Abstract
Enhanced rock weathering (ERW) is a scalable strategy for atmospheric carbon dioxide removal. The microbiome function critically regulates the below-ground cycle of carbon and nitrogen in terrestrial ecosystems. However, microbial functioning related to carbon and nitrogen under ERW remains elusive. Here we investigated how wollastonite addition affects key microbial carbon and nitrogen-cycles genes investigated using metagenomic, in a tropic rubber plantation. After two-year, ERW had enhanced the alpha diversity of biogeochemical cycling genes was mainly driven by increased soil pH. ERW led to an increase in the relative abundance of carbon-fixation genes, and a decrease in the carbon-degradation genes, providing microbiological evidence for carbon dioxide emissions. Additionally, the relative abundance of nitrogen-cycling functional genes, and available iron increased after wollastonite addition, indicating ERW may influence nitrous oxide emissions through biological and chemical processes. Altogether, our results illustrate how the effect of ERW alters microbial functioning, impacting soil organic matter dynamics.
Similar content being viewed by others
Introduction
Global warming is one of the most important environmental issues due to the increasing amount of greenhouse gas emissions (GHGs) released into the atmosphere. Despite efforts to reduce carbon dioxide (CO2) and other GHGs [e.g., nitrous oxide (N2O)], the total anthropogenic emissions of CO2 had increased from 16.9 Gt CO2 yr−1 (4.6 Gt C yr−1) to 40.9 Gt CO2 yr−1 (11.2 Gt C yr−1) between 1960s and 20231. The current trajectory of GHGs worldwide makes an overshoot of global temperature beyond 1.5 °C warming, which comes with an increased risk of reaching tipping points, including sea level rise, ecosystem collapse, and biodiversity loss2. Therefore, carbon dioxide removal (CDR) strategies are of growing urgency to reduce atmospheric CO2 concentrations3,4. Enhanced rock weathering (ERW) is a CDR technique that is based on speeding up the natural C sequestration processes caused by chemical weathering of silicate rocks5. Its global CDR potential in croplands was estimated to remove up to 2 Gt CO2 yr−1 globally6. Theoretically, ERW could be implemented in other types of vegetation, e.g., planted forests, which could increase the global CDR potential, due to the effect on the biogeochemical cycles of C, N, and phosphorus7,8. To date, ERW practices have been widely used in cropland6,9, but rarely in forests, and the impact of ERW on soil organic matter dynamics remains largely unexplored.
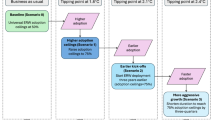
ERW can affect the soil GHG emissions depending on its effect on microbial-mediated processes, such as C degradation, N nitrification, and denitrification, but also on physical processes controlling soil organic matter stabilization7,10,11,12,13(Fig. 1). While soil CO2 emissions are related to C fixation and degradation processes, CH4 emissions arise from the methanogenesis and methane oxidation, N2O emissions are produced by nitrification and denitrification14. Few studies have attempted to investigate the effects of ERW on soil C and N cycling and their microbial attributes. ERW can increase microbial biomass and C use efficiency7, promote hyphae-forming bacteria, while reducing soil CO2 production15,16, and affect soil organic matter stabilization13,17. Whereas other studies found that ERW increased soil CO2 emissions, with increased soil pH, dissolved organic C, and microbial biomass C after incubation18. Additionally, the ERW efficiency depends on dissimilar types of silicate rock (e.g., basalt, wollastonite, dunite), with different chemical composition and common minerals (e.g., calcium (Ca), magnesium (Mg)), which may have a heterogeneous effect on ecosystem function19. Wollastonite, with its simple chemical composition (CaSiO3), minimal heavy metal, and rapid weathering characteristics, is appropriate for releasing calcium in soil and is widely used in simulated ERW experiments20,21. However, the potential impact of wollastonite power addition on the diversity and abundance of functional genes remains unclear22. Further research is necessary to investigate how functional gene diversity, composition, and assembly methods vary geographically and environmentally across various rates of ERW.
The abundance of functional genes in soil directly determines the biological capacity of C and/or N transformation and the emission of GHG23,24. For example, Peng et al.25 found a positive correlation between the abundance of the Calvin cycle gene (cbbL), nitrification gene (amoA), and the denitrification gene (nirS) with the rates of soil C fixation, N nitrification, and denitrification, respectively. Furthermore, previous studies have suggested that parallel26 or asymmetric27 responses between abundance and diversity of N transformation genes. Additionally, the variations in the microbial functional gene abundance can be mirrored in specific extracellular enzyme activities28,29, which are frequently regarded as the proxies of microbially mediated biochemistry cycling in the classical enzyme-driven Earth system models30. Together, combining gene diversity and abundance, and enzyme activities, offers a promising viewpoint for understanding the stability and rate of soil C and N metabolism. However, the relationship between gene abundance and diversity is currently unclear; meanwhile, studies on GHG emissions are still lacking.
In order to gain a better understanding of the abundance of functional genes involved in soil microbial C and N cycling and analyze the potential metabolic response to ERW, metagenomics was applied to a wollastonite addition experiment in a tropical rubber plantation in Xishuangbanna, Southwest China. We hypothesized that ERW would enhance microbial abundance and diversity through increased soil pH resulting from the input of alkaline cations (H1). Furthermore, we hypothesized that ERW increases the abundance and diversity of functional microbial genes and leading to increased GHG emissions (H2).
Results
Variations in the composition of soil functional genes via ERW
The functional genes involved in C degradation, methane metabolism, and N cycling with high wollastonite addition were greater than those of the low wollastonite addition and those of the control, respectively (Fig. 2b–d and Supplementary Fig. 1b–d). In contrast, the alpha diversities of C fixation-related functional genes did not change significantly following wollastonite addition (Fig. 2a and Supplementary Fig. 1a). Additionally, wollastonite addition had no significant effect on alpha diversity indices of all investigated functional genes, including the Shannon and Pielou_e index (Supplementary Fig. 2). However, wollastonite addition significantly increased the beta-diversity of C and N-cycling functional genes (KO level) based on Bray–Curtis distance (Kruskal-Walls test: all P < 0.05; Supplementary Fig. 3). Principal coordinates analysis confirmed that wollastonite addition significantly affected the functional genes involved in C and N cycling (ANOSIM test: all P < 0.05; Supplementary Fig. 4). These results found that wollastonite addition led to significant differences in the diversity of functional genes involved in C and N cycling.
Functional genes related to (a) carbon fixation, b carbon degradation, c methane metabolism, and d nitrogen cycling. The box plot displays the 25th percentile at its lower edge, the median at its center line, and the 75th percentile at its upper edge. The vertical lines extend to show the minimum and maximum data values, respectively. Different letters indicate a statistically significant difference at P < 0.05.
Specifically, in the C fixation group, the relative abundance of icd and cooC increased with wollastonite addition, while cbbR abundance decreased. For C degradation genes, the abundance of iam and xylH was significantly lower with wollastonite treatment (Fig. 3a and Supplementary Fig. 5). However, there were no significant changes observed in methane metabolism-related functional genes. The relative abundance of N cycling-related functional genes, including ureC, nasD, narB, and narG was higher in the wollastonite treatment compared to the control (Fig. 3b and Supplementary Fig. 5).
Functional genes associated with (a) carbon cycling, and b nitrogen cycling. Red solid lines indicate that the relative abundances of functional genes were increased with high wollastonite addition. Blue dashed lines indicate that the relative abundances of functional genes were decreased with high wollastonite addition. Gray lines indicate there are no significant differences in the relative abundances of functional genes with high wollastonite addition. Grey labels indicate functional genes that were not amplified during metagenomic sequencing.
Variations in the GHG emissions, enzyme activities, and soil properties via ERW
Soil CO2 emissions were significantly lower in the low wollastonite addition than in the control (Fig. 4a). Soil N2O emissions were significantly higher in the high wollastonite addition compared to the control (Fig. 4c). Soil CH4 uptake did not significantly vary among treatments (Fig. 4b). Wollastonite addition reduced the global warming potential (GWP) of GHGs (Supplementary Fig. 6). Enzyme activities related to C and N hydrolysis, namely β-glucosidase (BG) and acetylglucosaminidase (NAG) activities were higher in the high wollastonite addition, followed by the low wollastonite addition and those of the control (Supplementary Fig. 7a, c). However, no significant changes were observed in cellobiohydrolase (CBH) and leucine aminopeptidase (LAP) across treatments (Supplementary Fig. 7b, d). Soil pH, clay content, and cation exchange capacity (CEC), were significantly increased in the high wollastonite addition compared to the control. Soil organic C (SOC), dissolved organic C (DOC), dissolved organic N (DON), available iron, and exchangeable calcium were also enhanced under high wollastonite addition. Additionally, mineral-associated organic C (MAOC), microbial organic C (MBC), available silicon, and exchangeable magnesium increased with increasing wollastonite addition (Supplementary Table 1).
a CO2, b CH4, and c N2O. The box plot displays the 25th percentile at its lower edge, the median at its center line, and the 75th percentile at its upper edge. The vertical lines extend to show the minimum and maximum data values, respectively. Different letters indicate a statistically significant difference at P < 0.05.
Variations in the microbial community via ERW
At the phylum level, the microbial communities associated with C fixation process, methane metabolism, and N cycling were predominantly composed of Proteobacteria (relative abundance >38%), followed by Actinobacteria (relative abundance >19%) (Fig. 5a, c, d and Supplementary Fig. 8). The microbial community associated with C degradation process was dominantly composed of Actinobacteria (relative abundance >30%), Acidobacteria (relative abundance >22%), as well as Proteobacteria (relative abundance >18%) (Fig. 5b and Supplementary Fig. 8). Compared with control, the relative abundance of Proteobacteria increased with increasing wollastonite addition, while Actinobacteria decreased with increase wollastonite addition related to both C and N cycling (Fig. 5). Additionally, the relative abundance of Chloroflexi and Acidobacteria, which related to C fixation and degradation, increased through enhanced weathering. However, the relative abundance of Chloroflexi, related to methane metabolism, decreased with wollastonite addition (Fig. 5a, b).
Controls on GHG emissions and microbial functional genes
Partial Least Squares Path Modeling (PLS-PM) indicated that wollastonite addition affected soil edaphic factors, as well as the relative abundance and Shannon index of functional genes, which ultimately affected the GHG emissions (Fig. 6). Soil CO2 emission showed marginally positive correlations with the relative abundance of C-degradation genes and soil edaphic factors, while exhibiting negative associations with the alpha diversity of C-degradation genes due to enhanced rock weathering (Fig. 6a). Additionally, although soil CO2 emissions did not show a relationship with relative abundance of total C-fixation on PLS-PM (Fig. 6a), there were also significant negative correlations with accA, icd and korB genes (Supplementary Fig. 9). The relative abundance of C-fixation genes was positively correlated with wollastonite addition, whereas the relative abundance of C-degradation genes was negatively associated with wollastonite addition. The Shannon index of the C-degradation gene was positively affected by wollastonite addition (Fig. 6a).
Furthermore, the Shannon index of C degradation and methane metabolism genes showed strong correlations with soil properties such as soil pH, CEC, SOC, particulate organic C (POC), MBC, BG, NAG, available silicon, and exchange calcium (Supplementary Figs. 10 and 11). The Shannon index of N-cycling genes was positively associated with MBC, BG, NAG, and exchange sodium (Supplementary Fig. 11). Meanwhile, the relative abundance of the icd, and cooC had positive correlations, while cbbR and malQ genes had negative associations with soil pH. The relative abundance of iam and xylH genes had negative relationships with SOC, MBC, available silicon, and available iron (Supplementary Fig. 12). Soil CH4 emission showed marginally positive correlations with the relative abundance of methane metabolism genes and negative associations with soil edaphic factors. There were no relationships between wollastonite and the relative abundance or Shannon index of methane metabolism genes (Fig. 6b).
For soil N2O emission, positive correlations were found with the relative abundance of N-cycling genes after wollastonite addition (Fig. 6c), especially ureC, nasD, and narB genes (Supplementary Fig. 9). Meanwhile, soil N2O emission positively correlated with the available iron content and not related with available manganese (Supplementary Fig. 13). The relative abundance of N-cycling genes was positively correlated with soil edaphic factors, and genes involved in N cycling (i.e., ureC, nasD, narB, and nrfH) were positively associated with soil pH (Supplementary Fig. 12).
Discussion
ERW enhanced the diversity of microbial functional genes
In our present study, we found that the alpha diversities of functional genes related to C degradation, methane metabolism, and N cycling with a high rate of wollastonite addition were greater than those of the low wollastonite addition and the control (all P < 0.05; Fig. 2b–d and Supplementary Fig. 1b–d), which is in line with previous studies found that the diversity and community compositions of bacteria were strongly positively correlated with weathering indices31. Beyond, wollastonite addition did not significantly affect the alpha diversity of all investigated functional genes (both Shannon and Pielou_e index) (Supplementary Fig. 2). These results suggested that the diversity of core microbiota with specialized functions (alpha diversities of functional genes related to C or N cycling) might be more sensitive to response to wollastonite addition than broad functions (alpha diversities of total functional genes)32,33.
Consistent with our hypothesis 1, soil pH was positively linked with the alpha diversity of C degradation (Supplementary Figs. 10 and 11), which is consistent with other studies reporting that soil pH has a strong positive correlation with the diversity of soil biota groups34,35. Microbial growth, and soil microbial C and/or N cycling are often limited in highly acidic soils36,37,38, and thus the increased soil pH with ERW may favor soil microbial diversity39. Additionally, Domeignoz-Horta et al.40 found that microbial diversity is a strong predictor of soil C use efficiency. Thus, the increase in microbial diversity following wollastonite addition in our study may have contributed to the ERW enhancing C use efficiency, which was reported in the same experiment7.
Furthermore, wollastonite application increased SOC, MAOC, DOC and MBC, suggesting its potential to improve soil conditions for C sequestration and microbial activity and associated mineralization processes (Supplementary Table 1), which is in line with previous studies demonstrating that ERW accelerated C sequestration13,41. Positive relationships were observed between SOC, POC and MAOC, DOC and MBC with the alpha diversity of C degradation (Supplementary Fig. 11), which is consistent with previous study showing correlations between microbial alpha diversity and soil C pool42. The elevated C concentrations likely provide sufficient energy and substrate to support a wider range of microbial life, thereby promoting overall microbial diversity43.
Additionally, recent studies have demonstrated that weathering indices (silicon, magnesium, and calcium), which reflect the intensity of chemical weathering on parent materials, can directly influence biodiversity and subsequently affect ecosystem functions31. In this study, wollastonite (CaSiO₃) application enhanced microbial diversity through natural weathering processes, releasing primarily silicon and calcium ions into the soil system. This treatment increased concentrations of exchangeable Ca2+ and Mg2+, along with available silicon and iron (Supplementary Table 1). The observed positive correlations between alpha diversity of C degradation microorganisms and concentrations of available silicon, available iron, exchangeable calcium, and exchangeable sodium (Supplementary Fig. 11) further underscore the crucial role of silicate mineral weathering in maintaining microbial diversity and associated ecosystem functions.
ERW on the abundance of microbial functional genes and GHG emissions
We observed asymmetric responses of abundance and diversity of C-degradation genes (Figs. 2c and 3a and Supplementary Fig. 5), which is inconsistent with part of hypothesis 1, but similar to previous studies showing asymmetric responses of abundance and diversity of functional genes with precipitation change27. Additionally, for the N cycling genes affected by wollastonite addition, their response was symmetric. However, only a relatively limited number of N cycling genes were influenced by wollastonite addition. In this regard, previous studies have reported that rock weathering can directly and indirectly stimulate SOC sequestration44, reactive nitrogen45, and GHG emissions46, our findings tend to indicate that these changes may be largely controlled by soil microbes.
In detail, the trade-off between the increased relative abundance of C-fixation genes (icd and cooC) and the decreased relative abundance of C-degradation genes (iam and xylH) implied a reduction in soil CO2 release. This implies that wollastonite addition not only captured CO2 from a chemical reaction equation perspective (CaSiO3 + 2CO2 + H2O → Ca2+ + 2HCO3− + SiO2, the primary chemical formula of wollastonite is CaSiO3 in the current study)47,48, but also from affecting soil C cycling. As further confirmation of these patterns, we found that the CO2 emissions were decreased after wollastonite addition (Fig. 4a), thereby indicating microbiological evidence that confirmed ERW is a potential strategy to reduce CO2 emissions49. Meanwhile, our findings further support that ERW with wollastonite accelerated C sequestration, not only by stimulating microbial C pump through the formation of mineral-associated organic matter and macroaggregates in the same regions13, but also by reducing CO2 emissions through the activity of microorganisms. However, other studies found that silicate addition increased18 or decreased50 the soil CO2 efflux, likely due to differences in experimental duration, application frequency, and environmental conditions. The limited duration of our experiment may have constrained our ability to detect long-term GHG storage dynamics and fully capture temporal variations in GHG emissions and microbial responses to ERW. Therefore, extended field experiments combining high-frequency, multi-year GHG monitoring and microbial community are needed to elucidate the long-term temporal dynamics of C and N cycling and the mechanisms underlying climate feedback19.
The relationship between wollastonite application rates and CO2 reduction exhibited a distinct non-linear pattern, with lower application rates demonstrating proportionally greater reductions in CO2 emissions compared to higher treatment levels. These suggest that lower concentrations may potentially optimize the reactive surface area and weathering kinetics, thereby maximizing CO2 sequestration per unit of applied wollastonite51. This pattern suggested potential limitations in material utilization efficiency at elevated application rates. A sequestration of 39.3 t CO2 ha–1 had been reported20 when applying wollastonite at 221 t ha−1. While others reported a broader range of 32.8—62.4 t CO2 ha−1 under similar application rates21. These variations in sequestration efficiency across different experimental conditions highlight the complex interplay between application rates and material utilization efficiency. Consequently, to date, the optimal application of silicate rock remains a subject of ongoing scientific debate19.
The relative abundance of methane metabolism genes and soil CH4 uptake did not significantly vary under wollastonite addition (Figs. 3a and 4b and Supplementary Fig. 5). These results contrast with other studies showing that weathering processes can promote the growth of methanogenic microorganisms and ultimately stimulate CH4 production52. PLS-PM further elucidated that while CH4 uptake capacity showed a marginally positive correlation with methane metabolism gene abundance, as well as it showed a stronger negative association with soil edaphic factors (Fig. 6b). Increased environmental factors such as pH, DOC, and SOC stimulate CH4 production, in turn, may inhibit CH4 uptake, although these changes were not significant53. Furthermore, the absence of significant changes in the relative abundance of functional genes associated with methane metabolism likely contributed to the limited response of CH4 uptake to wollastonite addition (Fig. 3a and Supplementary Fig. 5).
Interestingly, different from the relative abundance of C-cycling genes, ERW increased the diversity and abundance of the N-cycling microbial community at functional levels and suggested an acceleration of soil N turnover. Soil N2O emissions were elevated following wollastonite addition (Fig. 4c), being inconsistent with previous studies showing that ERW mitigated N2O emissions from agricultural soil15,54. Furthermore, previous studies have shown that enrichment of Ca2+ would stimulate N mineralization36. In our study, NAG activities, which are considered to be responsible for the depolymerization of proteins and are a rate-limiting step of N mineralization driven by proteases55, increased following wollastonite addition (Supplementary Fig. 7). Additionally, ERW stimulated the abundance of assimilatory nitrate reduction genes, especially, nasD and narB, which participated in transporting NO3− to NO2− and NH4+, respectively. Meanwhile, wollastonite addition increased the relative abundance of narG (Fig. 3b and Supplementary Fig. 5), which encodes the first-rate step in denitrification, representing the capacity for the transformation of NO3− to NO2−56. These enrichments in N cycle enzymes and functional genes likely accelerated N cycle processes57, potentially contributing to increased N2O emissions.
However, we did not detect an increase in the abundance of key nitrifying (e.g., amoA) or denitrifying genes (e.g., nirS and nirK), are primarily responsible for N2O production, after wollastonite (Fig. 3). Although microbial communities are often assumed to dictate their functionality, leading to the expectation of a clear relationship between functional gene abundance and associated processes58, other studies have reported no consistent relationship between changes in soil N2O emissions and variations in the abundance of ammonia-oxidizing and denitrifying microorganisms59. Emerging studies suggest that N2O gas is emitted through both biological processes and chemical processes, including reactions involving N cycle intermediates (e.g., hydroxylamine, nitrous acid, nitrite, and nitric oxide), redox-active metals (e.g., iron and manganese), and soil organic matter (e.g., humic and fulvic acids)60,61. Moreover, the positive relationships between soil N2O emissions and available iron (Supplementary Fig. 13), compelling evidence supporting the hypothesis that biological processes are not the sole drivers of soil N2O emissions61. These findings suggest that ERW may also influence N2O emissions through chemical processes, and the role of these processes warrants further investigation. It is worth noting that the increase in N2O emissions resulting from wollastonite addition may partially offset the reduction in CO2 emissions achieved through this approach (Fig. 4). However, in our study, wollastonite addition ultimately reduced the GWP of GHGs (Supplementary Fig. 6), further confirming that ERW is a potential strategy for CDR.
The changes in C or N cycling genes with weathering were primarily ascribed to the changes in the relative abundance of major bacterial taxa. In particular, the relative abundance of Proteobacteria increased with wollastonite addition because they were the primary source of C and N-cycling genes (Fig. 5). Probably because Proteobacteria are copiotrophs and respond quickly to nutrient-rich environments, which could also help break down polymers by using lipids and proteins, which are components of microbes. (e.g., hemicellulose, cellulose)62,63. Additionally, the relative abundance of Chloroflexi and Acidobacteria related to C fixation and degradation increased with ERW (Fig. 5a, b). These oligotrophic microorganisms play important roles in C decomposition due to the mineralization of recalcitrant organic C and the production of extracellular enzymes, which might increase BG activities with wollastonite addition in our present study (Supplementary Fig. 7a). Moreover, the relative abundance of Actinobacteria related to both C and N cycling was decreased with wollastonite addition (Fig. 5), which was also able to depolymerize complex C compounds64. In this regard, Actinobacteria reductions might offset, to some extent, the increased relative abundance of Proteobacteria on the C and N cycles.
Uncertainties and Implications
Our study demonstrates that ERW influences microbial C and N cycling genes, thereby affecting ecosystem functions, particularly CO2 and N2O emissions. While ERW reduced soil CO2 emissions, it unexpectedly increased N2O emissions, which may offset the CO2 removal benefits achieved through ERW. Furthermore, the economic feasibility of ERW implementation (e.g., dust or slurry spreading) requires careful consideration, as the associated costs may outweigh the benefits of CO2 reduction8. Meanwhile, it should be noted that our findings are based on a 2-year wollastonite application study, and the observed effects may vary over longer timescales. Long-term field trials with high-frequency and multi-year GHG monitoring would be urgently needed to assess the weathering and CDR efficiency, which could provide more reliable insights10. Importantly, the current wollastonite application rates in our study may not represent optimal dosages for maximizing CDR benefits, as higher application rates do not necessarily result in proportional increases in weathering efficiency or linear responses in GHG emissions and functional gene expression65. Excessive application could potentially lead to nutrient imbalances and unintended ecological consequences, suggesting the need for more precise dosage optimization in future ERW implementations. Additionally, our study is based on X-ray diffraction and X-ray fluorescence to analyze and quantify the elemental composition of the crystalline phase, while scanning electron microscopy and energy-dispersive X-ray spectroscopy could provide three-dimensional morphological information, enabling more detailed monitoring of temporal changes following wollastonite addition. This approach would be valuable for assessing the weathering rate of wollastonite powder in soil and its broader impacts on the ecosystem41,66.
Conclusions
Based on a two-year wollastonite addition manipulation experiment, we found an asymmetric response of C gene abundance and diversity and a symmetric response of N gene abundance and diversity. The greater alpha diversity of C and N cycling was accompanied by an increase in soil pH with increased weathering. Changes in the relative abundance of C cycling genes (increased C fixation genes and decreased C degradation genes) provide microbiological evidence for observed responses of soil CO2 under wollastonite addition. However, ERW increased N2O emissions through both biological processes (e.g., by enhancing the abundance of N-cycling microbial functional genes and stimulating nitrogen mineralization) and chemical processes (e.g., through interactions with available iron). Furthermore, the observed increase in alpha diversity and the shift in microbial abundance associated with carbon fixation and degradation following ERW application provide additional evidence supporting our previous studies, which found enhanced C sequestration12 after wollastonite addition. Our findings highlight the response of microbial function to ERW and call for ecosystem-level GHG monitoring under ERW. Thereby, disentangling the effects of different GHG gases from enhanced weathering, and understanding the microbial mechanism, it is important to promote understanding of the long-term effects of ERW on the ecosystem’s C and N balance.
Materials and methods
Study site and experimental design
The field experiment was carried out in the rubber plantations (Hevea brasiliensis) of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (20°33′N, 101°28′E), which was located in Yunnan Province, China. These rubber plantations, which have been cultivated for ~40 years, were selected for their extensive distribution across tropical regions and their vital contributions to both the local ecosystem and economy. The elevational transit covers approximately between 880 and 950 m a.s.l. The region has a typical tropical monsoon climate, with a non-monsoon or dry period from October to May and a monsoon or rain period from June to September. The mean atmospheric temperature is around 21.9 °C. The average annual precipitation is 1528 mm. The soil pH was around 4.9, so the soil type was categorized as acidic oxisol according to the USDA (United States Department of Agriculture) soil taxonomy.
In May 2021, the ERW field experiment was established using a randomized complete block design in the rubber plantations. Nine plots (20 m × 20 m) were randomly arranged with more than 20 m buffers. Three ERW treatments (control: 0 kg m−2, low: 0.25 kg m−2, and high: 0.5 kg m−2) with three replicates of each level were included in this experiment (Supplementary Fig. 14). Wollastonite powder, sourced from the Diaobing Mountain commercial mine in Liaoning, China, was evenly spread across the forest floor, while the control plots received no additional material. In our study, wollastonite was applied at 5 t ha−1 with a cost of US$365, including procurement, mining, crushing, grinding, and field application. We anticipate that a single application will remain effective for five years, resulting in an average annual cost of US$75 over this period. X-ray diffraction and X-ray fluorescence were used to analyze and quantify the elemental composition of the crystalline phase inside the rock. Calcium metasilicate (CaSiO3, 69.4%) and silicon dioxide (SiO2, 14.8%) are the main minerals of the wollastonite by X-ray diffraction analysis using a Malvern Malvern Panalytical Empyrean Series 3 diffractometer with CoKa radiation (Supplementary Fig. 15). Calcium (30.36%) and silicon (24.29%) are the main chemical components of the wollastonite by X-ray fluorescence analysis using a plasma-atomic emission spectrometer. The particle size of the wollastonite powder was 90% between 10—57.53 µm, and all wollastonite powder was less than 100 µm using wet laser diffraction13.
Soil sampling, physicochemical analysis, and soil enzyme activity
Soil samples (0–10 cm) were taken from all nine plots after litter removal in April 2023. From each plot, four soil samples were collected randomly from each plot and combined into two mixed soil samples. A portion of the soil samples was stored at −80 °C for microbial analysis, while the remaining samples were stored at 4 °C for the soil physicochemical analysis. DOC and DON were extracted with a 1:5 soil-water mixture, and the concentrations were measured with a Vario TOC analyzer (Thermo Fisher Scientific Inc., USA). After drying at 60 °C, the concentration of soil organic C and total N was measured with an automatic TOC analyzer (Vario EL III; Elementar, Hanau, Germany). Carbonate was removed using 1 M HCl SOC67. POC and MAOC fractions were obtained using a combined density and size fractionation method for soil organic matter68. A soil core with a diameter of 5 cm was used to measure the bulk density at 0–10 cm. The soil pH was measured using a pH electrode (SevenEasy pH; Metler Toledo, Germany) in a 1:2.5 soil: water suspension. Microbial biomass C and N (MBC and MBN) were determined using the fumigation-extraction method69, as the difference between the extract solutions of fumigated and non-fumigated soils with a Vario TOC analyzer (Thermo Fisher Scientific Inc., USA).
Soil enzyme activities were evaluated using fluorometric techniques with high levels of 4-methylumbelliferone (MUB) and 7-amino-4-methylcoumarin (AMC)70. Briefly, 200 μl of soil suspension was dispensed into a 96-well black microplate, and then 50 μl fluorometric substrate solution was added (200 μΜ, saturating concentration, 4-MUB-β-D-glucoside for β-glucosidase (BG), 4-MUB-b-D-cellobioside for CBH, 4-MUB-N-acetyl-β-D-glucosaminide for NAG, L-leucine-7-amido-4-methylcoumarin for LAP) and incubated for 5 h at 25 °C. Eight replicates of each soil sample were set up on each plate. The reactions were stopped by adding 50 μL of 1 M NaOH. The amount of fluorescence was analyzed using a fluorometer (Tecan Infinite 200 PRO, TECAN Group Ltd., Mannedorf, Switzerland) with 365 nm excitation and 450 nm emission.
Soil DNA extraction and metagenomic sequencing
Metagenomic DNA was extracted from 0.5 g of fresh soil using the E. Z. N. A soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) following manufacturer's protocol. For sample homogenization, we employed a high-speed benchtop homogenizer (FastPrep-24 5 G, MP Biomedicals, USA) operating at 6 m s−1 for 40 seconds. The quantity and quality of DNA extraction were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and fragmented to a mean length of approximately 400 bp using the Illumina TruSeq Nano DNA LT Library Preparation Kit. Approximately 1 µg of DNA was sent to Majorbio Bio-pharm Technology Co., Ltd. (Shanghai, China) and analyzed with an Illumina HiSeq X-ten platform (Illumina Inc., San Diego, CA, USA) using the PE150 strategy. Metagenomic sequence data generated in this study have been uploaded to GenBank and are available in the NCBI Sequence Read Archive database with accession numbers PRJNA1072228.
Bioinformatic analysis
The fundamental data related to metagenomic sequencing is displayed in Supplementary Table 2. Quality control of the raw metagenomic sequences was performed in order to eliminate adapters by removing low-quality reads (length <50 bp or with a quality value < 20 or having N bases) using Fastp0.20.0. The acquired clean reads were put together into contigs using Megahit and the optimal k-mer parameter. Subsequently, the open reading frames (ORFs) of contigs were predicted using MetaGene, and the sequences greater than 100 bp were subsequently translated into amino acid sequences. All the predicted genes were grouped into nonredundant gene catalogs (90% coverage and 95% identity) using CD-HIT v4.6.1. High-quality reads were mapped to nonredundant gene catalogs to compute gene abundance (95% identity) using SOAPaligner v2.21.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/keeg/) was analyzed using Diamond (http://www.diamondsearch.org/index.php, v0.8.35) for the KEGG annotation. Raw sequencing data have been deposited at the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/) with accession number PRJNA1072228. For genome annotation, the functional genes involved in the C and N cycles were mainly involved in different categories, including C fixation, C degradation, methane metabolism, and N cycling23,71 (Supplementary Table 3). The annotations and relative abundance of functional genes were computed and listed as enzymes encoded by the genes at the KEGG Orthopedic, pathway, and module levels. The relative abundance of each taxon and functional gene was determined by adding up all the read counts associated with each taxon and function and dividing by the total read counts of the subjects.
GHG emissions analysis
The GHG emissions were measured after soil sampling using a cylindrical closed static non-transparent chamber. We conducted measurements over three consecutive days to minimize errors and ensure alignment with microbial activity at the same time points. The static chamber (PVC collars) was made up of two sections: a removable cover (20 cm diameter, 20 cm height) with a 10 cm long silicic tube (4 mm diameter) for air collection, and a cylindrical bottom pedestal (20 cm diameter, 5 cm height), half of which was placed into the soil. Each gas sample was taken between 9 and 11 AM to reduce the potential effects of diurnal variation. Thirty milliliter gas samples were drawn at 0, 0.25, and 0.5 h into the incubation period using a syringe, and then transferred to a 50 mL multi-layer foil sampling bag (Delin, Dalian China). The concentration of GHGs was determined using gas chromatography (Agilent 7890, Thermo Fisher Scientific Inc., USA). A linear least-squares fit was used to calculate the soil GHG emissions.
Where E is soil GHG emissions, dc is the gas concentration change in chamber, dt is the time difference in the chamber (h), M is the molecular weight of GHGs, 22.4 is the molar volume of gas at standard pressure and temperature. V is the chamber volume (m−3), A is the chamber area (m−2), 273 is the gas state equation constant, and T is the air temperature in the chamber. The GWP (kg CO2 eq m−2) was calculated using the following equation72:
where CH4, N2O, and CO2 denote the annual cumulative emission of CH4, N2O, and CO2 (kg m−2), respectively.
Statistical analysis
Differences in the alpha diversity (Shannon and Pielou_e index) of the functional gene categories as well as the relative abundance of functional genes involved in C and N cycling after wollastonite addition were calculated by a linear mixed effects model with the lme4 package: lmer (as wollastonite addition (control, low, and high) as categorical predictors, and plots as random effects). Principal coordinate analysis was used to visualize the differences in beta-diversity on the Bray-Curtis distance of functional genes, which were normalized using reads per million for data standardization, with the vegan package. The ANOSIM was further determined to examine how wollastonite addition affected the functional gene composition. A Mantel test was used to determine the significant effects of soil physicochemical properties on functional gene composition with the linkET and dplyr packages. Moreover, to investigate the direct and indirect effects of edaphic variables (e.g., clay, SOC, and DOC content) and microbial attributes on the diversity of functional genes, PLS-PM was implemented with the R package ‘plspm’ (Supplementary Table 4)73. The data were fitted to these models using the maximum likelihood estimation method. All statistical analyses were performed using R software (version 4.2.2, R Development Core Team, 2022).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets and Supplementary Tables are available through Figshare (https://doi.org/10.6084/m9.figshare.28587803.v2)74. Raw sequences have been uploaded to the National Center for Biotechnology Information (NCBI) Sequence Read Archive under the BioProject identifiers PRJNA1072228 (https://www.ncbi.nlm.nih.gov/sra/).
References
Friedlingstein, P. et al. Global Carbon Budget 2023. Earth Syst. Sci. Data 15, 5301–5369 (2023).
Moller, T. et al. Achieving net zero greenhouse gas emissions critical to limit climate tipping risks. Nat. Commun. 15, 6192 (2024).
Ganti, G. et al. Evaluating the near- and long-term role of carbon dioxide removal in meeting global climate objectives. Commun. Earth Environ. 5, 377 (2024).
Rockström, J. et al. A roadmap for rapid decarbonization. Science 84, 1269–1271 (2017).
Eufrasio, R. M. et al. Environmental and health impacts of atmospheric CO2 removal by enhanced rock weathering depend on nations’ energy mix. Commun. Earth Environ. 3, 106 (2022).
Beerling, D. J. et al. Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 583, 242–248 (2020).
Bi, B. et al. Enhanced rock weathering increased soil phosphorus availability and altered root phosphorus-acquisition strategies. Glob. Chang. Biol. 30, e17310 (2024).
Goll, D. S. et al. Potential CO2 removal from enhanced weathering by ecosystem responses to powdered rock. Nat. Geosci. 14, 545–549 (2021).
Baek, S. H. et al. Impact of climate on the global capacity for enhanced rock weathering on croplands. Earth’s. Future 11, e2023EF003698 (2023).
Beerling, D. J. et al. Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 4, 138–147 (2018).
Keuschnig, C. et al. Reduced methane emissions in former permafrost soils driven by vegetation and microbial changes following drainage. Glob. Chang. Biol. 28, 3411–3425 (2022).
Trivedi, P., Anderson, I. C. & Singh, B. K. Microbial modulators of soil carbon storage: integrating genomic and metabolic knowledge for global prediction. Trends Microbiol. 21, 641–651 (2013).
Xu, T. et al. Enhanced silicate weathering accelerates forest carbon sequestration by stimulating the soil mineral carbon pump. Glob. Chang. Biol. 30, e17464 (2024).
Guetlein, A., Gerschlauer, F., Kikoti, I. & Kiese, R. Impacts of climate and land use on N2O and CH4 fluxes from tropical ecosystems in the Mt. Kilimanjaro region, Tanzania. Glob. Chang. Biol. 24, 1239–1255 (2018).
Chen, H. et al. Wollastonite addition can significantly inhibit greenhouse gas emissions of freeze-thaw farmland soil. Environ. Technol. Inn. 34, 103547 (2024).
Shabtai, I. A. et al. Calcium promotes persistent soil organic matter by altering microbial transformation of plant litter. Nat. Commun. 14, 6609 (2023).
Buss, W., Hasemer, H., Ferguson, S. & Borevitz, J. Stabilisation of soil organic matter with rock dust partially counteracted by plants. Glob. Chang. Biol. 30, e17052 (2023).
Yan, Y. et al. Wollastonite addition stimulates soil organic carbon mineralization: evidences from 12 land-use types in subtropical China. Catena 225, 107031 (2023).
Abdalqadir, M., Hughes, D., Rezaei Gomari, S. & Rafiq, U. A state of the art of review on factors affecting the enhanced weathering in agricultural soil: strategies for carbon sequestration and climate mitigation. Environ. Sci. Pollut. Res. 31, 19047–19070 (2024).
Haque, F., Santos, R. M., Dutta, A., Thimmanagari, M. & Chiang, Y. W. Co-benefits of wollastonite weathering in agriculture: CO2 sequestration andpromoted plant growth. Acs Omega 4, 1425–1433 (2019).
Wood, C., Harrison, A. L. & Power, I. M. Impacts of dissolved phosphorus and soil-mineral-fluid interactions on CO2 removal through enhanced weathering of wollastonite in soils. Appl. Geochem. 148, 105511 (2023).
Wang, P.-L. et al. Microbial communities modulate chemical weathering and carbon dioxide cycling in an active orogen in Taiwan. Commun. Earth Environ. 5, 174 (2024).
Hu, X. et al. Metagenomics reveals divergent functional profiles of soil carbon and nitrogen cycling under long-term addition of chemical and organic fertilizers in the black soil region. Geoderma 418, 115846 (2022).
Kits, K. D. et al. Low yield and abiotic origin of N2O formed by the complete nitrifier Nitrospira inopinata. Nat. Commun. 10, 1836–1846 (2019).
Peng, Z. et al. Land conversion to agriculture induces taxonomic homogenization of soil microbial communities globally. Nat. Commun. 15, 3624 (2024).
Wang, P. et al. Biogeographical distributions of nitrogen-cycling functional genes in a subtropical estuary. Funct. Ecol. 36, 187–201 (2021).
Zhao, M. et al. Asymmetric responses of abundance and diversity of N-cycling genes to altered precipitation in arid grasslands. Funct. Ecol. 37, 2953–2966 (2023).
Chen, J. & Sinsabaugh, R. L. Linking microbial functional gene abundance and soil extracellular enzyme activity: Implications for soil carbon dynamics. Glob. Chang Biol. 27, 1322–1325 (2021).
Moore, J. A. M. et al. Fungal community structure and function shifts with atmospheric nitrogen deposition. Glob. Chang Biol. 27, 1349–1364 (2020).
Sulman, B. N. et al. Multiple models and experiments underscore large uncertainty in soil carbon dynamics. Biogeochemistry 141, 109–123 (2018).
Hu, A. et al. Mountain biodiversity and ecosystem functions: interplay between geology and contemporary environments. ISME J. 14, 931–944 (2020).
Delgado-Baquerizo, M. et al. Lack of functional redundancy in the relationship between microbial diversity and ecosystem functioning. J. Ecol. 104, 936–946 (2016).
Yang, F. et al. Keystone species affect the relationship between soil microbial diversity and ecosystem function under land use change in subtropical China. Funct. Ecol. 35, 1159–1170 (2021).
Hu, Z. et al. Nutrient-induced acidification modulates soil biodiversity-function relationships. Nat. Commun. 15, 2858 (2024).
Zhou, X. et al. Global analysis of soil bacterial genera and diversity in response to pH. Soil Biol. Biochem. 198, 109552 (2024).
Marinos, R. E., Groffman, P. M., Driscoll, C. T. & Bernhardt, E. S. Accelerated soil nitrogen cycling in response to a whole ecosystem acid rain mitigation experiment. Soil Biol. Biochem. 189, 109286 (2024).
Zhang, X. et al. Soil acidification as an additional driver to organic carbon accumulation in major Chinese croplands. Geoderma 366, 114234 (2020).
Zhong, Y., Yan, W., Canisares, L. P., Wang, S. & Brodie, E. L. Alterations in soil pH emerge as a key driver of the impact of global change on soil microbial nitrogen cycling: evidence from a global meta-analysis. Glob. Ecol. Biogeogr. 32, 145–165 (2023).
Ribeiro, I. D. A. et al. Use of mineral weathering bacteria to enhance nutrient availability in crops: a review. Front. Plant Sci. 11, 590774 (2020).
Domeignoz-Horta, L. A. et al. Microbial diversity drives carbon use efficiency in a model soil. Nat. Commun. 11, 3684 (2020).
Wang, F. et al. Wollastonite powder application increases rice yield and CO2 sequestration in a paddy field in Northeast China. Plant Soil 502, 589–603 (2024).
Yang, Z. et al. Soil carbon storage and accessibility drive microbial carbon use efficiency by regulating microbial diversity and key taxa in intercropping ecosystems. Biol. Fert. Soils 60, 437–453 (2024).
Bastida, F. et al. Soil microbial diversity-biomass relationships are driven by soil carbon content across global biomes. ISME J. 15, 2081–2091 (2021).
Liang, G., Stark, J. & Waring, B. G. Mineral reactivity determines root effects on soil organic carbon. Nat. Commun. 14, 4962 (2023).
Wan, J. et al. Bedrock weathering contributes to subsurface reactive nitrogen and nitrous oxide emissions. Nat. Geosci. 14, 217–224 (2021).
Vicca, S. et al. Is the climate change mitigation effect of enhanced silicate weathering governed by biological processes?. Glob. Chang Biol. 28, 711–726 (2022).
Abdalqadir, M., Gomari, S. R., Hughes, D., Sidiq, A. & Shifa, F. Process-based life cycle assessment of waste clay for mineral carbonation and enhanced weathering: A case study for northeast England, UK. J. Clean. Prod. 424, 138914 (2023).
Corbett, T. D. W. et al. Organic carbon source controlled microbial olivine dissolution in small-scale flow-through bioreactors, for CO2 removal. Npj Mat. Degrad. 8, 34 (2024).
Kantzas, E. P. et al. Substantial carbon drawdown potential from enhanced rock weathering in the United Kingdom. Nat. Geosci. 15, 382–389 (2022).
Xu, L., Deng, X., Ying, J., Zhou, G. & Shi, Y. Silicate fertilizer application reduces soil greenhouse gas emissions in a Moso bamboo forest. Sci. Total Environ. 747, 141380 (2020).
Taylor, L. L. et al. Enhanced weathering strategies for stabilizing climate and averting ocean acidification. Nat. Clim. Change 6, 402–406 (2015).
Zhang, D. et al. Enhanced rock weathering as a source of metals to promote methanogenesis and counteract CO2 sequestration. Environ. Sci. Technol. 58, 19679–19689 (2024).
Bodelier, P. L. E. & Steenbergh, A. K. Interactions between methane and the nitrogen cycle in light of climate change. Curr. Opin. Env. Sust. 9-10, 26–36 (2014).
Blanc-Betes, E. et al. In silico assessment of the potential of basalt amendments to reduce N2O emissions from bioenergy crops. GCB Bioenergy 13, 224–241 (2020).
Mooshammer, M. et al. Adjustment of microbial nitrogen use efficiency to carbon: nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 5, 3694–3700 (2014).
Zhu, X., Burger, M., Doane, T. A. & Horwath, W. R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 110, 6328–6333 (2013).
Wang, X. et al. Enhanced rock weathering boosts ecosystem multifunctionality via improving microbial networks complexity in a tropical forest plantation. J. Environ. Manag. 373, 123477 (2025).
Rocca, J. D. et al. Relationships between protein-encoding gene abundance and corresponding process are commonly assumed yet rarely observed. ISME J. 9, 1693–1699 (2015).
Zhang, Y. et al. Stimulation of ammonia oxidizer and denitrifier abundances by nitrogen loading: poor predictability for increased soil N2O emission. Glob. Chang Biol. 28, 2158–2168 (2022).
Chalk, P. M. & Smith, C. J. The role of agroecosystems in chemical pathways of N2O production. Agr. Ecosyst. Environ. 290, 106783 (2020).
Zhu-Barker, X., Cavazos, A. R., Ostrom, N. E., Horwath, W. R. & Glass, J. B. The importance of abiotic reactions for nitrous oxide production. Biogeochemistry 126, 251–267 (2015).
Leff, J. W. et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 112, 10967–10972 (2015).
Yang, Y. et al. Deciphering factors driving soil microbial life-history strategies in restored grasslands. iMeta 2, e66 (2022).
Cairns, J., Moerman, F., Fronhofer, E. A., Altermatt, F. & Hiltunen, T. Evolution in interacting species alters predator life-history traits, behaviour and morphology in experimental microbial communities. P R. Soc. B Biol. Scis 287, 1–10 (2020).
Daval, D. Carbon dioxide sequestration through silicate degradation and carbon mineralisation: promises and uncertainties. npj Mater. Degrad. 2, 11 (2018).
Su, C. et al. CO2 removal with enhanced wollastonite weathering in acidic and calcareous soils. Soil Ecol. Lett. 7, 240273 (2024).
Harris, D., Horwath, W. R. & van Kessel, C. Acid fumigation of soils to remove carbonates prior to total organic carbon or 13C isotopic analysis. Soil Sci. Soc. Am. J. 65, 1853–1856 (2001).
Lavallee, J. M., Soong, J. L. & Cotrufo, M. F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang Biol. 26, 261–273 (2020).
Vance, E. D., Brookes, P. C. & Jenkinson, D. S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987).
German, D. P. et al. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 43, 1387–1397 (2011).
Liu, J. et al. Conversion of steppe to cropland increases spatial heterogeneity of soil functional genes. ISME J. 17, 1872–1883 (2023).
Yuan, J. et al. Rapid growth in greenhouse gas emissions from the adoption of industrial-scale aquaculture. Nat. Clim. Change 9, 318–322 (2019).
Sanchez, G. PLS path modeling with R. R package v. 0.4.9. https://www.gastonsanchez.com/PLS_Path_Modeling_with_R.pdf (2013).
Chen, Q. et al. Divergent responses of carbon and nitrogen functional genes composition to enhanced rock weathering Data sets. figshare https://doi.org/10.6084/m9.figshare.28587803.v2 (2025).
Acknowledgements
This research was financially supported by the National Key Research and Development Program of China (2024YFF1306501), the National Natural Science Foundation of China (32171581, 32230067, 32401353, 32301361), the China Postdoctoral Science Foundation (2023M732865), the Postdoctoral Fellowship Program of CPSF (GZB20230979), the Natural Science Basic Research Program of Shaanxi Province (22JHQ036, 2024CYBQN-0221), the Department of Human Resources and Social Security of Shaanxi Province (2023BSHYDZZ59), and the Fundamental Research Funds for the Central Universities. We thank Jinchuan Duan and Jieru Hu for their assistance in creating the figure illustrations.
Author information
Authors and Affiliations
Contributions
Q.C: conceptualization, data curation; formal analysis; funding acquisition; investigation; methodology; visualization; writing—original draft; writing—review and editing. D.S.G., M.A.: supervision; writing—review and editing. X.H.: and Y.C.: writing—review and editing. G.L.: formal analysis; methodology. B.B. and T.X.: funding acquisition; investigation; methodology. C.L.: investigation; methodology. X.M.: investigation. Z.L.: funding acquisition. Y.F.: supervision. Z.H.: funding acquisition; supervision. Z.Y.: conceptualization; funding acquisition; investigation; methodology; supervision; writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Bikram K. Das, Aimeric Blaud and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: D’Arcy Meyer-Dombard, Somaparna Ghosh [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Q., Goll, D.S., Abdalqadir, M. et al. Divergent responses of carbon and nitrogen functional genes composition to enhanced rock weathering. Commun Earth Environ 6, 645 (2025). https://doi.org/10.1038/s43247-025-02455-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-025-02455-2