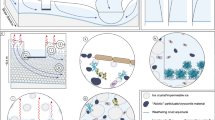
Abstract
Glacial lakes in high-altitude regions influence both water systems and greenhouse gas emissions, yet the effects of different hydrological recharge mechanisms on methane release and microbial ecosystems remain poorly understood. Here, we investigated methane fluxes and microbial community patterns in glacier-fed and non-glacier-fed lakes on the Tibetan Plateau during peak ablation stage. Our findings revealed that total methane flux from non-glacier-fed lakes were approximately three times higher than from glacier-fed lakes, with ebullition being the predominant emission pathway. Network analysis showed that the microbial community structure in glacier-fed lakes was more stable. The neutral community model confirmed deterministic processes mainly shape microbial assembly in glacial lakes with different recharge types. Microbial communities in glacier-fed lakes were more stable and tightly connected, while those in non-glacier-fed lakes were more fragmented. As glacier melting accelerates with climate change, more lakes may lose glacier input, potentially increasing methane emissions and disrupting microbial ecosystems.

Similar content being viewed by others
Introduction
Against the backdrop of global climate change, the aquatic ecosystems on the Tibetan Plateau are undergoing remarkable transformations1. As a critical component of high-altitude regions, glacial lakes play a key role not only in local hydrological cycles but also in influencing climate change and carbon cycling through their unique physical, chemical, and biological properties2,3, particularly in the releases of greenhouse gases (GHGs) such as methane (CH4)4. As a major GHG, CH4 has a global warming potential (GWP) 28 times higher than carbon dioxide, exerting a substantial impact on global climate change5. Although lakes cover only ~3.7% of the Earth’s non-glacier land area, they account for 14.9% of natural CH4 fluxes6, therefore being recognized as significant hotspots for CH4 fluxes7.
Over the past decades, rapid warming has led to widespread glacial retreats across the Tibetan Plateau, driving significant changes in the formation and expansion of glacial lakes8. Previous studies report a marked increase in both the number and total area of glacial lakes on the plateau, with the formation of over 1200 new lakes and an expansion of the total area exceeding 25%9,10. Newly formed glacier-fed lakes (GFLs) are emerging in recently deglaciated areas, while the older lakes are becoming increasingly disconnected from their glacial sources and transitioning into non-glacier-fed lakes (NGFLs). These NGFLs are mainly sustained by surface runoffs and precipitation11,12. Reduction of glacial meltwater input has profound ecological consequences, altering microbial diversity and biogeochemical process within glacial lakes13. CH4 production in glacial lakes is mainly governed by microbial metabolic activities14. Therefore, investigating the diversity patterns of microbial communities across different types of glacial lakes and their relationships with CH4 fluxes is crucial for understanding the stabilities of cold-region lake ecosystems and the role these ecosystems play in the global carbon cycle15.
The physicochemical properties of the glacial lakes, including water temperature (WT), pH value, electrical conductivity (EC), as well as nutrient concentrations, exert a decisive influence in determining CH4 fluxes and structures of microbial communities13,16,17. Studies have shown that the high-turbidity (TUR) glacial meltwater often carries large amounts of mineral particles18, which could reduce light penetration19, thus inhibiting the growth of primary producers20 and altering microbial community composition21. Simultaneously, the low water temperature and nutrient-poor conditions suppress activity of methanogens, resulting in low CH4 production rates22. However, under the influence of climate warming, non-glacier-fed lakes (NGFLs) that lack glacial meltwater inputs, undergo more intense solar heating during summer. This results in elevated water temperatures and more stable anoxic conditions in the lake-bottom sediment, thereby promoting increased CH4 production23,24. Seasonal input of glacial meltwater can deliver nutrients to glacial lakes. However, it could also dilute nutrient concentrations in lakes, leading to lower EC values and a weakening of microbial metabolic activities11,25. While numerous studies have assessed the impact of physicochemical conditions on microbial diversity in GFLs, there is still a lack of comparative research on the CH4 emission patterns and their driving factors between different recharge types (e.g., GFLs vs. NGFLs). Addressing this knowledge gap is crucial for understanding how glacial lake systems respond functionally to hydrological transformations caused by climate change.
The Tibetan Plateau, often referred to as the “Third Pole” and “Asia’s Water Tower”, hosts a vast number of glaciers at elevations above 5000 meters26. Glacial environments on the Tibetan Plateau harbor over 16 million functional genes, which represent an exceptionally high level of microbial biodiversity27. These microorganisms migrate and disperse across the atmosphere-glacier-river-lake-soil continuum of the plateau, and play a critical role in multiple spheres (e.g., cryosphere, hydrosphere, lithosphere), habitats, and ecosystems28,29,30. Cold-tolerant bacteria and archaea contribute to the biogeochemical processes by transforming organic matter, regulating GHGs emissions (e.g., CO2 and CH4), and influencing nutrient availability in downstream ecosystems31. Rapid global warming is reconfiguring the hydrological processes on the Tibet Plateau, triggering accelerated glacial retreat and a decrease in meltwater volume. These changes can have a substantial impact on microbial community structures of both GFLs and NGFLs. Microbial communities in GFLs are typically dominated by cold-tolerant taxa that are adapted to oligotrophic conditions32. Liu et al.13, found that the bacterial diversity in newly formed glacial lakes was much higher, and the bacterial co-occurrence networks were more complex. This phenomenon is likely attributed to increased input of glacier-derived bacteria. In contrast, microbial communities in NGFLs are characterized by typical lake-associated taxa, including Cyanobacteria, Chloroflexi, and Acidobacteriota. The composition of these taxa is significantly influenced by nutrient availability and hydrological dynamics33. Previous studies have indicated that bacterial communities in the aquatic systems of GFLs are assembled through deterministic processes, such as environmental filtering34,35. However, the mechanisms underlying the assembly of microbial communities in both GFLs and NGFLs remain poorly understood and insufficiently explored.
This study aimed to comprehensively investigate the differences in the microbial community structure of GFLs and NGFLs on the Tibet Plateau, as well as their influences of these communities on CH4 fluxes. Considering the significant biogeochemical changes that occur during the peak ablation stage, we systematically evaluated microbial diversity, microbial community compositions, and the relationships between these factors and CH4 fluxes in GFLs and NGFLs. Specifically, this study aimed to: (I) characterize the CH4 fluxes dynamics in lakes of different recharge types; (II) examine diversity features and compositional differences of microbial communities in lakes with different impacts from glaciers; (III) analyze the niche differentiation and environmental adaptability of microbial communities, thereby revealing the impact of environmental factor on microbial community structure; and (IV) explore correlation between microbial community structure and CH4 fluxes with the goal of elucidating the ecological functions of microbes in glacial lake ecosystems and their roles in the carbon cycle. As glacial lakes continue to undergo retreat, understanding the microbial community structures and CH4 fluxes dynamics in various lake types is crucial for enhancing our understanding of carbon cycling processes within the high-altitude lake ecosystems. This study represents one of the pioneering systematic comparisons of microbial composition, community assembly processes, and the CH4 fluxes pathways between GFLs and NGFLs on the Tibet Plateau. The results yield crucial insights into the biogeochemical feedback mechanism of the region in response to global climate change. Moreover, these findings furnish a scientific foundation for the formulation of targeted environmental protection measures and climate policies.
Results
Characteristics of physicochemical properties and CH4 fluxes in glacial lakes
GFLs were primarily impacted by glacial meltwater, whereas NGFLs were primarily replenished by precipitation and groundwater (Fig. 1). Significant differences in the meteorological and water quality characteristics were observed between GFLs and NGFLs (Fig. 2). The air temperature (AT) of NGFLs was significantly higher than that in GFLs (P = 0.008, ANOVA), whereas no significant differences were detected in atmospheric pressure (AP) and wind speed (WS) between the two types of lakes. Regarding water parameters, NGFLs showed higher values of WT, pH, dissolved oxygen (DO), salinity (Salt), and their EC was significantly greater than that of GFLs (P = 0.009, ANOVA). This indicated that NGFLs were more strongly influenced by evaporation and hydrological condition, likely leading to salt accumulation and changes in the physicochemical properties36. The TUR of GFLs was significantly higher than that in NGFLs (P = 0.032, ANOVA), suggesting that glacial meltwater introduced more suspended particles. Regarding the nutrients, phosphorus concentrations showed no substantial differences between the two lake types, but the nitrogen concentration was higher in NGFLs, with nitrate nitrogen (NO3--N) exhibiting significant difference (P = 0.042, ANOVA). Additionally, NGFLs had higher chlorophyll-a (Chl-a) concentrations, likely associated to elevated WT and increased nutrient availability. Higher WT facilitated phytoplankton growth and photosynthesis whereas the lower WT in GFLs driven by glacial meltwater input, restricted the phytoplankton development. The relatively high nitrogen and phosphorus concentrations in NGFLs further promoted phytoplankton growth, collectively contributing to the increased Chl-a concentration in NGFLs (Supplementary Table 2).
A Meteorological parameters and physicochemical properties of glacial lakes during different recharge types. Error bars represent the standard deviation (SD) of the measured values. B Total CH4 flux, C CH4 diffusion flux, and D CH4 ebullition flux in glacial lakes with different recharge types (**, P < 0.01; *, P < 0.05). The box plots with whiskers represent the 25–75% interquartile range (IQR) within 1.5 IQR, the black lines indicate the median, and the white squares represent the mean values.
Figure 2B–D illustrated the significant differences in CH4 fluxes characteristics between GFLs and NGFLs. The total CH4 flux, diffusive flux, and ebullitive flux in GFLs (n = 45) were 0.81 ± 0.92, 0.07 ± 0.02, and 0.74 ± 0.91 mmol·m−2·d−1, respectively, while for NGFLs (n = 20), the corresponding values for the total CH4 flux, diffusive flux, and ebullitive flux were 2.50 ± 2.53, 0.37 ± 0.23, and 2.13 ± 2.61 mmol·m-2·d-1, respectively. All these values were significantly higher than those observed in GFLs (P < 0.01, ANOVA). Additionally, it was observed that CH4 in glacial lakes was predominantly released into the atmosphere via ebullition (i.e., GFLs: 91.3%, NGFLs: 85.2%). The total CH4 flux in the NGFLs was approximately three times that of GFLs. These differences were probably closely linked to the variations in WT, nutrient availability, and hydrological characteristics. Based on these disparities in CH4 fluxes characteristics, we delved deeper into the influence of the formation time of glacial lakes on CH4 fluxes, as illustrated in Supplementary Fig. 2. As the formation time of glacial lakes advanced from prior to 1985 to after 2000, CH4 fluxes demonstrated a progressive decline. Glacial lakes that formed before 1985 had transformed into NGFLs which exhibited the highest total CH4 flux. GFLs, encompassing those formed between 1985 and 2000 and those formed after 2000, showed higher total CH4 fluxes when their formation times were earlier. In the meantime, the CH4 diffusion flux in GFLs from both of these time periods was relatively comparable, and the variations in total CH4 flux were primarily due to the ebullition flux.
Species diversity of prokaryotic microbial communities of glacial lakes
By analyzing multiple alpha diversity indices, as well as the OTU distribution, community composition, and relative abundance, large differences in microbial diversities and community structure are found between GFLs and NGFLs (Fig. 3). Specifically, the Chao1 index (Supplementary Table 3) for GFLs and NGFLs was 2145 ± 683 and 2785 ± 714, respectively, with NGFLs exhibiting a significantly higher Chao1 index than GFLs (P = 0.040, Wilcoxon rank sum test), indicating the greater species richness in NGFLs. However, the Pielou, Shannon, and Simpson indices did not show significant differences, suggesting that microbial communities in both lake categories were relatively evenly distributed and exhibited similar overall diversity levels (Fig. 3A and Supplementary Fig. 3). Owing to the disparities in the formation times of the glacial lakes, the microbial richness differed among these lakes. In GFLs, microbial richness was higher in lakes formed between 1985 and 2000 compared to those formed after 2000 (Supplementary Table 3). The Venn diagram showed that the GFLs and NGFLs shared 4715 OTUs, while NGFLs contained a larger number of unique OTUs (3735, accounting for 44.2%) (Fig. 3B). Non-metric multidimensional scaling analysis further revealed a distinct separation in the microbial community composition between GFLs and NGFLs, with a stress value of 0.064, an R-value of 0.610, and a P-value of 0.02. This result confirmed the differences in the community structure, as illustrated in Fig. 3C.
A Difference analysis of alpha diversity index of prokaryotic microbial communities (Wilcoxon rank sum test, **, P < 0.01; *, P < 0.05). The box plots with whiskers represent the 25–75% interquartile range (IQR) within 1.5 IQR, the black lines indicate the median, and the white squares represent the mean values. B Venn diagram showing the distribution of OTUs in glacial lakes with different replenishment types. C NMDS of microbial composition at the OTU level.
At the phylum level, Proteobacteria dominated in both lake types, followed by Bacteroidota and Actinobacteriota (Supplementary Fig. 4A). However, NGFLs showed a notably higher abundance of Chloroflexi and Acidobacteriota, while Cyanobacteria had a higher abundance in GFLs. At the genus level, Polaromonas, Rhodoferax, and Planktothrix were dominant in GFLs, and Cyanob- ium was more prevalent in NGFLs (Supplementary Fig. 4B). To gain a more comprehensive understanding of methane-cycling taxa, we conducted a screening of the taxonomic assignments for the well-known methanogens including Methanobacterium and Methanosarcina, and methanotrophs, including Methylovulum. The total relative abundance of methanogens was substantially higher in NGFLs than in GFLs (Supplementary Fig. 4C). In contrast, CH4-oxidizing bacteria were present at very low abundance in both GFLs and NGFLs. This distribution pattern aligns with the observed disparities in CH4 flux. It provides support for the idea that NGFLs host more active methanogenic communities, as depicted in Supplementary Fig. 4C.
The stability of prokaryotic microbial communities within glacial lakes
The microbial co-occurrence networks for GFLs and NGFLs revealed distinct interaction patterns among microbial taxa. In these networks, each node represented a microbial taxon, and different colors denoted different phylum-level classifications (Fig. 4). Comparatively, the network structure in GFLs was more complex, exhibiting a greater number of node and edges, indicating higher level of microbial diversity and stronger interspecies interactions (Supplementary Table 4). In contrast, the microbial co-occurrence network of NGFLs had displayed a more pronounced modular structure, characterized by dense intra-module connections and sparse inter-module interactions. Through topological role classification, it was found that the majority of nodes in both the GFLs and NGFLs networks were of a peripheral nature. However, the GFLs network contained several connectors (Pi > 0.62, Zi ≤ 2.5) and a small number of module hubs (Zi > 2.5), which indicated a greater degree of network integration. In sharp contrast, the NGFLs network had only one identified connector, and no module hubs were detected (Fig. 4B). Quantitative metrics further validated these disparities. GFLs showed significantly higher values for degree, betweenness, closeness, and eigenvector centrality (Fig. 4C), suggesting stronger connectivity and presence of more structurally influential microbial taxa. Specifically, the elevated degree centrality in GFLs signified that its nodes had a greater number of connections and the increased betweenness centrality indicated that these nodes functioned as essential bridges, facilitating communication between different modules within the network.
At the phylum level, keystone species in GFLs were predominantly affiliated with Proteobacteria, including OTU5999 (Ellin6067 and Nitrosomonadaceae), OTU2438 (Novosphingobium and Sphingomonadaceae), and OTU2893 (unclassified Hyphomicrobiaceae), all of which exhibited high centrality values and occupied key network positions. In addition, Cyanobacteria contributed one high-ranking keystone species, OTU2397 (Calothrix_KVSF5), associated with high connectivity within its module. In contrast, NGFLs contained only one taxon with inter-module connectivity: OTU3356 (Proteobacteria), the sole connector identified in that network. In microbial co-occurrence networks, both lake types exhibited around 75% positive correlations (Supplementary Table 4), indicating that microbial communities across lakes with different recharge types were predominantly governed by positive interactions, reflecting similar ecological niche preferences. Conversely, negative correlations, indicative of the potential competition or inhibition, constituted a relatively minor component within these microbial assemblages.
The assembly process of prokaryotic microbial communities in glacial lakes
To explore the potential factors driving variations in prokaryotic microbial communities, we carried out biogeographical analyses (Supplementary Fig. 5). These analyses were aimed at investigating the impacts of local environmental factors (i.e., deterministic processes) on the spatiotemporal distribution of biodiversity and the mechanisms that maintain such diversity. The two lake types, GFLs and NGFLs, exhibited distinct responses to environmental factors, revealing differences in their ecological niches and the adaptability of their species. To further investigate relative contributions of stochastic and deterministic processes, a null model based on βNTI was applied. Most βNTI values across the investigated lakes exceeded 2 (Fig. 5), with a more pronounced effect in NGFLs, indicating that the deterministic processes played a dominant role in shaping microbial community. Community structure modeling was employed to quantify the relative contribution of deterministic and stochastic processes to microbial community assembly37. For these lakes, heterogeneous selection was determined to be the most influential factor, molding 67% of the prokaryotic microbial community in GFLs and 74% in NGFLs (Fig. 5B). The Neutral community model (NCM) was used to fit the microbial communities of GFLs and NGFLs. A higher R2 value means a better fit to the neutral model, suggesting that the stochastic processes have a more significant role in community assembly. Conversely, lower R2 values imply that deterministic processes exert a dominant influence on community assembly38. Deterministic processes were found to be the dominant factor in shaping the community assembly (i.e., NGFLs: R2 = 0.210) (Fig. 5C, D). The migration rate (m) estimated using the neutral model serves as an indicator of species dispersal ability39. Both GFLs with a m value of 0.061 and NGFLs with an m value of 0.072 showed low migration rates. This means that species dispersal across the entire community was severely restricted40. Consequently, it further indicates that stochastic processes made only a minor contribution to the assembly of the microbial community, while deterministic processes were the dominant force, which is consistent with the findings from the null model analysis. In comparison, the assembly of the microbial community in GFLs adhered more closely to the neutral model, whereas that in NGFLs deviated from it. This divergence highlights that deterministic process had a more substantial impact on shaping the microbial communities in NGFLs.
A Changes in β nearest taxon index (βNTI) with replenishment type. B Contribution of different ecological processes in constructing prokaryotic microbial communities. C, D Neutral community modeling of eukaryotic planktonic microbial communities. The solid line indicates the best fit to the neutral community model, and the dashed line indicates the 95% confidence interval. m is the estimated mobility and R2 fit to the neutral community model.
Relationships between microbial communities, environmental factors, and FCH 4
After confirming that environmental factors primarily govern the distribution of prokaryotic microorganisms, we further examined the relationship between lake microbial communities and environmental variables. The Mantel test revealed that in GFLs, Chl-a exhibited the strongest correlation with the prokaryotic microbial community (i.e., r > 0.4, P < 0.01), whereas in NGFLs, TUR showed the strongest correlation (r > 0.4, P < 0.01) (Fig. 6A). Six environmental factors (i.e., WS, AT, WT, pH, ammonium nitrogen (NH4+-N), NO3--N) were negatively correlated with prokaryotic microbial composition in both GFLs and NGFLs. Meanwhile, eight factors (i.e., AP, TUR, Salt, total nitrogen (TN), total phosphorus (TP), total dissolved phosphorus (TDP), dissolved orthophosphate (PO43-), and Chl-a) exhibited significant correlations with microbial compositions in both lake types (i.e., r > 0.2, P < 0.05). To identify the key environment factors influencing microbial communities, we conducted the Spearman correlation analysis between the dominant bacterial phyla and environmental parameters. Across lakes with different recharge types, Bacteroidota was positively correlated with AP and NO3--N, whereas Chloroflexi was negatively correlated with AP, and Actinobacteriota showed a significant negative correlation with water TN (Fig. 6B). In the GFLs, Cyanobacteria, Actinobacteriota, and Chloroflexi were negatively correlated with water physical parameters and nutrients. Verrucomicrobiota was positively correlated with Salt, while Proteobacteria and Bacteroidota exhibited positive correlations with both physical parameters and nutrient concentrations. In NGFLs, Acidobacteriota showed significant positive correlations with Salt and EC, but a negative correlation with the Chl-a.
A Mantel test and correlation analysis between microorganisms and various factors and between various factors in glacial lakes with different recharge types (GFL vs NGFL). B Correlation between specific microorganisms and various environmental factors. C, D Correlation network between specific microorganisms and CH4 emission flux.
To further clarify how prokaryotic microbial communities affect CH4 fluxes in lakes with different recharge types and to explore the potential role of microorganisms in the GHG cycling, we performed microbial network correlation analyses (Fig. 6C, D). This analysis aimed to identify the regulatory differences of key microbial taxa on lake CH4 fluxes. In GFLs, the microbial community showed a highly interconnected association network with CH₄ emissions. Bacteroidota was significantly positively correlated with CH₄ emission. In contrast, Chloroflexi and Verrucomicrobiota showed significant negative correlations. The predominance of negative correlations indicated that CH4 fluxes in GFLs were mainly under inhibitory effects rather than stimulatory ones. In NGFLs, Actinobacteria, Bacteroidota, and Proteobacteria had been positively correlated with CH₄ emissions. Moreover, the negative correlations among microbial taxa were more pronounced, indicating that different microbial groups can impact CH4 fluxes via competitive interactions or resource-allocation mechanisms.
Discussion
The physicochemical characteristics of glacial lakes not only directly impact the production and emission of lake CH4 but also have an indirect influence by shaping microbial communities. This study demonstrated that NGFLs had higher WT and nutrient concentrations. In contrast, GFLs, marked by lower temperatures and restricted organic carbon availability, had CH4 fluxes that were merely one-third of those in NGFLs (Fig. 2). This difference can likely be attributed to high temperature sensitivities of methanogen metabolisms24. Warm, nutrient-rich condition promotes CH4 production in lakes (Fig. 6), while cold, oligotrophic conditions could impede methanogenesis41. Owing to the continuous inflow of glacial meltwaters, GFLs showed high water renewal rate and strong hydrodynamic condition. The significant influx of cold meltwater not only lowered water temperatures but also enhanced lake circulation and exchanges42. This dynamic hydrological regime led to a shorter water residence time, thus limiting accumulation of CH4 in the water columns. Frequent hydrodynamic disturbances further enabled the swift release of small gas bubbles from the sediments into the water column. This process inhibited CH4 from accumulating to the levels required for formation of large gas bubbles, consequently promoting continuous, low-intensity emissions43.
In contrast, due to the absence of external hydrological inputs, NGFLs presented a relatively enclosed and tranquil water environment44. With a long residence time, stable stratification, and reduced vertical mixing45, CH4 generated within NGFLs was more likely to accumulate in the water column. Once the CH4 concentration reached a critical threshold, it was abruptly released in the form of bubbles. Extended water retention time in NGFLs, combined with concentration effect caused by evaporation, led to a higher ionic concentration and an increase in EC. These factors, in turn, promoted CH4 fluxes (Figs. 2 and 6A). Additionally, the higher Chl-a concentration in NGFLs indicated higher primary productivity, signifying enhanced production of autochthonous organic matter. This organic matter then served as a substrate for sediment decomposition46. In anoxic environments, the breakdown of organic matter by microorganisms promoted the generation of CH447.
The environmental gradients generated by variations in physicochemical properties of glacial lakes have caused the microbial communities in GFLs and NGFLs to diverge. GFLs, dominated by glacial meltwater input, were characterized by cold, oligotrophic condition, whereas NGFLs received greater organic matter and nutrient input from precipitation runoff and groundwater, shaping distinct microbial community structures and then influencing CH4 production (Fig. 6). Specifically, taxa facilitating CH4 production include Bacteroidota, Actinobacteriota, and Proteobacteria. Bacteroidota contribute to degradation of organic matter, generating substrates necessary for methanogenesis48. Actinobacteriota decompose recalcitrant carbon, releasing some fermentable compounds49. Anaerobic Proteobacteria, particularly the sulfate-reducing groups, create favorable redox conditions for methanogenic activity50. In contrast, taxa that constrain CH4 fluxes include Chloroflexi and Verrucomicrobiota. Chloroflexi could inhibit methano-genesis by competing for shared substrates, such as hydrogen and acetate, or through syntrophic interactions. Verrucomicrobiota, including members of the genus Methylacidiphilum, possess the ability to oxidize CH4 under certain conditions. As a result, they function as biological sinks for CH4, playing a crucial role in regulating CH4 levels in their respective ecosystems51. These interpretations were consistent with the observed differences in the CH4-cycling microbial groups. As the results indicated, methanogens were more prevalent in NGFLs. This finding mirrored more conducive conditions for methanogenesis in these lakes, including higher temperatures, elevated nutrient concentrations, and extended periods of anoxia. Conversely, CH4-oxidizing bacteria were present at low abundances in both types of lakes, with only slightly higher levels in GFLs. This imbalance between CH4 production and consumption pathways likely accounts for the significantly higher emissions observed in NGFLs, underscoring the limited capacity of biological sinks to mitigate methane release in these ecosystems. Looking ahead, as climate change accelerates glacial retreat and diminishes the supplies of glacial meltwater, a transition from GFLs to NGFLs may take place in an increasing number of lakes. This shift has potential to enhance the CH4 fluxes potential across high-altitude lake ecosystems. In this regard, the formation time of glacial lakes exerts a substantial influence on the CH4 fluxes pattern. Older lakes, especially those formed prior to 1985, tend to exhibit higher CH4 fluxes. These older lakes probably experienced a more extended period of microbial community development and adaptation, leading to more vigorous methanogenesis52.
The diversity and ecological functions of microbial communities in the glacial lakes were significantly influenced by the type of recharge (Figs. 3 and 4). The alpha-diversity indicted in NGFLs were significantly higher than those in GFLs, likely due to the strong selective pressures imposed by the low temperature, high TUR, and low-nutrient conditions in GFLs. These extreme conditions limit microbial survival to only those taxa adapted to such harsh environments53. Beta-diversity analysis revealed that recharge type exerts a determinative role in shaping the microbial community composition54. This differentiation was primarily driven by variations in lake environmental conditions, a finding further supported by Mantel tests and Spearman correlation analyses (Fig. 6). In GFLs, the cold and oligotrophic environmental condition may lead to lower microbial diversity. However, the microbial interactions within the community were stronger, contributing to the community stability and enhanced resistance to disturbances. In contrast, NGFLs, influenced by higher exogenous nutrient inputs, exhibited overall weaker microbial network connectivity, but stronger synergistic interactions among the specific taxa within localized modules. This pattern shows a shift toward functional clustering driven by nutrient enrichment where cooperative interactions are enhanced within communities despite looser global connectivity. The topological structure of microbial networks played a crucial role in regulating the stability of lake ecosystem and the dynamics of microbial communities (Fig. 4B, C). In this study, the microbial network in GFLs exhibited stronger negative correlations and higher modularity, meaning that microbial communities in these lakes were more reliant on competitive interaction and environmental filtering. These factors can contribute to the long-term stability of communities, allowing specific functional microbial groups to maintain the essential ecological functions even under the extreme environmental conditions55,56. In contrast, NGFLs, characterized by a lower microbial network connectivity and looser inter-species association, likely exhibited weaker ecological stabilities57. In the eutrophic lakes, microbial communities were more dynamic, with community structures being more susceptible to nutrient inputs and environmental disturbances. The external organic matter supply could drive fluctuations in the microbial community dynamics, leading to greater structural variabilities in response to nutrient levels, which subsequently influenced CH4 production and carbon cycling processes58,59.
Through null model analysis and neutral model analysis, we found that the assembly of prokaryotic microbial communities in the glacial lakes of different recharge types (i.e., GFLs and NGFLs) was predominantly governed by the deterministic processes. Previous studies have confirmed that in cold or extreme environments, environmental filtering typically outweighs stochastic drift, driving the structure and succession patterns of microbial communities60. Similarly, in a study of the Yarlung Zangbo River, Yang, et al.61 observed that eukaryotic microbial assembly was primarily driven by the deterministic processes. In another typical extreme environment, terrestrial hot spring ecosystems, microbial community assembly was also found to be deterministically structured, with temperature identified as the primary factor62. However, in the Yangtze River Basin (spanning 4300 km), it was found that planktonic bacterial communities were more influenced by stochastic processes, with population dynamics primarily shaped by drift and dispersal rather than environmental selection63. This finding suggests that in hydrologically dynamic systems, microbial dispersal may weaken the effect of environment selection64, thereby leading to higher variabilities in the community structures. By contrast, microbial community assembly in the glacial lakes was more constrained by cold and oligotrophic conditions over the long term, leading to a greater reliance on specific adaptive strategies, which reinforced the dominance of deterministic processes65. Additionally, glacial meltwater input has been recognized as a key mechanism for microbial dispersal in alpine lakes, and the NCM effectively quantifies the impact of dispersal limitation on community assembly66. In this study, the migration rate of NGFLs (m = 0.065) was lower than that of the GFL (m = 0.075), indicating that absence of continuous glacial meltwater input in NGFLs led to greater constraints on microbial dispersal. Consequently, the absence of continuous glacial meltwater in NGFLs led to stronger dispersal limitation and reinforced environmental filtering, making the deterministic processes more dominant compared to GFLs.
Glacial lakes represent highly sensitive ecosystems under the background of global climate change, functioning not only as critical indicators of regional ecological shifts but also actively participating in and profoundly influencing global carbon cycling and GHGs emissions. With ongoing reductions of ice-covered area and accelerating glacier melts, alterations in ecological functions of glacial lakes are likely to enhance global emissions of GHGs, thereby reinforcing climate feedback mechanism and potentially contributing to an increased frequency of extreme climatic events. Furthermore, ecological transformations in glacial lakes could pose substantial threats to global freshwater availability, particularly for the downstream regions predominantly reliant on the glacial meltwater, where hydrological stability and ecosystem services are at risk. Concurrently, ecological changes in glacial lake systems have potential to disrupt biodiversity patterns. This disruption especially impacts species adapted to extreme environments and alters the microbial community structure. As a result, ecosystem functionality and service capacities are modified, and there is a risk of triggering the extensive ecological cascades. Since the glacial lake ecosystem changes are occurring widely across multiple alpine regions worldwide (e.g., the Himalayas, the Andes, and the Alps), the findings of this study could provide critical insight and valuable references for ecological managements and environmental policy formulation in similar regions globally.
Conclusions
This study provides a comprehensive assessment of microbial community structures and CH4 fluxes in the glacial lakes with different hydrological recharge types (i.e., GFLs and NGFLs) in extreme environment (with an altitude of 5000‒6000 m a.s.l). The results demonstrated that lake hydrological regimes largely influenced microbial diversity, network connectivity and CH4 fluxes, with NGFLs exhibiting markedly higher CH₄ flux than GFLs. Microbial communities in GFLs were mainly composed of cold-adapted and oligotrophic taxa. In contrast, the NGFLs supported microbial assemblages that were linked to greater nutrient availability and extended water residence times. Microbial co-occurrence networks suggested that in GFLs, microbial interactions were stronger, which enhanced community stability. On the other hand, NGFLs displayed a modular structure and the connectivity between modules was weaker. This indicates that the microbial community in NGFLs was more sensitive and adaptable to environmental fluctuations. Moreover, the community assembly processes varied between the two lake types. Although deterministic factors were the predominant drivers in both, NGFLs underwent more intense environmental filtering. This was due to restricted microbial dispersal, which shaped the composition and structure of their microbial communities. These findings underscore the significant ecological consequences of glacial retreat and hydrological changes in high-altitude lakes, providing valuable insights into how these fragile ecosystems could respond to ongoing environmental shifts.
Materials and methods
Study area
The study area was situated in the Lhasa River Valley on the Tibetan Plateau, encompassing a typical mountain glacier (29°51’N, 90°12’E) located on the southern edge of the Nyainqêntanglha Mountains, serving as a crucial headwater region for the Lhasa River67. This area, with an elevation ranging from 5000 to 6000 meters above sea level (a.s.l.), exhibits a trough-valley topography that descends from south to north. The glacier covers an area of 6.0 square kilometers, accounting for 19% of the total catchment area68. The distribution of lakes in the study area is shown in Fig. 1. Lakes L1 to L5 were classified as GFLs, while lakes L6 to L8 were categorized as NGFLs. This region is characterized by a typical high-altitude, low-temperature and low-oxygen extreme environment, displaying distinct climatic features69,70. The annual average air temperature in this region was 2.5 °C, with the maximum and minimum temperatures of 23.4 °C and −25.7 °C, respectively71. Several factors such as significant diurnal temperature fluctuations, intense ultraviolet radiation, low humidity, and low atmospheric pressure, have a unique impact on ecological environment and microbial communities72,73. The formation periods of the investigated lakes (Supplementary Table 1) were determined using satellite remote sensing images from Google Earth (www.google.com/earth/). Field observations had been conducted over a 2-month period, specifically during the peak ablation stage of glaciers from July to August 2023, on the eight glacial lakes. The geographical coordinates and elevation of the lakes were measured with a handheld GPS device (Triton-500, Magellan, USA). Supplementary information (Supplementary Table 1 and Fig. 1) provides detailed data on the sampling locations.
Sample collection and processing
During the sample collection process, water samples were retrieved from the surface, middle, and bottom layers of each lake by means of a 5-liter deep-water sampler (JC-800, manufactured by Juchuang Environment, China). All the water samples were initially filtered through a 200 μm nylon mesh to remove insoluble impurities. Subsequently, the filtered water was transferred into pre-rinsed 2-liter polyethylene bottles for the microbiological analyses and 500-milliliter polytetrafluoroethylene bottle for nutrients and Chl-a analyses74. A total of 24 microbiological samples were collected. In situ water quality parameters were measured using a portable multi-parameter water quality analyzer (HI 98195, Hanna Instruments) to determine lake WT, pH, EC, DO, Salt, and TUR. Concurrently, the local WS and AT were measured with a DLY-1602C anemometer (Delixi, China), and the AP was recorded by a DYM3 photosynthetic barometer (Longtuo, China). In the laboratory, the water samples were further filtered through 0.22 μm polycarbonate membranes. The filtered membranes were collected and stored in liquid nitrogen to maintain the integrity of microbial DNA for subsequent DNA extraction and high-throughput sequencing that was carried out at Majorbio (Shanghai, China). The concentrations of TN, NH4+-N, and NO3--N had been measured by means of spectrophotometry in combination with chemical oxidation, colorimetry, and ion chromatography, respectively. For TP, TDP, and PO43-, their concentrations were measured using ammonium molybdate spectrophotometric method.
Gas sampling, which is a complex and challenging task, was carried out at water-air interface through the use of the static flux chamber method (Supplementary Fig. 1). An optimally designed floating flux chamber was utilized to minimize the influences of environmental WS and turbulence, thereby ensuring effective sealing75. The chamber was gently immersed ~3 cm into the water and kept stable during the sampling process. At each sampling location, the chamber was positioned on the water surface, and the gas samples were collected in sequence. In order to guarantee the representativeness and accuracy of the data, gas samples were gathered at five specific time intervals (namely 0, 10, 20, 30, and 40 min), with 500 milliliters of gas being collected at each time point. This sampling procedure ensured a comprehensive capture of the gas dynamics, leading to a total of 65 gas samples being obtained.
DNA extraction and high-throughput sequencing
After thawing the samples for 24 h, total DNA was extracted using the E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, USA) based on the manufacturer’s instruction. The concentration and purity of the extracted DNA were evaluated utilizing a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA) to guarantee their suitability for the subsequent analyses. The V3-V4 region of the 16S rRNA gene was amplified by means of primers 515F (5′-GTGYCAG- CMGCCGCGGTAA-3′) and 806R (5′-GGACTACNVGGGTWTCTAAT-3′). The primers are designed to target both bacterial and archaeal 16S rRNA genes76. The PCR products had been examined via 2% agarose gel electrophoresis. Then, the qualified products were purified using the GeneJet Gel Recovery Kit and employed for library construction with the Illumina TruSeq DNA PCR-Free Sample Preparation Kit77. The constructed libraries were sequenced on the Illumina NovaSeq 6000 platform using 250 bp paired-end reads. The raw sequencing data underwent quality control via the Fastp software and were assembled using the Flash software. Then, the sequences were clustered into operational taxonomic units (OTUs) based on a 97% similarity threshold through the Uparse software, and the chimeric sequences were removed. Finally, species taxonomy annotation was carried out using the RDP classifier. It was based on the Silva 16S rRNA database (v138), and a confidence threshold of 0.7 was applied to obtain the species classification results78.
Estimations of CH4 fluxes in the glacial lakes
According to Henry’s law, the gas concentration in the gaseous phase was converted into the dissolved gas concentration in the liquid phase, while accounting for the potential background gas contamination79. To estimate the CH4 flux, a revised van Bergen diffusion flux equation80 was utilized. This equation estimated the flux by measuring the variations in CH4 concentration within the chamber headspace at specific time intervals, as depicted in Eq. 1.
where F denotes the total CH4 flux (mmol·m−2·d−1); ∆C is the difference in CH4 concentration (ppm) at the top of the flux chamber between times t and t0. P denotes the ambient atmospheric pressure on-site (Pa); V refers to the volume of the flux chamber (m3); A represents the contact area between the bottom of the chamber and the lake surface (m2); t is the sampling interval (min); R is the universal gas constant (8.314 m3·Pa−1·mol−1·K−1), and T denotes the AT recorded during measurement, expressed in Kelvin (K).
To estimate the relative contributions of diffusive and ebullitive CH4 fluxes to the total CH4 fluxes, the dual-layer model was employed81. The diffusive CH4 flux (Fd, mmol·m−²·d−¹) was calculated according to the field-measured WT, salinity, and the concentration gradient of CH4 between the headspace vial and the atmosphere82, as shown in Eq. 2. The ebullitive CH4 flux (Fe, mmol·m−2·d−1) was defined as the difference between total CH4 flux and diffusive CH4 flux, as specified in Eq. 3. The calculation process of other intermediate parameters was provided in Supplementary Note 1.
where KX represents the CH4 transfer rate (cm·h−1), and Cobs is the dissolved concentration of CH4 in surface water (μmol·L−1), calculated based on partial pressures of CH4 in the atmosphere and its Henry’s constant83; Csat is the saturated CH4 concentration in the water (μmol·L−1).
Statistical analyses
All data processing and calculations were performed using Excel 2021, and data visualization was conducted in the Origin 2021. One-way ANOVA was conducted using IBM SPSS Statistics 27, with statistical significance level set at P < 0.05 (*) and P < 0.01 (**). The distribution map of sampling sites was generated using ArcGIS 10.8. All microbial data analyses were conducted in the R (V 4.4.1). Alpha diversity indices of microbial communities were calculated using the boot and stats packages, and the significance was calculated using the Wilcoxon rank sum test. The ape, psych, vegan, and ade4 packages were employed for the non-metric multidimensional scaling (NMDS) based on Bray-Curtis distances and ANOSIM analysis to evaluate differences in bacterial community compositions84. Community bar plots were generated based on the data tables from the tax_summary_a folder. The vegan package was used for Mantel-test analysis and correlation assessment between environmental factors, with the results visualized in Mantel test network heatmap. The contribution network analysis was conducted using the microeco, magrittr, htmlwidgets, and networkD3 packages. The icamp package was applied to calculate the β-nearest taxon index (βNTI) within the null model. Neutral community model (NCM) was analyzed using the Hmisc, minpack.lm, and getopt packages. The correlation matrix plots were generated using the linkET, reshape2, and ggplot2 packages in R.
Data availability
All the Illumina sequence data have been deposited in the National Center for Biotechnology Information (NCBI), and the accession number is PRJNA1238012. The methane fluxes data and microbial co-occurrence network data have been deposited in the Zenodo repository under https://doi.org/10.5281/zenodo.15585715.
Change history
05 July 2025
A Correction to this paper has been published: https://doi.org/10.1038/s43247-025-02521-9
References
Pi, K. F. et al. The cold region critical zone in transition: responses to climate warming and land use change. Annu. Rev. Environ. Resour. 46, 111–134 (2021).
Williamson, C. E. et al. Lakes and reservoirs as sentinels, integrators, and regulators of climate change. Limnol. Oceanogr. 54, 2273–2282 (2009).
Hood, E. et al. Storage and release of organic carbon from glaciers and ice sheets. Nat. Geosci. 8, 91–96 (2015).
Rosentreter, J. A. et al. Half of global methane emissions come from highly variable aquatic ecosystem sources. Nat. Geosci. 14, 225–230 (2021).
Masson-Delmotte, V. et al. Climate change 2021: the physical science basis. Contribution Working Group I Sixth Assessment of the Intergovernmental Panel On Climate Change. Cambridge University Press. 2, 2391 (2021).
Liu, L. X. et al. Timescale dependence of environmental controls on methane efflux from Poyang Hu, China. Biogeosciences 14, 2019–2032 (2017).
Santoso, A. B. et al. High contribution of methane in greenhouse gas emissions from a eutrophic lake: a mass balance synthesis. N. Z. J. Mar. Freshw. Res. 55, 411–430 (2021).
Zhao, F. M. et al. Linking glacier retreat with climate change on the Tibetan Plateau through satellite remote sensing. Cryosphere 18, 5595–5612 (2024).
Shugar, D. H. et al. Rapid worldwide growth of glacial lakes since 1990. Nat. Clim. Chang. 10, 939–945 (2020).
Dou, X. Y. et al. Spatio-temporal evolution of glacial lakes in the Tibetan Plateau over the past 30 years. J. Remote. Sens. 15, 416 (2023).
Slemmons, K. E. et al. The influence of glacial meltwater on alpine aquatic ecosystems: a review. Environ. Sci. Process. Impacts 15, 1794–1806 (2013).
Yin, Y. et al. Characteristics and influence factors of the glacial lake changes in china from 1990 to 2020. J. Lake Sci. 35, 358–367 (2023).
Liu, K. S. et al. Glacier retreat induces contrasting shifts in bacterial biodiversity patterns in glacial lake water and sediment: bacterial communities in glacial lakes. Microb. Ecol. 87, 128 (2024).
Shirokova, L. S. et al. Biogeochemistry of organic carbon, CO2, CH4, and trace elements in thermokarst water bodies in discontinuous permafrost zones of Western Siberia. Biogeochem 113, 573–593 (2013).
Bastviken, D. et al. Freshwater methane emissions offset the continental carbon sink. Science 331, 50–50 (2011).
Yan, F. P. et al. Isotopic composition and emission characteristics of CO2 and CH4 in glacial lakes of the Tibetan Plateau. Environ. Res. Lett. 18, 094025 (2023).
Schrier-Uijl, A. P. et al. Release of CO2 and CH4 from lakes and drainage ditches in temperate wetlands. Biogeochem 102, 265–279 (2011).
Egholm, D. L. et al. Coupling the flow of ice, water, and sediment in a glacial landscape evolution model. Geormorphology 141, 47–66 (2012).
Liu, K. S. et al. Fate of glacier surface snow-originating bacteria in the glacier-fed hydrologic continuums. Environ. Microbiol. 23, 6450–6462 (2021).
Peter, H. et al. Changes in bacterioplankton community structure during early lake ontogeny resulting from the retreat of the Greenland Ice Sheet. ISME J. 12, 544–555 (2018).
Sommaruga, R. When glaciers and ice sheets melt: consequences for planktonic organisms. J. Plankton Res. 37, 509–518 (2015).
Mayr, M. J. et al. Niche partitioning of methane-oxidizing bacteria along the oxygen–methane counter gradient of stratified lakes. ISME J. 14, 274–287 (2020).
Kleinteich, J. et al. Glacier melt-down changes habitat characteristics and unique microbial community composition and physiology in alpine lake sediments. FEMS Microbiol. Ecol. 98, fiac075 (2022).
Jansen, J. et al. Global increase in methane production under future warming of lake bottom waters. Glob. Chang. Biol. 28, 5427–5440 (2022).
Liu, K. S. et al. Bacterial community changes in a glacial-fed Tibetan lake are correlated with glacial melting. Sci. Total Environ. 651, 2059–2067 (2019).
Yao, T. D. et al. Asian water tower change and its impacts. Bull. Chin. Acad. Sci. 34, 1203–1209 (2019).
Liu, Y. Q. et al. A genome and gene catalog of glacier microbiomes. Nat. Biotechnol. 40, 1341–1348 (2022).
Crump, B. C. et al. Microbial diversity in arctic freshwaters is structured by inoculation of microbes from soils. ISME J. 6, 1629–1639 (2012).
Echeverría-Vega, A. et al. Watershed-induced limnological and microbial status in two oligotrophic andean lakes exposed to the same climatic scenario. Front. Microbiol. 9, 00357 (2018).
Zhang, Z. H. et al. Supraglacial and subglacial ecosystems contribute differently towards proglacial ecosystem communities in Kuoqionggangri Glacier, Tibetan Plateau. Commun. Earth Environ. 5, 636 (2024).
Mackelprang, R. et al. Metagenomic analysis of a permafrost microbial community reveals a rapid response to thaw. Nature 480, 368–371 (2011).
Ruuskanen, M. O. et al. Microbial genomes retrieved from High Arctic lake sediments encode for adaptation to cold and oligotrophic environments. Limnol. Oceanogr. 65, S233–S247 (2020).
Ouyang, J. W. et al. Global warming induces the succession of photosynthetic microbial communities in a glacial lake on the Tibetan Plateau. Water Res. 242, 120213 (2023).
Wilhelm, L. et al. Microbial biodiversity in glacier-fed streams. ISME J. 7, 1651–1660 (2013).
Peter, H. & Sommaruga, R. Shifts in diversity and function of lake bacterial communities upon glacier retreat. ISME J. 10, 1545–1554 (2016).
Soued, C. et al. Salinity causes widespread restriction of methane emissions from small inland waters. Nat. Commun. 15, 717 (2024).
Yuan, H. Y. et al. Nexus of stochastic and deterministic processes on microbial community assembly in biological systems. Front. Microbiol. 10, 1536 (2019).
Dini-Andreote, F. et al. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl Acad. Sci. USA 112, 1326–1332 (2015).
Ricklefs, R. E. The unified neutral theory of biodiversity: do the numbers add up? Ecology 87, 1424–1431 (2006).
Zhang, Z. X. et al. Analysis of bacterial community structure, functional variation, and assembly mechanisms in multi-media habitats of lakes during the frozen period. Ecol. Environ. Saf. 284, 116903 (2024).
Xie, G. J. et al. Extreme trophic tales: deciphering bacterial diversity and potential functions in oligotrophic and hypereutrophic lakes. BMC Microbiol. 24, 348 (2024).
Peter, H. & Sommaruga, R. Alpine glacier-fed turbid lakes are discontinuous cold polymictic rather than dimictic. Inland Waters 7, 45–54 (2017).
Bastviken, D. et al. Methane emissions from lakes: dependence of lake characteristics, two regional assessments, and a global estimate. Glob. Biogeochem. Cycles 18, GB4009 (2004).
Gao, T. G. et al. Summer hydrological characteristics in glacier and non-glacier catchments in the Nam Co Basin, southern Tibetan Plateau. Environ. Earth Sci. 74, 2019–2028 (2015).
Walter, K. M. et al. Methane production and bubble emissions from arctic lakes: Isotopic implications for source pathways and ages. J. Geophys. Res. Biogeosci. 113, G00A08 (2008).
Mu, G. L. et al. Characteristics of nutrients and microbial communities in proglacial lakes on the Tibetan Plateau and their potential linkages associated with mercury. J. Hazard. Mater. 492, 138117 (2025).
Huang, M. Q. et al. Methane cycling in typical emerging proglacial lakes on the Tibetan Plateau: insights into the metabolic mechanisms mediated by microorganisms. Water Res. 280, 123533 (2025).
Huang, J. J. et al. Successional action of Bacteroidota and Firmicutes in decomposing straw polymers in a paddy soil. Environ. Microbiome. 18, 76 (2023).
Bao, Y. Y. et al. Important ecophysiological roles of non-dominant Actinobacteria in plant residue decomposition, especially in less fertile soils. Microbiome 9, 1–17 (2021).
Dar, S. A. et al. Competition and coexistence of sulfate-reducing bacteria, acetogens and methanogens in a lab-scale anaerobic bioreactor as affected by changing substrate to sulfate ratio. Appl. Microbiol. Biotechnol. 78, 1045–1055 (2008).
Schmitz, R. A. et al. The thermoacidophilic methanotroph Methylacidiphilum fumariolicum SolV oxidizes subatmospheric H2 with a high-affinity, membrane-associated [NiFe] hydrogenase. ISME J. 14, 1223–1232 (2020).
Yakimovich, K. M. et al. Lake characteristics influence how methanogens in littoral sediments respond to terrestrial litter inputs. ISME J. 14, 2153–2163 (2020).
Gao, C. & Guo, L. D. Progress on microbial species diversity, community assembly and functional traits (In Chinese). Biodivers. Sci. 30, 22429 (2022).
Mindl, B. et al. Factors influencing bacterial dynamics along a transect from supraglacial runoff to proglacial lakes of a high Arctic glacieri. FEMS Microbiol. Ecol. 59, 307–317 (2007).
Shu, W. S. & Huang, L. N. Microbial diversity in extreme environments. Nat. Rev. Microbiol. 20, 219–235 (2022).
Hernandez, D. J. et al. Environmental stress destabilizes microbial networks. ISME J. 15, 1722–1734 (2021).
Yuan, M. M. et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Chang. 11, 343–348 (2021).
Han, L. et al. Lake microbial communities and their mediated carbon cycling processes (In Chinese). Chin. Bull. Life Sci. 35, 1613–1629 (2023).
Langenheder, S. & Lindström, E. S. Factors influencing aquatic and terrestrial bacterial community assembly. Environ. Microbiol. Rep. 11, 306–315 (2019).
Wu, M. H. et al. Soil microbial distribution and assembly are related to vegetation biomass in the alpine permafrost regions of the Qinghai-Tibet Plateau. Sci. Total Environ. 834, 155259 (2022).
Yang, Q. et al. Distribution patterns and community assembly processes of eukaryotic microorganisms along an altitudinal gradient in the middle reaches of the Yarlung Zangbo River. Water Res. 239, 120047 (2023).
He, Q. et al. Temperature and microbial interactions drive the deterministic assembly processes in sediments of hot springs. Sci. Total Environ. 772, 145465 (2021).
Yi, M. L. et al. Distinct community assembly processes underlie significant spatiotemporal dynamics of abundant and rare bacterioplankton in the Yangtze River. Front. Environ. Sci. Eng. 16, 1–14 (2022).
Evans, S. et al. Effects of dispersal and selection on stochastic assembly in microbial communities. ISME J. 11, 176–185 (2017).
Lee, H. et al. Microbial assemblages and associated biogeochemical processes in Lake Bonney, a permanently ice-covered lake in the McMurdo Dry Valleys, Antarctica. Environ. Microbiome. 19, 1–15 (2024).
Sloan, W. T. et al. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 8, 732–740 (2006).
Yao, T. D. et al. Recent glacial retreat and its impact on hydrological processes on the Tibetan Plateau, China, and surrounding regions. Arct. Antarct. Alp. Res. 39, 642–650 (2007).
Yao, T. D. et al. Different glacier status with atmospheric circulations in Tibetan Plateau and surroundings. Nat. Clim. Chang. 2, 663–667 (2012).
Niu, T. et al. The characteristics of climate change over the Tibetan Plateau in the last 40 years and the detection of climatic jumps. Adv. Atmos. Sci. 21, 193–203 (2004).
Yang, M. X. et al. Permafrost degradation and its environmental effects on the Tibetan Plateau: a review of recent research. Earth-Sci. Rev. 103, 31–44 (2010).
Wang, B. et al. Tibetan Plateau warming and precipitation changes in East Asia. Geophys. Res. Lett. 35, L14702 (2008).
Zhang, G. Q. et al. An inventory of glacial lakes in the Third Pole region and their changes in response to global warming. Glob. Planet. Chang. 131, 148–157 (2015).
Merino, N. et al. Living at the extremes: extremophiles and the limits of life in a planetary context. Front. Microbiol. 10, 780 (2019).
Talukdar, P. et al. A review of water quality models and monitoring methods for capabilities of pollutant source identification, classification, and transport simulation. Rev. Environ. Sci. Biotechnol. 22, 653–677 (2023).
Li, J. H. et al. Summer greenhouse gases exchange flux across water-air interface in three water reservoirs located in different geologic setting in Guangxi, China (In Chinese). Environ. Sci. 36, 4032–4042 (2015).
Caporaso, J. G. et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl Acad. Sci. USA 108, 4516–4522 (2011).
Kozich, J. J. et al. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence Data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120 (2013).
Nguyen, N. P. et al. A perspective on 16S rRNA operational taxonomic unit clustering using sequence similarity. Biofilm. Microbiome. 2, 16004 (2016).
Musenze, R. S. et al. Methane and nitrous oxide emissions from a subtropical estuary (the Brisbane River estuary, Australia). Sci. Total Environ. 472, 719–729 (2014).
van Bergen, T. J. et al. Seasonal and diel variation in greenhouse gas emissions from an urban pond and its major drivers. Limnol. Oceanogr. 64, 2129–2139 (2019).
Bastviken, D. et al. Methane emissions from Pantanal, South America, during the low water season: toward more comprehensive sampling. Environ. Sci. Technol. 44, 5450–5455 (2010).
Turner, P. A. et al. Regional‐scale controls on dissolved nitrous oxide in the Upper Mississippi River. J. Geophys. Res. Lett. 43, 4400–4407 (2016).
Landschützer, P. et al. Decadal variations and trends of the global ocean carbon sink. J. Geophys. Res. Biogeosci. 30, 1396–1417 (2016).
Fuhrman, J. A. Microbial community structure and its functional implications. Nature 459, 193–199 (2009).
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Nos. U24A20640, 42377399, and 42122059), the Second Tibetan Plateau Scientific Expedition and Research Program (No. 2019QZKK0605), and the Key Research and Development Project of Xizang Autonomous Region (No. XZ202301ZY0021G). Dr. Yindong Tong was also funded by the Distinguished Professor Project in Tibet University. No special permissions were required to access the sampling sites on the Tibetan Plateau. The study was conducted in publicly accessible areas, and all necessary local permissions were obtained as required.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, visualization, and writing–original draft, S. Liu; investigation, and methodology, F.Y. Mai, X.D. Li, M.Q. Huang, and Q. Yang; investigation, L.Y. Lu and G.L. Mu; supervision, writing—review & editing, Q.G. Zhang, Y.W. Liu and Y.D. Tong; funding acquisition, Y.D. Tong.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Haihan Zhang and Alice Drinkwater. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, S., Mai, F., Li, X. et al. Glacier-fed lakes produce lower methane fluxes than non-glacier-fed lakes on the Tibetan Plateau. Commun Earth Environ 6, 465 (2025). https://doi.org/10.1038/s43247-025-02464-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-025-02464-1