Abstract
Oceanic submesoscale processes are believed to play a pivotal role in influencing phytoplankton growth and distribution, essentially influencing oceanic primary productivity and carbon cycling. However, our understanding of how phytoplankton respond to these dynamics remains fragmentary. Here, by combining surface drifter data and satellite observations, we show a rich geographic variability in the response of phytoplankton to submesoscale ageostrophic events over the global ocean. Substantial phytoplankton biomass and chlorophyll enrichments are observed during submesoscale processes in mid-high latitude regions and coastal upwelling systems. However, negligible phytoplankton biomass increases with notable chlorophyll increases are observed in the tropical ocean and subtropical gyres, suggesting that phytoplankton are likely undergoing physiological adjustments. Globally, about half of the chlorophyll growth driven by strong submesoscale ageostrophic events is due to physiological adjustments rather than biomass enrichment, calling for a reevaluation of the effects of submesoscale processes on oceanic productivity and carbon cycling.
Similar content being viewed by others
Introduction
Phytoplankton contribute to more than half of the primary production on the earth, forming the base of marine food webs and providing essential ecological functions for most marine life1,2. Typically, phytoplankton abundance and physiological states are sensitive to changes in physical and biogeochemical environments triggered by ocean dynamics at timescales of several days3. On one hand, the growth rate of phytoplankton can increase rapidly within a day when nutrient-replete water is transported into the sunlit ocean, resulting in phytoplankton blooms with substantial enhancements in oceanic primary productivity4. On the other hand, phytoplankton also adjust their cellular pigmentation in response to variations in light, nutrients, and temperature. These adjustments optimize photosynthesis, leading to apparent chlorophyll (Chl) variations without changes in phytoplankton biomass5,6,7,8. A full understanding of these phytoplankton responses to ocean dynamics is of great importance for a comprehensive understanding of their roles in regulating oceanic productivity and ecosystem functioning from local to global scales.
Submesoscale processes are widely documented as important ocean dynamics that alter the distribution and productivity of phytoplankton in the upper ocean. These dynamics have spatial scales of O (0.1–10) km. The timescales on which they act are of the order of O (0.1–10) days, similar to those of phytoplankton growth9,10,11. They can break down the geostrophic balance that suppresses vertical motions and support vertical velocities of 10–100 m day−1, playing a critical role in modulating phytoplankton living conditions such as nutrient supply and light availability12,13,14. The primary processes to generate submesoscale biological landscapes can be classified into the passive, the active, and the reactive15. The passive is the redistribution of phytoplankton by horizontal and vertical submesoscale velocities, with a negligible net impact on phytoplankton abundance16,17,18. The active is the change in phytoplankton growth rate caused by the vertical transport of nutrients upward into (or downward out of) the euphotic zone19. The reactive is the variation in the planktonic ecosystem due to changes in light or other living conditions, including changes in cellular pigmentation for photosynthesis optimization3,20.
Submesoscale phytoplankton features are shaped by a complex interplay of these processes. Quantifying the relative importance of each process in different regions of the global ocean is a challenging task due to the lack of observations that can fully resolve submesoscale dynamics. Recent advancements in Lagrangian analysis methods, which track changes in water properties along particle trajectories, have made progress in addressing this challenge21. By combining surface drifters, satellite ocean-color, and altimetry data, Zhang et al. (ref. 22) revealed a substantial Chl increase driven by submesoscale processes. However, an increase in Chl is not always equivalent to an increase in phytoplankton abundance, as Chl is also influenced by intracellular pigmentation arising from physiological adaption5,23. A key metric for assessing this factor is the ratio between phytoplankton carbon (CPhyto) and Chl (\(\theta\) = CPhyto/Chl)5,24. A decrease in \(\theta\) indicates a relative increase in intracellular pigmentation without changes in phytoplankton biomass. According to the observations of variations in \(\theta\), studies have revealed phytoplankton physiological adjustments in response to mesoscale eddies5,25,26,27. Yet, little is known about the potential response of phytoplankton physiology to submesoscale processes, making it formidable to quantify the influence of submesoscale processes on phytoplankton productivity and global carbon cycling.
In this study, we analyze the changes in phytoplanktonic physiological states and biomass within a Lagrangian framework by projecting ocean-color satellite observations onto drifter trajectories. This approach allows us to untangle the footprint of biological processes from that of horizontal stirring and mixing19,21,22,28. We leverage this method to explore phytoplankton responses to submesoscale ageostrophic motions and show a considerable decoupling between the Lagrangian variation rates of phytoplankton Chl and biomass during strong submesoscale ageostrophic motions, with pronounced spatial and seasonal variability.
Results
Decoupling between Chl and biomass during strong submesoscale ageostrophic motions
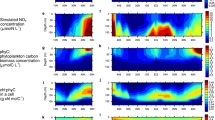
Two observational cases are presented to provide an insight into the diverse responses of phytoplankton to submesoscale processes (Fig. 1). For the case in the Arabian Sea, submesoscale filaments were associated with high Chl and CPhyto patchiness, where only minor \(\theta\) anomalies were observed simultaneously (Fig. 1b–d), demonstrating that the Chl increases were mainly due to the enrichment in phytoplankton biomass. However, for the case in the subtropical South Pacific Ocean (Fig. 1e–g), negligible CPhyto anomalies were detected along with high submesoscale Chl filaments. In comparison, negative \(\theta\) anomalies show high spatial consistency with the positive Chl anomalies, suggesting a predominant contribution of physiological adjustments to the increases in Chl. These contrasting features indicate that submesoscale Chl variations are not always representative of changes in phytoplankton abundance or productivity.
a The contribution of \(\theta\) anomaly to Chl anomaly (Supplementary Text 1). Magenta squares marked the regions of two cases. b–d Anomalies of Chl, CPhyto, and \(\theta\) between September 28, 2014, to October 7, 2014, in the Arabian Sea. e–g, same as (b–d) but in the subtropical South Pacific Ocean from August 10, 2008 to August 25, 2008. The superposed green contours are FSLE above 0.1 day–1 indicating the locations of frontal filaments on October 2, 2014 in (b–d) and on August 17, 2008 in (e–g), respectively.
To provide a global assessment of the influence of submesoscale processes on phytoplankton, we collocated ocean-color remote sensing data with simultaneously detected submesoscale ageostrophic motions estimated from surface drifters. This combination enables both the identification of strong submesoscale ageostrophic motions22,29 and a Lagrangian study of the associated variations in near-surface phytoplanktonic biomass and physiology. The results show that, at a global scale, the Lagrangian variation rate of Chl (\({r}_{{Chl}}\)) increases markedly from 0 to 1.5 × 10−2 day–1 on average with the rise in ageostrophic energy (Ea) from 0.1 m2 s−2 to 1 m2 s–2 (Fig. 2a), characterized by a nearly linear slope of 1.94 × 10–7 m–2 s. Similarly, the Lagrangian variation rate of CPhyto (\({r}_{{C}_{{Phyto}}}\)) also trends upward with the increase in Ea. These patterns indicate that strong submesoscale ageostrophic motions stimulate the growth of both phytoplankton Chl and biomass, consistent with the findings of previous studies13,30. However, the growth rate of \({r}_{{Chl}}\) is approximately twice that of \({r}_{{C}_{{Phyto}}}\) (~8.6 × 10–8 m–2 s), suggesting that only about 50% of the Chl increase is attributable to phytoplankton enrichment. Notably, the slope for \({r}_{{C}_{{Phyto}}}\) remains moderate (approximately 10–8 m–2 s) until Ea exceeds 0.6 m2 s–2. Beyond this threshold, this slope rises to approximately 9.3 × 10–8 m–2 s, indicating that a rapid biomass increase is primarily driven by more intense submesoscale ageostrophic motions. Conversely, the Lagrangian variation rate of \(\theta\) (\({r}_{\theta }\)) exhibits a declining trend with the rise in Ea, indicating that phytoplankton also undergo physiological adjustments (with an increase in cellular pigmentation) in response to strong ageostrophic motions9. This declining trend, with a slope of –8.6 × 10–8 m–2 s, generally mirrors the opposite trend of \({r}_{{C}_{{Phyto}}}\), demonstrating that about half of the Chl increase stems from physiological adjustments. The observations also show that, when Ea exceeds 0.8 m2 s–2, the downward slope of \({r}_{\theta }\) moderates to 5.7 × 10–9 m–2 s, indicating a weaker contribution of physiological adjustments than biomass growth to the increase in Chl during extremely intense submesoscale ageostrophic motions.
a Globally averaged curves of the rate of chlorophyll (\({r}_{{Chl}}\), blue), phytoplankton carbon biomass (\({r}_{{C}_{{Phyto}}}\), green), and the ratio between CPhyto and Chl (\({r}_{\theta }\), red) as functions of Ea. The shaded areas in lighter colors represent the standard error bars, respectively. The vertical dashed line denotes Ea = 0.1 m2 s–2, marking the threshold for strong submesoscale ageostrophic motions. b Histograms of \({r}_{{Chl}}\)(blue) and \({r}_{{C}_{{Phyto}}}\)(green) during strong submesoscale ageostrophic motions. b-d Geographic distributions of the mean value of (c) \({r}_{{Chl}}\), (d) \({r}_{{C}_{{Phyto}}}\) and (e) \({r}_{\theta }\) during strong submesoscale ageostrophic motions with positive \({r}_{{Chl}}\).
As shown in Fig. 2b, the distribution of \({r}_{{Chl}}\) exhibits thin tails during strong submesoscale ageostrophic motions, with a kurtosis of 26.71 and a mean value of 1.2 × 10–3 day–1. Seventeen percent of the strong submesoscale ageostrophic motions do not stimulate a positive \({r}_{{Chl}}\), potentially due to drifters encountering downwelling currents of cross-frontal circulations which pull phytoplankton downward into the dark ocean interior31. In contrast, the distribution of \({r}_{{C}_{{Phyto}}}\) has a higher kurtosis of 58.73 and a mean value of 2 × 10–4 day–1, indicating that these ageostrophic motions do not contribute to an equivalent increase in phytoplankton biomass compared to that in Chl globally. Only about 53% of the motions lead to biomass increases, supporting the notion that approximately half of the observed Chl enrichment is not attributable to biomass blooms.
Spatial and seasonal variability
Strong submesoscale ageostrophic motions that can induce Chl increases are mainly observed in the tropical ocean, the Indian Ocean, and the western basins of the Pacific and Atlantic oceans (Fig. 2c). The growth rates of Chl during these motions are similar among these regions (2.8 × 10–3 day–1 on average). However, the growth rate of phytoplankton biomass is nearly 55% lower in the tropical ocean and subtropical gyres (9 × 10–4 day–1) than in the western boundary current regions (2 × 10–3 day–1), indicating that a large portion of the Chl increase in the tropical ocean and subtropical gyres is not attributable to the increase in phytoplankton biomass (Fig. 2d). Instead, they are likely a result of the increase in cellular pigmentation, which is supported by the observed negative \({r}_{\theta }\) in these regions (–2 × 10–3 day–1 on average, Fig. 2e). In the western boundary currents, the mean of \({r}_{\theta }\) is about –1 × 10–3 day–1, implying that the role of physiological adjustments is not as important as in the tropical ocean and subtropical gyres. Some pixels in these regions show a positive average \({r}_{\theta }\), indicative of a decrease in cellular pigmentation, opposing the observed increase in Chl. This phenomenon is also detected in mid-high latitude oceans and coastal upwelling systems though available drifters are much less (Supplementary Fig. 1). In these regions, strong submesoscale ageostrophic motions trigger a phytoplankton biomass increase with an average rate of 2.8 × 10–3 day–1, close to the mean value of \({r}_{{Chl}}\), suggesting that the Chl increase during submesoscale ageostrophic motions in these regions is mainly caused by the accumulation of phytoplankton biomass.
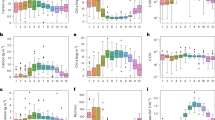
To further assess the regional variability in the sensitivity of phytoplankton response to submesoscale dynamics, we categorized the global ocean into two regions based on their physiological characteristics (Test S1, Fig. 3a). The first region, termed the biomass-dominated region, primarily includes mid-high latitude areas and coastal upwelling systems. The remaining is the physiology-dominated region, which is in turn classified into two regions by dynamic characteristics: western boundary current regions, subtropical gyres and the tropical ocean. In all of these regions, the average \({r}_{{Chl}}\) curves show a clear upward trend with increasing Ea. In the biomass-dominated region, both \({r}_{{C}_{{Phyto}}}\) and \({r}_{{Chl}}\) increase substantially, with a cosine similarity of 0.88 between the two curves (Fig. 3b). In contrast, \({r}_{\theta }\) remains relatively stable with a flat slope of –3.5 × 10–9 m–2 s, indicating that the Chl increases are primarily due to phytoplankton blooms. In subtropical gyres and the tropical ocean, conversely, \({r}_{{C}_{{Phyto}}}\) remains relatively flat, with a slope below 5.8 × 10–9 m–2 s (Fig. 3c). The response of \({r}_{\theta }\) to Ea is more notable, with a steep slope of –1.4 × 10–6 m–2 s, suggesting that Chl increases are largely driven by physiological adjustments rather than phytoplankton blooms. Only during instances of submesoscale processes with very high Ea values (above 0.7 m2 s–2) does phytoplankton biomass increase at a modest rate of 5 × 10−3 day−1. In the western boundary current region, the relationship between Ea and \({r}_{{C}_{{Phyto}}}\) contrasts with that in the subtropical gyres and the tropical ocean (Fig. 3d). As Ea is beyond 0.13 m2 s–2, \({r}_{{C}_{{Phyto}}}\) shows a rise, with a slope of 6.9 × 10–9 m–2 s, which is about half of the \({r}_{{Chl}}\) slope. Meanwhile, \({r}_{\theta }\) decreases with Ea with an opposite slope of –7.17 × 10–9 m–2 s, indicating that the Chl increases result from a combination of physiological adjustments and biomass growth during strong submesoscale ageostrophic motions in this region.
a Definition of the oceanic regions: biomass-dominated region, physiology-dominated region (consisting of subtropical gyres and the tropical ocean, and western boundary current region). b-d Fitting curves of \({r}_{{Chl}}\) (blue), \({r}_{{C}_{{Phyto}}}\) (green), and \({r}_{\theta }\) (red) as functions Ea for (b) Biomass-dominated region, (c) Subtropical gyres and the tropical ocean, and (d) Western boundary current region. Error bars are computed using the same method as in Fig. 2a.
The spatial distribution of \({r}_{{Chl}}\) remains relatively uniform throughout winter (December to March in the Northern Hemisphere, June to September in the Southern Hemisphere) and summer (June to September in the Northern Hemisphere, December to March in the Southern Hemisphere), except that \({r}_{{Chl}}\) values in the winter (averaging 2.5 × 10–3 day–1) are systemically higher than those in the summer (averaging 2.2 × 10–3 day–1, Fig. 4a, d). However, phytoplankton biomass responses to strong submesoscale ageostrophic motions demonstrate tremendous seasonal variations, particularly in the western boundary current region. During winter, phytoplankton biomass grows at an average rate of 2.9 × 10–3 day–1, aligning closely with \({r}_{{Chl}}\) (Fig. 4e). Both \({r}_{{Chl}}\) and \({r}_{{C}_{{Phyto}}}\) show a similar upward trend with increasing Ea (cosine similarity is 0.86 between their fitting curves, Supplementary Fig. 2). In contrast, \({r}_{{C}_{{Phyto}}}\) is 1.7 × 10–3 day–1 in summer, roughly half of the winter counterpart (Fig. 4b). Additionally, \({r}_{\theta }\) witnesses a positive-negative transition from winter to summer. This seasonal variation suggests that, in the western boundary current region, the main driver of Chl variation shifts from biomass blooms in winter to physiological adjustments in summer. In subtropical gyres and the tropical ocean, both biomass and \(\theta\) responses to strong submesoscale ageostrophic motions exhibit minimal seasonal variation. In the biomass-dominated region, while both \({r}_{{Chl}}\) and \({r}_{{C}_{{Phyto}}}\) exhibit a winter-peaked seasonality, \({r}_{\theta }\) maintains a positive average level, indicating that Chl increases are primarily driven by biomass growth in both seasons.
a-c Geographic distributions of (a) \({r}_{{Chl}}\), (b) \({r}_{{C}_{{Phyto}}}\) and (c) \({r}_{\theta }\) when strong submesoscale ageostrophic motions happen with positive \({r}_{{Chl}}\) in summer (June to September in the Northern Hemisphere, December to March in the Southern Hemisphere). d–f Same as (a–c) but for winter (December to March in the Northern Hemisphere, June to September in the Southern Hemisphere).
Discussion
Several studies have suggested that the shallow penetration of vertical velocity at some submesoscale fronts may not apparently promote the strength of vertical nutrient fluxes and may not drive surface blooms3,15. It is difficult to directly measure the vertical nutrient fluxes by submesoscale motions, thus we conducted a global estimate of the distance between the mixed layer and seasonal nutricline during strong submesoscale ageostrophic motions. The mixed layer depth (MLD) is estimated as the depth where density differs from the 10 dbar value by 0.03 kg m−3 from over 2 million quality-controlled CTD profiles between 1993 and 2019 collected by the World Ocean Database32,33. The seasonal nutricline depth is estimated as the depth within the upper 400 m where the gradient of nitrate concentration (from World Ocean Atlas) is maximal. As these discrete profile data do not support the estimation of Ea, we used geostrophic strain, computed from the altimetry products: \({S}_{g}=\sqrt{{\left(\frac{\partial {u}_{g}}{\partial x}-\frac{\partial {v}_{g}}{\partial y}\right)}^{2}+{\left(\frac{\partial {v}_{g}}{\partial x}+\frac{\partial {u}_{g}}{\partial y}\right)}^{2}.}\) We processed linear interpolations of Sg to match the spatial and temporal positions of the profiles. Those profiles with Sg above 1 × 10–5 s–2 are considered indicative of strong submesoscale ageostrophic motions since Ea is above 0.1 m2 s–2 on average when Sg exceeds this threshold22.
A crucial factor influencing vertical nutrient fluxes is the depth to which vertical velocities penetrate the nutricline15. In the tropical ocean and subtropical gyres, multi-year observations indicate a deeper nutricline compared to other regions (Supplementary Fig. 3). Also, submesoscale vertical velocities in these regions tend to penetrate shallowly34. These characteristics may lead to vertical velocities that are largely confined to the mixed layer above the nutricline35. Long-term observations also reveal that the seasonal nutricline is often deeper than the mixed layer during strong submesoscale ageostrophic motions (Supplementary Fig. 4a–c), implying that submesoscale processes contribute minimally to vertical nutrient flux15,36,37. In such a scenario, phytoplankton biomass may not increase rapidly in the near-surface (Fig. 5a). However, on the one hand, these vertical velocities might transport subsurface low-light acclimated phytoplankton (characterized by small \(\theta\) values) to the near-surface15,38. On the other hand, the vertical motions may expand the depth to which surface phytoplankton are mixed, resulting in shortened residence time of phytoplankton in the light-sufficient euphotic layer and thus decreased daily mean light exposure. This decrease in light exposure may stimulate the synthesis of Chl in phytoplankton cells to enhance light harvesting efficiency, thus leading to a decrease in \(\theta\)9.
In the biomass-dominated region, previous modeling studies39 indicate that submesoscale vertical motions are intense and extend to great depths (reaching around -500 m). Additionally, convective mixing and terrestrial nutrient input contribute to a shallow nutricline40 (Supplementary Fig. 3). These factors likely result in vertical velocities extending well below the nutricline (typically negative distance in Supplementary Fig. 4a), consistent with many case studies19,41,42. As a result, submesoscale processes often facilitate nutrient transport from the interior to the near-surface. In such circumstances (Supplementary Fig. 4b), phytoplankton growth responds to upward nutrient inputs driven by submesoscale vertical velocities over hours to days, leading to an apparent increase in phytoplankton biomass with higher Chl levels43.
In the western boundary current region, both vertical velocities and nutricline depth exhibit tremendous seasonal variations. Submesoscale vertical velocities tend to be larger and penetrate deeper in winter than in summer44. Meanwhile, the nutricline is shallower during winter compared to summer45. This contrasting seasonality suggests that submesoscale vertical velocities are more likely to reach the nutricline in winter, whereas they typically do not penetrate it in summer (the distance between the mixed layer and the seasonal nutricline is usually negative in winter but positive in summer, Supplementary Fig. 4b, c). This indicates that nutrient transport efficiency to the euphotic layer may be enhanced in winter, as enhanced nutrient fluxes contribute to biomass blooms and elevated Chl levels as aforementioned (Supplementary Fig. 4b). In summer, nutrient fluxes are minimally enhanced and thus do not support substantial biomass increases yet Chl still rises due to light adaption, similar to potential patterns in the subtropical gyres and the tropical ocean (Supplementary Fig. 4a).
We should note that variations in \(\theta\) are not only influenced by light exposure, but also by other factors, such as nutrient availability, and community composition9,46. Although the individual contributions of these factors are difficult to disentangle and quantify, recent studies have proposed a photoacclimation model that can separate the light-driven component from the others5 (Supplementary Text 2). The outputs of this model show negative \(\theta\) anomalies in tropical and subtropical gyres and western boundary current regions (approximately –0.22 on average, Supplementary Fig. 5a), geographically consistent with the satellite observations (Supplementary Fig. 5b). For ∼70% of these regions, the ratio of \(\theta\) anomalies between the model and satellite observation exceeds 50%, suggesting the dominance of light-driven physiological adjustments in the response of phytoplankton to submesoscale processes (Supplementary Fig. 5c). The remaining \(\theta\) anomalies might be generated from the stirring of low-\(\theta\) phytoplankton or stimulated by other reactive mechanisms. For instance, predators affect phytoplankton biomass through hunting behaviors47,48, and also exhibit preferences for certain phytoplankton species, which modulates differential loss rates among phytoplankton species4. These predator-prey interactions can lead to shifts in community composition, potentially affecting \(\theta\)49.
Assessing how vertical motions of submesoscale ageostrophic currents affect phytoplankton biomass and their physiological responses demands high-temporal resolution of depth-resolved biogeochemical observations. One possible way to achieve this is in situ observations from BGC-Argo. By evaluating the time series of BGC-Argo observations, recent studies have revealed phytoplankton biomass increases stimulated by ocean fronts in mid-high latitude regions19. Here we provide two observational cases from BGC-Argo demonstrating both phytoplankton biomass and physiological responses to submesoscale vertical motions. The first float (no. 4903274) was deployed in the Gulf of Mexico (64.1°W and 31.8°N), with a sampling rate of one profile per day during the winter of 2021 (Supplementary Fig. 6). During the observation period between January 19th and January 23rd, 2022, anomalous high horizontal buoyancy gradients were detected in the upper 200 m, with the average of absolute values of 2.8 × 10–7 s–2 (Fig. 6a), indicative of an intensive submesoscale event50. During this event, a rapid shoaling of the mixed layer from –100 m to –20 m was observed between January 19th and January 21st, accompanied with a notable nutrient upwelling from below –150 m to –50 m (Fig. 6b). This upward transport of nutrients into the euphotic layer dramatically promoted the growth of phytoplankton, resulting in a doubling of both the concentration and thickness of the subsurface phytoplankton biomass maximum layer and Chl maximum layer (Fig. 6c, d). Then, a deepening in the mixed layer by 50 m was observed between January 21st and January 23rd. This deepening in mixing transports subsurface phytoplankton to the near-surface51, resulting in an increase in surface phytoplankton biomass and Chl of 150%. In comparison, few changes in \(\theta\) values were observed during this whole process (Fig. 6e), suggesting a dominant response of phytoplankton abundance to submesoscale dynamics, in agreement with previous observations19.
a-e The observations of (a) horizontal buoyancy gradient (computed and normalized following the method proposed by Siegelman et al., ref. 50), (b) nitrate (normalized), (c) CPhyto, (d) Chl, and (e) \(\theta\) from the first BGC-Argo float (float1, no. 4903274). f–j Same as (a–e) but from the second float (float2, no. 2902755, right).
The second float (no. 2902755), with a sampling interval of 2 days, meandered in the Kuroshio (160.6°E, 40.8°N) during the summer of 2019 (Supplementary Fig. 6) when the mixed layer (~-30 m) was much shallower and submesoscale motions were weaker than in the winter Gulf of Mexico (Fig. 6f). During the two submesoscale events observed between August 6th and 8th and between August 25th and September 1st, the vertical nutrient flux was not intense enough to promote the growth of phytoplankton in the subsurface phytoplankton maximum layer (Fig. 6g, h). Instead, phytoplankton in this layer were uplifted to the near surface, resulting in an increase in surface phytoplankton biomass (by 80%, Fig. 6h). However, the increase in surface Chl (reaching up to 400%, Fig. 6i) was much greater than the increase in phytoplankton biomass, suggesting an increase in phytoplankton cellular pigmentation in response to decreased light exposure due to enhanced submesoscale mixing. This scenario was supported by the decrease in \(\theta\) both near the surface and in the subsurface phytoplankton maximum layer (Fig. 6j), which provides a direct demonstration of a dominant physiological response of phytoplankton to submesoscale dynamics.
Although these BGC-Argo observations provide valuable insight into the rich phytoplankton response to submesoscale processes, the currently accumulated BGC-Argo data are too scarce to support a global analysis52. Moreover, most of the deployed BGC-Argo floats have a relatively low temporal resolution of ~10 days, hindering their ability to capture submesoscale variations with a timescale of O (0.1–10) days. More depth-resolved high-temporal resolution biogeochemical observations, combined with high-resolution biogeochemical models, are needed to better understand the phytoplankton responses to vertical motions during these processes.
Summary
Uncovering phytoplankton responses to submesoscale events is of great importance for grasping the role of ocean dynamics in regulating oceanic primary productivity and carbon cycling. In this study, we reveal a rich geographic variability in phytoplankton responses to these events over the global ocean. In the oligotrophic tropical ocean and subtropical gyres, submesoscale events are relatively weak. The relatively shallow penetration of submesoscale vertical velocities is likely difficult to bring deep nutrients upward into the euphotic zone to facilitate the growth of near-surface phytoplankton. However, the deepened submesoscale mixing might reduce the average daily light exposure for these phytoplankton, leading to an increase in cellular pigmentation to improve light-harvesting efficiency. As a consequence, apparent increases in Chl without noticeable phytoplankton growth were observed during submesoscale processes. In contrast, mid-high latitude regions and coastal upwelling systems exhibit relatively shallow nutricline and intense submesoscale events. These submesoscale vertical velocities are more likely to reach the nutricline, substantially enhancing upward nutrient fluxes to the euphotic zone, which stimulates both phytoplankton biomass and Chl growth. In western boundary current regions, submesoscale vertical velocities are largely restricted to above the nutricline during summer, resulting in minimal vertical nutrient fluxes. In winter, however, these velocities extend deeper into the nutricline, leading to substantial nutrient transport. This seasonal variation in vertical nutrient fluxes may cause a winter-peaked seasonality in biomass. The main driver of Chl variation shifts from biomass blooms in winter to physiological adjustments in summer.
This study highlights the spatial and seasonal heterogeneity of phytoplankton responses to submesoscale processes, the underpinning for which is acclimation to different changing light, nutrients, and other conditions. These findings can offer a new perspective on the role of submesoscale processes in modifying phytoplankton populations and physiological states and provide valuable insights for reconceptualizing the ecological effects of these processes. Note that the drifter trajectories and satellite observations used in this study only captured surface variations. However, surface variations in phytoplankton are also influenced by the strong vertical component of submesoscale ageostrophic motions. Thus, there is an error introduced by restricting our analysis to 2D surface trajectories. Future research should prioritize collecting vertical data by semi-Lagrangian drifters28. This is expected to provide more detailed biogeochemical information and three-dimensional trajectories of water parcels, particularly regarding the variation in phytoplankton carbon-nitrogen ratio, which will contribute to a better understanding of how submesoscale processes influence phytoplankton carbon fixation efficiency53. Further integrated studies that combine in situ biogeochemical profile observations and satellite measurements will help fully uncover the response of phytoplankton to submesoscale processes throughout the upper ocean.
Methods
Surface drifter data
The surface drifter data used in this study is provided by the Drifter Data Assembly Center (DAC) of the National Oceanic and Atmospheric Administration (NOAA). The DAC provides uniform quality control of surface velocity data measured from over 30,000 drifters, spanning from the year 1998 to 2023. The derived surface velocities have been interpolated into 6-hour intervals using the kriging method54,55. Additionally, a wind-slip correction for the velocities is processed for drogue-lost drifters, following the method described by Huang et al56.
Satellite observations
This study used the SSALTO/DUACS delay-time altimetry product from CMEMS. These data contain global gridded surface geostrophic seawater velocity anomaly with a spatial resolution of 0.25° × 0.25° and daily temporal resolution, covering the period from 1998 to 2023. Multi-satellite merged ocean color products from the GlobColour project are utilized in this study. This global daily product contains Chl and backscattering coefficients (BBP), with a spatial resolution of 4 km × 4 km from 1998 to 202357. CPhyto has historically been estimated using indirect biomass proxies58,59,60, but recent in situ measurements of CPhyto have directly verified the satellite algorithm from BBP which is applied in this study: \({C}_{{Phyto}}=13,\!000\times \left({BBP}-0.00035\right)\)5,24. Additionally, the ratio between CPhyto and Chl (\(\theta\)) is defined as: \(\theta =\frac{{C}_{{Phyto}}}{{Chl}}\). The climatological maps of Chl, CPhyto, and \(\theta\) are shown in Supplementary Fig. 7.
Lagrangian variation rate
We performed linear interpolation in both temporal and spatial dimensions to access the along-drifter-trajectory distribution of Chl, CPhyto, and \(\theta\). Their Lagrangian variation rate can be calculated along the drifter trajectory:
where \(p\) is Chl, CPhyto, or \(\theta\). \({r}_{p}\) is multiplied by \(\frac{1}{{\log }_{10}e}\). There is a vital relationship between \({r}_{{Chl}}\), \({r}_{{C}_{{Phyto}}}\) and \({r}_{\theta }\):
That means, in the Lagrangian framework, changes in Chl can register variations in biomass and physiological adjustments. In this study, \(\frac{{dp}}{{dt}}\) represents the average rate of change in \(p\) over a day, i.e., the time scale of \({r}_{p}\) is 1 day. This time scale is selected according to the characteristic time-scale of the cell division (~1 day)4. Sensitivity tests comfirm that there is not a marked change of \({r}_{p}\) computed by monthly climatology data with the rise in Ea (Supplementary Fig. 8). These results hightlight that \({r}_{p}\) computed from daily data reflects fine-scale dynamics variability rather than artifacts of large-scale features.
Ageostrophic energy and strong submesoscale ageostrophic motion
Ageostrophic energy (\({E}_{a}=\frac{1}{2}{\vec{u}}_{a}^{2}\)) has been proven effective in evaluating the surface kinetic energy of submesoscale processes22,29,61. In this equation, \({\vec{u}}_{a}=\vec{u}-{\vec{u}}_{g}-{\vec{u}}_{0}\) is defined as ageostrophic velocity, where \({\vec{u}}_{0}\) is climatological mean surface velocity computed from the drifter absolute horizontal velocity \(\vec{u}\), and \({\vec{u}}_{g}=({u}_{g},{v}_{g})\) denotes geostrophic velocity anomaly derived via linear interpolation from altimetry products. Difters met a strong submesoscale ageostrophic motion when Ea > 0.1 m2 s–222,61. When Ea is computed from CMEMS global ocean eddy-resolving reanalysis product (GLORYS12v1), the relationship between \({r}_{p}\) and Ea exhibits a pattern similar to that shown in the ‘Results’ section (Supplementary Fig. 9). These findings indicate that our results are not extremely sensitive to the velocity dataset.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The satellite-measured chlorophyll, particulate backscattering coefficients, photosynthetically active radiation and attenuation coefficient for downwelling photosynthetically active radiation data are available on GlobColour (https://hermes.acri.fr/). The altimeter products and GLORYS12v1 data can be obtained from CMEMS (https://marine.copernicus.eu/). The drifter data can be accessed from this website (https://www.aoml.noaa.gov/phod/gdp/data.php/). The WOD data were made freely available (https://www.ncei.noaa.gov/products/world-ocean-database). The climatological Nitrate and mixed layer depth data can be accessed from World Ocean Atlas (https://www.ncei.noaa.gov/access/world-ocean-atlas-2018/). BGC-Argo data can be downloaded from Biogeochemical-Argo program (https://biogeochemical-argo.org/).
Code availability
Codes for the main results are available via GitHub at https://github.com/fredette0714/Submesoscale-Phytoplankton/.
References
Buitenhuis, E. T., Hashioka, T. & Quéré, C. L. Combined constraints on global ocean primary production using observations and models. Glob. Biogeochem. Cycles 27, 847–858 (2013).
Lévy, M., Jahn, O., Dutkiewicz, S., Follows, M. J. & d’Ovidio, F. The dynamical landscape of marine phytoplankton diversity. J. R. Soc. Interface 12, 20150481 (2015).
Lévy, M. et al. The impact of fine-scale currents on biogeochemical cycles in a changing ocean. Annu. Rev. Mar. Sci. 16, 191–215 (2024).
Behrenfeld, M. J. & Boss, E. S. Resurrecting the ecological underpinnings of ocean plankton blooms. Annu. Rev. Mar. Sci. 6, 167–194 (2014).
Behrenfeld, M. J. et al. Revaluating ocean warming impacts on global phytoplankton. Nat. Clim. Change 6, 323–330 (2016).
Geider, R. J. Light and temperature Dependence of the carbon to chlorophyll a ratio in microalgae and cyanobacteria: Implications for physiology and growth of phytoplankton. N. Phytologist 106, 1–34 (1987).
Macintyre, H., Kana, T., Anning, T. & Geider, R. Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. J. Phycol. 38, 17–38 (2002).
Ryan-Keogh, T. J., Thomalla, S. J., Monteiro, P. M. S. & Tagliabue, A. Multidecadal trend of increasing iron stress in Southern Ocean phytoplankton. Science 379, 834–840 (2023).
Halsey, K. H. & Jones, B. M. Phytoplankton strategies for photosynthetic energy allocation. Annu. Rev. Mar. Sci. 7, 265–297 (2015).
McWilliams, J. C. Submesoscale currents in the ocean. Proc. R. Soc. A: Math., Phys. Eng. Sci. 472, 20160117 (2016).
Thomas, L., Tandon, A. & Mahadevan, A. Submesoscale Processes and Dynamics. Washington DC American Geophysical Union Geophysical Monograph Series 177, (2008).
Mahadevan, A. The impact of submesoscale physics on primary productivity of plankton. Ann. Rev. Mar. Sci. 8, 161–184 (2016).
Ruiz, S. et al. Effects of oceanic mesoscale and submesoscale frontal processes on the vertical transport of phytoplankton. J. Geophys. Res. Oceans 124, 5999-6014 (2019).
Zhu, R. et al. Observations reveal vertical transport induced by submesoscale front. Sci. Rep. 14, 4407 (2024).
Lévy, M., Franks, P. J. S. & Smith, K. S. The role of submesoscale currents in structuring marine ecosystems. Nat. Commun. 9, 4758 (2018).
Lehahn, Y., d’Ovidio, F., Lévy, M. & Heifetz, E. Stirring of the Northeast Atlantic spring bloom: A Lagrangian analysis based on multisatellite data. J. Geophys. Res. Oceans 112, C08005 (2007).
Lehahn, Y. et al. Dispersion/dilution enhances phytoplankton blooms in low-nutrient waters. Nat. Commun. 8, 14868 (2017).
Sergi, S. et al. Interaction of the antarctic circumpolar current with seamounts fuels moderate blooms but vast foraging grounds for multiple marine predators. Front. Mar. Sci. 7, 416 (2020).
McKee, D. C. et al. Biophysical dynamics at ocean fronts revealed by bio-argo floats. J. Geophys. Res. Oceans 128, e2022JC019226 (2023).
Guo, M., Xiu, P. & Xing, X. Oceanic fronts structure phytoplankton distributions in the central South Indian Ocean. J. Geophys. Res. Oceans 127, e2021JC017594 (2022).
Kuhn, A. M. et al. A global comparison of marine chlorophyll variability observed in Eulerian and Lagrangian perspectives. J. Geophys. Res. Oceans 128, e2023JC019801 (2023).
Zhang, Z., Qiu, B., Klein, P. & Travis, S. The influence of geostrophic strain on oceanic ageostrophic motion and surface chlorophyll. Nat. Commun. 10, 2838 (2019).
Arteaga, L., Pahlow, M. & Oschlies, A. Modeled Chl:C ratio and derived estimates of phytoplankton carbon biomass and its contribution to total particulate organic carbon in the global surface ocean. Glob. Biogeochem. Cycles 30, 1791–1810 (2016).
Behrenfeld, M. J., Boss, E., Siegel, D. A. & Shea, D. M. Carbon-based ocean productivity and phytoplankton physiology from space. Global Biogeochem. Cycles 19, GB1006 (2005).
He, Q., Zhan, H., Cai, S. & Zhan, W. Eddy-induced near-surface chlorophyll anomalies in the subtropical gyres: Biomass or physiology?. Geophys. Res. Lett. 48, e2020GL091975 (2021).
Gaube, P., Chelton, D. B., Strutton, P. G. & Behrenfeld, M. J. Satellite observations of chlorophyll, phytoplankton biomass, and Ekman pumping in nonlinear mesoscale eddies. J. Geophys. Res. Oceans 118, 6349–6370 (2013).
Gaube, P., McGillicuddy, D. J. Jr., Chelton, D. B., Behrenfeld, M. J. & Strutton, P. G. Regional variations in the influence of mesoscale eddies on near-surface chlorophyll. J. Geophys. Res. Oceans 119, 8195–8220 (2014).
Lehahn, Y., d’Ovidio, F. & Koren, I. A satellite-based Lagrangian view on phytoplankton dynamics. Annu. Rev. Mar. Sci. 10, 99–119 (2018).
Zhang, Z. & Qiu, B. Evolution of submesoscale ageostrophic motions through the life cycle of oceanic mesoscale eddies. Geophys. Res. Lett. 45, 11,847–11,855 (2018).
Lévy, M., Ferrari, R., Franks, P. J. S., Martin, A. P. & Rivière, P. Bringing physics to life at the submesoscale. Geophys. Res. Lett. 39, L14602 (2012).
Omand, M. M. et al. Eddy-driven subduction exports particulate organic carbon from the spring bloom. Science 348, 222–225 (2015).
de Boyer Montégut, C., Madec, G., Fischer, A. S., Lazar, A. & Iudicone, D. Mixed layer depth over the global ocean: An examination of profile data and a profile-based climatology. J. Geophys. Res. Oceans 109, C12003 (2004).
He, Q. et al. Thermal imprints of mesoscale eddies in the global ocean. J. Phys. Oceanogr 54, 1991-2009 (2024).
Gula, J., Taylor, J., Shcherbina, A. & Mahadevan, A. Submesoscale processes and mixing. in 181–214 (2021) https://doi.org/10.1016/B978-0-12-821512-8.00015-3.
Lévy, M. et al. Large-scale impacts of submesoscale dynamics on phytoplankton: Local and remote effects. Ocean Model. 43–44, 77–93 (2012).
Callies, J., Ferrari, R., Klymak, J. M. & Gula, J. Seasonality in submesoscale turbulence. Nat. Commun. 6, 6862 (2015).
Ramachandran, S., Tandon, A. & Mahadevan, A. Enhancement in vertical fluxes at a front by mesoscale-submesoscale coupling. J. Geophys. Res. Oceans 119, 8495–8511 (2014).
Cullen, J. J. Subsurface chlorophyll maximum layers: Enduring enigma or mystery solved?. Annu. Rev. Mar. Sci. 7, 207–239 (2015).
Klein, P. & Lapeyre, G. The oceanic vertical pump induced by mesoscale and submesoscale turbulence. Annu. Rev. Mar. Sci. 1, 351–375 (2009).
Tanioka, T. et al. Global patterns and predictors of C:N:P in marine ecosystems. Commun. Earth Environ. 3, 1–9 (2022).
Hernández-Hernández, N. et al. Drivers of plankton distribution across mesoscale eddies at submesoscale range. Front. Mar. Sci. 7, 667 (2020).
Kurian, S. et al. Role of oceanic fronts in enhancing phytoplankton biomass in the eastern Arabian Sea during an oligotrophic period. Mar. Environ. Res. 160, 105023 (2020).
Stolte, W., McCollin, T., Noordeloos, A. A. M. & Riegman, R. Effect of nitrogen source on the size distribution within marine phytoplankton populations. J. Exp. Mar. Biol. Ecol. 184, 83–97 (1994).
Su, Z., Wang, J., Klein, P., Thompson, A. F. & Menemenlis, D. Ocean submesoscales as a key component of the global heat budget. Nat. Commun. 9, 775 (2018).
Richardson, K. & Bendtsen, J. Vertical distribution of phytoplankton and primary production in relation to nutricline depth in the open ocean. Mar. Ecol. Prog. Ser. 620, 33–46 (2019).
Thompson, P. The response of growth and biochemical composition to variations in daylength, temperature, and irradiance in the marine diatom thalassiosira pseudonana (bacillariophyceae). J. Phycol. 35, 1215–1223 (1999).
Behrenfeld, M. J. Abandoning Sverdrup’s critical depth hypothesis on phytoplankton blooms. Ecology 91, 977–989 (2010).
Boss, E. & Behrenfeld, M. In situ evaluation of the initiation of the North Atlantic phytoplankton bloom. Geophys. Res. Lett. 37, L18603 (2010).
Li, Q. P., Franks, P. J. S., Landry, M. R., Goericke, R. & Taylor, A. G. Modeling phytoplankton growth rates and chlorophyll to carbon ratios in California coastal and pelagic ecosystems. J. Geophys. Res. Biogeosci. 115, G04003 (2010).
Siegelman, L. et al. Enhanced upward heat transport at deep submesoscale ocean fronts. Nat. Geosci. 13, 50–55 (2020).
Cao, H. et al. Isopycnal submesoscale stirring crucially sustaining subsurface chlorophyll maximum in ocean cyclonic eddies. Geophys. Res. Lett. 51, e2023GL105793 (2024).
Roemmich, D. et al. On the future of argo: A global, full-depth, multi-disciplinary array. Front. Mar. Sci. 6, 439 (2019).
Ayata, S.-D. et al. Phytoplankton plasticity drives large variability in carbon fixation efficiency. Geophys. Res. Lett. 41, 8994–9000 (2014).
Elipot, S. et al. A global surface drifter data set at hourly resolution. J. Geophys. Res.: Oceans 121, 2937–2966 (2016).
Lumpkin, R. & Centurioni, L. Global Drifter Program quality-controlled 6-hour interpolated data from ocean surface drifting buoys. Global set. [Dataset]. NOAA National Centers for Environmental Information. (2019) https://doi.org/10.25921/7ntx-z961.
Huang, G., Zhan, H., He, Q., Wei, X. & Li, B. A Lagrangian study of the near-surface intrusion of Pacific water into the South China Sea. Acta Oceanol. Sin. 40, 15–30 (2021).
Maritorena, S. & Siegel, D. A. Consistent merging of satellite ocean color data sets using a bio-optical model. Remote Sens. Environ. 94, 429–440 (2005).
Westberry, T., Behrenfeld, M., Siegel, D. & Boss, E. Carbon-based primary productivity modeling with vertically resolved photoacclimation. Global Biogeochem. Cycles 22, GB2024 (2008).
Huot, Y., Morel, A., Twardowski, M. S., Stramski, D. & Reynolds, R. A. Particle optical backscattering along a chlorophyll gradient in the upper layer of the eastern South Pacific Ocean. Biogeosciences 5, 495–507 (2008).
Martinez-Vicente, V., Dall’Olmo, G., Tarran, G., Boss, E. & Sathyendranath, S. Optical backscattering is correlated with phytoplankton carbon across the Atlantic Ocean. Geophys. Res. Lett. 40, 1154–1158 (2013).
Zhang, Z. & Qiu, B. Surface chlorophyll enhancement in mesoscale eddies by submesoscale spiral bands. Geophys. Res. Lett. 47, e2020GL088820 (2020).
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2023YFC3108002), the National Natural Science Foundation of China (NSFC) (No. 42276186), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA0370101), the NSFC (Nos. 42494881 and 42276027), the Youth Innovation Promotion Association CAS (No. 2021343), the Natural Science Foundation of Guangdong (Nos. 2023A1515010966 and 2025A1515010871), the development fund of South China Sea Institute of Oceanology of the Chinese Academy of Sciences (Nos. SCSIO202204 and SCSIO20230Y02), and the China- Sri Lanka Joint Center for Education and Research, Chinese Academy of Sciences. W.Z. is supported by CAS Cultivating Young Scientists Foundation. The data analysis is supported by the High Performance Computing Division in the South China Sea Institute of Oceanology.
Author information
Authors and Affiliations
Contributions
H.Z., Q.H., and Y.L. designed the study. Y.L. performed the analysis, and wrote the manuscript. Q.H., W.Z., M.G., Y.Z., and H.Z. contributed to the discussion of the study. X.S. contribute to the data analysis. Q.H., W.Z., M.G., Y.Z., X.S. and H.Z. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Michael Stukel and Alice Drinkwater. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., He, Q., Zhan, W. et al. Heterogeneity of phytoplankton response to submesoscale processes in the global ocean. Commun Earth Environ 6, 386 (2025). https://doi.org/10.1038/s43247-025-02365-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-025-02365-3