Abstract
It remains uncertain whether and when rewetting of drained fen peatlands contributes to climate change mitigation by reducing carbon dioxide and methane emissions. Recolonization by emergent macrophytes is considered a key factor in this process. We present 5 years of carbon dioxide and methane emission data from a rewetted fen peatland in northeast Germany. Four automatic chambers were installed along a transect perpendicular to the shoreline of a lake formed after rewetting, capturing three stages of plant succession: open water (1), initial recolonization by emergent macrophytes (2), and a stable emergent macrophyte community (3). Net carbon dioxide fluxes decreased progressively throughout the successional stages, while methane emissions exhibited a wave-like pattern, with a pronounced short-term increase during stage 2. Excluding this emission peak can lead to considerable underestimation of net emissions. Our findings highlight the importance of accounting for all successional stages to accurately assess the climate effects of rewetting.

Similar content being viewed by others
Introduction
Extensive drainage has increased the climate impact of peatlands by accelerating the loss of peat carbon (C) stocks and creating significant greenhouse gas (GHG) emissions, primarily carbon dioxide (CO2) and nitrous oxide (N2O)1,2,3,4,5. This effect is particularly notable in nutrient-rich fen peatlands6,7. To mitigate these climate impacts and restore the peatland’s C accumulation function, multiple efforts are being made, and rewetting is increasingly employed to reverse drainage effects8,9,10. There is ample evidence that rewetting is a complex and prolonged process characterized by substantial shifts during the transitional phase from drained to stable rewetted conditions. In this study, we use the term “transitional phase” to refer to the full sequence of vegetation succession following rewetting, culminating in the establishment of a stable emergent macrophyte community. The transitional phase typically includes three successional stages: open water (stage 1), initial recolonization by emergent macrophytes (stage 2), and establishment of a stable emergent macrophyte community (stage 3).
Stage 1 is characterized by the die-off of the former grassland vegetation caused by sustained flooding, typically following surface subsidence due to prolonged drainage. This results in the formation of large open water areas that are initially colonized only by submerged macrophytes. Stage 2 is characterized by the recolonization of these open water areas by emergent macrophytes, such as Phragmites sp. or Typha sp., which have a very high net primary production compared to submerged species. This recolonization is often stimulated by temporary, weather-induced declines in water level11, allowing the establishment of emergent vegetation. Stage 3 involves the development of a longer-term, stable, and more species-rich reed community. This stage initially maintains high productivity, but over time net primary production tends to decline gradually as a result of decreasing nutrient supply12,13,14,15,16.
Previous studies have provided insights into the C dynamics and climate impact associated with stages 1 and 3 of vegetation succession. Open water areas (stage 1) are characterized by a pronounced net release of CH4 and CO2, and as a result, their net GHG emissions can even exceed those of drained peatlands, despite typically relatively low or negligible N2O emissions17,18,19,20. This is due to intensive microbial decomposition of dead plant material, which produces large amounts of CH4 and CO2, combined with limited CO2 uptake by submerged macrophytes due to their low gross and net primary production21,22,23. In contrast, stage 3 is associated with a significant long-term reduction in net GHG emissions and a resumption of organic matter accumulation in the form of sediment or peat. This reduction results from several factors: notably reduced N2O emissions, very strong CO2 uptake by emergent macrophytes with high net primary productivity, and markedly decreased CH4 release24,25,26,27,28. Although an increase in root biomass and rhizodeposits can stimulate CH4 production by methanogenic microorganisms, this effect is typically offset by enhanced CH4 oxidation by methanotrophs already present in the root zone. The latter is promoted by internal gas transport mechanisms, which transport atmospheric oxygen (O2) into the root zone. Under these conditions, CH4 oxidation often surpasses production29,30,31,32,33,34,35.
In contrast, to stages 1 and 3, little is currently known about GHG fluxes and net GHG emissions during the initial recolonization of open water areas by emergent macrophytes (stage 2). However, given the substantial differences in C dynamics between stage 1 (open water) and stage 3 (fully vegetated), it is reasonable to expect that major shifts and critical turning points occur during stage 2—the initial recolonization by emergent macrophytes. It remains unclear whether—and to what extent—stage 2 is associated with imbalances in key C formation and transformation processes such as net primary production, C input into the peat body, mineralization, CH4 formation, oxidation and release, or shifts in the microbial communities involved in these pathways29,30,31,33,36,37,38,39. So far, findings from short-term experiments on C transformation processes and CH4 release have been inconsistent and currently provide no clear evidence of such disruptions40,41,42.
Specifically accounting for stage 2, as well as the broader transitional phase from rewetting to the establishment of a stable emergent macrophyte community (stages 1–3), is of key importance for accurately assessing the effect of rewetting, especially when evaluating its long-term potential for reducing net GHG emissions. Our investigation aimed to determine whether, and to what extent, the transitional phase and its successional stages affect C dynamics and GHG exchange under in situ conditions at a rewetted, formerly drained fen grassland. We focused on answering the following questions: 1. How does recolonization of a shallow lake by emergent macrophytes on a formerly drained fen grassland influence the dynamics of GHG emissions, especially CO2 and CH4? 2. What are the implications of successional stages for peat C accumulation and net GHG emissions (i.e., the climate impact)? To answer these two questions, we conducted 5 years of CH4 and CO2 automatic chamber (AC) measurements. The study was carried out 10 years after the rewetting of a formerly drained fen grassland in northeast Germany. Following the rewetting, a shallow lake developed at the site. The AC measurements were conducted along a transect representing a spatial gradient, dominated by broadleaf cattail (Typha latifolia), a common emergent macrophyte in nutrient-rich shallow lakes14. The high-frequency AC measurements revealed a clear dynamic pattern of CO2, and in particular of CH4 emissions, during the three successional stages. Notably, stage 2 was associated with a strongly increased climatic effect due to enhanced CH4 emissions. These findings emphasize the need for integrating all successional stages when evaluating the climate impact of rewetting.
Results
Environmental conditions during the study period
Annual air temperature, WL, soil temperature at three depths, water temperature, and precipitation were continuously measured at the study site at 15 s intervals (Table 1). Throughout the study period, these variables exhibited clear seasonal and interannual variations (Fig. 1). The mean annual air temperature over the 5-year study period was 9.8 °C, 2016 being the coldest year (9.5 °C) and 2014 the warmest (10.2 °C). Soil temperature (measured at 2, 5, and 10 cm depth) and water temperature (at 10 cm depth) exhibited a slightly decreasing trend over time, with the highest annual temperature occurring in the first and the lowest in the final study year (Table 1). Mean annual precipitation during the study period was 471 mm, with annual precipitation as high as 570 mm and 662 mm in 2015 and 2017, respectively. These years were also those with the highest WL, reaching 53.9 cm in 2015 and 60.3 cm in 2017. In contrast, surface water along the chamber transect temporarily receded during the summer months of the particularly dry years 2016 and 2018, when annual precipitation was only 323 and 330 mm. These 2 years were also characterized by low WL, 38.5 cm in 2016 and 49.7 cm in 2018. The WL fluctuated substantially between the driest and wettest years: in 2015, the maximum value exceeded 1 m, while in the driest years (2016 and 2018), it temporarily declined to 0 cm. The difference in mean annual WL was nearly 22 cm between the wettest year (2017) and the driest year (2016).
Time series showing daily water level (WL, cm; light blue line) and precipitation (mm; dark blue bars) (a). Mean annual WL (light blue) and mean annual precipitation (dark blue) for the period 2014–2018 are shown in the top right corner. Temporal dynamics of mean daily temperatures are shown for air temperatures (b), water at 10 cm depth (c), and soil temperature at 2 cm depth (d). Gray areas indicate standard deviation (SD). In (b), mean daily air temperature is shown as a red line when above 0 °C and as a blue line when below 0 °C. Mean annual temperatures for 2014–2018 are shown in the top right corners of (b, c, d).
CO2 flux dynamics and annual emissions
Annual values of net ecosystem CO2 exchange (NEE) and other flux components (ecosystem respiration (Reco) and gross primary production (GPP)) varied across years and between chamber areas (Tables 2 and 3, Fig. 2). Weekly CO2 flux dynamics (g CO2-C g m−2 w−1) showed distinct spatial differences along the transect as well as temporal (diurnal, seasonal, and interannual) variability (Fig. 2, Tables 2 and 3). In the first chamber area (AC1), located closest to the shoreline, emergent macrophytes established in the first year of the study period (2014), resulting in sustained net CO2 uptake throughout the entire monitoring period (Tables 2 and 3). A similar pattern was observed in the second chamber area (AC2), where continuous net CO2 uptake began in 2016, coinciding with the establishment of emergent macrophytes. Similarly, in the third chamber area (AC3), net CO2 uptake increased substantially after emergent macrophyte recolonization in 2017. In contrast, the fourth chamber area (AC4), situated furthest from the shoreline and colonized by emergent macrophytes only in the final year of the study, showed limited changes in NEE compared to the other three chamber areas. Throughout the 5-year study period, only AC4 exhibited an overall positive trend of CO2 emissions, with a cumulative emission of 336 g CO2-C m−2 y−1. In comparison, the other three chamber areas acted as net CO2 sinks, with AC2 as the least productive (−364 g CO2-C m−2 y−1) to a more pronounced uptake in AC1 and AC3 (despite a 1-year data gap), which exhibited almost three times higher NEE values (−988 and −990 g CO2-C m −2 y−1, respectively).
Temporal dynamics of measured (blue dots) and modeled (dashed line) weekly CO2 fluxes (CO2-C g m−2 w−1) for the four chamber areas (AC1, AC2, AC3, and AC4) during the period 2014–2018. The gray area around the modeled values indicates the estimated errors from the gap-filling model. Cumulative modeled net ecosystem CO2 exchange (NEE; secondary axis) is shown as a solid blue line. Successional stages reflecting changes in dominant vegetation cover are given in the figure header: 1-open water, 2-initial recolonization by emergent macrophytes, 3-establishment of a stable emergent macrophyte community.
CH4 flux dynamics and annual emissions
Similar to CO2, annual values of modeled CH4 emissions and weekly CH4 flux dynamics (g CH4-C g m−2 w−1) exhibited both spatial and temporal variability (Table 2, Fig. 3). CH4 emissions peaked during summer and decreased significantly during colder periods. Interannual CH4 variability corresponded closely with the successional stages of the vegetation present in the chamber areas. On average, annual CH4 emissions from open-water areas accounted for 22.3% of total emissions, a proportion that was not significantly higher than that in chamber areas with an established stable emergent macrophyte community (21.3%). In contrast, areas undergoing initial recolonization by emergent macrophytes (2014 for AC1, 2016 for AC2, 2017 for AC3, and 2018 for AC4) contributed disproportionately to total CH4, accounting for over half (56.5%) of the total sum. Overall, all chamber areas showed net CH4 emissions during the study period. Despite a 1-year data gap, AC3 had the highest cumulative emissions, with a total of 99.1 g CH4-C m−2 over 4 years. The other three chamber areas had similar cumulative values of 90 ± 5 g CH4-C m−2 over 5 years, with the lowest average observed in AC1, followed by AC2 and AC4.
Temporal dynamics of measured (purple dots) and modeled (dashed line) weekly CH4 fluxes (g CH4-C m−2 w−1) in the four chamber areas (AC1, AC2, AC3, and AC4) during the period 2014–2018. The gray area around the modeled values represents error estimates from the gap-filling model. Cumulative modeled CH4 emissions (secondary axis) are shown as a solid blue line. Successional stages reflecting changes in dominant vegetation cover are given in the figure header: 1-open water, 2-initial recolonization by emergent macrophytes, 3-establishment of a stable emergent macrophyte community. Stacked bar plots on the right side of the figure show the relative contributions of each successional stage and year in each chamber, expressed as percentages.
NECB and net GHG emissions
Cumulative annual NECB values over the study period (Table 2, Fig. 4) revealed net C stock or peat accumulation in the first three chamber areas, with the highest accumulation rates (i.e., negative NECB) occurring in the years following the establishment of emergent macrophytes. Except for 2014 in AC2 and AC4, all years characterized by open water conditions showed net C losses. Substantial differences were observed between the successional stages, particularly between open water (stage 1) and a stable emergent macrophyte community (stage 3; p-value: <0.05; Wilcoxon test). Chamber areas with established emergent macrophyte communities exhibited consistently high negative cumulative NECB values and thus high net C accumulation, with an average of −240 g C m−2 y−1 (Fig. 4). In contrast, years representing the initial recolonization by emergent macrophytes (stage 2) showed more than two times lower C accumulation, averaging −115 g C m−2 y−1. Open water periods (stage 1) were characterized by net C losses, with an average NECB of +63 g C m−2 y−1. Average net GHG emissions for the three successional stages followed a pattern similar to that of CH4 emissions, peaking during the initial recolonization stage (stage 2), followed by a substantial decrease upon the establishment of a stable emergent macrophyte community (stage 3). Mean net GHG emissions for these three stages were 465 g CO2-eq m−2 y−1 for stage 1, 902 g CO2-eq m−2 y−1 for stage 2, and 143 g CO2-eq m−2 y−1 for stage 3 (Fig. 4).
Annual values of a methane emissions (CH4; g CH4-C m−2 y−1), b CO2 emissions as net ecosystem exchange (NEE; g CO2-C m−2 y−1), c net greenhouse gas emissions (GHG; g CO2-eq m−2 y−1), and d net ecosystem carbon balance (NECB; g CO2-C m−2 y−1). Data are grouped and averaged according to the three successional stages: 1-open water, 2-initial recolonization by emergent macrophytes, 3-establishment of a stable emergent macrophyte community.
Discussion
High-resolution CO2 and CH4 flux measurements during succession reveal differences in dynamics
Our study revealed distinct dynamic patterns of CO2 and CH4 fluxes during the three stages of plant succession in a flooded fen peatland. Net CO2 fluxes decreased continuously across the successional stages. While open water (stage 1) functioned as a strong CO2 source, the system transitioned into a moderate CO2 sink in stage 2 (initial recolonization by emergent macrophytes), and ultimately into a strong CO2 sink during the establishment of a stable emergent macrophyte community (stage 3). In contrast, CH4 emissions exhibited a wave-like pattern, with a pronounced increase during the transition from stage 1 to stage 2, followed by a marked decrease during the transition from stage 2 to stage 3 (Figs. 2, 3, 4a, b, Tables 2 and 3). These patterns were clearly recognizable despite the generally high variability of the CO2 and CH4 fluxes, which is most likely attributable to the AC measuring system employed in this study. It has been repeatedly shown that much more accurate results can be achieved with AC than with manual chamber measuring systems because of the capacity of AC for continuous and high-frequency measurements over extended periods43,44,45. In addition, the consistent and reproducible patterns of GHG emissions observed across the different chamber areas—particularly during the initial phase of cattail recolonization (stage 2), which occurred in different years in the respective chambers—support the reliability of our results (Figs. 2 and 3). The transect AC setup was a significant factor in capturing these dynamics. Owing to its high spatial resolution, it enabled repeated observation of the successional changes triggered by the cattail recolonization over several years.
However, it is noteworthy that GHG fluxes measured using AC may be biased due to, among other factors, disturbances of natural conditions during chamber placement, which can affect both the microclimate and atmospheric conditions within the chamber headspace46,47,48,49,50. Despite these potential biases, the high temporal resolution and small spatial scale of AC measurements likely enabled accurate detection of the pronounced and stage-different dynamics of CO2 and CH4 fluxes during plant recolonization. This is supported by the agreement between our CO2 and CH4 flux levels and similar flux data obtained using the eddy covariance method at the same site18,27. Therefore, we conclude that any methodological constraints do not compromise the validity of our research. Our results emphasize the effect of emergent macrophyte recolonization on CO2 and CH4 flux dynamics, net GHG emissions, and C-sink restoration in rewetted fen peatlands.
The causes of changes in CO2 and CH4 fluxes between successional stages
For CO2, the key factor was the drastic shift between the main CO2 fluxes—Reco and GPP—across the successional stages. During the open water stage, Reco was driven only by mineralization of newly washed-in litter21, while high GPP prevailed after T. latifolia recolonization (a species with a high net primary production, as already mentioned51,52). With the more profound rise of GPP compared to Reco (Table 3), an overall increase occurred in the net CO2 plant uptake, resulting in a shift from a CO2 source to a CO2 sink (Table 3, Fig. 4b).
A local peculiarity occurred in chamber area AC2, which exhibited lower Reco and GPP values (Table 3) and appeared less vegetated in field site photographs. The likely cause was a local depression in the peat surface, increasing the distance between the peat surface and the WL. This greater distance probably hindered canopy development and limited net primary production. In addition, WL fluctuations served as an indirect driver of the changes in CO2 dynamics during the transitional phase. As mentioned above, the widespread recolonization by T. latifolia of the open water only started after the temporary drying out of the riparian zone due to specific weather conditions17,53.
In contrast to the simple and rapid shift of CO2 fluxes into a CO2 sink, CH4 dynamics were much more complex. During the study period, CH4 emissions exhibited a fluctuating, non-linear, dynamic development pattern (“CH4 flux dynamics and annual emissions”; Fig. 3), with clear differences between the three stages: open water, initial recolonization by emergent macrophytes, and the establishment of a stable emergent macrophyte community (Fig. 4a). CH4 emissions peaked in each chamber area in different years but at the same successional stage, suggesting that recolonization itself drove CH4 emissions, exhibiting wave-like dynamics. This pattern likely resulted from a temporary imbalance between CH4 production and CH4-oxidizing processes, caused by a specific combination of relevant factors and conditions. A key factor was the substantial accumulation of decaying plant material (new sediment) on top of the old peat layer in the shallow lake, spanning the chamber transect. The continuous flooding of the polder following rewetting allowed for ongoing deposition of new, fresh organic matter transported from the River Peene to the study site, primarily by southwestern winds. Together with autochthonous plant litter from the existing aquatic plants, this newly formed thick sediment layer constituted a potent organic C source of CH4 emissions14,20,21,54,55,56. For example, litter from the submerged macrophyte Ceratophyllum demersum, which dominated the open water, has been shown to have a three times higher CH4 production potential than T. latifolia57, which colonized later. In addition, the CH4 production potential was increased by fresh assimilates—such as easily degradable rhizodeposits and root debris—introduced into the sediment layer by the Typha sp. at the onset of recolonization35,38,58. This high supply of readily degradable organic C compounds likely boosted CH4 production, supported by the often high abundance of methanogenic microorganisms in the sediment layer of rewetted fen peatlands59,60,61. A further but crucial mechanism likely leading to occasional peaks in CH4 emissions was the active internal gas transport system of T. latifolia. This species possesses aerenchymatic tissue that acts as a pathway for transport of the stored CH4 from anoxic sediment layers directly to the atmosphere, bypassing oxidation at the oxic sediment surface32,62,63. Therefore, all these interacting factors seem to be responsible for the initial CH4 emission peaks during stage 2: a high stock of easily degradable organic C compounds, high abundance of methanogenic microorganisms, and active internal gas transport of T. latifolia. The same factor also explained the increased CH4 emissions in a model study of Typha x glauca colonization on wetland soils reported for the Midwest (USA)40.
The second phase of the CH4 wave, i.e., the decline in emissions during stage 3 of vegetation succession, is most likely also attributable to a shift in the combination of the mentioned factors. Most notably, a new equilibrium between CH4 production and oxidation appears to have been established, with both processes occurring at lower intensities than in the earlier stages. This likely started with the continued spread of T. latifolia roots into the sediment as colonization of the open water advanced. Typha latifolia density increases via rhizomatic expansion64. It forms new ramets every year65, and thus develops new root systems over successive years. This would have intensified O2 uptake into the root system via the active gas transport system of the plants, as young roots in particular require O2 for growth31,33,34. The rapid transport of CH4 from the root environment into the atmosphere is likely a consequence of the need to maintain continuous internal gas circulation by balancing internal and external pressure differences32,33,34. However, a portion of the O2 transported into the roots diffuses into the surrounding rhizosphere, creating oxic conditions that promote CH4 oxidation66. In addition, increased abundance of methanotrophic microorganisms was found in the rhizosphere of Typha sp. species67. This suggests that with root system expansion, a growing proportion of the freshly produced CH4 is immediately oxidized within the sediment68. This mechanism probably also explains why mesocosm experiments with rewetted peat substrates have consistently shown lower CH4 fluxes in treatments with emergent macrophytes than in unvegetated controls41,42,54. Two additional processes may also have contributed to the decrease in CH4 emissions by reducing the input of readily microbially degradable C compounds into the sediment, thereby reducing the potential for CH4 production. First, starting in the second year of recolonization, the already established T. latifolia stands appear to have effectively reduced the input of allochthonous plant debris from the surrounding areas (see photographs; Fig. 5). Second, the exceptionally high CH4 production in the first year of recolonization may have led to a rapid decline in the stock of microbially degradable organic C compounds in the sediment69, leaving less material available for further CH4 production. Supporting these findings, a study from the Zarnekow polder revealed that the newly accumulated sediment was thicker in areas with newly established Typha spp. Stands compared to areas with older stands, with average values of 13.3 cm in open water, 11.5 cm in new stands, and 10.6 cm in old stands17,70.
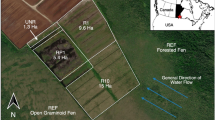
Experimental set-up showing the transect of four automatic chambers (AC1 to AC4, with AC1 located closest to the shoreline). A satellite image from 2006 of Polder Zarnekow (N53°52.5′, E12°53.3′) shows its condition two years after rewetting, when a shallow lake had formed. The squared area represents the chamber transect. The 2015 satellite image shows emergent vegetation (e.g., T. latifolia) in AC1, confirmed by field site photographs from the same year. In 2018, a satellite image confirmed the presence of emergent vegetation (e.g., T. latifolia) in all four chamber areas, as corroborated by ground-based photographs. The bottom panel illustrates the three successional stages: 1-open water, 2-initial recolonization by emergent macrophytes, and 3-establishment of a stable emergent macrophyte community.
As mentioned above, only a few studies have examined the effects of emergent macrophyte recolonization on CO2 and CH4 flux dynamics in rewetted fen peatlands27. To obtain more generalizable conclusions, investigations should be conducted under comparable conditions using the same macrophytes, the same time period, and the same site. In addition, sediment should be sampled from each chamber area and analyzed, especially the soil below the chambers, to better understand the causes of productivity differences, such as those noted in AC2. To gain a deeper understanding of the processes involved in CH4 production and oxidation, especially those driving emission peaks and declines, gas flux measurements and microbiological investigations should be combined with 13C- or 14C-isotope labeling techniques. The latter can, for example, be used to determine the origin of CH4 by distinguishing whether it originates from new plant litter or older soil organic matter using 14C-isotopes23. In addition, 13C labeling can help to: (1) identify the dominant microbial pathways of CH4 production—i.e., acetoclastic or hydrogenotrophic methanogenesis71,72, and (2) to assess the CH4 oxidation potential, as higher CH4 oxidation rates lead to increased accumulation of 13C enriched CH473.
The importance of successional stages for net GHG emissions and net peat C accumulation
Similar to the gas dynamics (see “The causes of changes in CO2 and CH4 fluxes between successional stages”), distinct patterns in net GHG emissions and net C accumulation were also observed, corresponding with the three successional stages following rewetting. Stage 1 (open water) showed relatively high net GHG emissions and functioned as a C source, characterized by both elevated net CO2 emission peaks and high CH4 emissions. Such open water areas and shallow lakes are generally recognized as net GHG sources to the atmosphere (Table 2; Fig. 4)18,53,74. Notably, net C accumulation was observed immediately upon the establishment of emergent macrophytes, predominantly T. latifolia, during stage 2 (initial recolonization). Despite significant CH4 emission peaks (p-value: <0.05; Wilcoxon), the C balance was offset by a pronounced net CO2 uptake75,76, leading to continued C sequestration or peat C accumulation, i.e., negative NECB values (Fig. 4d). This initial wave-like increase in CH4 emissions also caused net GHG emissions to peak during stage 2. In stage 3, increased biomass production further enhanced net or peat C accumulation (lowest NECB), maintaining the ecosystem as a CO2 sink, while CH4 emissions declined. Consequently, net GHG emissions were substantially reduced during this final stage.
Multiple studies provide data on CO2 and CH4 emissions, NECB, and overall net GHG emissions that align with our findings for stage 1 (dominant open water) and stage 3 (establishment of a stable emergent macrophyte community)18,52,74,77,78,79. However, none explicitly demonstrate a markedly increased climate impact like the one we observed during stage 2 (initial recolonization by emergent macrophytes), characterized by the wave-like CH4 emissions increase. While some researchers report high net GHG emissions from inundated peatlands vegetated by Typha spp., others find low net GHG emissions77,79,80. This broad range (especially of CH4 and net GHG emissions) may be due to the fact that most studies focus only on steady-state conditions rather than on successional stages, or that they rely on short-term, often 1-year measurements. Our results suggest that newly established T. latifolia stands show higher net GHG emissions during a short initial period, which subsequently decline once the emergent macrophyte community becomes well established. This wave-like pattern helps to explain the variability often reported in GHG emissions and underscores the importance of capturing transitional dynamics in rewetted fen peatlands. In addition, calculated average GHG emissions over a 20-year horizon indicate that excluding the transitional phase from analysis can result in misleading conclusions regarding GHG emission reduction following rewetting. Considering only the stage with a stable emergent macrophyte community yields an average net GHG emission of 143 g CO2-eq m−2 y−1, which is substantially lower than the estimates including the transitional phase. Including both the open water and the initial recolonization periods increases the average net GHG emission to 326 g CO2-eq m−2 y−1 for a 10-year transitional phase (as in AC1) and up to 406 g CO2-eq m−2 y−1 for a 14-year transitional phase (as in AC4). These findings highlight the critical importance of including the transitional phase in studies of rewetted fen peatlands like our study site. Here, over 30% of the area remains open water without stable emergent macrophyte communities, even 18 years after rewetting17.
The period of changes in GHG emissions and net C accumulation4,81 can differ. In our study, we observed that net or peat C accumulation occurred (negative NECB values) as soon as T. latifolia developed in our chamber areas. These negative values became more pronounced as the emergent macrophytes became well-established (stage 3). This suggests that net C accumulation was restored already in stage 2, preceding the decline in net GHG emissions. A sediment analysis from 2020 conducted at the same study site supports this finding by showing higher mean peat C accumulation in longer established T. latifolia stands (“old Typha” −53.63 t/ha) than in newly established stands (“new Typha” -45.8t/ha)70. However, the long-term effects of continued changes in vegetation composition and productivity on net GHG emissions and C accumulation need to be further elucidated in future studies26,28.
Overall, net GHG emissions were equivalent to 4.8 Mg CO2-eq ha−1 y−1 for open water, 9.4 Mg CO2-eq ha−1 y−1 during the initial recolonization by emergent macrophytes (stage 2), and 1.6 Mg CO2-eq ha−1 y−1 during the establishment of a stable emergent macrophyte community (stage 3). These values align with the emission factor of 5.5 Mg CO2-eq ha−1 y−1 currently used in German GHG inventories82 for rewetted peatlands. Before rewetting, the Zarnekow polder was a drained grassland, for which the national emission factor is 31.7 Mg CO2-eq ha−1 y−182. Thus, rewetting and subsequent flooding of the area led to a considerable reduction of net GHG emissions and associated climate impacts. Nevertheless, introduction of stage-specific emission factors for the transitional development phase of rewetted peatlands could be useful to enhance the accuracy of national inventories. If such stage differentiation is warranted depends on whether the observed wave-like progression of GHG emissions and rapid resumption of peat C accumulation during the establishment of site-adapted vegetation apply to other rewetted peatland types. This is a worthwhile target for further investigation, especially for the large-scale fen rewetting initiatives currently being planned.
Conclusions
We demonstrated that CO2 and CH4 fluxes in a shallow lake, established after rewetting a formerly drained fen peatland, reflected distinct successional stages during the transitional phase toward a more stable rewetted system: open water (stage 1), initial recolonization by emergent macrophytes (stage 2), and the establishment of a stable emergent macrophyte community (stage 3). Particularly noteworthy was that stage 2 showed a transient but significant increase in CH4 emissions, resulting in a short-term rise in net GHG emissions right after macrophyte recolonization. In contrast, net C accumulation was observed immediately following macrophyte recolonization. Notably, if stage 2 is overlooked and the immediate establishment of a stable emergent macrophyte community is assumed, net GHG emissions following rewetting may be substantially underestimated. For national GHG inventories, these results thus underscore the need to consider separate emission factors for the transitional phase as a whole (i.e., from stage 1 to stage 3) when developing national GHG inventories for rewetted fen peatlands.
Methods
Study site and experimental setup
The study site is located at the Zarnekow polder, an inundated former fen grassland within the River Peene valley in Mecklenburg-Western Pomerania, northeast Germany (N53°52.5′, E12°53.3′). The polder covers an area of 550 ha and has a peat thickness of up to 10.2 m17,83. The area experiences a moderately continental temperate climate21, with a mean annual air temperature of 9.2 °C and an annual precipitation of 583 mm (1991–2020, Teterow; German Meteorological Service, DWD). Drainage began in the 18th century, and intensive crop and grassland use in the last decades of the 20th century led to surface subsidence and a 0.3 m shrinkage of the upper peat layer57,83,84. As part of the mire restoration program, the polder was rewetted in fall 2004, resulting in the formation of a polytrophic shallow lake at the study site13. WLs were not controlled, with lake depths varying from 0.2 to 1.2 m57. The dominant vegetation prior to restoration, reed canary grass (Phalaris arundinacea), died off due to the new waterlogged conditions, forming a 30 cm thick sediment layer in the lake21,43,57. Subsequently, floating and submerged hydrophytes (e.g., Ceratophyllum sp. and Lemna sp.) became dominant, while emergent macrophytes, primarily T. latifolia, developed along the lake borders and spread toward its center during the study period13,17,21,57. In 2014, the study site was equipped with a climate station and an AC system to measure ecosystem C exchange (CO2 and CH4 emissions) over 5 years (2014–2018). The system consisted of four rectangular transparent chambers (AC1, AC2, AC3, and AC4; Lexan polycarbonate; 2 mm thickness), positioned along a spatial gradient from the lake shore to its center (Fig. 5). The chambers had a basal area of 1 m2 and a volume of 1.5 m3 and were secured with a wiring system, stabilized with a steel profile and an aluminum frame on the lake bottom. Chambers were raised and lowered using an electronically controlled cable winch atop the steel profile.
At the base of the chambers, a water sensor (capacitive limit switch KB 5004, efector150) enabled consistent 5 cm immersion into the water surface, maintaining airtight sealing and a constant chamber volume. Two tubes interconnected all chambers to a multiplexer, which directed data to a single Los Gatos fast greenhouse gas analyzer (911–0010, Los Gatos), operating with a gas flow rate of 5 L m-1. The analyzer measured air concentrations of CO2 and CH4. Each chamber was equipped with a fan to ensure homogeneous air mixing in the chamber headspace and a uniform air pressure. CO2 and CH4 fluxes were determined by continuously measuring air concentrations in the chamber headspace at 15-s intervals over a 10-min chamber closure period. Each chamber was lowered automatically, one at a time. During transitions between chambers, the connecting tubes underwent a 2-min venting process utilizing air from the next open chamber. After each measurement, the chamber was vented for 50 min before the next cycle, yielding one measurement per chamber every hour. To access the measurement site and to minimize disturbance of the water body and peat surface, a wooden boardwalk was installed.
Environmental parameters
Soil temperature at 2, 5, and 10 cm depth (°C) as well as water temperature at 10 and 20 cm depth (°C) were measured using thermocouples (T107, Campbell Scientific). A climate station (WXT52C, Vaisala) was installed at the study site to measure air temperature at 2 m height (°C), air pressure (hPA), photosynthetically active radiation (PAR; μmol m−2 s−1), relative humidity (%), wind speed, and precipitation (as a cm sum). Groundwater level (GWL) was monitored using a pressure probe (PDCR1830, Campbell Scientific). All data were stored using a data logger (CR 1000, Campbell Scientific) connected to a GPRS radio modem. Data gaps shorter than 2 h on air temperature and PAR were supplemented with data from linear interpolation. Data gaps longer than 2 h were filled using data from the DWD weather station in Teterow (air temperature) and the ZALF climate station near Dedelov (PAR, WXT52C, Vaisala; 60 km south-east of the study site). Soil temperature gaps were filled using linear interpolation.
CH4 flux calculation and gap-filling
CH4 flux calculations were performed using an adjusted R script44. CH4 flux separation was done according to Hoffmann et al.43, where fluxes are calculated twice to determine the contribution of diffusive and ebullitive fluxes to total CH4 emissions. First, CH4 total flux was calculated and then the diffusive component (R script settings were adjusted to exclude sudden concentration increases). According to Hoffmann et al43., a death band of 25% was used to exclude the initial portion of each measurement and thus avoid artifacts from chamber closing (outside ±0.25*IQR; interquartile range85). Furthermore, a minimum moving window of five data points was used for the diffusive flux calculation, while a 30-point window was applied for total fluxes. All CH4 fluxes \(({r}_{{{CH}}_{4}})\) were calculated over measurement period (t) using the equation below43 (Equation 1), where M is the molar mass of CH4, A and V are the basal area and chamber volume, while T stands for air temperature and P for air pressure. R denotes a constant (8.3143 m3 Pa K−1 mol−1):
To calculate total CH4 fluxes, the difference between the start and end concentrations of CH4 (δν) was determined over a moving window (MW) of 30 connected points (7.5 min). For diffusive CH4 flux estimates, δν was calculated as the slope of a linear regression fitted to each data subset. These diffusive fluxes were filtered based on three criteria defined by Hoffmann et al43.: (i) within-chamber air temperature range no greater than ±1.5 K; (ii) a significant regression slope (p ≤ 0.1); and (iii) non-significant tests (p > 0.1) for normality (Lilliefors’ adaption of the Kolmogorov–Smirnov test), homoscedasticity (Breusch-Pagan test), and linearity. Finally, if multiple flux values were available per measurement, the one with the initial CH4 start concentration closest to atmospheric levels was selected. An outlier test was applied, which excluded measurements that deviated significantly from the overall trend (±0.25 IQR43). The ebullitive fraction of total CH4 emissions was estimated by subtracting the CH4 diffusion flux from the total CH4 fluxes43. For further data processing and annual emission estimates, only total CH4 fluxes were used. Due to the substantial data gap in 2016 for chamber area 3 (AC3), CH4 data from this year and the chamber were excluded from further data processing.
For modeling annual emissions, the nonlinear regression model of Laine et al.86 was used, including both WL and temperature variability as predictors87,88 (see model equation in Laine et al.86). To identify the most influential temperature variable, a non-parametric Spearman’s correlation test was conducted. Soil temperature at 2 cm depth was highly correlated with the CH4 flux and was therefore selected for subsequent modeling. Weekly CH4 fluxes were modeled using corresponding weekly averages of environmental parameters (WL and 2 cm soil temperature). Model performance improved when data were treated according to vegetation dominance. Consequently, models were calculated separately for each year and chamber86,88. To avoid overestimations and negative CH4 values during WL or temperature peaks, maximum and minimum values for each year were determined and set as upper and lower bounds.
CO2 flux calculation and gap-filling
CO2 flux calculation and gap-filling were conducted using the R script developed by Hoffmann et al.44. Prior to calculation, less than 2% of all measurements were manually excluded as outliers. These outliers were typically associated with atmospheric layering and higher accumulation of CO2 near the surface in early morning/late evening, causing biased and high initial concentrations (e.g., linked to night-time respiration and decomposition, water column stratification, and stable atmospheric conditions limiting vertical mixing)50,89,90,91,92. The R script automatically removed 10% from the beginning and end of each measurement to exclude potential bias from chamber placement and to allow sufficient time for homogenizing the chamber headspace during each measurement44,93. Additionally, chamber measurements were checked for potential leakage by applying linear regression across the entire dataset. Assumptions of linear regression (linearity, homogeneity of variance, and normal distribution) were assessed.
Flux (f) calculation was conducted based on the ideal gas law (Equation 2):
where p represents ambient air pressure (Pa), V is the chamber volume, R is the gas constant (8.314 m3 PaK1 mol−1), T denotes inside chamber temperature (K), and A is the basal area (m−2). Finally, dc/dt (the rate of CO2 concentration change over time within the chamber headspace) is assessed using linear regressions applied to multiple data subsets. These subsets were generated with a variable MW with minimum eight consecutive data points (2 min), resulting in multiple calculated fluxes per measurement. These fluxes were subsequently filtered based on the following criteria: (i) compliance with the linear regression assumptions (normality, homoscedasticity, linearity), (ii) temperature stability with ±1.5 K (for both Reco and NEE), (iii) variation in photosynthetic active radiation (PAR) within the subset limited to ±20% (for NEE only), and (iv) absence of outliers, identified using a ±6xIQR threshold44,93,94. Chamber measurements where no flux values met the quality criteria were excluded from further analysis. If multiple flux values from one measurement passed all quality checks, the flux with the steepest slope (greatest rate of change) was selected. NEE was partitioned into two components: Reco and GPP (estimated as the difference between measured NEE and Reco)95,96. Flux separation and gap-filling were based on empirical modeling using temperature and PAR dependency functions for Reco97 and GPP98. Parameters for these dependency functions (Reco during night-time and GPP during daytime) were derived from fits generated through variable moving windows (7–21 measurement days)99. Reco parameters were obtained by fitting measured Reco fluxes with parallel measurements of soil temperatures at different depths and air temperatures (2 m height) to a modified Arrhenius-type exponential equation97. GPP parameter pairs were derived from GPP fluxes (modeled Reco fluxes subtracted from measured NEE fluxes) and simultaneously measured PAR using a non-rectangular hyperbolic light response function representing PAR dependency of GPP estimates (e.g., dark conditions assuming no CO2 uptake96,98). Model fitting prioritized parameter pairs with statistically significant values and the lowest Akaike information criterion (AIC)93,99. Daytime measurements were defined as those with PAR above or equal to 15 W/m2 and night-time measurements as those with PAR values below 15 W/m2. When no significant parameter pairs could be established, the average flux value for the respective CO2 flux component was used. For further data interpretation, CO2 flux components were aggregated into weekly values (Fig. 2). Due to the large data gap in 2016, CO2 values for chamber AC3 were excluded from further processing.
NECB and net GHG emissions
The net ecosystem C balance (NECB) is the sum of C import and export within an ecosystem, accounting for multiple origins95,100. As the main focus of our study was on CO2 and CH4 measurements in a system without C removal101, other sources were excluded from the NECB calculation. Although different sedimentation rates among chamber areas may explain some of the observed variability, DOC losses are considered minor in the C balance of grassland systems102. Therefore, NECB at our study site was the sum of GPP, ecosystem respiration (Reco), and net CH4-C emission. This approach serves as a simplified proxy for determining net or peat C stock changes in fen peatlands (net C accumulation). The climate impact quantification is based on net GHG emissions. Generally, net GHG emissions are calculated by multiplying CO2, CH4, and N2O fluxes with their corresponding CO2 equivalents and summing them up95. Specific global warming potentials (GWP) of the gases are used for these calculations. In our study, net GHG emissions were calculated by including NEE and CH4-C values, the latter being multiplied by a GWP of 28 for CH4 over the 100-year time horizon reported by the IPCC102,103. N2O emissions were excluded from net GHG emission calculation as they are expected to have insignificant impact on the overall trend. Previous studies have shown that N2O emissions from rewetted peatlands are minimal or below detection limits78,104,105,106. This is further supported by data from the exact same study site conducted using manual chamber measurements in the years immediately following rewetting, which found no substantial N2O emissions107.
Error calculation and statistical analyses
All calculations were performed using the statistical software R (version 4.4.2, R Core Team, 2023)108. Uncertainty in the CO2-based models of Reco, GPP, and NEE was estimated using error prediction algorithms44. Bootstrapping was applied to quantify uncertainty in measured CO2 and CH4 fluxes, derived ecosystem parameters (NEE, Reco, and GPP), and half-hourly modeled CO2 values44,93, as well as weekly modeled CH4 fluxes. Statistical differences among the three successional stages (1—open water, 2—initial recolonization by emergent macrophytes, 3—establishment of a stable emergent macrophyte community) were evaluated using averaged annual mean values of NEE, total CH4 emissions, NECB, and net GHG emissions through a two-sided Wilcoxon test (p < 0.05). The overall performance of the CH4 model was evaluated using leave-one-out cross-validation and the following metrics: RMSE (Root Mean Square Error), PBIAS (Percent Bias), R² (Coefficient of Determination), and KGE (Kling–Gupta Efficiency) (see Supplementary Table 1).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data from this study are freely available at https://doi.org/10.4228/ZALF-9Z35-T524.
Change history
21 August 2025
In this article the grant number VH-NG-821 relating to the Helmholtz Association of German Research Centres through a Helmholtz Young Investigators Group was omitted. The original article has been corrected.
References
Evans, C. D. et al. Overriding water table control on managed peatland greenhouse gas emissions. Nature 593, 548–552 (2021).
Evans, C. D., Renou-Wilson, F. & Strack, M. The role of waterborne carbon in the greenhouse gas balance of drained and re-wetted peatlands. Aquat. Sci. 78, 573–590 (2016).
Günther, A. et al. Prompt rewetting of drained peatlands reduces climate warming despite methane emissions. Nat. Commun. 11, 1644 (2020).
Hemes, K. S. et al. Assessing the carbon and climate benefit of restoring degraded agricultural peat soils to managed wetlands. Agric. Meteorol. 268, 202–214 (2019).
Jurasinski, G. et al. From understanding to sustainable use of peatlands: the WETSCAPES approach. Soil Syst. 4, 14 (2020).
Joosten, H. & Clarke, D. Wise Use of Mires and Peatlands: Background and Principles Including a Framework for Decision-Making (Internat. Mire Conservation Group [u.a.], 2002).
Wilson, D., Blain, D. & Couwenberg, J. Greenhouse gas emission factors associated with rewetting of organic soils. Mires Peat 1–28 https://doi.org/10.19189/MaP.2016.OMB.222 (2016).
Bianchi, A., Larmola, T., Kekkonen, H., Saarnio, S. & Lång, K. Review of greenhouse gas emissions from rewetted agricultural soils. Wetlands 41, 108 (2021).
Schwieger, S. et al. Wetter is better: rewetting of minerotrophic peatlands increases plant production and moves them towards carbon sinks in a dry year. Ecosystems 24, 1093–1109 (2021).
Tanneberger, F. et al. Mires in Europe—regional diversity, condition and protection. Diversity 13, 381 (2021).
Beyer, F. et al. Drought years in peatland rewetting: rapid vegetation succession can maintain the net CO2 sink function. Biogeosciences 18, 917–935 (2021).
Timmermann, T., Margóczi, K., Takács, G. & Vegelin, K. Restoration of peat-forming vegetation by rewetting species-poor fen grasslands. Appl. Veg. Sci. 9, 241–250 (2006).
Steffenhagen, P., Zak, D., Schulz, K., Timmermann, T. & Zerbe, S. Biomass and nutrient stock of submersed and floating macrophytes in shallow lakes formed by rewetting of degraded fens. Hydrobiologia 692, 99–109 (2012).
Zerbe, S. et al. Ecosystem service restoration after 10 years of rewetting peatlands in NE Germany. Environ. Manag. 51, 1194–1209 (2013).
Roth, S., Seeger, T., Poschlod, P., Pfadenhauer, J. & Succow, M. Establishment of helophytes in the course of fen restoration. Appl. Veg. Sci. 2, 131–136 (1999).
Schulz, K., Timmermann, T., Steffenhagen, P., Zerbe, S. & Succow, M. The effect of flooding on carbon and nutrient standing stocks of helophyte biomass in rewetted fens. Hydrobiologia 674, 25–40 (2011).
Antonijević, D. et al. The unexpected long period of elevated CH4 emissions from an inundated fen meadow ended only with the occurrence of cattail (Typha latifolia). Glob. Chang. Biol. 29, 3678–3691 (2023).
Franz, D., Koebsch, F., Larmanou, E., Augustin, J. & Sachs, T. High net CO2 and CH4 release at a eutrophic shallow lake on a formerly drained fen. Biogeosciences 13, 3051–3070 (2016).
Koebsch, F., Jurasinski, G., Koch, M., Hofmann, J. & Glatzel, S. Controls for multi-scale temporal variation in ecosystem methane exchange during the growing season of a permanently inundated fen. Agric. Meteorol. 204, 94–105 (2015).
McNicol, G., Knox, S. H., Guilderson, T. P., Baldocchi, D. D. & Silver, W. L. Where old meets new: an ecosystem study of methanogenesis in a reflooded agricultural peatland. Glob. Chang. Biol. 26, 772–785 (2020).
Hahn-Schöfl, M. et al. Organic sediment formed during inundation of a degraded fen grassland emits large fluxes of CH4 and CO2. Biogeosciences 8, 1539–1550 (2011).
Chamberlain, S. D. et al. Soil properties and sediment accretion modulate methane fluxes from restored wetlands. Glob. Chang. Biol. 24, 4107–4121 (2018).
Juutinen, S. et al. The contribution of Phragmites australis litter to methane (CH4) emission in planted and non-planted fen microcosms. Biol. Fertil. Soils 38, 10–14 (2003).
Wilson, D. et al. Carbon and climate implications of rewetting a raised bog in Ireland. Glob. Chang. Biol. 28, 6349–6365 (2022).
Schaller, C., Hofer, B. & Klemm, O. Greenhouse gas exchange of a new german peatland, 18 years after rewetting. J. Geophys. Res. Biogeosci. 127, e2020JG005960 (2022).
Schuster, L., Taillardat, P., Macreadie, P. I. & Malerba, M. E. Freshwater wetland restoration and conservation are long-term natural climate solutions. Sci. Total Environ. 922, 171218 (2024).
Kalhori, A. et al. Temporally dynamic carbon dioxide and methane emission factors for rewetted peatlands. Commun. Earth Environ. 5, 62 (2024).
Leifeld, J., Paul, S. M., Gross-Schmölders, M., Wang, Y. & Wüst-Galley, C. Crediting peatland rewetting for carbon farming: some considerations amidst optimism. Mitig. Adapt. Strateg. Glob. Change 30, 13 (2025).
Laanbroek, H. J. Methane emission from natural wetlands: interplay between emergent macrophytes and soil microbial processes. A mini-review. Ann. Bot. 105, 141–153 (2010).
Määttä, T. & Malhotra, A. The hidden roots of wetland methane emissions. Glob. Change Biol. 30, e17127 (2024).
Bansal, S., Johnson, O. F., Meier, J. & Zhu, X. Vegetation affects timing and location of wetland methane emissions. J. Geophys. Res. Biogeosci. 125, e2020JG005777 (2020).
Sebacher, D. I., Harriss, R. C. & Bartlett, K. B. Methane emissions to the atmosphere through aquatic plants. J. Environ. Qual. 14, 40–46 (1985).
Sutton-Grier, A. E. & Megonigal, J. P. Plant species traits regulate methane production in freshwater wetland soils. Soil Biol. Biochem. 43, 413–420 (2011).
Vroom, R. J. E., Van Den Berg, M., Pangala, S. R., Van Der Scheer, O. E. & Sorrell, B. K. Physiological processes affecting methane transport by wetland vegetation—a review. Aquat. Bot. 182, 103547 (2022).
Williams, C. J. & Yavitt, J. B. Temperate wetland methanogenesis: the importance of vegetation type and root ethanol production. Soil Sci. Soc. Am. J. 74, 317–325 (2010).
Bieniada, A., Hug, L. A., Parsons, C. T. & Strack, M. Methane cycling microbial community characteristics: comparing natural, actively extracted, restored and unrestored boreal peatlands. Wetlands 43, 83 (2023).
Gios, E. et al. Unraveling microbial processes involved in carbon and nitrogen cycling and greenhouse gas emissions in rewetted peatlands by molecular biology. Biogeochemistry 167, 609–629 (2024).
Jensen, A. B., Eller, F. & Sorrell, B. K. Comparative flooding tolerance of Typha latifolia and Phalaris arundinacea in wetland restoration: insights from photosynthetic CO2 response curves, photobiology and biomass allocation. Heliyon 10, e23657 (2024).
Ward, S. E. et al. Vegetation exerts a greater control on litter decomposition than climate warming in peatlands. Ecology 96, 113–123 (2015).
Lawrence, B. A., Lishawa, S. C., Hurst, N., Castillo, B. T. & Tuchman, N. C. Wetland invasion by Typha×glauca increases soil methane emissions. Aquat. Bot. 137, 80–87 (2017).
Vroom, R. J. E. et al. Typha latifolia paludiculture effectively improves water quality and reduces greenhouse gas emissions in rewetted peatlands. Ecol. Eng. 124, 88–98 (2018).
Quadra, G. R. et al. Removing 10 cm of degraded peat mitigates unwanted effects of peatland rewetting: a mesocosm study. Biogeochemistry 163, 65–84 (2023).
Hoffmann, M. et al. A simple calculation algorithm to separate high-resolution CH4 flux measurements into ebullition- and diffusion-derived components. Atmos. Meas. Tech. 10, 109–118 (2017).
Hoffmann, M. et al. Automated modeling of ecosystem CO 2 fluxes based on periodic closed chamber measurements: a standardized conceptual and practical approach. Agric. Meteorol. 200, 30–45 (2015).
Anthony, T. L. & Silver, W. L. Hot spots and hot moments of greenhouse gas emissions in agricultural peatlands. Biogeochemistry 167, 461–477 (2023).
Aben, R. C. H. et al. CO2 emissions of drained coastal peatlands in the Netherlands and potential emission reduction by water infiltration systems. Biogeosciences 21, 4099–4118 (2024).
Denmead, O. T. Approaches to measuring fluxes of methane and nitrous oxide between landscapes and the atmosphere. Plant Soil 309, 5–24 (2008).
Kutzbach, L. et al. CO2 flux determination by closed-chamber methods can be seriously biased by inappropriate application of linear regression. Biogeosciences 4, 1005–1025 (2007).
Lai, D. Y. F., Roulet, N. T., Humphreys, E. R., Moore, T. R. & Dalva, M. The effect of atmospheric turbulence and chamber deployment period on autochamber CO2 and CH4 flux measurements in an ombrotrophic peatland. Biogeosciences 9, 3305–3322 (2012).
Görres, C.-M., Kammann, C. & Ceulemans, R. Automation of soil flux chamber measurements: potentials and pitfalls. Biogeosciences 13, 1949–1966 (2016).
Rocha, A. V. & Goulden, M. L. Why is marsh productivity so high? New insights from eddy covariance and biomass measurements in a Typha marsh. Agric. Meteorol. 149, 159–168 (2009).
Van Den Berg, M. et al. A case study on topsoil removal and rewetting for paludiculture: effect on biogeochemistry and greenhouse gas emissions from Typha latifolia, Typha angustifolia, and Azolla filiculoides. Biogeosciences 21, 2669–2690 (2024).
Koebsch, F. et al. The impact of occasional drought periods on vegetation spread and greenhouse gas exchange in rewetted fens. Philos. Trans. R. Soc. B Biol. Sci. 375, 20190685 (2020).
Bhullar, G. S., Iravani, M., Edwards, P. J. & Olde Venterink, H. Methane transport and emissions from soil as affected by water table and vascular plants. BMC Ecol. 13, 32 (2013).
Bodmer, P., Vroom, R. J. E., Stepina, T., Del Giorgio, P. A. & Kosten, S. Methane dynamics in vegetated habitats in inland waters: quantification, regulation, and global significance. Front. Water 5, 1332968 (2024).
Vroom, R. J. E. et al. Species-dependent methane emissions in a Dutch peatland during paludiculture establishment. Mires Peat 31, 1–19 (2024).
Zak, D. et al. Changes of the CO2 and CH4 production potential of rewetted fens in the perspective of temporal vegetation shifts. Biogeosciences 12, 2455–2468 (2015).
Šantrůčková, H., Kubešová, J., Šantrůček, J., Kaštovská, E. & Rejmánková, E. The effect of p enrichment on exudate quantity and bioavailability—a comparison of two macrophyte species. Wetlands 36, 789–798 (2016).
Wang, H. et al. Linking transcriptional dynamics of peat microbiomes to methane fluxes during a summer drought in two rewetted fens. Environ. Sci. Technol. 57, 5089–5101 (2023).
Wen, X. et al. Predominance of methanogens over methanotrophs in rewetted fens characterized by high methane emissions. Biogeosciences 15, 6519–6536 (2018).
Unger, V. et al. Congruent changes in microbial community dynamics and ecosystem methane fluxes following natural drought in two restored fens. Soil Biol. Biochem. 160, 108348 (2021).
Chanton, J. P., Whiting, G. J., Happell, J. D. & Gerard, G. Contrasting rates and diurnal patterns of methane emission from emergent aquatic macrophytes. Aquat. Bot. 46, 111–128 (1993).
Lai, D. Y. F. Methane dynamics in northern peatlands: a review. Pedosphere 19, 409–421 (2009).
Bansal, S. et al. Typha (Cattail) invasion in north american wetlands: biology, regional problems, impacts, ecosystem services, and management. Wetlands 39, 645–684 (2019).
Grace, J. B. & Wetzel, R. G. Variations in growth and reproduction within populations of two rhizomatous plant species: Typha latifolia and Typha angustifolia. Oecologia 53, 258–263 (1982).
Duarte, V. P., Pereira, M. P., Corrêa, F. F., De Castro, E. M. & Pereira, F. J. Aerenchyma, gas diffusion, and catalase activity in Typha domingensis: a complementary model for radial oxygen loss. Protoplasma 258, 765–777 (2021).
Faußer, A. C., Hoppert, M., Walther, P. & Kazda, M. Roots of the wetland plants Typha latifolia and Phragmites australis are inhabited by methanotrophic bacteria in biofilms. Flora Morphol. Distrib. Funct. Ecol. Plants 207, 775–782 (2012).
Agethen, S., Sander, M., Waldemer, C. & Knorr, K.-H. Plant rhizosphere oxidation reduces methane production and emission in rewetted peatlands. Soil Biol. Biochem. 125, 125–135 (2018).
Van Der Nat, F.-J. W. A. & Middelburg, J. J. Seasonal variation in methane oxidation by the rhizosphere of Phragmites australis and Scirpus lacustris. Aquat. Bot. 61, 95–110 (1998).
Krabbe, K. Kohlenstoff-Akkumulation seit Wiedervernässung im Polder Zarnekow (Mecklenburg-Vorpommern). (Universität Greifswald, 2021).
Capooci, M. et al. High methane concentrations in tidal salt marsh soils: where does the methane go?. Glob. Chang. Biol. 30, e17050 (2024).
Knorr, K.-H., Glaser, B. & Blodau, C. Fluxes and13 C isotopic composition of dissolved carbon and pathways of methanogenesis in a fen soil exposed to experimental drought. Biogeosciences 5, 1457–1473 (2008).
Dorodnikov, M., Knorr, K.-H., Fan, L., Kuzyakov, Y. & Nilsson, M. B. A novel belowground in-situ gas labeling approach: CH4 oxidation in deep peat using passive diffusion chambers and 13C excess. Sci. Total Environ. 806, 150457 (2022).
Song, C. et al. Inland water greenhouse gas emissions offset the terrestrial carbon sink in the northern cryosphere. Sci. Adv. 10, eadp0024 (2024).
Peacock, M. et al. The full carbon balance of a rewetted cropland fen and a conservation-managed fen. Agric. Ecosyst. Environ. 269, 1–12 (2019).
Taillardat, P. et al. A carbon source in a carbon sink: carbon dioxide and methane dynamics in open-water peatland pools. Glob. Biogeochem. Cycles 38, e2023GB007909 (2024).
Günther, A., Huth, V., Jurasinski, G. & Glatzel, S. The effect of biomass harvesting on greenhouse gas emissions from a rewetted temperate fen. GCB Bioenergy 7, 1092–1106 (2015).
Hendriks, D. M. D., Van Huissteden, J., Dolman, A. J. & Van Der Molen, M. K. The full greenhouse gas balance of an abandoned peat meadow. Biogeosciences 4, 411–424 (2007).
Knox, S. H. et al. Agricultural peatland restoration: effects of land-use change on greenhouse gas (CO 2 and CH 4) fluxes in the Sacramento-San Joaquin Delta. Glob. Chang. Biol. 21, 750–765 (2015).
Buzacott, A. J. V. et al. A Bayesian inference approach to determine experimental Typha latifolia paludiculture greenhouse gas exchange measured with eddy covariance. Agric. Meteorol. 356, 110179 (2024).
Mander, Ü, Espenberg, M., Melling, L. & Kull, A. Peatland restoration pathways to mitigate greenhouse gas emissions and retain peat carbon. Biogeochemistry 167, 523–543 (2023).
Tiemeyer, B. et al. A new methodology for organic soils in national greenhouse gas inventories: data synthesis, derivation and application. Ecol. Indic. 109, 105838 (2020).
Schmidt, K. Neuregulierung des hydrologischen Systems im Polder Zarnekow-Upost. Ed. UmweltPlan GmbH Stralsund (2004).
Zak, D., Gelbrecht, J., Wagner, C. & Steinberg, C. E. W. Evaluation of phosphorus mobilization potential in rewetted fens by an improved sequential chemical extraction procedure. Eur. J. Soil Sci. 59, 1191–1201 (2008).
Hutchinson, G. L. & Livingston, G. P. Use of Chamber Systems to Measure Trace Gas Fluxes. in ASA Special Publications (eds. Harper, L. A., Mosier, A. R., Duxbury, J. M. & Rolston, D. E.) 63–78. https://doi.org/10.2134/asaspecpub55.c4(American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, 2015).
Laine, A., Wilson, D., Kiely, G. & Byrne, K. A. Methane flux dynamics in an Irish lowland blanket bog. Plant Soil 299, 181–193 (2007).
Kettunen, A. et al. Predicting variations in methane emissions from boreal peatlands through regression models. BOREAL Environ. Res. 115, 131 (2000).
Welpelo, C., Dubbert, M., Tiemeyer, B., Voigt, C. & Piayda, A. Effects of birch encroachment, water table and vegetation on methane emissions from peatland microforms in a rewetted bog. Sci. Rep. 14, 2533 (2024).
Godwin, C. M., McNamara, P. J. & Markfort, C. D. Evening methane emission pulses from a boreal wetland correspond to convective mixing in hollows. J. Geophys. Res. Biogeosci.118, 994–1005 (2013).
Maclntyre, S. et al. The critical importance of buoyancy flux for gas flux across the air-water interface. Geophys. Monogr. Ser. 127, 135–140 (2002).
Poindexter, C. M. & Variano, E. A. Gas exchange in wetlands with emergent vegetation: the effects of wind and thermal convection at the air-water interface. J. Geophys. Res. Biogeosci. 118, 1297–1306 (2013).
Riederer, M., Serafimovich, A. & Foken, T. Net ecosystem CO2 exchange measurements by the closed chamber method and the eddy covariance technique and their dependence on atmospheric conditions. Atmos. Meas. Tech. 7, 1057–1064 (2014).
Vaidya, S. et al. A novel robotic chamber system allowing to accurately and precisely determining spatio-temporal CO2 flux dynamics of heterogeneous croplands. Agric. Meteorol. 296, 108206 (2021).
Al Hamwi, W. et al. Technical note: A low-cost, automatic soil–plant–atmosphere enclosure system to investigate CO2 and evapotranspiration flux dynamics. Biogeosciences 21, 5639–5651 (2024).
Chapin, F. S., Matson, P. A. & Mooney, H. A. Principles of Terrestrial Ecosystem Ecology (Springer, 2002).
Huth, V. et al. Divergent NEE balances from manual-chamber CO2 fluxes linked to different measurement and gap-filling strategies: a source for uncertainty of estimated terrestrial C sources and sinks?. J. Plant Nutr. Soil Sci. 180, 302–315 (2017).
Lloyd, J. & Taylor, J. A. On the temperature dependence of soil respiration. Funct. Ecol. 8, 315 (1994).
Gilmanov, T. G. et al. CO2 uptake and ecophysiological parameters of the grain crops of midcontinent North America: estimates from flux tower measurements. Agric. Ecosyst. Environ. 164, 162–175 (2013).
Hoffmann, M. et al. Maize carbon dynamics are driven by soil erosion state and plant phenology rather than nitrogen fertilization form. Soil Tillage Res. 175, 255–266 (2018).
Ishtiaq, K. S. et al. Modeling net ecosystem carbon balance and loss in coastal wetlands exposed to sea-level rise and saltwater intrusion. Ecol. Appl. 32, e2702 (2022).
Elsgaard, L. et al. Net ecosystem exchange of CO2 and carbon balance for eight temperate organic soils under agricultural management. Agric. Ecosyst. Environ. 162, 52–67 (2012).
Tiemeyer, B. et al. High emissions of greenhouse gases from grasslands on peat and other organic soils. Glob. Chang. Biol. 22, 4134–4149 (2016).
Climate Change 2014: Synthesis Report (Intergovernmental Panel on Climate Change, 2015).
Maljanen, M. et al. Greenhouse gas balances of managed peatlands in the Nordic countries—present knowledge and gaps. Biogeosciences 7, 2711–2738 (2010).
Minke, M. et al. Water level, vegetation composition, and plant productivity explain greenhouse gas fluxes in temperate cutover fens after inundation. Biogeosciences 13, 3945–3970 (2016).
Wilson, D. et al. Multiyear greenhouse gas balances at a rewetted temperate peatland. Glob. Chang. Biol. 22, 4080–4095 (2016).
Augustin, J. & B. Chojnicki. ‘Austausch von klimarelevanten Spurengasen, Klimawirkung und Kohlenstoffdynamik in den ersten Jahren nach der Wiedervernässung von degradiertem Niedermoorgrünland.’ Berichte des Leibniz-Institut für Gewässerökologie und Binnenfischerei 26, 47–67 (2008).
R Core Team. R: A Language and Environment for Statistical Computing, version 4.4.2. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2023).
Acknowledgements
The authors would like to thank Hans-Joachim Schröder for his support with in-situ services. The investigations were funded as part of the project “Organic Soils in the Emission Reporting”, German Ministry of Education, BMBF, and the Thünen-Institute. This work was also supported by the interdisciplinary research project CarboZALF, the Helmholtz Association of German Research Centres through a Helmholtz Young Investigators Group grant to Torsten Sachs (grant VH-NG-821), and infrastructure funding through the Terrestrial Environmental Observatories Network (TERENO).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.A. designed the study, performed the research, and contributed to writing, reviewing, and editing the manuscript. M.S. was in charge of maintenance of the study site. D.A. analyzed the data and led the writing of the manuscript, with comments from all authors. M.H. was involved in data analysis and contributed to reviewing and editing the manuscript. D.Z., M.D., and A.P. contributed to reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Lifen Jiang and Mengjie Wang. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Antonijević, D., Hoffmann, M., Zak, D. et al. Impact of plant succession on greenhouse gas fluxes during the transition of a flooded fen peatland. Commun Earth Environ 6, 611 (2025). https://doi.org/10.1038/s43247-025-02607-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-025-02607-4