Abstract
The association between soil heterotrophic nitrification and plant nitrogen (N) uptake remains poorly understood. Here, we used 15N isotope tracing and numerical analysis to quantify the rate of ammonification (organic N to ammonium), heterotrophic nitrification (organic N to nitrate), and autotrophic nitrification (ammonium to nitrate) in soils, based on 104 lab observations across 11 plant species. Our results showed that plant cultivation stimulated heterotrophic nitrification while suppressing autotrophic nitrification, with effects varying by species and N preference. However, this stimulation disappeared rapidly after plant removal. Heterotrophic nitrification was strongly driven by labile substrates and positively associated with plant N uptake. In contrast, autotrophic nitrification, which was pH-driven, was negatively correlated with plant N uptake. Structural equation modeling further revealed that heterotrophic nitrification and ammonification promote total plant N acquisition. Plant-driven stimulation of heterotrophic nitrification demonstrates a critical mechanism by which plant-soil interactions regulate N cycling.
Similar content being viewed by others
Introduction
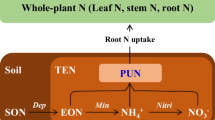
Nitrogen (N) mineralization is the microbial process that converts organic nitrogen in soil into inorganic forms, primarily ammonium \(\left({{{\rm{NH}}}}_{4}^{+}\right)\) and nitrate \(\left({{{\rm{NO}}}}_{3}^{-}\right)\), thereby making N available for plant uptake. Organic N is converted into mineral N through at least two processes: (1) ammonification, which transforms organic N into \({{{\rm{NH}}}}_{4}^{+}\), and can further be oxidized to \({{{\rm{NO}}}}_{3}^{-}\); and (2) heterotrophic nitrification, which directly oxidizes organic N to \({{{\rm{NO}}}}_{3}^{-}\). Heterotrophic nitrification is regarded as a mystery in the N cycle1, a discussion that has been ongoing since the late 19th century when it was first reported2,3,4. Microbes mediating the heterotrophic nitrification process can use both \({{{\rm{NH}}}}_{4}^{+}\) and organic N as substrates and produce \({{{\rm{NO}}}}_{3}^{-}\) as an end product, and are found in many environments5. Oxidation of \({{{\rm{NH}}}}_{4}^{+}\) can be mediated by autotrophic nitrifiers (i.e., autotrophic nitrification) or by heterotrophic nitrifiers1. However, heterotrophic nitrifiers are generally considered to primarily utilize organic N as its substrate, since \({{{\rm{NH}}}}_{4}^{+}\) in soil is predominantly oxidized to \({{{\rm{NO}}}}_{3}^{-}\) by autotrophic nitrifiers6,7. Studies attempt to quantify heterotrophic nitrification by applying chemical inhibitors8; however, these inhibitors often do not completely suppress autotrophic activity and may introduce unintended disturbances to soil, microbes, or plants. Furthermore, isotopic studies are frequently conducted using sieved soils without plants, thereby excluding plant-soil interactions. There is still a lack of quantitative assessments on the magnitude of plant-induced N transformations.
Heterotrophic nitrification is usually weak in many unplanted soils9, except in acidic or in soils with high organic carbon (C) content10. Additionally, heterotrophic nitrification is often lower than autotrophic nitrification, especially in cropland9,11. Fungi have been considered the primary agents of heterotrophic nitrification12,13, as they can oxidize both recalcitrant and labile organic N1,14. However, recent global data syntheses have failed to identify a consistent relationship between heterotrophic nitrification rates and soil pH11,15, suggesting that this process is not strictly pH-dependent. In contrast, the quantity and quality of available substrates may play a more critical role16. Abundant substrates can promote both ammonification and heterotrophic nitrification in soils9,11, but the effect of substrate quality on heterotrophic nitrification remains unclear. For example, several studies have reported increased heterotrophic nitrification with higher soil C:N ratios17,18, attributing this to enhanced microbial activity due to greater availability of labile C for fungi and bacteria13. However, this explanation may not apply to agroecosystems. As a previous analysis showed that increased C:N ratios significantly suppressed heterotrophic nitrification in global croplands11. Soil moisture is another important factor influencing heterotrophic nitrification. Some studies have shown that heterotrophic nitrification is the dominant nitrification pathway under moderate moisture conditions7,19, while it accounts for less than 10% of total nitrification under higher moisture levels19. In agricultural ecosystems, heterotrophic nitrification rates decreased significantly with increasing soil moisture20. In contrast, heterotrophic nitrification contributed up to 96% of total \({{{\rm{NO}}}}_{3}^{-}\) production in a rainforest21. A relevant understanding of heterotrophic nitrification is rapidly accumulating, but few studies have examined the effects of heterotrophic nitrification on plant N uptake.
Heterotrophic nitrification and its contribution to soil N cycling and plant N uptake are not well understood22,23,24. Limited studies have shown that the release of root exudates can significantly stimulate heterotrophic nitrification25,26,27. This may represent an important N acquisition strategy for \({{{\rm{NO}}}}_{3}^{-}\)-preferring plants27. This phenomenon could be linked to the release of mineral-associated organic matter (MAOM). Compared to particulate organic matter (POM) with high C/N ratio, MAOM with low C/N ratio is often highly recalcitrant due to its strong association with soil minerals28. However, specific organic acids in root exudates can disrupt these associations, releasing simple compounds that are subsequently decomposed and utilized29,30. Therefore, our first hypothesis is that plants can stimulate heterotrophic nitrification of organic N through the release of root exudates.
On the other hand, heterotrophic nitrification is also a component of total nitrification. Many plant root exudates contain biological nitrification inhibitors (BNIs), which suppress autotrophic nitrification by interfering with the activity of ammonia monooxygenase31. Unlike autotrophic nitrifiers, many heterotrophic nitrifiers (esp. fungi) lack this enzyme and are therefore less likely to be inhibited by BNIs7. However, studies that measure net \({{{\rm{NO}}}}_{3}^{-}\) accumulation often do not differentiate between autotrophic and heterotrophic nitrification32,33,34. As a result, \({{{\rm{NO}}}}_{3}^{-}\) is frequently assumed to originate solely from autotrophic nitrification. This assumption may partly explain the inconsistent effectiveness of BNIs observed in various studies. For instance, some research has found no impact of BNIs or of plant species with differing BNI capacities on soil nitrification rates33,34, although other studies have reported positive effects35,36. By distinguishing between autotrophic and heterotrophic nitrification, we propose the second hypothesis: plants are capable of selectively inhibiting autotrophic nitrification but not heterotrophic nitrification.
Here, by applying the Ntraceplant tool, we conducted 104 sets of 15N tracing studies in pot experiments to measure gross N transformations, including heterotrophic nitrification, as well as plant N uptake. These experiments involved 11 different plant species and various soil types (Supplementary Table 1). We primarily address two central questions: (1) How do plants regulate heterotrophic nitrification? (2) How does the heterotrophic nitrification process contribute to plant N uptake?
Results
N transformations under plant cultivation
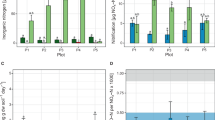
We found that plants significantly stimulated heterotrophic nitrification of organic N. The average rate of heterotrophic nitrification was initially low, but increased significantly after 30 days of short-term cultivation to 1.28 mg N kg−1 soil day−1 (median = 0.81 mg N kg−1 soil day−1), and reached 1.96 mg N kg−1 soil day−1 (median = 1.83 mg N kg−1 soil day−1) at 60 days in planted soils (Fig. 1A). In contrast, plant cultivation had no significant effect on the ammonification process (Fig. 1B). Notably, the rate of autotrophic nitrification exhibited an opposite trend, with plants significantly inhibiting autotrophic nitrification and significantly decreasing with planting time (Fig. 1C). Dissolved organic C (DOC) followed a pattern similar to heterotrophic nitrification, with increasing in DOC from 140 mg kg−1 soil (median = 154 mg kg−1 soil) in the unplanted control to 169 mg kg−1 soil (median = 180 mg kg−1 soil) under 60 days of cultivation (Fig. 1D).
A Heterotrophic nitrification. B ammonification. C autotrophic nitrification. D DOC. Unplanted refers to soil in which no plants are grown; Short refers to the soil in which plants are grown for one month; Long refers to the soil in which plants are grown for two months. Different letters on the box plots indicate significant differences after Kruskal–Wallis test, followed by Dunn’s post-hoc test. The box in the plot represents the interquartile range (IQR), with the center line indicating the median and the box limits showing the upper and lower quartiles. The whiskers extend to 1.5 times the IQR from the quartiles. The significance level was set at P < 0.05. N nitrogen, DOC dissolved organic carbon.
Plant effects on soil N transformations were species-dependent. For instance, although all plants stimulated heterotrophic nitrification, cucumber showed significantly stronger stimulation than maize (Supplementary Fig. 1A–C). Ammonification rates were generally consistent across species, though wheat exerted the strongest stimulation, while rice showed a tendency to suppress ammonification (Supplementary Fig. 1D–F). Autotrophic nitrification rates varied more noticeably, with cucumber, maize, rice, and tomato all exerting greater influence than wheat (Supplementary Fig. 1G–I).
In our pot experiments, heterotrophic nitrification rates were comparable across soils with different pH levels (Supplementary Fig. 2A–C). Although ammonification was lower in acidic soils than in alkaline soils, the effect of plant cultivation on ammonification did not vary significantly with pH (Supplementary Fig. 2D–F). Conversely, autotrophic nitrification was significantly higher in alkaline soils (Supplementary Fig. 2G). In absolute terms, the reduction in autotrophic nitrification due to plant presence was greater in alkaline soils (Supplementary Fig. 2H); however, the relative inhibition was stronger in acidic soils (Supplementary Fig. 2I).
Soils planted with \({{{\rm{NO}}}}_{3}^{-}\)-preferring species exhibited lower heterotrophic nitrification rates than those with \({{{\rm{NH}}}}_{4}^{+}\)-preferring species (Supplementary Fig. 3A). Although the absolute increase in heterotrophic nitrification stimulated by different N-preference plants was not significantly different (Supplementary Fig. 3B), \({{{\rm{NO}}}}_{3}^{-}\)-preferring species exhibited a significantly higher proportional stimulation, as indicated by their natural log response ratios (Supplementary Fig. 3C). Ammonification rates were similar between the two groups, although \({{{\rm{NO}}}}_{3}^{-}\)-preferring species exerted weaker suppression (Supplementary Fig. 3D–F). In contrast to heterotrophic nitrification, autotrophic nitrification was significantly lower under \({{{\rm{NH}}}}_{4}^{+}\)-preferring plants (Supplementary Fig. 3G), but the inhibitory effect on autotrophic nitrification was not associated with N preference (Supplementary Fig. 3I).
Drivers of heterotrophic nitrification of organic N
Regression analyses showed that plant N uptake was positively correlated with heterotrophic nitrification and ammonification (Fig. 2A, B), but not with autotrophic nitrification (Fig. 2C). Heterotrophic nitrification was uncorrelated with ammonification but negatively correlated with autotrophic nitrification (Fig. 2D, E). In contrast, autotrophic nitrification increased with increasing ammonification (Fig. 2F). Structural equation modeling further revealed that increasing soil pH simultaneously promoted ammonification (standardized coefficient = 0.18, P = 0.015) and autotrophic nitrification (standardized coefficient = 0.82, P < 0.001), while heterotrophic nitrification was significantly stimulated by DOC (standardized coefficient = 0.66, P = 0.037) (Fig. 3). Additionally, DOC (standardized coefficient = −0.59, P = 0.004) and heterotrophic nitrification (standardized coefficient = −0.39, P = 0.006) exerted suppressive effects on autotrophic nitrification. Plant N uptake was primarily driven by ammonification (standardized coefficient = 0.18, P < 0.001) and heterotrophic nitrification (standardized coefficient = 0.09, P = 0.029) but was negatively affected by soil pH (standardized coefficient = −0.11, P = 0.017). Together, these factors explained 37% of the variation in total plant N uptake. Overall, these results indicate that heterotrophic nitrification is stimulated by soil labile substrates and plays a facilitative role in plant N acquisition.
A Relationship of ln(heterotrophic nitrification) and ln(plant N uptake). B Relationship of ln(ammonification) and ln(plant N uptake). C Relationship of ln(autotrophic nitrification) and ln(plant N uptake). D Relationship of ln(ammonification) and ln(heterotrophic nitrification). E Relationship of ln(autotrophic nitrification) and ln(heterotrophic nitrification). F Relationship of ln(ammonification) and ln(autotrophic nitrification).
Blue arrows indicate positive effects, and orange arrows indicate negative effects. Numbers next to arrows indicate standardized coefficients. R2 indicates the degree of explanation of the corresponding variable for all covariates. All paths with P > 0.05 are hidden. N nitrogen, DOC dissolved organic carbon.
Effects of plant removal on heterotrophic nitrification
Our 15N tracing study found that 20-day-old maize had a heterotrophic nitrification of 0.79 mg N kg−1 day−1, which was significantly higher than the Removal-Day 3 (0.18 mg N kg−1 day−1), Removal-Day 7 (0.02 mg N kg−1 day−1), Dark-Day 2 (0.16 mg N kg−1 day−1) and Dark-Day 4 (0.13 mg N kg−1 day−1) treatments (Fig. 4A). Compared to the control group with normal plant growth, both plant removal and shading treatments led to a reduction in soil DOC (Fig. 4B). Consistent with the changes in heterotrophic nitrification rates, the abundance of heterotrophic nitrifying fungi also declined following plant removal and shading, with significantly lower levels observed under the Removal-Day 7 and Dark-Day 4 treatments relative to the control (Fig. 4C).
Heterotrophic nitrification (A), DOC (B), and abundance of heterotrophic nitrifying fungi C under different treatments. Control is the normal planting treatment; Removal−Day 3 and Removal−Day 5 are 15N tracing experiments on soil three and five days after plant removal, respectively; and Dark−Day 2 and Dark−Day 4 are 15N tracing experiments on soil after plants have been incubated in the dark for two and four days, respectively. Different letters on the box plots indicate significant differences after Kruskal–Wallis test followed by Dunn’s post-hoc test. The significance level was set at P < 0.05. DOC dissolved organic carbon.
Discussion
Heterotrophic nitrification is primarily plant-stimulated and substrate-regulated
Our results showed that plants significantly stimulated heterotrophic nitrification (Fig. 1A), while ammonification remained unchanged (Fig. 1B). We found that heterotrophic nitrification rates in the plant-soil system decreased significantly after the plant was in dark incubation (Fig. 4A), suggesting that photosynthates play an important role in stimulating heterotrophic nitrification. Together, these results confirm our first hypothesis
Some studies have reported that heterotrophic nitrification commonly occurs in acidic soils7,18,37. However, recent global analyses have shown no consistent relationship between soil pH and heterotrophic nitrification, suggesting that this process can occur across a broad range of pH conditions11,15. In contrast, substrates appear to be more influential. Notably, a high C:N ratio significantly suppresses heterotrophic nitrification in cropland11, consistent with our findings. We propose that labile substrates serve as key mediators promoting heterotrophic nitrification, as evidenced by their positive response to DOC (Figs. 3 and 4B). An indication of which organic compounds possibly stimulate heterotrophic nitrification is also provided by studies with various organic supplements, but without plants. Labile organic substrates, such as glycine, L-glutamic acid, and plant residues (straw), stimulated heterotrophic nitrification rates in the soil even in the absence of plants38,39. In the presence of plants, oxalic acid in root exudates serves as a key mediator. Due to its chelating capacity, oxalic acid can disrupt the associations between organic matter and soil minerals40, thereby releasing mineral-bound organic matter with a low C:N ratio that is readily decomposed28. Fungi, in particular, play a central role in this decomposition process (Fig. 4C). This mechanism may explain the lower heterotrophic nitrification observed in unplanted soils41, where MAOM is typically recalcitrant and its decomposition strongly depends on plant activity.
Our results showed that heterotrophic nitrification was independent of soil pH (Supplementary Fig. 2A, P > 0.05) and occurs in the soils of \({{{\rm{NH}}}}_{4}^{+}\) preferring plants (such as rice) and \({{{\rm{NO}}}}_{3}^{-}\) preferring plants (such as cucumber, maize, wheat, tomato) (Supplementary Fig. 3A and Supplementary Table 2). For \({{{\rm{NH}}}}_{4}^{+}\) preferring plants, \({{{\rm{NO}}}}_{3}^{-}\) originating from the heterotrophic nitrification is of particular importance to maintain the \({{{\rm{NH}}}}_{4}^{+}/{{{\rm{NO}}}}_{3}^{-}\) balance as mentioned above. For rice, a typical \({{{\rm{NH}}}}_{4}^{+}\) preferring plant, it has been shown that a partial supply of \({{{\rm{NO}}}}_{3}^{-}\) improves N nutrition42. This is even more important as it has also been reported that rice can secrete BNIs35 and inhibit ammonification43, which further limit the availability of \({{{\rm{NO}}}}_{3}^{-}\)31. As we observed, although the stimulation of heterotrophic nitrification by rice was the lowest, it was still positive (Supplementary Fig. 1A–C). Of course, the influence of soil moisture cannot be excluded (Supplementary Fig. 4). In this study, rice was grown under flooded conditions, while all other plant species were cultivated under non-flooded conditions. Flooding is also known to suppress both ammonification and autotrophic nitrification41.
Heterotrophic nitrification provides a way to ensure effective \({{{\rm{NO}}}}_{3}^{-}\) supply to rice. \({{{\rm{NO}}}}_{3}^{-}\) preferring plants take up more \({{{\rm{NO}}}}_{3}^{-}\) than \({{{\rm{NH}}}}_{4}^{+}\), but also take up \({{{\rm{NH}}}}_{4}^{+}\) to maintain the balance between \({{{\rm{NH}}}}_{4}^{+}\) and \({{{\rm{NO}}}}_{3}^{-}\)44. During rapid growth periods, plants are more competitive than nitrifiers when using \({{{\rm{NH}}}}_{4}^{+}\). \({{{\rm{NH}}}}_{4}^{+}\) uptake rates by plants were significantly higher than autotrophic nitrification rates (Supplementary Fig. 5). Thus, \({{{\rm{NO}}}}_{3}^{-}\) supply from ammonification and subsequent oxidation to \({{{\rm{NO}}}}_{3}^{-}\) would not be sufficient to maintain the \({{{\rm{NH}}}}_{4}^{+}/{{{\rm{NO}}}}_{3}^{-}\) balance for \({{{\rm{NO}}}}_{3}^{-}\) preferring plants even in the soils with strong nitrifying activity, because plants outcompete nitrifiers for \({{{\rm{NH}}}}_{4}^{+}\). This explains that heterotrophic nitrification also plays an essential role in maintaining the \({{{\rm{NH}}}}_{4}^{+}/{{{\rm{NO}}}}_{3}^{-}\) balance of \({{{\rm{NO}}}}_{3}^{-}\) preferring plants.
Heterotrophic nitrification promotes plant N uptake and helps understand plant effects on gross nitrification
The benefits of plant-microbe interactions on plant N uptake are realized by triggering heterotrophic nitrification and inhibiting autotrophic nitrification (Fig. 1A, C), which confirms our second hypothesis. Both regression analysis and structural equation modeling indicated that heterotrophic nitrification significantly promoted plant N uptake while autotrophic nitrification was not beneficial to plant N uptake (Figs. 2A, C and 3). Plants stimulated heterotrophic nitrification to mine \({{{\rm{NO}}}}_{3}^{-}\) from the soil on the one hand, and inhibited autotrophic nitrification to prolong the retention of \({{{\rm{NH}}}}_{4}^{+}\) produced by ammonification on the other. Plants satisfy their \({{{\rm{NH}}}}_{4}^{+}-{{{\rm{NO}}}}_{3}^{-}\) mixed nutrient requirements through these two mechanisms, thereby maintaining a suitable ionic balance and optimal growth42,45.
In this study, the negative correlation between heterotrophic and autotrophic nitrification under plant cultivation (Fig. 2E) may reflect a trade-off in N transformations within the plant-soil system. Autotrophic nitrification relies on \({{{\rm{NH}}}}_{4}^{+}\) supplied through ammonification, with both processes being stimulated by soil pH (Fig. 3). This suggests that the ammonification-autotrophic nitrification pathway represents a soil-dominate process with limited plant control. In contrast, heterotrophic nitrification is dependent on plant presence and stimulated by labile substrates. The resulting \({{{\rm{NO}}}}_{3}^{-}\) tends to accumulate in the rhizosphere, where it enhances plant N acquisition.
In addition, the opposite effect of plants on nitrification partly explains why some studies have found that BNIs do not alter net or gross nitrification. Net nitrification is usually considered to be a combination of nitrification and \({{{\rm{NO}}}}_{3}^{-}\) immobilization46, while gross nitrification is expressed as the sum of autotrophic nitrification and heterotrophic nitrification11. BNIs have an inhibitory effect on ammonia monooxygenase, which can limit autotrophic nitrifying microbes (e.g., ammonia-oxidizing bacteria and ammonia-oxidizing archaea)47. In contrast, many heterotrophic nitrifying microbes lack ammonia monooxygenase1 and can be stimulated by rhizodeposition. Plants (esp. rice) may also suppress autotrophic nitrification indirectly by limiting ammonification (Supplementary Fig. 1E, F). Regression analysis also indicated that autotrophic nitrification decreases significantly with reduction in ammonification (Fig. 2F). The ammonification-autotrophic nitrification pathway represents a diffusion-based N transformation mode, in which the produced \({{{\rm{NO}}}}_{3}^{-}\) is prone to loss from the soil system. Autotrophic nitrification is more active in soils away from the rhizosphere, where \({{{\rm{NO}}}}_{3}^{-}\) is not concentrated near roots. Moreover, root zones often experience reduced autotrophic nitrification due to the presence of BNIs. The \({{{\rm{NO}}}}_{3}^{-}\) produced via autotrophic nitrification in bulk soils is more likely to be lost through processes such as denitrification and leaching, which may explain the observed positive correlation between autotrophic nitrification and N2O emissions11, and its lack of association with plant N uptake (Fig. 2C). This contrasts with heterotrophic nitrification.
Limitations
This study was conducted under controlled pot conditions, which may not fully capture the complexity and heterogeneity of natural field environments. Differences in soil structure, root architecture, microbial interactions, and hydrological dynamics between pot systems and field settings could influence N transformation processes. However, pot experiments allow for precise control of environmental variables, enabling mechanistic analyses of plant, soil, and N dynamics. The plant-induced stimulation of heterotrophic nitrification observed in this study is consistent with findings from in situ experiments. For instance, a previous study reported a significant decrease in heterotrophic nitrification, but not ammonification, following root exclusion in forest soils48. Also, other studies have reported that ammonification rate is stimulated by plants49. Additionally, tree girdling is commonly employed to reduce root influence. For example, studies observed that girdling reduced rhizosphere ammonification in European beech50, while enhanced ammonification in two spruce forests51. These discrepancies may stem from differences in experimental conditions, plant functional types, or the quality of soil organic matter. In addition, many studies conducted in natural or minimally disturbed ecosystems have reported plant-induced stimulation of ammonification52,53,54. The lack of a significant plant effect on ammonification observed in our study may be partly attributed to differences between agricultural and natural soils. In agricultural systems, external N inputs (mainly urea) provide crops with substantial amounts of \({{{\rm{NH}}}}_{4}^{+}\), potentially reducing their reliance on ammonification55. In contrast, various allelochemicals released from crop exudates may stimulate the activity of heterotrophic nitrifiers through allelochemical effects56.
Moreover, the use of 15N tracings relies on assumptions such as equal utilization of 15N and 14N and the absence of re-mineralization of assimilated N, which may introduce potential bias46. Therefore, we acknowledge that the results of this study should be interpreted with caution. Future work should incorporate in situ experiments across diverse ecosystems to expand the applicability of these findings under more realistic conditions.
Methods
Preparation of plants and soils
A wide range of subject plants, such as grain crops like rice (Oryza sativa), maize (Zea mays), and wheat (Triticum aestivum); cash crops like sugarcane (Saccharum officinarum), tomato (Solanum lycopersicum), cucumber (Cucumis sativus), and tea (Camellia sinensis); and native or invasive plants such as lespedeza (Lespedeza bicolor Turcz.), Canada goldenrod (Solidago canadensis), common reed (Phragmites australis), and smooth cordgrass (Spartina alterniflora) were selected. The experiment comprised multiple groups per plant species, with each group representing the mean of three replicates. Specifically, the number of groups per species was as follows: rice (36), maize (33), wheat (9), cucumber (9), tomato (8), sugarcane (2), tea plant (2), Canada goldenrod (2), and one group each for lespedeza, common reed, and smooth cordgrass. The preparation procedures for seedlings varied among plant species. Rice seeds were surface-sterilized by soaking in 2% sodium hypochlorite for 15 min, rinsed with sterile water, and then incubated at 28 °C for germination. Germinated seeds were transferred to a flooded nursery tray and grown indoors for four weeks. Uniform seedlings at the four-week stage were selected for the subsequent 15N tracing experiment. In contrast, maize and wheat seeds were surface-sterilized, germinated, and directly sown into pots for growth. For tomato and cucumber, sterilized seeds were placed in Petri dishes lined with moist gauze and incubated in the dark at 30 °C for germination. Well-germinated seeds were then transferred to seedling trays containing nutrient-rich substrate. Once the first true leaves had expanded, uniform seedlings were selected and transplanted into pots. Sugarcane stem segments containing buds were surface-sterilized in 10% hydrogen peroxide, rinsed thoroughly with distilled water, and placed on soil-filled nursery beds for sprouting. Seedlings were transplanted into pots after three weeks of growth. For tea, one-year-old greenhouse-grown seedlings were directly transplanted into pots. Seedlings of Canada goldenrod, common reed, and smooth cordgrass were collected from the field, and uniform individuals were selected for pot cultivation. Lespedeza seeds were soaked in concentrated sulfuric acid for 10 min to break physical dormancy, then rinsed thoroughly with deionized water. The seeds were subsequently soaked in warm water (~60 °C) for 24 h to allow full imbibition. Swollen seeds were placed in Petri dishes for germination, and uniformly germinated seeds were transplanted into pots for further growth.
The soil samples were collected from multiple regions with varying environmental conditions (Supplementary Table 1). At each sampling site, five plots (1 × 1 m) were randomly selected, and subsequently top soil (0–20 cm) was collected from each plot and pooled together. The pooled soil samples were immediately transferred to sterile plastic bags and shipped to the laboratory within 48 h, sieved to 2 mm, and stored at 4 °C. Soil pH was determined in deionized water (1:2.5 ratio) using a DMP-2 mV/pH meter (Quark Ltd., Nanjing, China). Soil organic carbon was measured using the wet digestion method in an H2SO4-K2Cr2O7 solution. Total N was determined using the semi-micro Kjeldahl digestion method with Se and CuSO4 as the catalysts, and K2SO4 was added to increase the boiling point. The ratio of organic carbon to total N (C:N) was calculated. DOC was extracted using a soil-to-water ratio of 1:5, filtered through a 0.45 μm membrane, and subsequently measured using a Multi N/C Analyzer (Analytik Jena, Jena, Germany). All samples were taken in triplicate. Details regarding the plants and soils are provided in Supplementary Table 1.
15N isotopic tracing experiment
All plants were planted into pots containing 200–300 g soil after germination or field collection as detailed in Supplementary Table 2. Rice plants were subjected to continuous flooding, with a 2–3 cm water layer maintained above the soil surface throughout the growing period by daily watering. In contrast, all other plant species were watered daily to maintain soil moisture at 40–60% of the water holding capacity. All pots were randomly placed in a 25 °C climate chamber. The application of 15NH4NO3 and NH415NO3 (10 atom% 15N) after 30 days (or 60 days) of growth was in accordance with the usual fertilization practices, with \({{{\rm{NH}}}}_{4}^{+}\) and \({{{\rm{NO}}}}_{3}^{-}\) applied at a rate of 40 mg N kg−1 soil. The labeling solution was injected by using a 4-needle injection technique (Fig. S1). The soils were extracted with 1 M KCl solution using a ratio of 1:5. The whole plants (including roots) were harvested at 0.5, 24, 48, and 72 h after 15N application to determine the concentrations and isotopic compositions of \({{{\rm{NH}}}}_{4}^{+}\) and \({{{\rm{NO}}}}_{3}^{-}\), and N concentrations and isotopic compositions in plant biomass. All samples were taken in triplicate. The measured concentrations and 15N excess values (means ± standard deviations) of soil \({{{\rm{NH}}}}_{4}^{+}\) and \({{{\rm{NO}}}}_{3}^{-}\) and plant biomass from the two 15N treatments were analyzed by the Ntraceplant tool to quantify gross N transformation and plant N uptake rates by optimization against the observed data and expressed in units of mg N kg−1 soil day−1.
The Ntraceplant tool used in this study was based on the 15N tracing tool57, which combines the analysis model and a parameter estimation routine. The original model was extended by a sub-model for plant N uptake (Supplementary Fig. 6). Plant N uptake was divided into \({{{\rm{NH}}}}_{4}^{+}\) and \({{{\rm{NO}}}}_{3}^{-}\) uptake. Thus, in total, the Ntrace version used in this study consisted of 7 N pools and 14 processes. Compared to previous tool58, additional factors were introduced that governed whether \({{{\rm{NH}}}}_{4}^{+}\) and \({{{\rm{NO}}}}_{3}^{-}\) uptake was limited by plant biomass (plant N demand) (i.e., at early growth stages) or by the soil N supply (i.e., in later growth stages when soil N is depleted but plant N demand is high). Thus, the plant N uptake rates were based on both the soil mineral N concentrations as well as the total plant biomass. To obtain a continuous plant biomass, a polynomial function was fitted through the observed plant biomass by regression analysis. The concentrations of available \({{{\rm{NH}}}}_{4}^{+}\) and \({{{\rm{NO}}}}_{3}^{-}\), depicting the shift from plant to soil limitation, were set to 1 mmol N kg−1 soil and 0.8 mmol N kg−1 soil, for \({{{\rm{NH}}}}_{4}^{+}\) and \({{{\rm{NO}}}}_{3}^{-}\) uptake, respectively. Above this threshold, the kinetics were set to zero-order kinetics (i.e., governed by plant N demand), and below the threshold, the kinetics changed to first-order (i.e., soil N supply limited).
The gross rates of soil N transformations and plant N uptake over the entire experimental period were quantified by simultaneously optimizing the kinetic parameters of each N process by minimizing the misfit between modeled and observed \({{{\rm{NH}}}}_{4}^{+}\), \({{{\rm{NO}}}}_{3}^{-}\), and plant N concentrations and their 15N abundance using the Metropolis algorithm, a Markov Chain Monte Carlo method57. The advantage of Bayesian method is that they are less prone to over-parametrization and is the method of choice for large-scale inverse modeling problems (e.g., parameterization of ecosystem models)59. Assurance that the global minimum (i.e., a unique solution) was found for each optimization run was obtained via the Gelman reduction factor via analyses of three parallel sequences with randomly selected starting parameters. Moreover, comparisons with analytical approaches showed an excellent match60, providing additional assurance that the N tracing technique provides reliable results. The model calculations were performed in the software MATLAB (Version 7.1, The MathWorks Inc.) in combination with Simulink (Version 6.3, The MathWorks Inc.). To obtain the best parameter set, the kinetic settings of the individual processes were varied (i.e., using various combinations of zero-order, first-order, and Michaelis-Menten kinetics). The most suitable model was identified based on Akaikes Information Criterion for each model version. For N transformations following first-order kinetics, average gross N rates were calculated by integrating the gross N rates over the experimental period, divided by the total time. To evaluate plant feedback on soil N dynamics, gross N transformation rates in bulk soils (soils incubated without plants) were also measured concurrently in this study. The rates in bulk soils were calculated using the widely adopted Ntracebasic tool57. The incubation conditions, including temperature and moisture management, were kept consistent with those of the potted plants.
Soil heterotrophic nitrification under plant removal and dark incubation
Here, maize was used as the experimental plant, and the growth conditions were identical to those described in “Drivers of heterotrophic nitrification of organic N”. The 15N tracing experiments included five treatments. Control treatment: 15NH4NO3 and NH415NO3 (10 atom% 15N) were applied to intact plant-soil systems after 20 days of growth. Both \({{{\rm{NH}}}}_{4}^{+}\) and \({{{\rm{NO}}}}_{3}^{-}\) were applied at a rate of 40 mg N kg−1 soil, following the same protocols. Plant removal treatments: In two treatments, maize plants (including roots) were removed from the soil on day 20. 15N tracers were applied either 3 days (Removal–Day 3) or 7 days (Removal–Day 7) after plant removal. Dark incubation treatments: In another two treatments, plants were transferred to complete darkness at day 20 to suppress photosynthesis. 15N tracers were applied after 2 days (Dark–Day 2) or 4 days (Dark–Day 4) of dark incubation.
Soil samples were also used for the most probable number (MPN) determination of heterotrophic nitrifying microbes within 24 h61. Briefly, 10 g (fresh weight) of soil was suspended in 100 mL of sterile 0.9% NaCl solution and shaken on a gyrotary shaker at 130 rpm for 30 min at room temperature (20 °C) to facilitate microbial desorption. Serial dilutions were prepared up to 10−11. For each dilution step, five replicate test tubes containing 9 mL of potato dextrose agar were inoculated with 1 mL of the suspension. Tubes were sealed with Cap-O-Test caps and incubated at 28 °C in the dark under aerobic conditions for 7 days. The inoculated tubes were vortexed daily for 10 s. Nitrite production was tested daily using Griess reagent. Tubes showing transient nitrite accumulation (i.e., testing positive one day and negative the next) were scored as positive for heterotrophic nitrification. MPN values were calculated using standard statistical tables based on the number of positive tubes at each dilution. In addition, soil samples were also used for determining DOC.
Data analyses
We first performed the Kruskal–Wallis test, followed by Dunn’s post-hoc test, to compare the differences in gross N transformation rates among different planting times, plant species, soil pH, plant N preference, and soil moisture. A P value of <0.05, adjusted by Bonferroni correction, was considered statistically significant. Subsequently, to quantify the magnitude and direction of plant-driven fluxes of gross N transformation in the soil, we calculated the natural logarithmic response ratio (lnRR) using the following equation62:
\(\bar{{x}_{t}}\) and \(\bar{{x}_{c}}\) are mean values of the planted and unplanted soil, respectively.
We also added priming effect (PE) as a supplementary indicator. The equation is as follows:
\(\bar{{x}_{t}}\) and \(\bar{{x}_{c}}\) are mean values of the planted and unplanted soil, respectively.
We further examined the relationship between soil N transformations and plant N uptake by linear regression analysis. We also used structural equation modeling to explore the direct and indirect patterns of soil factors and N mineralization processes on plant N uptake. Prior to modeling, we first constructed pathways based on established relationships: soil properties can directly influence plant \({{{\rm{NH}}}}_{4}^{+}\) and \({{{\rm{NO}}}}_{3}^{-}\) uptake, or they can affect plant N uptake indirectly by modulating the strength of feedbacks from gross N transformations. Initially, we incorporated as many variables as possible, but the model was difficult to fit. We therefore carefully reduced the variables and tried them individually to generate the final structural equation modeling. The data used in the final model were normalized, and variance inflation factors (all < 2) were calculated to confirm the absence of multicollinearity (Supplementary Table 3). Structural equation modeling was implemented through the lavaan package63. Model fit was determined primarily through chi-square/df = 0.159, P = 0.690, GFI = 0.999. All statistical analyses were performed with R (4.3.3).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are available on Figshare (https://figshare.com/articles/dataset/__/29539964).
References
Martikainen, P. J. Heterotrophic nitrification-an eternal mystery in the nitrogen cycle. Soil Biol. Biochem. 168, 108611 (2022).
Warington, R. Six lectures on the investigations at Rothamsted experimental station delivered under the provision of the Lawes agricultual trust. U.S. Dept. of agriculture. Office of experiment stations. Experiment station bulletin (Govt. print. off. Washington, 1892). http://catalog.hathitrust.org/Record/100026438.
Stutzer, H. & Hartleb, R. Uber nitratbildung. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 22, 701 (1894).
Omelianski, V. L. Ueber die nitrifikation des organischen stickstoffs. Gent. Bakt. 5, 473–490 (1899).
Song, T. et al. A review of research progress of heterotrophic nitrification and aerobic denitrification microorganisms (HNADMs). Sci. Total Environ. 801, 149319 (2021).
Gao, W. et al. Heterotrophic ammonium oxidation is not active in acidic paddy soils. Soil Biol. Biochem. 182, 109046 (2023).
Gao, W. L. et al. Heterotrophic nitrification of organic nitrogen in soils: process, regulation, and ecological significance. Biol. Fertil. Soils 59, 261–274 (2023).
Stein, L. Y. Heterotrophic nitrification and nitrifier denitrification. Nitrification. 95-114 https://doi.org/10.1128/9781555817145.ch5 (Wiley, 2011).
Elrys, A. S. et al. Expanding agroforestry can increase nitrate retention and mitigate the global impact of a leaky nitrogen cycle in croplands. Nat. Food 4, 109–121 (2023).
Kuroiwa, M. et al. Gross nitrification rates in four Japanese forest soils: Heterotrophic versus autotrophic and the regulation factors for the nitrification. J. For. Res. 16, 363–373 (2011).
Elrys, A. S. et al. Global gross nitrification rates are dominantly driven by soil carbon-to-nitrogen stoichiometry and total nitrogen. Glob. Change Biol. 27, 6512–6524 (2021).
Lu, M. et al. Organic nitrogen addition causes decoupling of microbial nitrogen cycles by stimulating gross nitrogen transformation in a temperate forest soil. Geoderma 385, 114886 (2021).
Zhu, T. et al. Fungi-dominant heterotrophic nitrification in a subtropical forest soil of China. J. Soils Sediment. 15, 705–709 (2015).
Grosso, F., Bååth, E. & De Nicola, F. Bacterial and fungal growth on different plant litter in Mediterranean soils: effects of C/N ratio and soil pH. Appl. Soil Ecol. 108, 1–7 (2016).
Zhang, Y., Cai, Z., Zhang, J. & Müller, C. The controlling factors and the role of soil heterotrophic nitrification from a global review. Appl. Soil Ecol. 182, 104698 (2023).
Hodge, A., Robinson, D. & Fitter, A. Are microorganisms more effective than plants at competing for nitrogen?. Trends Plant Sci. 5, 304–308 (2000).
Zhang, J., Müller, C. & Cai, Z. Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils. Soil Biol. Biochem. 84, 199–209 (2015).
Zhu, T. et al. Nitrogen mineralization, immobilization turnover, heterotrophic nitrification, and microbial groups in acid forest soils of subtropical China. Biol. Fertil. Soils 49, 323–331 (2013).
Liu, R., Suter, H., He, J., Hayden, H. & Chen, D. Influence of temperature and moisture on the relative contributions of heterotrophic and autotrophic nitrification to gross nitrification in an acid cropping soil. J. Soils Sediment. 15, 2304–2309 (2015).
Chen, Z. et al. Importance of heterotrophic nitrification and dissimilatory nitrate reduction to ammonium in a cropland soil: evidences from a 15N tracing study to literature synthesis. Soil Biol. Biochem. 91, 65–75 (2015).
Huygens, D. et al. Mechanisms for retention of bioavailable nitrogen in volcanic rainforest soils. Nat. Geosci. 1, 543–548 (2008).
Canfield, D. E., Glazer, A. N. & Falkowski, P. G. The evolution and future of Earth’s nitrogen cycle. Science 330, 192–196 (2010).
Ollivier, J. et al. Nitrogen turnover in soil and global change. Fems Microbiol. Ecol. 78, 3–16 (2011).
Vlaeminck, S. E., Hay, A. G., Maignien, L. & Verstraete, W. In quest of the nitrogen-oxidizing prokaryotes of the early Earth. Environ. Microbiol. 13, 283–295 (2011).
He, M. et al. Understanding the stimulation of microbial oxidation of organic N to nitrate in plant soil systems. Soil Biol. Biochem. 190, 109312 (2024).
He, M. et al. Priming effects of maize growth and photosynthetic substrate supply on soil N mineralization-immobilization turnover. Plant Soil 508, 469–482 (2025).
He, X. et al. Plants with nitrate preference can regulate nitrification to meet their nitrate demand. Soil Biol. Biochem. 165, 108516 (2022).
Lavallee, J. M., Soong, J. L. & Cotrufo, M. F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 26, 261–273 (2020).
Keiluweit, M. et al. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Change 5, 588–595 (2015).
Li, H. et al. Simple plant and microbial exudates destabilize mineral-associated organic matter via multiple pathways. Environ. Sci. Technol. 55, 3389–3398 (2021).
Coskun, D., Britto, D. T., Shi, W. & Kronzucker, H. J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 3, 17074 (2017).
Li, Z. L. et al. Global patterns and controlling factors of soil nitrification rate. Glob. Change Biol. 26, 4147–4157 (2020).
Vázquez, E. et al. Gross nitrogen transformations in tropical pasture soils as affected by Urochloa genotypes differing in biological nitrification inhibition (BNI) capacity. Soil Biol. Biochem. 151, 108058 (2020).
Teutscherová, N. et al. Gross N transformation rates in soil system with contrasting Urochloa genotypes do not confirm the relevance of BNI as previously assessed in vitro. Biol. Fertil. Soils 58, 321–331 (2022).
Sun, L., Lu, Y., Yu, F., Kronzucker, H. J. & Shi, W. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. N. Phytol. 212, 646–656 (2016).
Chen, S. et al. Rice genotype affects nitrification inhibition in the rhizosphere. Plant Soil 481, 35–48 (2022).
Gao, W., Chen, M. & Xu, X. Tracing controls of autotrophic and heterotrophic nitrification in terrestrial soils. Eur. J. Soil Biol. 110, 103409 (2022).
Zhang, J., Sun, W., Zhong, W. & Cai, Z. The substrate is an important factor in controlling the significance of heterotrophic nitrification in acidic forest soils. Soil Biol. Biochem. 76, 143–148 (2014).
Zhang, Y. et al. The effect of C: N ratio on heterotrophic nitrification in acidic soils. Soil Biol. Biochem. 137, 107562 (2019).
Jilling, A. et al. Minerals in the rhizosphere: overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry 139, 103–122 (2018).
Chen, S. et al. Soil nitrogen dynamics regulate differential nitrogen uptake between rice and upland crops. J. Integrative Agric. https://doi.org/10.1016/j.jia.2025.03.014 (2025). In press.
Chen, J. et al. The optimal ammonia-nitrate ratio for various crops: a meta-analysis. Field Crops Res. 307, 109240 (2024).
Chen, S. et al. Enhancing rice nitrogen use efficiency via plant-microbe-soil interactions: Insights from 15N tracing. Appl. Soil Ecol. 207, 105931 (2025).
Vega-Mas, I. et al. Isotopic labelling reveals the efficient adaptation of wheat root TCA cycle flux modes to match carbon demand under ammonium nutrition. Sci. Rep. 9, 8925 (2019).
Feng, H., Fan, X., Miller, A. J. & Xu, G. Plant nitrogen uptake and assimilation: Regulation of cellular pH homeostasis. J. Exp. Bot. 71, 4380–4392 (2020).
Murphy, D. V. et al. Gross nitrogen fluxes in soil: theory, measurement and application of 15N pool dilution techniques. Adv. Agron. 79, 69–118 (2003).
Subbarao, G. V. et al. Suppression of soil nitrification by plants. Plant Sci. 233, 155–164 (2015).
Holz, M. et al. Gross nitrogen dynamics in the mycorrhizosphere of an organic forest soil. Ecosystems 19, 284–295 (2016).
Zhu, B. et al. Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol. Biochem. 76, 183–192 (2014).
Koranda, M. et al. Microbial processes and community composition in the rhizosphere of European beech – The influence of plant C exudates. Soil Biol. Biochem. 43, 551–558 (2011).
Zeller, B., Liu, J., Buchmann, N. & Richter, A. Tree girdling increases soil N mineralisation in two spruce stands. Soil Biol. Biochem. 40, 1155–1166 (2008).
Yin, L., Dijkstra, F. A., Wang, P., Zhu, B. & Cheng, W. Rhizosphere priming effects on soil carbon and nitrogen dynamics among tree species with and without intraspecific competition. N. Phytol. 218, 1036–1048 (2018).
Holz, M., Paterson, E. & Pausch, J. Rhizosphere carbon priming: a plant mechanism to enhance soil nitrogen accessibility?. Plant Soil 488, 175–185 (2023).
Dijkstra, F. A., Bader, N. E., Johnson, D. W. & Cheng, W. Does accelerated soil organic matter decomposition in the presence of plants increase plant N availability?. Soil Biol. Biochem. 41, 1080–1087 (2009).
Murphy, C. J., Baggs, E. M., Morley, N., Wall, D. P. & Paterson, E. Nitrogen availability alters rhizosphere processes mediating soil organic matter mineralisation. Plant Soil 417, 499–510 (2017).
Pulikova, E. P. et al. Heterotrophic nitrification in soils: approaches and mechanisms. Soil Biol. Biochem. 202, 109706 (2025).
Müller, C., Rütting, T., Kattge, J., Laughlin, R. J. & Stevens, R. J. Estimation of parameters in complex 15N tracing models by Monte Carlo sampling. Soil Biol. Biochem. 39, 715–726 (2007).
Inselsbacher, E., Wanek, W., Strauss, J., Zechmeister-Boltenstern, S. & Müller, C. A novel 15N tracer model reveals: plant nitrate uptake governs nitrogen transformation rates in agricultural soils. Soil Biol. Biochem. 57, 301–310 (2013).
Fer, I. et al. Linking big models to big data: efficient ecosystem model calibration through Bayesian model emulation. Biogeosciences 15, 5801–5830 (2018).
Zhang, Y. et al. Heterotrophic nitrification is the predominant NO3− production pathway in acid coniferous forest soil in subtropical China. Biol. Fertil. Soils 49, 955–957 (2013).
Papen, H. & von Berg, R. A. Most Probable Number method (MPN) for the estimation of cell numbers of heterotrophic nitrifying bacteria in soil. Plant Soil 199, 123–130 (1998).
Brinkman, E. P., Van der Putten, W. H., Bakker, E.-J. & Verhoeven, K. J. F. Plant-soil feedback: experimental approaches, statistical analyses and ecological interpretations. J. Ecol. 98, 1063–1073 (2010).
Rosseel, Y. lavaan: an R package for structural equation modeling. J. Stat. Softw. 48, 1–36 (2012).
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (2024YFD1501602) and Hainan Provincial Natural Science Foundation of China (425CXTD606). The study was carried out in close collaboration with the German research unit “Denitrification in Agricultural Soils: Integrated control and Modeling at various scales” (DASIM), funded by the German Research Foundation DFG (FOR2337). The authors also acknowledge the contribution of the International Atomic Energy Agency (IAEA) Vienna under Coordinated Research Project D1.50.16. of Soil and Water Management and Crop Nutrition Section, Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Department of Nuclear Sciences and Applications. The authors are very grateful to Richard Evershed in University of Bristol for providing us with detailed comments that helped to improve the manuscript. The authors appreciate the guidance and assistance of Qiaodong Chi, Xiaoxiang He, Chang Zhao, Xiaoqian Dan, Wenjie Wang, Qinying Zhu, Siwen Du, and Wenxuan Jiang in the 15N tracing experiments.
Author information
Authors and Affiliations
Contributions
The study and experiments were designed by Jinbo Zhang and Zucong Cai. The 15N tracing model was developed by Christoph Müller and Jinbo Zhang. The 15N tracing experiments and microbial experiments were mainly carried out by Shending Chen and Mengqiu He, with the help of Jun Zhao and Xinqi Huang. Data analysis was performed by Shending Chen and Ahmed S. Elrys. The initial manuscript was written by Shending Chen and Zucong Cai. Christoph Müller, Lei Meng, Jun Zhao, and Xinqi Huang provided comments and helped edit the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Peer review
Peer review information
Communications Earth and Environment thanks Wenlong Gao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: D’Arcy Meyer-Dombard, Somaparna Ghosh [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, S., Cai, Z., He, M. et al. Heterotrophic nitrification of organic nitrogen is stimulated by agricultural plants. Commun Earth Environ 6, 612 (2025). https://doi.org/10.1038/s43247-025-02617-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-025-02617-2