diversity and distribution in relation to phytoplankton community structure in the Ross Sea surface waters. Front. Microbiol. 13, (2022).
Crisafi, F. et al. Bacterial biofilms on medical masks disposed in the marine environment: a hotspot of biological and functional diversity. Sci. Total Environ. 837, (2022).
McMurdie, P. J. & Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8, e61217 (2013).
Shetty, S. & Lahti, L. Microbiome data science. J. Biosci. 44, 1–6 (2019).
Gu, Z., Gu, L., Eils, R., Schlesner, M. & Brors, B. Circlize implements and enhances circular visualization in R. Bioinformatics 30, 2811–2812 (2014).
Clarke, K. R. Non‐parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18, 117–143 (1993).
Acknowledgements
The authors thank the logistical and technical support of the Italian scientific base MZS for the qualified handling of our equipment during the campaign and for high-performance technical assistance in increasing the efficiency of thermal ice-penetration devices and their adaption to harsh climatic conditions occurred at the field camp during sampling. In addition, the authors thank Agnese Piacentini and the Advanced Centre for Microscopy “P. Albertano” of Tor Vergata University of Rome for CLSM analyses. This study was funded by the National Antarctic Research Program (Programma Nazionale di Ricerche in Antartide or PNRA) (grant no. PNRA16_00121), administered by the Italian Ministry of Education and Research. Thermal drilling approaches, used in this study, known as ‘clean hot water drills’, are fully compliant with the SCAR Code of Conduct for Subglacial Research and no specific sampling permit was required from the PNRA. Partially, this study was additionally supported by a grant from the FUTURENZYMES Project (Contract 101000327), funded by the European Union’s Horizon 2020 Research Program.
Author information
Authors and Affiliations
Contributions
Francesco Smedile: Conceptualization (equal); funding acquisition (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Violetta La Cono: Data curation (equal); investigation (equal); methodology (equal); writing – review and editing (supporting). Stefano Urbini: Conceptualization (equal); funding acquisition (equal); resources (equal); data curation (equal); formal analysis (equal); methodology (equal); writing – review and editing (equal). Giovanni Benedetti: Formal analysis (equal); methodology (equal). Gina La Spada: Data curation (equal); formal analysis (equal); methodology (equal); software (equal); visualization (equal). Francesca Crisafi: Data curation (equal); formal analysis (equal); methodology (equal); visualization (equal). Maurizio Azzaro: Supervision (equal); validation (equal); writing – review and editing (equal). Nunziatina Porcino: Formal analysis (equal); investigation (equal); validation (equal); writing – original draft (equal); writing – review and editing (equal). Stefano Fazi: Formal analysis (equal); validation (equal); writing – review and editing (equal). Stefano Amalfitano: Formal analysis (equal); methodology (equal); validation (equal); writing – review and editing (equal). Stefania Venturi: Formal analysis (equal); methodology (equal); validation (equal). Franco Tassi: Formal analysis (equal); methodology (equal); validation (equal); writing – review and editing (equal). Orlando Vaselli: Formal analysis (equal); methodology (equal); validation (equal); writing – review and editing (equal). Michael T. Madigan: Conceptualization (equal); writing – original draft (equal); writing – review and editing (equal). John E. Hallsworth: Conceptualization (equal); writing – original draft (equal); writing – review and editing (equal). Michail M. Yakimov: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); resources (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Shujuan Zhang, Somaparna Ghosh, Heike Langenberg. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Smedile, F., La Cono, V., Urbini, S. et al. The perennially ice-covered Lake Enigma, Antarctica supports unique microbial communities. Commun Earth Environ 5, 741 (2024). https://doi.org/10.1038/s43247-024-01842-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-024-01842-5