Abstract
To evaluate the path of anthropogenic mercury (Hg) from Asia to the Pacific Ocean, we report mercury stable isotopes in zooplankton from the East China Sea to the Bay of Bengal, and in the Philippine Sea and the Central Pacific. Here, we find that zooplankton mercury concentration decreases and δ202Hg and Δ199Hg increase with distances away from Asia, depicting anthropogenic mercury dilution. Anomalies of even mass-number isotopes (Δ200Hg), used to decipher between near-surface and tropospheric oxidation, suggest that 40–48% of anthropogenic Hg(0) is initially oxidized at the marine boundary layer or via terrestrial vegetation, and >50% of anthropogenic Hg(0) is circulated to the upper atmosphere for oxidation and removal to the Pacific. The fact that both near-surface and atmospheric Hg(0) oxidation supplies bioavailable Hg(II) strengthens the case for mitigating Hg(0) emissions. Recently reported climate-projected increase in riverine mercury fluxes may also benefit by reducing anthropogenic Hg(0) available for vegetative uptake.
Similar content being viewed by others
Introduction
Mercury (Hg) is considered to be a global pollutant, and is regulated by the United Nations Environment Programme Minamata Convention on Mercury1,2. Given that humans are primarily exposed to mercury through the consumption of marine fish3, it is important to understand sources and cycling pathways of Hg in coastal marine and open ocean sites. A recent literature finds that anthropogenic Hg emissions in the Northern Hemisphere have declined by more than 140 Mg year−1 (−0.011 ± 0.006 ng m−3 year−1) since 20054. Nonetheless, it remains challenging to quantify the response of recent anthropogenic Hg declines because the exact contribution and pathway of anthropogenic Hg to the marine food web remain inconclusive. This knowledge gap originates, in part, from not knowing how much of anthropogenic Hg travels via the atmosphere versus through continental/riverine export. It has long been regarded that oxidation of gaseous elemental Hg(0) emitted from natural and anthropogenic sources, followed by wet (Hg(II)) and dry deposition (particulate-bound mercury; PBM), explains >70% of the Hg deposited over the oceans5. A recent study has shown that, rather than wet and dry deposition, direct atmospheric Hg(0) invasion or dissolution into seawater explains 40−50% of Hg found in global seawater6. Apart from the atmospheric pathway, modeled estimates have reported spatially wide ranges in riverine Hg influences (6−70%) to coastal oceans, with its contribution to open ocean sites remaining uncertain7,8.
In addition to sources, the chemical form (Hg(0) vs. Hg(II)) in which Hg is transported and biogeochemically processed in coastal and open ocean has implications for assessing the spatial extent of anthropogenic migration and the processes that make Hg bioavailable. For instance, the previously underestimated effect of Hg(0) invasion in global seawater6 has raised widespread ecological and human health concerns. This is because >90% of Hg is emitted as Hg(0) from anthropogenic and natural sources, and Hg(0) has the ability to undergo long-range transport upon emission into the atmosphere5. Moreover, if estimates of Hg(0) invasion are correct, identifying processes and locations (i.e., marine boundary layer, seawater) at which Hg(0) becomes oxidized and methylated would be crucial since Hg enters the base of the food web as Hg(II) and methylmercury (MeHg)9. In the case of the West Pacific, a recent study has reported that Hg(0) oxidation mediated by anthropogenic short-lived halogens from continental Asia facilitates Hg(II) removal and prevents anthropogenic Hg outflow to the open ocean sites10. How all this information translates into Hg levels in marine biota is subject to large uncertainty, yet it is crucial for predicting future anthropogenic influences and for formulating effective policy actions to optimize human health concerns related to fish consumption.
We report Hg stable isotope signatures of mass-dependent fractionation (MDF; δ202Hg) and both even-mass and odd-mass-independent fractionation (even-MIF; Δ200Hg, Δ204Hg, odd-MIF; Δ199Hg, Δ201Hg) in zooplankton to illustrate the extent and pathway of anthropogenic Hg migration to coastal and open ocean sites. Given the absence of Hg isotope fractionation during bioaccumulation11, zooplankton, located at the base of the food web, are an ideal bioindicator for evaluating sources and processes involving Hg cycling in the atmosphere and in marine waters. For example, a study in the Arctic Ocean has utilized zooplankton δ202Hg and Δ199Hg to quantify the relative exposure of atmospheric and riverine Hg sources, and Δ200Hg to identify chemical forms of Hg and processes by which atmospheric Hg is oxidized and bioaccumulated from seawater12. Here, we report zooplankton mercury isotopes along an Asia transect (north to south), spanning from the East China Sea to the Bay of Bengal, as well as in the open ocean of the Philippine Sea. Samples along mainland China were collected either at the continental shelf break or in the pelagic region, while the south section (the Malacca Strait to the Bay of Bengal) includes shallower coastal samples. These locations are subject to increasing eutrophication, although the rates at which eutrophication occurs do not differ from other global coastal oceans13. The Philippine Sea represents a pelagic site located at the western boundary of the oligotrophic North Pacific Subtropical Gyre (NPSG). To evaluate the spatial extent and transport pathways of anthropogenic Hg from continental Asia to the Pacific Ocean, we compared our results with the industrially impacted coastal Bohai Sea14 and the North (Station ALOHA) and Equatorial Pacific Ocean (referred to as the Central Pacific Ocean)15,16. It is estimated that East and South Asia account for ~40% and 10% of total global anthropogenic Hg emissions, respectively17. Despite the limited Hg measurements along the Southeast Asian coasts (30 publications since 1984), a recent study has shown that artisanal gold mining in South Asia is responsible for local Hg contamination in seawater and sediments18. Recent climate-change projections have also shown that Chinese marginal seas and the Bay of Bengal will be subject to more prominent MeHg increase due to the presence of large riverine systems relative to other ocean basins19. Thus, it is of interest to discern riverine versus atmospheric Hg contribution to marine biota in this region. Our study explores how the present anthropogenic Hg emissions from Asia impact the greater Pacific to inform effective policies for marine fisheries under the Minamata Convention on Mercury and climate change.
Results and discussion
Anthropogenic Hg dilution from Asia to the Pacific
Zooplankton sampled in surface waters of the Asia transect (East China Sea to the Bay of Bengal; 68 ± 29 ng g−1, n = 11) and the Philippine Sea at the western boundary of the NPSGs (42 ± 14 ng g−1, n = 3) (Fig. 1, Supplementary Data 1) reveal elevated total Hg (THg) concentrations relative to those previously sampled at Station ALOHA (27 ± 6 ng g−1; n = 20)15 and from a Hawai’i to Samoa transect in the Central Pacific Ocean (30 ± 25 ng g−1; n = 42)20. The zooplankton THg from the Equatorial Pacific Ocean (49 ± 14 ng g−1, n = 16)16 are similar to the Philippine Sea but lower than the Asia transect. Zooplankton THg are expected to be lower in the NSPG (Philippine Sea, Station ALOHA) compared to the Equatorial Pacific, given the high water clarity21, enabling active photo-reduction and re-emission of Hg(0) from marine waters. The fraction of THg in the form of MeHg (% MeHg) in the surface zooplankton show an opposite trend to that of THg, in which elevated % MeHg values are found in the Philippine Sea (15−26%, n = 2) compared to the Asia transect of the China Seas (4−15%, n = 3) and the Malacca Strait and the Bay of Bengal (10−23%, n = 4) (Supplementary Data 1). Even higher % MeHg have been recorded in zooplankton from a Hawai’i to Samoa transect (2–44%, n = 42)20.
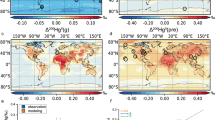
Map of zooplankton sampling locations and δ202Hg values. δ202Ηg of coal35,36, atmospheric samples of total gaseous mercury (TGM), particulate-bound mercury (PBM), and rain15,27,28,29,30,31,32,33,34, and zooplankton and fish14,15,16,21,26 from the Asia continent and the Central Pacific Ocean are shown in different symbols of star, heptagon, diamond, square, circle, and triangle, respectively. Tuna Hg concentrations are shown in blue circles37. Ocean Data View was used to illustrate the map of the figure64.
Sources and biogeochemical processes of Hg in marine waters can be assessed by zooplankton Hg isotope ratios. The δ202Hg values are used to express MDF22. While all known Hg biogeochemical processes, including microbial methylation23 and demethylation24, induce significant MDF, different types of natural and anthropogenic Hg sources have a wide range of δ202Hg values (−4.5 to +3.5‰)22, making it possible to distinguish between Hg sources across a large spatial scale. Δ199Hg and Δ201Hg denote odd-MIF, which are induced primarily via inorganic Hg photo-reduction and MeHg photodegradation25. The slope of Δ199Hg/Δ201Hg has been used to distinguish between inorganic Hg photo-reduction (1.0) and MeHg photodegradation (1.2−1.3) prior to bioaccumulation into aquatic food web14,21,26.
As illustrated in Fig. 2, zooplankton sampled across the Asia transect to the Philippine Sea show a gradual spatial change with increasing δ202Hg and Δ199Hg values (Supplementary Data 1). When including macroalgae from the Bohai Sea, located at the innermost region of the Chinese seas14, and zooplankton from the Central Pacific Ocean15,16, the spatial pattern breaks down somewhat with δ202Hg and Δ199Hg increasing from -3.22 ± 0.84‰ to 0.19 ± 0.17‰ and from 0.16 ± 0.08‰ to 1.46 ± 0.73‰, respectively. As compiled in Fig. 1, Hg emitted or affected by anthropogenic activities in the form of total gaseous Hg (TGM; −1.07 ± 0.48‰, n = 62)27,28,29,30,31 and Hg(II) (rain, PBM; −1.18 ± 0.79‰, n = 182)32,33,34 and coal used for combustion (−1.12 ± 0.85‰, n = 69)35,36 have negative δ202Hg values. Various anthropogenic Hg sources (liquid Hg, coal) and Hg emitted from industrial activities all have near-zero Δ199Hg values35. The negative δ202Hg (−0.90±0.46‰, n = 11) and near-zero Δ199Hg (0.30 ± 0.24‰) together with elevated THg levels in zooplankton sampled along the Asia transect suggest that anthropogenic Hg originated from Asia is bioaccumulated into the marine biota. As mentioned earlier, the macroalgae from the Bohai Sea have even lower δ202Hg, reflecting Hg(0) that has been oxidized at the coastal marine boundary layer or via terrestrial vegetation, which leaves a highly negative δ202Hg (MDF) in Hg(II) of foliage and other terrestrial organic matter14. As we move to the open ocean sites, zooplankton Hg isotopic compositions begin to increase toward the values of natural precipitation (Fig. 2). The more positive δ202Hg and Δ199Hg of the zooplankton relative to precipitation in the Central Pacific Ocean (δ202Hg; 0.11 ± 0.03‰, Δ199Hg; 0.28 ± 0.20‰, n = 11) has been attributed to a combination of microbial methylation and particle sorption of atmospheric Hg (MDF) followed by MeHg photodegradation (MIF) prior to bioaccumulation15,16,26.
Even after accounting for MDF and MIF caused by vegetative oxidation and biogeochemical processes (e.g., methylation, photodegradation) in the ocean, the gradual increase in zooplankton δ202Hg and Δ199Hg from nearshore systems to the Central Pacific Ocean depicts anthropogenic Hg dilution with increasing distances away from Asia. This is further verified by the significant negative relationships between zooplankton THg concentration and δ202Hg (r2 = 0.55, p = 0.0023) and Δ199Hg (r2 = 0.35, p = 0.026). The zooplankton Hg isotope pattern is also in strong agreement with elevated tuna THg concentrations reported in the northwest Pacific relative to the Central Pacific Ocean and the East Pacific37 (Fig. 1). These studies of tuna and our Hg isotope measurements in zooplankton highlight that anthropogenic Hg outflow from Asia extends to the West Pacific and that anthropogenic Hg influences are directly reflected in marine biota.
Mercury biogeochemical cycling in marine waters
Following Hg input to the ocean, the processes by which MeHg is produced (microbial methylation) or degraded (microbial demethylation, photodegradation) prior to bioaccumulation in marine waters can be evaluated using zooplankton sampled at varying ocean depths. This is because microbial methylation takes place across the water column16,26, while Hg photochemical reactions are isolated to the surface ocean26. To date, there are no vertical profiles of MeHg in zooplankton reported other than our Philippine Sea data. The vertical profiles of zooplankton Hg isotope ratios have been reported at Station ALOHA15,16, which we compare with the Philippine Sea since both are located within the NPSG.
There is an evident increase in % MeHg with water depth (from 15% to 43% MeHg) in all plankton size fractions in the Philippine Sea (Supplementary Fig. 1). The zooplankton of the Philippine Sea (1.17 ± 0.10) and Station ALOHA (1.21) exhibit Δ199Hg/Δ201Hg slopes consistent with MeHg photodegradation25, highlighting the presence of MeHg available for photodegradation. Moreover, while the zooplankton Δ199Hg decrease with depth, following the pattern of decreasing MeHg photodegradation below the euphotic zone, the δ202Hg values remain invariant in the Philippine Sea (−0.37 ± 0.12‰, n = 19) but decrease with depth at Station ALOHA (25 m: 0.15 ± 0.10‰, n = 6, 400 m: −0.13 ± 0.17‰, n = 6) (Fig. 3a). The δ202Hg reduction at Station ALOHA has been attributed to microbial methylation16,26, resulting in newly formed MeHg with a more negative δ202Hg relative to Hg(II)23. Based on the increasing zooplankton % MeHg with water depth, the uniform δ202Hg in the Philippine Sea can be explained by the presence of biogeochemical processes other than MeHg production (microbial demethylation, photodegradation, sorption), which counterbalance the zooplankton δ202Hg values.
a Vertical profiles of δ202Hg and Δ199Hg of the Philippine Sea (purple) and the Central Pacific Ocean (blue) zooplankton by size fraction15. Size fractions of 0.2–2 mm, 2–5 mm, and >5 mm zooplankton are represented by triangular, circular, and diamond symbols, respectively. b Δ199Hg versus δ202Hg of zooplankton from the Philippine Sea (purple triangles) and fish from the South China Sea (purple diamonds)38. The Δ199Hg/δ202Hg slopes represent zooplankton and fish from the Philippine Sea and the South China Sea (n = 37), zooplankton and fish from the Central Pacific (n = 63)15,26, and an experimentally determined MeHg photodegradation slope25. Atmospheric samples of total gaseous mercury (TGM), particulate-bound mercury (PBM), and rain are from continental Asia (black, gray, white squares) and the Central Pacific Ocean (blue square)15,27,28,29,30,31,32,33,34. South China Seawater is shown in orange circles38. Error bars plotted in the diagram indicate one standard deviation of each sample.
The linear relationship between δ202Hg and Δ199Hg (or the slope of Δ199Hg/δ202Hg) of aquatic biota has been used to assess the relative importance of photodegradation versus microbial demethylation of MeHg in marine waters16,26. For instance, the Δ199Hg/δ202Hg slope of zooplankton and fish from the Central Pacific Ocean (2.68 ± 0.3; n = 63)15,16,26 nearly match the experimental Δ199Hg/δ202Hg slope recorded during MeHg photodegradation (2.43)25 (Fig. 3b). By contrast, coastal ecosystems, where both photodegradation and microbial demethylation take place, have depressed Δ199Hg/δ202Hg slopes (Gulf of Mexico, Bohai Sea; ~0.4)22 since both of these processes leave a more positive δ202Hg in MeHg relative to Hg(II)24,25. This appears to be the case in the Philippine Sea zooplankton, and also for fish collected off the coast of Taiwan near the Philippine Sea38 (1.80 ± 0.3, n = 34; r2 = 0.78) (Fig. 3b). More interestingly, Hg sources subject to biogeochemical processes in the Philippine Sea, where the North Equatorial Current circulates from the east to the west NPSG, resemble those of the China marginal seas (anthropogenic) than the Central Pacific Ocean (natural precipitation). This is revealed by the linear regression of δ202Hg versus Δ199Hg of the Philippine Sea zooplankton and fish, which pass through the Hg isotopic compositions of seawater collected from the South China Sea38, which has negative δ202Hg and near-zero Δ199Hg values (Fig. 3b). Thus, unlike Station ALOHA, where precipitation Hg is subjected to methylation and photodegradation, anthropogenic Hg, characterized by low δ202Hg and Δ199Hg, appears to undergo microbial methylation-demethylation and photodegradation in the Philippine Sea. Despite the elevated THg concentrations, the presence of both microbial demethylation and photodegradation appears to result in less MeHg available for biotic uptake, as evident by the lower zooplankton % MeHg in the Philippine Sea compared to the Central Pacific Ocean.
Path of anthropogenic Hg migration to the Pacific
Finally, we address the path of anthropogenic Hg migration and the processes modulating Hg bioavailability in marine biota. Recently, several studies have utilized even-MIF to identify Hg depositional and exposure pathways to marine biota6,12,16. This is because significant even-MIF anomalies occur exclusively due to upper atmospheric (tropopause) Hg(0) photo-oxidation, leaving the product Hg(II) with a positive Δ200Hg and negative Δ204Hg value, and Hg(0) with a negative to near-zero Δ200Hg and positive Δ204Hg value39. Hg(0) oxidation is also a necessary step for microbial methylation and for plankton and macroalgae to assimilate Hg(II) via passive uptake through their cellular membrane9. Using Δ200Hg of seawater and biota measured from a few ocean basins, Jiskra et al.6 estimated that atmospheric Hg(0) invasion into seawater could explain 40−50% of Hg in global marine waters and biota. On the basis that Hg(0) has to be oxidized to become bioavailable, a study in the Arctic Ocean suggested that Hg(0) oxidation below the tropopause (via terrestrial vegetation and sea salt aerosols), followed by methylation in the water column, better explains the negative Δ200Hg observed in zooplankton12. In the Central Pacific Ocean, Hg(0) oxidation in the upper atmosphere, followed by wet/dry deposition, is regarded to be the dominant source subject to methylation and bioaccumulation, based on the positive Δ200Hg of zooplankton and fish15,26.
In the Asia transect and the Philippine Sea, where we record considerable anthropogenic Hg influences, we observe positive Δ200Hg values in zooplankton, reflecting Hg(II) (Fig. 4). Macroalgae and fish from the Bohai Sea exhibit negative Δ200Hg, reflecting Hg(0) (Fig. 4). Thus, in the Asia transect and the Philippine Sea, anthropogenically emitted Hg(0) appears to be transported to the upper atmosphere for photo-oxidation prior to deposition and biogeochemical processing in marine waters (see earlier section). In the nearshore Bohai Sea, anthropogenic Hg(0) is oxidized below the tropopause, thereby preserving Δ200Hg representative of anthropogenic Hg(0) emissions even upon biogeochemical processing in marine waters. In fact, there is a multitude of Hg(0) oxidation pathways below the tropopause that can produce Hg(II) without imparting significant even-MIF. Hg(0) oxidation via terrestrial vegetation40 and oxidation at the marine boundary layer29,41 are examples of this. Hg(0) oxidation via terrestrial vegetation, in particular, explains 76% of the global dry Hg deposition42. Field observations have also reported active in situ production of Hg(II) at the marine boundary layer of the Bohai Sea41.
Summary of Δ200Ηg of coal35,36, atmospheric samples of total gaseous mercury (TGM), particulate-bound mercury (PBM), and rain15,27,28,29,30,31,32,33,34, terrestrial litter27,46, sediment47,48,49,65, seawater14,38, and marine biota14,15,16,21,26,38,50,65 from the Asian continent-coastal and the Pacific Ocean. The box portion of the plot represents the interquartile range (IQR), while the upper whisker corresponds to the upper 25% of the data (above Q3), and the lower whisker corresponds to the lower 25% (below Q1), excluding outliers.
In the case of the Asia transect and the Philippine Sea, we reveal for the first time the importance of upper atmospheric photo-oxidation modulating anthropogenic Hg(0) removal and bioaccumulation to open ocean biota. Aircraft measurements have shown direct evidence for stratospheric-tropospheric Hg(0) oxidation, resulting in rapid Hg(II) removal43. A recent modeling study has also found that the regions of the largest atmospheric Hg(II) deposition, mediated via Hg(0) transport and oxidation above the tropopause, match well with the sites of high anthropogenic emissions, along the coast of Asia44. Note that our suggested mechanism differs from another modeling study, which reported that Hg(0) oxidation via anthropogenic short-lived halogens at the surface layer of the atmosphere explains ~20% of Hg deposited over the Chinese marginal seas and the Bay of Bengal10. While halogen species exacerbate Hg(0) oxidation, it has been widely regarded that Hg oxidized below the tropopause does not impart even-MIF anomalies12. Examples of this are the Arctic snowpack and zooplankton impacted by Atmospheric Mercury Depletion Events (AMDE) or halogen-induced oxidation, which have shown consistently near-zero to negative Δ200Hg values12,45. These studies illustrate that, even under the presence of abundant halogens, Hg(0) oxidized tens of meters above the snow and ocean surfaces do not impart even-MIF anomalies.
Given that near-surface (terrestrial vegetation, marine boundary layer) and upper atmospheric Hg(0), oxidation leave distinct even-MIF signatures in Hg(II), Δ200Hg can be used as a diagnostic of the path of Hg(II) migration from continental Asia to coastal and open ocean. As compiled in Fig. 4, the distinctly negative Δ200Hg (and δ202Hg) of macroalgae and fish from the Bohai Sea indicate that there is a presence of a highly negative Δ200Hg, originated from anthropogenic Hg(0) emissions, that is efficiently oxidized in the near-surface. The negative Δ200Hg of Bohai Sea macroalgae and fish14 resemble the Δ200Hg values of TGM27,28,29,30,31 and terrestrial litter27,46 compiled from various locations of China. There is a small dilution of the negative Δ200Hg in the riverine sediments47, illustrating the process of Hg(0) or TGM uptake and oxidation via terrestrial vegetation (litter) followed by riverine runoff. Marine sediments and biota from the Yellow Sea and other Chinese marginal seas38,48,49,50, by contrast, have positive Δ200Hg, with estuarine sediments48,49 falling in between the values of riverine and marine sediments. Thus, the overall increase in Δ200Hg across these environmental matrices suggests that riverine Hg, reflecting anthropogenic Hg(0) oxidized via terrestrial vegetation, diminishes from the nearshore region to the open ocean sites.
Using Δ200Hg and Δ204Hg values of the macroalgae in the Bohai Sea (Δ200Hg; −0.06 ± 0.06‰, Δ204Hg; 0.23 ± 0.16‰, n = 13)14 and precipitation from the Central Pacific (Δ200Hg; 0.14 ± 0.05‰, Δ204Hg; 0.26 ± 0.14‰, n = 11)15,16 as end-members, we quantify how much of Hg(0) is circulated to the tropopause for photo-oxidation relative to being oxidized in the near-surface environment before bioaccumulation. Two end-members representing Hg(0) oxidized at the marine boundary layer or via terrestrial vegetation prior to riverine export, and Hg(0) photo-oxidized in the upper atmosphere, are applied to a Bayesian Monte-Carlo isotope mixing model (see “Methods”). Based on the chosen end-members, the mixing model is constrained to reflect anthropogenic Hg influences from the Asian continent. Given the contrasting zooplankton Hg isotope signatures between the West and Central Pacific and low tuna Hg concentrations reported from the East Pacific Ocean37, it is unlikely that Hg point sources from the American continent influence the West Pacific Ocean. Estimates show that upper atmospheric photo-oxidation accounts for 52 ± 10% of THg found in the East and South China Sea zooplankton, 60 ± 14% in the Malacca Strait and the Bay of Bengal, and 59 ± 11% in the Philippine Sea, and the remaining proportion is oxidized in the near-surface environment. Since near-surface oxidation includes both Hg(0) oxidized at the coastal marine boundary layer and via terrestrial vegetation, we speculate that riverine Hg sources are not as widely transported as previously estimated via modeling studies of large riverine Hg fluxes to the West Pacific8,19. Alternatively, it is possible that riverine Hg sources or Hg bound to riverine/terrestrial particles may be less bioavailable for assimilation and/or methylation compared to atmospherically deposited Hg. The fact that zooplankton in the Asia transect and the Philippine Sea reflect anthropogenic Hg sources (MDF and odd-MIF signatures) suggests that much of anthropogenic Hg(0), upon emission from Asia, is actively circulated to the tropopause, consistent with the recent modeled reports on the global importance of stratospheric-tropospheric Hg(0) oxidation44. While 74 ± 3.6% of THg in the Central Pacific zooplankton originates from upper atmospheric photo-oxidation, the majority of Hg here represents natural precipitation, reflecting Hg(0) that has cycled through the atmosphere for an extended period prior to oxidation and scavenging by precipitation. Although the isotope mixing model does not include Hg point sources from the American continent, anthropogenic emissions, previously discussed as having a distinct Hg isotopic signature, could contribute to a small proportion of THg found in the Central Pacific Ocean biota. Overall, the spatial gradient of zooplankton Hg isotopic compositions (δ202Hg, Δ199Hg, Δ200Hg, Δ204Hg) showcase how the path of anthropogenic Hg(0) transitions from near-surface to upper atmospheric oxidation from emission sources across coastal Asia to the Pacific Ocean (Fig. 5). These oxidation processes also act as key initial steps for Hg biogeochemical processing (methylation, demethylation, photodegradation), bioavailability, and bioaccumulation in marine waters.
Implications for policies and Hg as a global pollutant
Our study uses Hg stable isotopes in zooplankton to illustrate the dissemination dynamics of Hg(0) from the source of emission to atmospheric and biogeochemical processing and bioaccumulation to marine biota. The dissemination dynamics of Hg(0) can be used to set informed Hg policies for marine fisheries and human health relevant to fish consumption. Until recently, much of the scientific discussions have been focused on identifying the relative importance of riverine and atmospheric Hg pathways to coastal and open ocean sites8,10,15,37. Especially, in the context of many recent Hg studies reported from the West Pacific, the initial sources and pathway governing the Asian Hg outflow and bioavailability at the base of the food web have largely been missing10,37. We find that the key sources and pathways, which ultimately supplies bioavailable Hg(II) to coastal and open ocean biota, are anthropogenic Hg(0) oxidized in the near-surface and the tropopause (Fig. 5). Effective Hg reduction in marine fisheries would, therefore, be achieved by targeting Hg(0) emissions from all anthropogenic sectors. Electrostatic precipitator (ESP) is known to be the most widely implemented particulate matter control device in Asia, which has a co-benefit of removing Hg from combustion sectors (coal-fired power plants, smelting and iron manufacturing industries)51. However, ESP generally captures Hg(II) and PBM, resulting in emissions that are predominantly in Hg(0)52. Since the northwest Pacific is the largest fishing ground, and therefore the Hg exporter in the world53, policies and technologies targeting Hg(0) emissions in Asia, which occupy >50% of the total global anthropogenic Hg emissions17, would bring health benefits to global populations. Moreover, while the effects of climate change have been projected to augment biomass production, nutrient productivity, and MeHg production in Asian rivers19, Hg-specific technologies and policies, implemented under the guidance of the Minamata Convention on Mercury, would contribute to lowering Hg(0) available for vegetative uptake, methylation, and reduce riverine Hg fluxes into coastal and open oceans.
Given the limited anthropogenic and riverine Hg influence on the Central Pacific Ocean, it would take much longer for Hg levels to decline via the legacy effect, consistent with relatively uniform Hg levels detected in the surface waters of the Pacific over the past 20 years54. It is interesting that Hg has a smaller spatiotemporal influence when compared to Asian emissions of reactive nitrogen, a pollutant not considered to be globally disseminated, as in the case of Hg deposition to the Central Pacific55. Mercury has been referred to as a global pollutant, not only due to its toxicity to all living organisms56, but also due to its pervasive nature, ability to cross continental boundaries, and the difficulty of linking sources into ecosystem exposure, as defined by the Minamata Convention on Mercury and the scientific community of the International Conference on Mercury as a Global Pollutant1,2. At least from a technical perspective, the fact that Hg stable isotopes can be used to track sources and cycling pathways of Hg emitted from Asia, both across space and ocean depths, calls for a re-evaluation of the concept of global pollutant.
Methods
Study locations
Surface-dwelling zooplankton were collected in the summer of 2017 (June 14th–26th) during the R/V ISABU at night in a transect starting from the East China Sea (30°57′ N and 126°57′ E) to the Bay of Bengal (6°03′ N 85°46′ E) (Supplementary Data 1). The East and South China Seas are the largest marginal seas in the western tropical Pacific Ocean, surrounded by fast-developing industrial Asian countries. The Malacca Straits are an important trade route linking the Pacific Ocean to the Indian Ocean, facing environmental degradation from the development of surrounding Southeast Asian countries57. The Malacca Straits are characterized by fringing coral reefs and seagrass beds along the coastlines. The transect concludes in the Bay of Bengal, a semi-enclosed basin located in the northeastern part of the Indian Ocean58.
Surface dwelling zooplankton from the Philippine Sea were collected between 17°51′ N and 127°0′ E to 21°06′ N and 134°10.21′ E in the summer of 2018 (August 29th to September 20th, 2018) during the R/V ISABU (Supplementary Data 1). In the same cruise, zooplankton were collected at a vertical profile (0–600 m) between 18°30′ N and 126°60′ E and 21°07′ N and 132°00′ E from four different MOCNESS (Multiple Opening/Closing Net and Environmental Sensing System) tows. The Philippine Sea is located at the western boundary of the NPSGs, an oligotrophic site with a relatively stable thermocline and halocline. During this study, the oxygen minimum zone was below 800 m.
Zooplankton sampling
Surface-dwelling zooplankton larger than 400 μm were collected during the transect and in the Philippine Sea using the CUFES (Continuous Underway Fish Egg Sampler). Fish eggs and larvae were removed from the samples and preserved with 95% ultraclean ethanol in acid-cleaned (10% w/v HCl acid) polyethylene bottles. At POSTECH, the transect samples from the East China Sea to the Bay of Bengal were filtered using an acid-cleaned 200 μm and 1080 μm nylon mesh, where the filtrate was collected onto a pre-combusted (5 h at 450 °C) glass fiber filter (GF/F) and dried in an oven at 40 °C for 24 h. Given the large amount of biomass needed for Hg isotope measurements, multiple filters were combined to reflect zooplankton from specific ocean basins along the transect (Fig. 1). The Philippine Sea CUFES samples were divided into three size fractions (0.5–2, 2–5, >5 mm) using sieves, and collected into pre-weighted acid-cleaned glass vials, frozen at −20 °C, and lyophilized for 48 h.
In the Philippine Sea, zooplankton were collected at a vertical profile using a 1 m2 MOCNESS equipped with a 200 μm mesh sampling net. Plankton were collected from four 8-h tows from 14–17 h over the following depth intervals of 0–50, 50–100, 100–200, 200–300, 300–400, and 400–600 m. Samples from afternoon casts were combined for Hg isotope analyses. These samples were also divided into three size fractions (0.5–2, 2–5, >5 mm) and processed similarly to the CUFES.
Total Hg and MeHg concentrations
All dried samples were measured for total Hg (THg) using atomic absorption spectroscopy (Nippon Instruments MA-3000 Hg analyzer) by combustion. All zooplankton samples were analyzed two or three times, and the standard reference material TORT-3 (lobster hepatopancreas) was measured between every six samples (average recovery 96 ± 7%, n = 15, 1 SD). A subset of samples was measured for MeHg concentrations by transferring 0.003–0.01 g of zooplankton biomass into an acid-clean 15 ml glass vial and digested in 30% trace-meal grade HNO3 for 12 h at 60 °C following the previous procedure20,59. Three hundred to four hundred microliters of the digested material were measured for MeHg by cold vapor atomic fluorescence spectroscopy coupled with gas chromatography. The standard reference material TORT-3 was also digested to assess the MeHg recoveries (average 102 ± 24%, n = 21, 1 SD). The THg and MeHg concentrations of all samples are shown in Supplementary Data 1.
Hg stable isotopes
Hg stable isotopes were measured using the Nu Plasma III multicollector inductively coupled plasma mass spectrometer (MC-ICP-MS) at POSTECH. The zooplankton and TORT-3 were combusted in a dual-stage thermal combustion furnace, and the released Hg(0) was trapped in a (1% w/w) KMnO4 solution in 10%(w/w) trace metal grade H2SO4 solution. Prior to isotopic analyses, the KMnO4 solution was measured for THg concentration to assess the recoveries. The THg yielded an average recovery of 92 ± 15% in all samples and TORT-3. All the samples were matched to the bracketing standard (SRM NIST 3133) and introduced into the MC-ICP-MS for isotope analysis. A TL reference standard (NIST SRM 997) was used for mass bias corrections.
MDF is reported as δ202Hg values in permil (‰) relative to NIST SRM 3133 (Eq. (1)), and mass-independent fractionation (MIF) is calculated as the difference between the measured δ202Hg value and those predicted based on mass dependence for a given isotope. MIF is reported as ΔxxxHg in ‰ (Eq. 2), where xxx is the mass of each Hg isotope, i.e., 199, 200, 201, 204, and β is the mass proportionality constant (0.2520, 0.5024, 0.7520, 1.493, respectively).

TORT-3 (combusted samples) and the standard reference material NIST 8610 (Hg isotopes in UM-Almaden mono-elemental secondary standard) were measured along with the samples. The long-term analytical uncertainty of the Hg isotopic composition of the samples was estimated using the ±2 SD of NIST 8610. Hg isotope ratios of all samples and standard reference materials are shown in Supplementary Data 1 and Supplementary Table 1.
Statistical analysis
To consider errors in both the X and Y axes in the three stable isotope plots linear regressions associated with Hg stable isotope data, we used the York regression in the RStudio package60.
To estimate the proportional contributions (%) from atmospheric and riverine Hg pathways to our zooplankton samples, we utilized the RStudio Bayesian Stable Isotope Mixing Models via the Markov chain Monte-Carlo package61. We used Δ200Hg and Δ204Hg values of the macroalgae in the Bohai Sea (Δ200Hg; −0.06 ± 0.06‰, Δ204Hg; 0.23 ± 0.16‰, n = 13, 1 SD)14 and precipitation from the Central Pacific (Δ200Hg; 0.14 ± 0.05‰, Δ204Hg; 0.26 ± 0.14‰, n = 11, 1 SD)15,16 as end-members. The multivariate hierarchical statistical approach accounts for the uncertainty and distribution of isotopic data, parameters that are omitted by traditional Hg isotope mixing models61,62,63, which can result in potential errors in source attribution.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated in this study are provided on Nature as Supplementary Data 1 and are available under accession code [https://doi.org/10.6084/m9.figshare.28518461]. Figure 1 was illustrated using the Ocean Data View program for the bathymetrical dataset (https://odv.awi.de/).
References
Driscoll, C. T., Mason, R. P., Chan, H. M., Jacob, D. J. & Pirrone, N. Mercury as a global pollutant: sources, pathways, and effects. Environ. Sci. Technol. 47, 4967–4983 (2013).
Secretariat of the Minamata Convention on Mercury. Minamata Convention on Mercury: Text and Annexes. 2023 Edition (United Nations Environment Programme, Geneva, 2023).
Sunderland, E. M. & Mason, R. P. Human impacts on open ocean mercury concentrations. Glob. Biogeochem. Cycles 21, 2006GB002876 (2007).
Feinberg, A. et al. Unexpected anthropogenic emission decreases explain recent atmospheric mercury concentration declines. Proc. Natl. Acad. Sci. USA 121, e2401950121 (2024).
Horowitz, H. M. et al. A new mechanism for atmospheric mercury redox chemistry: implications for the global mercury budget. Atmos. Chem. Phys. 17, 6353–6371 (2017).
Jiskra, M. et al. Mercury stable isotopes constrain atmospheric sources to the ocean. Nature 597, 678–682 (2021).
Zhang, Y. et al. Biogeochemical drivers of the fate of riverine mercury discharged to the global and Arctic oceans: RIVER MERCURY IN THE OCEAN. Glob. Biogeochem. Cycles 29, 854–864 (2015).
Liu, M. et al. Rivers as the largest source of mercury to coastal oceans worldwide. Nat. Geosci. 14, 672–677 (2021).
Mason, R. P., Reinfelder, J. R. & Morel, F. M. M. Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ. Sci. Technol. 30, 1835–1845 (1996).
Fu, X. et al. Anthropogenic short-lived halogens increase human exposure to mercury contamination due to enhanced mercury oxidation over continents. Proc. Natl. Acad. Sci. USA 121, e2315058121 (2024).
Kwon, S. Y. et al. Absence of fractionation of mercury isotopes during trophic transfer of methylmercury to freshwater fish in captivity. Environ. Sci. Technol. 46, 7527–7534 (2012).
Lim, S. H. et al. Near surface oxidation of elemental mercury leads to mercury exposure in the Arctic Ocean biota. Nat. Commun. 15, 7598 (2024).
Maúre, E. D. R., Terauchi, G., Ishizaka, J., Clinton, N. & DeWitt, M. Globally consistent assessment of coastal eutrophication. Nat. Commun. 12, 6142 (2021).
Meng, M. et al. Mercury isotope variations within the marine food web of Chinese Bohai Sea: implications for mercury sources and biogeochemical cycling. J. Hazard. Mater. 384, 121379 (2020).
Motta, L. C. et al. Mercury cycling in the North Pacific subtropical gyre as revealed by mercury stable isotope ratios. Glob. Biogeochem. Cycles 33, 777–794 (2019).
Motta, L. C. et al. Mercury isotopic evidence for the importance of particles as a source of mercury to marine organisms. Proc. Natl. Acad. Sci. USA 119, e2208183119 (2022).
UN Environment Programme. Global Mercury Assessment 2018 (UN Environment Programme, Chemicals and Health Branch, Geneva, 2019).
Tsui, M. T.-K., Wang, S. & Cheng, M. L.-H. Review of mercury pollution research in Southeast Asian marine environments. Mar. Pollut. Bull. 212, 117462 (2025).
Wang, Y., Wu, P. & Zhang, Y. Climate-driven changes of global marine mercury cycles in 2100. Proc. Natl. Acad. Sci. USA 120, e2202488120 (2023).
Gosnell, K. J. & Mason, R. P. Mercury and methylmercury incidence and bioaccumulation in plankton from the central Pacific Ocean. Mar. Chem. 177, 772–780 (2015).
Motta, L. C., Blum, J. D., Popp, B. N., Drazen, J. C. & Close, H. G. Mercury stable isotopes in flying fish as a monitor of photochemical degradation of methylmercury in the Atlantic and Pacific Oceans. Mar. Chem. 223, 103790 (2020).
Kwon, S. Y. et al. Mercury stable isotopes for monitoring the effectiveness of the Minamata Convention on Mercury. Earth Sci. Rev. 203, 103111 (2020).
Rodríguez-González, P. et al. Species-specific stable isotope fractionation of mercury during Hg(II) methylation by an anaerobic bacteria (Desulfobulbus propionicus) under dark conditions. Environ. Sci. Technol. 43, 9183–9188 (2009).
Kritee, K., Barkay, T. & Blum, J. D. Mass dependent stable isotope fractionation of mercury during mer mediated microbial degradation of monomethylmercury. Geochim. Cosmochim. Acta 73, 1285–1296 (2009).
Bergquist, B. A. & Blum, J. D. Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science 318, 417–420 (2007).
Blum, J. D., Popp, B. N., Drazen, J. C., Anela Choy, C. & Johnson, M. W. Methylmercury production below the mixed layer in the North Pacific Ocean. Nat. Geosci. 6, 879–884 (2013).
Yu, B. et al. Isotopic composition of atmospheric mercury in China: new evidence for sources and transformation processes in air and in vegetation. Environ. Sci. Technol. 50, 9262–9269 (2016).
Fu, X. et al. Isotopic compositions of atmospheric total gaseous mercury in 10 Chinese cities and implications for land surface emissions. Atmos. Chem. Phys. 21, 6721–6734 (2021).
Fu, X. et al. Isotopic composition of gaseous elemental mercury in the marine boundary layer of East China Sea. JGR Atmos. 123, 7656–7669 (2018).
Yu, B. et al. New evidence for atmospheric mercury transformations in the marine boundary layer from stable mercury isotopes. Atmos. Chem. Phys. 20, 9713–9723 (2020).
Qiu, Y. et al. Stable mercury isotopes revealing photochemical processes in the marine boundary layer. JGR Atmos. 126, e2021JD034630 (2021).
Liu, C. et al. Sources and transformation mechanisms of atmospheric particulate bound mercury revealed by mercury stable isotopes. Environ. Sci. Technol. 56, 5224–5233 (2022).
Das, R. et al. Mercury isotopes of atmospheric particle bound mercury for source apportionment study in urban Kolkata, India. Elem. Sci. Anthr. 4, 000098 (2016).
Huang, S. et al. Natural stable isotopic compositions of mercury in aerosols and wet precipitations around a coal-fired power plant in Xiamen, southeast China. Atmos. Environ. 173, 72–80 (2018).
Sun, R. et al. Historical (1850–2010) mercury stable isotope inventory from anthropogenic sources to the atmosphere. Elem. Sci. Anthr. 4, 000091 (2016).
Sun, R. et al. Mercury stable isotope signatures of world coal deposits and historical coal combustion emissions. Environ. Sci. Technol. 48, 7660–7668 (2014).
Médieu, A. et al. Evidence that Pacific tuna mercury levels are driven by marine methylmercury production and anthropogenic inputs. Proc. Natl. Acad. Sci. USA 119, e2113032119 (2022).
Yang, S. et al. Mercury isotope compositions in seawater and marine fish revealed the sources and processes of mercury in the food web within differing marine compartments. Water Res. 241, 120150 (2023).
Blum, J. D. & Johnson, M. W. Recent developments in mercury stable isotope analysis. Rev. Mineral. Geochem. 82, 733–757 (2017).
Demers, J. D., Blum, J. D. & Zak, D. R. Mercury isotopes in a forested ecosystem: implications for air-surface exchange dynamics and the global mercury cycle. Glob. Biogeochem. Cycles 27, 222–238 (2013).
Wang, C., Ci, Z., Wang, Z., Zhang, X. & Guo, J. Speciated atmospheric mercury in the marine boundary layer of the Bohai Sea and Yellow Sea. Atmos. Environ. 131, 360–370 (2016).
Zhou, J., Obrist, D., Dastoor, A., Jiskra, M. & Ryjkov, A. Vegetation uptake of mercury and impacts on global cycling. Nat. Rev. Earth Environ. 2, 269–284 (2021).
Lyman, S. N. & Jaffe, D. A. Formation and fate of oxidized mercury in the upper troposphere and lower stratosphere. Nat. Geosci. 5, 114–117 (2012).
Saiz-Lopez, A. et al. Role of the stratosphere in the global mercury cycle. Sci. Adv. 11, eads1459 (2025).
Sherman, L. S. et al. Mass-independent fractionation of mercury isotopes in Arctic snow driven by sunlight. Nat. Geosci. 3, 173–177 (2010).
Xia, S. et al. Latitudinal gradient for mercury accumulation and isotopic evidence for post-depositional processes among three tropical forests in Southwest China. J. Hazard. Mater. 429, 128295 (2022).
Liu, J., Feng, X., Yin, R., Zhu, W. & Li, Z. Mercury distributions and mercury isotope signatures in sediments of Dongjiang, the Pearl River Delta, China. Chem. Geol. 287, 81–89 (2011).
Meng, M. et al. An integrated model for input and migration of mercury in Chinese coastal sediments. Environ. Sci. Technol. 53, 2460–2471 (2019).
Yin, R. et al. Identifying the sources and processes of mercury in subtropical estuarine and ocean sediments using Hg isotopic composition. Environ. Sci. Technol. 49, 1347–1355 (2015).
Zhang, R. et al. Mercury isotope composition in open water corals of South China Sea: implication for atmospheric mercury deposition pathways into tropical oceans. Geophys. Res. Lett. 50, e2023GL105305 (2023).
Sloss, L. Legislation, Standards and Methods for Mercury Emissions Control (IEA Clean Coal Centre, 2012).
Wu, Y., Streets, D. G., Wang, S. X. & Hao, J. M. Uncertainties in estimating mercury emissions from coal-fired power plants in China. Atmos. Chem. Phys. 10, 2937–2946 (2010).
Lavoie, R. A., Bouffard, A., Maranger, R. & Amyot, M. Mercury transport and human exposure from global marine fisheries. Sci. Rep. 8, 6705 (2018).
Laurier, F. J. G., Mason, R. P., Gill, G. A. & Whalin, L. Mercury distributions in the North Pacific Ocean—20 years of observations. Mar. Chem. 90, 3–19 (2004).
Kim, I.-N. et al. Increasing anthropogenic nitrogen in the North Pacific Ocean. Science 346, 1102–1106 (2014).
World Health Organization. Exposure to Mercury: A Major Public Health Concern. Second Edition. Preventing Disease Through Healthy Environments (World Health Organization, Geneva, 2021).
Thia-Eng, C. et al. The Malacca Straits. Mar. Pollut. Bull. 41, 160–178 (2000).
Sarma, V. V. S. S. et al. Effects of freshwater stratification on nutrients, dissolved oxygen, and phytoplankton in the Bay of Bengal. Oceanography 29, 222–231 (2016).
Munson, K. M., Lamborg, C. H., Swarr, G. J. & Saito, M. A. Mercury species concentrations and fluxes in the Central Tropical Pacific Ocean. Glob. Biogeochem. Cycles 29, 656–676 (2015).
York, D., Evensen, N. M., Martı́nez, M. L. & De Basabe Delgado, J. Unified equations for the slope, intercept, and standard errors of the best straight line. Am. J. Phys. 72, 367–375 (2004).
Parnell, A. C. et al. Bayesian stable isotope mixing models. Environmetrics 24, 387–399 (2013).
Parnell, A. C., Inger, R., Bearhop, S. & Jackson, A. L. Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5, e9672 (2010).
Huang, S. et al. Application of an isotope binary mixing model for determination of precise mercury isotopic composition in samples with low mercury concentration. Anal. Chem. 91, 7063–7069 (2019).
Schlitzer, R. Ocean Data View. https://odv.awi.de (2023).
Jung, S., Kwon, S. Y., Li, M.-L., Yin, R. & Park, J. Elucidating sources of mercury in the west coast of Korea and the Chinese marginal seas using mercury stable isotopes. Sci. Total Environ. 814, 152598 (2022).
Acknowledgements
S.K. discloses support for this work from the research program of the Korea Institute of Ocean Science and Technology (KIOST) funded by the Ministry of Oceans and Fisheries [Grant number PE99883] and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) [Grant Number NRF-2021R1C1C1008429]. D.K. discloses support for this work, supported by Korea Institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Ocean and Fisheries, Korea [RS-2022-KS221662. PM64650].
Author information
Authors and Affiliations
Contributions
Laura C. Motta contributed to conceptualization and writing. Seung Hyeon Lim contributed to the analysis. Joel D. Blum contributed to conceptualization and writing. Youn-Ho Lee contributed to sample collection. Dong-Jin Kang contributed to funding and sample collection. Sae Yun Kwon contributed to conceptualization, writing, and funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Kathleen Gosnell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Somaparna Ghosh [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Motta, L.C., Lim, S.H., Blum, J.D. et al. Anthropogenic mercury migration from Asia to the open ocean. Commun Earth Environ 6, 669 (2025). https://doi.org/10.1038/s43247-025-02555-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-025-02555-z