Abstract
Bioarchaeology not only provides insights into human, animal, and environmental ecology, but also generates huge amounts of stable and radiogenic isotope data that are not well recognised by other disciplines. Here, we present potential avenues for the integration and interpretation of archaeological isotope data into environmental studies. We emphasise the large spatio-temporal scales on which isotope patterns can be observed, for example using isoscapes, the limitations and potential pitfalls that come with isotope data from archaeological research, and future cross-disciplinary collaborations between bioarchaeology and other palaeo-disciplines.
Similar content being viewed by others
Introduction
Isotope analyses were introduced in archaeological research in the late 1970s, when J. C. Vogel and N. J. van der Merwe applied carbon isotope analyses to investigate maize cultivation in North America1. In 1985, J. E. Ericson2 provided the basis for mobility studies using strontium isotopes. A few decades later, isotope analyses of carbon (δ13C), nitrogen (δ15N), oxygen (δ18O), radiogenic strontium (87Sr/86Sr)3,4,5 and increasingly sulphur (δ34S)6, hydrogen (δD)7, stable strontium (δ88Sr)8, zinc (δ66Zn)9, lead (206Pb/204Pb, 207Pb/204Pb, 208Pb/204Pb)10, neodymium (143Nd/144Nd)11 and also compound specific approaches12 have become common tools in archaeological research.
In bioarchaeology, i.e. the study of biological remains including humans, faunal and flora (but not restricted to osteoarchaeology), isotope sampling is limited to a fraction of sites (depending on preservation and access) and the research questions are formulated by archaeological interest. The resulting archives consist of individual high-quality records (Supplementary Note 1) in which 87Sr/86Sr, 208Pb/206Pb, and more recently 143Nd/144Nd isotopes are mostly dedicated to mobility studies10,11,13, δ18O and δD to mobility and climatic reconstruction14,15 and δ13C, δ15N, δ2H, δ66Zn16 and δ34S isotopes are applied to investigate dietary, social, environmental, climatic and agricultural changes17,18,19. The sample size strongly varies depending on the goals of the study: a single individual can represent an entire case study, whereas community-level targeted investigations can comprise sample sizes of many tens of individuals20. Various isotope systems are moreover increasingly combined into multi-proxy and multi-tissue approaches to improve the measured signals11,19,21, to contrast differing signals related to skeletal remodelling and isotope turnover processes22, and to minimise the negative effects of destructive sampling22,23,24. To our knowledge no study has yet integrated δ13C, δ15N, δ18O, δ34S, 87Sr/86Sr, and 208Pb/206Pb, although protocols exist to enable this25.
Several initiatives have started to create regional, temporal and thematic datasets to gather and centralise these fragmented datasets26,27,28,29,30. Moreover, some archaeological studies have taken advantage of such huge datasets to track large-scale cultural31,32, ecological33,34,35,36, climatic37,38 or environmental39,40 patterns. However, the scientific potential of this archive remains comparatively untapped, especially in the perspective of multidisciplinary research. This implies that palaeo-environmental, -ecological and -climatic research is restricted to areas where proxies such as ice cores, speleothems, tree-rings, molluscs or lake/marine sediments are available, which cannot necessarily be easily connected to human activity, while bioarchaeological samples have a nearly global coverage and intrinsically enable to investigate human-environment interactions. This paper hence suggests how bioarchaeological isotope data can be integrated into palaeo-studies as they provide complementary environmental and climatic information that is not available from other archives.
Recycling bioarchaeological isotope data in environmental research
Bioarchaeological studies have contributed to palaeoecological research by revealing migratory and dietary behaviour of domesticated (affected by agropastoral and husbandry practices) and wild species—including now extinct ones36,41,42—and by highlighting their interaction with humans43,44,45. In most cases, these isotope data are biased towards human activity (see Supplementary Note 1)—with the exception of studies conducted in collaboration with palaeontology46,47,48. But more importantly, isotope ratios from bioarchaeological tissues can serve as palaeoclimatic and palaeoenvironmental proxies, which is not recognised in other palaeo-disciplines. This section presents the palaeoclimatic and palaeoenvironmental potential of bioarchaeological isotope data using several examples and emphasising both the spatial and the temporal dimensions.
Environmental aspects
The δ2H and δ18O values of animal tissues are ultimately related to the δ2H and δ18O values of consumed environmental water, and thus reflect, e.g. latitude and longitude, altitude, distance to the moisture source and seasonality (see a review in O49 and H7). Other isotope systems can be targeted in faunal tissues reflecting other aspects of local palaeoclimatic conditions (see Stevens et al in press50 for a review). For example, plant δ13C, δ15N, δ34S and δ66Zn values are affected by local variability in humidity and aridity, salinity, daylight, or forest cover versus open landscapes. These ‘signatures’ are transferred through the food chain so that the isotopic composition of animal and human tissues reflect the organisms dietary choices and contain information about the palaeoenvironmental and palaeoecological context of their habitat9,49,50,51—provided that the archaeozoological material is not biased towards grazers or browsers only, or that mixed-feeding herbivores are available from the site. Such isotope data can thus be used to retrace palaeoenvironmental diversity at a given archaeological site during a specific time frame21,38, or to reconstruct surrounding ecosystems, for example in coastal areas52,53, wetlands54, or steppe21 (Fig. 1). This approach also had particular success for reconstructing environmental change, for example associated with MIS 3, when the Neanderthals became extinct55, or the Last Glacial Maximum (LGM) and refugia regions34,56.
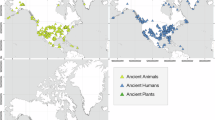
The bioarchaeological samples are (from left to right): hair, nail, tooth, bones with short or long turnover rates, bones from young animals/individuals, and charred grains. The temporal depth varies between proxies, yet the palaeontology and bioarchaeology hardly provide any continuous time series and the bioarchaeological sample frequency is considerably reduced towards the oldest samples. The dating uncertainty or precision can reach one calendar year for tree-rings, speleothems, varved lake’s sediments, speleothems, young ice cores, corals and molluscs, but it can be decadal to millennial for most proxies depending on the temporal depth and the datation method. The proxy resolution varies from sub-annual to decadal for most proxies, it can be (multi-)centennial for marine sediments and other layers that form slowly. The global spatial distribution vary between proxies: ice cores depend on global glacier distribution (light blue signature; source: https://wgms.ch/downloads/DOI-WGMS-FoG-2025-02b.zip); speleothems depend on karst regions (light brown signature; source: https://download.bgr.de/bgr/grundwasser/whymap/shp/WHYMAP_WOKAM_v1.zip); corals are restricted to tropical areas (dark blue signature; source: https://databasin.org/datasets/b983863c0a1a41e8839383b40ade437d/); molluscs are restricted to water bodies or archaeological sites, marine and lakes sediments originate from the respective water bodies, tree-rings depend on past forest covers and to some extent on archaeological sites (green signature: global tree ring record distribution; source: https://gis.ncdc.noaa.gov/kml/paleo_tree.kmz, last data access: 06th June 2025), palaeontological and bioarchaeological samples have a global extent but depend on past human activities, excavated sites and preservation conditions. The spatial scale reflected by each proxy goes from the local to the global scale, but even local proxies may inform about supra-regional processes when considered altogether. These characteristics are summarised in Supplementary Table 1. Figure © Michael Kempf 2025.
Several examples thus underline the ecological and palaeoenvironmental potential of bioarchaeological isotope data. In C3-dominated ecosystems under peri-arctic, boreal and temperate conditions, contrast in δ13C and δ15N values reflect the habitat and foraging preferences of herbivores. A striking example of niche partitioning based on specialised diet is observed among large ungulates of the mammoth steppe during the Last Glacial Period, a pattern that persisted across broad temporal and geographical scales. Reindeer exhibit the highest δ13C values due to their specific and substantial consumption of lichen, showing few if any overlap with horses, which have among the lowest δ13C values36. Mammoths show the highest δ15N values, likely due to grazing on more mature plants and possibly coprophagy, while woolly rhinoceroses and large bovids present intermediate δ15N values50,57. Change in the δ15N baseline is associated with complex, though sometimes decipherable, environmental conditions. In particular, the increase in δ15N across Europe during the Lateglacial to early Holocene transition may be linked indirectly to rising temperatures35,58,59,60,61. Geographical variation in δ15N values of large ungulates during the Lateglacial period is best explained by local environmental conditions, through the lingering effects of permafrost on N-cycling in soils35,59,62. A decline in isotopic niche partitioning reflects the changing post-LGM conditions, as seen in the 15N-depleted mammoths in Central Europe63 and the increasing overlap in δ13C and δ15N values among large ungulates62,64.
From a palaeoclimatic perspective, archaeological charcoals and molluscan shells represent further well-suited materials for investigating seasonal patterns and climate change on the site scale65. At the global scale, bioarchaeological proxies further allow for tracking larger climatic processes such as El Niño66 or variability in the monsoon67. When considered separately, archaeological sites thus offer site-specific or micro-regional insights into the effects of large-scale phenomena, while the accumulation of bioarchaeological data from one or several regions provides itself a record of large-scale phenomena (see Figs. 1, 2 and Section 3.2). Moreover, isotope data from diverse species further reflect different trends depending on their individual niches34, while palaeoenvironmental changes are not always reflected across all niches, such as the impact of LGM on ibex, but not on red deer dietary ecology in Northern Spain56, or the impact of the Heinrich 4 (~39/40 Ka BP) event on reindeer feeding ecology at Les Cottés (France) relative to other species36. Bioarchaeological isotope data are therefore complementary datasets to fill gaps among proxies from the environmental sciences.
The upper panel shows decreasing climatic and ecological scales from GCMs (Global Climate Models) to supraregional ecozones, regional habitats, and local and micro niches (realised niches). The lower panel shows increasing spatial operations, including small-scale fragmentation, simplification such as regional aggregation and binning, and estimation processes such as interpolation. Figure © Michael Kempf 2025.
This also applies in terms of geographical distribution of the records, as (zoo-)archaeological remains often survive when other environmental proxies do not. Stable isotope analysis of faunal remains provides a proxy for local environmental conditions, when many other proxies such as ice cores, sea cores, and speleothems are often distant from these locations and/or do not necessarily reflect local climatic conditions68. Zooarchaeological remains that originate from human activity can even provide contrasting evidence to other site-specific proxies that may not be contemporary to human site-use. For example, δ18O data from bovid teeth at the site of La Ferrassie, France, demonstrated that Neanderthals used the site during milder phases of a (broadly) cool period69. The data contrasted with wider regional environmental records for the period, as well as certain sediment records at the site itself, which also attested to these cooler conditions. This highlights the diverse scales of the different proxies, and the unique insights afforded through the isotope analysis of anthropogenically-derived faunal remains in reconstructing climate at the scale of human behaviour69. When combining and comparing different proxy datasets, the use of modern data is also important for testing the application of these proxies and feed current models in terms of spatial representativity70. This further offers the potential to use bioarchaeological isotope data to model phenomena for which proxies exist only in recent times71. Combining either contemporaneous records from different regions or diachronic records from a defined geographical area thus represent invaluable records for past processes that are otherwise recovered only in specific contexts or restricted geographical areas72,73.
When working with archaeological datasets, wider consideration needs to be given to the impact of anthropogenic activity on the stable isotope record. Concerning hunter-gatherer populations, the faunal record is a product of hunting strategies employed, and reflect times when a site was occupied by human populations, potentially reflecting seasonal occupation preferences or occupation only during particular climatic conditions, as seen at the Middle Palaeolithic site of Axlor in Northern Spain74. When working with material pertaining to (agro-)pastoralist populations the application of manure to fodder crops can impact on observed values75,76 as well as keeping animals in pens or enclosures77, transhumance77,78,79 the provision of specific80 or unusual fodder or graze such as salt-marsh, seaweed and marine resources81,82, or the use of diverse or distinctive pasture locations such as forests83 can all impact on the stable isotope record. In contrast to palaeontological material84,85,86 the potential of such bioarchaeological samples to reflect palaeoenvironmental conditions would be biased by the human impact on feeding habits. Small mammals such as rodents from the archaeological context are good alternatives to target the local environment87,88. However, as explained above, the targeting of anthropogenically-derived remains of prey-species (specifically large obligate-drinking ungulates) can conversely be viewed as an inherent bias that is favourable, resulting in a climatic proxy that is directly linked to human site-use.
Moreover, a certain degree of mobility of humans, animals (e.g. for food, transport), food resources and animal fodder can always be expected, in particular for the historical periods18,89. Likewise, trading of resources between rural and urban locations and between central places and hinterlands90 can result in the displacement of remains. In such cases, the isotope composition of bioarchaeological material would not be representative for local conditions and hence could not be used for reconstructing the local palaeoenvironment. It must be further kept in mind that regardless of the economy (hunter-gatherer, pastoralists, farmer), the material found at an archaeological site results from human selection and is biased towards what survived until today due to cultural choices or preservation issues. It might therefore not represent all biomes from the past local ecosystem—which is, however, a problem known from modern samples as well91. Working with archaeologists who understand such a record can help to ensure that consideration is given to some of these nuances and how they might be influencing any trends observed.
Temporal and seasonal aspects
Isotope analysis of incrementally developed structures such as speleothems68,73, tree-rings72,92, shells93, and stratified sequences like lake sediments94, provide multifaceted archives of past climatic and environmental conditions. Such records are valuable taken separately or combined, producing complementary proxy datasets95,96. Data from these archives cover different chronological and geographical scales on varying temporal resolutions with proxy-inherent limitations (Fig. 1). The analysis of speleothems and tree rings, for example, captures two different seasonal signals: mean annual or cooler season (fall to spring) precipitation in speleothems68,73 and the growing season signal in tree rings92. Climatic variability deriving from one or the other is a characterisation of a specific period of the year and an enhanced drought signal in one proxy does not necessarily occur in the other—triggering an overestimation of climatic extremes across a multi-annual period.
In comparison, the measured isotopic composition of bioarchaeological samples informs on contexts as old as several millions of years, with a resolution varying from a few weeks to several decades depending on the tissue and the sampling method (Table 1 and Supplementary Note 1). For example, δ2H isotope analysis of bone collagen can provide insight into time-averaged (i.e. supra-annual) precipitation97. Similarly, δ18O values from ‘bulk’ enamel samples (when undertaken to ensure a full year of growth is sampled) can allow the reconstruction of mean annual temperatures98,99. On the other hand, high-resolution intra-tooth micro-sampling on animal teeth offers continuous data for the period during which a particular tooth formed, covering both the fall/winter and the spring/summer seasons100, thus permitting the reconstruction of seasonal palaeoclimatic conditions14,38 over a full year. This approach has been applied at a number of Neanderthal and early modern human sites in Europe. For example, at Ilsenhöhle in Ranis, Germany, intra-tooth sampling and O isotope analysis of horses (combined with δ13C and δ15N analysis of faunal collagen) revealed decreasing temperatures throughout the sequence but also that extreme seasonality and coolest winter temperatures coincided with earliest modern human use of the site (the initial Upper Palaeolithic)21.
However, such approaches rely on dating and clear stratigraphic association, and the precision of the sample’s absolute dating depends on the period considered, and on the method used, e.g. radiocarbon dating101, luminescence102, dendrochronology92, stratigraphy, typology103. In contrast to the possibility of dating tree-rings, ice cores or speleothems to one specific calendar year, the associated chronological uncertainty among bioarchaeological samples (despite radiocarbon dating, for example) can vary dramatically and stratigraphic (or other) relationships at sites remain pivotal. The duration of the occupation might further be difficult to estimate104. The absolute dating resolution of bioarchaeological samples may be as large as several hundreds of years in the case of the oldest samples, when there is a prolonged lack of change in archaeological material culture, or when the calibration curve for radiocarbon dates hits a plateau105,106,107,108, while it can reach a few years or decades when methods are combined and/or when the archaeological context provides a high-resolution chronology such as during the Early Middle Ages109,110. Nevertheless, bioarchaeological research can provide time sequences in the same way as ice cores or lake varves.
Following the tree-ring approach by Loader and colleagues111 and by Black et al. on molluscs112 it may be possible to compare large sequential isotope datasets from animal teeth to, e.g. tree-ring and/or speleothem isotope data, and thus to integrate these bioarchaeological records into existing time series via cross-dating. A machine learning approach at the global scale and over long chronologies113, would facilitate linking of bioarchaeological sequential isotope data to palaeoclimatic and palaeoenvironmental parameters and completion or testing of existing models. This will imply further treatment of the data (i.e. interpolation, imputation, averaging), creating a loss of information (see also Fig. 2). Introducing various modelling procedures represents a first step to deal with these issues.
Following metadata guidelines provided by environmental sciences96 may further facilitate the integration of bioarchaeological isotope data into existing datasets such as the global Iso2k database, expanding the latter to the BCE time scale (see also ref. 26 and the Pandora initiative since 2023). Despite being discontinuous and uneven sequences, they would fill temporal and seasonal gaps and provide new insights and dimensions on phenomena recorded elsewhere. Yet a preliminary cross-disciplinary discussion is required to make sure that workflows align not only within72 but also between disciplines. Targeted sampling strategies within an area where comparable proxy data from speleothems, tree rings or others are available—and where a satisfying criterion regarding sample size can be met—would allow testing of this approach.
Isoscapes and the spatial dimension of isotope variability
The production of isotope data covering a wide geographical area provides the potential for generating isoscapes, which are detailed maps of isotope distributions across landscapes. These are fundamental isotope baselines for archaeological and ecological research, food sciences, and forensics in order to investigate mobility patterns and provenance. This is crucial when the isotope composition varies considerably over space in a predictable way according to known factors10,114,115,116. In parallel, some isotope systems are particularly sensitive to environmental and climatic changes and/or to human activities: their isoscapes thus allow for tracking spatial variations in environmental settings at a specific time—or over time61,117. In this context, bioarchaeological research has strongly contributed to the production of isoscapes, as O, S, Sr, and Pb isoscapes are purposely created to interpret the bioarchaeological isotope data in terms of mobility patterns5,10,117,118. This unique and extended dataset can be used in the past as it is in ecological research, since it provides a reference framework for several regions of the world, enabling the investigation of animal ecology and migration patterns119,120. It may also contribute to baselines used to establish the authenticity of product origins121,122. As a by-product of isotope data accumulation in bioarchaeology, isoscapes for C and N isotopes can now also be produced, enabling to track spatial and temporal variations in environmental parameters35. Yet the anthropocentric questions triggering the production of these bioarchaeological isoscapes and the diversity of approaches applied in this making process need to be considered to facilitate the reusability of isoscapes generated in bioarchaeological research.
Archives for targeted isoscapes
The development of isoscapes relies on primary or secondary data that can be anchored to a specific region, terrain or environment. The breadth of potential archives for these data across the lithosphere, biosphere, hydrosphere and atmosphere is almost limitless, yet bioarchaeological research targets specific samples to meet specific needs, as the modern ecosphere may differ considerably from the past. It is not only modern chemical contamination (which is a key part of the provenancing process123), but also natural chemical cycling and environmental processes that cause these chronological shifts in certain isotope proxies (e.g. δ15N61; 87Sr/86Sr124, δ34S40, and Stevens et al.50 in for a general review). The most work on this issue relates to 87Sr/86Sr analysis (see summary in Holt et al.115), but most considerations are applicable across isotope systems.
The impact of these processes varies across isotope systems, and archives for bioarchaeological research are selected accordingly. For example, to overcome the problem of Pb pollution in the modern environment—whose impact is noticeable since the advent of metallurgy10 and in particular from the Roman time125—base geological samples are generally favoured in Pb isoscapes instead of environmental samples (e.g. plants, soils and water), with prehistoric faunal samples providing an additional useful source10. Environmental samples are of greater value for 87Sr/86Sr, but the bioarchaeological approach prefers samples from ‘pristine’ landscapes, away from roads, urban areas or arable agriculture to avoid modern contamination126. Even traditional agricultural practices, such as the use of seaweed as a fertiliser, may shift biosphere signatures—i.e. closer to marine values in Sr127 and S128.
Whenever the site location and its surroundings are still accessible, plant sampling from landscapes with little anthropogenic impact has become the mainstay for 87Sr/86Sr isoscapes built on primary data115,129. Environmental alternatives for 87Sr/86Sr include surface water sources130, soils and soil leachates131 and land molluscs65. However, concerns have been raised in relation to contamination and the degree to which these archives are consistently representative of the bioavailable Sr of the local area132. As the base of the food chain, and since their local provenance can be assured, plants have many benefits for isoscape production115. However, they do not provide a homogenised signature for an area and are susceptible to extreme values, which are generally most likely in shallow rooted plants. Therefore, dense sampling is advantageous and assessing results from shallow, medium and deep-rooted plants is a good approach to characterise variability133. Homogenising plant samples from the same locality provides an alternative, cost-effective approach134, but reduces resolution to some degree.
Some diversity in taxa is of value due to plant-specific environmental processes that can impact local values (e.g. the forest-effect on 87Sr/86Sr87). Plants are also used for δ34S mapping114, but can often be subject to the impact of modern pollution135, though this problem is much reduced in many areas due to clean air legislation54. Faunal sources such as sheep wool136 or archaeological bone54 provide alternatives. Archaeological fauna is a good alternative in areas where pollution remains an issue as long as there is strong evidence for local origin. Therefore, small-bodied wild mammals (such as small rodents) with a limited home range are a good choice137, though these are generally sparse in faunal assemblages. Because the currently only global δ34S isoscape is based on bioarchaeological remains118, which may show the limits mentioned above, it is advisable to complete it with other samples such as plants114 to test for the local reliability—although the non-local and diagenetically altered samples were sorted out before producing the isoscape.
Using larger mammals, whether modern or ancient, provides homogenised values and helps to characterise a landscape rather than pinpointing extreme values138. They moreover sometimes represent the only available resource, especially at sites within modern urban areas or which are not accessible anymore due to building activities or some other restrictions. However, using this material requires a dialogue with experts on this archaeo(zoo)logical context, as animals may have had substantial grazing ranges, or may have been involved in transhumance practices and carcass parts found on sites may have been transported from elsewhere by humans, meaning they would not represent local bioavailability. In this context, using environmental analyses to model the site’s catchment area is an efficient way to determine the extent of environmental similarities or differences represented by the faunal sample compared to other proxies24,139.
Using bone or dentine in archaeological animals for 87Sr/86Sr is further ill-advised as these results represent a blend of biogenic (life) and diagenetic (burial) and are therefore non diagnostic140. In bioarchaeology, infants also have some potential for providing homogenised local values141, provided that early mobility can be excluded. δ18O isoscapes are invariably generated on baseline water sources, typically precipitation and/or groundwater116,142 as these sources dominate the O in organisms and the complexities of variable fractionation mean other archives are problematic. However, these are also subject to environment-specific impacts, for example through variable fractionation effects associated with evaporation depending on the depth of surface waters142, and the results may vary depending on the conversion equation used to compare tissue, drinking, and environmental water143.
Bioarchaeological isoscapes in ecological and palaeo-research
The geographical scale of isoscapes generated in bioarchaeological research depends strongly on the scope of the study, spanning from the site-specific or micro-regional54,139 to the regional and supra-regional10,129,144 or the global scale145. In addition, regional Sr isoscapes are getting increasingly produced with the only goal of serving as isotope background for future research, reflecting the main geological and environmental units of the area129, yet remaining a first step for bioarchaeological—and ecological or forensic—investigations. Depending on the research question, denser sampling might be required to complete this first approximation of the overall isotope diversity within the targeted area.
The geographical scale has in turn an impact on the resolution of the isoscape. At a reduced geographical scale, the isotope diversity of the local environment can be as detailed as to represent every ecological unit within a site’s catchment area using an important diversity of samples and sampling locations53,146—although bioarchaeological isoscapes often target places suitable for anthropogenic land-use17,139. On the other hand, large-scale isoscapes rather interpolate more scattered measurements based on the geological background, topography, hydrology, distance to the coast, or any relevant variable10,144,145. Importantly, local isotope values do not necessarily match the values predicted by global isoscapes (e.g. Ireland129,145), because increasing the geographical area often leads to a loss of resolution among the parameters used for the interpolation, while the relevant environmental parameters vary between ecosystems and local specificities thus cannot be considered at the large scale (Fig. 2). In this context, bioarchaeology contributes significantly to characterising the isotope variability in context- and region-specific environmental conditions. In this context, it is essential to create models that integrate multiple environmental and climatic variables to account for all factors influencing isotope variability at the investigated scale17,139,147. For more transparency in the interpolation process, isoscapes (of mean or median values) are meanwhile frequently published together with a map showing the error ranges129.
By integrating bioarchaeological isoscape data in palaeo-research, the discrepancy in terms of geographical and temporal resolution of the various proxies may require the use of categorised data or average values, which necessarily leads to a loss of information. In this context, it is worth engaging a cross-disciplinary discussion regarding targeted processes and gaps that need to be filled in terms of isoscapes, to determine which proxies and sample types are required and suitable. Yet with the large diversity of proxies derived from all disciplines as well as their great temporal and geographical dimension, it may be possible to disentangle locally from globally driven triggers of isotope variability within various ecological processes. It would be also important to discuss issues related to disturbed environments and their impact on local isotope composition.
For example, in temperate zones such as the UK areas of high precipitation in the west, the bioavailable 87Sr/86Sr is shifted from the geological value towards the value of rainfall146. Similarly, contexts of marine inundation, seaspray (causing increasing values) and waterlogging (generally reducing values), altering bioavailable δ34S, δ13C, δ18O and 87Sr/86Sr52,53,54, require specific attention. And since the deposition of aeolian dust can have a major impact in altering bioavailable 87Sr/86Sr away from underlying lithology132, bioarchaeological samples may track previous stages of the landscape. Such an attempt has been done in the Peak District, England, where the considerable change in 87Sr/86Sr over time may be due to the expansion of blanket peat and/or of leaching148. A bioarchaeological study in Sweden further demonstrated the potential variation of 87Sr/86Sr over time in areas affected by melting glaciers149.
Formal isoscapes tend not to be developed for δ13C and δ15N due to the manifold factors dictating variability. However, there is evidence that cultural practices such as dietary habits (e.g. marine versus terrestrial food, C3 versus C4 crops, high versus low-protein diet) or agricultural strategies (e.g. manuring) as well as geographical, environmental and climatic settings such as latitude, lithology or moisture have an impact on these isotope ratios, with archaeological fauna providing a useful archive to explore large-scale patterns35,37,61,150. The exponential amount of isotope data produced in bioarchaeology thus allows the investigation of changes in ecological, geological, and geomorphological processes in the long-term and recent studies have taken advantage of both the diachronic perspective and the large geographical distribution of bioarchaeological isotope data to determine new indicators for large-scale evolution of environmental conditions35,40,151. By combining bioarchaeological isotope data with further approaches and proxies152,153, there might be, for instance, potential to investigate changes in forest cover using the canopy effect154.
This opportunity to translate the huge amount of isotope data into isoscapes is further useful for the investigation of habitats and animal ecologies155. It can also be used to track mobility patterns, because specific geographically constrained environmental conditions, agricultural practices, or dietary habits produce typical isotopic signatures—especially when considering multiple isotopic systems150,156. It may be a challenge, however, to correlate changes in isotope baselines with changes in environmental conditions, especially when one needs to consider and quantify a potential anthropogenic impact on the isotope variability – including manuring fields or consuming boiled, stewed or fermented food and drinks, which can considerably alter the original local stable isotope ratio75,157. A first step in this direction would be to constrain the research area in terms of period and region, in which we can rule out some factors of variability (targeting especially periods at which the human impact was absent or negligible), to focus on the herbivores (to avoid animals with more flexible dietary habits), and to integrate different proxies, although it remains key to consider the potential effect of both mobility and seasonality on isotope variability158 as well as the time lag and varying isotope accumulation rate related to tissue formation159.
Conclusions and perspectives
Bioarchaeological research has achieved considerable advances in the understanding of past human and animal behaviours and how this has shaped or impacted on the environment. It hence actively contributed to palaeoenvironmental and ecological research, yet this contribution is mainly restricted to the acknowledgment of resulting narratives and created knowledge. On the contrary, the tremendous amount of isotope data derived from a large diversity of bioarchaeological samples and contexts remains underexploited. With this paper, we present potential and limits of integrating bioarchaeological isotope data into other palaeo-disciplines and identify future directions and strategies enabled by a cross-disciplinary collaboration, starting from the research designs and models.
The huge diversity of sampled tissues and materials provides complementary information about various aspects of past ecologies and environments and can contribute to track processes such as precipitation patterns, climate change, or the evolution of palaeoenvironmental conditions in regions where other proxies are not available. In this context, there is an urgent need to start integrating these data into existing models to test how they can be used, for instance, in predictive/inductive models. More importantly, the large geographical distribution of bioarchaeological data and their deep chronology allow for tracking large-scale and long-term processes. In turn, the diversity and density of the existing dataset make it possible to question the impact of large-scale processes as well as of short-term or long-term changes at the local or regional scale. As the data is easily related to human activity, one can address questions related to human-environment interactions over the long term.
In this context, it is paramount to recognise the complexity derived from bioarchaeological research, and to consider the different meanings of the various samples, to accurately deal with uncertain or imprecise chronologies, and to acknowledge the bias in the sample related to archaeological research procedures. This paper offers a wide spectrum of answers to these issues. It would, however, be of great value for greater engagement between bioarchaeologists and other palaeo-scientists to foster collaboration and design joint research programs that benefit from analytical advances and interdisciplinary methodological transfer. There is a great potential in targeting common research areas of interest, identifying gaps in terms of chronology, location or types of data/proxies (i.e. the types of sampled tissue/material), creating joint monitoring programs, and testing for instance physiological, climatic, ecological, and environmental models. Based on this, one could adapt future sampling procedures and hence produce data and proxies as meaningful and reusable as possible for the various disciplines.
References
Vogel, J. C. & van der Merwe, N. J. Isotopic evidence for early maize cultivation in New York State. Am. Antiq. 42, 238–242 (1977).
Ericson, J. E. Strontium isotope characterization in the study of prehistoric human ecology. J. Hum. Evol.14, 503–514 (1985).
Ambrose, S. H. Isotopic analysis of paleodiets: methodological and interpretative considerations. In Investigations of Ancient Human Tissue. Chemical Analyses in Anthropology (ed. Sandford, M.K.) 59–130 (Gordon and Breach, 1993).
Lee-Thorp, J. A. On isotopes and old bones. Archaeometry 50, 925–950 (2008).
Bentley, R. A. Strontium Isotopes from the Earth to the archaeological skeleton: a review. J. Archaeol. Method Theory 13, 135–187 (2006).
Nehlich, O. The application of sulphur isotope analyses in archaeological research: a review. Earth Sci. Rev. 142, 1–17 (2015).
Reynard, L. M. & Hedges, R. Stable hydrogen isotopes of bone collagen in palaeodietary and palaeoenvironmental reconstruction. J. Archaeol. Sci. 35, 1934–1942 (2008).
Guiserix, D. et al. Simultaneous analysis of stable and radiogenic strontium isotopes in reference materials, plants and modern tooth enamel. Chem. Geol. 606, 121000 (2022).
Junqueira, T. P. et al. Applications of zinc stable isotope analysis in environmental and biological systems: a review. GEEA 24; https://doi.org/10.1144/geochem2024-003 (2024).
Evans, J. A. et al. Applying lead (Pb) isotopes to explore mobility in humans and animals. PloS ONE 17, e0274831 (2022).
Plomp, E. Neodymium isotopes in modern human dental enamel: an exploratory dataset for human provenancing. Data Brief 38, 107375 (2021).
Evershed, R. P., Dudd, S. N., Copley, M. S. & Mutherjee, A. Identification of animal fats via compound specific δ13C values of individual fatty acids: assessments of results for reference fats and lipid extracts of archaeological pottery vessels. Doc. Praeh. 29, 73–96 (2002).
Depaermentier, M. L. C., Kempf, M., Bánffy, E. & Alt, K. W. Tracing mobility patterns through the 6th-5th millennia BC in the Carpathian Basin with strontium and oxygen stable isotope analyses. PloS ONE 15, e0242745 (2020).
Reade, H., O’connell, T. C., Barker, G. & Stevens, R. E. Increased climate seasonality during the late glacial in the Gebel Akhdar, Libya. Quat. Sci. Rev. 192, 225–235 (2018).
France, C. A., Qi, H. & Kavich, G. M. Combined influence of meteoric water and protein intake on hydrogen isotope values in archaeological human bone collagen. J. Archaeol. Sci. 96, 33–44 (2018).
Jaouen, K., Beasley, M., Schoeninger, M., Hublin, J.-J. & Richards, M. P. Zinc isotope ratios of bones and teeth as new dietary indicators: results from a modern food web (Koobi Fora, Kenya). Sci. Rep. 6, 26281 (2016).
Depaermentier, M. L. C., Kempf, M., Bánffy, E. & Alt, K. W. Cultural diversity shaped neolithic subsistence in the Carpathian Basin. Sci. Rep. 15, 4281 (2025).
Alexander, M. M., Gerrard, C. M., Gutiérrez, A. & Millard, A. R. Diet, society, and economy in late medieval Spain: stable isotope evidence from Muslims and Christians from Gandía, Valencia. Am. J. Phys. Anthropol. 156, 263–273 (2015).
van der Sluis, L. G., Reimer, P. J. & Ogle, N. Adding hydrogen to the isotopic inventory—combining δ 13 C, δ 15 N and δ 2 H stable isotope analysis for palaeodietary purposes on archaeological bone. Archaeometry 61, 720–749 (2019).
Pearson, J. & Grove, M. Counting sheep: sample size and statistical inference in stable isotope analysis and palaeodietary reconstruction. World Archaeol. 45, 373–387 (2013).
Pederzani, S. et al. Stable isotopes show Homo sapiens dispersed into cold steppes ~45,000 years ago at Ilsenhöhle in Ranis, Germany. Nat. Ecol. Evol. 8, 578–588 (2024).
Gruchy et al. Bone of contention: intra-element variability in remodelling of human femora based on histomorphometric and isotope analyses. PloS ONE 19, e0305089 (2024).
Leggett, S., Rose, A., Praet, E. & Le Roux, P. Multi-tissue and multi-isotope (δ13 C, δ15 N, δ18 O and 87/86 Sr) data for early medieval human and animal palaeoecology. Ecology 102, e03349 (2021).
Depaermentier, M. L. C. et al. Bioarchaeological analyses reveal long-lasting continuity at the periphery of the late antique Roman Empire. iScience 26, 107034 (2023).
Esposito, C. et al. NOthing goes to WAste (NOWA): a protocol to optimise sampling of ancient teeth. J. Archaeol. Sci. 171, 106087 (2024).
Pilaar Birch, S. E. & Graham, R. W. A Stable isotope data repository as part of neotoma, a paleoecological database. BioScience 65, 953 (2015).
Salesse, K. et al. IsoArcH.eu: an open-access and collaborative isotope database for bioarchaeological samples from the Graeco-Roman world and its margins. J. Archaeol. Sci. Rep. 19, 1050–1055 (2018).
Shipley, O. N. et al. Design, development, and implementation of IsoBank: a centralized repository for isotopic data. PloS ONE 19, e0295662 (2024).
Ebert, C. E. et al. The Caribbean and Mesoamerica Biogeochemical Isotope Overview (CAMBIO). Sci. Data 11, 349 (2024).
Billings, T. N. et al. The North American repository for archaeological isotopes. Sci. Data 12, 50 (2025).
Wang, T. et al. Tianshanbeilu and the Isotopic millet road: reviewing the late neolithic/bronze age radiation of human millet consumption from north China to Europe. Natl. Sci. Rev. 6, 1024–1039 (2019).
Leggett, S. A hierarchical meta-analytical approach to western European dietary transitions in the first millennium AD. Eur. J. Archaeol. 1–21; https://doi.org/10.1017/eaa.2022.23 (2022).
van Klinken, G. J., Richards, M. P. & Hedges, R. E. M. An overview of causes for stable isotopic variations in past european human populations. environmental, ecophysiological, and cultural effects. In Biogeochemical Approaches to Paleodietary Analysis. Advances in Archaeological and Museum Science (eds Ambrose, S.H. & Katzenberg, M.A.) 39–63 (New York, London, 2002).
Drucker, D. G. et al. Ecology of large ungulates in the northeastern Iberian Peninsula during the Upper Palaeolithic through stable isotopes and tooth wear analysis. Quat. Environ. Hum. 2, 100011 (2024).
Reade, H. et al. Nitrogen palaeo-isoscapes: changing spatial gradients of faunal δ15N in late Pleistocene and early Holocene Europe. PloS ONE 18, e0268607 (2023).
Britton, K. et al. Multi-isotope analysis of bone collagen of Late Pleistocene ungulates reveals niche partitioning and behavioural plasticity of reindeer during MIS 3. Sci. Rep. 13, 15722 (2023).
van Klinken, G. J., van der Plicht, J. & Hedges, R. E. M. Bone 13C/12C ratios reflect (palaeo) climatic variations. Geophys. Res. Lett. 21, 445–448 (1994).
Pederzani, S. et al. Subarctic climate for the earliest Homo sapiens in Europe. Sci. Adv. 7, eabi4642 (2021).
Drucker, D. G., Bocherens, H. & Billiou, D. Evidence for shifting environmental conditions in Southwestern France from 33,000 to 15,000 years ago derived from carbon-13 and nitrogen-15 natural abundances in collagen of large herbivores. Earth Planet. Sci. Lett. 216, 163–173 (2003).
Stevens, R. et al. Major excursions in sulfur isotopes linked to permafrost change in Eurasia during the last 50,000 Years. Research Square; https://doi.org/10.21203/rs.3.rs-2556240/v1 (2023).
Crowley, B. E. & Godfrey, L. R. Strontium isotopes support small home ranges for extinct lemurs. Front. Ecol. Evol. 7; https://doi.org/10.3389/fevo.2019.00490 (2019).
Drucker, D. G. et al. Tracking possible decline of woolly mammoth during the Gravettian in Dordogne (France) and the Ach Valley (Germany) using multi-isotope tracking (13C, 14C, 15N, 34S, 18O). Quat. Int. 359-360, 304–317 (2015).
Pilaar Birch, S. E., Miracle, P. T., Stevens, R. E. & O’connell, T. C. Late Pleistocene/Early Holocene migratory behavior of ungulates using isotopic analysis of tooth enamel and its effects on forager mobility. PloS ONE 11, e0155714 (2016).
Baumann, C. et al. Dietary niche partitioning among Magdalenian canids in southwestern Germany and Switzerland. Quat. Sci. Rev. 227, 106032 (2020).
Kveiborg, J. et al. Paleodiet reconstructions and human utilization of middle Holocene Equus ferus in northwest Europe. Palaeogeogr. Palaeoclimatol. Palaeoecol. 649, 112334 (2024).
Richards, M. P. et al. Isotopic evidence for omnivory among European cave bears: late Pleistocene Ursus spelaeus from the Peştera cu Oase, Romania. PNAS 105, 600–604 (2008).
Bocherens, H. et al. Isotopic evidence for dietary ecology of cave lion (Panthera spelaea) in North-Western Europe: prey choice, competition and implications for extinction. Quat. Int. 245, 249–261 (2011).
Robinson, J. R., Rowan, J., Barr, W. A. & Sponheimer, M. Intrataxonomic trends in herbivore enamel δ13C are decoupled from ecosystem woody cover. Nat. Ecol. Evol. 5, 995–1002 (2021).
Pederzani, S. & Britton, K. Oxygen isotopes in bioarchaeology: principles and applications, challenges and opportunities. Earth Sci. Rev. 188, 77–107 (2019).
Stevens, R. E., Pederzani, S., Britton, K. & Wexler, S. Bones and teeth isotopes as archives for palaeoclimatic, palaeoenvironmental and palaeoecological data. Quat. Sci. Rev. 357, 109320 (2025).
Hedges, R. E., Stevens, R. E. & Richards, M. Bone as a stable isotope archive for local climatic information. Quat. Sci. Rev. 23, 959–965 (2004).
Göhring, A., Hölzl, S., Mayr, C. & Strauss, H. Identification and quantification of the sea spray effect on isotopic systems in α-cellulose (δ13C, δ18O), total sulfur (δ34S), and 87Sr/86Sr of European beach grass (Ammophila arenaria, L.) in a greenhouse experiment. Sci. Total Environ. 856, 158840 (2023).
Göhring, A., Hölzl, S., Mayr, C. & Strauss, H. Multi-isotope fingerprints of recent environmental samples from the Baltic coast and their implications for bioarchaeological studies. Sci. Total Environ. 874, 162513 (2023).
Lamb, A. L., Chenery, C. A., Madgwick, R. & Evans, J. A. Wet feet: developing sulfur isotope provenance methods to identify wetland inhabitants. R. Soc. Open Sci. 10, 230391 (2023).
Bocherens, H., Drucker, D. G. & Madelaine, S. Evidence for a (15)N positive excursion in terrestrial foodwebs at the middle to upper Palaeolithic transition in south-western France: implications for early modern human palaeodiet and palaeoenvironment. J. Hum. Evol. 69, 31–43 (2014).
Jones, J. R., Marín-Arroyo, A. B., Straus, L. G. & Richards, M. P. Adaptability, resilience and environmental buffering in European Refugia during the late Pleistocene: insights from La Riera Cave (Asturias, Cantabria, Spain). Sci. Rep. 10, 1217 (2020).
Drucker, D. G. The isotopic ecology of the mammoth steppe. Annu. Rev. Earth Planet. Sci. 50, 395–418 (2022).
Drucker, D. G., Kind, C.-J. & Stephan, E. Chronological and ecological information on Late-glacial and early Holocene reindeer from northwest Europe using radiocarbon (14C) and stable isotope (13C, 15N) analysis of bone collagen: case study in southwestern Germany. Quat. Int. 245, 218–224 (2011).
Stevens, R. E. et al. Nitrogen isotope analyses of reindeer (Rangifer tarandus), 45,000 BP to 9,000 BP: palaeoenvironmental reconstructions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 262, 32–45 (2008).
Drucker, D. G., Madelaine, S. & Morala, A. Les derniers rennes de Dordogne. Paleo, 85–100; https://doi.org/10.4000/paleo.2087 (2011).
Stevens, R. E. & Hedges, R. E. Carbon and nitrogen stable isotope analysis of northwest European horse bone and tooth collagen, 40,000BP–present: Palaeoclimatic interpretations. Quat. Sci. Rev. 23, 977–991 (2004).
Drucker, D. G., Bridault, A. & Cupillard, C. Environmental context of the Magdalenian settlement in the Jura Mountains using stable isotope tracking (13C, 15N, 34S) of bone collagen from reindeer (Rangifer tarandus). Quat. Int. 272-273, 322–332 (2012).
Drucker, D. G. et al. Collagen stable isotopes provide insights into the end of the mammoth steppe in the central East European plains during the Epigravettian. Quat. Res. 90, 457–469 (2018).
Reiss, L. et al. Changing food webs before and during the Last Glacial Maximum based on stable isotopes of animal bone collagen from Lower Austria. J. Quat. Sci. 38, 1337–1356 (2023).
Prendergast, A. L. & Stevens, R. E. Molluscs (isotopes): analyses in environmental archaeology. In Encyclopedia of Global Archaeology (ed. Smith, C.) 7332–7340 (Springer International Publishing, 2020).
Sandweiss, D. H. et al. Archaeological climate proxies and the complexities of reconstructing Holocene El Niño in coastal Peru. PNAS 117, 8271–8279 (2020).
Vaiglova, P. et al. Past rainfall patterns in Southeast Asia revealed by microanalysis of δ18O values in human teeth. J. Archaeol. Sci. 162, 105922 (2024).
Tian, Y. et al. Holocene climate change in southern Oman deciphered by speleothem records and climate model simulations. Nat. Commun. 14, 4718 (2023).
Pederzani, S. et al. Reconstructing Late Pleistocene paleoclimate at the scale of human behavior: an example from the Neandertal occupation of La Ferrassie (France). Sci. Rep. 11, 1419 (2021).
Luterbacher, J. & Zorita, E. Analysis and Interpretation: spatial climate field reconstructions. In The Palgrave Handbook of Climate History (eds White, S., Pfister, C. & Mauelshagen, F.) 131–139 (Palgrave Macmillan UK, 2018).
Suraprasit, K. et al. Long-term isotope evidence on the diet and habitat breadth of Pleistocene to Holocene Caprines in Thailand: implications for the extirpation and conservation of Himalayan Gorals. Front. Ecol. Evol. 8; https://doi.org/10.3389/fevo.2020.00067 (2020).
Büntgen, U. et al. The influence of decision-making in tree ring-based climate reconstructions. Nat. Commun. 12, 3411 (2021).
Fleitmann, D. et al. Palaeoclimatic interpretation of high-resolution oxygen isotope profiles derived from annually laminated speleothems from Southern Oman. Quat. Sci. Rev. 23, 935–945 (2004).
Pederzani, S. et al. Late Pleistocene Neanderthal exploitation of stable and mosaic ecosystems in northern Iberia shown by multi-isotope evidence. Quat. Res. 116, 108–132 (2023).
Larsson, M., Bergman, J. & Olsson, P. A. Soil, fertilizer and plant density: exploring the influence of environmental factors to stable nitrogen and carbon isotope composition in cereal grain. J. Archaeol. Sci. 163, 105935 (2024).
Bogaard, A., Heaton, T., Poulton, P. & Merbach, I. The impact of manuring on nitrogen isotope ratios in cereals: archaeological implications for reconstruction of diet and crop management practices. J. Archaeol. Sci. 34, 335–343 (2007).
Shahack-Gross, R., Simons, A. & Ambrose, S. H. Identification of pastoral sites using stable nitrogen and carbon isotopes from bulk sediment samples: a case study in modern and archaeological pastoral settlements in Kenya. J. Archaeol. Sci. 35, 983–990 (2008).
Diaz, L. Unraveling Ancient Andean Camelid Herding Strategies Using Multi-Isotope Analyses. In Arts & Sciences Electronic Theses and Dissertations. Vol. 3335, https://doi.org/10.7936/ktkp-h909 (2024).
Melton, M. A. et al. Reconstructing middle horizon camelid diets and foddering practices: microbotanical and isotope analyses of dental remains from Quilcapampa, Peru. Lat. Am. Antiq. 34, 783–803 (2023).
Somerville, A. D. Human-animal interactions in the pre-colonial Americas: insights from stable carbon isotope analysis. In Exploring Human Behavior Through Isotope Analysis (eds Beasley, M.M. & Somerville, A.D.) 181–205 (Springer International Publishing, 2023).
Britton, K., Müldner, G. & Bell, M. Stable isotope evidence for salt-marsh grazing in the Bronze Age Severn Estuary, UK: implications for palaeodietary analysis at coastal sites. J. Archaeol. Sci. 35, 2111–2118 (2008).
Jones, J. R. & Mulville, J. Isotopic and zooarchaeological approaches towards understanding aquatic resource use in human economies and animal management in the prehistoric Scottish North Atlantic Islands. J. Archaeol. Sci. Rep. 6, 665–677 (2016).
Gillis, R. E. et al. Diverse prehistoric cattle husbandry strategies in the forests of Central Europe. Nat. Ecol. Evol. 9, 87–98 (2025).
Szabó, P. et al. Pliocene—early Pleistocene continental climate and vegetation in Europe based on stable isotope compositions of mammal tooth enamel. Quat. Sci. Rev. 288, 107572 (2022).
Nguy, W. H. & Secord, R. Middle Miocene paleoenvironmental reconstruction in the central Great Plains, USA, from stable carbon isotopes in ungulates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 594, 110929 (2022).
Cullen, T. M., Zhang, S., Spencer, J. & Cousens, B. Sr-O-C isotope signatures reveal herbivore niche-partitioning in a Cretaceous ecosystem. Palaeontology 65; https://doi.org/10.1111/pala.12591 (2022).
Samec, C. T. & Yacobaccio, H. D. Chinchillidae exploitation during the first half of the Holocene in the Argentinian Puna: a contribution from zooarchaeology and stable isotope analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 648, 112298 (2024).
Berto, C., Arnaud, J., López-García, J. M., Luzi, E. & Arzarello, M. Analysis of the early Pleistocene small mammals from Pirro Nord 13 (Apricena, southern Italy) and their implications for reconstructing the palaeoenvironment of the early human occupation in Europe. Palaeogeogr. Palaeoclimatol. Palaeoecol. 647, 112251 (2024).
Chidimuro, B. et al. North and South: exploring isotopic analysis of bone carbonates and collagen to understand post-medieval diets in London and northern England. Am. J. Biol. Anthropol. 182, 126–142 (2023).
Stevens, R. E., Lightfoot, E., Hamilton, J., CUNLIFFE, B. & Hedges, R. E. Stable isotope investigations of the Danebury hillfort pit burials. Oxf. J. Archaeol. 29, 407–428 (2010).
Norwood, A. L., Wang, B. & Kingston, J. D. Linking African herbivore community enamel isotopes and environments: challenges, opportunities, and paleoecological implications. Oecologia 204, 467–489 (2024).
Fritts, H. C. Tree Rings and Climate (Elsevier, 1976).
Zong, X. et al. Climatic significance of modern minute land snail shells δ13C and δ18O on the Chinese Loess Plateau. Ecol. Indic. 145, 109733 (2022).
Leng, M. J. & Marshall, J. D. Palaeoclimate interpretation of stable isotope data from lake sediment archives. Quat. Sci. Rev. 23, 811–831 (2004).
Dee, S. et al. Water isotopes, climate variability, and the hydrological cycle: recent advances and new frontiers. Environ. Res. Clim. 2, 22002 (2023).
Konecky, B. L. et al. The Iso2k database: a global compilation of paleo- δ18 O and δ2 H records to aid understanding of common era climate. Earth Syst. Sci. Data 12, 2261–2288 (2020).
Reynard, L. M. et al. Mediterranean precipitation isoscape preserved in bone collagen δ2H. Sci. Rep. 10, 8579 (2020).
Britton, K. et al. Oxygen isotope analysis of Equus teeth evidences early Eemian and early Weichselian palaeotemperatures at the Middle Palaeolithic site of Neumark-Nord 2, Saxony-Anhalt, Germany. Quat. Sci. Rev. 226, 106029 (2019).
Pryor, A. J., Stevens, R. E., O’connell, T. C. & Lister, J. R. Quantification and propagation of errors when converting vertebrate biomineral oxygen isotope data to temperature for palaeoclimate reconstruction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 412, 99–107 (2014).
Sharp, Z. D. & Cerling, T. E. Fossil isotope records of seasonal climate and ecology: straight from the horse’s mouth. Geology 26, 219 (1998).
Hajdas, I. Radiocarbon dating and its applications in quaternary studies; https://doi.org/10.23689/FIDGEO-988 (2008).
Gliganic, L. A., McDonald, J. & Meyer, M. C. Luminescence rock surface exposure and burial dating: a review of an innovative new method and its applications in archaeology. Archaeol. Anthropol. Sci. 16; https://doi.org/10.1007/s12520-023-01915-0 (2024).
Grechko, D. Western frontier of the Archaic Scythia: typo-chronology vs radiocarbon dating. Spraw. Arch. 75, 405–436 (2023).
Rodríguez Ramos, R., Rodríguez López, M. & Pestle, W. J. Revision of the cultural chronology of precolonial Puerto Rico: a Bayesian approach. PloS ONE 18, e0282052 (2023).
Wu, X. et al. Early pottery at 20,000 years ago in Xianrendong Cave, China. Science 336, 1696–1700 (2012).
Shennan, S. (ed.) The First Farmers of Europe (Cambridge University Press, 2018).
Potter, B. A. et al. Current understanding of the earliest human occupations in the Americas: evaluation of Becerra-Valdivia and Higham (2020). PaleoAmerica 8, 62–76 (2022).
Hajdas, I. et al. Radiocarbon dating. Nat. Rev. Methods Primers 1; https://doi.org/10.1038/s43586-021-00058-7 (2021).
Hines, J. & Bayliss, A. (eds) Anglo-Saxon Graves and Grave Goods of the 6th And 7th centuries AD: A Chronological Framework (Society for Medieval Archaeology, 2013).
Jacobsson, P. et al. Refining the Hallstatt Plateau: short-term 14 C variability and small scale offsets in 50 consecutive single tree-rings from Southwest Scotland Dendro-dated to 510–460 BC. Radiocarbon 60, 219–237 (2018).
Loader, N. J. et al. Tree ring dating using oxygen isotopes: a master chronology for central England. J. Quat. Sci. 34, 475–490 (2019).
Black, B. A. et al. The revolution of crossdating in marine palaeoecology and palaeoclimatology. Biol. Lett. 15, 20180665 (2019).
Savard, M. M. & Daux, V. An overview on isotopic divergences—causes for instability of tree-ring isotopes and climate correlations. Climate 16, 1223–1243 (2020).
Tarrant, D. & Richards, M. P. Modern plants and sulfur isoscapes - a review, discussion, and construction of a pilot δ34S isoscape for mobility and provenance studies. Rapid Commun. Mass Spectrom. RCM 38, e9908 (2024).
Holt, E., Evans, J. A. & Madgwick, R. Strontium (87Sr/86Sr) mapping: a critical review of methods and approaches. Earth Sci. Rev. 216, 103593 (2021).
Bowen, G. J. & Revenaugh, J. Interpolating the isotopic composition of modern meteoric precipitation. Water Resour. Res. 39; https://doi.org/10.1029/2003WR002086 (2003).
Bowen, G. J. Isoscapes: Spatial pattern in isotopic biogeochemistry. Annu. Rev. Earth Planet. Sci. 38, 161–187 (2010).
Bataille, C. P. et al. Triple sulfur-oxygen-strontium isotopes probabilistic geographic assignment of archaeological remains using a novel sulfur isoscape of western Europe. PloS ONE 16, e0250383 (2021).
Holder, P. W. et al. Isotopes and trace elements as natal origin markers of Helicoverpa armigera-an experimental model for biosecurity pests. PloS ONE 9, e92384 (2014).
Brlík, V. et al. Animal tracing with sulfur isotopes: spatial segregation and climate variability in Africa likely contribute to population trends of a migratory songbird. J. Anim. Ecol. 92, 1320–1331 (2023).
Rossmann, A. et al. The potential of multielement stable isotope analysis for regional origin assignment of butter. Eur. Food Res. Technol. 211, 32–40 (2000).
Almeida, C. M. & Vasconcelos, M. T. S. D. ICP-MS determination of strontium isotope ratio in wine in order to be used as a fingerprint of its regional origin. J. Anal. Spectrom. 16, 607–611 (2001).
Durante, C. et al. An analytical approach to Sr isotope ratio determination in Lambrusco wines for geographical traceability purposes. Food Chem. 173, 557–563 (2015).
Johnson, L., Evans, J., Montgomery, J. & Chenery, C. The forest effect: biosphere 87Sr/86Sr shifts due to changing land use and the implications for migration studies. Sci. Total Environ. 839, 156083 (2022).
Silva-Sánchez, N. & Armada, X.-L. Environmental impact of roman mining and metallurgy and its correlation with the archaeological evidence: an European perspective. Environ. Archaeol. 1–25; https://doi.org/10.1080/14614103.2023.2181295 (2023).
Thomsen, E. & Andreasen, R. Agricultural lime disturbs natural strontium isotope variations: implications for provenance and migration studies. Sci. Adv. 5, eaav8083 (2019).
Blanz, M. et al. Seaweed fertilisation impacts the chemical and isotopic composition of barley: implications for analyses of archaeological skeletal remains. J. Archaeol. Sci. 104, 34–44 (2019).
Blanz, M., Gröcke, D. R., Martin, P. & Church, M. J. The effect of seaweed fertilisation on sulfur isotope ratios (δ34S) and grain size in barley: implications for agronomy and archaeological research. Front. Environ. Archaeol. 3; https://doi.org/10.3389/fearc.2024.1465082 (2024).
Snoeck, C. et al. Towards a biologically available strontium isotope baseline for Ireland. Sci. Total Environ. 712, 136248 (2020).
Frei, K. M. & Frei, R. The geographic distribution of strontium isotopes in Danish surface waters – a base for provenance studies in archaeology, hydrology and agriculture. Appl. Geochem. 26, 326–340 (2011).
Hoogewerff, J. A. et al. Bioavailable 87Sr/86Sr in European soils: a baseline for provenancing studies. Sci. Total Environ. 672, 1033–1044 (2019).
Hartman, G. & Richards, M. Mapping and defining sources of variability in bioavailable strontium isotope ratios in the Eastern Mediterranean. Geochim. Cosmochim. Acta 126, 250–264 (2014).
Sengeløv, A. et al. From plants to patterns: constructing a comprehensive online strontium isoscape for Belgium (IsoBel) using high density grid mapping. Geoderma 453, 117123 (2025).
Macheridis, S. et al. Preliminary strontium isotope (87Sr/86Sr) baselines for the Bjäre Peninsula and Halland in southern Sweden. Front. Environ. Archaeol. 3; https://doi.org/10.3389/fearc.2024.1379055 (2024).
Zhao, F. J., Spiro, B., Poulton, P. R. & McGrath, S. P. Use of sulfur isotope ratios to determine anthropogenic sulfur signals in a grassland ecosystem. Environ. Sci. Technol. 32, 2288–2291 (1998).
Zazzo, A. et al. Isotopic composition of sheep wool records seasonality of climate and diet. Rapid Commun. Mass Spectrom. RCM 29, 1357–1369 (2015).
Perry, M. A., Coleman, D. & Delhopital, N. Mobility and exile at 2nd century A.D. khirbet edh-dharih: strontium isotope analysis of human migration in western Jordan. Geoarchaeology 23, 528–549 (2008).
Bentley, R. A. & Knipper, C. Geographical patterns in biologically available strontium, carbon and oxygen isotope signatures in prehistoric SW Germany. Archaeometry 47, 629–644 (2005).
Depaermentier, M. L. C., Kempf, M., Bánffy, E. & Alt, K. W. Modelling a scale-based strontium isotope baseline for Hungary. J. Archaeol. Sci. 135, 1–16 (2021).
Madgwick, R., Lewis, J., Grimes, V. & Guest, P. On the hoof: exploring the supply of animals to the Roman legionary fortress at Caerleon using strontium (87Sr/86Sr) isotope analysis. Archaeol. Anthropol. Sci. 11, 223–235 (2019).
Esposito, C. et al. Intense community dynamics in the pre-Roman frontier site of Fermo (ninth-fifth century BCE, Marche, central Italy) inferred from isotopic data. Sci. Rep. 13, 3632 (2023).
Darling, W. G., Bath, A. H. & Talbot, J. C. The O and H stable isotope composition of freshwaters in the British Isles. 2. Surface waters and groundwater. Hydrol. Earth Syst. Sci. Discuss. 7, 183–195 (2003).
Pellegrini, M., Lee-Thorp, J. A. & Donahue, R. E. Exploring the variation of the δ18Op and δ18Oc relationship in enamel increments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 310, 71–83 (2011).
Serna, A., Prates, L., Valenzuela, L. O. & Salazar-García, D. C. Back to the bases: building a terrestrial water δ18O baseline for archaeological studies in North Patagonia (Argentina). Quat. Int. 548, 4–12 (2020).
Bataille, C. P., Crowley, B. E., Wooller, M. J. & Bowen, G. J. Advances in global bioavailable strontium isoscapes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 555, 109849 (2020).
Montgomery, J. Passports from the past: investigating human dispersals using strontium isotope analysis of tooth enamel. Ann. Hum. Biol. 37, 325–346 (2010).
Jones, J. & Britton, K. Multi-scale, integrated approaches to understanding the nature and impact of past environmental and climatic change in the archaeological record, and the role of isotope zooarchaeology. J. Archaeol. Sci. Rep. 23, 968–972 (2019).
O’Regan, H. J., Wilkinson, D. M., Wagner, D. & Evans, J. Why so high?’ Examining discrepancies between the Sr biosphere map and archaeological tooth data from the Peak District, England. J. Archaeol. Sci. 157, 105826 (2023).
Boethius, A., Kielman-Schmitt, M. & Robson, H. K. Mesolithic Scandinavian foraging patterns and hunting grounds targeted through laser ablation derived 87Sr/86Sr ratios at the Early-Mid Holocene site of Huseby Klev on the west coast of Sweden. Quat. Sci. Rev. 293, 107697 (2022).
Hülsemann, F. et al. Global spatial distributions of nitrogen and carbon stable isotope ratios of modern human hair. Rapid Commun. Mass Spectrom. RCM 29, 2111–2121 (2015).
McAllister, M. S., Morley, M. W., Tyler, J. J., McInerney, F. A. & Blyth, A. J. Investigating the palaeoenvironmental context of Late Pleistocene human dispersals into Southeast Asia: a review of stable isotope applications. Archaeol. Anthropol. Sci. 14; https://doi.org/10.1007/s12520-022-01540-3 (2022).
Vitali, V. et al. High-frequency stable isotope signals in uneven-aged forests as proxy for physiological responses to climate in Central Europe. Tree Physiol. 41, 2046–2062 (2021).
Di Matteo, G., Nardi, P. & Fabbio, G. On the use of stable carbon isotopes to detect the physiological impact of forest management: the case of Mediterranean coppice woodland. For. Ecol. Manag. 389, 158–166 (2017).
Drucker, D. G., Bridault, A., Hobson, K. A., Szuma, E. & Bocherens, H. Can carbon-13 in large herbivores reflect the canopy effect in temperate and boreal ecosystems? Evidence from modern and ancient ungulates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 266, 69–82 (2008).
Hobson, K. A. & Kardynal, K. J. Multi-isotope (δ2H, δ13C, δ15N) feather profiles and morphometrics inform patterns of migratory connectivity in three species of North American swallows. Mov. Ecol. 11, 48 (2023).
Knipper, C. et al. Coalescing traditions-Coalescing people: community formation in Pannonia after the decline of the Roman Empire. PloS ONE 15, e0231760 (2020).
Brettell, R., Montgomery, J. & Evans, J. Brewing and stewing: the effect of culturally mediated behaviour on the oxygen isotope composition of ingested fluids and the implications for human provenance studies. J. Anal. At. Spectrom. 27, 778 (2012).
Balasse, M. Reconstructing dietary and environmental history from enamel isotopic analysis: time resolution of intra-tooth sequential sampling. Int. J. Osteoarchaeol. 12, 155–165 (2002).
Balasse, M., Ambrose, S. H., Smith, A. B. & Price, T. D. The seasonal mobility model for prehistoric herders in the south-western Cape of South Africa assessed by isotopic analysis of sheep tooth enamel. J. Archaeol. Sci. 29, 917–932 (2002).
Acknowledgements
M.L.C.D. thanks the Basel Doctoral Program in Ancient Civilisations (DBAW) for financing the workshop at the University of Basel during which the concept and preparation of this paper were initiated. M.K.’s research is funded by the Swiss National Science Foundation (SNSF/SNF): Project EXOCHAINS - Exploring Holocene Climate Change and Human Innovations across Eurasia (SNSF grant number: TMPFP2_217358). K.B. thanks ERC-selected/UKRI-funded project EP/Y023641/1 for salary support during production of this manuscript. U.B. received funding from the Czech Science Foundation (# 23-08049S; Hydro8), the ERC Advanced Grant (# 882727; Monostar), and the ERC Synergy Grant (# 101118880; Synergy-Plague). We further thank Judith Sealy, Noreen Tuross, Chris Baumann, Michelle Alexander, Claudia Gerling, Dominik Fleitmann, Stéphane Affolter, Ansgar Kahmen, and Daniel Nelson for their input during the related workshop. Open Access publication is supported by the Swiss National Science Foundation (SNF) and the EXOCHAINS project awarded to MK.
Author information
Authors and Affiliations
Contributions
Margaux L. C. Depaermentier: concept, writing, and revision. Michael Kempf: concept, writing, revision and visualisation. Marc Vander Linden: concept and writing. Kate Britton: concept, writing and revision. Richard Madgwick: concept and writing, Ulf Büntgen: writing and revision. Dorothée G. Drucker: concept, writing and revision. Jennifer R. Jones: concept, writing and revision. Christophe Snoeck: concept and writing. Rhiannon E. Stevens: concept, writing and revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth and Environment thanks Penny Bickle and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Aliénor Lavergne. [A peer review file is available.]
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Depaermentier, M.L.C., Kempf, M., Vander Linden, M. et al. The palaeoenvironmental potential of bioarchaeological isotope data. Commun Earth Environ 6, 501 (2025). https://doi.org/10.1038/s43247-025-02507-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-025-02507-7