Abstract
The German waters contain 1.6 million tons of munitions, mostly dumped after the World Wars. The structure and composition of epifauna were investigated on the dumped munition in the Lübeck Bay (Baltic Sea) within the area with regular hypoxic conditions using a remotely operated vehicle (ROV). Objects were identified as warheads from the Fieseler flying bomb (Fi103, known as V1). Eight species of epifauna were found, with a mean density of 43,184 ind. m−2, mostly concentrated on the metal parts. Surrounding sediment had a significantly lower abundance of only 8213 ind. m−2. Chemical analysis of water sampled directly near several warheads showed high concentrations of explosive compounds, with, e.g., trinitrotoluene (TNT) levels of 2.73 mg/l. These concentrations approach toxicity thresholds for aquatic organisms. However, the epifauna develops on breached munitions in numbers comparable to natural hard substrata. In the future, munitions should be replaced with safe, hard substrates.

Similar content being viewed by others
Introduction
Coastal areas of many countries are contaminated with the dumped munitions. In the German EEZ, the total amount of munitions is estimated at around 1.6 million tons, mostly dumped during the demilitarization after World War II (WW2)1,2,3. Within Lübeck Bay (Baltic Sea), several official dumpsites are marked, including “Haffkrug” in the western part of the bay, and “Pelzerhaken” in the central part4. However, according to recent mapping during several national and internationally funded projects (e.g., UDEMM, https://udemm.geomar.de; BASTA, https://www.basta-munition.eu; ExPloTect, https://www.explotect.eu; and the ongoing CONMAR, https://conmar-munition.eu/), many munition items are found outside of the designated areas5. During the past years, a number of studies were published, focusing on the potential influence of munition chemicals (explosive compounds, ECs; organic explosives and their derivatives) on the surrounding ecosystem. Conventional munition objects contain solid explosives, primarily 2,4,6-trinitrotoluene (TNT)6,7. These ECs exhibit varying degrees of toxicity, and for laboratory tested aquatic organisms, the LC-50 (50% lethality) for TNT ranges from 1 to 10 mg l−1 for different invertebrates and fishes8,9. Specifically, at the munition dumpsites, elevated oxidative stress under exposure to EC was observed in blue mussels10,11,12. Other biological responses known include lower lysosome membrane stability and altered micronuclei formation13,14,15, though these responses are mostly caused by chemical warfare rather than by conventional munitions. Although the dumped munition possesses a clear threat to the benthic ecosystems of the Baltic Sea16, no clear influence of EC concentrations has been shown at the ecosystem level on the overall spatial structure of the benthic infauna17. Nonetheless, both individual munition objects and piles of munition items represent hard substrate that drives increased faunal abundance and diversity up to several meters distance around each munition object (Vedenin et al. 2025)18. Despite the potential negative effects of the toxic munition compounds, published underwater images show dense populations of algae, hydroids, mussels, and other epifauna on the munition objects, including mines, torpedo heads, bombs, and wooden crates5,7,19. However, the composition and structure of the epifaunal communities found on the underwater munition have not yet been investigated directly.
There are a lot of published studies on other kinds of artificial hard substrata, represented by ship-wrecks, offshore wind farms, oil rigs, pipe-lines, concrete bases for future coral reefs, etc.20,21,22,23,24. Both natural and artificial hard substrata are known to provide a habitat and attract various species, like algae, invertebrates, and fish. Comparisons of natural hard substrata (e.g., stones and boulders) with artificial ones showed controversial results; sometimes artificial structures provide higher epifaunal abundance and diversity25 and sometimes—the natural substrates26. The factors influencing epifauna abundance and diversity include the surface of the substrate, its heterogeneity, material, and shape—e.g., elevated artificial metal structures with larger relative surface area and varying orientation may support denser populations of sedentary organisms compared to rounded boulders and stones. However, the recently submerged artificial structures usually show lower abundance and diversity compared to natural substrata, which have been present in the area for hundreds or thousands of years27,28.
In October 2024, one location between the dedicated munition dumpsites “Haffkrug” and “Pelzerhaken,” initially suspected in multibeam data to hold munition objects, was investigated with a remotely operated vehicle (ROV) equipped with two camera systems (Figs. 1 and 2). The resolution of video data allowed us to visually identify and count epifauna species, inhabiting both the munition objects and the surrounding sediment.
A German Baltic Sea with munition dumpsites outlined; B enclosed area of the Lübeck Bay with shown position of the investigated site; C multibeam imagery (depth values) of the investigated area with the track of the ROV, colored according to the dive time; D AUV photomosaic of the investigated area with marked sediment tracks and ROV-observed objects classified according to their degradation stage; dark trapezoid—intact; white trapezoid—partly degraded; I-shaped contour—fully degraded.
In this study, for the first time, the composition and structure of epifauna on the surface of marine munitions are described. The appearance, size, and potential origin of the munition objects and their content are analyzed. We hypothesized that the structure and composition of the epifauna on the munition is different from surrounding areas and from hard substrata of natural origin described in the literature. More specific goals were to understand which species and at which abundance can be found on the investigated munition, and whether epifauna are likely to be exposed to toxic levels of explosive compounds. Materials and Methods are described in detail in the last section.
Results
Characteristics and identification of the munition objects
The overall dimensions were consistent for every object and were, correspondingly, 130–140 cm in length and 90–100 cm in width, depending on the corrosion rate and epifauna layer (Figs. 3 and 4A). The central cylinder appeared to be slightly conical, although the difference between the wider and thinner ends was only around 10 cm, almost negligible for measuring it with the ROV laser points. The ends of each object were covered with large metal hoops that probably supported the overall structure and allowed rolling. The hoops were connected with each other through four (although no more than three were visible) thin metal shafts.
A–C Observed objects at varying stage of degradation with estimations of their unburied surface area based on the ROV measurements; D modified scheme of the transporting device for the warhead of Fi 103 flying bomb31; D modified scheme of the Fi 103 warhead structure (version Fi 103 A-2) with published measurements30.
Only two out of nine examined objects were intact; for the rest, the metal casing of the central cylinder was in varying stages of degradation, exposing the explosive filling. The filling was yellowish and had a very specific “cheesy” structure with multiple concaves, holes, and cavities (Fig. 3A, I). The filling of three of the investigated objects was mostly gone, exposing a 6–8 cm thick tube along the central axis, identified as a fuse pocket.
According to the literature29, several kinds of WW2 munitions have similar sizes, including heavy bombs, depth charges, and mines. However, considering the slightly conical shape and a longitudinal fuse pocket, the most probable candidate is the warhead from the Fi 103 A-2 flying bomb (also known as V1); its dimensions follow our measurements almost exactly (Fig. 4A, E30). The information about the storage and transport of the German munition during the demilitarization is scarce, though there is one drawing of the so-called “Transportgerät” (=transporting device) designed specifically for the Fi 103 warhead (Fig. 4D31) that follows our description of hoops, connected with thin shafts. The surface area estimated for the observed objects varied from 3.23 ± 0.54 m2 for Item 9 to 5.67 ± 0.94 m2 for Item 3.
Epifauna community structure
A total of eight species (five species of invertebrate epifauna and three fish species) were identified in the ROV videos, with 1–6 species present at each track and item observed (Fig. 5). From the distance the dominant taxa visible were cnidarians (Campanulariidae hydroids and Metridium dianthus anemones) and echinoderms (Asterias rubens starfishes). During close observations, the polychaetes (Polydora ciliata, identifiable by tubes) dominated, reaching over 90% of the total abundance. Diversity values were low, including the evenness and Shannon-Wiener index. Abundance values for the species and diversity values are shown in Table 1.
Cluster analysis revealed two distinct clusters at both, distant and close observations as well as the sediment tracks (Fig. 6). The species composition was very different between the two clusters, as is shown in the shade plots (Fig. 6). Two types of sample groups were further tested with PERMDIST and PERMANOVA routines—1. sediment tracks vs. items (two groups) and 2. sediment tracks vs. items at different degradation stages (four groups). PERMDISP showed no significant difference in the dispersion between the groups (except for sediment tracks vs. items at different degradation stages—there, the sample size was not enough for calculations). The type of the munition part and stage of degradation appeared to have no effect on the dendrogram topology, although the PERMANOVA test showed that, at least for the distant observations, the distinction between the intact, partly rusted, and fully degraded objects was significant (Fig. 6).
A Distant observation; B close observation. Symbols of items at the bottom are the same as in Fig. 2. Unresolved nodes and branches on the dendrogram (according to the SIMPROF routine with threshold = 0.05) are marked with dotted red lines. PERMDISP and PERMANOVA results are shown in boxes below with F values and p values for PERMDISP (=F; p) and Statistics values and p values for PERMANOVA (=St.; p); Track vs. Item—comparison between tracks and individual objects (two groups); Track vs. Degrad. stage—comparison between tracks and objects at three different stages of degradation (four groups).
Individual species distribution within the revealed clusters varied. Specifically, on the sediment, the only species present were Asterias rubens starfish from a distance and, additionally, Polydora ciliata polychaetes during close observations. Among various items, the species were distributed similarly, although Metridium dianthus anemones were slightly more abundant on intact or partly rusted items, rather than on completely degraded objects (Fig. 6).
On the individual objects, the majority of epifauna was found on metal carcasses, while the exposed explosive was usually free of visible overgrowth. On rare occasions, individual starfish Asterias rubens or crabs Carcinus maenas were seen crawling on the surface of the explosive, or smaller Polydora ciliata colonies developed in crevices filled with sediment (Fig. S1). One exception was object eight, with several larger chunks of the explosive lying freely on the nearby sediment. Those chunks were covered with multiple starfish A. rubens, both adult and juvenile (a total of 45 individuals, Figs. 5E and S5). Specifically, a total of 25 and 12A. rubens individuals were found on two chunks with approximate areas of 0.07 and 0.04 m2 (i.e., 357 and 300 ind. m−2 density, respectively). Due to a small sample size, no significant conclusions can be made, though an unequal variance t-test between the A. rubens on these chunks and A. rubens at the rest of the close observations shows values of t = −7.76 and p = 0.01.
Dissolved explosive chemicals
Concentrations of EC in samples collected near breached Fi 103 objects varied widely, across some five orders of magnitude, with values up to 2.7 mg/l for TNT and 0.5 mg/l for RDX (Table 2). The wide range in EC concentrations probably reflects the plume nature of emissions from these point source objects. TNT concentrations at all but one of the targets were detected at levels above 100 ng/l, far higher than typically observed in the Lübeck Bay water column or elsewhere in the southwest Baltic Sea32. Concentrations of TNT were higher than any other EC, and the highest observed concentration of nearly 3 mg/l is similar to levels measured directly at the explosive surface at another dumpsite in the Baltic Sea33. Samples in the present study were collected at a number of locations around the Fi 103 munition objects, but were not intended to systematically investigate individual munition sources. The wide range in chemical concentrations likely reflects variations in current direction and ROV position during sampling, as chemical plumes from munition objects are heterogeneous and can decline rapidly with distance due to mixing and dilution6,34. Detailed mapping of munition chemical plumes is challenging due to the limited number of samples that can be collected on one dive, but the current dataset shows the range of exposure concentrations for fauna at such sites.
Discussion
Our identification of the studied munition objects as Fi 103 warheads gives possible explanations for the observed cavitated structure of the explosive fillings. On previously published photos of chunks of explosive material, the surface was smoother and lacked such “cheesy” structure6,35. In the literature, there is contradictory information about the specific type of explosives in Fi 103 warheads. The mixtures reported include “Trialen 106” (70% TNT, 15% RDX, 15% aluminum powder), “Amatol 40/60” (60% TNT, 40% ammonium nitrate), “Amatol 39A” (=“52A+,” varying mixture of RDX, dinitrobenzene, calcium nitrate and ammonium nitrate), “Myrol” (67% methyl nitrate and 33% methanol) and “Donarit” (11–16% TNT, 55–84% ammonium nitrate, up to 22% of nitroglycerin)29,30,36 [https://www.fortlitroz.ch]. Direct measurements at the “Pelzerhaken” dumpsite obtained from similar objects showed that dissolved EC near these objects includes TNT, RDX, and DNB (Table 2), consistent with reported explosive fill types, and which may reflect different mixtures used in the warheads present in Lübeck Bay. According to scattered information regarding the factory process of filling the warhead shells, the density of the liquefied explosives prepared for pouring was semifluid or pasty (whatever mixture was used) just above the melting point for faster further solidification. By longer cooling from higher temperatures, a gravitational separation of components was possible due to the large volume (~850 kg for Fi 103), which was tried to be avoided. Therefore, some air bubbles could remain in the charge, resulting in the observed cavitated structure of the overall filling37,38 [http://v1armedudesespoir.free.fr]. Another explanation would be a poorly stirred mixture, so that components of varying solubility could dissolve at a different rate, creating holes.
Our data on epifaunal abundance roughly correspond with other studies on the natural hard substrata in the South-Western Baltic Sea, where different values of 5000–40,000 ind. m−2 were reported39,40,41. Comparisons of our observational numbers with published data are shown in Table 3. The species composition was often different, with, e.g., barnacles Amphibalanus improvisus dominating in the Gulf of Gdansk40; ascidians Dendrodoa grossularia dominating in the western Lübeck Bay (the only available investigation performed close to our study area, <15 km away39)—and barnacles Semibalanus balanoides and mussels Mytilus edulis—dominating in the Øresund41. None of the mentioned organisms were found at our study location in Lübeck Bay. A likely explanation is the regular oxygen deficiency happening in the deeper parts of the Lübeck Bay42, including the vicinity of the study area (Fig. 7), that is tolerable for only a few taxa. Different taxa dominating different areas of the Baltic Sea vary in their biological traits, including their relative size and gregarious settling pattern, resulting in almost one order of abundance deviation. The percentage of epifauna coverage can be an alternative to simple abundance values (i.e., individuals per area), but this parameter is almost impossible to assess through video data (our data43). One more reason for the different abundance values reported is seasonality—in the Baltic, the highest values of benthic abundance are usually observed in March-July44. Additional observations in spring could add more information regarding epifauna dynamics on the dumped munition. The difference in salinity also plays a role, although all the mentioned taxa are distributed across the majority of the South-Western Baltic Sea, including the Lübeck Bay (GBIF, www.gbif.org).
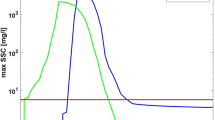
A Temporal profile through the 0–20 m water depth; B values for the 20 m depth. Data from ref. 70.
The dominant species identified in this study, primarily Polydora ciliata polychaetes and Metridium dianthus anemones, are typical for the areas of the South-Western Baltic Sea that experience regular oxygen deficiency. In the deeper Arkona Basin with similar conditions, a community dominated by Metridum dianthus anemones, Asterias rubens starfish, and hydrozoans was found at depths down to 46 m43. P. ciliata was not mentioned in that study, apparently due to a larger distance of the towed camera from the seafloor. Mobile species like fish or crabs are less vulnerable to periodical oxygen deficiencies, as they can move from the area of decreasing oxygen. M. dianthus, considered to be nearly immobile, can remarkably survive for many days in completely anoxic conditions, as was shown in experiments in Flensburg Fjord, reaching densities of over 200 ind. m−2 in 15 m depth, similar to our study location45. Specifically, the survival rate on the 25th day of anoxia is still 50%46. We have no directly measured data on how long the low-oxygen periods last within the study area. According to Copernicus Marine data, deeper parts of the Lübeck Bay (~1.8 km south from the study area) experience regular oxygen deficiency (Fig. 7). Specifically, in the 2022 the oxygen levels remained below 50 mmol m−3 with only a few short increases from the beginning of August till the end of October, which is probably beyond the tolerance limits of the M. dianthus. However, in 2023, these low levels stayed for less than 2 weeks. We don’t have the data for 2024, but it is possible that the low-oxygen period was short enough for M. dianthus to maintain the population during the last two years. P. ciliata polychaetes do not have such tolerance to anoxic conditions. However, they can develop rapidly, with full metamorphosis from hatching to maturity lasting 9–11 days47; they can thus form a dense population within less than a month48,49. In addition, their tubes can remain long after the animal dies, and it is possible that some of the tubes counted in our data were actually empty39. noticed that for 33,000 tubes for 1 m2 there are usually 5000–6000 living individuals, so the actual abundance of living P. ciliata in our observations might be several times less than observed.
The majority of the epifauna was developed on the metal carcasses, on the transport parts, and the remaining shell of the warhead, as well as on fuse pockets if they were exposed. The EC concentrations measured near the Fi 103 objects (0.13 ± 0.58 mg/l of TNT) often exceed water quality thresholds for aquatic organisms, especially at object AL622_75-Btl4 with TNT values > 2.7 mg/l—above the known LC-50 threshold for fishes (Table 26). However, this exposure clearly does not prevent epifauna from settling and growing on the metal housing surrounding the explosive, where EC concentrations reach the mg/l level. These concentrations can also induce fauna behavioral responses. For example, mussels exposed to TNT concentrations above 0.6 mg/L tended to keep their shells closed, interfering with normal filtering behavior11. Such effects would be likely to have negative effects on faunal health, but no significant differences were observed between the epifauna on the metal surface and reported communities on natural hard substrata within the regular oxygen deficiency zones43. This suggests that the high measured EC concentrations are not sustained long-term, or that they, in fact, do not have a major negative effect on nearby organisms. Descriptions of the epifauna found on stones or artificial hard substrata of non-military origin near the study area will support this observation, although no large stones or boulders are present in the direct vicinity (<180 m) of the study site (more potential munition objects can be seen). In the South-Western Baltic Sea and in Lübeck Bay in particular, hard surfaces are rare, due to so-called “stone-fishing”—commercial exploitation of stones and boulders from the seafloor during the nineteenth to twentieth centuries5,50.
The solid explosive surface was mostly free of organisms, with the rare exception of starfish and crabs. The receding edge of the dissolving explosives may prevent the attachment of sessile organisms directly to the surface. However, the polychaete Polydora ciliata is known to inhabit a large variety of surfaces, building their tubes on rocks, and even drilling through carbonate structures such as mollusk shells and burrowing into silt and dense mud51,52. Water trapped in tunnels in solid explosives is likely to reach saturation concentrations (>100 mg/l), as is the case for stagnant water in slightly breached munitions53, and such high concentrations would be acutely toxic to polychaetes54.
On one occasion, a dense aggregation of A. rubens starfish was observed on several chunks of the explosives lying exposed on the sediment (Figs. 5E and S5). Considering that was a single observation, it is hard to assess whether such numbers of A. rubens can be random. However, simple two-sample statistics (observed aggregations vs. the rest of the available footage) suggest that it is highly unlikely to be random, with ~330 ind. m−2 on the chunks and ≤100 ind. m−2 at every other site. Starfish aggregate either during the reproductive period (when they gather on elevated substrates for dispersing the gametes) or during feeding (e.g., feeding on mussel beds)55,56. In October 2024, neither the season nor their positions were consistent with reproductive behavior. The explosive surfaces were bare, with no evidence of macro-epifauna overgrowth. For some starfish species, grazing on biofilms is described, especially for younger individuals57. For A. rubens, no such behavior was reported; in situ and experimental observations show direct predation on individual micro- and macrobenthic organisms for this species58, although anecdotal reports of juveniles feeding on some bacterial or algal films are published55. We can only suggest that either A. rubens is feeding on the biofilms growing on the explosives, or they are chemically attracted to the exposed chunks by some components in the explosives. At the nearby Kolberger Heide munitions dumpsite, the TNT content of tissue in A. rubens exceeded 500 µg/g, several hundred times higher than any other biota analyzed from the site59. Thus, starfish behavioral patterns, grazing or otherwise, appear to lead to exceptionally high levels of EC exposure. Additional observations are required, including replicative footage or deploying time-lapse cameras at the study area and/or other known Fi 103 locations in the Lübeck Bay.
The sediment epifauna described in this study is poor, both in terms of abundance and diversity. This corresponds with previous data, where the abundance, biomass, and diversity are known to be higher on hard substrata and near it compared to the surrounding soft sediment60,61.
Overall, the epifaunal community on the dumped munition in the study area reaches a high density, with the elevated metal structures providing a suitable habitat for benthic organisms. Previously, it was shown that even on the scale of several meters around the dumped munition, the infauna abundance, biomass, and diversity tend to increase (Vedenin et al. 2025)18. Therefore, it should be considered to replace the munition after remediation with other “safe” hard substrata objects. Although adding new hard substrates to the marine ecosystems can be questionable due to altering the surrounding habitat and even providing a possible refuge for non-native species27,28, in the particular case of the German Baltic Sea, new hard substrata can be a conservation tool and lead to conditions closer to initial natural conditions. Before dumping, the sea-bottom landscape of the bay was much richer with stones and boulders of glacial origin before the “stone-fishing” took the high volume of the existing hard substratum, which became prohibited only in 197650,62.
Conclusions
Our study presents a novel evaluation of epifauna community structure on dumped munitions in the Baltic Sea. This previously unknown dumpsite consisting of Fi 103 (=V1) warheads in transport frames is located in an area with regular oxygen deficiency that lasts from weeks to 3 months per year. However, the surface of the munition objects was found to be densely covered by epifauna. Epifauna diversity was not particularly high, with only 8 species identified, but the densities of over 40,000 ind. m−2 are much higher than, e.g., macrofauna densities reported from different parts of the Lübeck Bay.
Dissolved EC concentrations near the Fi 103 objects reached mg/l levels, a threshold likely to be toxic to aquatic organisms. However, the high levels of chemical exposure apparently do not prevent the development of a dense epifauna community on the metal shells, fuse pockets, and transport cases centimeters from the explosive filling. The bare explosive, however, remains mostly free from epifauna, even from the Polydora polychaetes that are known to inhabit a vast variety of substrates. A possible reason for the explosives remaining free from epifauna is extremely high concentrations of dissolved ECs at the boundary layer that, in this case, could be acutely toxic for all fauna. In this study, only a single site was investigated, which makes extrapolation of our results to other dumpsites within the Lübeck Bay and other areas speculative. Further investigations are required after our first glimpse at the epifauna communities developing on the dumped munition. The described methods of observation can be used at other dumpsites as well as at the hard substrates of natural origin to provide a direct comparison between the Fi 103 warheads investigated in this study and other areas.
Materials and methods
Study area
We have discovered and investigated one previously undescribed dumpsite located in the northern part of the Lübeck Bay, between the designated dumping areas of “Haffkrug” and “Pelzerhaken” in 19 to 21 m water depth, covering ~500 m2 (Fig. 1A, B). The sediments in the area are represented by fine mud. Temperature and salinity vary during the year with near-bottom values of 4–15 °C and 16–24 psu63. The near-bottom currents are rather weak, not exceeding 0.3 m s−1 64 and median values of 0.1 m s−1 (ADCP, unpublished data). The majority of deeper areas of the bay are experiencing regular oxygen deficiencies, due to strong thermal stratification and limited vertical water exchange42. The oxygen content in the sediment is also low, as confirmed by darker sediment with an intense smell of H2S.
A total of 12 objects lying within the area were initially unknown large metal cylinders ~130–140 cm long and ~90–100 cm in diameter with varying stages of degradation (Fig. 1C). Some were almost intact, others—rusted out with the explosive content strongly dissolved. The objects resembled, by shape and content, similar objects from the “Pelzerhaken” area described by ref. 65 that were identified as Fi 103 “flying bomb” warheads. The relative distribution and stages of degradation of the investigated objects are shown on the photomosaic compiled from multiple AUV-photos (model Girona 500, obtained in expedition AL628 with RV “Alkor” in March 2025, Fig. 1D).
Video data analysis
The study area (~300 m2/14 by 22 m) was investigated using the BlueRobotics ROV named “Käpt’n Blaubär” (model BlueROV2) during cruise AL622 with RV “Alkor” on 19. October 2024. The ROV is equipped by default with a tiltable HD camera and LED lights in front, and accurate USBL underwater navigation. Additionally, a laser pointer system was installed (distance 40 cm) and a fixed-angle GoPro3 (4K) was fixed on top of the ROV (Fig. 2).
A total of nine munition objects and two sediment track profiles were filmed. The relative distribution of the investigated objects is shown in Fig. 1D. An overview image of each object from 2 to 3 m is shown in Fig. 3. Eight objects were filmed from a distance of ~2 m (further referred to as “distant observations”). Three of these objects and one additional object were filmed from a distance of ~30 cm, allowing for the identification and counting of smaller epifauna organisms (further referred to as “close observation”).
Distant-observations
Video-track of each observed item was divided into separate frames (2704 × 1520 px resolution). Several frames of the best quality (i.e., when the ROV was not moving fast) were chosen from each object at ~90° between each other to avoid marking the same organism multiple times. All visible organisms were identified and counted manually at each frame and summarized (same individuals from different frames were counted once). The entire filmed area of each object was used for fauna analysis.
Close-observations
Smaller organisms visible only by close observations were not accounted for in the distant observations to avoid scale bias. Epifauna abundance calculations of each close observation were performed based on a single frame with the best quality of the image chosen. The close observation frames are shown in the Supplementary Material with calculations (Figs. S1–S5).
The area of the objects (both total and observed by distant and close observations) was estimated, and the abundance per m2 of each species was calculated based on the laser points reference and approximations of the observed objects to simple geometrical figures (Supplementary 1). A summary of the investigated objects and their surface area is presented in Table 1. The approximate shapes of the objects, depending on their stage of degradation and corresponding surface areas, are shown in Fig. 3. At some stages of degradation, the explosive core was exposed, which did not allow a good estimation of the surface area due to multiple holes and cracks (e.g., Fig. 3I). In such cases, the surface of the object was assumed to be flat.
The abundance values were calculated separately for the distant observations and for the close observations, as smaller individuals were unrecognizable by distant observations. Taxonomical names are reported according to the World Register of Marine Species database (https://marinespecies.org). The number of species per object, Pielou’s evenness, and Shannon-Wiener index were used as diversity indicators.
The similarity between the samples was estimated using Bray-Curtis similarity based on abundance66. Raw data were square-root transformed to reduce the dominant taxa bias. Hierarchical cluster analysis was performed with the UPGMA (=group average) algorithm with the similarity profile routine (SIMPROF) at the significance level of 0.0567. Shade plots were used for visualization. Identified groups of samples were further tested with Distance-based test for homogeneity of multivariate dispersions (PERMDISP) and Permutational multivariate analysis of variance (PERMANOVA)68.
Statistical analyses were performed using the Primer v6 and Microsoft Excel 2010 software67. Maps, diversity indices, PERMANOVA analysis, correlations, CCA, and models were run and plotted using the original Python 3.8 scripts (with Basemap, Matplotlib, NetCDF4, NumPy, Pandas, Pygam, Scikit-bio, Seaborn packages69).
Environmental parameters
Oxygen data modeled for the last 3 years were taken from the Copernicus Marine database70 from the nearest available coordinates (~1800 m south of the study area). The values are plotted in Fig. 7. The depth for comparison was chosen to be 20 m, as a mean for the study area. Data are presented in mmol m−3 with values below 62.5 mmol m−3 being considered as hypoxic, and below 67 mmol m−3—sublethal for most benthic organisms71.
Discrete water samples (n = 22) were collected with polycarbonate water sampling bottles mounted on the ROV (Fig. 2). The ROV sampler consists of four 0.5 L custom-built bottles that are deployed open and are flushed by a pump before being individually closed. One bottle was modified temporarily with a sampling tube to collect several samples from depressions in exposed solid explosives. Water samples were collected near several similar-type objects at the Pelzerhaken area during cruises in July and October 2024. These data are considered together here as representative of chemical levels around these breached munitions. A technical problem with the mass spectrometer, unfortunately, prevented measurement of DNB during the October cruise.
Samples were measured onboard using the Xplotector lab-in-a-box system72. The system consists of a fluidics module coupled to an analytical module. The fluidics module uses several HPLC pumps and switching valves to automate preconcentration of EC from seawater, including inline particle filtration (0.5 µm; sintered stainless steel, Swagelok). For internal standardization, 20 ng of heavy isotope-labeled TNT and DNB (as described by ref. 73; Cambridge Isotopes) was infused by automated syringe pump into the sample water stream of each sample during preconcentration. The preconcentrated sample was then injected into the analytical module, which performs HPLC chromatography and analysis by mass spectrometry. The Xplotector system was improved from the original system with a higher-sensitivity compact atmospheric-pressure chemical ionization (APCI) mass spectrometer (expression CMS; Advion Interchim Scientific), with improved sensitivity for TNT (limit of quantification, LOQ, 3.3x the limit of detection: 1 ng/l) and detection capabilities also for ADNT, RDX, and DNB (LOQ: 8, 14, and 8 ng/l, respectively). System blanks using high-purity water showed no detectable munition compounds, so detection limits were calculated from the residuals of the matrix-matched calibration curves following ref. 74. Water column samples collected away from munitions objects show no evidence of carryover in the ROV sampler, likely helped by flushing of the open bottles during every deployment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Raw video data will be available at the PANGAEA repository (https://www.pangaea.de/), PDI-41838. The exact location of the investigated munition is considered to be sensitive information and therefore cannot be disclosed in a publicly accessible repository.
References
Böttcher, C. et al. Munitionsbelastung der deutschen Meeresgewässer: Bestandsaufnahme und Empfehlungen 174 (Bundesamt für Seeschifffahrt und Hydrographie (BSH), Sekretariat Bund/Länder-Messprogramm für die Meeresumwelt von Nord-und Ostsee (BMLP), 2011).
GICHD. A Guide to Survey and Clearance of Underwater Explosive Ordnance. (GICHD, 2016).
Wehner, D. & Frey, T. Offshore unexploded ordnance detection and data quality control—a guideline. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 15, 7483–7498 (2022).
Munition Cadaster “AmuCad”, EGEOS GmbH. www.amucad.org.
Kampmeier, M., van der Lee, E. M., Wichert, U. & Greinert, J. Exploration of the munition dumpsite Kolberger Heide in Kiel Bay, Germany: Example for a standardised hydroacoustic and optic monitoring approach. Cont. Shelf Res. 198, 104108 (2020).
Lotufo, G. R. et al. Review and Synthesis of Evidence Regarding Environmental Risks Posed by Munitions Constituents (MC) in Aquatic Systems 0254 (US Army Engineer Research and Development Center, Environmental Laboratory, 2017).
Beck, A. J. et al. Spread, behavior, and ecosystem consequences of conventional munitions compounds in coastal marine waters. Front. Mar. Sci. 5, 141 (2018).
Nipper, M., Carr, R. S. & Lotufo, G. R. Aquatic toxicology of explosives. in Ecotoxicology of Explosives (eds Sunahara, G. I., Lotufo, G. R., Kuperman, R. G. & Hawari, J.) 77–115 (CRC Press, 2009).
Lotufo, G. R., Rosen, G., Wild, W. & Carton, G. Summary Review of the Aquatic Toxicology of Munitions Constituents (US Army Engineer Research and Development Center, 2013).
Strehse, J. S., Brenner, M., Kisiela, M. & Maser, E. The explosive trinitrotoluene (TNT) induces gene expression of carbonyl reductase in the blue mussel (Mytilus spp.): a new promising biomarker for sea dumped war relicts? Arch. Toxicol. 94, 4043–4054 (2020).
Schuster, R. et al. Exposure to dissolved TNT causes multilevel biological effects in Baltic mussels (Mytilus spp.). Mar. Environ. Res. 167, 105264 (2021).
Brenner, M., Schuster, R. & Binder, F. Biological effects of munition left on war wrecks on the health of caged blue mussels (Mytilus edulis, L.) in the southern North Sea. In EGU General Assembly Conference Abstracts (EGU-2746). https://doi.org/10.5194/egusphere-egu23-2746 (2023).
Bełdowski, J. et al. CHEMSEA Findings–Results from the CHEMSEA project (Chemical Munitions Search and Assessment). (IOPAN, 2014).
Bełdowski, J. et al. Chemical munitions search & assessment—an evaluation of the dumped munitions problem in the Baltic Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 128, 85–95 (2016).
Baršienė, J. et al. Environmental genotoxicity assessment along the transport routes of chemical munitions leading to the dumping areas in the Baltic Sea. Mar. Pollut. Bull. 103, 45–53 (2016).
Dobrzycka-Krahel, A. & Bogalecka, M. The Baltic Sea under anthropopressure—the sea of paradoxes. Water 14, 3772 (2022).
Vedenin, A. A. et al. Spatial structure and biodiversity of macrofauna around marine munition dumpsites—a case study from the Baltic Sea. Mar. Pollut. Bull. 198, 115865 (2024).
Vedenin A. et al. Influence of dumped munition on the benthic macrofauna: Relation to munition object proximity and explosive compounds content. Mar. Pollut. Bull. 220, 118379 (2025).
Greinert, J. Practical Guide for Environmental Monitoring of Conventional Munitions in the Seas-Results from the BMBF Funded Project UDEMM “Umweltmonitoring für die Delaboration von Munition im Meer” Version 1.1, Nr. 54 (Berichte aus dem GEOMAR Helmholtz-Zentrum für Ozeanforschung, 2019).
Svane, I. B. & Petersen, J. K. On the problems of epibioses, fouling and artificial reefs, a review. Mar. Ecol. 22, 169–188 (2001).
Walker, S. J., Schlacher, T. A. & Schlacher-Hoenlinger, M. A. Spatial heterogeneity of epibenthos on artificial reefs: fouling communities in the early stages of colonization on an East Australian shipwreck. Mar. Ecol. 28, 435–445 (2007).
Zintzen, V., Norro, A., Massin, C. & Mallefet, J. Spatial variability of epifaunal communities from artificial habitat: Shipwrecks in the Southern Bight of the North Sea. Estuar., Coast. Shelf Sci. 76, 327–344 (2008).
Schutter, M. et al. Oil and gas platforms as artificial substrates for epibenthic North Sea fauna: Effects of location and depth. J. Sea Res. 153, 101782 (2019).
Lacey, N. C. & Hayes, P. Epifauna associated with subsea pipelines in the North Sea. ICES J. Mar. Sci. 77, 1137–1147 (2020).
Balazy, P. & Kuklinski, P. Arctic field experiment shows differences in epifaunal assemblages between natural and artificial substrates of different heterogeneity and origin. J. Exp. Mar. Biol. Ecol. 486, 178–187 (2017).
Brzana, R., Peschke, M. B. & Janas, U. Biodiversity and functioning of benthic macrofauna associated with natural and artificial hard substrate in the Gulf of Gdańsk (Baltic Sea). Mar. Environ. Res. 199, 106592 (2024).
Wilhelmsson, D. & Malm, T. Fouling assemblages on offshore wind power plants and adjacent substrata. Estuar., Coast. Shelf Sci. 79, 459–466 (2008).
Coolen, J. W. North Sea Reefs: Benthic Biodiversity of Artificial and Rocky Reefs in the Southern North Sea Doctoral dissertation, Wageningen University and Research (2017).
German Explosive Ordnance, Vol. 1 (Navy Department Bureau of Ordnance, 1946).
Hölsken, D. V-Missiles of the Third Reich, the V-1 and V-2 (Monogram Aviation Pub, 1994).
Dienstgebrauch Lüftwaffe. FZG 76 Geräte-Handbuch. T. 2076, Teil I (1944).
Beck, A. J. et al. Widespread environmental contamination from relic munitions in the southwestern Baltic Sea. Chemosphere 372, 144115 (2025).
Beck, A. J. et al. In situ measurements of explosive compound dissolution fluxes from exposed munition material in the Baltic Sea. Environ. Sci. Technol. 53, 5652–5660 (2019).
Rosen, G., Lotufo, G. R., Belden, J. B. & George, R. D. Environmental characterization of underwater munitions constituents at a former military training range. Environ. Toxicol. Chem. 41, 275–286 (2022).
Maser, E. & Strehse, J. S. Can seafood from marine sites of dumped World War relicts be eaten? Arch. Toxicol. 95, 2255–2261 (2021).
Hellmold, W. Die V1: eine Dokumentation: mit 104 technischen Darstellungen und 136 Fotos (Bechtle, 1988).
Young, J. Military explosives of to-day. Lect. Iii. J. R. Soc. Arts 66, 733–742 (1918).
Voigt, H. W. Process for suspending particulate additives in molten TNT. Patent number: US4000021A (1976).
Gulliksen, B. The macrobenthic fauna of rocks and boulders in the Lübeck Bay (Western Baltic Sea) investigated from the Underwater Laboratory “Helgoland”. Helgol. Wiss. Meeresunters. 27, 439–449 (1975).
Grzelak, K. & Kuklinski, P. Benthic assemblages associated with rocks in a brackish environment of the southern Baltic Sea. J. Mar. Biol. Assoc. U. K. 90, 115–124 (2010).
Grabowska, M., Grzelak, K. & Kukliński, P. Rock encrusting assemblages: structure and distribution along the Baltic Sea. J. Sea Res. 103, 24–31 (2015).
Piehl, S. et al. Modeling of water quality indicators in the Western Baltic Sea: Seasonal oxygen deficiency. Environ. Model. Assess. 28, 429–446 (2023).
Beisiegel, K., Tauber, F., Gogina, M., Zettler, M. L. & Darr, A. The potential exceptional role of a small Baltic boulder reef as a solitary habitat in a sea of mud. Aquat. Conserv. Mar. Freshw. Ecosyst. 29, 321–328 (2019).
Włodarska-Kowalczuk, M., Jankowska, E., Kotwicki, L. & Balazy, P. Evidence of season-dependency in vegetation effects on macrofauna in temperate seagrass meadows (Baltic Sea). PLoS ONE 9, e100788 (2014).
Wahl, M. In-situ Versuche zum Vorkommen von Metridium senile in der Flensburger Förde. Diplomarbeit, Universität Kiel, FRG (1982).
Wahl, M. The fluffy sea anemone Metridium senile in periodically oxygen depleted surroundings. Mar. Biol. 81, 81–86 (1984).
Anger, K., Anger, V. & Hagmeier, E. Laboratory studies on larval growth of Polydora ligni, Polydora ciliata, and Pygospio elegans (Polychaeta, Spionidae). Helgol. Meeresunters. 40, 377–395 (1986).
Harms, J. & Anger, K. Seasonal, annual, and spatial variation in the development of hard bottom communities. Helgol. Meeresunters.36, 137–150 (1983).
Wahl, M. Metridium senile: dispersion and small scale colonization by the combined strategy of locomotion and asexual reproduction (laceration). Marine Ecology Progress Series, 271–277 (1985).
Schwarzer, K., Bohling, B. & Heinrich, C. Submarine hard-bottom substrates in the western Baltic Sea–human impact versus natural development. J. Coast. Res. 70, 145–150 (2014).
Dorsett, D. A. The behaviour of Polydora ciliata (Johnst.). Tube-building and burrowing. J. Mar. Biol. Assoc. U. K. 41, 577–590 (1961).
De-Bastos, E. S. R. & Hill, J. Polydora sp. tubes on moderately exposed sublittoral soft rock. in Marine Life Information Network: Biology and Sensitivity Key Information Reviews (eds Tyler-Walters, H. & Hiscock, K.) https://doi.org/10.17031/marlinhab.247.1 (Marine Biological Association of the United Kingdom, 2016).
Porter, J. W., Barton, J. V. & Torres, C. Ecological, radiological, and toxicological effects of naval bombardment on the coral reefs of Isla de Vieques, Puerto Rico. in Warfare Ecology: A New Synthesis for Peace and Security 65–122 (Springer Netherlands, 2011).
Nipper, M. et al. Development of marine toxicity data for ordnance compounds. Arch. Environ. Contam. Toxicol. 41, 308–318 (2001).
Barker, M. F. & Nichols, D. Reproduction, recruitment and juvenile ecology of the starfish, Asterias rubens and Marthasterias glacialis. J. Mar. Biol. Assoc. U. K. 63, 745–765 (1983).
Scheibling, R. E. & Lauzon-Guay, J. S. Feeding aggregations of sea stars (Asterias spp. and Henricia sanguinolenta) associated with sea urchin (Strongylocentrotus droebachiensis) grazing fronts in Nova Scotia. Mar. Biol. 151, 1175–1183 (2007).
Maganhe, B. L., Andrade, L. S., Camilo, L. D. O., Neto, H. G. & Sanches, E. G. Food-related substrate preference in juveniles seastar Echinaster (Othilia) brasiliensis (Müller & Troschel, 1842) in captivity. Zoo. Biol. 42, 675–682 (2023).
Anger, K., Rogal, U., Schriever, G. & Valentin, C. In-situ investigations on the echinoderm Asterias rubens as a predator of soft-bottom communities in the western Baltic Sea. Helgol. Wiss. Meeresunters. 29, 439–459 (1977).
Beck, A. J. et al. Explosives compounds from sea-dumped relic munitions accumulate in marine biota. Sci. Total Environ. 806, 151266 (2022).
Balazy, P., Copeland, U. & Sokołowski, A. Shipwrecks and underwater objects of the southern Baltic–Hard substrata islands in the brackish, soft bottom marine environment. Estuar., Coast. Shelf Sci. 225, 106240 (2019).
Uhlenkott, K., Vink, A., Kuhn, T. & Martinez Arbizu, P. Predicting meiofauna abundance to define preservation and impact zones in a deep-sea mining context using random forest modelling. J. Appl. Ecol. 57, 1210–1221 (2020).
Bock, G., Thiermann, F., Rumohr, H. & Karez, R. Ausmaß der Steinfischerei an der schleswig-holsteinischen Ostseeküste. Jahresber. Landesamt Nat. Umw. des. Landes Schlesw. Holst. 2003, 111–116 (2003).
Baltic Sea Physics Analysis and Forecast. Copernicus Marine Service. https://doi.org/10.48670/moi-00010.
Bundesamt für Seeschifffahrt und Hydrographie. https://www2.bsh.de/.
Greinert, J., Kampmeier, M., Buck, V. & Frey, T. Marine dumped munition. Hydrogr. Nachr. 128, 34–41 (2024).
Clarke, K. R., Somerfield, P. J. & Chapman, M. G. On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray–Curtis coefficient for denuded assemblages. J. Exp. Mar. Biol. Ecol. 330, 55–80 (2006).
Clarke, K. R. & Warwick, R. M. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation 2nd edn (PRIMER-E, 2001).
Anderson, M., Gorley, R. & Clarke, K. P. For PRIMER: Guide to Software and Statistical Methods (PRIMER-E Ltd, 2008).
Van Rossum, G. & Drake, F. L. Python 3 Reference Manual. CreateSpace. https://www.python.org/downloads/release/python-380/ (2009).
Baltic Sea Biogeochemistry Reanalysis. Copernicus Marine Service. https://doi.org/10.48670/moi-00012.
Levin, L. A. et al. Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences 6, 2063–2098 (2009).
Esposito, M., Beck, A. J., Martinez-Cabanas, M., Gledhill, M. & Achterberg, E. P. Rapid, At-Sea Detection of Munition Compounds in Coastal Waters Using a Shipboard System. ACS EST Water 3, 2890–2898 (2023).
Gledhill, M., Beck, A. J., Stamer, B., Schlosser, C. & Achterberg, E. P. Quantification of munition compounds in the marine environment by solid phase extraction–ultra high performance liquid chromatography with detection by electrospray ionisation–mass spectrometry. Talanta 200, 366–372 (2019).
Wenzl, T., Johannes, H., Schaechtele, A., Robouch, P. & Stroka, J. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food, EUR 28099 EN ISBN 978-92-79-61768-3, 10.2787/8931, JRC102946 (Publications Office of the European Union, 2016).
Anthony, K. R. & Svane, I. B. Effects of flow-habitat on body size and reproductive patterns in the sea anemone Metridium senile in the Gullmarsfjord, Sweden. Marine ecology progress series. Oldendorf 113, 257–269 (1994).
Acknowledgements
We greatly appreciate the assistance from the crew of R/V “Alkor.” Special thanks to Dr. Jörn Scharsack for helping us in species identification and to Iason-Zois Gazis for providing the AUV photomosaic. This work was funded by the German Federal Ministry of Education and Research (BMBF, grant numbers 03F0912E and 03F0912A) through the DAM (Deutsche Allianz Meeresforschung) sustainMare project CONMAR (CONcepts for conventional MArine Munition Remediation in the German North and Baltic Seas) and Senckenberg Gesellschaft für Naturforschung. No special permissions were required to access the study area.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
V.A.: conceptualization, data curation, formal analysis, investigation, validation, visualization, writing—original draft, writing—review and editing; K.I.: project administration, data curation, writing—review and editing; W.T.: investigation, visualization, writing—review and editing; N.G.: investigation, visualization, writing—review and editing; K.M.: formal analysis, data curation, writing—review and editing; B.A.J.: investigation, formal analysis, validation, writing—original draft, writing—review and editing; E.M.: investigation, formal analysis, validation writing—review and editing; G.J.: project administration, data curation, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Jacek Bełdowski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alice Drinkwater. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vedenin, A., Kröncke, I., Weiß, T. et al. Sea-dumped munitions in the Baltic Sea support high epifauna abundance and diversity. Commun Earth Environ 6, 749 (2025). https://doi.org/10.1038/s43247-025-02593-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-025-02593-7